Significance
In order for animals to successfully navigate the world, they must constantly shape and reshape motor outputs to best reflect the demands of their environment. When environmental demands suddenly change, the reshaping of motor outputs to adaptively meet the new constraints of the environment requires dynamic subsecond changes in action plans. Decades of imaging and modeling research in humans have implicated the anterior cingulate cortex (ACC) in the evaluation of situational demands and the hypothesized alteration of downstream nodes such as the dorsal medial striatum that facilitate appropriate action selection. Here, we show that the ACC is necessary for proactively and reactively modulating response selection in rats both within and across trials at the level of single neurons and behavior.
Keywords: conflict, action planning, anterior cingulate, inhibition, striatum
Abstract
Previous research has focused on the anterior cingulate cortex (ACC) as a key brain region in the mitigation of the competition that arises from two simultaneously active signals. However, to date, no study has demonstrated that ACC is necessary for this form of behavioral flexibility, nor have any studies shown that ACC acts by modulating downstream brain regions such as the dorsal medial striatum (DMS) that encode action plans necessary for task completion. Here, we performed unilateral excitotoxic lesions of ACC while recording downstream from the ipsilateral hemisphere of DMS in rats, performing a variant of the STOP-signal task. We show that on STOP trials lesioned rats perform worse, in part due to the failure of timely directional action plans to emerge in the DMS, as well as the overrepresentation of the to-be-inhibited behavior. Collectively, our findings suggest that ACC is necessary for the mitigation of competing inputs and validates many of the existing theoretical predictions for the role of ACC in cognitive control.
Humans and animals must be able to rapidly adapt their behaviors in response to sudden changes in their environment. When stopped at a stoplight, being able to stop oneself from accelerating when the light turns green in response to a pedestrian suddenly crossing in front of your car is just one example of how response selection and reshaping is an important component of daily life. In order to avoid hitting the pedestrian, your brain must rapidly process the change in the environment and quickly move to cancel the initial action plan (i.e., accelerate; move to right pedal), in favor of a more appropriate action plan (i.e., stop; move to left pedal). This drastic change in motor output, when successful, is remarkably fast, occurring within a few hundred milliseconds, and requires the coordinated efforts of multiple brain systems.
Across species and in clinical populations, a common paradigm used to test cognitive control is the STOP-signal task, where participants are required to inhibit (i.e., “STOP”) an automatic response (“GO” response) for a relatively small percentage of trials (∼20 to 30%) (1). Cognitive control is assessed both in terms of accuracy on GO versus STOP trials as well as in terms of how well participants adapt their behavior following difficult or errant trials (i.e., slow responding to increase accuracy, conflict adaptation). Much like the stoplight example above, one crucial component of the STOP-signal task is the involvement of quick, automatic processing of a prepotent stimulus feature that then has to be rapidly overturned or inhibited when a second stimulus is presented. In other words, subjects must quickly recognize the change in environmental constraints and reshape or cancel their preexisting action plan in favor of a more adaptive one.
While the exact function of the anterior cingulate cortex (ACC) has been described as perennially controversial (2), and as being a Rorschach test for cognitive neuroscience (3), a large body of literature has suggested that ACC may generally be important for recognizing instances in which sudden changes in the environment require the rapid reshaping of motor outputs (2–6). Positron emission tomography (7) and functional magnetic resonance imaging (8, 9) studies in humans have shown that changes in metabolic or hemodynamic activity in ACC are consistently greater when task-relevant sensory features are incongruent with other stimuli presented in close proximity to the relevant stimulus. Collectively, these findings have led to countless theories and models where ACC recognizes the need for cognitive control and then modulates activity in downstream brain regions responsible for guiding behavior (2). However, no study has fully examined the necessity of ACC in this process and whether ACC is actually responsible for changing action plans in conjunction with unexpected or sudden changes in the environment.
The reason for the lack of studies investigating the necessity of ACC in modulating response selection is that the majority of work has been done in humans, thus determining if ACC is necessary for modulating response selection has been difficult (i.e., difficult to perturb ACC in humans). Even in animal studies, proof that ACC is critical for this function is limited. There is some evidence that ACC is important for inhibiting behavior or altering behavior after difficult/error trials (10) as well as under conditions of uncertainty (6), but no study has shown that ACC is critical when an errant action needs to be canceled in favor of another. To better study this phenomenon in rats, we have recently developed a directional STOP-change task where rats must update and reconcile responses between left and right movements to obtain a liquid sucrose reward. In this task, for a minority of trials (20%) rats were first instructed to perform one action (e.g., move left) and then, unexpectedly, a light cue appeared telling the rat to move in the opposite direction (e.g., move right). During these trials, response units that encode the first and second signals were activated in close proximity to one another. In our task, cues were presented sequentially, which allows us to better resolve the timing of neural signals related to the two processes and also better reflects real life scenarios, such as the stoplight example, where environmental cues suddenly change after an action plan has likely been formed and moved toward initiation. It also captures the ability of animals to adapt action plans based on previous experience (e.g., after nearly hitting a pedestrian, a driver is likely to use more caution).
The development of this task in rats enables us to determine if ACC is necessary for the reshaping of neural signals associated with response selection for several reasons. First, we know that rats are slower and less accurate during trials where left and right signals have to be reconciled. Furthermore, rats modulate response selection across trials, slowing down their response times and improving their accuracy in trials that immediately follow trials where action plans were adapted (i.e., conflict adaptation). Second, during performance of our STOP-change task, firing of 26% of single neurons in ACC and overall population firing is higher in trials during presentation of the second cue light when rats successfully changed their initial action plan in order to correctly complete the behavioral trial (11). Thus our task demonstrates construct validity, tapping into functions that strongly activate ACC; moreover, at present, no other task has been able to demonstrate ACC activation at the level of the single neuron (11). Finally, our task provides a behavioral readout demonstrating that the task induces the need to change action plans, as well as a neural readout in that, when recording from dorsal medial striatum (DMS), an area involved in motor planning, we can monitor how the neural correlates of response direction encoding emerge prior to completion of the behavioral response. We have shown that, much like behavior, neural signals in DMS take longer to signal the appropriate response direction in trials that are preceded by trials requiring a change in response selection, and, when DMS fails to signal the correct direction, rats produce errors (12). Given that ACC is highly active in STOP-change trials during the same period of time when DMS neurons reconcile directional signals to accurately represent the correct behavioral output, we predicted that excitotoxic ACC lesions would disrupt the development of accurate directional signals in DMS in rats performing this task.
Results
To determine if directional response selectivity in DMS is dependent on ACC, we unilaterally lesioned ACC and recorded from DMS in the same hemisphere [connections are largely ipsilateral (13)] in rats performing the STOP-change task. We chose unilateral lesions to limit the impact on behavior and to minimize the potential recruitment of redundant systems likely involved in task performance (14). Rats (n = 16) were trained on the directional STOP-change task prior to surgery to ensure that basic task training would be unaffected by ACC lesions. After training, rats received two unilateral, 0.2-µL injections of either ibotenic acid (0.6 M; Tocris; n = 8) or saline into ACC (n = 8, injection 1: anterior–posterior [AP]: +1.2 mm; medial–lateral [ML]: ±0.6 mm; dorsal–ventral [DV]: −2.2 mm; injection 2: AP: +0.2 mm; ML: ±0.6 mm; DV: −2.2 mm) (Fig. 1C). All rats were implanted with eight-channel drivable recording electrodes in DMS (AP: −0.4 mm; ML: ±0.5 mm; DV: −3.5 mm) (Fig. 1C). Rats were randomly assigned to treatment conditions, and no differences in the number of trials performed (t(14) = 0.4149, P = 0.6845) or the percentage of correct trials performed (t(14) = 0.807, P = 0.4332) were observed prior to surgery between the two treatment groups.
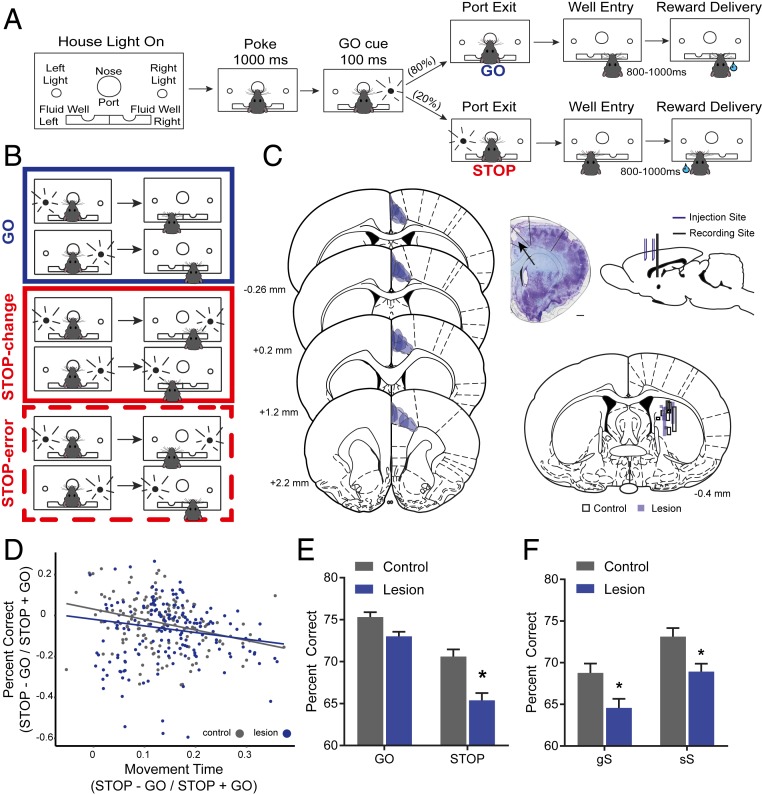
Fig. 1.
Unilateral ACC lesions impair response selection. (A) Illustration of STOP-change task. Rats were required to nose-poke and remain in the port for 1,000 ms before one of two directional lights (left or right) were flashed for 100 ms. For 80% of the time, the cue light directs the rat toward the correct fluid well (GO trials). For 20% of the time, following port withdrawal (0 to 100 ms), the cue light opposite the first cue light turns on and remains on, instructing the rat to inhibit its response to the first cue light in favor of responding to the second cue light (STOP-change trials). (B) Illustration of GO (blue), STOP-change correct (red), and STOP-change error (dotted red) trials. (C, Left) Stereotaxic overlays detailing the extent of the lesion for all eight rats (four females, four males) tested. Lesions were primarily constrained to rodent Cg1 and showed little overlap with other frontal regions (35). (C, Middle) Photomicrograph of Nissl-stained tissue showing representative lesion (arrow). Overlay approximately matched to section (∼+1.6 mm anterior from bregma). (Scale bar, 1 mm.) (C, Top Right) Sagittal view of two injection sites in ACC and the targeted recording site in DMS. (C, Bottom Right) Overlays detailing the tracks of all 16 electrode tracks in the DMS. Shaded blue tracks signify lesioned animals, while black borders signify controls. Injections and electrodes were placed in either the right or the left hemisphere, and placement was counterbalanced across treatment groups. Note: placements are shown on the same hemisphere to reflect the unilateral nature of the manipulations. (D) Scatter plot showing the percentage of correct responses as a function of movement time. Black and blue dots indicate data from individual sessions for controls and lesions, respectively. (E) Percentage of correct scores on GO and STOP-change trials by condition averaged over recording sessions. (F) Percentage of correct scores based on sequence effects averaged over recording sessions. Lowercase letters indicate previous trial type; uppercase letters indicate current trial type (gS reads as “GO trial preceded a STOP-change trial”). A single asterisk (*) indicates significance at Bonferroni-adjusted P < 0.05.
ACC Lesions Impair the Ability to Inhibit and Redirect Behavior.
The task structure is illustrated in Fig. 1 A and B. On one wall of the recording chamber, a central port was located above two adjacent fluid wells. Directional lights were located next to the fluid wells. Each trial began with the illumination of house lights that instructed the rat to nose-poke into the central port. After 1 s, a directional light to the rats left or right was flashed for 100 ms. On 80% of trials (GO trials) presentation of this directional cue light instructed the rat to exit the port and respond in the direction of the light in order to receive reward. On the remaining 20% of trials, the initial sequence of events was identical; however, a second directional cue light was illuminated in the opposite direction 0 to 100 ms after the rat exited the port. During these trials, rats had to respond in the direction of the second light in order to receive the reward.
Unilateral lesions of ACC made rats perform worse on STOP-change trials. This is illustrated in Fig. 1E, which shows the average percentage of correct scores for both GO and STOP-change trials. A two-way ANOVA across sessions revealed significant main effects for treatment (F(1,1438) = 1.692, P < 0.0001) and trial type (F(1,1438) = 4.532, P < 0.0001) as well as a significant interaction (F(1,1438) = 0.253, P = 0.0464). To explore this interaction, we performed Bonferroni-corrected pairwise t tests comparing lesions to controls on GO and STOP-change trials. On GO trials, there was no significant difference between controls and lesions (Bonferroni-adjusted: P = 0.1687). We found that both control (Bonferroni-adjusted: P < 0.0001) and lesioned (Bonferroni-adjusted: P < 0.001) rats were worse on STOP-change trials relative to GO trials and that lesioned rats performed significantly worse on STOP-change trials compared to controls (Bonferroni-adjusted: P < 0.0001) (Fig. 1E). Thus, ACC lesions impaired the ability of rats to inhibit and redirect behavior, but left responding on GO trials intact.
In addition to lesioned rats performing worse on STOP-change trials, they were also slower on STOP-change trials compared to GO trials. Fig. 1D plots the difference between movement times on STOP-change and GO trials and its relationship with the percentage of correct responses over sessions. Distributions for percent correct (t(719) = −2.9087, P = 0.0037) and movement times (t(719) = −2.877, P = 0.0041) were more strongly shifted after lesions when compared to controls, demonstrating that lesioned rats were worse on STOP-change trials compared to GO trials and took longer to alter action plans on STOP-change trials across sessions. Furthermore, there was a significant correlation between the movement time and percent correct indices for both groups (control: r = −0.3069, P < 0.05; lesion: r = −0.175, P < 0.05) indicating that, in sessions where rats performed poorly, they took longer to adapt their action plan.
Next, we determined if ACC lesions impacted the ability of rats to adapt to the need to change action plans on trials subsequent to STOP-change trials. As described previously, rats were more accurate on STOP-change trials following a STOP-change trial (i.e., conflict adaption) (11). We replicated this result here by comparing performance on STOP-change trials when the STOP-change trial was preceded by either a GO (gS) or a STOP (sS) trial. We observed a significant main effect of lesion (F(1,1432) = 15.84, P < 0.0001) and previous trial-type (F(1,1432) = 16.97, P < 0.0001), but no significant interaction (F(1,1438) = 0.00002, P = 0.9959), demonstrating that, although ACC lesioned rats perform worse on STOP-change trials overall, they still retain the ability to adapt their behavior after experiencing response conflict (Fig. 1F).
Finally, to determine if single hemisphere lesions differentially impacted the rats’ ability to inhibit behavior for movements made contralateral versus ipsilateral to the lesion we asked whether the percent correct and time needed to accurately perform STOP-change trials differed between the two directions. For percent correct, we found that rats with ACC lesions performed significantly worse on STOP-change trials for both contralateral (t(718) = 2.4588; P = 0.0142) and ipsilateral (t(719) = 3.0394; P = 0.0025) responses. Next, we investigated whether responses made in the direction that was contralateral to the lesioned hemisphere of ACC were more impaired than responses made in the ipsilateral direction. We compared STOP-change reaction times (i.e., movement times on STOP-change trials minus movement times on GO trials) across treatment conditions for when the correct response required a movement that was either contralateral or ipsilateral to the lesion. We found that, in rats with ACC lesions, movements in the direction that was contralateral to the lesion took significantly longer to redirect on STOP-change trials (t(718) = 2.8404; P = 0.0046), but no differences were observed when the correct response was being made in the direction that was ipsilateral to the lesion (t(718) = 1.4737; P = 0.1410). Collectively, these findings suggest that ACC contributes to both the inhibition and the redirection of behavior for STOP-change trials in both response directions.
Anterior Cingulate Cortex Is Necessary for Resolving Neural Signals Associated with Response Selection in the DMS.
We next examined population firing of all DMS neurons (n = 97) in control rats that increased firing on correct trials during the response epoch (i.e., port exit to well entry) (11, 12) (Fig. 2B). Thick and thin lines represent firing when responses were made correctly into and away from the response field of each neuron, respectively. Trials are aligned to the nose-port exit which is a common event between GO and STOP-change trial types (i.e., there is no second cue for GO trials; later plots are aligned to multiple events). On GO trials, population firing rapidly increased in response to the first cue light, prior to exiting the nose port (i.e., time 0 = first light on) for behavioral responses into the response field. The quick development of this signal is consistent with the swift behavioral response observed on GO trials.
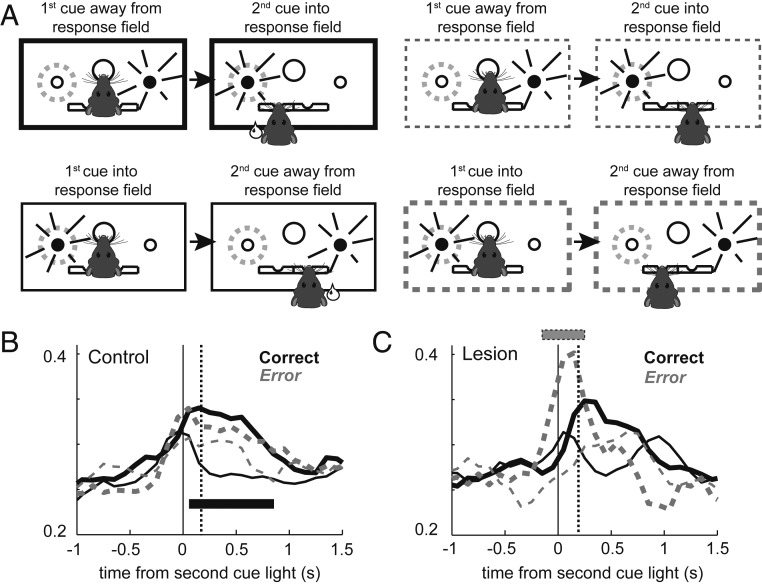
Fig. 2.
Unilateral ACC lesions delay response selection in the DMS on STOP-change trials. (A) Illustration of trial types as they relate to movement into and away from a neuron’s response field for GO (blue) and STOP (red) trials as defined by firing in response to the first cue light. (B and E) Population histogram for control (B; n = 97) and lesioned (E; n = 53) rats aligned to port exit. Blue lines represent GO trials; red lines represent correct STOP-change trials. Thin lines represent movements away from a neuron’s response field (nonpreferred direction); thick lines represent movement into a neuron’s response field (preferred direction). Preferred direction was defined by the direction that elicited the strongest response averaged over correct trial types. Vertical dashed line represents the SCRT as computed by subtracting movement times on GO trials from STOP trials. (C and F) Directional indices (into − away/into + away) for firing related to the first cue-light epoch (first cue-light onset to port exit) in controls (C) and lesioned (F) rats. (D and G) Directional indices for firing related to the second cue-light epoch (second cue light to well entry) in controls (D) and lesioned (G) rats. A single asterisk (*) indicates significance at Wilcoxon P < 0.05.
On STOP-change trials, rats were significantly slower to respond and were less accurate (Fig. 1 D and E). Presumably, this is because neural signals related to encoding response direction first represent the direction associated with the first cue, which then must be inhibited or changed, so that activity reflecting the second light can emerge and behavior can be redirected. Indeed, this is exactly what we see in the neural signals recorded in DMS; that is, when the first and second cue are presented into and away from the response field (Fig. 2B, thin red line), respectively, DMS firing first increases in response to the first light and then decreases in response to the second light. The opposite pattern was observed when the first and second cues were presented into and away from the response field, respectively (Fig. 2B, thick red line). Importantly, resolution of the directional signal preceded the estimated time necessary to redirect behavior [STOP-change reaction time (SCRT) = STOP minus GO movement times; gray dashed line] on correct trials as previously reported (12) (Fig. 2B).
To quantify these effects, we computed a directional index by subtracting firing for correct movements away from each cell’s response field from firing for correct movements to be made into the response field divided by the sum (into − away/into + away). These indices were computed for two epochs. The first index examines firing from first light onset until port exit, thus capturing firing related to the first light and associated motor signals. The second epoch examines firing from the second light until fluid-well entry, thus capturing firing related to the second light and the correct movement into the fluid well. Fig. 2 C and D illustrate the distribution of indices during first and second light epochs for controls. During the first light epoch the distribution significantly shifts below zero (Fig. 2C; Wilcoxon; n = 97; µ = −0.13; P < 0.0001), indicating that the majority of neurons signaled the direction of the first light. During the second light epoch, the distribution was significantly shifted in the opposite direction (Fig. 2D; Wilcoxon; n = 97; µ = 0.05; P = 0.003), indicating that the majority of neurons altered firing to accurately represent the direction in which the animal needed to move to obtain a reward. Of the 28 neurons (black bars in Fig. 2D) that significantly encoded direction during the second light epoch (Wilcoxon; P < 0.05), the firing of 21 neurons accurately reflected the response associated with the second light (21 vs. 7; χ2 = 6.90; P = 0.008). Thus, in controls, the majority of single neurons redirected firing after presentation of the second light to accurately represent the behavior necessary to obtain the reward despite initially encoding the direction of the first light. Next, we asked if directional signals in DMS can be similarly resolved in the absence of ACC.
In ACC lesioned rats, similar patterns of firing were observed, with activity tracking the location of the first and second lights (Fig. 2E). During the first light epoch, as in controls, the distribution of indices significantly shifted in the negative direction (Fig. 2F; Wilcoxon; n = 53; µ = −0.11; P < 0.0001); however, in response to the second light, the activity of the majority of neurons did not accurately represent the direction of the response necessary to obtain the reward. The distribution of indices did not significantly shift in the positive direction (Fig. 2G; Wilcoxon; n = 53; µ = 0.0283; P = 0.2743), and the counts of neurons that fired significantly more strongly for actions into the response field did not significantly outnumber those made away from the response field (Fig. 2G, black bars; 6 vs. 11; χ2 = 1.41; P = 0.23). Thus, the population of DMS neurons failed to represent the accurate response after ACC lesions.
Firing in DMS Is Overly Active during Errant Responses after ACC Lesions.
These results suggest that reduced directional encoding contributes to longer latencies to inhibit behavior on correct trials and selection of incorrect behavior on error trials. Above, we focused on correct trials; here we plot average firing on errant STOP trials aligned to the presentation of the second light cue (Fig. 3 B and C) during sessions where there was at least one stop error in each response direction. As above, firing tracked the location of second light on correct trials, and significant directional signals emerged shortly after cue onset and prior to the SCRT (Fig. 3B; black tick marks prior to SCRT; t tests every 100 ms; P < 0.01) in the control group but not in the lesion group (Fig. 3C).
Fig. 3.
Firing in DMS is overly active during errant responses after ACC lesions. (A) Illustration of behavioral responses on STOP-change correct (solid) and STOP-change error (dashed) trials. (B and C) Population histograms for control (B; n = 97) and lesion (C; n = 53) rats aligned to onset of the second cue light. Solid lines represent firing on STOP-change trials where the rat made the correct response, and dashed lines represent STOP-change trials where the rat committed an error. Thick and thin lines represent firing into (thick) or away from (thin) a neuron’s preferred direction. Tick marks (gray) represent significance when comparing thick to thin lines within trial type.
On error trials, DMS neurons in control rats fired similarly for responses to be made into and away from the response field, suggesting that, on error trials, DMS failed to form an appropriate motor plan (Fig. 3B; gray dashed line). In the lesion group, DMS firing also failed to represent the appropriate action but also fired significantly more strongly for errant responses in the direction cued by the first light, presumably driving behavior in the incorrect direction (Fig. 3C). Tick marks indicating significant differences between thick and thin gray dashed lines (t tests every 100 ms; P < 0.01) in the population demonstrate that the average firing was significantly stronger in lesioned rats for errant behaviors made into the response field. Notably, errant selectivity emerged prior to illumination of the second cue light (Fig. 3C), suggesting that ACC contributes to the dampening of the firing that prevents errant responses in the likelihood that the second cue might be presented. This issue is further addressed in the following section.
Impact of ACC Lesions on DMS Firing during Conflict Adaptation.
Above we report results suggesting that unilateral ACC lesions do not impair conflict adaptation at the level of behavior; that is, after ACC lesions, rats were still more accurate on sS trials compared to gS trials. However, unilateral lesions may have altered processing in the DMS without impacting overall behavior, suggesting that the ACC contributes to this function even in the absence of a behavioral effect, perhaps due to redundancy in the system and/or intact ACC processing in the other hemisphere. Theoretically, conflict adaptation can arise from mechanisms by which the brain is better equipped to reshape behavior in response to the second cue light and/or mechanisms that reduce automatic prepotent tendencies to follow the first cue light, thus making it easier to inhibit and redirect behavior. In controls, average neural firing in DMS suggests that both are engaged to a degree.
This is illustrated in Fig. 4, which shows the average firing aligned to first cue light on (Fig. 4 C and F), port exit (Fig. 4 D and G), and second cue light on (Fig. 4 E and H). Tick marks indicate significant differences between firing for responses made in (thick line) and away (thin line) from the neuron’s response field (t tests every 100 ms; P < 0.01), and directional indices were computed for each cell (into − away/into + away). The only difference in this analysis is that we split STOP-change trials into sS (orange) and gS (red) trial types.
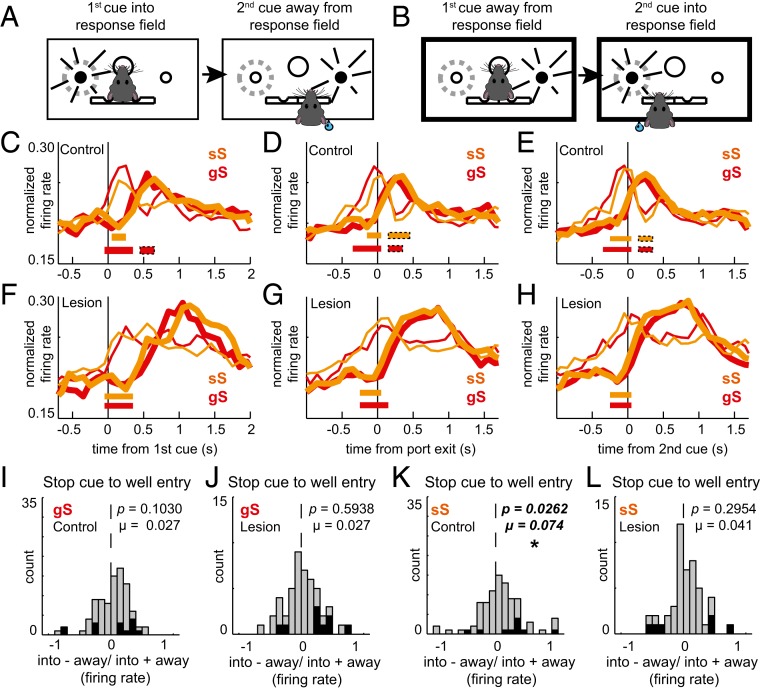
Fig. 4.
Unilateral ACC lesions disrupt STOP-change trial performance regardless of previous experience. (A and B) Illustrations of responding into and away from a neuron’s response field. (C and F) Population histograms comparing sequence effects in control (C; n = 89) and lesioned (F; n = 48) rats. Red lines represent gS trials, and orange lines represent sS trials. Thin lines represent movement away from the response field. Thick lines represent movement into the response field. The histogram is aligned to the first cue. Tick marks represent significance when comparing thick to thin lines within a trial type. (D and G) Population histograms comparing sequence effects between control (D) and lesioned (G) rats when aligned to port exit. (E and H) Population histograms comparing sequence effects between control (E) and lesioned (H) rats when aligned to the second cue. (I–L) Distributions of directional indices (into − away/into + away) during the second cue-light epoch (stop cue to well entry) for gS (I and J) and sS trials (K and L) for control (I and K) and lesioned (J and L) rats. The single asterisk (*) indicates significance at Wilcoxon P < 0.05.
When examining directional selectivity to the first cue light, the directional signal emerged earlier in gS trials (Fig. 4C, red; first 100-ms bin) compared to sS trials (Fig. 4C, orange; second 100-ms bin) and persisted longer in controls. This suggests that processing of the first cue light is diminished on sS trials in DMS, likely contributing to better performance on sS trials. Notably, this effect was not present in lesioned animals. Firing in DMS became directionally selective for both sS and gS within 100 ms after onset of the first light (Fig. 4F; t tests every 100 ms; P < 0.01). When comparing the two population histograms (Fig. 4 C and F), differences in the strength of the directional signal between control and lesion groups appear to rise from a reduction in firing to the presentation of the first cue light when it directed movement into the response field during sS trials (thin orange line) in controls. Indeed, when directly comparing the strength of this difference (i.e., gS – sS) during the first cue light epoch, we found that the difference was significantly stronger in controls compared to lesioned rats (Mann–Whitney U test = 2,013, control: µ = 0.7951; lesion: µ = −1.4809, P = 0.0285).
The above results suggest that ACC contributes to mechanisms that reduce automatic prepotent tendencies to follow the first cue light. Next, we asked whether ACC lesions impacted the frequency of neurons that accurately encoded response direction during conflict adaptation (i.e., sS trials), after presentation of the first cue light, in response to presentation of the second cue light (i.e., STOP signal). We found that for both control and lesioned rats that the distributions of directional indices did not significantly shift above zero on gS trials (control: Wilcoxon, n = 89, µ = 0.027, P = 0.1030; lesion: Wilcoxon, n = 48, µ = 0.027, P = 0.5938) and that there was no difference between groups (Wilcoxon, control: n = 89, µ = 0.027; lesion: n = 48, µ = 0.027, z = −0.3265, P = 0.5360). For sS trials (i.e., conflict adaptation), we found that the distribution of directional indices significantly shifted above zero for control rats only (Fig. 4J; control: Wilcoxon, n = 89, µ = 0.074, P = 0.0262; lesion: Wilcoxon, n = 48, µ = 0.041, P = 0.2954); however, there was no significant difference between controls and lesions (Wilcoxon, z = −0.3265, P = 0.4203).
Discussion
Adapting existing motor plans to better reflect the constraints of the environment is an essential component of complex behavior. While many studies have implicated the ACC in detecting the need to adapt and reshape motor plans, this study shows that ACC is necessary for adjusting action plans at the levels of behavior and single neuron firing.
To demonstrate this, we performed unilateral ACC lesions with ipsilateral DMS recordings to determine if the resolution of directional signals in DMS is dependent on ACC [connections are largely ipsilateral (13)]. Instead of bilateral lesions, we performed unilateral lesions to minimize the impact on behavior (i.e., the degree of impairment is likely less because one hemisphere remained intact) to assess changes in DMS firing without excessive changes to behavior or potential engagement of redundant systems (14). The task was selected because previous work has shown that single neurons in ACC are highly active prior to and during the resolution of directional signals in DMS (11). We found that, after ACC lesions, firing in DMS did not accurately represent the appropriate response direction on STOP-change trials and that, when rats made errors, firing strongly represented the incorrect, unresolved response. Consistent with these neural response patterns, rats with ACC lesions showed a significant reduction in accuracy on STOP-change trials and were slower to shift to a new response. This demonstrates that the ACC is necessary for appropriate response signaling in downstream regions as proposed by several computational and theoretical models (15–23).
Although our results strongly suggest that impairment of ACC function negatively impacts the ability to reactively modulate response selection at both the behavioral and the neural levels, alternative explanations should be considered. First, it might be argued that ACC lesions result in a decrease in the willingness to exert effort (2, 23). This interpretation seems unlikely because control and lesioned rats performed the same total number of trials (t(724) = 1.011, P = 0.3123), and latencies to initiate trials did not significantly differ (t(724) = 0.6124, P = 0.5405), suggesting that rats from both groups displayed similar levels of motivation and effort. Another possibility would be that rats are just better at GO trials after lesions, thus making inhibition and redirection to the second light more difficult. This seems unlikely given that accuracy on GO trials did not significantly differ between groups and previous recordings show that firing in ACC is relatively weak on GO trials (11). This result also suggests that basic motor functions remain intact. A third possibility is that ACC lesions impact mechanisms of arousal or attention. This is possible, but also seems unlikely because rats responded to houselights with a similar speed (t(724) = 0.6124, P = 0.5405) and did not show impairments on GO trials. Taken together, these findings suggest that ACC is necessary for the detection and mitigation of conflict between two competing action plans. That is not to say that ACC does not contribute to other functions, or that other brain areas do not contribute to these functions, but instead, these findings demonstrate that bother behavior and DMS activity relay on ACC to accomplish these particular features of executive control.
Although we saw general deficits on STOP-change trials, we did not observe disruption in behavioral measures related to conflict adaptation. Interestingly, however, at the neural level, firing to the first cue took longer to emerge and was attenuated on sS trials in control rats but not in rats with ACC lesions. Thus, it appears that ACC contributes to the modulation of downstream neural signals after having experienced a trial that required adjustment, either directly, through an intermediate, or in parallel with other structures [e.g., medial prefrontal cortex, orbitofrontal cortex (24, 25)]. Consistent with this observation, when ACC was lesioned, firing in DMS during error trials for responses made into the response field was not dampened prior to illumination of the second cue. Overall, these results suggest that, in addition to ACC modulating DMS to reactively adjust directional signals after presentation of STOP cues, ACC also acts by putting a brake on downstream targets to attenuate responding to the first cue light.
Although numerous lesion or ACC manipulation studies have linked ACC function with decision-making and outcome evaluation particularly within the context of foraging behavior (4, 20, 21, 26–29), few have supported its role in conflict monitoring (26–30) despite the historical attribution of ACC to this function (2, 22, 31, 32). In particular, decision-making studies using ACC lesions or disruptions have often yielded a surprisingly high number of negative results (26–30), making our results potentially surprising. We think that there are a variety of reasons that may account for this apparent discrepancy. First, many animal-based tasks rely on oculomotor movements, which are relatively ballistic, and offer fewer degrees of freedom when compared to the whole-body movements required for our task. Similarly, many variants of the stop-signal task require subjects to simply refrain from completing an action, rather than actually change the direction or outcome of an action after having already initiated an errant response.
The prevalence of negative results may also highlight anatomical discrepancies or differences in homology that may exist across species and even across studies within the same species. While there is debate about the degree to which rodents share homologous frontal lobe structures such as the ACC with humans (33–35), recent evidence suggests that there may be a rodent homolog of this region when looking at striatal-cortical connectivity (36); still other studies have highlighted high degrees of functional homology (33). Importantly, in our study, we targeted lesions to the same region of the ACC that exhibited increased activity on STOP trials (11). Thus, even if there is not an exact anatomical homolog in rats, our lesions targeted functionally homologous signals in the ACC to examine the role that they play at the level of behavior and downstream firing, which may have been key in obtaining the results described here.
Other features of the task may also account for discrepancies between our results and the existing literature. Many of the studies that report minimal changes in conflict monitoring with ACC disruption employ choice-based paradigms where subjects must adapt their behavior based on changes in the reward probabilities associated with different actions (26, 28, 29). On these choice-based tasks, errors stem from subjects’ inability to correctly detect and/or represent changes in reward value. In our task both GO and STOP trials are equally rewarded, and errors in performance stem from a failure to follow cues, rather than from a miscalculation of the likelihood of reward. While both forms of error could be argued to represent a failure in conflict monitoring, the latter would appear to rely more heavily on the subject’s perception of reward value, which may impact the “type” or severity of conflict that arises, thus potentially impacting the degree of ACC involvement.
While we do think that our results fit nicely within the conflict-monitoring framework, we recognize that one limitation of our study is that we were unable to manipulate reward value explicitly in our task and that reward signals may be tracked simultaneously with conflict-monitoring information. Previous theoretical arguments for ACC function certainly allow for the possibility that ACC may process multiple forms of information, if not serve, multiple functions (31, 32). Moreover, recent evidence from the orbital frontal cortex describes the discrete and simultaneous encoding of both inhibitory control and value information on a stop-signal task (37), suggesting that the encoding of control and value signals by a single brain region is at least possible. At present, we feel that our data provide a foundation for attempting to link animal and human accounts of ACC function, but we also feel strongly that future research specifically looking at foraging tasks while employing a similar methodology to ours may help to further elucidate the role(s) of ACC in guiding behavior. The use of more temporally precise or cell-type–specific techniques, such as optogenetics, may allow for the further validation of the findings presented here. Specifically, being able to manipulate ACC and DMS in different hemispheres during the presentation of the first and second cues, respectively, would help clarify whether ACC is responding to conflict specific to visual inputs in the task versus motor plans. Answering this is crucial for understanding specifically what ACC is responding to as well as in directing future research regarding the downstream regulation and implementation of cognitive control processes.
Materials and Methods
Animals.
Sixteen Long–Evans rats (eight females, eight males) were obtained at 175 to 200 g from Charles River Laboratories and weighed an average of 426.13 ± 36.7 g (males) and 286.38 ± 29.86 g (females) at the time of surgery. All rats were maintained on a 12-h light/dark schedule with lights on at 6 am. Food was provided ad libitum, but mice were water-restricted to 35 mL of water per day throughout training and testing. During weekends and the postsurgery recovery periods rats were provided with ad libitum access to food and water. All experimental procedures were approved by the University of Maryland Animal Care and Use Committee and conformed to the guidelines set forth by the National Research Council Guide for the Care and Use of Laboratory Animals (38).
Surgical Procedures.
Stereotaxic injection.
All surgical procedures were conducted using an aseptic technique. Rats (eight females, eight males) were randomly assigned to either the ibotenic acid (four females, four males) or saline treatment (four females, four males) conditions prior to surgery. Behavioral performance during training was assessed for the last 5 d of training prior to surgery in order to verify that groups showed no difference in behavioral performance prior to surgery. Prior to surgery, there were no differences in the average number of trials completed in a session (t(14) = 0.4149, P = 0.6845) or the in percentage of correct trials performed (t(14) = 0.807, P = 0.4332) between the groups. In order to investigate the role of the ACC in modulating motor outcomes and stopping behavior, rats, regardless of treatment condition, received two unilateral stereotactic injections spaced 1 mm apart targeting the ACC at the following coordinates relative to bregma (injection 1: AP, +0.2 mm; ML: ±0.5 mm; DV: −2.2 mm; injection 2: AP, +1.2 mm; ML: ±0.5 mm; DV: −2.2 mm). Coordinates were chosen based on a previous recording study targeting the same area (11) and in consultation with the authors of two previous ibotenic acid lesion studies that also targeted this brain region (10, 39). For each injection site, a beveled 33-ga, 5-µL Neuros Syringe (Hamilton) was lowered slowly over the course of 5 min to its final depth. Care was taken to ensure that the bevel of the needle was positioned away from the midline of the brain (10, 39). Rats were unilaterally infused with either 0.2 µL of 0.6 M ibotenic acid in saline or 0.2 µL of 0.9% saline per site over the course of 3 min (approximately 125 nL/min). Needles were left in place for 5 min before being slowly removed over the course of an additional 5 min in order to minimize the risk of tissue damage and backflow. Holes were loosely filled with sterile bonewax prior to beginning electrode implantation.
Electrode implantation.
Unilateral electrode implantation procedures were carried out after stereotaxic injection, and electrodes were implanted in the same hemisphere that was targeted by injection. Hemispheres were counterbalanced across groups, and the methods for implantation have been described in detail previously (11, 12, 24, 25, 40, 41). Rats were chronically implanted with a drivable bundle of 10, 25-µm diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) into either the left or right hemisphere of the DMS using the following coordinates relative to bregma (AP: −0.4 mm; ML: ±2.4 mm; DV: −3.5 mm). Coordinates were chosen based on our previous results investigating the role of the DMS using the STOP-change task (12). Immediately prior to implantation, wires were freshly cut with surgical scissors to extend ∼1 mm beyond the cannula and were electroplated with platinum (H2Cl6Pt) to an impedance of ∼300 kOhms. Cephalexin (15 mg/kg per os) was administered twice daily for 7 d following surgery to prevent infection.
Behavioral Task.
Recordings were conducted in custom-built aluminum chambers ∼18″ on each side with downward sloping walls narrowing to an area of 12″ × 12″ at the bottom. On one wall, the central port was located above two adjacent fluid wells with directional lights located directly next to each fluid well (Fig. 1A). House lights were located just above the panel. Task control was implemented via computer, and port-entry and well-entry times were monitored by photobeams.
The basic trial design is illustrated in Fig. 1A. Each trial began with the illumination of house lights that instructed the rat to nose-poke into the central port. Nose-poking initiated a 1,000-ms precue delay period. At the end of the precue delay period, a directional light located to the right or left of the rat was flashed for 100 ms. If the rat exited the port at any time prior to presentation of the directional cue, the trial was aborted and the house lights were extinguished. On 80% of trials, presentation of either the left or the right cue light signaled the direction of the fluid well in which the rat was to respond in order to obtain a liquid reward. On the remaining 20% of trials, the first cue was presented, but after a variable delay between 0 and 100 ms (selected with replacement from uniform distribution), the light opposite the location of the first cue was turned on and remained on until a behavioral response was made. These trials were referred to as STOP-change trials and were randomly interleaved with GO trials (i.e., the other 80% of trials) (Fig. 1 A and B). On STOP-change trials, rats were required to stop their initial movement signaled by the first cue and to respond in the direction of the second light in order to receive reward. Upon correct responding, rats were required to remain in the fluid well for a variable time period between 800 and 1,000 ms (prefluid delay) before a reward was delivered (10% sucrose solution). Intertrial intervals (ITI) for correct and incorrect responses was held at 4 and 7 s, respectively. Error trials (i.e., response in the incorrect direction) were immediately followed by the extinguishing of the house lights and ITI onset.
Trials were presented in a pseudorandom sequence such that left and right trials were presented in roughly equal numbers. The time necessary to stop and redirect a motor action (SCRT) on STOP trials was computed using the difference between the average movement time on correct STOP and Go trials (11, 12, 24, 25, 40). While we recognize that there are multiple ways to estimate the timing necessary to inhibit a movement (42), we chose to use SCRT because we have access to STOP trial movement time distributions and we varied the STOP-signal delay systematically across sessions, making SSRT-mean and integration methods inappropriate for our dataset (1, 42).
Single-Unit Recordings.
The procedures for single-unit recordings have been described previously (11, 12, 24, 25, 40). Wires were screened daily for activity; if no activity was detected, rats were removed from the testing box and the electrode assembly was advanced 40 to 80 µm. If activity was detected, the session occurred as usual and the electrode assembly was advanced 40 to 80 µm at the end of testing. Neural activity was recorded using four identical Multichannel Acquisition Processor systems (Plexon). Signals from electrode wires were amplified 20× by an op-amp headstage located on the electrode array. Immediately outside the testing chamber, signals were passed through a differential preamplifier (Plexon) where single units were amplified 50× and filtered at 150 to 9,000 Hz. Single-unit signals were then sent to the Multichannel Acquisition Processor box, where they were further filtered at 250 to 8,000 Hz, digitized at 40 kHz, and amplified at 1 to 32×. Waveforms (>2.5:1 signal to noise) were extracted from active channels and recorded to disk by an associated workstation with event timestamps provided by the behavioral computer.
Histology.
Following the completion of testing, rats were overdosed on isoflurane and transcardially perfused with 4% paraformaldehyde (PFA). The electrode assembly was removed from the skull and brains were extracted. Brains were postfixed for 48 h in 4% PFA before being moved to a 30% sucrose solution for cryoprotection. Following cryoprotection, brains were blocked, flash-frozen in alcohol, and sectioned on a freezing microtome. The 40-µm coronal sections were cut throughout the extent of the ACC and DMS. Sections were collected, mounted to positively charged Superfrost slides, and underwent Nissl staining. Slides were viewed under a light microscope, and the extent of the lesion and presence or absence of electrode tracks were verified and cross-referenced with score sheets demarcating electrode assembly advancement. Traces of the lesion and electrode tracks were placed on coordinate matched printouts of stereotaxic space.
Data Analysis and Statistics.
Units were sorted offline via Offline Sorter Version 3.3 software (Plexon) using a template-matching algorithm and analyzed using Neuroexplorer Version 4 software (Plexon) and Matlab (Mathworks; 2018b). Activity was examined during two response epochs: during the period of time following presentation of the first cue light until port exit and the period of time following presentation of the second cue light until well entry (stop-change trials only). Activity in the population histograms was normalized by dividing by the maximal firing rate of each neuron; however, statistical procedures were conducted using raw firing rates. Unless otherwise specified, behavioral data were analyzed using a two-way ANOVA where each data point represents a session average. To capture activity that differentiated based on a previous trial, we examined firing rates on GO and STOP trials that followed either a GO or STOP trial. This analysis allows for the examination of sequence effects as well as comparisons between trials that were not preceded by a need to adapt behavior (i.e., when a STOP follows a GO) versus trials that were preceded by a need to adapt behavior (i.e., when a STOP follows a STOP). For analysis of single units, we computed distributions of difference scores based on the raw firing rates (spikes) for each neuron. Distributions were deemed significant if they differed from either zero or one another via Wilcoxon sign-rank and rank sum/Mann–Whitney U tests, respectively.
Materials and Data Availability.
All Matlab data files and relevant documentation used in the analyses presented here have been archived and uploaded to the Digital Repository at the University of Maryland and are freely available in ref. 43.
Acknowledgments
This work was supported by National Institute of Mental Health Grant MH1117836 (to A.T.B.) and National Institute on Drug Abuse Grant DA031695 (to M.R.R.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All Matlab data files and relevant documentation used in the analyses presented here have been archived and uploaded to the Digital Repository at the University of Maryland and are freely available at https://drum.lib.umd.edu/handle/1903/25346.
References
- 1.Verbruggen F., et al. , A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 8, e46323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenhav A., Cohen J. D., Botvinick M. M., Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci. 19, 1286–1291 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Ebitz R. B., Hayden B. Y., Dorsal anterior cingulate: A Rorschach test for cognitive neuroscience. Nat. Neurosci. 19, 1278–1279 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kolling N., et al. , Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci. 19, 1280–1285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stolyarova A., et al. , Dissociable roles for anterior cingulate cortex and basolateral amygdala in decision confidence and learning under uncertainty. bioRxiv, 10.1101/655860 (2019). [DOI] [PMC free article] [PubMed]
- 6.van Holstein M., Floresco S. B., Dissociable roles for the ventral and dorsal medial prefrontal cortex in cue-guided risk/reward decision making. Neuropsychopharmacology, 10.1038/s41386-019-0557-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo J. V., Pardo P. J., Janer K. W., Raichle M. E., The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. U.S.A. 87, 256–259 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter C. S., et al. , Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–749 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Carter C. S., Mintun M., Cohen J. D., Interference and facilitation effects during selective attention: An H215O PET study of Stroop task performance. Neuroimage 2, 264–272 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Newman L. A., Creer D. J., McGaughy J. A., Cognitive control and the anterior cingulate cortex: How conflicting stimuli affect attentional control in the rat. J. Physiol. Paris 109, 95–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryden D. W., et al. , Single neurons in anterior cingulate cortex signal the need to change action during performance of a stop-change task that induces response competition. Cereb. Cortex 29, 1020–1031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryden D. W., Burton A. C., Kashtelyan V., Barnett B. R., Roesch M. R., Response inhibition signals and miscoding of direction in dorsomedial striatum. Front. Integr. Neurosci. 6, 69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi K., Holly E. N., Davatolhagh M. F., Beier K. T., Fuccillo M. V., Integrated anatomical and physiological mapping of striatal afferent projections. Eur. J. Neurosci. 49, 623–636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaidya A. R., Pujara M. S., Petrides M., Murray E. A., Fellows L. K., Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 23, 653–671 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botvinick M. M., Cohen J. D., The computational and neural basis of cognitive control: Charted territory and new frontiers. Cogn. Sci. 38, 1249–1285 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Vassena E., Holroyd C. B., Alexander W. H., Computational models of anterior cingulate cortex: At the crossroads between prediction and effort. Front. Neurosci. 11, 316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holroyd C. B., McClure S. M., Hierarchical control over effortful behavior by rodent medial frontal cortex: A computational model. Psychol. Rev. 122, 54–83 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Holroyd C. B., Yeung N., Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 16, 122–128 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Alexander W. H., Brown J. W., Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolling N., Behrens T. E. J., Mars R. B., Rushworth M. F. S., Neural mechanisms of foraging. Science 336, 95–98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolling N., Behrens T., Wittmann M. K., Rushworth M., Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol. 37, 36–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenhav A., Botvinick M. M., Cohen J. D., The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenhav A., Straccia M. A., Cohen J. D., Botvinick M. M., Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat. Neurosci. 17, 1249–1254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryden D. W., Roesch M. R., Executive control signals in orbitofrontal cortex during response inhibition. J. Neurosci. 35, 3903–3914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryden D. W., et al. , Prenatal nicotine exposure impairs executive control signals in medial prefrontal cortex. Neuropsychopharmacology 41, 716–725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fouragnan E. F., et al. , The macaque anterior cingulate cortex translates counterfactual choice value into actual behavioral change. Nat. Neurosci. 22, 797–808 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima K., Tanji J., Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335–1338 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Buckley M. J., et al. , Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science 325, 52–58 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Kennerley S. W., Walton M. E., Behrens T. E. J., Buckley M. J., Rushworth M. F. S., Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 9, 940–947 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Fellows L. K., Farah M. J., Is anterior cingulate cortex necessary for cognitive control? Brain 128, 788–796 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., Cohen J. D., Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Botvinick M. M., Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356–366 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Brown V. J., Bowman E. M., Rodent models of prefrontal cortical function. Trends Neurosci. 25, 340–343 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Laubach M., Amarante L. M., Swanson K., White S. R., What, if anything, is rodent prefrontal cortex? eNeuro 5, ENEURO.0315-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise S. P., Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 31, 599–608 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heilbronner S. R., Rodriguez-Romaguera J., Quirk G. J., Groenewegen H. J., Haber S. N., Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasubramani P. P., Pesce M. C., Hayden B. Y., Activity in orbitofrontal neuronal ensembles reflects inhibitory control. Eur. J. Neurosci., 10.1111/ejn.14638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Research Council , Guide for the Care and Use of Laboratory Animals. (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 39.Newman L. A., McGaughy J., Attentional effects of lesions to the anterior cingulate cortex: How prior reinforcement influences distractibility. Behav. Neurosci. 125, 360–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tennyson S. S., Brockett A. T., Hricz N. W., Bryden D. W., Roesch M. R., Firing of putative dopamine neurons in ventral tegmental area is modulated by probability of success during performance of a stop-change task. eNeuro 5, ENEURO.0007-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashtelyan V., Tobia S. C., Burton A. C., Bryden D. W., Roesch M. R., Basolateral amygdala encodes upcoming errors but not response conflict. Eur. J. Neurosci. 35, 952–959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbruggen F., Logan G. D., Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 12, 418–424 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brockett A. T., Roesch M. R., Anterior cingulate cortex is necessary for adaptation of action plans. Digital Repository at the University of Maryland. 10.13016/lhzo-v5qc. Deposited 24 January 2020. [DOI] [PMC free article] [PubMed]