Significance
Polyglutamine expansion within the N-terminal region of huntingtin encoded by exon 1 (httex1) results in accumulation of httex1 aggregates in neurons, leading to Huntington’s disease. Profilin is a ubiquitous intracellular protein that reduces aggregation and toxicity of httex1. Prenucleation, transient oligomerization of the httex1 N-terminal amphiphilic domain proceeds along two branches: an on-pathway, “productive” branch resulting in a helical coiled-coil tetramer that supports nucleation of the polyglutamine tract and subsequent aggregation, and an off-pathway “nonproductive” branch that does not proceed beyond a partially helical dimer. Using NMR, we show that profilin binding to the proline-rich domain of httex1 blocks the on-pathway oligomerization pathway while leaving the off-pathway branch unaffected, thereby providing a mechanistic basis for profilin inhibition of httex1 aggregation.
Keywords: relaxation-based NMR, short-lived excited states, oligomerization, binding kinetics, negative cooperativity
Abstract
Human profilin I reduces aggregation and concomitant toxicity of the polyglutamine-containing N-terminal region of the huntingtin protein encoded by exon 1 (httex1) and responsible for Huntington’s disease. Here, we investigate the interaction of profilin with httex1 using NMR techniques designed to quantitatively analyze the kinetics and equilibria of chemical exchange at atomic resolution, including relaxation dispersion, exchange-induced shifts, and lifetime line broadening. We first show that the presence of two polyproline tracts in httex1, absent from a shorter huntingtin variant studied previously, modulates the kinetics of the transient branched oligomerization pathway that precedes nucleation, resulting in an increase in the populations of the on-pathway helical coiled-coil dimeric and tetrameric species (τex ≤ 50 to 70 μs), while leaving the population of the off-pathway (nonproductive) dimeric species largely unaffected (τex ∼750 μs). Next, we show that the affinity of a single molecule of profilin to the polyproline tracts is in the micromolar range (Kdiss ∼ 17 and ∼ 31 μM), but binding of a second molecule of profilin is negatively cooperative, with the affinity reduced ∼11-fold. The lifetime of a 1:1 complex of httex1 with profilin, determined using a shorter huntingtin variant containing only a single polyproline tract, is shown to be on the submillisecond timescale (τex ∼ 600 μs and Kdiss ∼ 50 μM). Finally, we demonstrate that, in stable profilin–httex1 complexes, the productive oligomerization pathway, leading to the formation of helical coiled-coil httex1 tetramers, is completely abolished, and only the pathway resulting in “nonproductive” dimers remains active, thereby providing a mechanistic basis for how profilin reduces aggregation and toxicity of httex1.
Huntington’s disease is a fatal, autosomal-dominant, neurodegenerative condition arising from expansion of the polyglutamine (polyQ) tract beyond 35 repeats within exon 1 of the huntingtin (HTT) gene (1, 2). Proteolysis (3) or incomplete mRNA splicing of the HTT gene (4) results in pathogenic mutated N-terminal fragments encoded by exon 1 (5) that self-associate into polymorphic aggregates of oligomers and fibers (6, 7) to form neuronal inclusion bodies (8). The polypeptide encoded by exon 1, httex1, comprises three domains: a 16-residue N-terminal amphiphilic sequence (NT domain), a polyQ tract of variable length, and a proline-rich domain (PRD) comprising two polyproline repeats (P11 and P10) separated by a 17-residue linker.
From a structural perspective, httex1 assembles into fibrils consisting of a polyQ β-hairpin core surrounded by the NT and PRD domains that retain a significant degree of inherent mobility (9–12). The kinetics of aggregation is modulated by the regions on either side of the polyQ tract: The NT domain promotes fibril formation, while the PRD reduces aggregation propensity (9, 11, 13–15). Additional factors affecting aggregation include interaction of the NT domain with lipid membranes (16), posttranslational modifications (17–19), and binding to various ligands (20, 21). In this regard, human profilin I, a ubiquitous eukaryotic protein that binds actin, poly-l-proline, and phosphatidylinositol 4,5-biphosphate (22), has been shown to significantly reduce aggregation and toxicity of httex1 (23, 24) by binding to the PRD (25).
Recently, we investigated the transient (submillisecond) prenucleation events involved in the early stages of oligomerization of a minimalistic construct of httex1 (httNTQ7) comprising the NT and polyQ domains but lacking the PRD (26). Using relaxation-based NMR measurements, we were able to show that oligomerization of the N-terminal domain of httNTQ7 involves a branched pathway: one (on-pathway) leading to a “productive” helical coiled-coil dimer that further self-associates into a tetramer (comprising a dimer of dimers), and the other (off-pathway) leading to a “nonproductive,” partially helical dimer (or ensemble of dimers) that does not undergo further oligomerization. Here, using NMR, we investigate the effects of human profilin I on the oligomerization of the full-length exon 1 Huntingtin protein, httex1. We first show that the branched oligomerization pathway is preserved in the presence of the PRD. Next, we show that profilin binds specifically to the two polyproline tracts (P11 and P10) within the PRD, and quantitatively characterize the binding equilibria involved, as well as the lifetime of a 1:1 complex. Finally, we show that binding of profilin completely abrogates the productive oligomerization pathway that leads to the helical coiled-coil tetramer, while leaving the nonproductive pathway, which does not extend beyond a dimer, unaffected. These results provide a molecular basis for the mechanism whereby profilin binding reduces the aggregation propensity and toxicity of httex1.
Results and Discussion
Impact of the PRD Domain on the Kinetics of Transient httex1 Oligomerization.
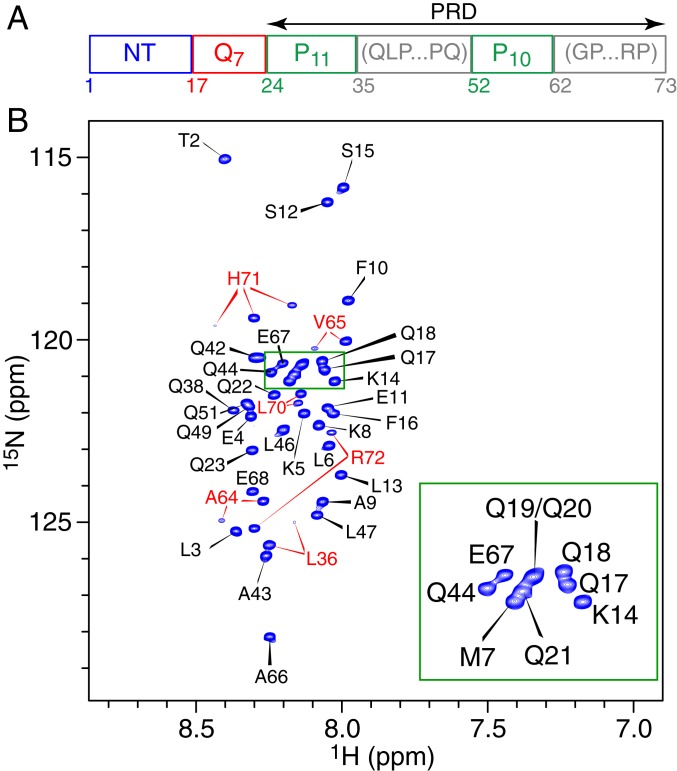
The domain architecture of the 73-residue httex1 construct used in the present work is shown in Fig. 1A. The PRD domain comprises two polyproline tracts, P11 and P10, separated by a 17-residue linker and followed by a 12-residue C-terminal sequence. In the current work, we have chosen to keep the length of the polyQ tract at seven glutamines to both facilitate comparison with our earlier work on httNTQ7 (26), as well as to ensure that the construct remains largely monomeric and stable during the course of the NMR experiments (several weeks). Sedimentation velocity experiments (SI Appendix, Fig. S1) confirm that the major, directly observable species of httex1 is monomeric (with a single peak at 0.74 S corresponding to an estimated mass of 7.9 kDa). The 1H–15N heteronuclear single quantum coherence (HSQC) spectrum of httex1 exhibits very limited 1H chemical shift dispersion (Fig. 1B) characteristic of an intrinsically disordered polypeptide.
Fig. 1.
Domain organization and NMR characterization of httex1. (A) Schematic representation of the domain architecture of the httex1 construct used in the current study. (B) 1H–15N HSQC spectrum of httex1 (600 MHz; 5 °C). The portion of the spectrum enclosed in the green box is zoomed in the Bottom Right corner of the figure. Cross-peaks are labeled with the assigned residue numbers in black, except for residues labeled in red that display additional cross-peaks as a result of proximity to at least one proline undergoing cis/trans isomerization that is slow on the chemical shift timescale. These residues are located either in the linker connecting the two polyproline tracts (Leu36) or in the region C-terminal to the second polyproline tract (Ala64, Val65, Leu70, His71, and Arg72). The additional cross-peaks are much weaker than the main correlations and constitute less than 5% of the total intensity, in agreement with similar observations on other unfolded proteins (37). Three correlations are observed for His71, which is proximal to two proline residues at positions i − 2 and i + 2. Almost complete (97%) chemical shift assignments of 15N, 13Cα, 13C′, and 13Cβ nuclei of all nonproline residues of 15N/13C-labeled httex1 were obtained using standard three-dimensional triple-resonance NMR experiments (SI Appendix, Materials and Methods).
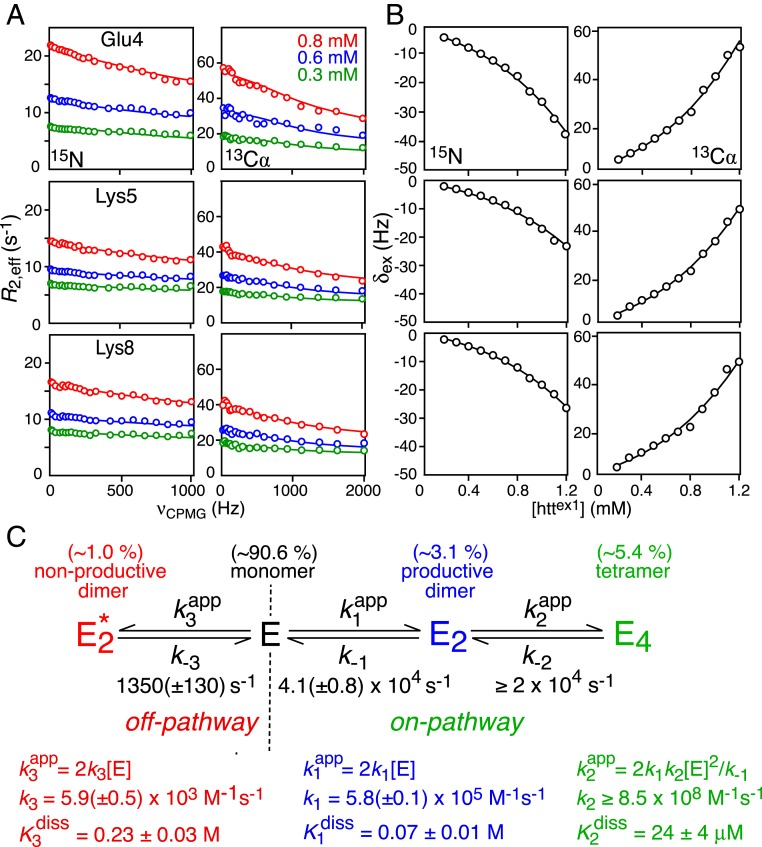
Quantitative characterization of the transient prenucleation oligomerization events involving the submillisecond interconversion between monomeric and sparsely populated multimeric species of httex1 was probed using the same experimental approach and data analysis employed previously for httNTQ7 (26). The experimental data comprised 15N and 13Cα Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersions (27, 28) at three concentrations (0.3, 0.6, and 0.8 mM), and 15N and 13Cα exchange-induced shifts (δex) (29) at 12 concentrations ranging from 50 μM to 1.2 mM (Fig. 2 A and B and SI Appendix, Fig. S2). The NMR data were analyzed simultaneously within the framework of the branched kinetic scheme shown in Fig. 2C, which features on-pathway (productive) and off-pathway (nonproductive) self-association branches. The major observable species is the monomeric state E. The on-pathway pathway leads to the formation of an excited state tetramer E4 via the productive dimer E2. (Note that direct conversion of monomer to tetramer is not only physically unrealistic but leads to a steeper concentration dependence of δex.) In the case of the off-pathway branch, the resulting dimer represents an “end state” that does not undergo further oligomerization. Details of the kinetic model, and data fitting procedures, as well as the assumptions and approximations used in the data analysis, are provided in SI Appendix.
Fig. 2.
Quantitative analysis of transient oligomerization of httex1. Examples of (A) 15N (Left) and 13Cα (Right) CPMG relaxation dispersion profiles at three concentrations (0.3, 0.6, and 0.8 mM); and (B) 15N (Left) and 13Cα (Right) exchange-induced shifts (δex) over concentrations ranging from 200 μM to 1.2 mM (referenced relative to the shifts at 50 μM httex1). Experimental data, recorded at 900 MHz and 5 °C, are displayed as circles, and the best-fit curves obtained from a global fit to the kinetic scheme in C are shown as solid lines. (C) Minimal kinetic model for prenucleation transient oligomerization of httex1 that accounts for all of the experimental NMR data. The kinetic scheme comprises two branches: an on-pathway branch that leads to a helical coiled-coil tetramer (E4) via a “productive” coiled-coil helical dimer, E2; and an off-pathway branch that terminates in a “nonproductive” partially helical dimer, . The populations of the various species at [httex1] = 1.2 mM, the highest concentration used in the NMR experiments, are provided above each state. The complete set of data used in the global fit is provided in SI Appendix, Fig. S2. For errors of 0.3 Hz and 0.6 s−1 for δex and R2,eff, respectively, the reduced χ2 is 2.3.
The 13Cα and 15N chemical shift differences (SI Appendix, Table S1) between the monomer and the on-pathway dimer and tetramer (which are assumed to be the same) are fully consistent with the formation of a helical coiled-coil comprising residues 3 to 16 of the NT domain, while those between the monomer and off-pathway dimer are consistent with the formation of an ensemble of partially helical states of the NT domain, as described previously for httNTQ7 (26). The overall interconversion between E and E4 is fast on the chemical shift timescale (τex ≤ 50 to 70 μs; SI Appendix, Fig. S3) and tetramerization is responsible for the curvature of the concentration dependence of the 15N/13Cα-δex data (Fig. 2B). The off-pathway interconversion between E and proceeds on a much slower timescale (τex ∼ 750 μs). Both on- and off-pathway processes contribute to the CPMG relaxation dispersion data, with the contribution from the latter being suppressed at CPMG fields in excess of about 600 Hz; the contribution, however, of off-pathway dimerization to the concentration dependence of 15N/13Cα-δex is negligible (SI Appendix, Fig. S4). No significant CPMG relaxation dispersions or concentration-dependent changes in 15N/13Cα-δex values are observed beyond the polyQ7 tract (SI Appendix, Figs. S5 and S6), indicating that the PRD does not participate directly in the intermolecular interactions that drive the transient, prenucleation oligomerization events.
The rate constants for the on- and off-pathway kinetic steps are broadly comparable (within a factor of ∼2) to those observed for the shorter httNTQ7 construct (26). However, at the highest concentration of 1.2 mM used in both studies, the populations of the on-pathway dimer (E2) and tetramer (E4) are significantly increased (∼1.5- and ∼2.5-fold, respectively) for httex1 relative to httNTQ7, which is reflected in proportionately larger δex values. Furthermore, the overall equilibrium dissociation constant of the tetramer into monomer, given by , is reduced by ∼35% for httex1 (1.7 μM2) relative to httNTQ7 (2.6 μM2). These findings might be explained by the presence of additional transient interactions between the NT and PRD domains and in the case of the tetramer between the PRD domains as well. The population of the off-pathway dimer , however, is the same for the two constructs (∼1% at 1.2 mM).
Binding of Profilin to httex1.
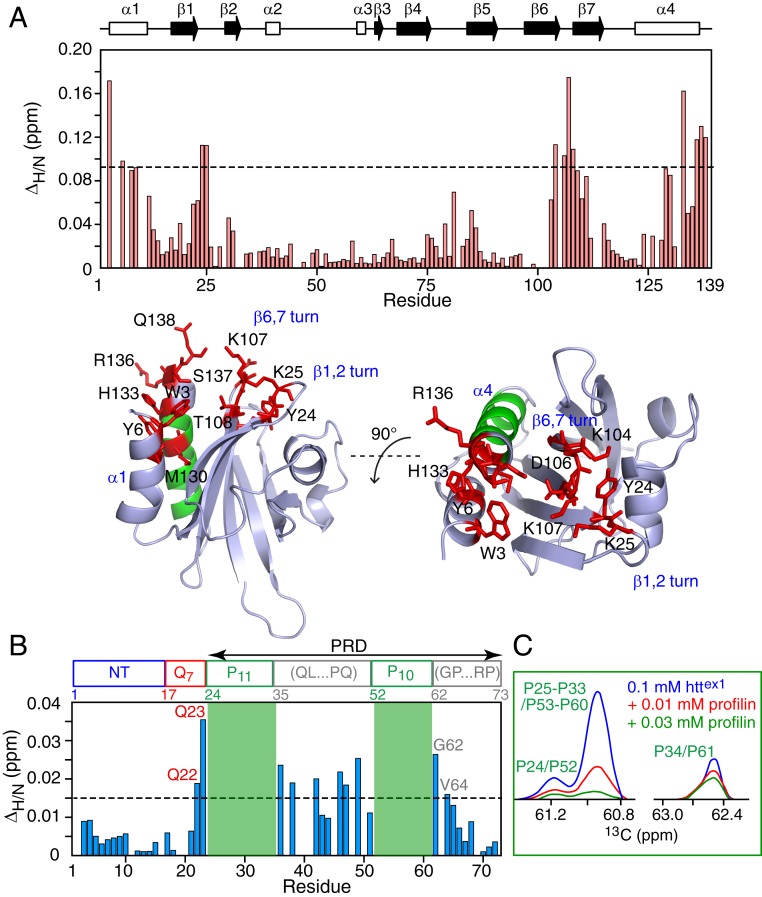
The httex1 binding site on profilin I was delineated by 1HN/15N chemical shift perturbation mapping in which the positions of cross-peaks in a series of 1H–15N HSQC spectra of 15N-labeled profilin were monitored upon titration with unlabeled httex1. A contiguous, predominantly hydrophobic, binding surface, characterized by ΔH/N chemical shift perturbations in excess of 0.09 ppm upon addition of 0.9 mM httex1 to 0.4 mM profilin, is formed by the N-terminal end of helix α1 (residues 3 and 6), the C-terminal end of helix α3 (residues 130, 131, and 133 to 138) and the turns connecting strands β1 and β2 (residues 24 and 25) and strands β5 and β6 (residues 106 to 108) (Fig. 3A). The httex1 binding surface on profilin corresponds to that for poly-l-proline (23, 30, 31), and partially overlaps with the site of profilin self-association (helix α4) (32). However, at the concentration of profilin employed (0.4 mM), the population of dimeric and tetrameric states of profilin is insignificant (32).
Fig. 3.
1HN/15N chemical shift perturbation mapping of the profilin–httex1 binding interface. Weighted 1HN/15N chemical shift perturbation (ΔH/N) profiles measured on (A) 0.4 mM 15N-labeled profilin in the presence of 0.9 mM unlabeled httex1 (Top) and (B) 0.1 mM 15N-labeled httex1 in the presence of 0.8 mM unlabeled profilin. (As the backbone nitrogen of proline is not bonded to a proton, the two polyproline tracts, shaded in green, are not detectable in 1H–15N HSQC correlation maps.) The data were obtained from 1H–15N HSQC spectra recorded at 600 MHz and 5 °C. ΔH/N is the weighted chemical shift difference (38) in parts per million given by (ΔδH2 + ΔδN2/25)1/2, where ΔδH and ΔδN are the 1HN and 15N chemical shift differences in parts per million observed between the 1H–15N HSQC spectra of the 15N-labeled binding partner in the absence and presence of the unlabeled binding partner. A ribbon representation of the structure of human profilin I [PBD code: 1PFL (39)] is shown in A (Bottom), with the regions involved in httex1 binding (ΔΗ/Ν > 0.09 ppm) and oligomerization (α-helix α4) shown in red and green, respectively. (C) Cross-section through the 13C indirect dimension of a 1H–13C HSQC spectrum of 0.1 mM 15N/13C-labeled httex1 in the presence of varying amounts of unlabeled profilin (0, 0.01, and 0.03 mM profilin in blue, red, and green, respectively) illustrating line broadening of the 1Hα/13Cα proline cross-peaks within the two polyproline tracts upon addition of profilin. Note that the 1Hα/13Cα cross-peaks of the N- and C-terminal prolines are distinct from the other prolines, whose 1Hα/13Cα cross-peaks are completely overlapped (including the 1Hα/13Cα proline cross-peaks of the two polyproline tracts).
1HN/15N chemical shift perturbation mapping of 15N-labeled httex1 upon titration with unlabeled profilin reveals chemical shift perturbations for residues immediately adjacent to the N- and C-terminal ends of both polyproline tracts as well as residues between the two polyproline tracts (Fig. 3B). Furthermore, the 1H–13Cα cross-peaks for the two polyproline tracts are broadened out very early on during the course of the titration (Fig. 3C). Thus, one can conclude that profilin binds to the P11 and P10 polyproline repeats.
Quantitative Analysis of Profilin–httex1 Binding Equilibria.
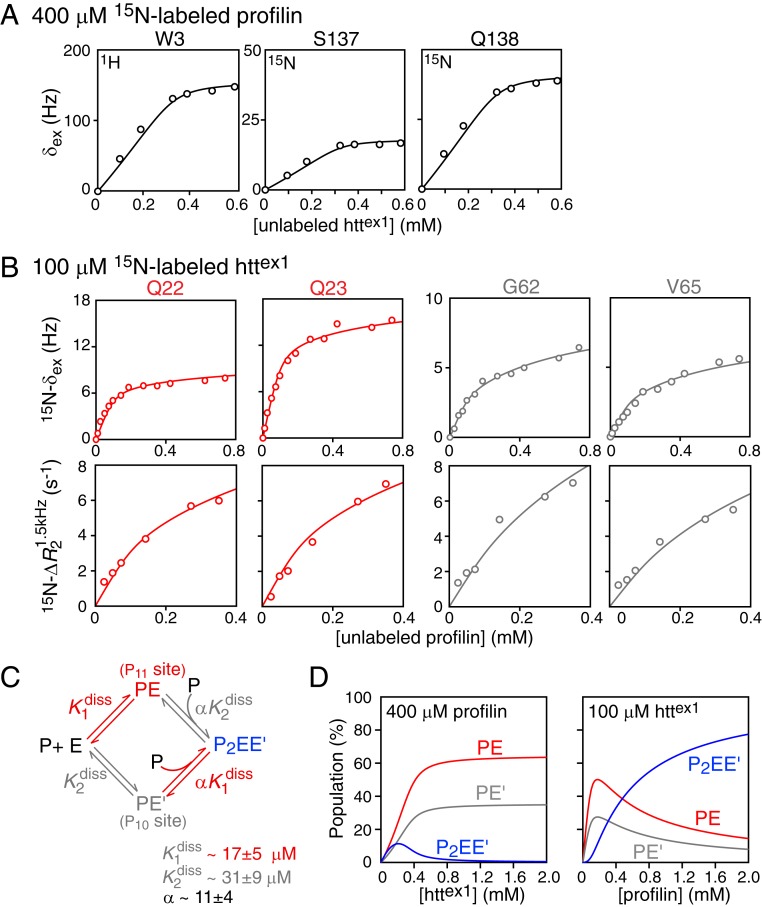
To obtain a quantitative description of the equilibria involving the binding of httex1 to profilin, we globally fit 15N exchange-induced shift (15N-δex) data for 0.4 mM 15N-labeled profilin upon titration with unlabeled httex1 (Fig. 4A), and 15N-δex and 15N lifetime line broadening data for 0.1 mM 15N-labeled httex1 upon titration with unlabeled profilin (Fig. 4B) to the minimalistic binding scheme depicted in Fig. 4C (see SI Appendix for details of the global fitting procedure). Under these experimental conditions, the populations of oligomeric httex1 species (Fig. 2) are sufficiently low to be neglected. Initially, a single molecule of profilin (P) binds to either one of the two polyproline tracts of httex1 (E) to form two possible singly occupied complexes, PE (profilin-P11) and PE′ (profilin-P10), characterized by equilibrium dissociation constants, and , respectively; binding of a second molecule of profilin to the previously unoccupied polyproline tract then results in a doubly occupied complex, P2EE′, characterized by two equilibrium dissociation constants, and = , where α is a cooperativity factor. The titration data for 15N-labeled profilin report on all binding events since profilin contains only a single polyproline binding site (23), and the two polyproline tracts of httex1 differ by only a single proline in length. In the case of httex1, however, binding of profilin to the two polyproline tracts can be monitored independently by making use of data from residues immediately preceding P11 (Gln22/Gln23) or following P10 (Gly62/Val65).
Fig. 4.
Quantitative characterization of the binding equilibria involved in the interaction of profilin with httex1. (A) Exchange-induced shifts for selected residues of 0.4 mM 15N-labeled profilin upon titration with unlabeled httex1. (B) Exchange-induced shifts, δex (Top row) and lifetime line broadening (Bottom row) for Gln22 and Gln23 (red; monitoring binding to P11) and for Gly62 and Val65 (gray; monitoring binding to P10) of 0.1 mM 15N-labeled httex1 upon titration with unlabeled profilin. All experimental data were recorded at 600 MHz and 5 °C. (C) Scheme for the binding of profilin to the two polyproline tracts, P11 and P10, of httex1. The experimental data in A and B are shown by circles, and the best-fit curves from the global fit to the binding scheme shown in C are represented by solid lines. (D) Simulation of species’ populations during the course of the titrations using the best-fit parameters for the equilibrium dissociation constants and cooperativity factor. For errors of 0.3 Hz and 0.6 s−1 for the δex and R2 data, the value of the reduced χ2 is 0.81.
For all of the data used in the analysis of profilin–httex1 binding equilibria, exchange was assumed to be fast on the chemical shift timescale. For 15N-labeled profilin, δex,i for residue i as a function of unlabeled httex concentration (chtt) is given by δex,i(chtt) = Δωi(pPE + pPE′ + pP2EE′), where pj is the fractional population of each of the complexes, and the chemical shift differences between free and bound profilin (Δωi) are assumed to be the same for all complexes. For 15N-labeled httex1, δex,i as a function of unlabeled profilin concentration (cprof) is expressed by two separate relationships: δex,i(cprof) = Δωi(pPE + pP2EE′) for Gln22/Gln23 preceding the P11 tract and δex(cprof) = Δωi(pPE′ + pP2EE′) for Gly62/Val65 following the P10 tract, respectively. In both instances, the chemical shifts of the doubly occupied species, P2EE′, are assumed to be the same as those of singly occupied ones (ΔωP2EE′,i = ΔωPE,i or ΔωP2EE′,i = ΔωPE′,i). The increase in the 15N transverse relaxation rate, , where a 1.5-kHz spin-lock field is used to suppress line broadening arising from chemical exchange, arises from the substantial increase in molecular weight of the singly and doubly occupied complexes relative to free httex1. In the fast exchange approximation, as a function of profilin concentration is given by for Gln22/Gln23 and by for Gly62/Val65, where , , , and are the transverse relaxation rates for residue i of free httex1, the two singly occupied PE and PE′ complexes, and the doubly occupied P2EE′ complex, respectively (see SI Appendix, Fig. S7, for validation of the fast exchange approximation in this instance). and are assumed to be equal, and is scaled by a factor of 1.65 relative to according to the ratio of the molecular weights of the doubly (∼38 kDa) and singly (∼23 kDa) occupied complexes. Further details of the fitting procedure are provided in SI Appendix, and the fitted values of the residue-specific parameters are given in SI Appendix, Table S2.
The equilibrium dissociation constants for the binding of profilin to the P11 and P10 polyproline tracts, and , obtained from the global fit, have values of 17 ± 5 and 31 ± 9 μM, respectively. The binding of the second profilin molecule to either of the P11 or P10 tracts (formation of the P2EE′ complex) is negatively cooperative with α = 11 ± 4. Since the linker connecting the P11 and P10 polyproline tracts is not excessively long (17 residues), negative cooperativity may possibly be attributed to partial occlusion of the second polyproline tract once the first polyproline tract is occupied by profilin.
The dependence of the fractional populations of the PE, PE′, and P2EE′ complexes on the total concentrations of profilin and httex1, calculated using the experimental concentrations and the binding parameters obtained from the global fit, are shown in Fig. 4D. When 0.4 mM 15N-labeled profilin is titrated with unlabeled httex1, the population of the doubly occupied P2EE′ complex reaches a maximum at ∼0.2 mM httex1, and at the end of the concentration series (0.6 mM httex1), P2EE′ is minimally populated and the predominant bound species are the two singly occupied complexes, PE and PE′ (Fig. 4 D, Left). Given the relatively tight binding ( in the 15 to 30 μM range) of the first molecule of profilin to httex1, these simulations explain why saturation is achieved close to 0.4 mM added httex1 (Fig. 4A). For the reverse titration, where unlabeled profilin is added to 0.1 mM 15N-labeled httex1, all three bound species are significantly populated (Fig. 4 D, Right) at the highest concentration (0.4 mM) of profilin employed in the experiments.
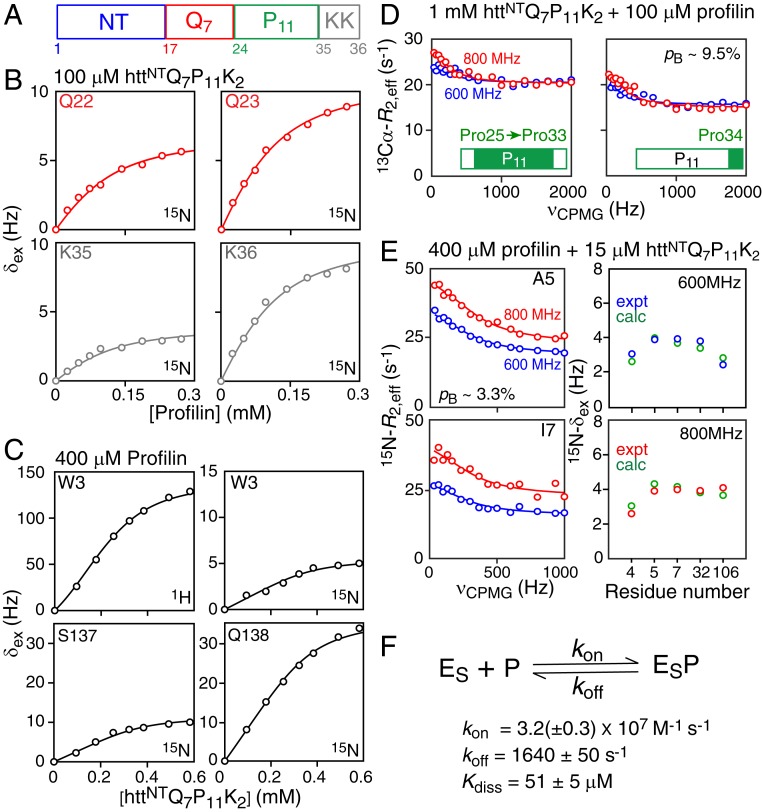
Kinetics of Profilin Binding to httNTQ7P11K2.
To probe the kinetics of the interaction of profilin binding to the polyproline tracts of httex1, we made use of a shorter construct, httNTQ7P11K2 (Fig. 5A, and SI Appendix, Fig. S8), comprising only the first polyproline tract, P11, followed by two lysines, as it would be problematic to determine the rate constants for the four-state binding scheme involving full-length httex1. Global analysis was based on exchange-induced shifts observed on 15N-labeled httNTQ7P11K2 upon titration with unlabeled profilin (Fig. 5B), exchange-induced shifts observed on 15N/13C-labeled profilin upon titration with unlabeled httNTQ7P11K2 (Fig. 5C), 13Cα-CPMG relaxation dispersion profiles for the prolines of 15N/13Cα-labeled httNTQ7P11K2 in the presence of a small amount of unlabeled profilin (Fig. 5D), and 15N-CPMG relaxation dispersion profiles and exchange-induced shifts for 15N/13C-labeled profilin in the presence of a small amount of unlabeled httNTQ7P11K2 (Fig. 5E). Of note, the residues of httNTQ7P11K2 and profilin that exhibit exchange-induced shifts shown in Fig. 5 B and C, respectively, do not display any 15N-CPMG relaxation dispersions (SI Appendix, Fig. S9 A and B); 13Cα-CPMG dispersion profiles, however, are observed for the polyproline tract of httex1 (Fig. 5D), and several residues of profilin also show 15N-CPMG dispersions (Fig. 5E and SI Appendix, Fig. S9C). These exchange conditions benefit from combined analysis of relaxation dispersion and exchange-induced shift data (29). Details of the fitting procedure are provided in SI Appendix, and the best-fit values of the residue-specific parameters are listed in SI Appendix, Table S3.
Fig. 5.
Binding kinetics of the profilin–httNTQ7P11K2 interaction. (A) Schematic representation of the httNTQ7P11K2 domain architecture. (B) Exchange-induced 15N shifts (δex) at 600 MHz for residues immediately N-terminal (Gln22/Gln23, red) and C-terminal (Lys35/Lys36, gray) to the P11 polyproline tract observed for 0.1 mM 15N/13Cα-labeled httNTQ7P11K2 upon titration with unlabeled profilin. (C) Exchange-induced 1HN and 15N shifts at 500 MHz observed for 0.4 mM 15N/13C-labeled profilin upon titration with httNTQ7P11K2. (D) 13Cα-CPMG relaxation dispersion profiles of the spectrally overlapped prolines (Pro25–Pro33) (Left) and the C-terminal proline (Pro34) (Right) of the P11 polyproline tract measured on 1 mM 15N/13Cα-labeled httNTQ7P11K2 in the presence of 0.1 mM unlabeled profilin at 600 (red) and 800 (blue) MHz. (E) 15N-CPMG relaxation dispersion profiles (Left) and 15N-δex shifts (Right) measured on 0.4 mM 15N/3C-labeled profilin in the presence of 15 μM httNTQ7P11K2. All experimental data were recorded at 5 °C. (F) Kinetic scheme for the binding of profilin (P) to httNTQ7P11K2 (ES). The experimental data in B–D are shown as circles, and the best-fit curves to the kinetic scheme are shown as solid lines, with the exception of the best-fit 15N-δex values shown in D, which are shown as green circles. The population of the bound complex (pB) for the observed species is indicated in D and E. For errors of 0.3 Hz, 2 Hz, and 0.6 s−1 for 15N-δex, 1HN-δex, and 15N/13Cα R2,eff, respectively, the value of the reduced χ2 is 2.56.
The lifetime of the profilin–httNTQ7P11K2 complex under the conditions of the CPMG relaxation dispersion experiments is ∼600 μs with a Kdiss value of ∼51 μM. The association rate constant, kon, is ∼3 × 107 M−1⋅s−1, consistent with a diffusion-limited reaction. The threefold weaker binding to the P11 polyproline tract of httNTQ7P11K2 relative to that of httex1 may possibly be due to end effects: namely, the absence of the linker as well as the second P10 polyproline tract in httNTQ7P11K2. The values of 15N-Δω obtained from the global fits for the residues of httNTQ7P11K2 and profilin that show 15N exchange-induced shifts, but no 15N-CPMG relaxation dispersions, are <0.6 ppm, while those residues of profilin that show 15N-CPMG relaxation dispersions are in the range 1.3 to 2.8 ppm (SI Appendix, Table S3), explaining why the exchange process occurs in different regimes on the chemical shift timescale for these sites. The values of |13Cα-Δω| for Pro25–Pro33 and Pro34, obtained from the 13Cα-CPMG relaxation dispersion data, are ∼0.3 ppm, and inspection of the corresponding cross-peaks in the 1H–13Cα HSQC spectra of 1 mM httNTQ7P11K2, in the absence and presence of 100 μM profilin (SI Appendix, Fig. S10), indicates that the exchange-induced shifts for the proline 1Hα and 13Cα nuclei are negative in sign, characteristic of a propensity toward PPII helix formation (33). In addition, the very weak field dependence of the observed 15N-δex shifts for profilin in the presence of httNTQ7P11K2 (right column in Fig. 5E) is fully consistent with the results of our analysis as explained in detail in SI Appendix, Fig. S11.
Extrapolation of the results of the kinetic study of httNTQ7P11K2–profilin binding to full-length httex1–profilin interactions, allows us to conclude that the interconversion between the free and bound species occurs on a moderately fast timescale (τex ∼ 600 μs with kex ≥ 4ΔωN for the majority of httex1 or profilin sites), thus validating the approach used for analysis of httex1–profilin binding equilibria.
On-Pathway Transient Oligomerization of httex1 Is Inhibited by Binding of Profilin.
Thioflavin T assays on httex1 constructs containing up to a 40-residue polyglutamine tract have shown that addition of profilin reduces the rate of httex1 fibrillation (25). We hypothesized that binding of profilin to the polyproline tracts of httex1 also modulates prenucleation, transient oligomerization of the NT domain.
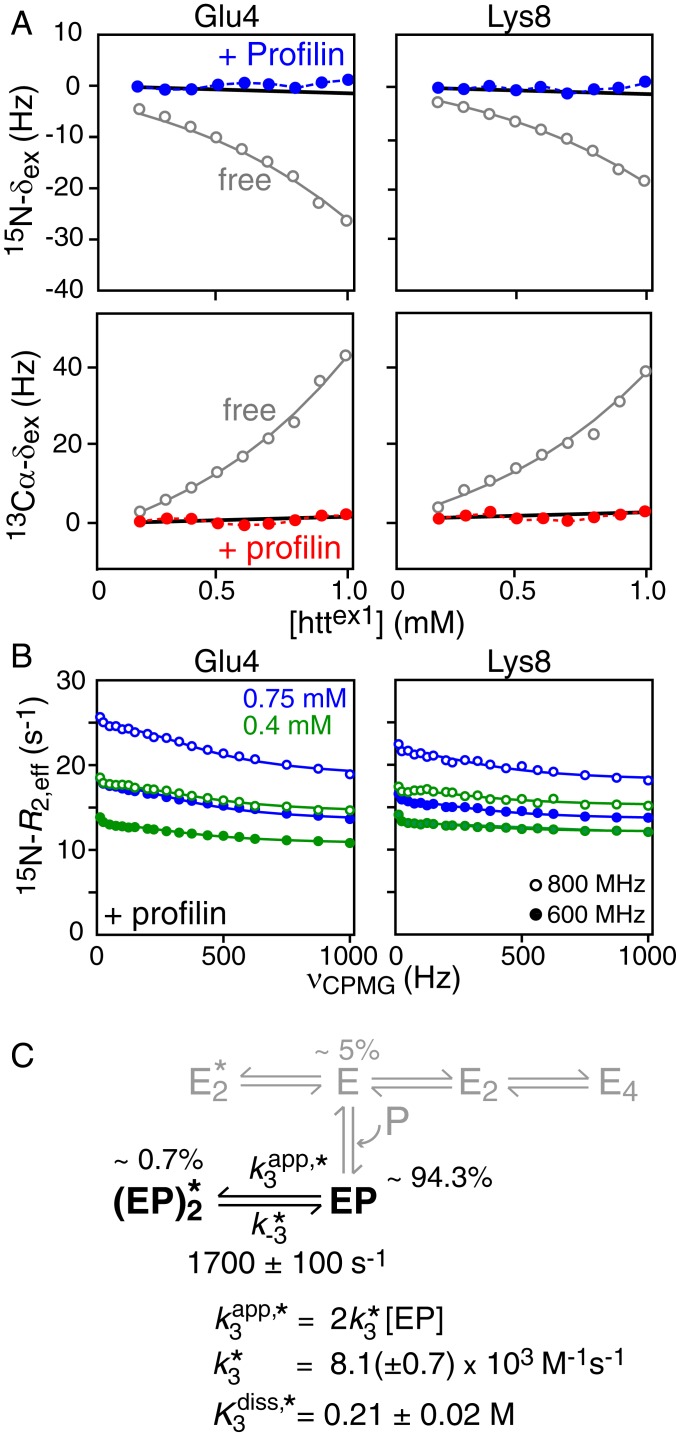
To test the above hypothesis we examined the concentration dependence of 15N and 13Cα exchange-induced shifts (from 50 μM to 1 mM) and 15N-CPMG relaxation dispersions (at 0.4 and 0.75 mM) for httex1 in the presence of a fixed (4.8 mM) concentration of profilin, ensuring close to complete (∼95%) saturation of httex1 with profilin. The large exchange-induced shifts seen in the absence of profilin are completely abolished in the presence of profilin (Fig. 6A). These data indicate that the on-pathway leading to the formation of a tetramer via a productive dimer is completely inhibited. 15N-CPMG relaxation dispersion, however, for residues within the NT domain (which does not bind profilin) are still observed (Fig. 6B and SI Appendix, Fig. S12) and arise exclusively from off-pathway dimerization.
Fig. 6.
Effect of profilin on prenucleation, transient oligomerization of the NT domain httex1. (A) Concentration dependence of 15N (Top, blue circles) and 13Cα (Bottom, red circles) exchange-induced shifts of 15N/13Cα-labeled httex1 in the presence of 4.8 mM profilin (referenced relative to the shifts at 50 μM httex1). The 15N- and 13Cα-δex values obtained for httex1 in the absence of profilin are reproduced in gray from Fig. 2B for comparison. All measurements were performed at 800 MHz and 5 °C. (B) Examples of 15N-CPMG relaxation dispersion profiles acquired at 600 (filled-in circles) and 800 (open circles) MHz and 5 °C on 0.4 (green) and 0.75 (blue) mM 15N-labeled httex1 in the presence of 4.8 mM profilin. (C) Overall kinetic scheme for the oligomerization of httex1 bound to profilin (EP). (Note the singly and doubly bound species denoted as PE, PE′, and P2EE′ in Fig. 2 are combined into a single species EP for the purpose of this analysis.) Only the off-pathway leading to the “nonproductive” dimer remains active when profilin is bound. The population of free httex1 in the presence of 4.5 mM profilin is only 5%, and hence, the oligomeric species of free httex1 shown in gray, whose populations are <0.05%, do not make any measurable contribution to either the 15N-CPMG relaxation dispersion profiles or the 15N/13Cα exchange-induced shifts. Furthermore, the residues analyzed, all of which are located in the NT domain, do not show changes in chemical shifts upon binding to profilin, and hence the binding of profilin to free httex1 does not contribute to the CPMG relaxation dispersions either. The species populations listed in the figure correspond to those at the highest concentration of httex1 used in 15N-CPMG relaxation dispersion experiments (0.75 mM). The solid lines in B are the best-fit curves (reduced χ2 = 0.77 for errors of 0.3 s−1 for R2,eff) obtained from global fitting of the 15N-CPMG relaxation dispersion data to the two-state exchange system depicted in black in C. The thick black solid lines in A are the backcalculated 15N (Top) and 13Cα (Bottom) exchange-induced shifts for 15N and 13Cα Δω values of 2 and 3 ppm, respectively. The 15N-Δω values were taken from the fits to the 15N-CPMG relaxation dispersion data (SI Appendix, Table S4); in the case of 13Cα-Δω, 3 ppm is comparable to the largest 13Cα-Δω value observed for free httex1 (SI Appendix, Table S1).
The impact of profilin on the kinetic scheme for httex1 oligomerization is summarized in Fig. 6C. In terms of analysis, the only exchange process that contributes to the 15N-CPMG relaxation dispersions is the one between profilin-bound httex1 monomer EP and off-pathway dimer. This is because the residues analyzed are located within the NT domain and do not show changes in chemical shifts upon binding to profilin; hence exchange between free and profilin-bound httex1 monomer (shown in gray in Fig. 6C) does not generate 15N-CPMG dispersions. Furthermore, since httex1 is ∼95% saturated with profilin, the populations of the free oligomeric species (shown in gray in Fig. 6C) will be too low (<0.05%) to make any measurable contribution to either the 15N-CPMG relaxation dispersion profiles or the 15N/13Cα exchange-induced shifts.
Best fitting of the 15N-CPMG relaxation dispersion data (see SI Appendix for details) reveals that the overall interconversion rate (τex ∼ 600 μs) between the profilin-bound httex1 monomer, EP, and the profilin-bound off-pathway dimer, , is comparable to that in the absence of profilin (τex ∼ 750 μs; Fig. 2C). The equilibrium dissociation constant ( ∼ 0.2 M) for the off-pathway dimer is also largely unaffected by binding of profilin. The backcalculated 15N- and 13Cα exchange-induced shifts (thick black lines in Fig. 6A) expected for Δω values of 2 and 3 ppm, respectively, at 800 MHz are too small to be experimentally measurable over the 50 μM to 1 mM concentration range studied. The |15N-Δω| values for are ∼2 ppm (SI Appendix, Table S4), about twofold larger than the corresponding values in the absence of profilin (SI Appendix, Table S1), possibly suggesting that the ensemble of partially helical states for the off-pathway dimer may be somewhat more ordered in the presence of profilin.
Concluding Remarks.
We have investigated the impact of human profilin I on the prenucleation, transient oligomerization events involving the full-length exon 1 huntingtin protein, httex1, comprising the N-terminal oligomerization domain (NT), a short seven-residue polyglutamine tract, and a polyproline rich domain (PRD) containing two polyproline tracts. We show that when at least one profilin molecule is bound to the PRD of httex1, the on-pathway (productive) oligomerization pathway, leading to the formation of a transient, helical coiled-coil tetramer of the NT domain, is effectively abolished, and only the off-pathway leading to a nonproductive, partially helical NT dimer (that does not oligomerize further) is preserved (Fig. 6C). This result provides a clear mechanism whereby binding of profilin to the PRD inhibits fibrillation and subsequent aggregation and toxicity of httex1 (23–25). Furthermore, the fact that the on-pathway for early-stage oligomerization of httex1 is eliminated by profilin, validates the branched oligomerization scheme first proposed for the shorter httNTQ7 construct (26). Indeed, it is difficult to conceive a mechanism whereby the absence of an off-pathway leading to a nonproductive dimer of httex1 would be possible if only the latter is retained in the profilin–httex1 complex.
The above seemingly simple result required nonetheless a considerable amount of auxiliary investigations aimed at a quantitative description of transient oligomerization of the free (unliganded) full-length httex1, as well as the binding equilibria and kinetics of httex1–profilin interactions. First, using a combination of the state-of-the-art NMR techniques for the characterization of chemical exchange and binding equilibria, including CPMG relaxation dispersion, exchange-induced chemical shifts, and lifetime line broadening, we quantitatively characterized the impact of the C-terminal PRD domain, absent from the shorter huntingtin variant httNTQ7 studied earlier (26), on the prenucleation transient, oligomerization events involving free httex1. We found that, although the equilibrium dissociation constant of httex1 tetramers into on-pathway (productive) dimers (Fig. 2C) is preserved between the shorter and full-length huntingtin exon 1 variants, the presence of the PRD in full-length httex1 somewhat stabilizes both on- and off-pathway dimeric species relative to the monomer. Second, we quantitatively described the equilibria involved in the binding of profilin to the two distinct polyproline tracts, P11 and P10, within the PRD using a four-state binding model. The equilibrium dissociation constants for the binding of the first molecule of profilin are in the 15 to 30 μM range, but binding of a second profilin molecule is ∼11-fold weaker, indicative of negative cooperativity, possibly due to partial steric hindrance between spatially close profilin molecules. In this regard, we note that the intracellular concentration of profilin ranges from 10 to 40 μM (34), while that for huntingtin within whole brain is around 0.15 μM (35) and the estimated concentration of soluble httex1 fragments within neuronal inclusion bodies is ∼10 μM (15). Hence, significant occupancy of profilin bound to httex1 can be achieved in vivo. The kinetics of profilin binding were investigated using a shorter huntingtin construct containing only a single polyproline tract. Exchange between the free proteins and the complex occurs on a moderately fast timescale (τex ∼ 600 μs) relative to the observed chemical shift changes upon binding.
Building upon the quantitative information on the early stages of httex1 oligomerization as well as profilin–httex1 interactions, we were able to unambiguously demonstrate that early-stage on-pathway oligomerization events in the aggregation of httex1 leading to tetramer formation are abrogated by binding of profilin to the polyproline tracts. Since the PRD does not participate directly in the intermolecular interactions that stabilize the on-pathway dimer and tetramer formed by the NT domain (26), how does profilin binding exert its inhibitory effect? A possible explanation may lie in steric hindrance from the relatively large profilin significantly reducing the probability of forming the site-specific contacts required to form the on-pathway helical coil-coiled dimer and tetramer; the off-pathway dimer, however, does not appear to constitute a single structure but an ensemble of conformations with different registers and degrees of overlap (26), and hence the formation of the off-pathway dimer is only minimally impeded by profilin binding.
Experimental Methods
A detailed description of expression, purification, and isotope labeling of httex1 and human profilin I, experimental details of NMR and analytical ultracentrifugation measurements, and details of all global data fitting procedures are provided in SI Appendix.
Data Availability Statement.
All experimental relaxation dispersion, exchange-induced shift, and transverse relaxation data discussed in this paper are provided either in the main text or SI Appendix. The experimental data in digital format, together with MatLab scripts used in global fitting, have been deposited on Figshare (DOI: 10.6084/m9.figshare.11887860). In addition, the backbone chemical shifts for httex1 have been deposited in the Biological Magnetic Resonance Data Bank (36).
Supplementary Material
Acknowledgments
We thank Drs. Enrico Rennella and Lewis E. Kay (University of Toronto) for providing backbone chemical shift assignments of human profilin I. We acknowledge the technical assistance of Drs. Dusty Baber, Dan Garrett, and Jinfa Ying (National Institute of Diabetes and Digestive and Kidney Diseases, NIH). This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (to G.M.C., DK029023-19).
Footnotes
The authors declare no competing interest.
Data deposition: The backbone chemical shifts of httex1 reported in this paper have been deposited in the Biological Magnetic Resonance Data Bank (BMRB), http://www.bmrb.wisc.edu (accession no. 50122). The experimental data in digital format, together with MatLab scripts used in global fitting, have been deposited on Figshare (DOI: 10.6084/m9.figshare.11887860). Note that the backbone assignments are explicitly indicated on the 1H–15N correlation spectrum shown in Fig. 1. There are no atomic coordinates or structural restraints associated with the current submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922264117/-/DCSupplemental.
References
- 1.Andresen J. M., et al. ; US–Venezuela Collaborative Research Group ; HD MAPS Collaborative Research Group , The relationship between CAG repeat length and age of onset differs for Huntington’s disease patients with juvenile onset or adult onset. Ann. Hum. Genet. 71, 295–301 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Bates G. P., et al. , Huntington disease. Nat. Rev. Dis. Primers 1, 15005 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Ratovitski T., et al. , Mutant huntingtin N-terminal fragments of specific size mediate aggregation and toxicity in neuronal cells. J. Biol. Chem. 284, 10855–10867 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neueder A., et al. , The pathogenic exon 1 HTT protein is produced by incomplete splicing in Huntington’s disease patients. Sci. Rep. 7, 1307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro B. A., et al. , Comparative study of naturally occurring huntingtin fragments in Drosophila points to exon 1 as the most pathogenic species in Huntington’s disease. Hum. Mol. Genet. 24, 913–925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duim W. C., Jiang Y., Shen K., Frydman J., Moerner W. E., Super-resolution fluorescence of huntingtin reveals growth of globular species into short fibers and coexistence of distinct aggregates. ACS Chem. Biol. 9, 2767–2778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherzinger E., et al. , Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: Implications for Huntington’s disease pathology. Proc. Natl. Acad. Sci. U.S.A. 96, 4604–4609 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saudou F., Humbert S., The biology of huntingtin. Neuron 89, 910–926 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Hoop C. L., et al. , Polyglutamine amyloid core boundaries and flanking domain dynamics in huntingtin fragment fibrils determined by solid-state nuclear magnetic resonance. Biochemistry 53, 6653–6666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isas J. M., Langen R., Siemer A. B., Solid-state nuclear magnetic resonance on the static and dynamic domains of huntingtin exon-1 fibrils. Biochemistry 54, 3942–3949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoop C. L., et al. , Huntingtin exon 1 fibrils feature an interdigitated β-hairpin-based polyglutamine core. Proc. Natl. Acad. Sci. U.S.A. 113, 1546–1551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H. K., et al. , Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core. Nat. Commun. 8, 15462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur A. K., et al. , Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat. Struct. Mol. Biol. 16, 380–389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crick S. L., Ruff K. M., Garai K., Frieden C., Pappu R. V., Unmasking the roles of N- and C-terminal flanking sequences from exon 1 of huntingtin as modulators of polyglutamine aggregation. Proc. Natl. Acad. Sci. U.S.A. 110, 20075–20080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M., Wolynes P. G., Aggregation landscapes of Huntingtin exon 1 protein fragments and the critical repeat length for the onset of Huntington’s disease. Proc. Natl. Acad. Sci. U.S.A. 114, 4406–4411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceccon A., et al. , Interaction of huntingtin exon-1 peptides with lipid-based micellar nanoparticles probed by solution NMR and Q-band pulsed EPR. J. Am. Chem. Soc. 140, 6199–6202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrnhoefer D. E., Sutton L., Hayden M. R., Small changes, big impact: Posttranslational modifications and function of huntingtin in Huntington disease. Neuroscientist 17, 475–492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cariulo C., et al. , Phosphorylation of huntingtin at residue T3 is decreased in Huntington’s disease and modulates mutant huntingtin protein conformation. Proc. Natl. Acad. Sci. U.S.A. 114, E10809–E10818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccon A., Tugarinov V., Clore G. M., TiO2 nanoparticles catalyze oxidation of huntingtin exon 1-derived peptides impeding aggregation: A quantitative NMR study of binding and kinetics. J. Am. Chem. Soc. 141, 94–97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J. P., Hughes R. E., “Protein interactions and target discovery in Huntington’s disease” in Neurobiology of Huntington’s Disease: Applications to Drug Discovery, Lo D. C., Hughes R. E., Eds. (CRC Press, Boca Raton, FL, 2011), chap. 3. [PubMed] [Google Scholar]
- 21.Bochicchio A., Rossetti G., Tabarrini O., Krauβ S., Carloni P., Molecular view of ligands specificity for CAG repeats in anti-Huntington therapy. J. Chem. Theory Comput. 11, 4911–4922 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Machesky L. M., Poland T. D., Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 3, 381–385 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Mahoney N. M., Rozwarski D. A., Fedorov E., Fedorov A. A., Almo S. C., Profilin binds proline-rich ligands in two distinct amide backbone orientations. Nat. Struct. Biol. 6, 666–671 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Shao J., Welch W. J., Diprospero N. A., Diamond M. I., Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol. Cell. Biol. 28, 5196–5208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posey A. E., et al. , Profilin reduces aggregation and phase separation of huntingtin N-terminal fragments by preferentially binding to soluble monomers and oligomers. J. Biol. Chem. 293, 3734–3746 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotler S. A., et al. , Probing initial transient oligomerization events facilitating Huntingtin fibril nucleation at atomic resolution by relaxation-based NMR. Proc. Natl. Acad. Sci. U.S.A. 116, 3562–3571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer A. G., 3rd, NMR characterization of the dynamics of biomacromolecules. Chem. Rev. 104, 3623–3640 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Mittermaier A., Kay L. E., New tools provide new insights in NMR studies of protein dynamics. Science 312, 224–228 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Vallurupalli P., Bouvignies G., Kay L. E., Increasing the exchange time-scale that can be probed by CPMG relaxation dispersion NMR. J. Phys. Chem. B 115, 14891–14900 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Archer S. J., Vinson V. K., Pollard T. D., Torchia D. A., Elucidation of the poly-l-proline binding site in Acanthamoeba profilin I by NMR spectroscopy. FEBS Lett. 337, 145–151 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Metzler W. J., Bell A. J., Ernst E., Lavoie T. B., Mueller L., Identification of the poly-l-proline-binding site on human profilin. J. Biol. Chem. 269, 4620–4625 (1994). [PubMed] [Google Scholar]
- 32.Rennella E., Sekhar A., Kay L. E., Self-assembly of human profilin-1 detected by Carr-Purcell-Meiboom-Gill nuclear magnetic resonance (CPMG NMR) spectroscopy. Biochemistry 56, 692–703 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Treviño M. A., et al. , The singular NMR fingerprint of a polyproline II helical bundle. J. Am. Chem. Soc. 140, 16988–17000 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Zigmond S. H., Beginning and ending an actin filament: Control at the barbed end. Curr. Top. Dev. Biol. 63, 145–188 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Macdonald D., et al. , Quantification assays for total and polyglutamine-expanded huntingtin proteins. PLoS One 9, e96854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceccon A., Tugarinov V., Clore G. M., Backbone 1H, 13C and 15N chemical shift assignments for full length exon-1 huntingtin protein. Biological Magnetic Resonance Data Bank. http://www.bmrb.wisc.edu/data_library/summary/index.php?bmrbid=50122. Deposited 12 December 2019.
- 37.Alderson T. R., Lee J. H., Charlier C., Ying J., Bax A., Propensity for cis-proline formation in unfolded proteins. ChemBioChem 19, 37–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzesiek S., Stahl S. J., Wingfield P. T., Bax A., The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35, 10256–10261 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Metzler W. J., et al. , Refined solution structure of human profilin I. Protein Sci. 4, 450–459 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All experimental relaxation dispersion, exchange-induced shift, and transverse relaxation data discussed in this paper are provided either in the main text or SI Appendix. The experimental data in digital format, together with MatLab scripts used in global fitting, have been deposited on Figshare (DOI: 10.6084/m9.figshare.11887860). In addition, the backbone chemical shifts for httex1 have been deposited in the Biological Magnetic Resonance Data Bank (36).