Significance
Multiple fungal lineages have independently evolved carnivorous behaviors, preying on a diversity of nematodes as an adaptation for survival in low-nutrient environments. The edible oyster mushroom Pleurotus ostreatus is known to paralyze nematode prey, but the mechanism was unclear. We show that P. ostreatus triggers a massive calcium influx and rapid cell necrosis in the neuromuscular system of C. elegans via that nematode’s sensory cilia—a mode of action that is conserved across nematodes. Our study reveals a rapid killing mechanism that has not been described previously and is distinct from that employed by common anthelmintic drugs, representing a potential route for targeting parasitic nematodes. It also establishes a paradigm for studying cell death in C. elegans.
Keywords: C. elegans, oyster mushroom, predator–prey interactions, paralysis, cilia
Abstract
Fungal predatory behavior on nematodes has evolved independently in all major fungal lineages. The basidiomycete oyster mushroom Pleurotus ostreatus is a carnivorous fungus that preys on nematodes to supplement its nitrogen intake under nutrient-limiting conditions. Its hyphae can paralyze nematodes within a few minutes of contact, but the mechanism had remained unclear. We demonstrate that the predator–prey relationship is highly conserved between multiple Pleurotus species and a diversity of nematodes. To further investigate the cellular and molecular mechanisms underlying rapid nematode paralysis, we conducted genetic screens in Caenorhabditis elegans and isolated mutants that became resistant to P. ostreatus. We found that paralysis-resistant mutants all harbored loss-of-function mutations in genes required for ciliogenesis, demonstrating that the fungus induced paralysis via the cilia of nematode sensory neurons. Furthermore, we observed that P. ostreatus caused excess calcium influx and hypercontraction of the head and pharyngeal muscle cells, ultimately resulting in rapid necrosis of the entire nervous system and muscle cells throughout the entire organism. This cilia-dependent predatory mechanism is evolutionarily conserved in Pristionchus pacificus, a nematode species estimated to have diverged from C. elegans 280 to 430 million y ago. Thus, P. ostreatus exploits a nematode-killing mechanism that is distinct from widely used anthelmintic drugs such as ivermectin, levamisole, and aldicarb, representing a potential route for targeting parasitic nematodes in plants, animals, and humans.
Predators are known to evolve extraordinary adaptations to capture their prey. They employ diverse strategies, ranging from mechanical to chemical, to hunt effectively for their food. Athletic species such as leopards and cheetahs rely on their greater power and speed to catch their prey (1) whereas animals that are not athletic, such as cone snails, produce a large variety of peptide toxins that target ion channels and receptors in the neuromuscular systems of their prey (2).
Multiple fungal lineages of the Ascomycetes, Basidiomycetes, and Zygomycetes have independently evolved diverse strategies to prey on nematodes, the most abundant animals in soils (3), to supplement their nitrogen intakes. Nematode-trapping fungi such as Arthrobotrys oligospora (Ascomycota) and other closely related species are known to develop adhesive traps and constricting rings to mechanically catch their prey (4, 5). In contrast, the oyster mushroom Pleurotus ostreatus (Basidiomycota) produces chemicals to paralyze its nematode prey within a few minutes of contact (6, 7). Given the potent nematicidal activities of the Pleurotus species, these mushrooms may be used to control parasitic nematodes (8, 9). However, an effective management approach has not yet been established, partly because of the lack of knowledge of the basic biology of mushroom–nematode interactions in this microscopic predator–prey system. For instance, it had been unclear how P. ostreatus triggers paralysis in nematodes. Thus, we set out to investigate the cellular and molecular mechanisms of the Pleurotus-triggered paralysis in the model nematode Caenorhabditis elegans. We demonstrate that P. ostreatus paralyzes C. elegans via a previously unreported mechanism that is evolutionarily conserved across different nematode species. Through unbiased genetic screens, we found that the toxins produced by the Pleurotus mushrooms could only exert their nematicidal activity via the sensory cilia of C. elegans, triggering massive intracellular calcium influx and hypercontraction of the pharyngeal and body wall muscles, ultimately inducing cell necrosis in the neuromuscular system of the entire organism.
Results
Pleurotus Mushrooms Can Paralyze a Diversity of Nematode Species and Cause Massive Calcium Influx in C. elegans Pharyngeal and Body Wall Muscles.
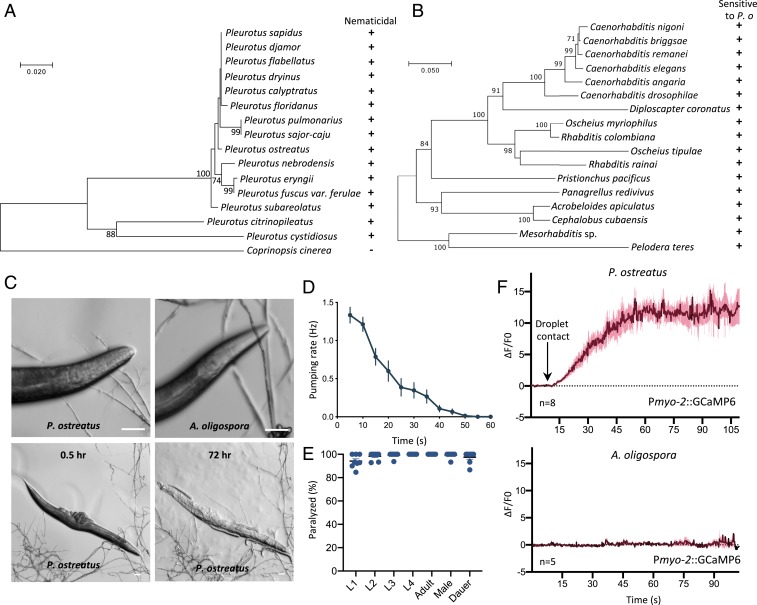
To assess how conserved is the predator–prey interaction between Pleurotus mushrooms and nematodes, we tested 15 species of Pleurotus for their ability to paralyze C. elegans. We cultured Pleurotus species on low-nutrient medium (LNM) and directly exposed C. elegans to the fungal hyphae. We observed that C. elegans were quickly paralyzed within a few minutes of contacting the fungal hyphae (Fig. 1A and SI Appendix, Fig. S1A). Next, we investigated if the oyster mushroom P. ostreatus could paralyze a diversity of nematode species and found that nematodes of the genera Caenorhabditis, Diploscapter, Oscheius, Rhabditis, Pristionchus, Panagrellus, Acrobeloides, Cephalobus, Mesorhabditis, and Pelodera were all paralyzed and ultimately consumed (Fig. 1 B and C and SI Appendix, Fig. S1B). These results demonstrate that the predator–prey relationship between Pleurotus fungi and nematodes is highly conserved.
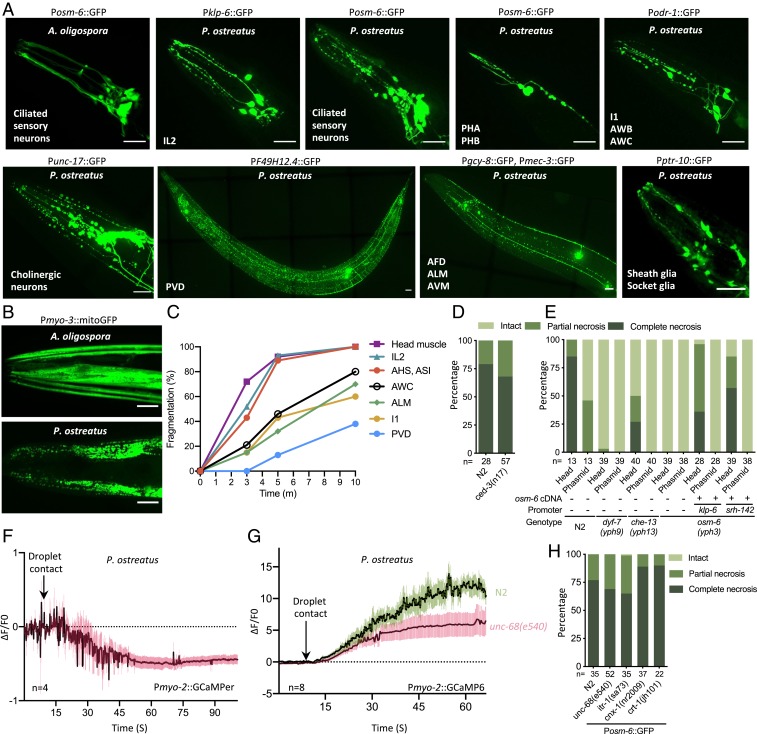
Fig. 1.
Pleurotus mushrooms trigger paralysis and muscle hypercontraction in nematodes, including C. elegans. (A) Phylogenetic tree of the ITS region of 15 Pleurotus species. “+” indicates paralysis and “−” indicates no effect on C. elegans wild-type strain N2. Coprinopsis cinerea was selected as an outgroup. (B) Phylogenetic tree of the small-subunit rDNA regions of 17 nematode species, all of which were paralyzed by P. ostreatus. (C) Adult N2 nematode interacting with P. ostreatus and A. oligospora hyphae. (Scale bars, 50 µm.) (D) Pharyngeal pumping rate of adult N2 on P. ostreatus (mean ± SEM; n = 15). (E) Quantification of the rate of paralysis for different developmental stages of N2. Each dot represents 15 to 20 animals (mean ± SEM, n = 8). (F) GCaMP6 signal of the pharyngeal corpus of adult N2 in response to P. ostreatus and A. oligospora hyphae (mean ± SEM; n shown above the x axis).
We then used the model nematode C. elegans to dissect the molecular mechanisms underlying P. ostreatus-induced paralysis. When C. elegans came into contact with fungal hyphae, we observed marked hypercontraction of the head muscles and cessation of pharyngeal pumping. Nematodes became immobilized almost immediately after the nose of the nematode touched the spherical droplet-like structure on the fungal hyphae (Fig. 1 C and D and Movie S1). This acute paralysis response differs considerably from the outcome when C. elegans encounters the nematode-trapping fungus A. oligospora, whereby the nematodes are attracted to the fungal hyphae and continue to move for several hours after triggering fungal trap morphogenesis via their ascaroside pheromones (10, 11). We found that all developmental stages of C. elegans are sensitive to P. ostreatus and they become paralyzed upon contacting the P. ostreatus hyphae (Fig. 1E). To further characterize the observed hypercontraction of the head muscle cells and cessation of pharyngeal pumping, we expressed in C. elegans the calcium indicator GCaMP6 (12) under the myo-2 and myo-3 promoters. We found that the calcium levels were massively increased (ΔF/F0 > 10) in the corpus region of the pharynx and in the head muscles (ΔF/F0 > 2.5) upon nematodes coming into contact with the fungal hyphae (Fig. 1F, SI Appendix, Fig. S1C, and Movies S2 and S3).
Forward Genetic Screens Revealed That Mutants Defective in Ciliogenesis Are Resistant to P. ostreatus-Induced Paralysis.
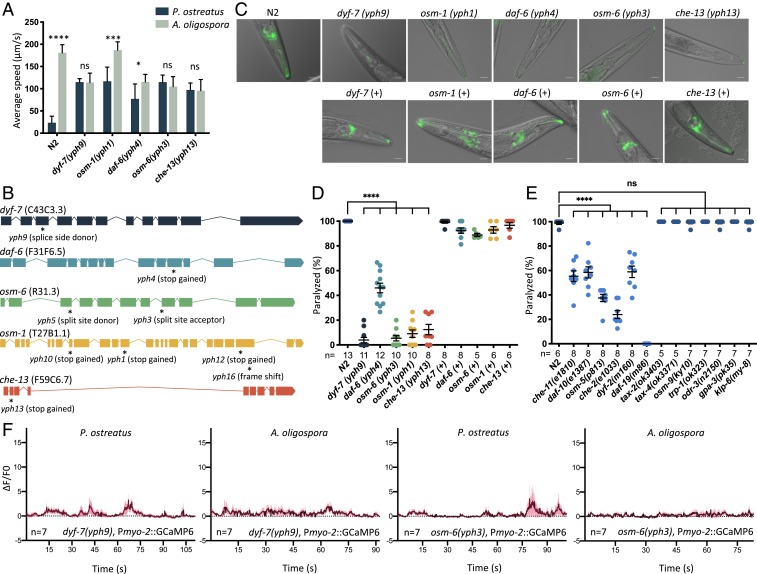
To gain molecular insights into the acute paralysis response of C. elegans, we conducted random ethyl methanesulfonate mutagenesis screens to identify C. elegans mutants resistant to P. ostreatus-induced paralysis. We conducted several rounds of mutagenesis to attain genome coverage of ∼200,000 and isolated a dozen mutants that exhibited locomotory ability on P. ostreatus hyphae. Quantitative measurements of the locomotion demonstrated that these mutants moved with a speed on P. ostreatus hyphae comparable to movements on the nonparalyzing nematode-trapping fungus A. oligospora (Fig. 2A and Movie S4). To identify the mutations responsible for this phenotype, we employed single-nucleotide polymorphism (SNP) mapping (13) and whole-genome sequencing (WGS) by CloudMap (14) and identified 9 independent alleles representing loss-of-function mutations in the C. elegans dyf-7 (15), daf-6 (16), osm-6 (17), osm-1 (18), and che-13 (19) genes (Fig. 2B and SI Appendix, Fig. S2). All of these genes have been extensively studied in C. elegans. dyf-7, osm-6, osm-1, and che-13 are required for the development of sensory cilia, whereas daf-6 is expressed in glial socket and sheath cells and is required for amphid channel morphogenesis (20, 21). A phenotypic hallmark of C. elegans mutants defective in ciliogenesis is the lack of dye uptake in the amphid sensory neurons when stained with the lipophilic dye DiI (22). Accordingly, when we applied DiI to our mutants, they failed to incorporate this fluorescent dye (Fig. 2C). By individually expressing the genomic fragments containing the respective wild-type genes in our mutants, we recovered their susceptibility to P. ostreatus-induced paralysis, as well as their ability to take up DiI dye in ciliated sensory neurons, demonstrating that resistance to fungal-induced paralysis was indeed caused by the loss-of-function mutations in these genes (Fig. 2 C and D). We further tested other C. elegans mutants that are known to exhibit defects in the development of ciliated sensory neurons (20)—namely the che-11, daf-10, osm-5, che-2, dyf-2, and daf-19 mutants—and found that all of these mutants exhibited various degrees of resistance to the paralysis induced by contact with P. ostreatus hyphae (Fig. 2E), supporting our conclusion that intact cilia of nematode sensory neurons are required for the fungus to induce paralysis in C. elegans. To establish if the signaling function of these sensory neurons is required to trigger the paralysis response, we examined the sensitivity to P. ostreatus of mutants deficient in signaling and function of the ciliated sensory neurons. We found that mutant lines exhibiting impaired neuronal signaling (23)—such as due to mutations of the cyclic nucleotide gated channels tax-2 and tax-4, G proteins odr-3 and gpa-3, and transient receptor potential (TRP) channels osm-9 and trp-1 or the kinesin-like protein klp-6—were all paralyzed by P. ostreatus to the same extent as the wild-type nematodes (Fig. 2E). These results demonstrate that the structure of nematode sensory cilia must be intact for the acute paralysis response but the signaling function of the sensory cilia is dispensable for responding to P. ostreatus. Furthermore, we also observed that the calcium level in the pharyngeal muscles of mutants resistant to P. ostreatus-induced paralysis (i.e., dyf-7 and osm-6) did not massively increase upon hyphal contact (Fig. 2F).
Fig. 2.
Genetic screens reveal that mutants defective in ciliogenesis are resistant to P. ostreatus-induced paralysis. (A) Movement of wild-type (N2) and P. ostreatus-resistant C. elegans mutants on P. ostreatus and A. oligospora (mean ± SD; n > 8). (B) Genetic mapping and whole-genome sequencing reveal the causative mutations in P. ostreatus-resistant mutants (marked by asterisks). Boxes and lines represent exons and introns, respectively. (C) Images of DiI-stained P. ostreatus-resistant mutants and the respective complemented strains. (+ indicates mutants harboring the reintroduced wild-type genomic locus; Materials and Methods). (Scale bars, 20 µm.) (D and E) Quantification of rates of paralysis for wild-type, mutant, and complemented strains of C. elegans on P. ostreatus hyphae. Each dot represents 15 to 20 animals (mean ± SEM; n shown below the x axis). (F) GCaMP6 signal of the pharyngeal corpus of P. ostreatus-resistant mutants in response to P. ostreatus and A. oligospora hyphae (mean ± SEM; n shown above the x axis). *P < 0.05, ***P < 0.001, ****P < 0.0001; ns, not significant.
Restoring Ciliogenesis of a Single Class of Nematode Neurons Whose Cilia Endings Are Externally Exposed Is Sufficient to Trigger Paralysis.
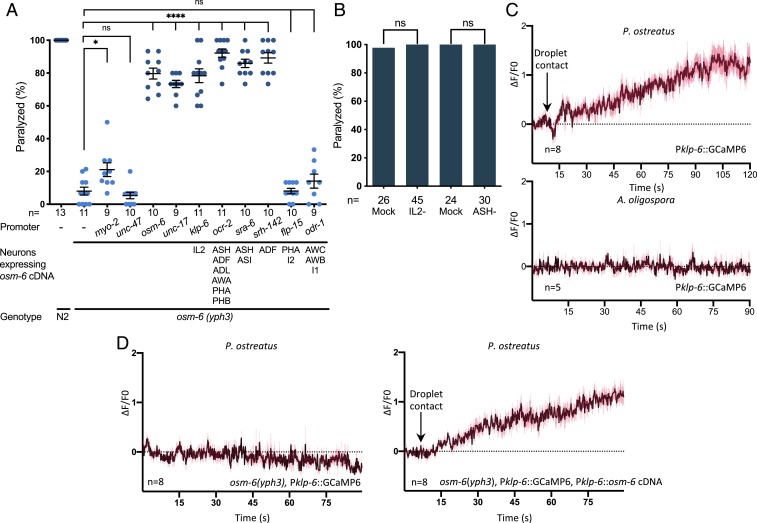
C. elegans possess ∼60 ciliated sensory neurons. To investigate if a single or multiple neurons are involved in triggering paralysis, we performed cell-specific rescue experiments in the osm-6(yph3) mutant background by using different promoters to drive expression of wild-type osm-6 complementary (c)DNA in various neurons (Fig. 3A and SI Appendix, Fig. S3). We found that if osm-6 expression was recapitulated in 1 or multiple head sensory neurons that have externally exposed ciliated dendritic endings (such as the IL2, ADF, ASH, and ASI neurons), the nematodes regained susceptibility to paralysis by P. ostreatus (Fig. 3A). In contrast, expressing osm-6 in the phasmid neurons of the tail, or in the olfactory AWB and AWC neurons that are enclosed in sheath cells, failed to recover the paralysis response to P. ostreatus hyphae (Fig. 3A). We then used laser ablation to kill the IL2 and ASH neurons to determine if they are required for paralysis and found that the neuron-ablated nematodes were still sensitive to P. ostreatus (Fig. 3B). These results demonstrate that multiple ciliated sensory neurons are involved in the paralysis triggered by P. ostreatus, and that certain neurons possessing externally exposed ciliated dendritic endings (such as IL2) are sufficient for the paralysis response. To monitor the activity of the IL2 neurons, we expressed GCaMP6 under the klp-6 promoter. We observed that after only 2 min of C. elegans touching P. ostreatus hyphae, there was a 2-fold increase in the GCaMP6 signals of nematode IL2 neurons (Fig. 3C and Movie S5), suggesting that the IL2 neurons had been activated by the fungal hyphae. However, activation of the IL2 neurons was not due to the touch response, because the GCaMP6 signal of IL2 neurons did not increase in response to touching A. oligospora hyphae. Furthermore, the activation of IL2 was dependent on osm-6, and could be complemented in a respective mutant line by expressing osm-6 cDNA under an IL2-specific promoter (Fig. 3D).
Fig. 3.
Multiple ciliated sensory neurons mediate responses to P. ostreatus hyphae. (A) Quantification of the rates of paralysis in wild-type C. elegans (N2), the osm-6 mutant, and cell-specific rescue lines expressing osm-6 cDNA under various promoters (mean ± SEM; n shown along the x axis). (B) Quantification of P. ostreatus-induced paralysis in mock and laser-ablated nematodes (n shown along the x axis). (C) GCaMP6 signal of IL2 neurons in adult N2 in response to P. ostreatus or A. oligospora hyphae (mean ± SEM; n shown above the x axis). (D) GCaMP6 signal of IL2 neurons in the osm-6 mutant (Left) and a cell-specific rescue line (Right) in response to P. ostreatus hyphae (mean ± SEM; n shown above the x axis). *P < 0.05, ****P < 0.0001.
Neuronal Activity Is Not Required for the Muscle Hypercontraction Triggered by P. ostreatus.
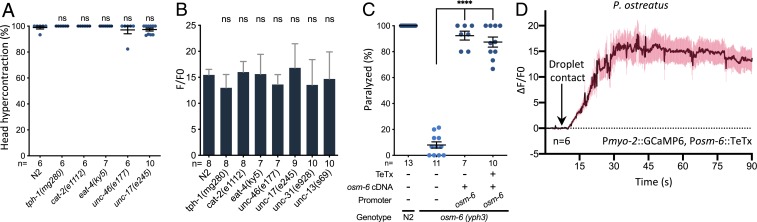
To establish if neuronal activity is required for the phenotypes we observed when C. elegans encountered P. ostreatus hyphae, we examined head muscle hypercontraction and monitored the pharyngeal calcium levels in tph-1 (serotonin), cat-2 (dopamine), eat-4 (glutamate), unc-46 (GABA), unc-17 (acetylcholine), unc-13 (synaptic vesicle fusion), and unc-31 (dense-core vesicle fusion) mutants. We found that these mutants exhibited comparable phenotypes compared with the wild-type animals, suggesting that these neurotransmitters are not required for the calcium influx observed in the pharyngeal muscle cells (Fig. 4 A and B). Moreover, when we blocked synaptic transmission by expressing tetanus toxin (TeTx) in ciliated sensory neurons, we found that it did not significantly affect paralysis or the increase in pharyngeal calcium levels (Fig. 4 C and D). These results suggest that neuronal activities are dispensable for the observed calcium influx in the pharynx muscles upon contacting Pleurotus hyphae.
Fig. 4.
Neuronal activity is not required for the paralysis induced by P. ostreatus hyphae. (A) Quantification of the head muscle hypercontraction phenotype in wild-type C. elegans (N2) and various neurotransmitter mutant lines (mean ± SEM; n shown along the x axis). (B) Quantification of the GCaMP6 signal in the pharyngeal corpus of the neurotransmitter mutant lines in response to P. ostreatus hyphae. (C) Quantification of paralysis in wild-type N2, osm-6 mutants, and mutants expressing osm-6 cDNA and the tetanus toxin under the osm-6 promoter (mean ± SEM; n shown along the x axis). (D) GCaMP6 signal of the pharyngeal corpus in wild-type N2 expressing TeTx in ciliated sensory neurons in response to P. ostreatus hyphae (n = 6). ****P < 0.0001.
P. ostreatus Triggers Rapid Cell Necrosis in Multiple Tissues of C. elegans.
When we monitored the GCaMP6 signals in the IL2 neurons in response to contact with P. ostreatus hyphae, we observed that the neuronal processes became fragmented within a few minutes of exposure to the fungal hyphae. Therefore, we systematically examined different types of neurons by exposing multiple C. elegans neuronal reporter lines to P. ostreatus hyphae for 10 min and then observed the resulting neuronal morphology. Strikingly, we observed massive neuron degeneration across the entire nervous system, including of the amphid and phasmid ciliated sensory neurons, cholinergic motor neurons, mechanosensory neurons, as well as glia cells (Fig. 5A and Movie S6). Moreover, all developmental stages of C. elegans showed pronounced fragmentation of the neuronal processes and swollen cell bodies, representing morphological features of neuronal necrosis (24, 25), in all developmental stages of C. elegans, and this massive neuronal necrosis was independent of neurotransmission (SI Appendix, Fig. S4 A and B). Furthermore, the muscle cells also exhibited prominent signs of necrosis after contacting the P. ostreatus hyphae (Fig. 5B). Approximately 90% of C. elegans presented signs of necrosis in the head sensory neurons with externally exposed ciliated dendrites 5 min after coming into contact with P. ostreatus hyphae, suggesting that the fungus exerts its paralytic activity very rapidly (Fig. 5C). We do not consider that the observed cell death is a form of apoptosis because ced-3 is not required (Fig. 5D). Next, we introduced the Posm-6::GFP reporter into the P. ostreatus-resistant dyf-7, che-13, and osm-6 mutants isolated from our genetic screens, and found that the neurons were largely intact even after 10 min of exposure to P. ostreatus hyphae (Fig. 5E). We again observed massive cell necrosis when we complemented osm-6 expression in a single class of ciliated sensory neurons (either IL2 or ADF), supporting our conclusion that the intact cilia structure of a single class of neurons is sufficient to trigger paralysis (Fig. 5E).
Fig. 5.
P. ostreatus triggers rapid cell necrosis via a mechanism that requires intact ciliated sensory neurons. (A) Images of cell necrosis in various neurons (indicated at the bottom left of each image) and glia cells. (Scale bars, 20 µm.) (B) Images of cell necrosis in the head or body wall muscle (Pmyo-3::mitoGFP). (Scale bars, 20 µm.) (C) Rates of cell fragmentation in various reporter lines that label specific neurons and head muscle (n > 20 for each time point). (D and E) Quantification of necrosis of ciliated sensory neurons (Posm-6::GFP) in wild-type N2 and ced-3 mutants (D), or Pleurotus-resistant mutants and cell-specific rescue lines expressing osm-6 cDNA under various promoters (E). (F) GCaMPer signal of the pharyngeal corpus of adult N2 in response to P. ostreatus hyphae (mean ± SEM; n shown above the x axis). (G) GCaMP6 signal of the pharyngeal corpus of adult N2 and unc-68(e540) mutants in response to P. ostreatus hyphae (mean ± SEM; n shown above the x axis). (H) Quantification of necrosis of ciliated sensory neurons in mutants in which calcium release is modulated from the ER.
Multiple pathways can contribute to necrotic cell death in C. elegans. The hyperactive degenerin ion channels (26, 27) and other stress signals converge to markedly increase intracellular Ca2+ levels (28), signaling cell death. Since we had observed a greater than 10-fold increase in GCaMP6 signal in nematode pharyngeal muscles, we tested if this massive calcium influx is the major factor that led to cell death. To examine if the source of Ca2+ influx originated from the sarcoplasmic reticulum in the pharyngeal muscles, we imaged the sarcoplasmic reticulum Ca2+ store by expressing a low-affinity GCaMP3 variant (GCaMPer) that had been designed to attach to the lumen of the endoplasmic reticulum (ER) (29). We observed that calcium levels decreased in the sarcoplasmic reticulum upon hyphal contact (Fig. 5F). Furthermore, when we monitored the Pmyo2::GCaMP6 signals in the unc-86 (ryanodine receptor) mutant background, we observed that the massively increased GCaMP signal upon hyphal contact was reduced to less than 50% of the wild-type nematodes, demonstrating that calcium influx into the pharynx was considerably diminished (Fig. 5G). Next, we examined if the ciliated sensory neurons and pharyngeal muscles of the unc-68 mutant also underwent necrosis upon contact with P. ostreatus hyphae and found that the level of cell necrosis was comparable to that of the wild-type C. elegans (Fig. 5H and SI Appendix, Fig. S4C). In addition, cell necrosis was also not affected in the itr-1, crt-1, and cnx-1 mutants that are known to regulate and block the necrotic cell death induced by the dominant MEC-4(d) channel (Fig. 5H and SI Appendix, Fig. S4 C and D) (30). Together, these results suggest that although the ER is the source of Ca2+ influx, the downstream mechanism may be distinct from previously well-characterized hyperactivated channel-mediated neurotoxicity in C. elegans (30).
Cilia-Dependent Paralysis by P. ostreatus Is Evolutionarily Conserved and the Mechanism Is Distinct from That of Known Anthelmintic Drugs.
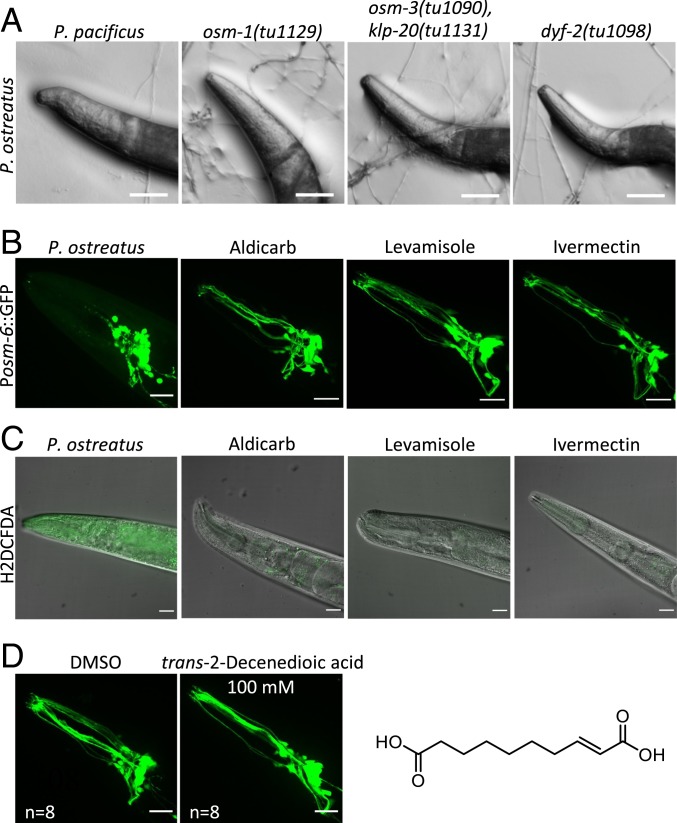
Since we observed that P. ostreatus could paralyze a diversity of nematode species, we wondered if the cilia-dependent mechanism is evolutionarily conserved across nematode lineages. Many genes required for ciliogenesis are highly conserved in nematodes. Therefore, we examined the osm-1, dyf-2, and osm-3 klp20 double mutants of the nematode Pristionchus pacificus (31) for their response to P. ostreatus hyphae. P. pacificus diverged from C. elegans 280 to 430 million y ago, but we found that these P. pacificus mutants were also resistant to P. ostreatus, demonstrating that the cilia-dependent mechanism of nematode paralysis is evolutionarily conserved (Fig. 6A and Movie S7).
Fig. 6.
Mechanism of paralysis triggered by P. ostreatus hyphae is evolutionarily conserved among nematodes and is distinct from that of well-known anthelmintic drugs. (A) Images of wild-type P. pacificus and cilia-defective mutants interacting with the hyphae of P. ostreatus. (Scale bars, 50 µm.) (B) Images of ciliated sensory neurons (Posm-6::GFP) in C. elegans treated with P. ostreatus, aldicarb, levamisole, or ivermectin. (Scale bars, 20 µm.) (C) Images of the head regions of paralyzed C. elegans stained with an indicator for reactive oxygen species (H2DCFDA). (Scale bars, 20 µm.) (D) Images of ciliated sensory neurons (Posm-6::GFP) in C. elegans treated with trans-2-decenedioic acid (100 mM) or DMSO control. (Scale bars, 20 µm.) The chemical formula of trans-2-decenedioic acid is also shown.
We then tested if the cilia-mediated neuronal necrosis evoked by P. ostreatus hyphae is a feature distinct from the effect elicited by anthelmintic drugs that can also paralyze nematodes, such as ivermectin, aldicarb, and levamisole, which also paralyze C. elegans. Research over the past 4 decades has revealed that these anthelmintic drugs act at neuromuscular junctions (32, 33), targeting nicotinic acetylcholine receptors (levamisole) (34), glutamate-gated chloride channels (ivermectin) (35), and acetylcholinesterase (aldicarb) (36). We examined if any of these drugs cause necrosis of the ciliated sensory neurons, but there were no indications of neuronal necrosis in C. elegans that were paralyzed by aldicarb, levamisole, or ivermectin (Fig. 6B). Furthermore, when we stained the paralyzed C. elegans with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), which detects reactive oxygen species (ROS), we only observed strong fluorescent signals in nematodes paralyzed upon contact with P. ostreatus. However, ivermectin, aldicarb, and levamisole, did not trigger ROS accumulation in the paralyzed nematodes (Fig. 6C). These data indicate that P. ostreatus paralyzes nematodes through a mechanism that is distinct from that elicited by popular anthelmintic drugs.
Does P. ostreatus produce a nematicidal toxin and what might be its chemical nature? P. ostreatus has been reported to produce the nematicidal toxin trans-2-decenedioic acid (7), and application of 330 ppm of this toxin to a population of nematodes immobilized 95% of them within an hour in vitro (7), unlike the fast-acting paralysis response of nematodes in contact with the hyphae of P. ostreatus in vivo. Therefore, we suspect that P. ostreatus produces other yet to be identified nematicidal toxins. To test if trans-2-decenedioic acid (7) is responsible for the paralysis phenotype we observed in C. elegans, we synthesized this compound and tested if it induces paralysis and neuronal necrosis. We observed that C. elegans locomotion was not affected even by a high concentration (100 mM) of trans-2-decenedioic acid and that the ciliated sensory neurons of the nematodes looked healthy (Fig. 6D). These results suggest that trans-2-decenedioic acid is not the compound produced by P. ostreatus responsible for rapid paralysis and systemic necrosis in C. elegans.
Discussion
Toxin-Induced Necrotic Responses in C. elegans.
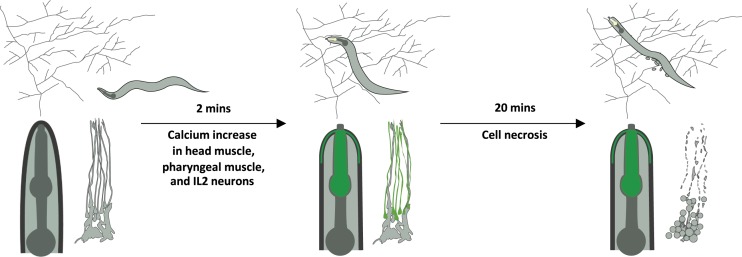
Many animal predators produce toxins to incapacitate their prey, and the responses of various nematodes to Pleurotus hyphae we present here strongly indicate that these mushrooms have independently evolved a similar strategy. Our genetic analyses clearly demonstrate that the purported toxin enters the nematode body via the sensory cilia to cause rapid and systemic necrosis in multiple tissues throughout the organism (Fig. 7). Several bacterial pathogens, including Enterococcus faecalis, Photorhabdus luminescens, and Bacillus thuringiensis, are known to elicit necrotic responses in C. elegans (37, 38). B. thuringiensis produces crystal pore-forming toxins that bind to glycolipids and are lethal to both nematodes and insects (39, 40). It was demonstrated recently that the crystal protein Cry6Aa triggers the necrosis pathway mediated by the aspartic protease ASP-1 (37). In the case of infections caused by Erwinia carotovora or P. luminescens, necrosis follows the bacterial infection and aggravates nematode pathology (38). These bacteria-triggered necrotic responses shared certain characteristics with the P. ostreatus-triggered necrosis we observed. However, P. ostreatus-induced necrosis occurred within minutes, whereas the bacterial infection-triggered necrosis took place only 24 h postinfection (38).
Fig. 7.
Contact with P. ostreatus hyphae induces massive cell calcium influx and cell necrosis in nematodes via ciliated sensory neurons.
Convergent Evolution of the Predatory Fungal Mechanism Targeting Sensory Cilia of Nematode Prey.
Primary cilia are sensory organelles essential for cells to sense their environments and transduce external signals (41). Studies over the past 20 y have revealed that malfunctioning cilia can give rise to many diseases (ciliopathies), and C. elegans has proven to be a valuable model that has contributed to our understanding of cilia biology and to the molecular basis of ciliopathies such as polycystic kidney disease and nephronophthisis (42–46). The only ciliated cell type in C. elegans is the sensory neuron (20). C. elegans have ∼60 neurons possessing diverse ciliate structures, and it is well-established that mutants exhibiting defective cilia development or function display severely compromised sensory responses, such as in chemosensation, osmosensation, mechanosensation, thermosensation, and mating (22, 43, 47). Therefore, it is likely that these mutants are inviable in nature.
Predators are known to eavesdrop on essential communication cues of their prey (48). Such strategies have evolved in animal predators, as well as carnivorous plants and fungi. For example, Venus flytraps, sundews, and pitcher plants produce fruity or flowery scents to attract their insect prey (49, 50). In our previous studies, we demonstrated that the nematode-trapping fungus A. oligospora not only eavesdrops on the nematode ascaroside pheromones but also lures the nematode prey by producing odorants that mimic food and sex cues that are highly attractive to many nematode species (10, 11). The chemotactic behavior of C. elegans depends on functional cilia and neurons so sensory cilia are also the target for nematode-trapping fungi when they seek to capture nematodes. In this study, we have demonstrated that a distinct nematicidal mechanism, which also exploits nematode sensory cilia, has independently evolved in the carnivorous oyster mushroom P. ostreatus. Both predatory fungi, P. ostreatus and A. oligospora, target the sensory cilia of nematodes to lure or capture their nematode prey.
Future Applications of P. ostreatus Treatment for Parasitic Nematode Control.
In recent years, anthelmintic resistance has become a major global problem for controlling livestock parasites and has also been observed in patients suffering from river blindness (51, 52). Thus, new anthelmintic compounds with novel mechanisms of action are urgently required. Our results demonstrate that the hyphae of P. ostreatus can paralyze nematodes via a mechanism that is distinct from those of several currently available anthelmintics, including ivermectin, which is the most widely used antiparasitic drug in human and veterinary medicine (53). Furthermore, since P. ostreatus is highly effective against a wide range of nematode species, we believe that the paralysis-inducing agent produced by P. ostreatus has far-reaching potential as a novel type of drug that could be used in the future to target the parasitic nematodes of plants, animals, and humans.
Materials and Methods
Strains.
Nematode strains were maintained at room temperature on nematode growth medium plates seeded with Escherichia coli, OP50. Transgenic animals were generated using standard microinjection techniques (54). Fungal cultures were grown on potato dextrose agar (Difco) or low-nutrient mineral salts medium (2% agar, 1.66 mM MgSO4, 5.4 µM ZnSO4, 2.6 µM MnSO4, 18.5 µM FeCl3, 13.4 mM KCl, 0.34 µM biotin, and 0.75 μM thiamin). The nematode and fungal strains used in this study and their sources are listed in SI Appendix.
Genetic Screen, Single-Nucleotide Polymorphism Mapping, and Whole-Genome Sequencing.
Ethyl methanesulfonate mutagenesis was conducted following a standard protocol (55). Semisynchronized F2 populations were screened on P. ostreatus cultures. Mutants that exhibited a resistant phenotype were singled out and their progeny were subjected to rescreening. Approximately 200,000 genomes were screened, and ∼30 mutants were isolated. Nine mutants which exhibited a strong resistant phenotype were subjected to further genetic mapping and WGS. Rapid SNP mapping with the Hawaiian strain was conducted as described (13). Genomic DNA was extracted from the F2 progeny of the mutant and Hawaiian crosses and subjected to Illumina WGS at 11× to 13× depth of coverage. WGS data were analyzed using the CloudMap pipeline (14).
C. elegans Response Assays.
C. elegans locomotion was recorded and analyzed using WormLab (MBF Bioscience). One-day-old adult hermaphrodites were transferred to 14-d LNM cultures of P. ostreatus and A. oligospora. After 10 min of fungal exposure, their behavior was recorded for 2 min. To quantify the rate of paralysis, 15 to 20 adult animals were transferred to P. ostreatus for 10 min. Hypercontraction of the head muscles and cessation of pharyngeal pumping defined the paralysis phenotype. To assess the C. elegans pumping rate in contact with P. ostreatus, 1-d-old adult N2 individuals were placed on P. ostreatus and the pumping rate was scored by eye using a stereo dissecting microscope (Nikon; SMZ745). The pumping rate was determined by the number of pumping events every 5 s.
Calcium Imaging.
Transgenic animals expressing GCaMP6 under various promoters were transferred to P. ostreatus or A. oligospora cultures and the calcium responses were recorded with a Zeiss SteREO V20 microscope with an Andor Zyla 5.5 sCMOS camera. The fluorescence intensity of the region of interest (ROI) was automatically drawn and analyzed via a customized module in MetaXpress. F is the fluorescence intensity of the ROI-subtracted background fluorescence. F0 is the average F of ∼80 frames at the beginning of the video.
Cell-Specific Rescue.
osm-6 cDNA was PCR-amplified from N2 cDNA, subcloned into a pSM plasmid using the In-Fusion HD Cloning Kit (Takara). Various promoter sequences were further subcloned into the plasmid using SphI and AscI. The plasmids were injected into the osm-6 mutant (yph3) background. One-day-old adults of the transgenic animals were subjected to C. elegans response assay and dye-filling assay.
Neuronal Necrosis Assay.
Nematodes were transferred to P. ostreatus or A. oligospora for 10 min, and subjected to a 3% agar pad on glass slides with 500 mM NaN3. The neuronal necrosis phenotype was quantified and imaged by compound microscopy 20 min later. To image the neuronal necrosis, the nematodes were analyzed with an Andor Revolution WD with a Nikon Ti-E automatic microscope with a Lambda 60× oil lens. Images were captured with an iXon Ultra 888 EMCCD (Andor), and analyzed with MetaMorph.
Statistics.
Two-tailed Student’s t test was performed to determine the statistical difference between the control and remaining samples using GraphPad Prism 8. In the figures, asterisks denote statistical significance as calculated by Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Data Availability.
All data are available in the article, the SI Appendix, and Movies S1–S7. Additional methods can be found in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetic Center (CGC), WormBase, P.W.S., Chun-Liang Pan, Yi-Chun Wu, and Ralf Sommer for sharing the nematode strains; and the Bioresource Collection and Research Center, Sung-Yuan Hsieh, and Ursula Kües for sharing the fungal strains. We thank Brandon Harvey for providing the GCaMPer construct. We are grateful to Chun-Liang Pan, John O’Brien, and Meng-Chao Yao for their comments and suggestions on the manuscript; and for the technical assistance of Ling-Mei Hsu and A-Mei Yang in the laboratory. We thank Ching-Che Lee for designing the graphic model. The CGC is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by Academia Sinica Career Development Award AS-CDA-106-L03 and the Foundation for the Advancement of Outstanding Scholarship Young Scholars’ Creativity Award (to Y.-P.H.).
Footnotes
Competing interest statement: P.W.S. provided some materials for this work.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918473117/-/DCSupplemental.
References
- 1.Wilson A. M., et al. , Biomechanics of predator-prey arms race in lion, zebra, cheetah and impala. Nature 554, 183–188 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Olivera B. M., et al. , Peptide neurotoxins from fish-hunting cone snails. Science 230, 1338–1343 (1985). [DOI] [PubMed] [Google Scholar]
- 3.van den Hoogen J., et al. , Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Nordbring-Hertz B., Nematophagous fungi: Strategies for nematode exploitation and for survival. Microbiol. Sci. 5, 108–116 (1988). [PubMed] [Google Scholar]
- 5.Barron G. L., The Nematode-Destroying Fungi (Canadian Biological Publications, 1977). [Google Scholar]
- 6.Thorn R. G., Barron G. L., Carnivorous mushrooms. Science 224, 76–78 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Kwok O. C. H., Plattner R., Weisleder D., Wicklow D. T., A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 18, 127–136 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Hahn M. H., De Mio L. L. M., Kuhn O. J., Duarte H. D. S., Nematophagous mushrooms can be an alternative to control Meloidogyne javanica. Biol. Control 138, 104024 (2019). [Google Scholar]
- 9.Okorie C. C., Ononuju C. C., Okwujiako I. A., Management of Meloidogyne incognita with Pleurotus ostreatus and P. tuberregium in soybean. Int. J. Agric. Biol. 13, 401–405 (2011). [Google Scholar]
- 10.Hsueh Y. P., Mahanti P., Schroeder F. C., Sternberg P. W., Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 23, 83–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh Y. P., et al. , Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 6, e20023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T. W., et al. , Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M. W., et al. , Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genom. 6, 118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., CloudMap: A cloud-based pipeline for analysis of mutant genome sequences. Genetics 192, 1249–1269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiman M. G., Shaham S., DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137, 344–355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perens E. A., Shaham S., C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev. Cell 8, 893–906 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Collet J., Spike C. A., Lundquist E. A., Shaw J. E., Herman R. K., Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148, 187–200 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell L. R., Stone S., Yochem J., Shaw J. E., Herman R. K., The molecular identities of the Caenorhabditis elegans intraflagellar transport genes dyf-6, daf-10 and osm-1. Genetics 173, 1275–1286 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haycraft C. J., Schafer J. C., Zhang Q., Taulman P. D., Yoder B. K., Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp. Cell Res. 284, 251–263 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Inglis P. N., Ou G., Leroux M. R., Scholey J. M., The sensory cilia of Caenorhabditis elegans. WormBook, 1–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oikonomou G., et al. , Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans. PLoS Biol. 9, e1001121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G., Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 (1986). [DOI] [PubMed] [Google Scholar]
- 23.Bargmann C. I., Chemosensation in C. elegans. WormBook, 1–29 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall D. H., et al. , Neuropathology of degenerative cell death in Caenorhabditis elegans. J. Neurosci. 17, 1033–1045 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikoletopoulou V., Tavernarakis N., Necrotic cell death in Caenorhabditis elegans. Methods Enzymol. 545, 127–155 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Driscoll M., Chalfie M., The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349, 588–593 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Chalfie M., Wolinsky E., The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345, 410–416 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Kourtis N., Tavernarakis N., Non-developmentally programmed cell death in Caenorhabditis elegans. Semin. Cancer Biol. 17, 122–133 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Henderson M. J., et al. , A low affinity GCaMP3 variant (GCaMPer) for imaging the endoplasmic reticulum calcium store. PLoS One 10, e0139273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu K., Tavernarakis N., Driscoll M., Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 31, 957–971 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Moreno E., et al. , Regulation of hyperoxia-induced social behaviour in Pristionchus pacificus nematodes requires a novel cilia-mediated environmental input. Sci. Rep. 7, 17550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazie S. M., Jin Y., Pharming for genes in neurotransmission: Combining chemical and genetic approaches in Caenorhabditis elegans. ACS Chem. Neurosci. 9, 1963–1974 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holden-Dye L., Walker R. J., Anthelmintic drugs and nematicides: Studies in Caenorhabditis elegans. WormBook, 1–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis J. A., Wu C. H., Berg H., Levine J. H., The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95, 905–928 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dent J. A., Smith M. M., Vassilatis D. K., Avery L., The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 97, 2674–2679 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen M., Alfonso A., Johnson C. D., Rand J. B., Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140, 527–535 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F., et al. , Bacillus thuringiensis crystal protein Cry6Aa triggers Caenorhabditis elegans necrosis pathway mediated by aspartic protease (ASP-1). PLoS Pathog. 12, e1005389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong D., Bazopoulou D., Pujol N., Tavernarakis N., Ewbank J. J., Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 8, R194 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffitts J. S., et al. , Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307, 922–925 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Cappello M., et al. , A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc. Natl. Acad. Sci. U.S.A. 103, 15154–15159 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nachury M. V., Mick D. U., Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 20, 389–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jauregui A. R., Nguyen K. C. Q., Hall D. H., Barr M. M., The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J. Cell Biol. 180, 973–988 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barr M. M., Sternberg P. W., A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386–389 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Sengupta P., Cilia and sensory signaling: The journey from “animalcules” to human disease. PLoS Biol. 15, e2002240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nechipurenko I. V., Sengupta P., The rise and fall of basal bodies in the nematode Caenorhabditis elegans. Cilia 6, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., et al. , C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol. 24, 519–525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bargmann C. I., Hartwieg E., Horvitz H. R., Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527 (1993). [DOI] [PubMed] [Google Scholar]
- 48.Siemers B. M., Kriner E., Kaipf I., Simon M., Greif S., Bats eavesdrop on the sound of copulating flies. Curr. Biol. 22, R563–R564 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Jurgens A., El-Sayed A. M., Suckling D. M., Do carnivorous plants use volatiles for attracting prey insects? Funct. Ecol. 23, 875–887 (2009). [Google Scholar]
- 50.Di Giusto B., et al. , Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. J. Ecol. 98, 845–856 (2010). [Google Scholar]
- 51.Kaplan R. M., Vidyashankar A. N., An inconvenient truth: Global worming and anthelmintic resistance. Vet. Parasitol. 186, 70–78 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Osei-Atweneboana M. Y., et al. , Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 5, e998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laing R., Gillan V., Devaney E., Ivermectin—Old drug, new tricks? Trends Parasitol. 33, 463–472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kutscher L. M., Shaham S., Forward and reverse mutagenesis in C. elegans. WormBook, 1–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the article, the SI Appendix, and Movies S1–S7. Additional methods can be found in SI Appendix, Materials and Methods.