Significance
The acid dissociation constant (pKa) and protonation state of active-site residues plays a critical role in enzyme catalysis. Elucidating how these properties may be influenced by substrate/inhibitor binding is crucial to a deep understanding of enzyme function and inhibition. In this study, we focus on class A β-lactamases, one of the most common mediators of β-lactam antibiotic resistance in gram-negative bacteria. Through a series of high-resolution crystal structures of the class A β-lactamase CTX-M-14 in complex with the β-lactamase inhibitor avibactam at varying pHs, combined with pKa calculations and NMR, we examine how this inhibitor affects active-site residues’ pKa values and proton transfer. The results offer important insights into the mechanism of avibactam inhibition and the substrate influence on β-lactamase catalysis.
Keywords: serine beta-lactamase, avibactam, beta-lactamase inhibitor, acyl–enzyme, pKa perturbation
Abstract
Gram-negative bacteria expressing class A β-lactamases pose a serious health threat due to their ability to inactivate all β-lactam antibiotics. The acyl–enzyme intermediate is a central milestone in the hydrolysis reaction catalyzed by these enzymes. However, the protonation states of the catalytic residues in this complex have never been fully analyzed experimentally due to inherent difficulties. To help unravel the ambiguity surrounding class A β-lactamase catalysis, we have used ultrahigh-resolution X-ray crystallography and the recently approved β-lactamase inhibitor avibactam to trap the acyl–enzyme complex of class A β-lactamase CTX-M-14 at varying pHs. A 0.83-Å-resolution CTX-M-14 complex structure at pH 7.9 revealed a neutral state for both Lys73 and Glu166. Furthermore, the avibactam hydroxylamine-O-sulfonate group conformation varied according to pH, and this conformational switch appeared to correspond to a change in the Lys73 protonation state at low pH. In conjunction with computational analyses, our structures suggest that Lys73 has a perturbed acid dissociation constant (pKa) compared with acyl–enzyme complexes with β-lactams, hindering its function to deprotonate Glu166 and the initiation of the deacylation reaction. Further NMR analysis demonstrated Lys73 pKa to be ∼5.2 to 5.6. Together with previous ultrahigh-resolution crystal structures, these findings enable us to follow the proton transfer process of the entire acylation reaction and reveal the critical role of Lys73. They also shed light on the stability and reversibility of the avibactam carbamoyl acyl–enzyme complex, highlighting the effect of substrate functional groups in influencing the protonation states of catalytic residues and subsequently the progression of the reaction.
The β-lactamases are bacterial enzymes that catalyze the hydrolysis of β-lactam antibiotics (1, 2). Class A, C, and D β-lactamases belong to a broadly diverse family of enzymes known as serine β-lactamases (3). In contrast, the remaining class B β-lactamases are metalloenzymes, which require divalent zinc ions for activity (1). Class A β-lactamases are the most prevalent β-lactamases in multidrug-resistant gram-negative bacteria, including the KPC-2 carbapenemase that inactivates nearly all β-lactam compounds, and represent an important target for antimicrobial drug discovery efforts (1, 4–8).
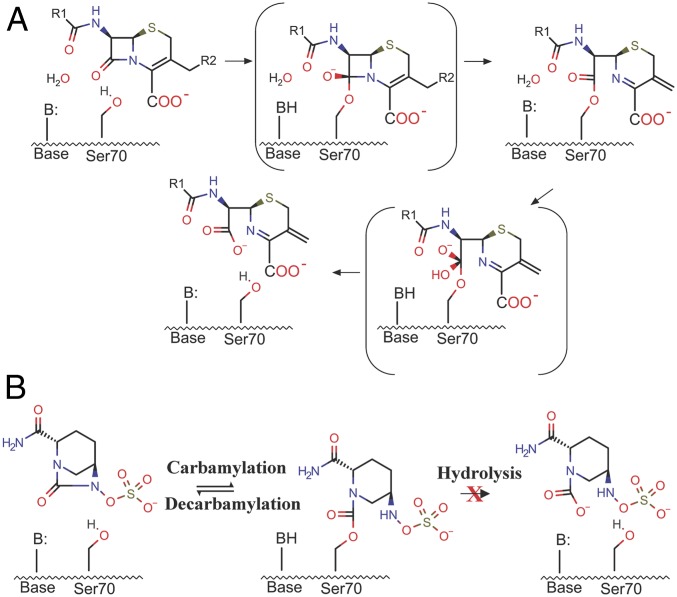
All class A β-lactamases catalyze β-lactam hydrolysis in a two-step acylation–deacylation reaction (Fig. 1A) (9). Upon the formation of a precovalent enzyme-substrate complex, the acylation reaction is initiated through a general base-mediated activation of Ser70, which subsequently behaves as nucleophile to attack the carbonyl carbon of the β-lactam ring to form an acyl–enzyme intermediate. Concomitantly, a proton is transferred to the substrate ring nitrogen. For cephalosporins, this proton is subsequently lost together with the C3 side chain during the ring isomerization process (10). In the second step of class A β-lactamase catalysis, the acyl–enzyme intermediate undergoes nucleophilic attack via a general base-activated hydrolytic water molecule, leading to deacylation and release of a hydrolyzed and inactivated β-lactam product (9, 11).
Fig. 1.
General mechanism of β-lactam hydrolysis and avibactam inhibition of class A β-lactamases. The high-energy TSs are indicated by brackets. (A) Hydrolysis of β-lactam substrates such as cephalosporins proceeds through a precovalent enzyme–substrate complex, acylation TS, an acyl–enzyme intermediate, deacylation TS, and finally a hydrolyzed β-lactam product. (B) Avibactam reversibly reacts with the active site via carbamylation. The reverse decarbamylation reaction is slow but more thermodynamically favorable than the hydrolysis reaction seen for β-lactam substrates.
Although ultrahigh-resolution crystal structures and quantum mechanical/molecular mechanical (QM/MM) simulations have been carried out on class A β-lactamases, there is not a clear consensus on the acylation mechanism, concerning the proton transfer from Ser70 ultimately to the leaving group ring nitrogen during the progression of the acylation reaction and the subsequent transition into the deacylation step (12, 13). As the acyl–enzyme complex of class A β-lactamases is the key intermediate in this hydrolysis reaction, a detailed understanding of the molecular interactions in this complex is essential to elucidating the catalytic mechanism of class A β-lactamases. For β-lactam substrates, the acyl–enzyme complex is a transient intermediate. However, the recently developed β-lactamase inhibitor avibactam traps the enzyme in a stable carbamoyl acyl–enzyme state. Avibactam is a non–β-lactam β-lactamase inhibitor that exhibits excellent activity against serine β-lactamases mainly belonging to classes A and C (14–16). It consists of a diazabicyclooctane (DBO) core scaffold that contains an amide group that reacts with the nucleophilic serine of β-lactamases via a unique reversible carbamylation/decarbamylation reaction that allows the inhibitor to be recycled (Fig. 1B) (15–18). A high-resolution complex crystal structure of CTX-M-15 with avibactam revealed valuable information about avibactam inhibition, especially a protonated Glu166 (16, 19). QM/MM calculations also provided insights into the free energy landscape underlying the unique reaction involving β-lactamase and avibactam, where recyclization is favored over hydrolysis (Fig. 1B) (14, 20). However, key questions, particularly the lack of a clear mechanistic understanding, remain as to why the covalent carbamoyl–ester bond avibactam forms with serine β-lactamases is very stable against hydrolysis and why Glu166 remains neutral, despite the presence of a water molecule perfectly positioned to attack the covalent bond with the inhibitor and the configuration of active-site residues being almost identical to that in previous β-lactam acyl–enzyme complexes (16, 18). In comparison, in these other previously developed β-lactam inhibitors such as cefoxitin complex structures show that the water molecule is displaced or blocked from attacking the acyl–enzyme linkage (21–25). A deep understanding of the avibactam inhibition mechanism will be crucial to the continuing development of DBO-type compounds, which are currently being intensely studied as new antibacterial reagents not only against β-lactamases but also against penicillin-binding proteins (PBPs), the original targets of β-lactam antibiotics (26, 27).
In this study, we probed the protonation state and acid dissociation constant (pKa) of Lys73 using high-resolution crystal structures of the class A β-lactamase CTX-M-14 determined at both basic and acidic pHs, combined with pKa determination by NMR. By capturing ligand conformation and protein protonation state not observed in previous studies, these results have shed important light on class A β-lactamase acylation and the mechanism of avibactam inhibition, as well as the influence of substrate functional groups on enzyme catalysis in general.
Results and Discussion
Subangstrom-Resolution Complex Structure of Avibactam and CTX-M-14 at pH 7.9.
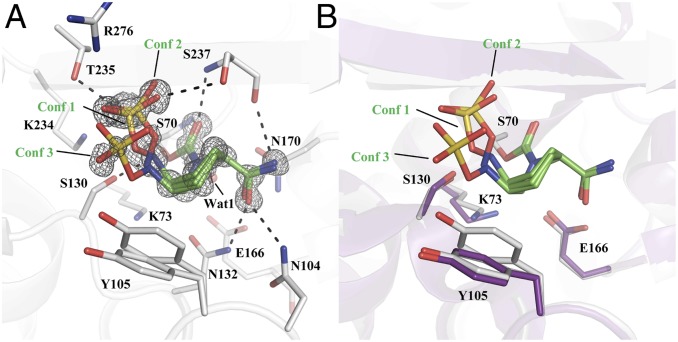
The crystal structure of avibactam covalently bound to CTX-M-14 was determined to 0.83-Å resolution with a refined Rwork/Rfree of 0.1168/0.1267. CTX-M-14 crystallized in the P21 space group with two molecules in the asymmetric unit (ASU). Analysis of the Fo – Fc and 2Fo – Fc maps showed three conformations of avibactam (monomer A: conformation 1, 2, and 3 at 0.52, 0.29, and 0.19 occupancy, respectively; monomer B: 0.57, 0.17, and 0.26 occupancy, respectively) (Fig. 2A). The major conformation, conformation 1, adopts an orientation similar to the previously determined CTX-M-15/avibactam complex (Protein Data Bank [PDB] ID code 4HBU) (16). Avibactam is covalently attached to the nucleophilic Ser70 residue, where its carbonyl oxygen atom is in the oxyanion hole formed by the backbone –NH of Ser70 and Ser237. As expected, the carboxamide group of avibactam is within hydrogen bond (HB) distance to the side chains of Asn104 (3.0 Å) and Asn132 (2.8 Å). The carboxamide group also forms a weak HB (3.2 Å) with the backbone carbonyl oxygen of Ser237.
Fig. 2.
Complex crystal structure of avibactam and CTX-M-14 at pH 7.9. (A) The simulated annealing composite Fo – Fc omit map (gray) contoured at 3 σ. The multiple avibactam conformations (Conf 1, 2, and 3) are labeled and colored in green. Black dashed lines depict HBs. (B) Superimposition of the complex structure (gray) with apo structure (purple), showing the movement of K73 and Y105.
One of the most striking differences between the CTX-M-14/avibactam complex at pH 7.9 and avibactam solved in previously determined class A β-lactamases (over a range of pHs, from 4.1 to 8.5) is the conformation of the hydroxylamine-O-sulfonate group. Conformation 1 (refined occupancy: 0.52 [monomer A, same below]) positions the sulfonate moiety in a binding pocket to form HBs with the side chains of Thr235 and Ser237 and electrostatic interactions with Lys234 and Arg276. This is the same binding pocket that coordinates the C3/C4 carboxylate of β-lactam substrates (28). The N6 atom of conformation 1 is forming an HB (2.9 Å) with the side chain of Ser130, which is hypothesized to serve as the general acid to protonate the substrate leaving nitrogen during avibactam carbamylation or β-lactam acylation (19). The sulfonate group of conformation 2 (refined occupancy: 0.29) forms interactions similar to conformation 1 with Thr235, Ser237, Lys234, and Arg276. However, the sulfonate group is positioned closer to the Ser130 Oγ (3.3 Å) versus conformation 1 (3.6 Å). In addition, the N6 atom is slightly further away from the Ser130 Oγ at 3.1 Å. The sulfonate group of conformation 3 (refined occupancy: 0.19) swings out of the active site, abolishing the HB contacts with Thr235 and Ser237 and weakening its interactions with Lys234 and Arg276. In one of the monomers in the ASU, this new pose allows the sulfonate group to form a potential salt bridge with Arg222 from the neighboring protein monomer. The N6 atom’s HB with Ser130 Oγ has also been significantly weakened with the distance between the two groups at 3.3 Å. Overall, the presence of three conformations of the hydroxylamine-O-sulfonate group suggests significant flexibility of this moiety, which is most likely more pronounced at room temperature than the cryogenic conditions of X-ray diffraction data collection (∼100 K) and may thus impact its interactions with protein residues. On the protein side, the binding of avibactam induces a conformational shift in Tyr105, a residue that has been suggested to play a role in substrate recognition (Fig. 2B) (29–31). The minor Tyr105 conformation (refined occupancy: 0.34) has shifted toward the piperidine ring to form a favorable nonpolar interaction, whereas the major Tyr105 conformation (refined occupancy: 0.66) retains the configuration usually seen in class A β-lactamases (Fig. 2B) (32). In comparison to the apo protein and similar to previous observations, Lys73 also moves away from Glu166 and closer to Ser130 (33, 34).
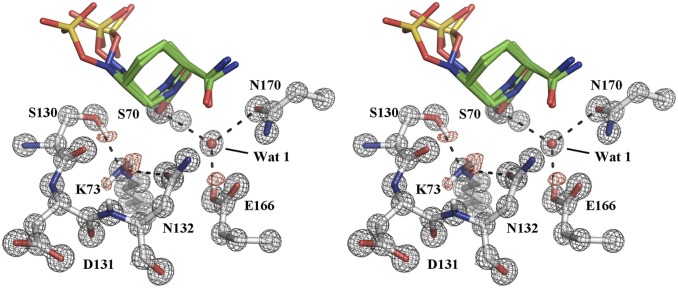
The 0.83-Å resolution of the CTX-M-14/avibactam complex at pH 7.9 allowed for the visualization of hydrogen atoms on the catalytically important residues Lys73, Ser130, and Glu166. The previously determined CTX-M-15/avibactam complex solved at 1.1 Å shows a protonated and neutral Glu166. However, the protonation state of Lys73 could not be clearly assigned. Based on its conformation, Lys73 is within HB distance (2.8 Å) to Ser130 (16). Our ultrahigh-resolution CTX-M-14/avibactam structure clears the ambiguity surrounding the protonation state of Lys73. In the structure at a basic pH of 7.9, the omit Fo – Fc electron density map shows that Lys73 has two protons and is in its neutral form (Fig. 3). One of the protons on Lys73 is engaged in an HB (2.9 Å) with the side chain of Asn132. The second proton has no hydrogen bonding partners with appropriate angles. However, Lys73Nζ is 2.9 and 3.0 Å away from Ser130O and Ser130C, respectively, and appears to form favorable interactions with π electrons on one side of the planar peptide bond between Ser130 and Asp131 (Fig. 3). Most importantly, the structure also shows a hydrogen on the Ser130Oγ, which serves as an HB (2.8 Å) donor to the neutral Lys73. In agreement with the 1.1-Å resolution CTX-M-15/avibactam complex, Glu166 is protonated and participates with Ser70 and Asn170 to coordinate the catalytic water molecule.
Fig. 3.
Hydrogen atoms revealed by subangstrom-resolution diffraction in stereo view. The unbiased Fo – Fc map contoured at 2 σ showing hydrogen atom positions on K73, S130, and E166 is colored red and the 2Fo – Fc map contoured at 3 σ around active-site residues is colored gray.
Complex crystal structure of avibactam with CTX-M-14 at acidic pHs.
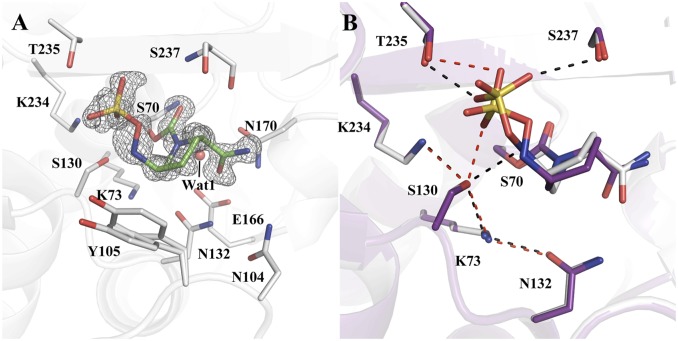
To investigate the influence of the crystallization buffer pH on the residue protonation states, we prepared the CTX-M-14/avibactam complex crystal in buffers with pHs 5.3, 6.3, 7.4, 8.4, and 10. All structures were determined at ∼1-Å resolution and shared the same active-site configurations as the pH 7.9 complex, except for the one at pH 5.3. The CTX-M-14/avibactam complex at pH 5.3 was solved to 1.0-Å resolution with a final Rwork/Rfree of 0.1420/0.1601 (Fig. 4A). Due to the lower resolution of this structure, hydrogen atoms were not as visible as in the 0.83-Å structure. Overall, the CTX-M-14/avibactam complex solved at pH 5.3 is similar to the CTX-M-14/avibactam complex solved at pH 7.9, but with some notable changes in the hydrogen bonding pattern between the inhibitor and active-site residues (Fig. 4B). The most interesting observation was the presence of a single inhibitor conformation versus three inhibitor conformations modeled at the higher pH. Under acidic condition, the negatively charged hydroxylamine-O-sulfonate group adopts a conformation that puts it within a hydrogen bonding distance of 2.6 Å to the hydroxyl side chain of Ser130. Ser130 maintains an HB with Lys73, with a distance between the two groups now at 3.0 Å versus 2.8 Å in the pH 7.9 structure. Because the avibactam sulfonate group can only serve as an HB acceptor, this HB network suggests that Ser130 donates an HB to the sulfonate oxygen and accepts a hydrogen in its HB with Lys73, reversing the donor/acceptor role compared with the pH 7.9 structure. Also, we observed that the HB between the N6 nitrogen and Ser130 had been abolished due to the flipping of the hydroxylamine-O-sulfonate group, with the side chain Oγ being 3.4 Å away from the N6 nitrogen.
Fig. 4.
Complex crystal structures of avibactam with CTX-M-14 at pH 5.3. (A) The composite simulated annealing Fo – Fc omit map (gray) contoured at 3 σ of the CTX-M-14/avibactam complex at pH 5.3. (B) Superimposition of CTX-M-14/avibactam complexes at pH 5.3 (purple) and 7.9 (gray). Red dashed lines represent HBs in the pH 5.3 complex and black dashed lines represent HBs in the pH 7.9 complex. Only the major conformation of avibactam is shown for the pH 7.9 complex.
The acyl–enzyme complexes between β-lactamases and avibactam have been studied extensively (18, 35–37). In most previous crystal structures with closely related class A β-lactamases, similar pH-dependent hydrogen bonding patterns were observed, that is, an HB between the sulfonate group and Ser130 occurred at low pH (38, 39) and was abolished at high pH (16). We hypothesize that this change of HB pattern may have originated from a positively charged Lys73, as compared to a neutral Lys73 observed in the pH 7.9 structure. Lys73 is the only functional group that can undergo protonation state change in the active site between pH 7.9 and pH 5.3. Glu166 and Lys234 are both protonated at pH 7.9, while the pKas of avibactam’s largely solvent-exposed sulfonate and –NH group are far below pH 5 as calculated by MarvinSketch, a program commonly used to provide pKa predictions for small molecules (40). As the pH 6.3 structure is identical to the pH 7.9 complex, this indicates the pKa of Lys73 is likely between pH 5.3 and 6.3, significantly lower than the estimate of 8.0 to 8.5 in previous studies of β-lactam hydrolysis (41). Additionally, computational predictions indicated similar disparity in Lys73 pKa between the acyl–enzyme complexes of avibactam and β-lactam inhibitor cefoxitin (6.24 versus 7.82; SI Appendix, Table S2) (11, 42). Although these calculations were performed without using thermodynamic integration based on extensive explicit-solvent molecular dynamics simulations as done by others and were thus less accurate (41), they suggested that it would be of interest to further probe Lys73 pKa.
CTX-M lysine protonation state and pKa probed by NMR.
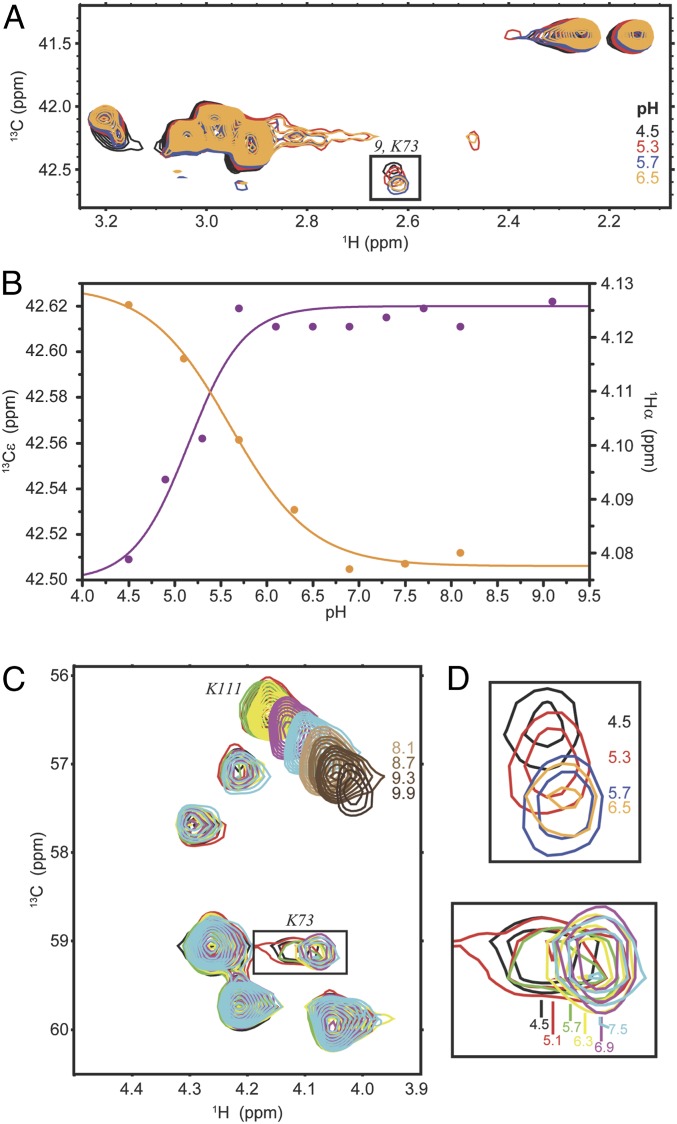
We sought to experimentally determine lysine pKa values using NMR chemical shifts as an independent observable. The ε position of lysines in particular makes an excellent probe to monitor the protonation state of lysine side chains, because it lies adjacent to the Nζ group and therefore changes in Cε chemical shift as a function of pH report a direct effect of altered Nζ protonation states. For this experiment, we used a His-tagged CTX-M-9 for the ease of purification. As described above, CTX-M-14 and CTX-M-9 are identical to one another in the active-site structure, and the two proteins exhibit essentially the same biochemical activity (43). The 1H-13C heteronuclear single-quantum correlation (HSQC) spectrum of free, [13Cε]-Lys selectively labeled CTX-M-9 acquired at pH 5.7 accounts for 9 of the 10 lysines expected based on the sequence of CTX-M-9 (SI Appendix, Fig. S1). Addition of avibactam results in a prominent shift for one of these signals (K9) and a subtle shift for a set of two signals originating from a single lysine (K1), while all other signals remain unaffected. Based on the available structure of the complex between CTX-M-14 and avibactam, K9 is therefore tentatively assigned to one of Lys73 and Lys234, the two lysines lining the catalytic site of CTX-M enzymes (Fig. 2A). From the pH dependence of the 13C chemical shifts for signals K1 through K9 (Fig. 5 and SI Appendix, Fig. S1B), it is evident that signal K9 corresponds to a lysine with a pKa of 5.2 ± 0.2, which is in agreement with the deprotonated state of Lys73 observed in the pH >5.3 structures, while all other signals correspond to pKa values greater than 10.0. To obtain site-specific information we also monitored the pH dependence of the Cα–Hα groups. Although the chemical shifts of the Cα–Hα group are not as sensitive to Nζ deprotonation as of the Cε–Hε group, detection of even small chemical shift changes that report coincidental pH titration curves, and thus pKa values, can be used to assign these pKa values to specific residues. Assignment for the Cα of Lys73 was obtained in a sequential manner using a set of triple resonance experiments, while the position of its Cα–Hα on a 1H-13C HSQC was identified again through a comparison with the spectrum of free CTX-M-9 acquired at the same pH (SI Appendix, Fig. S2). Using the pH dependence of the Hα chemical shift as a reporter, a pKa of 5.6 ± 0.2 is obtained for Lys73, which is consistent with the pKa determined using the Cε chemical shift, as well as the pH-dependent crystal structures.
Fig. 5.
NMR titration data of CTX-M-9 in complex with avibactam. (A) 1H-13C HSQC spectrum of avibactam-bound [13Cε]-Lys–labeled CTX-M-9 at pH 4.5 (black), 5.3 (red), 5.7 (blue), and 6.5 (orange). The boxed signal is expanded in D (Top) and is tentatively assigned to the Cε–Hε group of Lys73. (B) The chemical shift dependence for the Cε of lysine “9” (magenta) and for the tentatively assigned Hα of Lys73 (orange). The curves are nonlinear least square fits to Eq. 1. (C) The Cα–Hα region of avibactam-bound [U-13C]-Lys–labeled CTX-M-9 at pH 4.5 (black), 5.1 (red), 5.7 (green), 6.3 (yellow), 6.9 (magenta), and 7.5 (cyan). The boxed signal, which is tentatively assigned to the Cα–Hα group of Lys73, is expanded in D (Bottom) and is broadened beyond detection at pH 8.7. The signal corresponding to the Cα–Hα group of Lys111 is also shown at higher pH. It shifts throughout the titration (pH 4.5 to 9.9) and it does not follow a monophasic pH dependence, presumably due to the presence of other ionizable groups in its vicinity (SI Appendix, Fig. S2C). (D) Expanded regions shown boxed in A (Top) and C (Bottom).
Mechanism of proton transfer in class A β-lactamases catalysis.
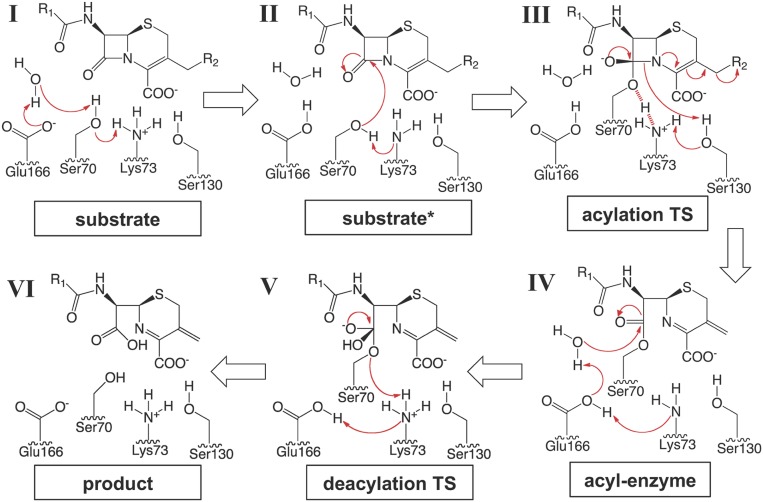
The high-resolution crystal structures of class A β-lactamases trapped with avibactam have allowed us to visualize the protonation states of key catalytic residues in the acyl–enzyme complex of a wild-type class A β-lactamase. Together with previous subangstrom-resolution crystal structures, we can now track the changes of residue protonation states throughout the entire acylation process and gain deep insights into the proton transfer mechanism of these enzymes (Fig. 6). In combination with the biochemical analyses and the QM/MM calculations by Mobashery and coworkers (44), these data demonstrate the central role of Lys73, acting concertedly with Glu166, in activating both Ser70 in the acylation step and subsequently the catalytic water in the deacylation reaction.
Fig. 6.
A proposed mechanism of proton transfer for the class A β-lactamase catalysis. A cephalosporin is used as a representative β-lactam substrate. Electron transfers are indicated by arrows. Based on QM/MM calculations and experimental data, the reaction of class A β-lactamases follows a concerted base catalysis mechanism involving Lys73 and Glu166. (I) Substrate binding triggers a series of proton transfers, changing the protonation states of both Lys73 and Glu166 from being charged to neutral. (II) A neutral Lys73 deprotonates and activates Ser70 for nucleophilic attack on the β-lactam ring. (III) The reaction proceeds to a high-energy tetrahedral intermediate, where Ser70 is covalently attached to the substrate while being stabilized by a potential LBHB with Lys73 (indicated by red dashed lines). The shared proton of the LBHB is subsequently transferred to Lys73, which protonates Ser130 after the serine residue transfers its own proton to the ring nitrogen atom. Isomerization of the cephalosporin dihydrothiazine ring results in cleavage of the C3 side chain, a reaction unique for many cephalosporins. (IV) A neutral Lys73 takes a proton from Glu166. A negatively charged Glu166 serves as the base for the deacylation process and activates the catalytic water molecule for its attack on the acyl–enzyme linkage. (V) Lys73 protonates Ser70 during the collapse of the tetrahedral deacylation TS and then takes a proton from Glu166. (VI) The protonation states of Lys73 and Glu166 return to those of the free enzyme, and the hydrolyzed product is released.
In the apo protein, both Lys73 and Glu166 are charged (Fig. 6, stage I). The binding of the substrate initiates proton transfers from Ser70 to the catalytic water and from the water to Glu166. Both experimental and computational data suggest that a third, at least partial proton transfer also takes place between the positively charged Lys73 and the negatively charged Ser70, before the formation of the transition state (TS). In the QM/MM calculations, a complete proton transfer leads to a neutral Lys73 and a neutral Ser70, enabling Lys73 to be the general base for the acylation reaction (Fig. 6, stage II) (44). Meanwhile, in a noncovalent complex crystal structure with CTX-M-14, a low-barrier HB (LBHB) is observed between Lys73 and Ser70, with an HB length of 2.53 Å and the hydrogen atom equally shared between the two (45).
An LBHB is a special type of HB occurring between polar functional groups with comparable pKas and has been proposed to play an important role in enzyme catalysis, including general acid/base catalysis due to their extraordinary strength compared with standard HBs (46–49). An LBHB between Lys73 and Ser70 can significantly lower the energy of the TS, allowing Lys73 to carry out the role of a general base where the proton transfer is en route at the acylation TS (Fig. 6, state III). In comparison, the deprotonation of Ser70 by Glu166 and the catalytic water is most likely completed before the TS. The hydrogen shared between Lys73 and Ser70 is ultimately transferred to Lys73, resulting again in a positively charged Lys73 during the collapse of the acylation TS (Fig. 6, stage III), as shown by the 0.83-Å-resolution crystal structure of CTX-M-14 with a boronic acid TS analog (45). In this TS analog structure, both Lys234 and Lys73 serve as HB donors to Ser130, which in turn donates a hydrogen in an HB with the boronic acid oxygen mimicking the ring nitrogen. The structures of the TS analog and of our latest avibactam complex captures the stages immediately before and after the proton transfer from Ser130 to the ring nitrogen, respectively (Fig. 6, stages III and IV). Whereas it has been debated whether Lys234 or Lys73 replenishes the proton on Ser130 (50), our structure clearly shows that it is the latter. In addition, the avibactam structure supports previous QM/MM calculations showing that a neutral Lys73 is also in a commanding position to initiate the deacylation process by activating Glu166 (Fig. 6, state IV) (44), although the proton transfer from Glu166 to Lys73 is impeded in the avibactam complex (51). For β-lactam substrates, a negatively charged Glu166 would then deprotonate the catalytic water, which attacks and cleaves the acyl–enzyme linkage. This results in a second tetrahedral TS where Lys73 protonates Ser70 while taking a proton from Glu166 (Fig. 6, state V). The collapse of this deacylation TS leads to the formation of the hydrolyzed product and the regeneration of the free enzyme (Fig. 6, state VI).
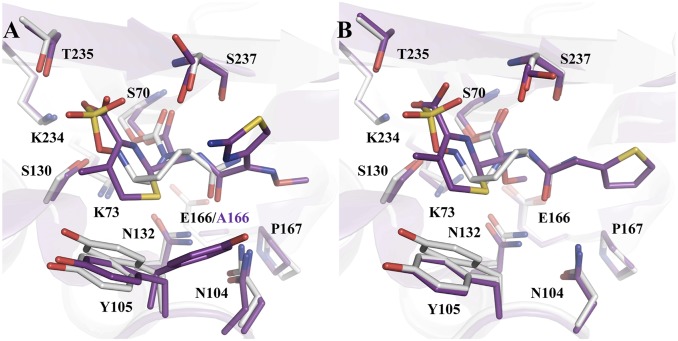
Class A β-lactamases have evolved from PBPs, acquiring Glu166 to help catalyze the deacylation reaction (52–54). Previous subangstrom-resolution acyl–enzyme and product complex structures using E166A mutants demonstrate a similar HB network involving the substrate, Ser130 and Lys73 (Fig. 7A) (11, 55). Together with the apo PBP structures, they suggest that Lys73 may play a similar role in the β-lactam acylation process in PBPs in the absence of Glu166.
Fig. 7.
Comparison of acyl–enzyme complexes with β-lactams and avibactam. (A) Superimposition of CTX-M-14/avibactam at pH 7.9 (gray) with Toho-1/cefotaxime E166A (purple) (PDB ID code 5A92). (B) Superimposition of CTX-M-14/avibactam at pH 7.9 (gray) with CTX-M-9/cefoxitin (purple) (PDB ID code 1YMX).
Influence of substrate on catalytic residue pKa and enzymatic reaction.
The pKa of the catalytic residues in class A β-lactamases has been studied both experimentally and computationally using TEM-1 as a model system (34, 41, 44, 56). It is hypothesized that the binding of a β-lactam substrate can influence the pKa of active-site residues. Although earlier computational studies did not show such effect (41), it was later supported by the protonation state changes of Lys73 and Glu166 upon ligand binding, captured by both computational and experimental studies, as described above (11, 44, 45). Our studies herein have shed light on how the deacylation process can also be subjected to the influence of substrate/inhibitor functional groups on catalytic residue pKa.
Compared with β-lactam substrates, the acyl–enzyme complexes of avibactam and β-lactamases are more stable against hydrolysis. This originates at least partially from the chemical difference of the two acyl–enzyme linkages, a carbamoyl for avibactam and an ester for β-lactams (38). However, previous QM/MM calculations also suggest that in the acyl–enzyme complex with avibactam, the proton transfer from Glu166 to Lys73 has an activation barrier of ∼30 kcal/mol (51), in comparison to a spontaneous and barrierless process in the complexes with β-lactam substrates (44). The higher proton transfer barrier could make it more difficult for Lys73 to activate Glu166, and subsequently the catalytic water, for the deacylation reaction. Our analyses provide a mechanistic understanding for this difference between avibactam and β-lactams.
We propose that the proton transfer is impaired due to the particular properties of avibactam’s hydroxylamine-O-sulfonate side chain, compared with β-lactams, through both direct influence of electrostatic interactions and indirect effect via Ser130. Lys73Nζ is ∼5 to 6 Å away from the negatively charged oxygen atoms of Glu166 and β-lactam C3/4 carboxylate group in the acyl–enzyme complex (Fig. 7). Previous studies have shown that the introduction of Glu166 increases the pKa of Lys73 from 6 to 8, making it a stronger base (41, 44, 50, 57). The β-lactam carboxylate group is likely to have a similar effect on Lys73 pKa, but such influence by the avibactam sulfonate would be significantly diminished. Both the β-lactam carboxylate group and the avibactam sulfonate group have one net negative charge, distributed between the two oxygen atoms of a carboxylate group, and the three oxygens in the sulfonate group. Comparing the major conformation of the avibactam crystal structure with β-lactam complexes, the sulfonate group is more solvent-exposed, which could further affect the localization of the negative charge (Fig. 7). Furthermore, the position of the avibactam sulfonate group in the active site is relatively unstable due to the flexibility of the hydroxylamine chain. Most notably, in the avibactam complex structure, one of the sulfonate conformations resides outside the active-site pocket and away from Lys73. Such a conformation further decreases the electrostatic influence of the sulfonate charge on Lys73 and is mostly likely much more dominant at 37 °C in solution compared with −170 °C cryogenic conditions and the crystalline environment. All these effects could potentially reduce the influence of the sulfonate group on the pKa of Lys73, compared with the β-lactam carboxylate group.
Aside from direct electrostatic influence, the ligand may affect Lys73 pKa through Ser130. In the avibactam conformation with the sulfonate inside the active site, the N6 nitrogen of the hydroxylamine-O-sulfonate group functions as a HB donor in its interaction with Ser130, which in turn donates a hydrogen in its HB with Lys73. Such interactions may also favor a neutral Lys73, particularly considering the N6 hydrogen may have more positive partial charge than the β-lactam ring –NH group in acyl–enzyme complexes with penicillins or monobactams. In acyl–enzyme complexes with cephalosporins, the ring nitrogen loses the hydrogen during dihydrothiazine ring isomerization (Fig. 6, step III). Interestingly, in the acyl–enzyme complexes of Toho-1 E166A mutant Lys73 adopts two conformations in the cefotaxime complex lacking the HB between the ring nitrogen and Ser130 (55), in comparison to one conformation for the penicillin-G (31) and aztreonam (11) structures with such an HB. Similar observations were also made in the CTX-M-9 acyl–enzyme complex structure with cefoxitin, a cephalosporin, suggesting a potential influence of the substrate –NH group on Lys73 through Ser130, particularly in the avibactam complex (21, 56). Similarly, the avibactam sulfonate group and the β-lactam carboxylate moiety can potentially affect the position of the hydrogen on the Ser130 hydroxyl group to varied extents, and indirectly influence Lys73 pKa as well, as demonstrated by the different hydrogen bonding network involving the sulfonate group, Ser130, and Lys73 in the avibactam complex crystal structures determined at different pHs. Taken together, the charge distribution, flexibility, and HB capability of the avibactam side chain may ultimately be responsible for perturbing Lys73’s pKa, making it an unfavorable process to accept a proton from Glu166.
Conclusion
The 0.83-Å-resolution complex crystal structure of CTX-M-14 and avibactam captures the acyl–enzyme at a state where both Lys73 and Glu166 are neutral. Compared with β-lactam acyl–enzyme complexes, the proton transfer from Glu166 to Lys73 is hindered by avibactam. Based on multiple crystals at both basic and acidic pHs, as well as computations and NMR, our results suggest that the unique hydroxylamine-O-sulfonate side chain of avibactam may perturb the pKa of Lys73 differently than the C3/4 carboxylate group of β-lactam compounds, making Lys73 a weaker base in the avibactam acyl–enzyme complex. By elucidating the residue protonation states of a key intermediate of class A β-lactamase catalysis, these studies offer important insights into the proton transfer mechanism of β-lactam hydrolysis and the influence of substrate functional groups on the progression of enzymatic reactions in general. In particular, together with previous results, we now have a complete experimental picture of the central role of Lys73, acting concertedly with Glu166, in activating both the acylation and deacylation steps of the reaction, as suggested by previous QM/MM calculations (44). Finally, whereas X-ray crystallography, computational predictions and NMR are in overall agreement about Lys73 pKa in the avibactam complex, future investigation into the small differences among these methods, especially by using more sophisticated calculations, will offer additional insights into the interpretation of residue protonation states in crystal structures and the development of new computational approaches.
Materials and Methods
Construct Design.
The CTX-M-14 gene (residues 23 to 284) was cloned into a modified plasmid vector pET-9a. The ligation product was transformed into NEB 5-alpha–competent Escherichia coli and plated onto lysogeny broth (LB) agar containing 50 µg/mL kanamycin. A single colony was isolated and grown overnight at 37 °C in LB media containing 50 µg/mL kanamycin. Cells were harvested, and plasmid DNA was obtained using a miniprep kit. The CTX-M-14 gene sequence was verified.
Protein Expression and Purification.
BL21(DE3) E. coli cells transformed with the plasmid pET-blaCTX-M-14 were cultured in LB broth containing kanamycin at 100 μg/mL at 37 °C for 5 h. Overexpression of CTX-M-14 was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) overnight at 20 °C. Cells were harvested by centrifugation (10,000 × g for 10 min at 4 °C) and then disrupted by ultrasonic treatment (four times for 30 s, each time at 20 W). The extract was clarified by centrifugation at 48,000 × g for 60 min at 4 °C. After addition of 2 μg of DNase I (Roche), the supernatant was dialyzed overnight against 20 mM MES–NaOH (pH 6.0). The purification was carried out by ion-exchange chromatography on a fast-flow CM column (Amersham Pharmacia Biotech) in 20 mM MES buffer (pH 6.0) and eluted with a linear 0 to 0.15 M NaCl gradient. The enzyme was more than 95% homogeneous as judged by Coomassie blue staining after sodium dodecyl sulfate polyacrylamide gel electrophoresis. The purified protein was dialyzed against 5 mM Tri⋅HCl buffer (pH 7.0) and concentrated to 20 mg/mL for crystallization.
Protein Crystallization.
CTX-M-14 crystals were grown in 1.0 M potassium phosphate. A 200 mM stock of avibactam in dimethyl sulfoxide was used to make a soaking solution of 5 mM avibactam in 1.0 M potassium phosphate. Crystals were soaked for 30 min in different pH ranges then cryoprotected with 1.0 M potassium phosphate and 30% (wt/vol) sucrose before flash cooling in liquid nitrogen.
Data Collection and Structure Determination.
Data for the CTX-M-14 avibactam complex structures were collected at the Advanced Photon Source beamlines SBC 19-ID-D and GM/CA 23-ID-B. Diffraction data were indexed and integrated with iMosflm (58) or HKL2000 (59) and scaled with Aimless (60) from the CCP4 suite (61) or HKL2000. Phasing was performed using molecular replacement with the program Phaser (62). Structure refinement was performed using phenix.refine (63) of the PHENIX suite (64) and model building in WinCoot (65). The program eLBOW in PHENIX was used to obtain geometry restraint information (66). For data collection and refinement statistics see SI Appendix, Table S1.
Protein Expression and Purification for NMR.
Isotopically labeled CTX-M-9 for NMR measurements was expressed from a pODc29 vector encoding for an N-terminal His-tag using BL21(DE3) E. coli cells. U-[13C/15N] labeling was performed in M9 media supplemented with U-13C glucose and 15N-NH4Cl as the sole carbon and nitrogen source. Protein expression was induced at an optical density at 600 nm of ∼0.7 by the addition of 0.5 mM IPTG and was allowed to proceed overnight at 20 °C. Selectively [13Cε]- or U-[13C]-Lys–labeled CTX-M-9 was produced in unlabeled M9. The [13Cε]- or U-[13C]–labeled lysine was added 15 min prior to induction and overexpression was allowed to proceed for 3 h at 20 °C. Cells were resuspended in 20 mM Tris, pH 8.4, 300 mM NaCl, 20 mM imidazole, 10% glycerol, and 5 mM 2-mercaptoethanol and lysed by sonication. The lysate was clarified by centrifugation and loaded on a Ni Sepharose column. After extensive washing the protein was eluted in the same buffer at a 400 mM imidazole concentration and the tag was removed by tobacco etch virus cleavage and a second affinity column. The protein was finally purified through a Superdex 200 gel-filtration column.
NMR Spectroscopy.
NMR pH titrations of avibactam-bound [13Cε]- or U-[13C]–labeled CTX-M-9 were followed by acquiring 1H-13C HSQC spectra; 14 pH points were acquired for the [13Cε]-Lys–labeled (4.5, 4.9, 5.3, 5.7, 6.1, 6.5, 6.9, 7.3, 7.7, 8.1, 9.1, 10.1, 11.1, and 12.1) and 13 pH points (4.5, 5.1, 5.7, 6.3, 6.9, 7.5, 8.1, 8.7, 9.3, 9.9, 10.65, 11.7, and 12.3) for the U-[13C]-Lys–labeled sample. Sequence-specific backbone resonance assignment of CTX-M-9 in the avibactam-bound state at pH 5.5 was transferred from a partial resonance assignment of free CTX-M-9 obtained at the same pH, using a set of HNCA and HNCOCA experiments. pKa values were calculated by three-parameter least-square fitting of the chemical shift data (δobs) obtained from the titration experiments into the following equation:
| [1] |
where δU is the chemical of the deprotonated state and Δδ is the chemical shift difference between the protonated and deprotonated states. n, the Hill coefficient commonly introduced to account for interactions between titratable residues was held constant to 1, because of the lack of a clear plateau at low pH values. Only pH titration data below pH 10.0 were used to determine pKa values as a significant amount of precipitation was observed at pH >10.0.
Data Availability.
Structure coordinates and factors have been deposited in the Protein Data Bank (PDB), https://www.wwpdb.org/, under ID codes 6MZ1 (CTX-M-14/avibactam pH 5.3) and 6MZ2 (CTX-M-14/avibactam pH 7.9).
Supplementary Material
Acknowledgments
Support for this project was given by National Institutes of Health grants AI103158 and AI147654 (Y.C.), GM129519 (H.L.W.), and GM115854 (I.G.) and NSF grant CHE-1464946 (H.L.W.). F.L.K. thanks the NSF Graduate Research Fellowship Program (project number 3900101301) for support. We thank Forest Research Institute for the kind gift of avibactam, Dr. Thomas Durand-Reville for insightful discussion, and Dr. Eric Lewandowski for assistance with manuscript preparation. Diffraction data were collected at the Advanced Photon Source (APS), Argonne National Laboratory, particularly at the 19-ID-D beamline of the Structural Biology Center (SBC) and 23-ID-B beamline of GM/CA@APS. The use of beamlines at SBC was supported in part by the US Department of Energy, Office of Biological and Environmental Research grant DE-AC02-06CH11357. GM/CA@APS has been funded in whole or in part with federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). Computations were performed at the University of South Florida Research Computing Center and using Extreme Science and Engineering Discovery Environment computational resources (MCB120133); both centers are greatly appreciated. The Materials and Methods section on protein purification for crystallization was previously included in the graduate thesis of O.A.P.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.M. is a guest editor invited by the Editorial Board.
Data deposition: Structure coordinates and factors have been deposited in the Protein Data Bank, https://www.wwpdb.org (PDB ID codes 6MZ1 [CTX-M-14/avibactam pH 5.3] and 6MZ2 [CTX-M-14/avibactam pH 7.9]).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922203117/-/DCSupplemental.
References
- 1.Bush K., Jacoby G. A., Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54, 969–976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queenan A. M., Bush K., Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 20, 440–458 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philippon A., Slama P., Dény P., Labia R., A structure-based classification of class A β-lactamases, a broadly diverse family of enzymes. Clin. Microbiol. Rev. 29, 29–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K., New beta-lactamases in gram-negative bacteria: Diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32, 1085–1089 (2001). [DOI] [PubMed] [Google Scholar]
- 5.El Salabi A., Walsh T. R., Chouchani C., Extended spectrum β-lactamases, carbapenemases and mobile genetic elements responsible for antibiotics resistance in Gram-negative bacteria. Crit. Rev. Microbiol. 39, 113–122 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Massova I., Kollman P. A., pKa, MM, and QM studies of mechanisms of beta-lactamases and penicillin-binding proteins: acylation step. J. Comput. Chem. 23, 1559–1576 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Mehta S. C., Rice K., Palzkill T., Natural variants of the KPC-2 carbapenemase have evolved increased catalytic efficiency for ceftazidime hydrolysis at the cost of enzyme stability. PLoS Pathog. 11, e1004949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strynadka N. C., et al. , Molecular structure of the acyl-enzyme intermediate in beta-lactam hydrolysis at 1.7 A resolution. Nature 359, 700–705 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Tomanicek S. J., et al. , Neutron and X-ray crystal structures of a perdeuterated enzyme inhibitor complex reveal the catalytic proton network of the Toho-1 β-lactamase for the acylation reaction. J. Biol. Chem. 288, 4715–4722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page M. I., Proctor P., Mechanism of. beta.-lactam ring opening in cephalosporins. J. Am. Chem. Soc. 106, 3820–3825 (1984). [Google Scholar]
- 11.Vandavasi V. G., et al. , Active-site protonation states in an acyl-enzyme intermediate of a class A β-lactamase with a Monobactam substrate. Antimicrob. Agents Chemother. 61, e01636-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty S., Enumerating pathways of proton abstraction based on a spatial and electrostatic analysis of residues in the catalytic site. PLoS One 7, e39577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan X., He Y., Lei J., Huang X., Zhao Y., Crystallographic snapshots of class A β-lactamase catalysis reveal structural changes that facilitate β-lactam hydrolysis. J. Biol. Chem. 292, 4022–4033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahiri S. D., et al. , Avibactam and class C β-lactamases: Mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob. Agents Chemother. 58, 5704–5713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman K., Diazabicyclooctanes (DBOs): A potent new class of non-β-lactam β-lactamase inhibitors. Curr. Opin. Microbiol. 14, 550–555 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Lahiri S. D., et al. , Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: Avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob. Agents Chemother. 57, 2496–2505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehmann D. E., et al. , Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl. Acad. Sci. U.S.A. 109, 11663–11668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King D. T., King A. M., Lal S. M., Wright G. D., Strynadka N. C., Molecular mechanism of avibactam-mediated β-lactamase inhibition. ACS Infect. Dis. 1, 175–184 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Winkler M. L., Papp-Wallace K. M., Taracila M. A., Bonomo R. A., Avibactam and inhibitor-resistant SHV β-lactamases. Antimicrob. Agents Chemother. 59, 3700–3709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H., Paton R. S., Park H., Schofield C. J., Investigations on recyclisation and hydrolysis in avibactam mediated serine β-lactamase inhibition. Org. Biomol. Chem. 14, 4116–4128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Shoichet B., Bonnet R., Structure, function, and inhibition along the reaction coordinate of CTX-M beta-lactamases. J. Am. Chem. Soc. 127, 5423–5434 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nukaga M., et al. , Inhibition of class A beta-lactamases by carbapenems: Crystallographic observation of two conformations of meropenem in SHV-1. J. Am. Chem. Soc. 130, 12656–12662 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca F., et al. , The basis for carbapenem hydrolysis by class A β-lactamases: A combined investigation using crystallography and simulations. J. Am. Chem. Soc. 134, 18275–18285 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Smith C. A., et al. , Structural basis for progression toward the carbapenemase activity in the GES family of β-lactamases. J. Am. Chem. Soc. 134, 19512–19515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuey-Ching Y., Hecht D., Tsai J. J., Hu R. M., Cefoxitin is both an inhibitor of class A beta-lactamase of Xanthomonas campestris pv. campestris str. 17 and an inducer of its gene. Res. Microbiol. 163, 550–556 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Durand-Réville T. F., et al. , ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat. Microbiol. 2, 17104 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Levy N., et al. , Structural basis for E. coli penicillin binding protein (PBP) 2 inhibition, a platform for drug design. J. Med. Chem. 62, 4742–4754 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Tondi D., et al. , Targeting class A and C serine β-lactamases with a broad-spectrum boronic acid derivative. J. Med. Chem. 57, 5449–5458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doucet N., De Wals P. Y., Pelletier J. N., Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in TEM-1 beta-lactamase. J. Biol. Chem. 279, 46295–46303 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Doucet N., Savard P. Y., Pelletier J. N., Gagné S. M., NMR investigation of Tyr105 mutants in TEM-1 beta-lactamase: Dynamics are correlated with function. J. Biol. Chem. 282, 21448–21459 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Langan P. S., et al. , The structure of Toho1 β-lactamase in complex with penicillin reveals the role of Tyr105 in substrate recognition. FEBS Open Bio 6, 1170–1177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langan P. S., et al. , Substrate binding induces conformational changes in a class A beta-lactamase that prime it for catalysis. ACS Catal. 8, 2428–2437 (2018). [Google Scholar]
- 33.Chen Y., Bonnet R., Shoichet B. K., The acylation mechanism of CTX-M beta-lactamase at 0.88 a resolution. J. Am. Chem. Soc. 129, 5378–5380 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargis J. C., White J. K., Chen Y., Woodcock H. L., Can molecular dynamics and QM/MM solve the penicillin binding protein protonation puzzle? J. Chem. Inf. Model. 54, 1412–1424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvopiña K., et al. , Structural/mechanistic insights into the efficacy of nonclassical β-lactamase inhibitors against extensively drug resistant Stenotrophomonas maltophilia clinical isolates. Mol. Microbiol. 106, 492–504 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Nukaga M., et al. , Probing the mechanism of inactivation of the FOX-4 Cephamycinase by avibactam. Antimicrob. Agents Chemother. 62, e02371-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohans C. T., et al. , 13C-Carbamylation as a mechanistic probe for the inhibition of class D β-lactamases by avibactam and halide ions. Org. Biomol. Chem. 15, 6024–6032 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Xu H., Hazra S., Blanchard J. S., NXL104 irreversibly inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry 51, 4551–4557 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan N. P., Nguyen N. Q., Papp-Wallace K. M., Bonomo R. A., van den Akker F., Inhibition of Klebsiella β-lactamases (SHV-1 and KPC-2) by avibactam: A structural study. PLoS One 10, e0136813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ChemAxon, MarvinSketch (version 18.16, ChemAxon, Budapest, Hungary, 2018).
- 41.Golemi-Kotra D., et al. , The importance of a critical protonation state and the fate of the catalytic steps in class A beta-lactamases and penicillin-binding proteins. J. Biol. Chem. 279, 34665–34673 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimamura T., et al. , Acyl-intermediate structures of the extended-spectrum class A beta-lactamase, Toho-1, in complex with cefotaxime, cephalothin, and benzylpenicillin. J. Biol. Chem. 277, 46601–46608 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Chen Y., Delmas J., Sirot J., Shoichet B., Bonnet R., Atomic resolution structures of CTX-M beta-lactamases: Extended spectrum activities from increased mobility and decreased stability. J. Mol. Biol. 348, 349–362 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Meroueh S. O., Fisher J. F., Schlegel H. B., Mobashery S., Ab initio QM/MM study of class A beta-lactamase acylation: Dual participation of Glu166 and Lys73 in a concerted base promotion of Ser70. J. Am. Chem. Soc. 127, 15397–15407 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Nichols D. A., et al. , Ligand-induced proton transfer and low-barrier hydrogen bond revealed by X-ray crystallography. J. Am. Chem. Soc. 137, 8086–8095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cleland W. W., Kreevoy M. M., Low-barrier hydrogen bonds and enzymic catalysis. Science 264, 1887–1890 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Graham J. D., Buytendyk A. M., Wang D., Bowen K. H., Collins K. D., Strong, low-barrier hydrogen bonds may be available to enzymes. Biochemistry 53, 344–349 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Frey P. A., Whitt S. A., Tobin J. B., A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science 264, 1927–1930 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Nadal-Ferret M., Gelabert R., Moreno M., Lluch J. M., Are there really low-barrier hydrogen bonds in proteins? The case of photoactive yellow protein. J. Am. Chem. Soc. 136, 3542–3552 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Lewandowski E. M., et al. , Mechanisms of proton relay and product release by Class A β-lactamase at ultrahigh resolution. FEBS J. 285, 87–100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sgrignani J., Grazioso G., De Amici M., Colombo G., Inactivation of TEM-1 by avibactam (NXL-104): Insights from quantum mechanics/molecular mechanics metadynamics simulations. Biochemistry 53, 5174–5185 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Bush K., Jacoby G. A., Medeiros A. A., A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39, 1211–1233 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher J. F., Mobashery S., Three decades of the class A beta-lactamase acyl-enzyme. Curr. Protein Pept. Sci. 10, 401–407 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massova I., Mobashery S., Kinship and diversification of bacterial penicillin-binding proteins and beta-lactamases. Antimicrob. Agents Chemother. 42, 1–17 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vandavasi V. G., et al. , Exploring the mechanism of β-Lactam ring protonation in the class A β-lactamase acylation mechanism using neutron and X-ray crystallography. J. Med. Chem. 59, 474–479 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Hargis J. C., Vankayala S. L., White J. K., Woodcock H. L., Identification and characterization of noncovalent interactions that drive binding and specificity in DD-peptidases and β-lactamases. J. Chem. Theory Comput. 10, 855–864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W., Shi Q., Meroueh S. O., Vakulenko S. B., Mobashery S., Catalytic mechanism of penicillin-binding protein 5 of Escherichia coli. Biochemistry 46, 10113–10121 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Battye T. G., Kontogiannis L., Johnson O., Powell H. R., Leslie A. G., iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otwinowski Z., Minor W., Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Evans P., Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Winn M. D., et al. , Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCoy A. J., et al. , Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afonine P. V., et al. , Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams P. D., et al. , PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moriarty N. W., Grosse-Kunstleve R. W., Adams P. D., Electronic Ligand Builder and Optimization Workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structure coordinates and factors have been deposited in the Protein Data Bank (PDB), https://www.wwpdb.org/, under ID codes 6MZ1 (CTX-M-14/avibactam pH 5.3) and 6MZ2 (CTX-M-14/avibactam pH 7.9).