Abstract
Objective
To elucidate the expression and function of miR-34a in rat osteoarthritic cartilage cells, and further to explore its mechanism.
Material and Methods
Rat model of osteoarthritis was constructed and knee joint cartilage cells were isolated in vitro. Immunocytochemical staining was used for identification. qRT-PCR was used to detect the expression of miR-34a in cartilaginous tissues and cartilage cells. Cartilage cells were divided into blank control (BC), negative control (NC), miR-34a inhibitor (34aI), osteoarthritis model (OA), osteoarthritis model + negative control (OA + NC) and osteoarthritis model + miR-34a inhibitor (OA + 34aI) groups. Cell proliferation was detected by CCK-8 and colony formation assays. Cell apoptosis was studied by flow cytometry and Western blot. PI3K/AKT-pathway-related proteins were also analyzed by Western blot. To further validate the effect of miR-34a on the PI3K/Akt pathway, the cartilage cells were divided into blank control (BC), osteoarthritis model (OA), osteoarthritis model + miR-34a inhibitor (OA + 34aI), osteoarthritis model + PI3K activator (OA + IGF-1) and osteoarthritis model + miR-34a inhibitor + PI3K inhibitor (OA + 34aI + LY) groups, the experiments above were repeated.
Results
The expression of miR-34a in cartilaginous tissues and cells of osteoarthritis model was significantly higher than that in normal (p < 0.05). After silencing miR-34a gene, the cell proliferation and proteins expression of PI3K/Akt pathway were increased, while the apoptosis rate and expression of apoptosis-related proteins were decreased. Addition of PI3K activator also evidently promoted proliferation and inhibited apoptosis. The protein expression of Bax, Cleaved caspase-3 and Cleaved caspase-9 were dramatically decreased, while the ratios of p-PI3K/PI3K and p-Akt/Akt were increased in OA + IGF-1 group.
Conclusion
Downregulation of miR-34a regulated proliferation and apoptosis of cartilage cells by activating PI3K/Akt pathway, providing a potential therapeutic approach for the treatment of osteoarthritis.
Keywords: miR-34a, proliferation, apoptosis, osteoarthritis, PI3K/Akt pathway
Introduction
Osteoarthritis is a degenerative joint disease that can ultimately lead to joint damage.1 Articular cartilage degeneration, subchondral sclerosis and synovial inflammation are the characteristics of osteoarthritis.2 The etiology of osteoarthritis is multifactorial, including aging, strain, trauma, infection, obesity, joint congenital anomalies, metabolic disease, and so on.3,4 Osteoarthritis increases with the prolongation of lifespan and has become one of the global clinical problems that burden patients and health care systems.5 Although osteoarthritis has received attention from researchers in terms of drug development and physical exercise, the therapeutic effect is still unsatisfactory.6,7 Therefore, it would be of great clinical value to find new molecularly targeted drugs or new therapeutic target that can effectively treat osteoarthritis.
MicroRNAs (miRNAs) are short noncoding RNAs molecules (19–25 nucleotides length) that regulate the expression of many human-protein-coding genes.8 miRNA plays an important role in a variety of biological processes by bind to the complementary sequences in the 3ʹ untranslated region to involve the post-transcriptional regulation.9,10 miR-34a is a member of the miR-34 family and is the most significantly regulated downstream miRNA of the p53 pathway.11 Numerous studies indicate that the dysregulation of P53 is related to the progression of various diseases, miR-34a influences p53-mediated cell apoptosis, cell-cycle arrest in the G1 phase and silences in several types of cancer.12–14 The crucial role of miRNA in various diseases is related to their regulation of essential cellular processes and pathways.15 Previous studies have demonstrated that an apoptosis activation by the intrinsic and extrinsic way might be due to a protection mechanism after sublethal injury.16,17 The phosphatidylinositol-3-kinase-protein kinase B (PI3K/Akt) mediated signaling is one of the most critical pathways in regulation of cellular survival, proliferation, differentiation and apoptosis.18 Growth factors and hormones trigger PI3K phosphorylation events, which in turn coordinate cell growth, cell cycle entry, cell migration and cell survival.19 Moreover, the PI3K pathway inhibits the cell cycle progression by repressing downstream molecule Akt.19 These findings together build a strong rationale for the miR-34a and PI3K/AKT pathway in order to achieve a better outcome for osteoarthritis patients.
Therefore, we hypothesized that miR-34a may be effective in the treatment of osteoarthritis. This study aimed to evaluate the effects of miR-34a in rat model of osteoarthritis, and explored the mechanism of inhibition of miR-34a on cartilage cells proliferation and apoptosis.
Materials and Methods
Experimental Animal
Thirty male Sprague Dawley (SD) rats (250 ± 30g, 8–10 weeks old) were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd., license number SCXK (Beijing) 20160006. Rats were raised in the specific pathogens free (SPF) room with relative humidity 55 ± 5% at 23 ± 2°C and free to water and food. All experiments follow the NIH guidelines (NIH Pub. No. 85–23, revised 1996) and have been reviewed and approved by the Animal Protection and Use Committee of The 3rd People’s Hospital of Qingdao.
Establishment of Osteoarthritis Model
Rats were randomly divided into OA model group (Model) and normal control group (Normal), 15 in each group. An osteoarthritis model was established based on previous researches.20,21 The rats in the Model group were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (50 mg/kg). After a 2 cm incision was made in the longitudinal direction of the knee joint in the aseptic condition, sterile ophthalmic scissor was used to cut the medial collateral ligament and anterior and posterior cruciate ligaments. Then, the incision was sutured and the injured limb was not fixed to establish the OA model. The rats in Normal group were no special treatment. All rats were intramuscularly injected with 2 x 104 U/kg penicillin daily for 3 days. One week later, all rats were driven daily for 30 mins, 3 weeks consecutively.
Isolation and Primary Culture of Cartilage Cells
After anesthetized by intraperitoneal injection of 3% sodium pentobarbital (50 mg/kg), rats were sacrificed by dislocation. Rat bilateral knee articular cartilage was taken and stored in a refrigerator at −80°C. The other part was cut into small sections (<1 mm3) and washed in phosphate buffer containing double antibody. After digested with 0.2% trypsin and 0.2% type II collagenase for 30 mins and 2 hrs, respectively, the sections were centrifuged at 300 x g for 10 mins. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Rockville, MD, USA) supplemented with 20% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin-streptomycin in a 37°C, 5% CO2 incubator (Thermo Fisher Scientific, Waltham, USA).
Immunocytochemical Staining
Cells grown on glass coverslips were fixed with paraformaldehyde for 30 mins, broken by 0.5% Triton X-100 for 20 mins and added into 3% H2O2 for 15–20 mins. Each step was followed by the procedure washed with PBS 2–3 times. After blocking in serum protein at 37°C for 30 mins, cells in positive group were incubated in rabbit anti-rat Collagen II antibody (1:200, orb235107, Biorbyt, Cambridge, UK) at 37°C for 30 mins and the cells in negative group were incubated with PBS. Then, horseradish peroxidase-labeled goat anti-rabbit IgG (Proteintech, USA) was added at 37°C for 30 mins and washed with PBS 2–3 times. After incubated with SABC for 30 mins, the cells were colored with DAB, slightly re-stained with hematoxylin, dehydrated in ethanol gradient, transparent, mounted in neutral tree lipid. Finally, cells were observed under a 400X microscope (Olympus, Japan) and identified the cartilage cells.
Cell Transfection and Grouping
The cells were passaged one day before transfection, cultured in a 6-well plate, and transfected at a confluency of 40%. Lipofectamine TM2000 (Invitrogen, Carlsbad, CA) and plasmid (GenePharma, Shanghai, China) were added to a sterile centrifuge tube containing 200 μL DMEM, respectively. After standing at room temperature for 5 mins, plasmid mix was added to the Lipofectamine TM2000. Then, the mix was added to a 6-well plate and incubated for 6 hrs. The plate was gently shaken every 1 hr to make the liposomes and plasmids to fully interact with the cells. The OA and Normal cells were divided into 6 groups: the cells in the blank control group (BC) and Model group (OA) were not treated, the cells in the negative control group (NC) and Model + negative control group (OA+NC) were transfected with negative miR-34a, the cells in the miR-34a Inhibitor group (34aI) and Model + miR-34a Inhibitor group (OA + 34aI) were transfected with miR-34a Inhibitor.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
After 48 hrs, qRT-PCR was used to detect the expression of miR-34a in cartilaginous tissues and cartilage cells, and verify cell transfection. TRIzol kit (Takara, Dalian, China) was used to extract total RNA, the OD260/OD280 between 1.8 and 2.0 indicated that the purity was qualified. RNA was then transcribed into cDNA using the reverse transcription kit (Applied Biosystems, Waltham, MA, USA). qRT-PCR was performed using Mastercycler® nexus X2 (Eppendorf, Hamburg, Germany). Primer sequences (Shanghai Shenggong Bioengineering Technology Service Co., Ltd., Shanghai, China) are as follows: miR-34a, Forward: 5′-GCCTGGCAGTGTCTTAGCT-3′ and Reverse: 5′- GTGCAGGGTCCGAGGTC −3ʹ. U6, Forward: 5′- GACCTCTATGCCAACACAGT −3ʹ and Reverse: 5′- AGTACTTGCGCTCAGGAGGA −3ʹ. The reaction conditions were: 95°C for 15 s, 60°C for 60 s and 72°C for 40 s (35 cycles). U6 mRNA as an internal parameter and data were processed by the 2−ΔΔCt method.
Cell Counting Assay Kit-8 (CCK-8) Assay
Logarithmic growth cells were planted into 96-well plates at a density of 2×104 cells/mL, 100 μL per well. The cells were incubated in a 37°C, 5% CO2 incubator, 10 μL CCK-8 solution (PA137267, Pierce) was added to each well after 24 hrs, 48 hrs, 72 hrs and 96 hrs. After cultured for another 4 hrs, Enzyme-linked immunosorbent assay was used to measure absorbance (OD) at 450 nm.
Colony Formation Assay
After digested with 0.25% trypsin, logarithmic growth phase cells were blown into individual cell. Then, cells were plated in a 6-well plate at 500 cells/well and cultured at 37°C, 5% CO2 for 2–3 weeks, fresh medium were changed every three days. After fixed by methanol, cells were stained with 1 mL Giemsa working fluid for 30 mins. Finally, the plates were washed in ultrapure water and air-dried. The inverted microscope (Olympus, Japan) was used to count colonies.
Flow Cytometry
Cells were collected and resuspended in 1 x PBS at 4°C. After centrifuging at 1000 rpm for 5–10 mins, cells were resuspended with 300 μL of 1 x Binding Buffer. 5 μL of FITC-labeled annexin-V (Beyotime Biotechnology, Shanghai, China) was added to the tube and the cells were incubated for 15 mins in the dark. Then, 5 μL of propidium iodide solution was added and incubated in the dark for 5 mins. Two hundred μL of 1 x Binding Buffer was added prior to analysis by flow cytometer (Beckman Coulter, Brea, CA, USA) and Cell Quest 5.1 software (BD Bioscience, San Diego, CA).
Western Blot
Forty-eight hours after cell transfection, bicinchoninic acid (BCA) protein assay kit (Beijing Suolabao Biotech, Beijing, China) was used to quantitate the protein. Then, the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane. After blocking with 5% fat-free dried milk for 2 hrs at room temperature, the membranes were incubated overnight at 4°C with primary rabbit anti-rat antibodies against Bax (1:500, orb224426, Biorbyt, Cambridge, UK), Bcl-2 (1:500, orb226346, Biorbyt, Cambridge, UK), Cleaved caspase-3 (1:500, ab2302, Abcam), Cleaved caspase-9 (1:400, ab2324, Abcam), PI3K (1:1000, orb137259, Biorbyt, Cambridge, UK), p-PI3K (1:1000, orb338965, Biorbyt, Cambridge, UK), Akt (1:1000, orb213545, Biorbyt, Cambridge, UK), p-Akt (1:1000, orb222951, Biorbyt, Cambridge, UK) and β-actin (1:1000, ABIN2854709, antibodies-online, Aachen, Germany). Subsequently, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:1000, #7074, Cell Signaling Technology, USA) was used. The bands were observed and recorded using enhanced chemiluminescence (ECL) system (ImageQuant LAS 4000, General Electric Company, Fairfield, CT, USA), and the gray scale scanning and quantification were performed by Image J (National Institutes of Health) software.
To further validate the effect of miR-34a on the PI3K/Akt pathway, the pathway activator (IGF-1, 100 ng/mL, Shanghai Hengyuan Biotechnology Co., Ltd., Shanghai, USA) and inhibitor (LY294002, 30 μM, Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China) were used. The cartilage cells were divided into blank control (BC), osteoarthritis model (OA), osteoarthritis model + miR-34a inhibitor (OA + 34aI), osteoarthritis model + PI3K activator (OA + IGF-1) and osteoarthritis model + miR-34a inhibitor + PI3K inhibitor (OA + 34aI + LY) groups, the experiments above were repeated.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL). Mean ± SD was used to measure the data. Significant differences between two groups were assessed by t-test. Pairwise comparisons were performed with the one-way analysis of variance (ANOVA) test and LSD analysis used to further analysis. P < 0.05 at least is considered as statistically significant.
Results
The Identification of Cartilage Cells and Gene Expression of miR-34a in Cartilaginous Tissues and Cartilage Cells
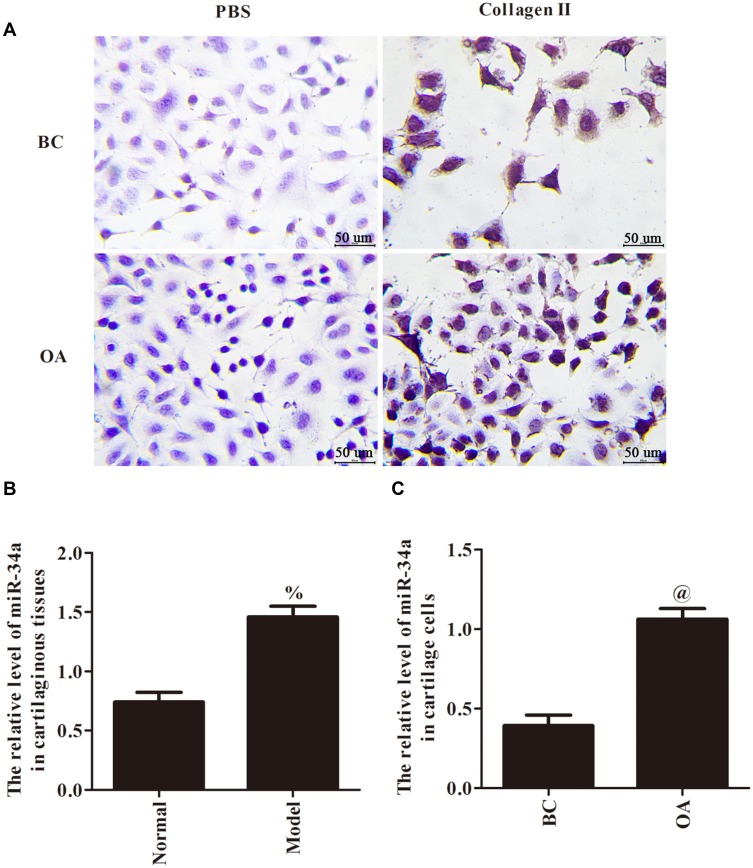
Immunocytochemical staining has shown that the cytoplasmic area of the negative group was transparent, and the cytoplasmic area of the experimental group was brownish yellow (Figure 1A). Compared with Normal group, the mRNA expression of miR-34a was significantly increased in Model group (p < 0.05, Figure 1B). Meanwhile, compared with BC group, the mRNA expression of miR-34a was also significantly increased in OA group (p < 0.05, Figure 1C). Those results indicated that the expression of miR-34a was elevated in cartilaginous tissues and cartilage cells of osteoarthritis.
Figure 1.
The identification of cartilage cells (A, ×400 and scale bars=50 µm) and gene expression of miR-34a in cartilaginous tissues (B) and cartilage cells (C). %p < 0.05 compared with Normal group; @p < 0.05 compared with BC group.
Effects of miR-34a on the Proliferation of Cartilage Cells
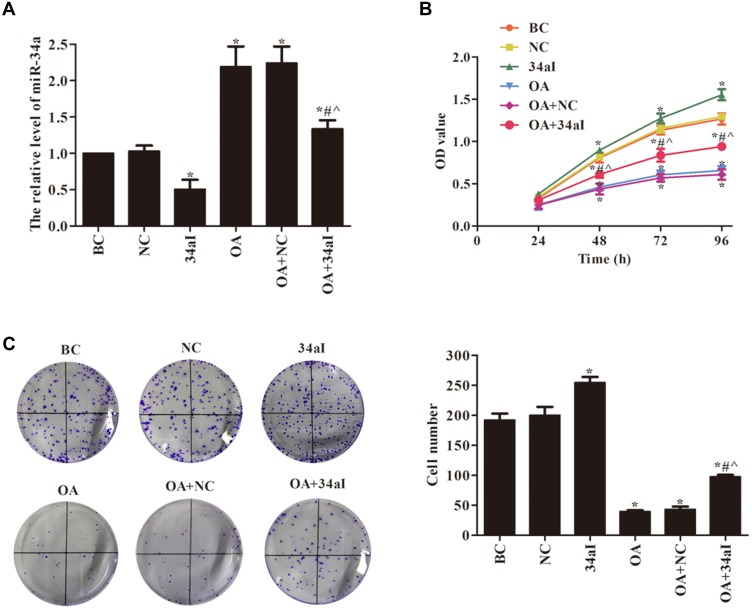
The miR-34a interfering and overexpressing cartilage cell line were constructed, and the transfection effect was verified by qRT-PCR. The results of Figure 2A show that the transfection is successful. Cell proliferation ability was detected by CCK-8 and colony formation assays (Figure 2B and C). There was no significant difference in cell proliferation ability between BC and NC groups and between OA and OA+NC groups (p > 0.05). Compared with BC group, the cell proliferation and colony formation were significantly increased in 34aI group (p < 0.05). While, compared with BC group, the cell proliferation ability was significantly decreased in OA, OA + NC and OA + 34aI groups (p < 0.05). In addition, compared with 34aI group, the proliferation ability was significantly decreased in OA + 34aI group (p < 0.05). However, compared with OA + NC group, cell proliferation and colony formation were significantly increased in OA + 34aI group (p < 0.05). Taken together, these results suggested that the downregulation of miR-34a could promote proliferation of rat osteoarthritic cartilage cells.
Figure 2.
Effects of miR-34a on the proliferation of cartilage cells. (A) qRT-PCR was used to detect the expression of miR-34a; (B) CCK8 and (C) cloning formation assays were used to detect the cell proliferation. *p < 0.05 compared with BC group; #p < 0.05 compared with OA + NC group; ^p < 0.05 compared with 34aI group.
Effects of miR-34a on the Apoptosis of Cartilage Cells
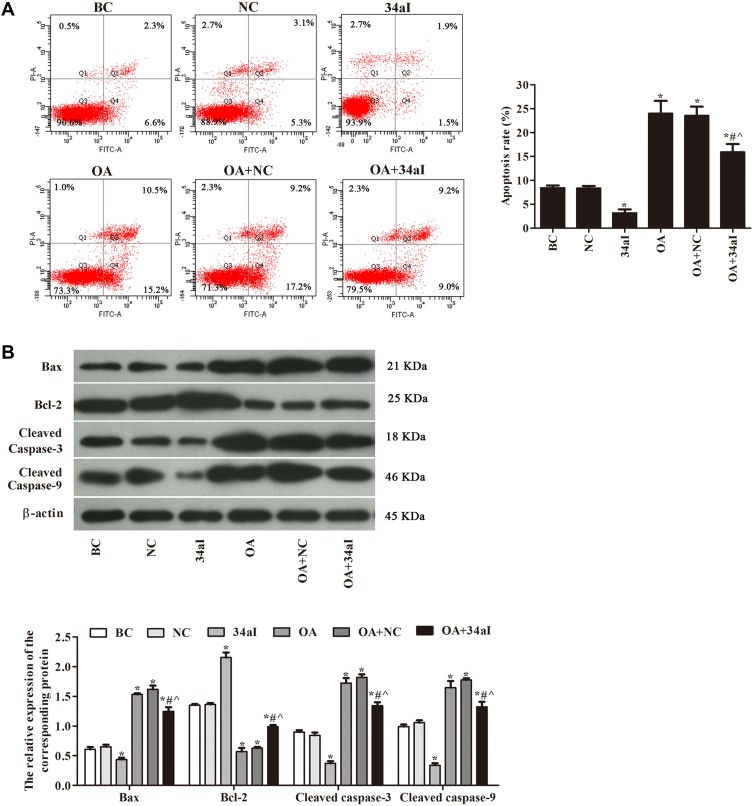
As shown in Figure 3, the apoptosis rate and proteins expression of Bax, Cleaved caspase-3 and Cleaved caspase-9 were dramatically decreased after silencing miR-34a gene, while the protein expression of Bcl-2 was increased. Compared with 34aI group, the apoptosis rate and expression of apoptosis-related proteins were significantly increased in OA + 34aI group (p < 0.05). However, compared with OA + NC group, the apoptosis rate and expression of apoptosis-related proteins were significantly decreased in OA + 34aI group (p < 0.05). These results indicated that downregulation of miR-34a could inhibit apoptosis of rat osteoarthritic cartilage cells.
Figure 3.
Effects of miR-34a on the apoptosis of cartilage cells. (A) Flow cytometry was used to detect apoptosis rate; (B) Western blot was used to detect the expression of apoptosis-related proteins. *p < 0.05 compared with BC group; #p < 0.05 compared with OA + NC group; ^p < 0.05 compared with 34aI group.
Effect of miR-34a on PI3K/Akt-Signaling-Pathway-Related Proteins in Cartilage Cells
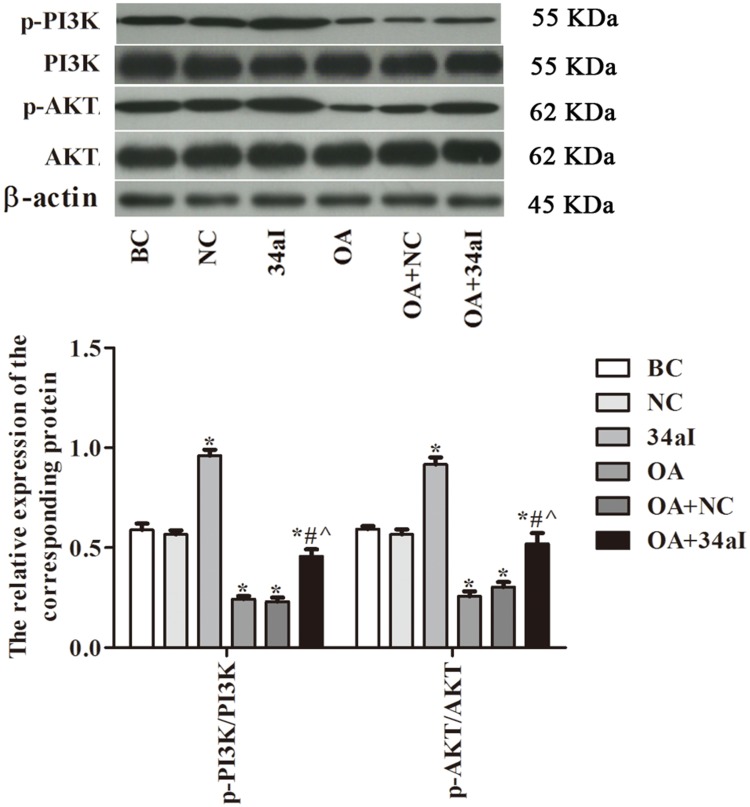
The protein expression of PI3K, Akt and their phosphorylated forms in different group were assessed by Western blot (Figure 4). The results showed that the ratios of p-PI3K/PI3K and p-AKT/Akt in 34aI group were significantly higher than the BC group (P < 0.05). However, those protein ratios in OA, OA + NC and OA + 34aI groups were significantly lower than that of BC group (P < 0.05). Compared with the 34aI group, the expression of PI3K/Akt-signaling-pathway-related proteins were significantly decreased in OA + 34aI group (p < 0.05). While, compared with OA + NC group, the expression of PI3K/Akt-signaling-pathway-related proteins were significantly increased in OA + 34aI group (p < 0.05). These findings indicated that downregulation of miR-34a could activate PI3K/Akt pathway.
Figure 4.
Effect of miR-34a on PI3K/Akt-signaling-pathway-related proteins in cartilage cells. *p < 0.05 compared with BC group; #p < 0.05 compared with OA + NC group; ^p < 0.05 compared with 34aI group.
miR-34a Regulates the Proliferation of Cartilage Cells by Regulating PI3K/Akt Pathway
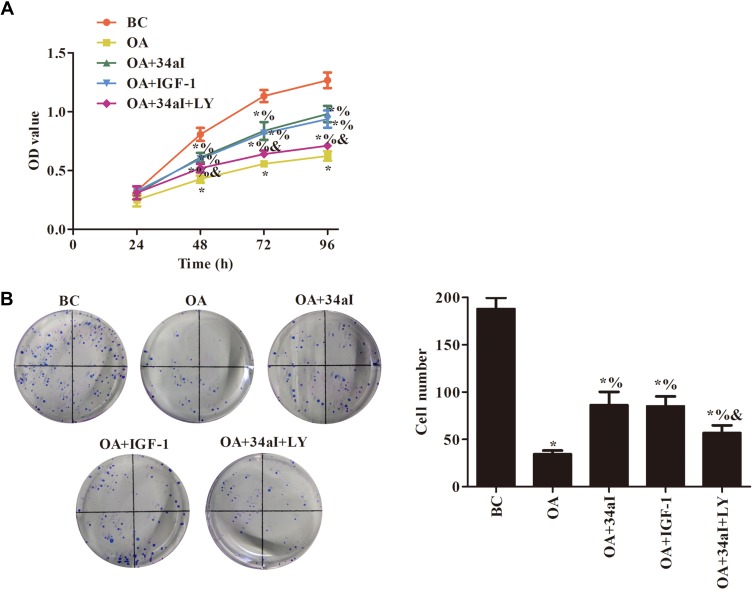
As shown in Figure 5, compared with BC group, the cell proliferation ability of the other groups was decreased with different degrees (p < 0.05). When compared with OA group, the cell proliferation ability was significantly increased in OA + 34aI, OA + IGF-1 and OA + 34aI + LY groups (p < 0.05). While, compared with OA + 34aI group, the cell proliferation ability was significantly decreased in OA + 34aI + LY group (p<0.05). These findings suggested that downregulation of miR-34a regulates the proliferation of cartilage cells by regulating PI3K/Akt pathway.
Figure 5.
miR-34a regulates the proliferation of cartilage cells by regulating PI3K/Akt pathway. (A) CCK8 and (B) cloning formation assays were used to detect the cell proliferation. *p < 0.05 compared with BC group; %p < 0.05 compared with OA group; &p < 0.05 compared with OA + 34aI group.
miR-34a Regulates the Apoptosis of Cartilage Cells by Regulating PI3K/Akt Pathway
Flow cytometry and Western blot were used to investigate the cell apoptosis (Figure 6). Compared with BC group, the apoptosis rate and expression of apoptosis-related proteins including Bax, Cleaved caspase-3 and Cleaved caspase-9 in the other groups were increased to varying degrees, while the expression of Bcl-2 protein was significantly decreased (p < 0.05). After treatment with miR-34a inhibitor or PI3K activator/inhibitor, there was a significantly decreased in the apoptosis rate and the expressions of apoptosis-related proteins, with a significantly increased in the expression of Bcl-2 (p < 0.05). Compared with OA + 34aI group, the apoptosis rate and expression of apoptosis-related proteins were significantly increased, and the expression of Bcl-2 protein was significantly decreased in OA + 34aI + LY group (p < 0.05). These results demonstrated that downregulation of miR-34a regulates the apoptosis of cartilage cells by regulating PI3K/Akt pathway.
Figure 6.
miR-34a regulates the apoptosis of cartilage cells by regulating PI3K/Akt pathway. (A) Flow cytometry was used to detect apoptosis rate; (B) Western blot was used to detect the expression of apoptosis-related proteins. *p < 0.05 compared with BC group; %p < 0.05 compared with OA group; &p < 0.05 compared with OA + 34aI group.
miR-34a Regulates Proliferation and Apoptosis of Cartilage Cells by Activating PI3K/Akt Pathway
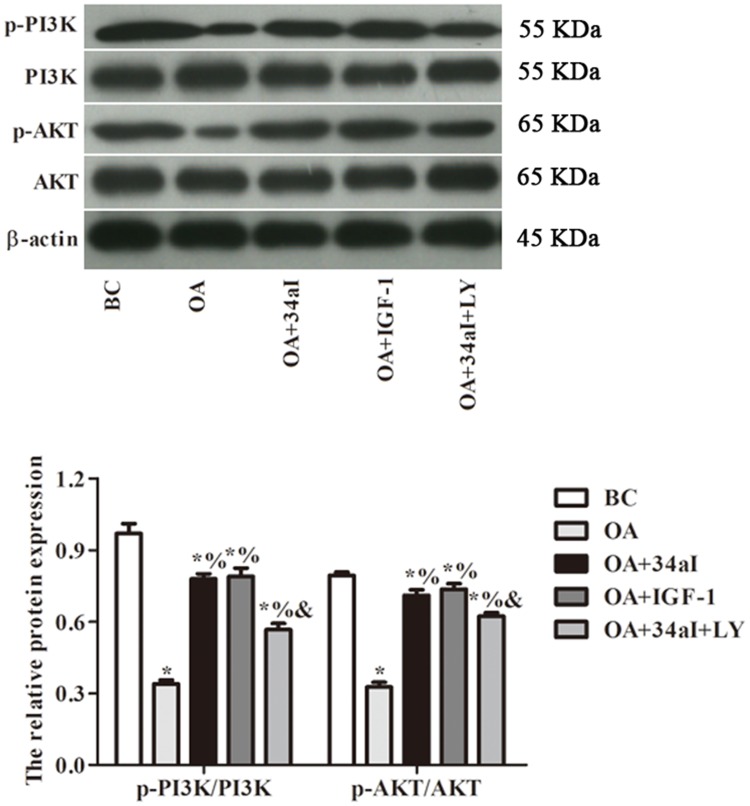
As shown in Figure 7, compared with BC group, others all have a significantly decreased in the ratios of p-PI3K/PI3K and p-AKT/Akt. Moreover, those protein ratios in OA + 34aI, OA + IGF-1 and OA + 34aI + LY groups were significantly higher than that of OA group (p < 0.05). While, compared with OA + 34aI group, the expression of PI3K/Akt-signaling-pathway-related proteins were significantly decreased in OA + 34aI + LY group (p < 0.05). Taken together, these results revealed that downregulation of miR-34a regulates proliferation and apoptosis of cartilage cells by activating PI3K/Akt pathway.
Figure 7.
miR-34a regulates proliferation and apoptosis of cartilage cells by activating PI3K/Akt pathway. *p < 0.05 compared with BC group; %p < 0.05 compared with OA group; &p < 0.05 compared with OA + 34aI group.
Discussion
Until now, the exact cause of osteoarthritis remain mysterious, various modalities were used for the treatment of osteoarthritis. However, the therapeutic effect is still unsatisfactory. Previous study has demonstrated that specific biomechanical stimuli and intercellular interactions produce intracellular signals that are powerful inducers or suppressors of pro-inflammatory genes in chondrocytes.22 Thus, it is crucial that studying the key areas of osteoarthritis pathogenesis to define the spectrum of therapeutic targets that to reducing the symptomatic and structural effects of osteoarthritis.
miR-34a is the result of miR-34 gene mutation, located on human chromosome 1p36.23 Studies have indicated that miR-34a is frequently lost or downregulated in most tissues.24–26 Therefore, this study hypothesized that miR-34a also plays an important role in rat osteoarthritic cartilage cells. We first established an osteoarthritis rat model and found that the expression of miR-34a was elevated in cartilaginous tissues and cartilage cells of osteoarthritis. This indicates that miR-34a plays a suppressive role in osteoarthritic cartilage cells proliferation. Previous studies already confirmed that miR-34a can induce cell cycle arrest and growth inhibition by activating apoptosis.27,28 We then used CCK8 and cloning formation assays to detect the cell proliferation. Furthermore, flow cytometry and Western blot were used to evaluate the influences of miR-34a on the cell apoptosis. The results show that downregulation of miR-34a promotes proliferation and inhibits apoptosis of rat osteoarthritic cartilage cells. All those findings demonstrated that miR-34a plays a crucial role in the pathogenesis and development of osteoarthritic and maybe a new therapeutic target for osteoarthritis.
The mechanisms involved in the osteoarthritis have not yet been completely elucidated. While the other protective signaling pathways prevent cells from apoptosis, a number of studies found that PI3KAKT pathway plays an important role in preventing cell apoptosis.18 PI3K links to an extraordinarily diverse group of cellular functions via the activation of Akt.29 To study the molecular mechanisms by which miR-34a protects rat osteoarthritic cartilage cells, the protein expression of PI3K, AKT, and their phosphorylated were detected by Western blot. We found that after silencing miR-34a gene, the ratios of p-PI3K/PI3K and p-AKT/Akt were increased, which indicated that downregulation of miR-34a could activate PI3K/Akt pathway. The phosphorylation of PI3K/Akt pathway could protect cells via activation of Bcl-2 family and inhibition of caspase family.19 To further define whether miR-34a regulates rat osteoarthritic cartilage cells through the PI3K/Akt signaling pathway, the pathway activator and inhibitor were used. The results showed that the addition of PI3K activator evidently increased the ratios of p-PI3K/PI3K and p-Akt/Akt and the expression of Bcl-2, while the expression of Bax, Cleaved caspase-3 and Cleaved caspase-9 were dramatically decreased.
In conclusion, we found that downregulation of miR-34a could promote proliferation and inhibit apoptosis of rat osteoarthritic cartilage cells. The possible mechanisms may be by activating PI3K/Akt pathway. Our study demonstrated that miR-34a was a potential therapeutic approach on osteoarthritis treatment, which provides a new insight for the clinical treatment of osteoarthritis.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declared no conflicts of interest in this work.
References
- 1.Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS One. 2018;13(4):e0196625. doi: 10.1371/journal.pone.0196625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalunian KC. Current advances in therapies for osteoarthritis. Curr Opin Rheumatol. 2016;28(3):246–250. doi: 10.1097/BOR.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 3.Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage. 2015;23(11):1989–1998. doi: 10.1016/j.joca.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Lane NE, Nevitt MC. Osteoarthritis, bone mass, and fractures: how are they related? Arthritis Rheum. 2002;46(1):1–4. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 5.Ondrésik M, Azevedo Maia FR, da Silva Morais A, et al. Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng. 2017;114(4):717–739. [DOI] [PubMed] [Google Scholar]
- 6.Szychlinska MA, Castrogiovanni P, Trovato FM, et al. Physical activity and mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur J Nutr. 2019;58(2):565–581. doi: 10.1007/s00394-018-1632-2 [DOI] [PubMed] [Google Scholar]
- 7.Castrogiovanni P, Di Rosa M, Ravalli S, et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci. 2019;20(3):511. doi: 10.3390/ijms20030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Wang W, Huang K, Wang Y, Li J, Yang X. MicroRNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget. 2017;8(67):111258–111270. doi: 10.18632/oncotarget.22770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 10.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–524. doi: 10.1016/j.cell.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacombe J, Zenhausern F. Emergence of miR-34a in radiation therapy. Crit Rev Oncol Hematol. 2017;109:69–78. doi: 10.1016/j.critrevonc.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarasov V, Jung P, Verdoodt B, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6(13):1586–1593. doi: 10.4161/cc.6.13.4436 [DOI] [PubMed] [Google Scholar]
- 14.Xie L, Zhou J, Zhang S, et al. Integrating microRNA and mRNA expression profiles in response to radiation-induced injury in rat lung. Radiat Oncol. 2014;9:111. doi: 10.1186/1748-717X-9-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Dahlberg JE, Tam W. MicroRNAs in tumorigenesis: a primer. Am J Pathol. 2007;171(3):728–738. doi: 10.2353/ajpath.2007.070070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musumeci G, Loreto C, Carnazza ML, Martinez G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):307–313. doi: 10.1007/s00167-010-1215-0 [DOI] [PubMed] [Google Scholar]
- 17.Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci. 2013;14(8):15767–15784. doi: 10.3390/ijms140815767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song HP, Chu ZG, Zhang DX, Dang YM, Zhang Q. PI3K-AKT pathway protects cardiomyocytes against hypoxia-induced apoptosis by MitoKATP-mediated mitochondrial translocation of pAKT. Cell Physiol Biochem. 2018;49(2):717–727. doi: 10.1159/000493037 [DOI] [PubMed] [Google Scholar]
- 19.Xu T, Pang Q, Wang Y, Yan X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int J Mol Med. 2017;40(6):1669–1678. doi: 10.3892/ijmm.2017.3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szychlinska MA, Trovato FM, Di Rosa M, et al. Co-expression and co-localization of cartilage glycoproteins CHI3L1 and lubricin in osteoarthritic cartilage: morphological, immunohistochemical and gene expression profiles. Int J Mol Sci. 2016;17(3):359. doi: 10.3390/ijms17030359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Rosa M, Szychlinska MA, Tibullo D, Malaguarnera L, Musumeci G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur J Histochem. 2014;58(3):2423. doi: 10.4081/ejh.2014.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knobloch TJ, Madhavan S, Nam J, Agarwal S Jr, Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18(2):139–150. doi: 10.1615/CritRevEukarGeneExpr.v18.i2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng PS, Wen WX, Fadlullah MZ, et al. Identification of germline alterations in breast cancer predisposition genes among Malaysian breast cancer patients using panel testing. Clin Genet. 2016;90(4):315–323. doi: 10.1111/cge.2016.90.issue-4 [DOI] [PubMed] [Google Scholar]
- 24.Sukata T, Sumida K, Kushida M, et al. Circulating microRNAs, possible indicators of progress of rat hepatocarcinogenesis from early stages. Toxicol Lett. 2011;200(1–2):46–52. doi: 10.1016/j.toxlet.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Tian J, Xian W, Xie T, Yang X. miR-34a inhibits proliferation and invasion of bladder cancer cells by targeting orphan nuclear receptor HNF4G. Dis Markers. 2015;2015:879254. doi: 10.1155/2015/879254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun TY, Xie HJ, Li Z, et al. miR-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Am J Transl Res. 2017;9(1):103–114. [PMC free article] [PubMed] [Google Scholar]
- 27.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–752. doi: 10.1016/j.molcel.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y, Lu S, Li J, Deng L. Shikonin-mediated up-regulation of miR-34a and miR-202 inhibits retinoblastoma proliferation. Toxicol Res (Camb). 2018;7(5):907–912. doi: 10.1039/C8TX00079D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilic U, Caglayan AB, Beker MC, et al. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 2017;12:657–665. doi: 10.1016/j.redox.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]