This systematic review and meta-analysis assesses whether there is an association between placebo and erectile dysfunction outcomes among men enrolled in placebo-controlled, phosphodiesterase 5 inhibitor trials.
Key Points
Question
Was there an association between placebo and improved erectile function among men enrolled in studies of phosphodiesterase 5 inhibitors?
Findings
This systematic review and meta-analysis of 12 564 men found a significant association between placebo and improved erectile function, with the effect size being larger among men with posttraumatic stress disorder. There was no difference between response to placebo and phosphodiesterase 5 inhibitors in men with erectile dysfunction after prostate surgery.
Meaning
The findings suggest that contextual factors are important in the delivery of care to patients with sexual dysfunction, and the lack of difference in response between placebo and phosphodiesterase 5 inhibitors in certain patient subgroups suggests that clinical practice should change.
Abstract
Importance
Placebo responses in the treatment of erectile dysfunction (ED) are poorly described in the literature to date.
Objective
To quantify the association of placebo with ED outcomes among men enrolled in placebo-controlled, phosphodiesterase 5 inhibitor (PDE5I) trials.
Data Sources
For this systematic review and meta-analysis, a database search was conducted to identify double-blind, placebo-controlled studies using PDE5Is for the treatment of ED published from January 1, 1998, to December 31, 2018, within MEDLINE, Embase, Cochrane Library, and Web of Science. Only articles published in the English language were included.
Study Selection
Double-blind, placebo-controlled randomized clinical trials of PDE5Is for ED were included. Studies were excluded if they did not provide distribution measures for statistical analysis. Study selection review assessments were conducted by 2 independent investigators. A total of 2215 studies were identified from the database search, and after review, 63 studies that included 12 564 men were analyzed.
Data Extraction and Synthesis
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in abstracting data and assessing validity. Data were extracted from published reports by 2 independent reviewers. Quality assessment was performed using the Jadad scale. Data were pooled using a random-effects model.
Main Outcomes and Measures
The main outcome was improvement in the erectile function domain of the International Index of Erectile Function questionnaire in the placebo arm of the included studies. Effect size was reported as bias-corrected standardized mean difference (Hedges g). The hypothesis was formulated before data extraction.
Results
A total of 63 studies that included 12 564 men (mean [SD] age, 55 [7] years; age range, 36-68 years) were included. Erectile function was significantly improved among participants in the placebo arm, with a small to moderate effect size (Hedges g [SE], 0.35 [0.03]; P < .001). Placebo effect size was larger among participants with ED associated with posttraumatic stress disorder (Hedges g [SE], 0.78 [0.32]; P = .02) compared with the overall analysis. No significant difference was found between placebo and PDE5Is for ED after prostate surgery or radiotherapy (Hedges g [SE], 0.30 [0.17]; P = .08).
Conclusions and Relevance
In this study, placebo was associated with improvement of ED, especially among men with ED-related posttraumatic stress disorder. No difference was found between placebo and PDE5I among men treated for ED after prostate surgery.
Introduction
In the past few decades, few drugs have achieved the same mythologic status as sildenafil. Approved in 1998 for treatment of erectile dysfunction (ED), it was soon accompanied by tadalafil and vardenafil, and more recently a few additional drugs within the class of phosphodiesterase 5 inhibitors (PDE5Is) entered the market. Sildenafil was originally developed to relieve symptoms of angina pectoris. What was first considered an adverse effect turned out to be beneficial for increasing blood flow in other areas as well.1 Anecdotes from early clinical trials describe study participants being unwilling to return unused pills because of the positive erectile effects of the study drug. Despite its specific effect on blood flow through relaxing the penile cavernosal smooth muscle cells, the reputation of the drug has become synonymous with potency, increased virility, and improved sexual performance in general. A previous study2 reported that sildenafil and other equivalent drugs have been used recreationally by men (both adults and adolescents) without ED. Given the reputation of this class of drugs, it seems possible that some of the effects of the drugs may be related to the power of belief.
Erectile function can be divided into a central component that influences the sympathetic outflow from the thoracolumbar region of the spinal cord and a peripheral reflexogenic erectile function mediated by nitrergic nerves that project from the sacral region of the spinal cord.3 Erectile dysfunction can be caused by many adversities, such as cardiovascular disease, diabetes, smoking, hypogonadism, iatrogenesis due to pelvic surgery, and adverse effects of medication, but it can also be of psychogenic origin. It has been estimated that approximately 80% of ED is peripheral in origin, although psychogenic factors is likely associated with ED in these cases too. The severity of ED is commonly diagnosed using the International Index of Erectile Function questionnaire (IIEF).4,5 A previous study6 performed in the United States estimated the prevalence of ED to be 44% to 70% in men 60 years and older, with increased prevalence with advancing age. A European study7 has estimated that ED prevalence ranges from 6% to 64% beginning at 40 years of age and becoming more prevalent in the upper range of the age span.
In any clinical trial, improvement in the placebo arm is common. Placebo effects have been demonstrated in many conditions, such as Parkinson disease, pain disorders, anxiety disorders, depression, asthma, and irritable bowel syndrome.8 Several neurobiological mechanisms have been proposed to underlie placebo effects, including involvement of endogenous opioids,9,10 endogenous cannabinoids,11 and activation of dopaminergic neurons.12,13 The placebo effect has also been demonstrated in neuroimaging studies,14,15 which have found increased neural activations in brain structures involved in reduction of, for example, pain or motor symptoms.
We conducted a systematic review and meta-analysis on the use of PDE5Is for ED. The primary goal was to quantify the change in erectile function among patients in the placebo arm of randomized clinical trials as measured by the erectile function domain of the IIEF questionnaire. To our knowledge, this is the first comprehensive meta-analysis that quantifies the association of placebo with ED outcomes in randomized clinical trials of PDE5I.
Methods
Data Sources and Searches
The review protocol was preregistered in the PROSPERO database for meta-analyses (CRD42018109553), including a full account of searches, inclusion and exclusion criteria, main outcomes, and an analysis plan. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Data were obtained in collaboration with the Karolinska Institute Library. Searches in MEDLINE, Embase, Cochrane Library, and Web of Science Core Collection were performed for all randomized clinical trials published between January 1, 1998, and December 31, 2018, that focused on PDE5Is for ED treatment.
The most common measure to evaluate erectile function is the erectile function domain of the IIEF (IIEF-EF). Along with the IIEF-EF, the other domains of the IIEF are orgasmic function (IIEF-OF), intercourse satisfaction (IIEF-IS), sexual desire (IIEF-SD), and overall satisfaction (IIEF-OS). The IIEF-EF domain has a top score of 30, with a score below 14 indicating clinically impaired ED function and recommending use of a PDE5I.4,5 A change of 4 points or more is considered clinically meaningful. The IIEF-EF domain was chosen as the primary outcome of this meta-analysis. Some studies used the abridged 5-question version of the IIEF, Sexual Health Inventory for Men.
Study Selection
The selection of studies to be included in our analysis was independently conducted by 2 of us (A.S. and C.A.) in a blinded fashion using the Rayyan software for meta-analyses (Qatar Computing Research Institute). The inclusion criteria were double-blind, placebo-controlled randomized clinical trials that used PDE5I for ED treatment or had ED as a comorbidity for another condition and that were reported in English. After unblinding with the Rayyan software, any discrepancies between the 2 reviewers were reconciled in a consensus meeting. The study selection procedure adhered to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline to ensure adequate quality of included studies. A flowchart of study selection is given in Figure 1.
Figure 1. Flowchart for the Trials Included in the Meta-analysis.
ED indicates erectile dysfunction.
Statistical Analysis
Data extraction was independently conducted by 2 of us (A.S. and M.P.). Studies were excluded from the meta-analysis if they did not contain any IIEF questionnaire data, did not report any separate data for drug and placebo arms, provided incomplete data, did not allow the calculation of an effect size, or used mixed treatments with proposed effects for ED in the treatment regimen (eg, testosterone). The quality of included studies was graded with Jadad scores16 and was included in the risk of bias assessments at the study and outcome levels.
The Comprehensive Meta-Analysis software, version 3.0, was used for data management and statistical calculations of bias-corrected standardized mean differences (Hedges g). Effect size is commonly interpreted as small (Hedges g, 0.2), moderate (Hedges g, 0.5), or large (Hedges g, 0.8). Treatment response data from various domains of the IIEF questionnaire were analyzed separately per treatment arm as measured from before to after treatment, as well as the difference in outcomes between patients receiving active treatment and those receiving placebo. All analyses were performed with a random-effects approach using a 2-tailed α = . 05.
Studies that contained arms with different doses of active drug treatment were averaged to a mean treatment response. In line with a prespecified protocol, subanalyses were performed to explain effect size heterogeneity. Moderators included mean age of participants, study duration, evidence of financial interest, study drug, Jadad scale (range of 0-5, with higher numbers indicating higher quality), and comorbidities. A random-effect metaregression analysis was conducted on mean age of study participants, study drug, study duration, and influence of financial interest by study sponsor or investigators.
Results
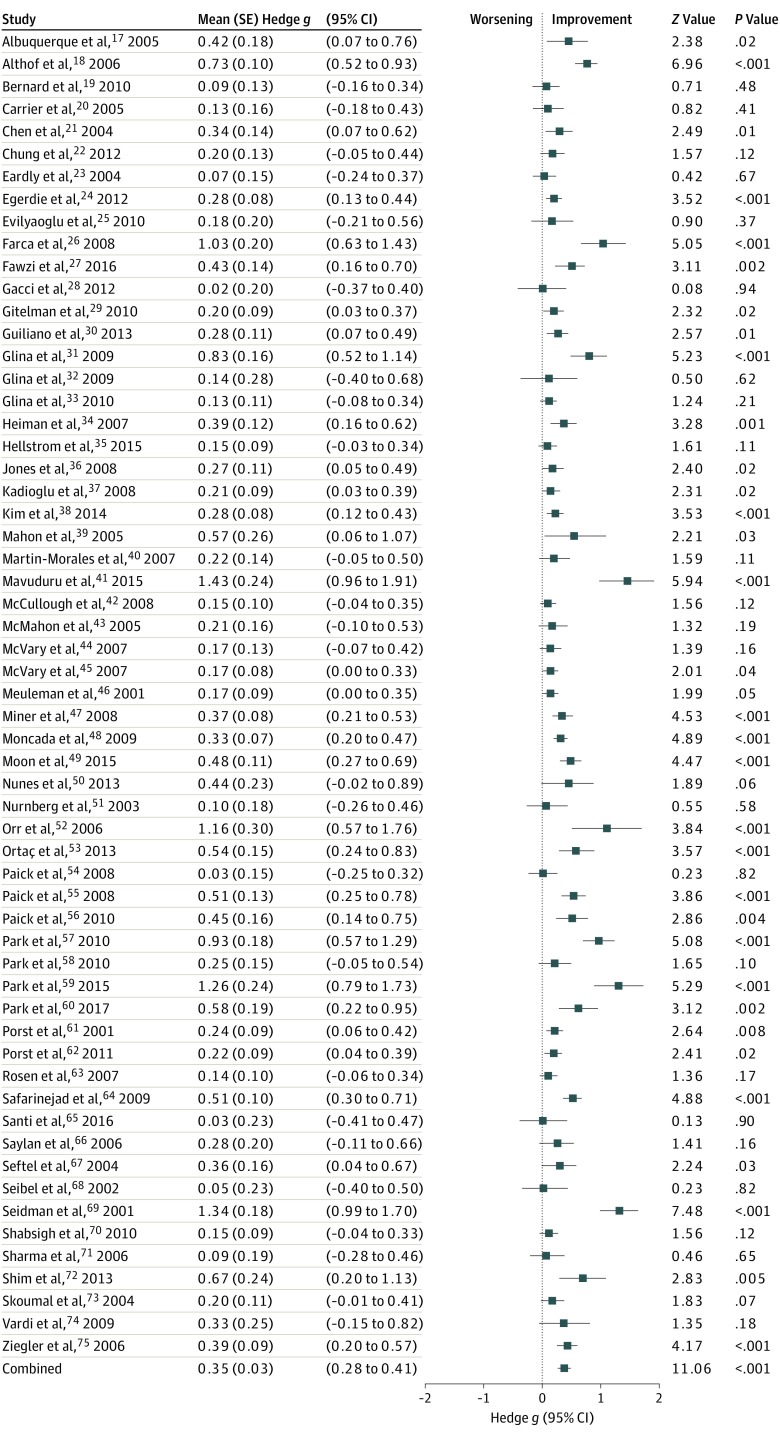
A total of 63 studies fulfilled the inclusion criteria; 59 studies were included in the main analysis that assessed the effect size before and after treatment for the various domains of IIEF. Four studies were analyzed separately because PDE5Is were used for treatment of ED after prostate cancer treatment and the design and patient groups differed considerably from the other studies (end of treatment scores indicate worsened erectile function compared with baseline because of surgery or radiotherapy). The treatment effect size in this subset of studies was calculated in a traditional drug vs placebo comparison. The methodologic quality of studies had a mean (SD) of 3.6 (0.88) points on the Jadad scale.16 A funnel plot was created in the Comprehensive Meta-Analysis software and revealed no risks of publication bias. The 63 included trials (Table 1) included a total of 12 564 men with ED (mean [SD] age, 55 [7] years; age range, 36-68 years). Some studies reported the cause of ED, but in general, studies represented a mix of peripheral and centrally mediated causes of ED, which was commonly referred to in the literature as organic and psychogenic. The mean duration of a treatment trial was 14 weeks (range, 4-104 weeks). A calculation on the combined effect size within the drug and placebo arms was conducted for all included studies in the main analysis (k = 59) excluding the 4 studies in patients with prostate surgery. The effect size in the placebo arm showed a small to moderate improvement of erectile function (Hedges g [SE], 0.35 [0.03]; I2 = 70.48; P < .001). The overall effect size in the drug arm showed a large response (Hedges g [SE], 1.25 [0.07]; I2 = 93.34; P < .001). A comparison between responses in the drug and placebo arms revealed a large difference in favor of active drug (Hedges g [SE], 1.04 [0.08]; I2 = 92.38; P < .001) (Figure 2).
Table 1. Studies Included in the Meta-analysis.
| Source | Study drug | No. of patients | Jadad score | Financial interest | Mean age, y | Study duration, wk |
|---|---|---|---|---|---|---|
| Albuquerque et al,17 2005 | Sildenafil | 87 | 2 | Yes | 60.0 | 8 |
| Althof et al,18 2006 | Sildenafil | 282 | 3 | Yes | 55.0 | 12 |
| Bernard et al,19 2010 | Sildenafil | 162 | 3 | Yes | 50.0 | 8 |
| Carrier et al,20 2005 | Tadalafil | 239 | 4 | Yes | 59.0 | 12 |
| Chen et al,21 2004 | Tadalafil | 194 | 4 | Yes | 59.5 | 12 |
| Chung et al,22 2012a | Mirodenafil | 134 | 3 | Yes | 55.5 | 12 |
| Eardly et al,23 2004 | Tadalafil | 215 | 3 | Yes | 53.5 | 12 |
| Egerdie et al,24 2012 | Tadalafil | 583 | 4 | Yes | 62.5 | 12 |
| Evilyaoglu et al,25 2010 | Tadalafil | 50 | 3 | No | 42.5 | 12 |
| Farca et al,26 2008 | Sildenafil | 89 | 3 | Yes | 56.0 | 12 |
| Fawzi et al,27 2016a | Sildenafil | 131 | 5 | No | 66.0 | 24 |
| Gacci et al,28 2012a | Vardenafil | 59 | 5 | No | 67.0 | 12 |
| Gitelman et al,29 2010 | Vardenafil | 327 | 3 | Yes | 62.0 | 12 |
| Guiliano et al,30 2013 | Tadalafil | 211 | 3 | Yes | 63.0 | 12 |
| Glina et al,31 2009 | Sildenafil | 129 | 3 | Yes | 53.5 | 12 |
| Glina et al,32 2009 | Lodenafil | 60 | 4 | Yes | 54.5 | 4 |
| Glina et al,33 2010 | Lodenafil | 319 | 4 | Yes | 55.5 | 4 |
| Heiman et al,34 2007 | Sildenafil | 176 | 5 | Yes | 58.0 | 12 |
| Hellstrom et al,35 2015 | Avanafil | 414 | 3 | Yes | 58.0 | 8 |
| Jones et al,36 2008 | Sildenafil | 202 | 3 | Yes | 52.0 | 10 |
| Kadioglu et al,37 2008 | Sildenafil | 294 | 2 | Yes | 45.0 | 6 |
| Kim et al,38 2014 | Tadalafil | 592 | 4 | Yes | 58.0 | 12 |
| Mahon et al,39 2005 | Sildenafil | 16 | 3 | Yes | 53.0 | 12 |
| Martin-Morales et al,40 2007 | Vardenafil | 121 | 3 | Yes | 53.0 | 12 |
| Mavuduru et al,41 2015a | Tadalafil | 82 | 4 | No | NA | 4 |
| McCullough et al,42 2008 | Sildenafil | 260 | 3 | Yes | 52.5 | 8 |
| McMahon et al,43 2005 | Tadalafil | 133 | 5 | Yes | 59.5 | 26 |
| McVary et al,44 2007 | Tadalafil | 156 | 3 | Yes | 61.5 | 12 |
| McVary et al,45 2007 | Sildenafil | 351 | 4 | Yes | 60.0 | 12 |
| Meuleman et al,46 2001 | Sildenafil | 315 | 3 | Yes | 54.5 | 26 |
| Miner et al,47 2008 | Vardenafil | 386 | 3 | No | 54.5 | 12 |
| Moncada et al,48 2009 | Sildenafil | 817 | 3 | Yes | 56.0 | 12 |
| Moon et al,49 2015 | Udenafil | 346 | 5 | Yes | 58.5 | 24 |
| Nunes et al,50 2013 | Lodenafil | 48 | 5 | No | 37.5 | 8 |
| Nurnberg et al,51 2003 | Sildenafil | 77 | 5 | Yes | 45.0 | 6 |
| Orr et al,52 2006 | Sildenafil | 42 | 4 | Yes | 45.0 | 4 |
| Ortaç et al,53 2013 | Udenafil | 118 | 5 | Yes | 43.5 | 8 |
| Paick et al,54 2008 | Udenafil | 164 | 3 | Yes | 55.0 | 12 |
| Paick et al,55 2008 | Mirodenafil | 222 | 3 | Yes | 53.5 | 12 |
| Paick et al,56 2010 | Mirodenafil | 107 | 3 | Yes | 57.5 | 12 |
| Park et al,57 2010 | Udenafil | 103 | 3 | Yes | 53.0 | 4 |
| Park et al,58 2010 | Mirodenafil | 108 | 2 | Yes | 56.5 | 12 |
| Park et al,59 2015a | Udenafil | 73 | 5 | No | 55.0 | 12 |
| Park et al,60 2017 | Avanafil | 158 | 4 | Yes | 56.5 | 8 |
| Porst et al,61 2001 | Vardenafil | 580 | 4 | Yes | 52.0 | 12 |
| Porst et al,62 2011 | Tadalafil | 300 | 3 | Yes | 65.0 | 12 |
| Rosen et al,63 2007 | Vardenafil | 216 | 4 | Yes | 58.0 | 8 |
| Safarinejad et al,64 2009 | Sildenafil | 242 | 4 | No | 48.0 | 4 |
| Santi et al,65 2016 | Vardenafil | 42 | 5 | No | 55.5 | 24 |
| Saylan et al,66 2006 | Tadalafil | 132 | 3 | Yes | 50.5 | 12 |
| Seftel et al,67 2004 | Tadalafil | 205 | 4 | Yes | 59.0 | 12 |
| Seibel et al,68 2002 | Sildenafil | 41 | 5 | No | 47.5 | 4 |
| Seidman et al,69 2001 | Sildenafil | 136 | 3 | Yes | 56.0 | 12 |
| Shabsigh et al,70 2010 | Sildenafil | 266 | 3 | Yes | 40.0 | 8 |
| Sharma et al,71 2006 | Sildenafil | 32 | 4 | No | 40.0 | 8 |
| Shim et al,72 2013a | Udenafil | 49 | 3 | No | 60.0 | 8 |
| Skoumal et al,73 2004 | Tadalafil | 403 | 3 | Yes | 52.0 | 12 |
| Vardi et al,74 2009 | Sildenafil | 53 | 2 | Yes | 55.0 | 4 |
| Ziegler et al,75 2006 | Vardenafil | 302 | 4 | Yes | 50.3 | 12 |
| Prostate cancer trials | ||||||
| Zelefsky et al,76 2014a | Sildenafil | 181 | 2 | Yes | NA | 104 |
| Incrocci et al,77 2001 | Sildenafil | 60 | 3 | Yes | 68.0 | 12 |
| Padma-Nathan et al,78 2008 | Sildenafil | 76 | 5 | Yes | 56.0 | 48 |
| Pisansky et al,79 2014 | Tadalafil | 96 | 4 | Yes | 63.0 | 52 |
Abbreviation: NA, not available.
Studies that used the abridged 5-question version of the International Index of Erectile Function (Sexual Health Inventory for Men).
Figure 2. Forest Plot of the Association Between Placebo and Erectile Disfunction Outcomes.
Erectile dysfunction outcomes were measured using the Erectile Function Domain of the International Index of Erectile Function. A low to moderate improvement was seen in the placebo arm, as indicated by the bias-corrected standardized mean difference (Hedges g [SE], 0.35 [0.03]; P < .001).
An analysis was performed on the other domains of the IIEF (ie, the IIEF-OF, IIEF-IS, IIEF-SD, and IIEF-OS). Not all included studies reported data on all domains; therefore, the number of studies per domain in this follow-up analysis was reduced. All results of IIEF subdomains are given in Table 2. For the effect size on orgasmic function (IIEF-OF; n = 31), the drug arm showed a high response, whereas the placebo arm showed a lower response, and between-group analysis showed a moderate response in favor of the drug arm. For intercourse satisfaction (IIEF-IS; n = 40), the drug arm showed a large response, the placebo arm showed a moderate response, and the between-group analysis showed a large effect size that favored the study drug. For sexual desire (IIEF-SD; n = 30), the drug arm showed a moderate response, and the placebo arm showed a low to moderate response. The between-group analysis was in favor of the study drug, with a moderate effect size. For overall satisfaction (IIEF-OS; n = 39), the drug arm showed a large response, whereas the placebo arm showed a low to moderate response. The between-group analysis showed a large effect size that favored the drug arm.
Table 2. Results From All IIEF Survey Domains Other Than Erectile Functiona.
| Domain | Studies, No. | Hedges g, (SE) | P value |
|---|---|---|---|
| Intercourse satisfaction (IIEF-IS) | |||
| Drug arm | 40 | 1.27 (0.09) | <.001 |
| Placebo arm | 40 | 0.52 (0.05) | <.001 |
| Between | 40 | 0.88 (0.08) | <.001 |
| Overall satisfaction (IIEF-OS) | |||
| Drug arm | 39 | 1.17 (0.08) | <.001 |
| Placebo arm | 39 | 0.35 (0.04) | <.001 |
| Between | 39 | 0.89 (0.09) | <.001 |
| Sexual desire (IIEF-SD) | |||
| Drug arm | 30 | 0.60 (0.06) | <.001 |
| Placebo arm | 30 | 0.25 (0.25) | <.001 |
| Between | 30 | 0.40 (0.04) | <.001 |
| Orgasmic function (IIEF-OF) | |||
| Drug arm | 31 | 0.75 (0.05) | <.001 |
| Placebo arm | 31 | 0.21 (0.03) | <.001 |
| Between | 31 | 0.57 (0.05) | <.001 |
Abbreviations: IIEF, International Index of Erectile Function; IS, intercourse satisfaction; OF, orgasmic function; OS, overall satisfaction; SD, sexual desire.
Data are given in descending order of effect size in the placebo arm. Not all studies used all subscales, which accounts for the different number of studies. Effect sizes are reported as bias-corrected standardized mean difference (Hedges g).
The effect size for studies in which ED was associated with posttraumatic stress disorder (PTSD; n = 2) indicated a large response for the drug arm (Hedges g [SE], 1.12 [0.39]; I2 = 77.79; P = .004) and a large response in the placebo arm (Hedges g [SE], 0.77 [0.32]; I2 = 76.15; P = .02). The between-group analysis yielded a moderate effect size in favor of the study drug (Hedges g [SE], 0.40 [0.17]; I2 = 27.64; P = .02).
Using a regression model, we assessed the association of moderators with ED treatment responses. In an overall analysis including data from the drug and placebo arms combined, no significant association of PDE5I drug type with treatment responses was found (q = 10.02; df = 6; I2 = 94.44; P = .12). The association was significant when the placebo arm was analyzed separately (q = 15.96; df = 6; I2 = 68.63; P = .01), but no significant association was seen in the drug arm (q = 11.14; df = 6; I2 = 92.83; P = .08) or in the between-group comparisons (q = 11.79; df = 6; I2 = 92.71; P = .07). There was a high correlation between treatment responses in the drug and placebo arm (r = 0.67), and without avanafil, a drug assessed in only 2 of the 63 studies, the correlation was higher (r = 0.94). There was no significant association of any of the other moderators with ED treatment responses (ie, financial interest, study duration, mean study participant age, or Jadad score). For these moderators, there were no significant associations for drug and placebo combined, for drug and placebo arms separately, or in a traditional drug vs placebo comparison.
An explorative analysis was conducted on the studies using PDE5Is as aid in recovery of erectile function after prostate surgery or radiotherapy (n = 4). This between-group analysis of the effect size did not show a significant response in favor of drug vs placebo (Hedges g [SE], 0.29 [0.17]; I2 = 59.35; P = .08).
Discussion
This study found a significant association between placebo treatment and IIEF-EF scores in patients with ED, but the potential mechanisms are still unexplored. One possibility is that the association between placebo effect and erectile function are mediated by an increased nervous tone in the thoracolumbar tract because this nerve tract is mainly influenced by arousal mechanisms.3 Several neurobiological mechanisms have been proposed to underlie placebo effects. Endogenous opioids and cannabinoids have been proposed to mediate the placebo effect in various conditions.9,10,11 Given that these 2 substrates (opioid and cannabinoid) are mainly involved in a negative association with sexual arousal, it seems unlikely that they are associated with the placebo effect in treatment for ED. Another commonly proposed neural substrate for the placebo effect, the dopaminergic system,13 would be more likely to be involved in the association between placebo effect and erectile function because dopamine has a positive association with sexual arousal.80 The dopaminergic hypothesis is supported by dopamine agonists having been used in the treatment of ED.81,82,83
There was a significant association of the active drug with erectile function scores in patients with ED. Given that the site of action of PDE5Is is the smooth muscle cells that influence the blood flow necessary to achieve erection, it seems plausible that the effect of the active drug would vary depending on the cause of the ED. If the problem were mainly vascular or endocrinologic, such as in atherosclerosis, diabetes, or hypogonadism, it seems plausible that the PDE5Is would have a strong effect because they address the underlying pathophysiological cause. If the nerves to the penis have been severed or severely damaged (as in the 4 prostate cancer trials in this analysis), the PDE5Is cannot amplify the nervous signal to the smooth muscle cells; thus, PDE5Is would have no specific effect.
Other domains of the IIEF questionnaire showed variations in effect sizes, in which responses in the drug arm were lower for orgasmic function (IIEF-OF) and sexual desire (IIEF-SD) compared with erectile function (IIEF-EF). This finding was expected because orgasmic function and especially sexual desire are associated with numerous factors other than the ability to achieve erection. There were also variations in effect size in the placebo arm, in which the response was lower for IIEF-OF and IIEF-SD compared with IIEF-EF. This finding was most likely attributable to the same factors as the differences among domains in the drug arm. Of interest, the response for intercourse satisfaction (IIEF-IS) was higher compared with the IIEF-EF response in the placebo arm. This result was not concordant with the result in the drug arm, and because the degree of the association with intercourse satisfaction may be more affected by psychological factors, this result supports separate mechanisms for placebo and drug improvements in patients with ED.
The 2 studies52,64 on treatment for PTSD-associated ED had a lower effect size for the drug response compared with results from the main analysis. The effect size of the placebo response in PTSD studies was markedly higher compared with the main analysis. Assuming that ED in this patient group was associated with psychological stress caused by traumatic experiences, the large improvement in the placebo arm could indicate that psychological factors might be associated with ED symptom improvements. This finding is supported by clinical studies84,85 that found comparable ED improvements for psychological interventions and PDE5Is, or increased improvements when combined (compared with PDE5Is alone).86 However, neither the drug nor placebo arm in the 2 studies52,64 on ED associated with PTSD reached a median IIEF-EF score, indicating normal erectile function, although clinically significant improvements were observed.
A metaregression analysis revealed a significant association between PDE5I drug type and treatment responses in the placebo arm and high correlation between the treatment response in the drug and placebo arms. The association of PDE5I drug type with erectile function in individuals given placebo suggests that differences in subjective perception of the different drugs exist because they differ in brand names, marketing, and visual appearance. Previous research shows that placebo effects are associated with labels87 and marketing,88 and it is possible that such differences contributed to the results in the present study.
No significant association of study duration, participant age, Jadad score, or financial interest with treatment effect size was found in the drug or placebo arm. Study duration is difficult to compare with previous studies of placebo longevity because PDE5Is are taken when needed, and the effects may be different from placebo responses in long-term use of, for example, antidepressants.89 Furthermore, there was little variation in participant age among studies, and new studies are needed to determine whether placebo responses in ED treatment may differ depending on participant age. The lack of an association between documented financial interests and treatment responses suggests that the trials included in this meta-analysis were not biased by the potential influence of a study sponsor.
The Jadad score reflects the quality of the clinical trials included in the meta-analysis. The lack of an association between Jadad scores and treatment outcomes indicates that the results in this meta-analysis are not biased by study quality.
Usually, PDE5Is are only taken before sexual intercourse. It has been theorized that daily long-term treatment with PDE5Is after prostate surgery and radiotherapy can aid the healing of damaged nerves through an amplification of the nervous input to the smooth muscles of the cavernous body.90 Previous research4 regarding the usefulness of this practice has been inconclusive. The results from the present meta-analysis showed no statistically significant differences between the response in the drug and placebo arms among patients who underwent treatment for prostate cancer. Individual studies91,92 that are not part of this meta-analysis have found stronger associations of erectile function and active drug compared with placebo. However, differences in the degree of nerve damage in the pelvic area may represent a confounding factor by which patients with less severe nerve damage after surgery or radiotherapy might receive a short-term benefit from PDE5Is (unrelated to the proposed effects of long-term treatment). The results in this meta-analysis suggest that PDE5Is have no significant association with the recovery of erectile function after prostate surgery or radiotherapy. Until robust differences between drug and placebo have been demonstrated, the practice of prescribing daily intake of PDE5Is after prostate cancer treatment may not be considered evidence, questioning the long-term use of PDE5Is for these patients.
Limitations
This study has limitations. This meta-analysis is limited by the inability to compare improvements in the placebo arm with no-treatment data from the included study populations. In any placebo-controlled drug trial, the inclusion of a no-treatment group will help understanding of how much of the treatment response is attributable to the drug itself, how much is attributable to the placebo effect, and how much of the treatment response is attributable to factors such as spontaneous remission and regression to the mean. We cannot exclude that the observed placebo response was partly associated with spontaneous ED improvements. The inclusion of a no-treatment control group in randomized clinical trials of ED is warranted because it would potentially lead to a better understanding of placebo effects in PDE5I treatment trials.
Conclusions
This systematic review and meta-analysis found a significant association of placebo and ED outcomes, with larger effect sizes among men with PTSD-associated ED. No difference in erectile function was found between those who received placebo vs PDE5I for ED after prostate surgery.
References
- 1.Goldstein I, Burnett AL, Rosen RC, Park PW, Stecher VJ. The serendipitous story of sildenafil: an unexpected oral therapy for erectile dysfunction. Sex Med Rev. 2019;7(1):-. doi: 10.1016/j.sxmr.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Delate T, Simmons VA, Motheral BR. Patterns of use of sildenafil among commercially insured adults in the United States: 1998-2002. Int J Impot Res. 2004;16(4):313-318. doi: 10.1038/sj.ijir.3901191 [DOI] [PubMed] [Google Scholar]
- 3.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379-395, v. v. doi: 10.1016/j.ucl.2005.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830. doi: 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 6.Pastuszak AW. Current diagnosis and management of erectile dysfunction. Curr Sex Health Rep. 2014;6(3):164-176. doi: 10.1007/s11930-014-0023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona G, Lee DM, Forti G, et al. ; EMAS Study Group . Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. 2010;7(4, pt 1):1362-1380. doi: 10.1111/j.1743-6109.2009.01601.x [DOI] [PubMed] [Google Scholar]
- 8.Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36(1):339-354. doi: 10.1038/npp.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauro MD, Greenberg RP. Endogenous opiates and the placebo effect: a meta-analytic review. J Psychosom Res. 2005;58(2):115-120. doi: 10.1016/j.jpsychores.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484-494. doi: 10.1523/JNEUROSCI.19-01-00484.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228-1230. doi: 10.1038/nm.2435 [DOI] [PubMed] [Google Scholar]
- 12.de la Fuente-Fernández R, Lidstone S, Stoessl AJ. Placebo effect and dopamine release. J Neural Transm Suppl. 2006;(70):415-418. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente-Fernández R. The placebo-reward hypothesis: dopamine and the placebo effect. Parkinsonism Relat Disord. 2009;15(suppl 3):S72-S74. doi: 10.1016/S1353-8020(09)70785-0 [DOI] [PubMed] [Google Scholar]
- 14.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162-1167. doi: 10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- 15.Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227-236. doi: 10.1016/j.neuroimage.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 17.Albuquerque DC, Miziara LJ, Saraiva JF, Rodrigues US, Ribeiro AB, Wajngarten M. Efficacy, safety and tolerability of sildenafil in Brazilian hypertensive patients on multiple antihypertensive drugs. Int Braz J Urol. 2005;31(4):342-353. doi: 10.1590/S1677-55382005000400008 [DOI] [PubMed] [Google Scholar]
- 18.Althof SE, O’leary MP, Cappelleri JC, et al. ; International SEAR Study Group . Sildenafil citrate improves self-esteem, confidence, and relationships in men with erectile dysfunction: results from an international, multi-center, double-blind, placebo-controlled trial. J Sex Med. 2006;3(3):521-529. doi: 10.1111/j.1743-6109.2006.00234.x [DOI] [PubMed] [Google Scholar]
- 19.Bénard F, Carrier S, Lee JC, Talwar V, Defoy I. Men with mild erectile dysfunction benefit from sildenafil treatment. J Sex Med. 2010;7(11):3725-3735. doi: 10.1111/j.1743-6109.2010.02015.x [DOI] [PubMed] [Google Scholar]
- 20.Carrier S, Brock GB, Pommerville PJ, et al. Efficacy and safety of oral tadalafil in the treatment of men in Canada with erectile dysfunction: a randomized, double-blind, parallel, placebo-controlled clinical trial. J Sex Med. 2005;2(5):685-698. doi: 10.1111/j.1743-6109.2005.00097.x [DOI] [PubMed] [Google Scholar]
- 21.Chen KK, Jiann BP, Lin JS, et al. Efficacy and safety of on-demand oral tadalafil in the treatment of men with erectile dysfunction in Taiwan: a randomized, double-blind, parallel, placebo-controlled clinical study. J Sex Med. 2004;1(2):201-208. doi: 10.1111/j.1743-6109.2004.04029.x [DOI] [PubMed] [Google Scholar]
- 22.Chung JH, Kang DH, Oh CY, et al. Safety and efficacy of once daily administration of 50 mg mirodenafil in patients with erectile dysfunction: a multicenter, double-blind, placebo controlled trial. J Urol. 2013;189(3):1006-1013. doi: 10.1016/j.juro.2012.08.243 [DOI] [PubMed] [Google Scholar]
- 23.Eardley I, Gentile V, Austoni E, et al. Efficacy and safety of tadalafil in a Western European population of men with erectile dysfunction. BJU Int. 2004;94(6):871-877. doi: 10.1111/j.1464-410X.2004.05049.x [DOI] [PubMed] [Google Scholar]
- 24.Egerdie RB, Auerbach S, Roehrborn CG, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. J Sex Med. 2012;9(1):271-281. doi: 10.1111/j.1743-6109.2011.02504.x [DOI] [PubMed] [Google Scholar]
- 25.Evliyaoğlu Y, Yelsel K, Kobaner M, Alma E, Saygılı M. Efficacy and tolerability of tadalafil for treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Urology. 2011;77(5):1137-1141. doi: 10.1016/j.urology.2010.12.036 [DOI] [PubMed] [Google Scholar]
- 26.Zonana Farca E, Francolugo-Vélez V, Moy-Eransus C, Orozco Bravo A, Tseng LJ, Stecher VJ. Self-esteem, confidence and relationship satisfaction in men with erectile dysfunction: a randomized, parallel-group, double-blind, placebo-controlled study of sildenafil in Mexico. Int J Impot Res. 2008;20(4):402-408. doi: 10.1038/ijir.2008.24 [DOI] [PubMed] [Google Scholar]
- 27.Fawzi A, Kamel M, Salem E, et al. Sildenafil citrate in combination with tamsulosin versus tamsulosin monotherapy for management of male lower urinary tract symptoms due to benign prostatic hyperplasia: a randomised, double-blind, placebo-controlled trial. Arab J Urol. 2016;15(1):53-59. doi: 10.1016/j.aju.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gacci M, Vittori G, Tosi N, et al. A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med. 2012;9(6):1624-1633. doi: 10.1111/j.1743-6109.2012.02718.x [DOI] [PubMed] [Google Scholar]
- 29.Gittelman M, McMahon CG, Rodríguez-Rivera JA, Beneke M, Ulbrich E, Ewald S. The POTENT II randomised trial: efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction. Int J Clin Pract. 2010;64(5):594-603. doi: 10.1111/j.1742-1241.2010.02358.x [DOI] [PubMed] [Google Scholar]
- 30.Giuliano F, Oelke M, Jungwirth A, et al. Tadalafil once daily improves ejaculatory function, erectile function, and sexual satisfaction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia and erectile dysfunction: results from a randomized, placebo- and tamsulosin-controlled, 12-week double-blind study. J Sex Med. 2013;10(3):857-865. doi: 10.1111/jsm.12039 [DOI] [PubMed] [Google Scholar]
- 31.Glina S, Damião R, Abdo C, Afif-Abdo J, Tseng LJ, Stecher V. Self-esteem, confidence, and relationships in Brazilian men with erectile dysfunction receiving sildenafil citrate: a randomized, parallel-group, double-blind, placebo-controlled study in Brazil. J Sex Med. 2009;6(1):268-275. doi: 10.1111/j.1743-6109.2008.01026.x [DOI] [PubMed] [Google Scholar]
- 32.Glina S, Toscano I, Gomatzky C, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy in erectile dysfunction: a phase II clinical trial. J Sex Med. 2009;6(2):553-557. doi: 10.1111/j.1743-6109.2008.01079.x [DOI] [PubMed] [Google Scholar]
- 33.Glina S, Fonseca GN, Bertero EB, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy of erectile dysfunction: a phase III clinical trial. J Sex Med. 2010;7(5):1928-1936. doi: 10.1111/j.1743-6109.2010.01711.x [DOI] [PubMed] [Google Scholar]
- 34.Heiman JR, Talley DR, Bailen JL, et al. Sexual function and satisfaction in heterosexual couples when men are administered sildenafil citrate (Viagra) for erectile dysfunction: a multicentre, randomised, double-blind, placebo-controlled trial. BJOG. 2007;114(4):437-447. doi: 10.1111/j.1471-0528.2006.01228.x [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom WJ, Kaminetsky J, Belkoff LH, et al. Efficacy of avanafil 15 minutes after dosing in men with erectile dysfunction: a randomized, double-blind, placebo controlled study. J Urol. 2015;194(2):485-492. doi: 10.1016/j.juro.2014.12.101 [DOI] [PubMed] [Google Scholar]
- 36.Jones LA, Klimberg IW, McMurray JG, Padula R, Tseng LJ, Stecher VJ. Effect of sildenafil citrate on the male sexual experience assessed with the Sexual Experience Questionnaire: a multicenter, double-blind, placebo-controlled trial with open-label extension. J Sex Med. 2008;5(8):1955-1964. doi: 10.1111/j.1743-6109.2008.00879.x [DOI] [PubMed] [Google Scholar]
- 37.Kadioglu A, Grohmann W, Depko A, Levinson IP, Sun F, Collins S. Quality of erections in men treated with flexible-dose sildenafil for erectile dysfunction: multicenter trial with a double-blind, randomized, placebo-controlled phase and an open-label phase. J Sex Med. 2008;5(3):726-734. doi: 10.1111/j.1743-6109.2007.00701.x [DOI] [PubMed] [Google Scholar]
- 38.Kim ED, Seftel AD, Goldfischer ER, Ni X, Burns PR. A return to normal erectile function with tadalafil once daily after an incomplete response to as-needed PDE5 inhibitor therapy. J Sex Med. 2014;11(3):820-830. doi: 10.1111/jsm.12253 [DOI] [PubMed] [Google Scholar]
- 39.Mahon A, Sidhu PS, Muir G, Macdougall IC. The efficacy of sildenafil for the treatment of erectile dysfunction in male peritoneal dialysis patients. Am J Kidney Dis. 2005;45(2):381-387. doi: 10.1053/j.ajkd.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 40.Martin-Morales A, Meijide F, García N, Artes M, Muñoz A. Efficacy of vardenafil and influence on self-esteem and self-confidence in patients with severe erectile dysfunction. J Sex Med. 2007;4(2):440-447. doi: 10.1111/j.1743-6109.2006.00426.x [DOI] [PubMed] [Google Scholar]
- 41.Pattanaik S, Kaundal P, Mavuduru RS, Singh SK, Mandal AK. Endothelial dysfunction in patients with erectile dysfunction: a double-blind, randomized-control trial using tadalafil. Sex Med. 2019;7(1):41-47. doi: 10.1016/j.esxm.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCullough AR, Steidle CP, Klee B, Tseng LJ. Randomized, double-blind, crossover trial of sildenafil in men with mild to moderate erectile dysfunction: efficacy at 8 and 12 hours postdose. Urology. 2008;71(4):686-692. doi: 10.1016/j.urology.2007.12.025 [DOI] [PubMed] [Google Scholar]
- 43.McMahon CG, Stuckey BG, Lording DW, et al. A 6-month study of the efficacy and safety of tadalafil in the treatment of erectile dysfunction: a randomised, double-blind, parallel-group, placebo-controlled study in Australian men. Int J Clin Pract. 2005;59(2):143-149. doi: 10.1111/j.1742-1241.2005.00451.x [DOI] [PubMed] [Google Scholar]
- 44.McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177(4):1401-1407. doi: 10.1016/j.juro.2006.11.037 [DOI] [PubMed] [Google Scholar]
- 45.McVary KT, Monnig W, Camps JL Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177(3):1071-1077. doi: 10.1016/j.juro.2006.10.055 [DOI] [PubMed] [Google Scholar]
- 46.Meuleman E, Cuzin B, Opsomer RJ, et al. A dose-escalation study to assess the efficacy and safety of sildenafil citrate in men with erectile dysfunction. BJU Int. 2001;87(1):75-81. doi: 10.1046/j.1464-410x.2001.00998.x [DOI] [PubMed] [Google Scholar]
- 47.Miner M, Gilderman L, Bailen J, et al. Vardenafil in men with stable statin therapy and dyslipidemia. J Sex Med. 2008;5(6):1455-1467. doi: 10.1111/j.1743-6109.2008.00820.x [DOI] [PubMed] [Google Scholar]
- 48.Moncada I, Martínez-Jabaloyas JM, Rodriguez-Vela L, et al. Emotional changes in men treated with sildenafil citrate for erectile dysfunction: a double-blind, placebo-controlled clinical trial. J Sex Med. 2009;6(12):3469-3477. doi: 10.1111/j.1743-6109.2009.01514.x [DOI] [PubMed] [Google Scholar]
- 49.Moon KH, Ko YH, Kim SW, et al. Efficacy of once-daily administration of udenafil for 24 weeks on erectile dysfunction: results from a randomized multicenter placebo-controlled clinical trial. J Sex Med. 2015;12(5):1194-1201. doi: 10.1111/jsm.12862 [DOI] [PubMed] [Google Scholar]
- 50.Nunes LV, Lacaz FS, Bressan RA, Nunes SO, Mari JdeJ. Adjunctive treatment with lodenafil carbonate for erectile dysfunction in outpatients with schizophrenia and spectrum: a randomized, double-blind, crossover, placebo-controlled trial. J Sex Med. 2013;10(4):1136-1145. doi: 10.1111/jsm.12040 [DOI] [PubMed] [Google Scholar]
- 51.Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S. Treatment of antidepressant-associated sexual dysfunction with sildenafil: a randomized controlled trial. JAMA. 2003;289(1):56-64. doi: 10.1001/jama.289.1.56 [DOI] [PubMed] [Google Scholar]
- 52.Orr G, Weiser M, Polliack M, Raviv G, Tadmor D, Grunhaus L. Effectiveness of sildenafil in treating erectile dysfunction in PTSD patients: a double-blind, placebo-controlled crossover study. J Clin Psychopharmacol. 2006;26(4):426-430. doi: 10.1097/01.jcp.0000227701.33999.b3 [DOI] [PubMed] [Google Scholar]
- 53.Ortaç M, Çayan S, Çalişkan MK, et al. Efficacy and tolerability of udenafil in Turkish men with erectile dysfunction of psychogenic and organic aetiology: a randomized, double-blind, placebo-controlled study. Andrology. 2013;1(4):549-555. doi: 10.1111/j.2047-2927.2013.00085.x [DOI] [PubMed] [Google Scholar]
- 54.Paick JS, Kim SW, Yang DY, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5(4):946-953. doi: 10.1111/j.1743-6109.2007.00723.x [DOI] [PubMed] [Google Scholar]
- 55.Paick JS, Ahn TY, Choi HK, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008;5(11):2672-2680. doi: 10.1111/j.1743-6109.2008.00945.x [DOI] [PubMed] [Google Scholar]
- 56.Paick JS, Kim JJ, Kim SC, et al. Efficacy and safety of mirodenafil in men taking antihypertensive medications. J Sex Med. 2010;7(9):3143-3152. doi: 10.1111/j.1743-6109.2010.01926.x [DOI] [PubMed] [Google Scholar]
- 57.Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010;7(6):2209-2216. doi: 10.1111/j.1743-6109.2010.01817.x [DOI] [PubMed] [Google Scholar]
- 58.Park HJ, Choi HK, Ahn TY, et al. Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Sex Med. 2010;7(8):2842-2850. doi: 10.1111/j.1743-6109.2010.01888.x [DOI] [PubMed] [Google Scholar]
- 59.Park SY, Choi GS, Park JS, Kim HJ, Park JA, Choi JI. Efficacy and safety of udenafil for the treatment of erectile dysfunction after total mesorectal excision of rectal cancer: a randomized, double-blind, placebo-controlled trial. Surgery. 2015;157(1):64-71. doi: 10.1016/j.surg.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 60.Park HJ, Kim SW, Kim JJ, et al. A randomized, placebo-controlled, double-blind, multi-center therapeutic confirmatory study to evaluate the safety and efficacy of avanafil in Korean patients with erectile dysfunction. J Korean Med Sci. 2017;32(6):1016-1023. doi: 10.3346/jkms.2017.32.6.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porst H, Rosen R, Padma-Nathan H, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res. 2001;13(4):192-199. doi: 10.1038/sj.ijir.3900713 [DOI] [PubMed] [Google Scholar]
- 62.Porst H, Kim ED, Casabé AR, et al. ; LVHJ study team . Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60(5):1105-1113. doi: 10.1016/j.eururo.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 63.Rosen RC, Wincze J, Mollen MD, Gondek K, McLeod LD, Fisher WA. Responsiveness and minimum important differences for the erection quality scale. J Urol. 2007;178(5):2076-2081. doi: 10.1016/j.juro.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 64.Safarinejad MR, Kolahi AA, Ghaedi G. Safety and efficacy of sildenafil citrate in treating erectile dysfunction in patients with combat-related post-traumatic stress disorder: a double-blind, randomized and placebo-controlled study. BJU Int. 2009;104(3):376-383. doi: 10.1111/j.1464-410X.2009.08560.x [DOI] [PubMed] [Google Scholar]
- 65.Santi D, Granata AR, Guidi A, et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, prospective trial. Eur J Endocrinol. 2016;174(4):513-522. doi: 10.1530/EJE-15-1100 [DOI] [PubMed] [Google Scholar]
- 66.Saylan M, Khalaf I, Kadioglu A, et al. Efficacy of tadalafil in Egyptian and Turkish men with erectile dysfunction. Int J Clin Pract. 2006;60(7):812-819. doi: 10.1111/j.1742-1241.2006.00993.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seftel AD, Wilson SK, Knapp PM, Shin J, Wang WC, Ahuja S. The efficacy and safety of tadalafil in United States and Puerto Rican men with erectile dysfunction. J Urol. 2004;172(2):652-657. doi: 10.1097/01.ju.0000132857.39680.ce [DOI] [PubMed] [Google Scholar]
- 68.Seibel I, Poli De Figueiredo CE, Telöken C, Moraes JF. Efficacy of oral sildenafil in hemodialysis patients with erectile dysfunction. J Am Soc Nephrol. 2002;13(11):2770-2775. doi: 10.1097/01.ASN.0000034201.97937.3E [DOI] [PubMed] [Google Scholar]
- 69.Seidman SN, Roose SP, Menza MA, Shabsigh R, Rosen RC. Treatment of erectile dysfunction in men with depressive symptoms: results of a placebo-controlled trial with sildenafil citrate. Am J Psychiatry. 2001;158(10):1623-1630. doi: 10.1176/appi.ajp.158.10.1623 [DOI] [PubMed] [Google Scholar]
- 70.Shabsigh R, Kaufman J, Magee M, Creanga D, Russell D, Budhwani M. A multicenter, double-blind, placebo-controlled trial to assess the efficacy of sildenafil citrate in men with unrecognized erectile dysfunction. Urology. 2010;76(2):373-379. doi: 10.1016/j.urology.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 71.Sharma RK, Prasad N, Gupta A, Kapoor R. Treatment of erectile dysfunction with sildenafil citrate in renal allograft recipients: a randomized, double-blind, placebo-controlled, crossover trial. Am J Kidney Dis. 2006;48(1):128-133. doi: 10.1053/j.ajkd.2006.04.061 [DOI] [PubMed] [Google Scholar]
- 72.Shim YS, Pae CU, Cho KJ, Kim SW, Kim JC, Koh JS. Effects of daily low-dose treatment with phosphodiesterase type 5 inhibitor on cognition, depression, somatization and erectile function in patients with erectile dysfunction: a double-blind, placebo-controlled study. Int J Impot Res. 2014;26(2):76-80. doi: 10.1038/ijir.2013.38 [DOI] [PubMed] [Google Scholar]
- 73.Skoumal R, Chen J, Kula K, et al. Efficacy and treatment satisfaction with on-demand tadalafil (Cialis) in men with erectile dysfunction. Eur Urol. 2004;46(3):362-369. doi: 10.1016/j.eururo.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 74.Vardi Y, Appel B, Ofer Y, Greunwald I, Dayan L, Jacob G. Effect of chronic sildenafil treatment on penile endothelial function: a randomized, double-blind, placebo controlled study. J Urol. 2009;182(6):2850-2855. doi: 10.1016/j.juro.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 75.Ziegler D, Merfort F, Van Ahlen H, Yassin A, Reblin T, Neureither M. Efficacy and safety of flexible-dose vardenafil in men with type 1 diabetes and erectile dysfunction. J Sex Med. 2006;3(5):883-891. doi: 10.1111/j.1743-6109.2006.00295.x [DOI] [PubMed] [Google Scholar]
- 76.Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874. doi: 10.1016/j.juro.2014.02.097 [DOI] [PubMed] [Google Scholar]
- 77.Incrocci L, Koper PC, Hop WC, Slob AK. Sildenafil citrate (Viagra) and erectile dysfunction following external beam radiotherapy for prostate cancer: a randomized, double-blind, placebo-controlled, cross-over study. Int J Radiat Oncol Biol Phys. 2001;51(5):1190-1195. doi: 10.1016/S0360-3016(01)01767-9 [DOI] [PubMed] [Google Scholar]
- 78.Padma-Nathan H, McCullough AR, Levine LA, et al. ; Study Group . Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20(5):479-486. doi: 10.1038/ijir.2008.33 [DOI] [PubMed] [Google Scholar]
- 79.Pisansky TM, Pugh SL, Greenberg RE, et al. Tadalafil for prevention of erectile dysfunction after radiotherapy for prostate cancer: the Radiation Therapy Oncology Group [0831] randomized clinical trial. JAMA. 2014;311(13):1300-1307. doi: 10.1001/jama.2014.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506-1533. doi: 10.1111/j.1743-6109.2009.01309.x [DOI] [PubMed] [Google Scholar]
- 81.Montorsi F, Perani D, Anchisi D, et al. Brain activation patterns during video sexual stimulation following the administration of apomorphine: results of a placebo-controlled study. Eur Urol. 2003;43(4):405-411. doi: 10.1016/S0302-2838(03)00053-8 [DOI] [PubMed] [Google Scholar]
- 82.Mulhall JP. Sublingual apomorphine for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2002;11(2):295-302. doi: 10.1517/13543784.11.2.295 [DOI] [PubMed] [Google Scholar]
- 83.Montorsi F, Perani D, Anchisi D, et al. Apomorphine-induced brain modulation during sexual stimulation: a new look at central phenomena related to erectile dysfunction. Int J Impot Res. 2003;15(3):203-209. doi: 10.1038/sj.ijir.3900999 [DOI] [PubMed] [Google Scholar]
- 84.Melnik T, Abdo CH. Psychogenic erectile dysfunction: comparative study of three therapeutic approaches. J Sex Marital Ther. 2005;31(3):243-255. doi: 10.1080/00926230590513465 [DOI] [PubMed] [Google Scholar]
- 85.McCabe MP, Price E, Piterman L, Lording D. Evaluation of an internet-based psychological intervention for the treatment of erectile dysfunction. Int J Impot Res. 2008;20(3):324-330. doi: 10.1038/ijir.2008.3 [DOI] [PubMed] [Google Scholar]
- 86.Aubin S, Heiman JR, Berger RE, Murallo AV, Yung-Wen L. Comparing sildenafil alone vs. sildenafil plus brief couple sex therapy on erectile dysfunction and couples’ sexual and marital quality of life: a pilot study. J Sex Marital Ther. 2009;35(2):122-143. doi: 10.1080/00926230802712319 [DOI] [PubMed] [Google Scholar]
- 87.Kam-Hansen S, Jakubowski M, Kelley JM, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6(218):218ra5. doi: 10.1126/scitranslmed.3006175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. JAMA. 2008;299(9):1016-1017. doi: 10.1001/jama.299.9.1016 [DOI] [PubMed] [Google Scholar]
- 89.Khan A, Redding N, Brown WA. The persistence of the placebo response in antidepressant clinical trials. J Psychiatr Res. 2008;42(10):791-796. doi: 10.1016/j.jpsychires.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 90.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol. 2004;171(2, pt 1):771-774. doi: 10.1097/01.ju.0000106970.97082.61 [DOI] [PubMed] [Google Scholar]
- 91.Raina R, Lakin MM, Agarwal A, et al. Long-term effect of sildenafil citrate on erectile dysfunction after radical prostatectomy: 3-year follow-up. Urology. 2003;62(1):110-115. doi: 10.1016/S0090-4295(03)00157-2 [DOI] [PubMed] [Google Scholar]
- 92.Ohebshalom M, Parker M, Guhring P, Mulhall JP. The efficacy of sildenafil citrate following radiation therapy for prostate cancer: temporal considerations. J Urol. 2005;174(1):258-262. doi: 10.1097/01.ju.0000164286.47518.1e [DOI] [PubMed] [Google Scholar]