Abstract
Preamble
The past year has brought many new concepts and an abundance of new data on the nature, management, and outcome of heart failure. The pace of change is accelerating. We look forward to an exciting new decade of research. The prognosis of cardiovascular disease is determined to a large extent by the ability to delay or prevent the development and progression of heart failure.1 Accordingly, attention is shifting to earlier diagnosis of and intervention for heart failure. Patients with type-2 diabetes mellitus (T2DM)2 or coronary artery disease (CAD)3 have a relatively good prognosis unless plasma concentrations of natriuretic peptides are increased, indicating important cardiac or renal dysfunction. Adoption of a simple ‘Universal Definition’ of heart failure based on natriuretic peptides would facilitate early diagnosis and treatment but lead to an enormous increase in its prevalence and demand upon medical services.4 We need to prepare for the impending shock.
Epidemiology and prevention
In cardiology, the term prevention is often used to mean delaying the onset of disease; in other words, procrastination. Failure to appreciate the difference between prevention and procrastination leads to problems in projecting future healthcare needs and costs. Older people have more co-morbid conditions that complicate management but may also offer more opportunities for intervention; consequently, more time and resources are required to manage older patients well.
A detailed report on heart failure in the UK shows that the median age of onset has risen to about 80 years, consistent with improvements in the treatment of hypertension and other risk factors for atherosclerosis and better management of myocardial infarction.5 Unfortunately, data on left ventricular ejection fraction (LVEF) were not available for this report. Analyses of the diagnostic pathway in primary care in the UK suggest that key investigations are often not done.6–8 Similar data from other countries are urgently required. Several large epidemiological surveys9,10 and analyses of large trials11,12 have recently been published that allow the demographics, aetiology, and management of heart failure to be compared internationally.
Mineralocorticoid receptor antagonists (MRAs) are effective anti-hypertensive agents that also improve the prognosis of patients with heart failure and a reduced (HFrEF) and possibly preserved (HFpEF) LVEF.13 Whether MRAs have specific effects on reducing other potential drivers of the progression to heart failure such as inflammation and fibrosis is currently under investigation.14,15
Genetic propensity to greater body fat was associated with the risk of developing heart failure in an analysis on 367 703 UK Biobank participants.16 However, the incidence of heart failure was only 1% (4803 patients), the diagnostic criteria were not robust, and the increase in risk was modest (odds ratio 1.22; 95% CI 1.06–1.41). Further analyses on this population showed a strong relationship between cardio-respiratory fitness and grip strength and future incidence of heart failure.17 A study of 4403 people considered for bariatric surgery in Sweden and followed for 22 years, found that 188 (9%) of the 2003 who had surgery (25–35 kg weight loss; BMI 1 year after surgery 32 kg/m2) developed heart failure compared with 266 (13%) of 2030 who did not (BMI after 1 year observation 40 kg/m2).18 Although these data suggest links between obesity and the risk of developing heart failure, it is possible that obesity just provokes similar symptoms. Once heart failure has developed, obesity is associated with a lower mortality, but this may also reflect earlier diagnosis rather than a protective effect.19 Randomized controlled trials (RCTs) of effective interventions for obesity are required to demonstrate whether weight loss improves symptoms (likely) and clinical outcomes (less certain).
A report from ‘the Atherosclerosis Risk in Communities’ (ARIC) study confirmed the association between influenza epidemics and hospitalizations for heart failure, reinforcing guideline-recommendations for vaccination20; an RCT is underway.21 Extended follow-up (median 18.9 years) of the Women’s Health Initiative Hormone Therapy trials, which randomized 27 347 women to various hormone replacement regimens, showed that they had no effect on the incidence of HFrEF or pEF.22
The ISCHEMIA trial (presented at the American Heart Association 2019) compared strategies of early coronary revascularization, predominantly percutaneous, with conservative management for stable CAD, some of whom had mild symptoms of heart failure and/or a reduced LVEF. Revascularization did not reduce the risk of myocardial infarction or death but increased the risk of stroke almost four-fold and did not reduce new-onset heart failure over the following 4 years.
Diagnosis
The Heart Failure Association of the European Society of Cardiology has proposed a new scoring system for the diagnosis of HFpEF.23 Its practical utility awaits confirmation.24 Simpler approaches may be preferred.4
Congestion
Congestion lies at the heart of failure.25–27 Imaging has long been used to identify dilation of the atria and venous system, which might be termed haemodynamic congestion, for which natriuretic peptides are a useful biomarker.25 More recently imaging has been used to identify accumulation of fluid in tissues (tissue congestion),25,28–32 which may be associated with increases in the biomarker, (bio)-adrenomedullin.33 Imaging and biomarkers in combination are both sensitive and specific for detecting a failing heart, a useful guide to the severity of congestion and prognosis and a potential therapeutic target indicating successful management. Imaging remains the preferred method for identifying the cause of heart failure. If congestion is central to the management of heart failure, then better monitoring34 and more effective (diuretic) interventions (perhaps acetazolamide?35) should improve outcome (Take home figure).
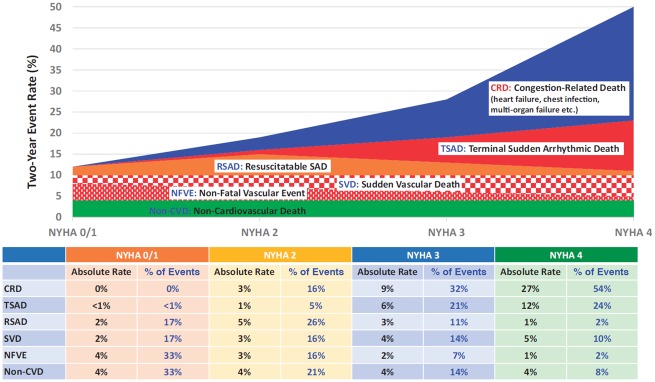
Take home figure.
Two-year cause-specific mortality and non-fatal vascular events for patients with cardiovascular disease according to New York Heart Association (NYHA) class. Numbers and proportions are a conceptual representation of absolute and relative risk and are not strictly evidence-based. Note that for patients in NYHA Class 4, interventions for sudden arrhythmic death may be ineffective or fail to lead to a meaningful prolongation of life because the patient is likely soon to die of worsening heart failure. CRD, congestion-related death, otherwise called death due to worsening heart failure; NFVE, non-fatal vascular event (e.g. myocardial infarction and stroke; note that events are more likely to be suddenly fatal as heart failure progresses); non-CVD, non-cardiovascular death; RSAD, resuscitatable sudden arrhythmic death; SVD, sudden vascular death; TSAD, terminal (non-resucitatable) sudden arrhythmic death. Reproduced with permission from ref.59
Age and prognosis
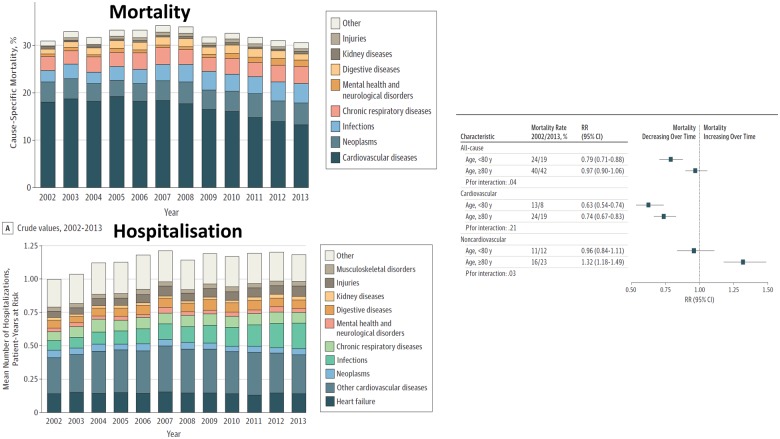
Analysis of a large primary care database suggested that the cardiovascular (CV) prognosis of new-onset heart failure improved substantially between 2002 and 2014 [hazard ratio (HR): 0.73; 95% CI 0.68–0.80] for patients above and below the age of 80 years.5 However, in those aged >80 years, the fall in CV mortality was entirely offset by non-CV mortality. In other words, treatment changed the way that elderly patients died but not overall mortality (Figure 1). Unfortunately, information on LVEF was not available; many patients will have had HFpEF and, therefore, caution should be exercised in attributing the reduction in CV mortality to treatment of heart failure. A systematic review of survey and registry data also suggested that the prognosis of heart failure had improved; important determinants of outcome were age and cardiology input to management.36 Frailty, which might be considered a biological rather than chronological measure of age, may be an even more powerful predictor of disability and death.37
Figure 1.
Changes in cause-specific mortality and hospitalizations for patients with incident heart failure in the UK between 2002 and 2013. Reproduced with permission from ref.5
Guideline-recommendations for the treatment of HFrEF do not discriminate by age. The Swedish Heart Failure Registry found that prescription of ACE inhibitors or beta-blockers to patients with HFrEF aged >80 years was associated with a lower mortality.38,39 However, observational associations have many explanations other than a therapeutic effect.40 An individual patient-data meta-analysis of three RCTs of MRA (RALES, EMPHASIS, and TOPCAT-Americas)13 suggested that MRAs exerted a similar reductions in mortality (by about ∼25%) for patients with HFrEF above and below age 75 years but benefit was less certain for HFpEF.
The diversity of heart failure phenotypes
Precision-medicine, which should also be accurate, requires patients to be classified in a way that informs management. For oncology, this has focused on the genetic cause, tumour location, and spread. For heart failure, a multi-system disorder, it is much more complex.41–47
Current, therapeutically relevant classifications of heart failure include the severity of congestion (based on symptoms, signs, blood biomarkers, and imaging), CAD, heart rate and rhythm and QRS duration, blood pressure, serum potassium, renal function, indices of iron deficiency, mitral regurgitation, infiltrative myocardial disease (e.g. amyloid), and ventricular phenotype.41,48 Optimal management of heart failure, with a few rare exceptions, requires only a modest amount of information but this still creates many thousands of patient-subgroups or clusters that might have different therapeutic needs.45,46 Such subgroups will increase exponentially with the introduction of each new class of treatment. Despite this heterogeneity of substrate and wealth of interventions, precision-medicine is in its infancy in heart failure.
One therapeutically relevant classification of heart failure is by LVEF, a surrogate for left ventricular (LV) dilation. Prior to the 1980s, imaging of cardiac function was available only in expert centres. Clinical trials relied on the chest X-ray rather than the echocardiogram to support a diagnosis of heart failure. The success of trials such as SOLVD, MERIT, and CHARM, which all had a reduced LVEF as an inclusion criterion, led to the adoption of LVEF <40% as the European Society of Cardiology (ESC) Guideline definition for HFrEF.49 Values ≥40% were termed HFpEF, comprising patients with a mid-range or mildly-reduced (HFmrEF), normal (HFnEF) and, perhaps, supra-normal (HFsnEF) LVEF.50 Analyses of >350 000 routinely collected echocardiograms suggested that the nadir of risk, whether or not the patient has a diagnosis of heart failure, lies in the range 60–65% both for men and women. Interestingly, an LVEF of >70% was associated with similar risk as an LVEF of 30–40% (Figure 2).50
Figure 2.
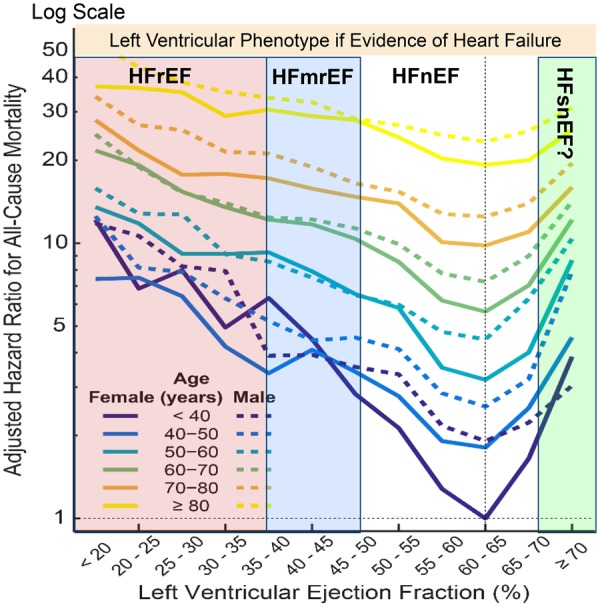
All-cause mortality according to left ventricular ejection fraction reported on >350 000 routine echocardiograms stratified by age and sex. HFmrEF, heart failure with mildly reduced ejection fraction; HFnEF, heart failure with normal ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFsnEF, heart failure with supra-normal ejection fraction. Reproduced with permission from ref.50
The ESC Guidelines of 2016 introduced the concept of HFmrEF, for two main reasons. Firstly, because of imprecision, an echocardiographic measurement could not reliably distinguish between two measurements of LVEF within 10% of each other. Creating a buffer-zone between HFrEF and HFnEF meant that misclassification was less likely. This innovation meant that a trial of HFpEF could not claim benefit for all patients with an LVEF >40% based solely on an effect in those with an LVEF 40–49%. Secondly, the introduction of HFmrEF challenged the convention that an LVEF <40% was the correct threshold for HFrEF. Some analyses subsequent to the ESC 2016 Guideline suggest that patients with an LVEF <50% may respond to treatment similarly to those with an LVEF <40%.51 However, this interpretation could reflect confirmation-bias amongst enthusiastic proponents of HFmrEF (Table 1). The evidence is not so consistent when looked at in its entirety, especially if mortality is considered a key outcome. In the future, many trials will probably include both HFrEF and HFmrEF, others will include HFmrEF, HFnEF, and HFsnEF, but NT-proBNP should be used routinely to stratify risk and potentially exclude low-risk patients who have little to gain from yet another ‘pill’. Assuming we continue to use LVEF to classify patients, which seems likely since we cannot undo the past, then the major issue is where to set thresholds. For HFrEF, these have ranged from <25% in COPERNICUS, <30% in MADIT-II, and RAFT to <35–40% for the bulk of other trials.51 For HFpEF, LVEF has generally been set at >40% or >45% with no upper limit. Analyses of recent trials have led some to suggest that, for patients with an elevated NT-proBNP, the upper limit of LVEF for HFmrEF should be increased to 55% or even 60% but this seems premature until consistency is demonstrated across multiple interventions and end-points and measurement precision for LVEF improves.
Table 1.
Evidence supporting or refuting the benefits of treatments for heart failure with a left ventricular ejection fraction in the “mid-range” (HFmrEF: 40–49%)
| LVEF | Symptoms | Hospitalization for heart failurea | CV death or HFHa | CV mortality | All-cause mortality | |
|---|---|---|---|---|---|---|
| Diuretics | ||||||
| Perindopril | Improved | 0.38 (0.19–0.75) b | ||||
| Candesartan | Improved | 0.72 (0.55–0.95)∏ | 0.76 (0.61–0.96) | 0.81 (0.60–1.11) | 0.79 (0.60–1.04) | |
| Irbesartan | 0.98 (0.85–1.12)Δ | |||||
| ARNI (Sac/Val) vs. Valc | Improved | 0.77 (0.58–1.02) | 0.81 (0.64–1.03) | 0.94 (0.69–1.28) | NYR | |
| MRA (overall)c | 0.76 (0.46–1.27) | 0.72 (0.50–1.05) | 0.69 (0.43–1.12) | 0.73 (0.49–1.10) | ||
| MRA (Americas)c | 0.60 (0.32–1.10) | 0.55 (0.33–0.91) | 0.46 (0.23–0.94) | 0.58 (0.34–0.99) | ||
| ß-Blocker (SR) | Improved | 0.95 (0.68–1.32) | 0.83 (0.60–1.13) | 0.48 (0.24–0.97) | 0.59 (0.34–1.03) | |
| ß-Blocker (AF) | Improved | 1.15 (0.57–2.32) | 1.06 (0.58–1.94) | 0.86 (0.36–2.03) | 1.30 (0.63–2.67) | |
| Ivabradine | ||||||
| Digoxin | 0.80 (0.63–1.03) | 0.96 (0.79–1.17) | 1.24 (0.94–1.64) | 1.08 (0.85–1.37) | ||
| Rivaroxaban vs. aspirin | 0.65 (0.40–1.05) | 0.75 (0.53–1.06) | ||||
| Rivaroxaban+Aspirin vs. aspirin | 0.87 (0.56–1.35) | 0.63 (0.44–0.90) | ||||
| CRT | ||||||
| ICD | ||||||
| BNP-guided therapy | Reduction from 67% to 44% patients with an event |
Statistically significant results are shown in bold on a blue background. Blank cells indicate no relevant information reported. Other data shown are not significant, although may not be heterogeneous with the effect in patients with a reduced left ventricular ejection fraction (HFrEF). Data for sacubitril/valsartan taken from reference for LVEF >42.5% to 52.5%.98
AF, atrial fibrillation; ARNI, angiotensin receptor-neprilysin inhibitors; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRA, Mineralocorticoid receptor antagonist; SR, sinus rhythm.
Recurrent event analyses used when available.
The PEP-CHF trial specified inclusion of patients with LVEF 40–49% as was LVEF >49% but did not report effects in this subgroup. However, it did report effects in patients with a prior myocardial infarction who were more likely to have HFmrEF.
Stronger effect in women.
In a substantial observational study of patients with HFpEF and pulmonary hypertension, progression of right rather than left ventricular dysfunction was observed and was associated with an increased risk of atrial fibrillation (AF) and death.52 Although right ventricular (RV) dysfunction is a powerful prognostic marker, remarkably few trials focusing on RV dysfunction have been done (SERENADE: https://clinicaltrials.gov/ct2/show/NCT03153111).
Atrial fibrillation
About a third of outpatients, perhaps more for those with HFpEF,53 and more than half of those admitted with heart failure will be in AF, which is associated with an adverse prognosis even after correcting for age and other risk factors.54 Controversy continues over whether medical management focused on rate control or restoration of sinus rhythm is the better strategy for AF and heart failure. In practice, the strategy needs to be tailored to the patient. When AF is the driver of symptoms and worsening cardiac function, restoration of sinus rhythm might be appropriate but when AF reflects the progression of underlying cardiac dysfunction, it may not.55 For new-onset or paroxysmal AF associated with a clear deterioration in symptoms, restoration of sinus rhythm may be warranted to improve symptoms. For long-standing AF and heart failure with markedly dilated atria, sustained restoration of sinus rhythm and atrial contraction is less likely. Optimal pharmacological management includes anticoagulation, avoiding toxic anti-arrhythmic agents and lenient ventricular rate control. Beta-blockers are the agent of choice for rate control, a resting day-time ventricular rate of 70–90 b.p.m. is preferred,49 which may require only modest doses; digoxin should be used sparingly, if at all. Unfortunately, RCTs of rate vs. rhythm control for AF have failed to optimize the rate control strategy in the above fashion.
A meta-analysis of RCTs of rate vs. rhythm control included four trials (n = 2486) comparing pharmacological rhythm to rate control found no difference in mortality or thromboembolic events but an increase in hospitalizations, often due to recurrent AF, in the rhythm control group.56 Six trials (n = 1112) comparing AF ablation with rate control reported reductions in mortality (0.51; 95% CI 0.36–0.74), hospitalizations (0.44; 95% CI 0.26–0.76), and stroke (0.59: 95% CI 0.23–1.51) and an improved quality of life.56 However, none of the trials individually had a robust result, patients were highly selected and the rate control strategy was not optimal. As such, this meta-analysis should be considered hypothesis generating. Further trials are required with greater involvement of heart failure physicians.
Implanted electrical devices
The controversy over the role of high-energy devices for heart failure continues. Long-term follow-up of cardiac resynchronization therapy (CRT) in a French Registry showed a low rate of sudden death amongst patients who received CRT-Pacing (without a defibrillator).57–59 A systematic review of observational studies and RCTs reported that differences in the rate of sudden death with CRT-Pacing and CRT-D were narrowing.58 RCTs comparing CRT-Pacing and CRT-D are underway59 (Take home figure). Whether myocardial scar found on cardiac magnetic resonance imaging identifies patients with more to gain from an implantable cardioverter defibrillator (ICD) is also under investigation60 (CMR_GUIDE; https://clinicaltrials.gov/ct2/show/NCT01918215). Retrospective analysis of SCD-HeFT found that patients with T2DM did not benefit from an ICD.61 An individual patient-data meta-analysis confirmed a reduction in sudden death with MRA.62 A systematic review identified 22 studies with post-mortem interrogation of ICDs; the analysis suggested that 24% of sudden deaths were not arrhythmic.63 A substantial multi-point pacing trial failed, so far, to show improvements in the clinical or echocardiographic response to CRT.64
Mitral regurgitation
COAPT suggested that a percutaneously delivered mitral clip could reduce functional (secondary) regurgitation with a subsequent substantial improvement in morbidity and mortality that was moderately cost-effective in a US healthcare context (US$40 361 per life-year gained and $55 600 per quality-adjusted life year).65–68 Two-year follow-up of MITRA.fr suggested no benefit.69 A possible explanation for the apparent discrepancy could be the ratio of the severity of LV dysfunction to the severity of mitral regurgitation. When regurgitation is disproportionate to the severity of LV dysfunction it may drive disease progression and correction may improve outcome.70,71 When regurgitation is proportionate to the severity of LV dysfunction, fixing the mitral regurgitation may be less useful because myocardial dysfunction drives disease progression. The concept is simple and plausible but application in practice may be difficult. Mitral regurgitation offloads the LV and may mask dysfunction. It is also likely that there is a spectrum of primary and secondary mitral regurgitation, with some patients having a mixed picture. More experience and further data from RCTs may improve patient selection (RESHAPE-HF2: https://clinicaltrials.gov/ct2/show/NCT02444338). However, optimizing guideline-recommended therapy, including diuretic dose, may cause mitral regurgitation secondary to dilation of the LV and mitral ring to improve or resolve. Other technologies for secondary mitral72 and tricuspid regurgitation73,74 are being developed.
Coronary artery disease
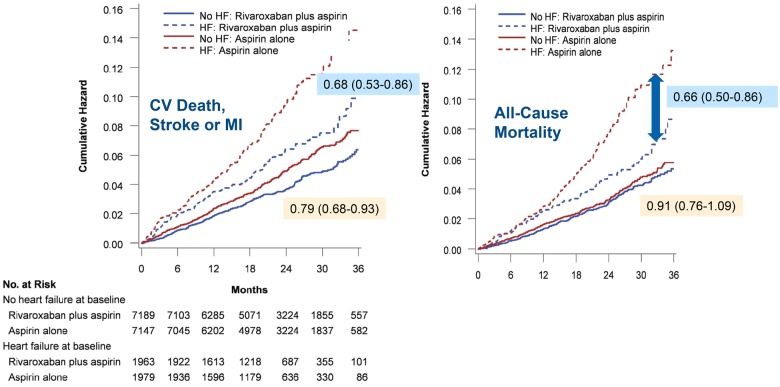
In COMPASS (n = 27 395), 5902 with CAD, in sinus rhythm and with a diagnosis of heart failure (predominantly HFpEF) were randomly assigned them to aspirin 100 mg/day, rivaroxaban 5 mg bd or aspirin and rivaroxaban 2.5 mg bd.75,76 The study was stopped early for benefit on the primary endpoint (a composite of CV death, stroke, or myocardial infarction) with the combination compared with aspirin alone. Further analysis suggested a reduction in all-cause mortality for patients with heart failure, especially HFpEF, assigned to combination therapy (HR: 0.63; 0.44–0.90) or rivaroxaban alone (HR: 0.75; 0.53–1.06) with an estimated 4% absolute difference at 2 years; rather similar to the magnitude of effect in HFrEF for sacubitril-valsartan77 or dapagliflozin78 (Figure 3). This suggests that coronary events might be an important driver of death in HFpEF (Take home figure), although effects of rivaroxaban on endothelial function, inflammation, and fibrosis should not be discounted. The analysis also suggests that those who do not have heart failure have little to gain from additional treatment with rivaroxaban.
Figure 3.
Effect of rivaroxaban 2.5 mg bd and aspirin 100 mg/day compared with aspirin alone for stable CAD, sinus rhythm and heart failure (predominantly heart failure with preserved ejection fraction) in COMPASS-HF. Reproduced with permission from ref.75
However, for patients with HFrEF, CAD in sinus rhythm with a recent hospital discharge for worsening heart failure, addition of rivaroxaban 2.5 mg bd to background anti-platelet therapy did not improve overall prognosis, although a composite of vascular outcomes (stroke, myocardial infarction, and sudden death) was reduced, driven mainly by a reduction in stroke.79,80 This suggests that for patients with stable CAD and more advanced heart failure, hospitalizations, and deaths due to worsening heart failure are not greatly influenced by anti-thrombotic therapy (Take home figure).
Angiotensin receptor-neprilysin inhibitors
Heart failure with reduced ejection fraction
As experience in the implementation of angiotensin receptor-neprilysin inhibitors (ARNIs) grows, both in clinical trials and in clinical practice, there is a strong argument to consider them as first-line agents, rather than angiotensin converting-enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), for the treatment of HFrEF. In PIONEER-HF,81 881 patients with an LVEF ≤40% who were hospitalized for worsening heart failure were randomly assigned, without a run-in period, to sacubitril/valsartan or enalapril prior to discharge and followed for 8 weeks to determine the effect on plasma concentrations of NT-proBNP; about one-third had new-onset heart failure. Sacubitril-valsartan exerted a greater reduction in NT-proBNP. Reductions in markers of myocardial injury or stress, high-sensitivity cardiac troponin-T and soluble ST2, were also observed. These effects appeared early after randomization (within 1–4 weeks). Moreover, patients assigned to sacubitril/valsartan were less likely to experience adverse outcomes within the first 8 weeks. TRANSITION82 randomly assigned 1002 patients to pre- or post-discharge initiation of sacubitril/valsartan, showing no adverse consequences to earlier administration.
EVALUATE83 compared the effects of sacubitril/valsartan and enalapril on aortic stiffness in HFrEF most of whom were already chronically treated with an ACEi or ARB. After 24 weeks treatment, no differences in aortic stiffness were observed but slightly greater reductions in LV end-diastolic and systolic volumes were observed with sacubitril/valsartan compared with enalapril, although changes in LVEF were similar. Mitral E-velocity and left atrial volume declined, consistent with a fall in left atrial pressure. PROVE-HF,84 an observational study, had similar findings and showed that most of the decline in NT-proBNP occurred within 14 days consistent with the rapid onset of clinical benefit observed with sacubitril/valsartan in trials and clinical practice. PRIME85 was an RCT (n = 118) comparing the effects of sacubitril/valsartan or valsartan on functional mitral regurgitation in patients with an LVEF between 25% and 49% who were already receiving an ACEi or ARB. Those assigned to sacubitril/valsartan had greater reductions in mitral regurgitation and LV end-diastolic and left atrial volumes but LVEF increased by a similar small amount in each group (about 2.5%).
Further reports from PARADIGM-HF suggest that, compared with enalapril, sacubitril/valsartan may improve markers of collagen metabolism, in particular, decreasing synthesis of type-I collagen, which makes an important contribution to myocardial stiffness.86 In I-PRESERVE, irbesartan (an ARB) did not affect collagen biomarkers compared with placebo.87
Heart failure with preserved ejection fraction
PARAGON-HF investigated the effect of sacubitril/valsartan compared to valsartan alone on morbidity and mortality in patients with HFpEF (defined as an LVEF >45%).88 It was the first RCT since PEP-CHF89 to require patients to be treated with diuretics, the first-line treatment for the relief of symptoms and signs of congestion, and to have echocardiographic evidence of cardiac dysfunction. It was also the first large trial of HFpEF to require all patients to have raised plasma concentrations of natriuretic peptides, the most powerful, widely available prognostic marker in HFpEF. Sacubitril/valsartan was compared with valsartan rather than placebo because many patients eligible for PARAGON-HF had indications for ACE inhibitors and ARBs such as hypertension and CAD. The only trial comparing valsartan to placebo in HFpEF was of modest size and neutral.90 Previous RCTs of other ARBs, including candesartan (CHARM-Preserved) and irbesartan (I-PRESERVE) failed to show substantial benefit for HFpEF.88 Patients had to tolerate, sequentially, both valsartan and sacubitril/valsartan at half the intended target dose before randomization. This simulates clinical practice (doctors do not usually prescribe medicines to patients unwilling or unable to take them) and reduces the risk of a neutral trial-outcome due to low adherence. Of 10 539 patients screened, 4822 were randomized.
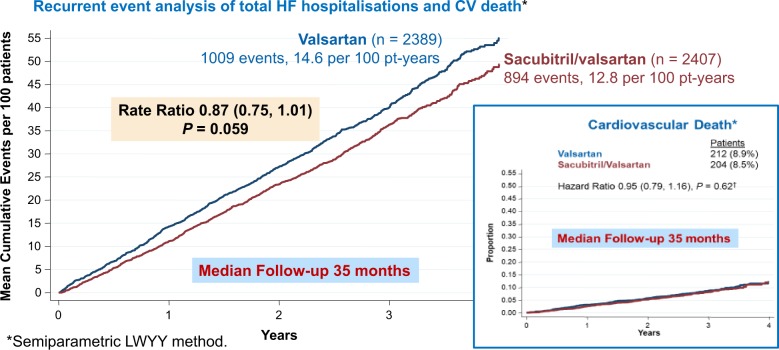
PARAGON-HF was neutral for its primary endpoint (CV death or the total number of recurrent hospitalizations for heart failure91; Figure 4). Some have argued that the P-value was very close to 0.05 and that it was ‘almost’ positive. This misses the point. The trial shows that the size of the potential benefit of sacubitril/valsartan for HFpEF is modest, regardless of the P-value and that the treatment is, overall, unlikely to be cost-effective. Accordingly, we should look for more effective treatments or, more controversially, subgroups that obtain greater benefit. After a median follow-up of 35 months, 23% of patients experienced a primary event but the annual incidence of CV and all-cause mortality were, respectively, only about 3% and 5%, which is similar to those for previous trials of HFpEF and for elderly patients with resistant hypertension assigned to placebo in HYVET.92 Although <3% of patients were reported to have heart failure in HYVET, a combination of indapamide and perindopril reduced all-cause mortality and cut the incidence of heart failure by >50%. Many of these patients probably had undiagnosed HFpEF prior to randomization. Higher rates of hospitalization for heart failure in trials of HFpEF compared to hypertension may well reflect ascertainment bias, as clinicians who are interested or expert in the management of heart failure are more likely to diagnose or report heart failure events. Overall, these trials suggest that the mortality rate and possibly the rates of cardiovascular and all-cause hospitalization may be similar in patients with and without a diagnosis of HFpEF, if they have a similar burden of co-morbidities. However, it is also likely that many patients with hypertension, CAD and T2DM have undiagnosed heart failure.
Figure 4.
Effect of sacubitril/valsartan compared with valsartan for heart failure with preserved ejection fraction in PARAGON-HF. Reproduced with permission from ref.91
Subgroup analysis suggested that the effect of sacubitril/valsartan on the primary endpoint was greater for patients with an LVEF below the median (57%), but this was driven almost entirely by an effect on hospitalization for heart failure rather than on CV death.93 The effect of sacubitril/valsartan on the primary endpoint was also greater for women and this was true throughout the studied range of LVEF, but again this was driven by a difference in hospitalization for heart failure and not CV mortality.94 Reductions in NT-proBNP were similar for each sex. Sacubitril/valsartan appeared to have a favourable effect on quality of life for men but not for women. Patients with a recent heart failure hospitalization may also have benefited more.95 These observations should be interpreted in the light of a trial that was neutral for its primary endpoint. No effect was observed on mortality and the benefits of treatment on quality of life and hospitalizations for heart failure according to sex were inconsistent. In PARADIGM-HF, no difference in treatment effect according to sex was observed. A further sizeable RCT in HFpEF, PARALLAX-HF, investigating the effects of sacubitril/valsartan on quality of life and exercise capacity will provide more evidence in 2020 (https://clinicaltrials.gov/ct2/show/NCT03066804).
Do women and men respond differently to treatment?
An analysis of 12 058 patients with HFrEF in two large trials found that women had more severe symptoms, similar LVEF but a substantially better prognosis than men, even after adjusting for key prognostic variables including aetiology and NT-proBNP (HR: 0.68; 0.62–0.89).96 A combined analysis of PARAGON-HF and PARADIGM-HF suggested that patients with HFrEF and HFpEF had similarly impaired quality of life but that women generally reported a worse quality of life than men.97 In an observational analysis of patients with HFrEF, the BIOSTAT survey also found that women generally had a better prognosis than men despite being prescribed lower doses of beta-blockers and ACE inhibitors.98 Interestingly, men and women had the same heart rate, the pharmacodynamic marker of beta-blocker dose. For patients with HFpEF in the TOPCAT trial, reductions in mortality, but not hospitalizations for heart failure, were greater for women, although the interaction was statistically significant only for all-cause mortality.99 In the PARAGON-HF trial (HFpEF), women obtained greater benefit than men throughout the studied range of LVEF but the difference was driven by differences in the rate of hospitalization for heart failure rather than mortality.94 One obvious difference between men and women, on average, is size. Cardiac resynchronization therapy is reputed to be more effective in women than men, but differences disappear once adjusted for height.100 Many medicines are cleared by the kidney. Estimated glomerular filtration rate (eGFR) is indexed to body surface area (BSA) but doses of treatment are usually not. A woman (or small man) weighing 64 kg and 160 cm tall has BSA of 1.67 m2 using the Dubois formula and a man (or large woman) weighing 85 kg and 180 cm tall has a BSA 2.05 m2. If both have an eGFR of 60 mL/kg/m2, then the woman (or small man) has an un-indexed eGFR of 100 mL/min and the man (or large woman) has an un-indexed eGFR of 123 mL/min. If a medicine is cleared by the kidney then perhaps smaller people require lower doses to achieve the same plasma therapeutic concentration and clinical benefit?
Sodium-glucose cotransporter-2 inhibitors
Sodium-glucose cotransporter protein-2 (SGLT2) is found mainly in the proximal renal tubule and to a lesser extent in other organs. SGLT1 is abundant in the intestine and myocardium. SGLT2 inhibitors (SGLT2i) cause glycosuria, improving glycaemia, which led to their development for the treatment of T2DM, and an osmotic diuresis, leading to a contraction of plasma volume.101,102 SGLT1 inhibitors reduce intestinal glucose absorption, which can cause diarrhoea but might have favourable effects on myocardial energy-utilization.103 Most SGLT2i are highly selective, including dapagliflozin and empagliflozin, but sotagliflozin is less selective.103
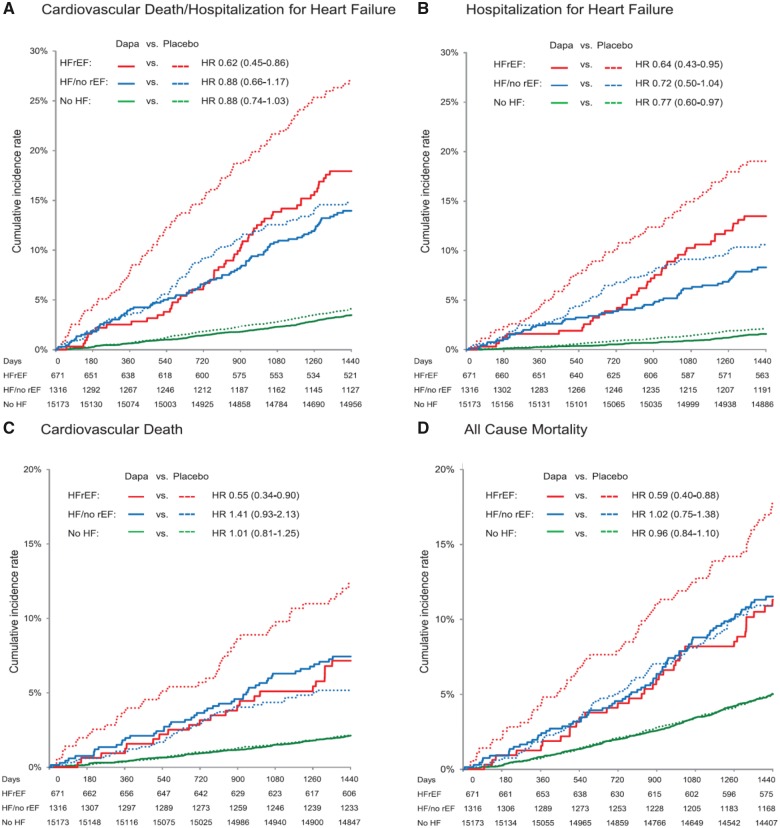
EMPA-REG enrolled 7020 patients with T2DM, about 10% of whom had heart failure (LVEF was not measured) and showed that empagliflozin reduced the risk of hospitalization for heart failure and mortality.104 Within a few weeks of initiating empagliflozin, body weight, and blood pressure fell and haematocrit rose, consistent with a diuretic effect. Subsequent RCTs of other SGLT2i in T2DM had similar findings. Meta-analyses suggested that SGLT2i were the hypoglycaemic agents most likely to reduce incident heart failure,105–107 whilst observational data raises concerns about insulin therapy.108 A meta-analysis of RCTs of empagliflozin, canagliflozin, and dapagliflozin for T2DM, including >30 000 patients, showed benefit, at least for those with established CV disease.109 For the outcome of hospitalization for heart failure or CV death, the annual rate was about 0.6% for the 13 672 patients with multiple risk factors but without established CV disease, about 3% for the 20 650 patients with established atherosclerotic disease and about 6% for 3891 patients with heart failure at baseline; the relative risk reductions with SGLT2i in these populations were 16%, 24%, and 29%, respectively, without evidence of heterogeneity amongst agents. The largest of these trials, DECLARE,110 included 17 160 patients of whom 671 had HFrEF and 1316 had HFpEF or an unspecified LVEF. In a subgroup analysis,111 dapagliflozin reduced hospitalizations for heart failure and CV mortality for HFrEF but not for other patient-groups (Figure 5).
Figure 5.
Effect of dapagliflozin compared with placebo in type-2 diabetes mellitus in patients with heart failure with reduced ejection fraction, heart failure with preserved ejection fraction, or without heart failure in DECLARE. Reproduced with permission from ref.111
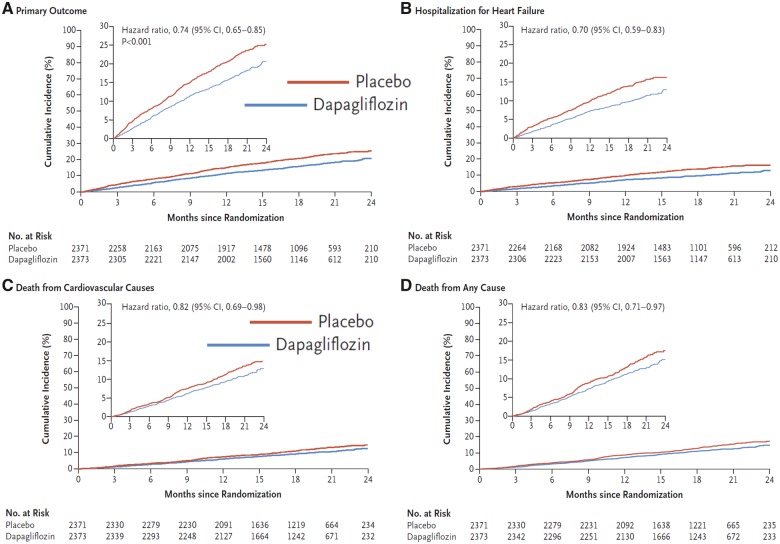
DAPA-HF78,112 enrolled 4744 patients and followed them for a median of 18.3 months, demonstrating that addition of dapagliflozin to guideline-recommended therapy for HFrEF-reduced hospitalizations for heart failure by 30% and mortality (mainly cardiovascular) by 18%, preventing 3–5 hospitalizations and 1–2 deaths per 100 patients treated per year (Figure 6). Patients were somewhat less likely to experience serious adverse events, especially renal, with dapagliflozin compared with placebo. The benefits appeared consistent across subgroups, although patients with evidence of more severe congestion (worse NYHA class or higher NT-proBNP) may have received less benefit. Importantly, benefits were similar for those with and without T2DM and regardless of age.113 Dapagliflozin also improved quality of life,114 an effect that was confirmed in a smaller RCT (DEFINE)115 that followed 263 patients for 12 weeks; about one in six patients got a meaningful benefit, either prevention of worsening or an improvement in symptoms, compared with placebo.
Figure 6.
Effect of dapagliflozin compared with placebo in patients with heart failure with reduced ejection fraction, with or without type-2 diabetesmellitus in DAPA-HF. Reproduced with permission from ref.78
In DAPA-HF, the placebo-corrected decline in weight between baseline and 8 months was 0.87 kg and this was associated with a small fall in NT-proBNP and systolic blood pressure and a small increase in haematocrit and serum creatinine. These findings are again consistent with the belief that SGLT2i exert at least some of their benefits by enhancing diuresis, either through an osmotic effect of glycosuria or by interfering with sodium-hydrogen exchange in the nephron.116 The effects of SGLT2i appear early, consistent with an immediate haemodynamic effect. However, alternative or additional explanations for the effect of SGLT2i have been proposed. A small RCT suggested that empagliflozin stimulated production of erythropoietin leading to a rise in haematocrit and a fall in ferritin, a marker of inflammation and iron deficiency, although not transferrin saturation, a marker of iron deficiency alone.117 However, administration of exogenous erythropoietin did not reduce morbidity or mortality in the RED-HF trial.118 Others have suggested that SGLT2i increase the production of ketones, which may be a more efficient myocardial energy substrate, or block myocardial sodium–hydrogen exchanger-3, which may improve myocardial function and reduce fibrosis.119,120 An RCT of empagliflozin in patients with T2DM but not heart failure121 suggested little effect on cardiac function or remodelling; RCTs of the effects of SGLT2i on cardiac function in patients with HFrEF and HFpEF are awaited. Future trials will confirm whether the benefit observed in DAPA-HF is a class effect and whether they are effective for HFpEF or when congestion is severe.122,123
Acute heart failure
Two large RCTs of serelaxin failed to confirm the results of the original RELAX-AHF trial. RELAX-AHF-EU,124 an open-label RCT (n = 2688), reported a similar and low rate for mortality (≤2%) and re-admissions for heart failure (<1%) at 14 days for patients assigned placebo or serelaxin, despite a reduction in worsening heart failure at day 5 [6.7–4.5% (P < 0.008)]. The RELAX-AHF-2 trial,125 a double-blind RCT (n = 6545), reported that the rates of worsening heart failure in the first 5 days (about 7%) and 180-day mortality (about 11%) were similar for placebo and serelaxin. The failure of so many short-term interventions for AHF may reflect failed therapeutic concepts, ineffective interventions, or problems with trial design. RCTs of AHF are difficult to implement, especially if conducted double-blind. Indeed, GALACTIC, a trial of personalized, early intensive and sustained vasodilation with nitrates and hydralazine, also failed to show benefit, calling into question the concept of vasodilator therapy for the routine management of acute heart failure.126 Many patients present with acute breathlessness in the middle of the night. It is difficult to have research staff available ‘24/7’ when there is no ‘gateway’ similar to a coronary care unit or catheter laboratory. Compassionate investigators may also be unwilling to enrol frail elderly patients who are most at risk of adverse outcomes. Moreover, breathlessness usually responds to oxygen and diuretics within hours,127 especially for patients with a systolic blood pressure ≥125 mmHg, as required in the serelaxin trials. On the other hand, patients with extensive peripheral oedema,26 renal dysfunction, and a low blood pressure, who often do not constitute an acute emergency have a poor prognosis and an unmet need for more effective interventions; pharmacological, or device.127,128
Stem cell therapy
Intra-myocardial injection of stem cells failed to improve weaning from left ventricular assist devices.130
Heart failure in patients with cancer
Interest in cardio-oncology reflects increasing survival after treatment for cancer, growing awareness of the CV toxicity associated with both established and new treatments for cancer, and interest in personalized risk-profiling prior to chemotherapy. People with cardiomyopathy-related gene mutations may be more prone (7.5% of those with compared to 1.1% of those without a titin gene mutation) to develop ventricular dysfunction after the administration of chemotherapy.131
Interruption of trastuzumab is associated with a higher risk of cancer recurrence in women with early invasive HER2+ve breast cancer; about 60% of interruptions are for cardiotoxicity.132 An observational study showed that of 30 women receiving HER2-targeted therapies who developed an LVEF of 40–49% and were treated prospectively with beta-blockers and ACE inhibitors, only three went on to develop severe heart failure or a LVEF <35%.133 Cardiac function rarely returned to normal after completion of treatment, challenging the view that trastuzumab-related LV dysfunction is usually reversible. A recent study reported high rates of CV events, especially heart failure, amongst patients with multiple myeloma receiving potent proteasome inhibitors, such as carfilzomib and bortezomib,134 which were associated with much poorer survival. Risk factors for developing a CV event included elevated pre-treatment NT-proBNP or an increase during treatment. A systematic review of prophylactic use of renin–angiotensin–aldosterone antagonists and beta-blockers identified 22 relevant RCTs, of which the largest had only 206 patients,135,136 but found no convincing evidence of clinical efficacy
Implementation of therapy
Analyses of administrative data from primary care in the UK suggest that implementation of therapy has improved substantially over the last decade, with 72% now prescribed a beta-blocker, although many patients remain on less than target doses.6 Amongst hospital discharges in England and Wales, 89% of those with HFrEF were discharged on a beta-blocker (https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-Report-final.pdf), which is very similar to that observed in patients with HFrEF selected for enrolment in the ESC-EURObservational Heart Failure Long-Term Registry.137 However, an analysis of Medicare beneficiaries in the USA found that only 51% of patients with HFrEF were prescribed a beta-blocker after a first or recurrent hospitalization for heart failure and only 12% received at least ≥50% of the target dose by 1 year.138 This suggests that the organization of care for HFrEF makes an important difference to treatment and, consequently, outcome. However, a cluster RCT (n = 2494) of service redesign aiming to improve hospital-to-home transition, which included self-care education, a structured hospital discharge summary, family physician follow-up within 1 week, and, for high-risk patients, home-visits, did not substantially improve patient well-being or outcome.139 An RCT (n = 110) showed that frequent (several times per month) visits to participating community pharmacies could improve medication adherence and well-being.140 An RCT of 450 patients found benefits of e-Health intervention on self-care behaviour and quality of life in the first 3 months after initiation but not thereafter,141 with no effect on hospitalizations or mortality. There are many reasons why RCTs of complex interventions fail including inadequate power, suboptimal trial design, already excellent or unintended improvements in care for the control group, lack of long-term engagement and motivation of staff and patients, inclusion of patients for whom pharmacological intervention is largely ineffective (e.g. HFpEF) but sometimes we just have to admit that what should work does not. More evidence is required; learning from past experience.142
Rehabilitation
Systematic reviews suggest that exercise-based rehabilitation can improve patients’ well-being and exercise capacity and reduce heart failure-related and all-cause hospitalization but may not reduce mortality, despite potentially improving adherence to treatment.143–147 The best and most cost-effective service-model is a topic of active research.148,149
Palliative care
Morphine relieves chronic breathlessness in patients with chronic lung disease but data for heart failure are sparse. An RCT of 45 patients failed to demonstrate important clinical benefits of morphine administration to patients with HFrEF or HFpEF predominantly in NYHA functional class III.150
Withdrawing treatment for heart failure after recovery
Withdrawing treatment from patients with idiopathic or genetically determined dilated cardiomyopathy who have experienced full recovery of ventricular function should be done with great caution if at all.151 Although patients with a recovered LVEF (HFrcEF) may have a better prognosis, it may still not be good.152 Further research is required for peripartum and other specific types of cardiomyopathy. A recent report from an old trial (DIG), suggested that withdrawal of digoxin was associated with an increased risk of hospitalization for heart failure but did not affect mortality.153 An RCT of 188 patients with stable heart failure from Brazil suggested that 75% of patients could be withdrawn from loop diuretics for at least 90 days without deterioration in symptoms, need for reinstitution of diuretic therapy, or a rise in plasma NT-proBNP.154 This is in stark contrast to a smaller RCT from the UK, where withdrawal of diuretics and other therapies for 48 h led to a doubling of plasma concentrations of NT-proBNP, an increase in LV and left atrial volumes and worsening symptoms.155
Conclusion
Great progress in the understanding and management of heart failure has been made over the last year. New controversies and new evidence challenge many old assumptions. As ever, some will resist progress and others will embrace it. You, the reader, must help our professions and patients find the correct balance between reckless enthusiasm and diagnostic and therapeutic inertia.
Funding
The British Heart Foundation Cardiovascular Research Centre at the University of Glasgow is supported by a Centre of Research Excellence grant from the British Heart Foundation (RE/18/6/34217).
Conflict of interest: Dr J.G.C. reports grants and personal fees from Amgen, Bayer, Novartis, Vifor, and Pharmacosmos; personal fees and non-financial support from Medtronic; personal fees from Abbott, outside the submitted work. Dr A.R.L. reports personal fees from Servier, Novartis, Roche, Takeda, Boehringer Ingelheim, Amgen, Clinigen Group, Ferring Pharmaceuticals, Eli Lily, Bristol Myers Squibb, and Eisai Ltd; grants and personal fees from Pfizer, outside the submitted work. T.M. reports honoraria from Vifor. J.J.M. reports non-financial support and other from AstraZeneca, during the conduct of the study; other from Bayer, non-financial support and other from Cardiorentis, non-financial support and other from Amgen, non-financial support and other from Oxford University/Bayer, non-financial support and other from Theracos, non-financial support and other from Abbvie, other from DalCor, other from Pfizer, other from Merck, non-financial support and other from Novartis, non-financial support and other from Glaxo Smith Kline (GSK), other from Bristol Myers Squibb (BMS), non-financial support and other from Vifor-Fresenius, non-financial support and other from Kidney Research UK (KRUK), non-financial support and other from Novartis, outside the submitted work.
References
- 1. Torabi A, Rigby AS, Cleland J.. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol 2009;55:79–81. [DOI] [PubMed] [Google Scholar]
- 2. Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL.. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014;130:1579–1588. [DOI] [PubMed] [Google Scholar]
- 3. Zhang C, Jiang L, Xu L, Tian J, Liu J, Zhao X, Feng X, Wang D, Zhang Y, Sun K, Xu B, Zhao W, Hui R, Gao R, Yuan J, Song L.. Implications of N-terminal pro-B-type natriuretic peptide in patients with three-vessel disease. Eur Heart J 2019;40:3397–3405. [DOI] [PubMed] [Google Scholar]
- 4. Cleland JGF, Pellicori P, Clark AL.. Prevention or procrastination for heart failure? Why we need a universal definition of heart failure. J Am Coll Cardiol 2019;73:2398–2400. [DOI] [PubMed] [Google Scholar]
- 5. Conrad N, Judge A, Canoy D, Tran J, Pinho-Gomes AC, Millett ERC, Salimi-Khorshidi G, Cleland JG, McMurray JJV, Rahimi K.. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol 2019;4:1102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conrad N, Judge A, Canoy D, Tran J, O’Donnell J, Nazarzadeh M, Salimi-Khorshidi G, Hobbs FDR, Cleland JG, McMurray JJV, Rahimi K.. Diagnostic tests, drug prescriptions, and follow-up patterns after incident heart failure: a cohort study of 93,000 UK patients. PLoS Med 2019;16:e1002805.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bottle A, Kim D, Aylin P, Cowie MR, Majeed A, Hayhoe B.. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104:600–605. [DOI] [PubMed] [Google Scholar]
- 8. Kim D, Hayhoe B, Aylin P, Majeed A, Cowie MR, Bottle A.. Route to heart failure diagnosis in English primary care: a retrospective cohort study of variation. Br J Gen Pract 2019;69:e697–e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filippatos G, Angermann CE, Cleland JGF, Lam CSP, Dahlström U, Dickstein K, Ertl G, Hassanein M, Hart KW, Hu D, Lindsell CJ, Perrone SV, Guerin T, Ghadanfar M, Schweizer A, Obergfell A, Collins SP. Global differences in acute heart failure patient characteristics, precipitants, point of hospital entry and inpatient management: an analysis from REPORT-HF, a worldwide, prospective heart failure disease registry. JAMA Cardiol 2019;in press. [Google Scholar]
- 10. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, Palileo-Villaneuva L, Lopez-Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley-Cote E, Balasubramanian K, Islam S, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Bangdiwala SI, Yusuf S; INTER-CHF Investigators. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–e672. [DOI] [PubMed] [Google Scholar]
- 11. Dewan P, Rorth R, Jhund PS, Ferreira JP, Zannad F, Shen L, Kober L, Abraham WT, Desai AS, Dickstein K, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray J.. Income inequality and outcomes in heart failure: a global between-country analysis. JACC Heart Fail 2019;7:336–346. [DOI] [PubMed] [Google Scholar]
- 12. Dewan P, Jhund PS, Shen L, Petrie MC, Abraham WT, Atif AM, Chen CH, Desai AS, Dickstein K, Huang J, Kiatchoosakun S, Kim KS, Kober L, Lai WT, Liao Y, Mogensen UM, Oh BH, Packer M, Rouleau JL, Shi V, Sibulo AS Jr, Solomon SD, Sritara P, Swedberg K, Tsutsui H, Zile MR, McMurray J.. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail 2019;21:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira JP, Rossello X, Eschalier R, McMurray JJV, Pocock S, Girerd N, Rossignol P, Pitt B, Zannad F.. MRAs in elderly HF patients: individual patient-data meta-analysis of RALES, EMPAHSIS-HF, and TOPCAT. JACC Heart Fail 2019;7:1012–1021. [DOI] [PubMed] [Google Scholar]
- 14. Ferreira JP, Rossignol P, Pizard A, Machu JL, Collier T, Girerd N, Huby AC, Gonzalez A, Diez J, Lopez B, Sattar N, Cleland JG, Sever PS, Zannad F.. Potential spironolactone effects on collagen metabolism biomarkers in patients with uncontrolled blood pressure. Heart 2019;105:307–314. [DOI] [PubMed] [Google Scholar]
- 15. Pellicori P, Ferreira JP, Mariottoni B, Brunner-La Rocca H-P, Ahmed FZ, Verdonschot J, Collier T, Cuthbert JJ, Petutschnigg J, Mujaj B, Girerd N, González A, Clark AL, Cosmi F, Staessen JA, Heymans S, Latini R, Rossignol P, Zannad F, Cleland JGF. Effects of spironolactone on serum markers of fibrosis in people at high risk of developing heart failure: rationale, design and baseline characteristics of a proof-of-concept, randomised, precision-medicine, prevention trial. The Heart OMics in AGing (HOMAGE) trial. Eur J Heart Failure 2019;in press. [DOI] [PubMed] [Google Scholar]
- 16. Larsson SC, Back M, Rees JMB, Mason AM, Burgess S.. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sillars A, Celis-Morales CA, Ho FK, Petermann F, Welsh P, Iliodromiti S, Ferguson LD, Lyall DM, Anderson J, Mackay DF, Pellicori P, Cleland J, Pell JP, Gill JMR, Gray SR, Sattar N.. Association of fitness and grip strength with heart failure: findings from the UK biobank population-based study. Mayo Clin Proc 2019;94:2230–2240. [DOI] [PubMed] [Google Scholar]
- 18. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K.. Surgical obesity treatment and the risk of heart failure. Eur Heart J 2019;40:2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, Cleland J.. Body mass index and all-cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose-response meta-analysis. Clin Res Cardiol 2019;108:119–132. [DOI] [PubMed] [Google Scholar]
- 20. Kytomaa S, Hegde S, Claggett B, Udell JA, Rosamond W, Temte J, Nichol K, Wright JD, Solomon SD, Vardeny O.. Association of influenza-like illness activity with hospitalizations for heart failure: the atherosclerosis risk in communities study. JAMA Cardiol 2019;4:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loeb M, Dokainish H, Dans A, Palileo-Villanueva LM, Roy A, Karaye K, Zhu J, Liang Y, Goma F, Damasceno A, AlHabib KF, Yonga G, Mondo C, Almahmeed W, Al MA, Yusuf S.. Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): rationale and design. Am Heart J 2019;212:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Klein L, Eaton C, Panjrath G, Martin LW, Chae CU, Greenland P, Lloyd-Jones DM, Wactawski-Wende J, Manson JE.. Menopausal hormone therapy and risks of first hospitalized heart failure and its subtypes during the intervention and extended postintervention follow-up of the women's health initiative randomized trials. J Card Fail 2019;doi: 10.1016/j.cardfail.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 23. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G.. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 24. Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Zampierollo GA, Jarolim P, Pappagianopoulos PP, Malhotra R, Nayor M, Lewis GD.. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka-Bennett A, Clark AL, Cleland J.. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019;21:904–916. [DOI] [PubMed] [Google Scholar]
- 26. Shoaib A, Mamas MA, Ahmad QS, McDonagh TM, Hardman SMC, Rashid M, Butler R, Duckett S, Satchithananda D, Nolan J, Dargie HJ, Clark AL, Cleland J.. Characteristics and outcome of acute heart failure patients according to the severity of peripheral oedema. Int J Cardiol 2019;285:40–46. [DOI] [PubMed] [Google Scholar]
- 27. Shoaib A, Farag M, Nolan J, Rigby A, Patwala A, Rashid M, Kwok CS, Perveen R, Clark AL, Komajda M, Cleland J.. Mode of presentation and mortality amongst patients hospitalized with heart failure? A report from the First Euro Heart Failure Survey. Clin Res Cardiol 2019;108:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Platz E, Solomon SD, McMurray J.. Lung ultrasound: monitoring congestion in patients with heart failure. Eur J Heart Fail 2019;doi: 10.1002/ejhf.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray J.. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 2019;7:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Platz E, Jhund PS, Girerd N, Pivetta E, McMurray JJV, Peacock WF, Masip J, Martin-Sanchez FJ, Miro O, Price S, Cullen L, Maisel AS, Vrints C, Cowie MR, DiSomma S, Bueno H, Mebazaa A, Gualandro DM, Tavares M, Metra M, Coats AJS, Ruschitzka F, Seferovic PM, Mueller C; on behalf of the Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association and the Heart Failure Association of the European Society of Cardiology. Expert consensus document: reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail 2019;21:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E; on behalf of the Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019;21:754–766. [DOI] [PubMed] [Google Scholar]
- 32. Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J.. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 2019;doi: 10.1002/ejhf.1604. [DOI] [PubMed] [Google Scholar]
- 33. Ter Maaten JM, Kremer D, Demissei BG, Struck J, Bergmann A, Anker SD, Ng LL, Dickstein K, Metra M, Samani NJ, Romaine SPR, Cleland J, Girerd N, Lang CC, Van Veldhuisen DJ, Voors AA.. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur J Heart Fail 2019;21:732–743. [DOI] [PubMed] [Google Scholar]
- 34. Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, Capodilupo R, Adamson PB, Roberts G, Dalal N, Desai AS, Benza RL.. Association of ambulatory hemodynamic monitoring of heart failure with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiol 2019;4:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, Tang WHW, Droogne W, Mullens W.. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail 2019;21:1415–1422. [DOI] [PubMed] [Google Scholar]
- 36. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ.. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019;21:1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwok CS, Zieroth S, Van Spall HGC, Helliwell T, Clarson L, Mohamed M, Mallen C, Duckett S, Mamas MA.. The Hospital Frailty Risk Score and its association with in-hospital mortality, cost, length of stay and discharge location in patients with heart failure short running title: frailty and outcomes in heart failure. Int J Cardiol 2019;doi: 10.1016/j.ijcard.2019.09.064. [DOI] [PubMed] [Google Scholar]
- 38. Savarese G, Dahlstrom U, Vasko P, Pitt B, Lund LH.. Association between renin-angiotensin system inhibitor use and mortality/morbidity in elderly patients with heart failure with reduced ejection fraction: a prospective propensity score-matched cohort study. Eur Heart J 2018;39:4257–4265. [DOI] [PubMed] [Google Scholar]
- 39. Stolfo D, Uijl A, Benson L, Schrage B, Fudim M, Asselbergs FW, Koudstaal S, Sinagra G, Dahlstrom U, Rosano G, Savarese G.. Association between beta-blocker use and mortality/morbidity in older patients with heart failure with reduced ejection fraction. A propensity score-matched analysis from the Swedish Heart Failure Registry. Eur J Heart Fail 2019;doi: 10.1002/ejhf.1615. [DOI] [PubMed] [Google Scholar]
- 40. Rush CJ, Campbell RT, Jhund PS, Petrie MC, McMurray J.. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J 2018;39:3417–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de WL, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW.. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019;40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Boer RA, De KG, Bauersachs J, Brutsaert D, Cleland JG, Diez J, Du XJ, Ford P, Heinzel FR, Lipson KE, McDonagh T, Lopez-Andres N, Lunde IG, Lyon AR, Pollesello P, Prasad SK, Tocchetti CG, Mayr M, Sluijter JPG, Thum T, Tschope C, Zannad F, Zimmermann WH, Ruschitzka F, Filippatos G, Lindsey ML, Maack C, Heymans S.. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail 2019;21:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Packer M. The epicardial adipose inflammatory triad: coronary atherosclerosis, atrial fibrillation, and heart failure with a preserved ejection fraction. Eur J Heart Fail 2018;20:1567–1569. [DOI] [PubMed] [Google Scholar]
- 44. Pellicori P, Zhang J, Cuthbert J, Urbinati A, Shah P, Kazmi S, Clark AL, Cleland JGF.. High sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes and mode of death. Cardiovasc Res 2020;116:91–100. [DOI] [PubMed] [Google Scholar]
- 45. Tromp J, Ouwerkerk W, Demissei BG, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Van Veldhuisen DJ, Zannad F, Zwinderman AH, Voors AA, van der Meer P.. Novel endotypes in heart failure: effects on guideline-directed medical therapy. Eur Heart J 2018;39:4269–4276. [DOI] [PubMed] [Google Scholar]
- 46. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J.. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015;17:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao TH, Jones DJL, Voors AA, Quinn PA, Sandhu JK, Chan DCS, Parry HM, Mohan M, Mordi IR, Sama IE, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Metra M, Ponikowski P, Samani NJ, Van Veldhuisen DJ, Zannad F, Lang CC, Ng LL.. Plasma proteomic approach in patients with heart failure: insights into pathogenesis of disease progression and potential novel treatment targets. Eur J Heart Fail 2019;doi: 10.1002/ejhf.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cleland JGF, Van Veldhuisen DJ, Ponikowski P.. The year in cardiology 2018: heart failure. Eur Heart J 2019;40:651–661. [DOI] [PubMed] [Google Scholar]
- 49. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 50. Wehner GJ, Jing L, Haggerty CM, Suever JD, Leader JB, Hartzel DN, Kirchner HL, Manus JNA, James N, Ayar Z, Gladding P, Good CW, Cleland JGF, Fornwalt BK.. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J 2019;doi: 10.1093/eurheartj/ehz550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS.. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 52. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA.. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pellicori P, Urbinati A, Kaur K, Zhang J, Shah P, Kazmi S, Capucci A, Cleland JGF, Clark AL.. Prevalence and incidence of atrial fibrillation in ambulatory patients with heart failure. Am J Cardiol 2019;124:1554–1560. [DOI] [PubMed] [Google Scholar]
- 54. Anderson SG, Shoaib A, Myint PK, Cleland JG, Hardman SM, McDonagh TA, Dargie H, Keavney B, Garratt CJ, Mamas MA.. Does rhythm matter in acute heart failure? An insight from the British Society for Heart Failure National Audit. Clin Res Cardiol 2019;108:1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Packer M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J 2019;40:1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen S, Purerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z, Liu S, Yin Y, Martinek M, Kiuchi MG, Schmidt B, Chun KRJ.. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz443. [DOI] [PubMed] [Google Scholar]
- 57. Barra S, Duehmke R, Providencia R, Narayanan K, Reitan C, Roubicek T, Polasek R, Chow A, Defaye P, Fauchier L, Piot O, Deharo JC, Sadoul N, Klug D, Garcia R, Dockrill S, Virdee M, Pettit S, Agarwal S, Borgquist R, Marijon E, Boveda S.. Very long-term survival and late sudden cardiac death in cardiac resynchronization therapy patients. Eur Heart J 2019;40:2121–2127. [DOI] [PubMed] [Google Scholar]
- 58. Barra S, Providencia R, Narayanan K, Boveda S, Duehmke R, Garcia R, Leyva F, Roger V, Jouven X, Agarwal S, Levy WC, Marijon E.. Time trends in sudden cardiac death risk in heart failure patients with cardiac resynchronization therapy: a systematic review. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz773. [DOI] [PubMed] [Google Scholar]
- 59. Cleland JGF, Hindricks G, Petrie M.. The shocking lack of evidence for implantable cardioverter defibrillators for heart failure; with or without cardiac resynchronization. Eur Heart J 2019;40:2128–2130. [DOI] [PubMed] [Google Scholar]
- 60. Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, Gregson J, Alpendurada F, Frenneaux MP, Cook SA, Cleland JGF, Pennell DJ, Prasad SK.. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 2019;12:1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rorth R, Dewan P, Kristensen SL, Jhund PS, Petrie MC, Kober L, McMurray J.. Efficacy of an implantable cardioverter-defibrillator in patients with diabetes and heart failure and reduced ejection fraction. Clin Res Cardiol 2019;108:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJV, Van Veldhuisen DJ, Pitt B, Zannad F.. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: an individual patient-level meta-analysis of three randomized-controlled trials. Clin Res Cardiol 2019;108:477–486. [DOI] [PubMed] [Google Scholar]
- 63. Nikolaidou T, Johnson MJ, Ghosh JM, Marincowitz C, Shah S, Lammiman MJ, Schilling RJ, Clark AL.. Postmortem ICD interrogation in mode of death classification. J Cardiovasc Electrophysiol 2018;29:573–583. [DOI] [PubMed] [Google Scholar]
- 64. Leclercq C, Burri H, Curnis A, Delnoy PP, Rinaldi CA, Sperzel J, Lee K, Calo L, Vicentini A, Concha JF, Thibault B.. Cardiac resynchronization therapy non-responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE-CRT MPP) study: results from Phase I. Eur Heart J 2019;40:2979–2987. [DOI] [PubMed] [Google Scholar]
- 65. Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ.. Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol 2019;74:2969–2979. [DOI] [PubMed] [Google Scholar]
- 66. Baron SJ, Wang K, Arnold SV, Magnuson EA, Whisenant B, Brieke A, Rinaldi M, Asgar AW, Lindenfeld J, Abraham WT, Mack MJ, Stone GW, Cohen DJ; On behalf of the COAPT Investigators. Cost-effectiveness of transcatheter mitral valve repair versus medical therapy in patients with heart failure and secondary mitral regurgitation: results from the COAPT trial. Circulation 2019;140:1881–1891. [DOI] [PubMed] [Google Scholar]
- 67. Arnold SV, Chinnakondepalli KM, Spertus JA, Magnuson EA, Baron SJ, Kar S, Lim DS, Mishell JM, Abraham WT, Lindenfeld JA, Mack MJ, Stone GW, Cohen DJ.. Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation: COAPT trial. J Am Coll Cardiol 2019;73:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ.. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 69. Iung B, Armoiry X, Vahanian A, Boutitie F, Mewton N, Trochu JN, Lefèvre T, Messika-Zeitoun D, Guerin P, Cormier B, Brochet E, Thibault H, Himbert D, Thivolet S, Leurent G, Bonnet G, Donal E, Piriou N, Piot C, Habib G, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Saint Etienne C, Leroux L, Gilard M, Samson G, Rioufol G, Maucort-Boulch D, Obadia JF, Obadia JF; on behalf of the MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail 2019;doi: 10.1002/ejhf.1616. [Google Scholar]
- 70. Grayburn PA, Sannino A, Packer M.. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging 2019;12:353–362. [DOI] [PubMed] [Google Scholar]
- 71. Packer M, Grayburn PA.. Contrasting effects of pharmacological, procedural, and surgical interventions on proportionate and disproportionate functional mitral regurgitation in chronic heart failure. Circulation 2019;140:779–789. [DOI] [PubMed] [Google Scholar]
- 72. Witte KK, Lipiecki J, Siminiak T, Meredith IT, Malkin CJ, Goldberg SL, Stark MA, von Bardeleben RS, Cremer PC, Jaber WA, Celermajer DS, Kaye DM, Sievert H.. The REDUCE FMR trial: a randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail 2019;7:945–955. [DOI] [PubMed] [Google Scholar]
- 73. Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Nabauer M, Dahou A, Hahn RT.. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019;394:2002–2011. [DOI] [PubMed] [Google Scholar]
- 74. Taramasso M, Benfari G, van der Bijl P, Alessandrini H, Attinger-Toller A, Biasco L, Lurz P, Braun D, Brochet E, Connelly KA, de BS, Denti P, Deuschl F, Estevez-Loureiro R, Fam N, Frerker C, Gavazzoni M, Hausleiter JR, Ho E, Juliard JM, Kaple R, Besler C, Kodali S, Kreidel F, Kuck KH, Latib A, Lauten A, Monivas V, Mehr M, Muntane-Carol G, Nazif T, Nickening G, Pedrazzini G, Philippon F, Pozzoli A, Praz F, Puri R, Rodes-Cabau J, Scha FU, Schofer J, Sievert H, Tang GHL, Thiele H, Topilsky Y, Rommel KP, Delgado V, Vahanian A, von Bardeleben RS, Webb JG, Weber M, Windecker S, Winkel M, Zuber M, Leon MB, Hahn RT, Bax JJ, Enriquez-Sarano M, Maisano F.. Transcatheter versus medical treatment of symptomatic severe tricuspid regurgitation. J Am Coll Cardiol 2019;74:2998–3008. [DOI] [PubMed] [Google Scholar]
- 75. Branch KR, Probstfield JL, Eikelboom JW, Bosch J, Maggioni AP, Cheng RK, Bhatt DL, Avezum A, Fox KAA, Connolly SJ, Shestakovska O, Yusuf S.. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease: the COMPASS trial. Circulation 2019;140:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cleland JGF, Pellicori P.. Myocardial dysfunction and coronary artery disease as therapeutic targets in heart failure. Circulation 2019;140:538–541. [DOI] [PubMed] [Google Scholar]
- 77. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR.. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 78. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 79. Greenberg B, Neaton JD, Anker SD, Byra WM, Cleland JGF, Deng H, Fu M, La Police DA, Lam CSP, Mehra MR, Nessel CC, Spiro TE, Van Veldhuisen DJ, Vanden Boom CM, Zannad F.. Association of rivaroxaban with thromboembolic events in patients with heart failure, coronary disease, and sinus rhythm: a post hoc analysis of the COMMANDER HF trial. JAMA Cardiol 2019;4:515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mehra MR, Vaduganathan M, Fu M, Ferreira JP, Anker SD, Cleland JGF, Lam CSP, Van Veldhuisen DJ, Byra WM, Spiro TE, Deng H, Zannad F, Greenberg B.. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J 2019;40:3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morrow DA, Velazquez EJ, DeVore AD, Prescott MF, Duffy CI, Gurmu Y, McCague K, Rocha R, Braunwald E.. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J 2019;40:3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bohmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Buraiki AL, Gniot J, Mozheiko M, Lelonek M, Noe A, Schwende H, Bao W, Butylin D, Pascual-Figal D.. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019;21:998–1007. [DOI] [PubMed] [Google Scholar]
- 83. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF.. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2019;doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD.. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ.. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019;139:1354–1365. [DOI] [PubMed] [Google Scholar]
- 86. Zile MR, O’Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, Packer M, McMurray JJV, Shi V, Lefkowitz M, Rouleau J.. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol 2019;73:795–806. [DOI] [PubMed] [Google Scholar]
- 87. Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, Carson PE, Komajda M, Massie BM, McKelvie RS, McMurray JJ, Zile MR, Anand IS.. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I-PRESERVE collagen substudy. Circ Heart Fail 2011;4:561–568. [DOI] [PubMed] [Google Scholar]
- 88. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverria Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Kober L, Lelonek M, Linssen G, Lund LH, O'Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS Jr, Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimsky J Jr, Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV.. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 89. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; on behalf of PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 90. Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM.. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 2009;11:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP.. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 92. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ.. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 93. Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer MA, Desai A, Lund LH, Koeber L, Anand I, Sweitzer NK, Linssen G, Merkely B, Arango JL, Vinereanu D, Chen CH, Senni M, Sibulo A, Boytsov S, Shi V, Rizkala A, Lefkowitz M, McMurray J.. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2019;doi: 10.1161/CIRCULATIONAHA.119.044586. [DOI] [PubMed] [Google Scholar]
- 94. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, Van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer NK, Vardeny O, Claggett B, Jhund PS, Solomon SD.. Effects of sacubitril-valsartan, versus valsartan, in women compared to men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2019;doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PubMed] [Google Scholar]
- 95.Vaduganathan M, Claggett BL, Desai AS, Anker SD, Perrone SV, Janssens S, Milicic D, Arango JL, Packer M, Shi VC, Lefkowitz MP, McMurray JJV, Solomon SD. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol 2019. Nov 6. pii: S0735-1097(19)38299-3. doi: 10.1016/j.jacc.2019.11.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dewan P, Rorth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Kober L, Mogensen UM, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray J.. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 97. Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, Lefkowitz MP, Shi VC, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV, Solomon SD.. Health-related quality of life in heart failure with preserved ejection fraction: the PARAGON-HF trial. JACC Heart Fail 2019;7:862–874. [DOI] [PubMed] [Google Scholar]
- 98. Santema BT, Ouwerkerk W, Tromp J, Sama IE, Ravera A, Regitz-Zagrosek V, Hillege H, Samani NJ, Zannad F, Dickstein K, Lang CC, Cleland JG, Ter Maaten JM, Metra M, Anker SD, van der Harst P, Ng LL, van der Meer P, van Veldhuisen DJ, Meyer S, Lam CSP, Voors AA, Richards AM, Lam CSP, Anand I, Hung C-L, Ling LH, Liew HB, Narasimhan C, Ngarmukos T, Park SW, Reyes E, Siswanto BB, Shimizu W, Zhang S.. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019;394:1254–1263. [DOI] [PubMed] [Google Scholar]
- 99. Merrill M, Sweitzer NK, Lindenfeld J, Kao DP.. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail 2019;7:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Linde C, Cleland JGF, Gold MR, Claude DJ, Tang ASL, Young JB, Sherfesee L, Abraham WT.. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual-patient data meta-analysis. Eur J Heart Fail 2018;20:780–791. [DOI] [PubMed] [Google Scholar]
- 101. Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Moller M, Jorgensen NR, Faber J, Danser AHJ, Gansevoort RT, Rossing P, Persson F, Heerspink H.. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med 2019;8:E779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dekkers CCJ, Sjostrom CD, Greasley PJ, Cain V, Boulton DW, Heerspink H.. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cefalo CMA, Cinti F, Moffa S, Impronta F, Sorice GP, Mezza T, Pontecorvi A, Giaccari A.. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc Diabetol 2019;18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 105. Yang DY, He X, Liang HW, Zhang SZ, Zhong XB, Luo CF, Du ZM, He JG, Zhuang XD, Liao XX.. Comparative outcomes of heart failure among existent classes of anti-diabetic agents: a network meta-analysis of 171, 253 participants from 91 randomized controlled trials. Cardiovasc Diabetol 2019;18:47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS.. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 107. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray J.. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 108. Shen L, Rorth R, Cosmi D, Kristensen SL, Petrie MC, Cosmi F, Latini R, Kober L, Anand IS, Carson PE, Granger CB, Komajda M, McKelvie RS, Solomon SD, Staszewsky L, Swedberg K, Huynh T, Zile MR, Jhund PS, McMurray J.. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS.. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 110. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 111. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause-Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD.. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019;139:2528–2536. [DOI] [PubMed] [Google Scholar]
- 112. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD; on behalf of the DAPA‐HF Committees and Investigators. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402–1411. [DOI] [PubMed] [Google Scholar]
- 113. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz-Piasecka M, Bengtsson O, Sjostrand M, Langkilde AM, Jhund PS, McMurray J.. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation 2019;doi: 10.1161/CIRCULATIONAHA.119.044133. [DOI] [PubMed] [Google Scholar]
- 114. Kosiborod MN, Jhund P, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Koeber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjostrand M, Langkilde AM, McMurray J.. Effects of dapagliflozin on symptoms, function and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2019;doi: 10.1161/CIRCULATIONAHA.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, Mc-Guire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld J, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M; On behalf of the DEFINE-HF Investigators. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation 2019;140:1463–1476. [DOI] [PubMed] [Google Scholar]
- 116. Packer M, Anker SD, Butler J, Filippatos G, Zannad F.. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2017;2:1025–1029. [DOI] [PubMed] [Google Scholar]
- 117. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Juni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S.. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation 2019;doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 118. Swedberg K, Young JB, Anand S, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor CM, Pfeffer MA, Solomon SD, Sun Y, Tendera M, Van Veldhuisen DJ; RED-HF Committees, RED-HF Investigators. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013;368:1210–1219. [DOI] [PubMed] [Google Scholar]
- 119. Packer M. Do sodium-glucose co-transporter-2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes Metab 2018;20:1361–1366. [DOI] [PubMed] [Google Scholar]
- 120. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Bøtker HE, Wiggers H.. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al-Omran M, Gilbert RE, Bhatt DL, Leiter LA, Juni P, Zinman B, Connelly KA; For the EMPA-HEART CardioLink-6 Investigators. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART cardiolink-6 randomized clinical trial. Circulation 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 122. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F; on behalf of the EMPEROR‐Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 123. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M; on behalf of the EMPEROR‐Preserved Trial Committees and Investigators. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail 2019;21:1279–1287. [DOI] [PubMed] [Google Scholar]
- 124. Maggioni AP, Lopez-Sendon J, Nielsen OW, Hallen J, Aalamian-Mattheis M, Wang Y, Ertl G.. Efficacy and safety of serelaxin when added to standard of care in patients with acute heart failure: results from a PROBE study, RELAX-AHF-EU. Eur J Heart Fail 2019;21:322–333. [DOI] [PubMed] [Google Scholar]
- 125. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias-Mendoza A, Avendano P, Bacal F, Bohm M, Bortman G, Cleland JGF, Cohen-Solal A, Crespo-Leiro MG, Dorobantu M, Echeverria LE, Ferrari R, Goland S, Goncalvesova E, Goudev A, Kober L, Lema-Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva-Cardoso J, Spinar J, Squire I, Stepinska J, van MW, von LD, Wikstrom G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsody P, Gimpelewicz C; RELAX-AHF-2 Committees Investigators. Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019;381:716–726.31433919 [Google Scholar]
- 126.Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, Gualandro DM, de Oliveira Junior MT, Sabti Z, Müller B, Noveanu M, Socrates T, Ziller R, Bayés-Genís A, Sionis A, Simon P, Michou E, Gujer S, Gori T, Wenzel P, Pfister O, Conen D, Kapos I, Kobza R, Rickli H, Breidthardt T, Münzel T, Erne P, Mueller C; GALACTIC Investigators. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: The GALACTIC Randomized Clinical Trial. JAMA 2019;322:2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Courtney DM, Hasa J, Spinar J, Masip J, Peacock WF, Sliwa K, Gayat E, Filippatos G, Cleland JGF, Gheorghiade M.. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 2010;31:832–841. [DOI] [PubMed] [Google Scholar]
- 128. Biegus J, Zymlinski R, Siwolowski P, Testani J, Szachniewicz J, Tycińska A, Banasiak W, Halpert A, Levin H, Ponikowski P.. Controlled decongestion by reprieve therapy in acute heart failure: results of the TARGET-1 and TARGET-2 studies. Eur J Heart Fail 2019;21:1079–1087. [DOI] [PubMed] [Google Scholar]
- 129. Keeble TR, Karamasis GV, Rothman MT, Ricksten SE, Ferrari M, Hullin R, Schersten F, Reitan O, Kirking ST, Cleland JGF, Smith EJ.. Percutaneous haemodynamic and renal support in patients presenting with decompensated heart failure: a multi-centre efficacy study using the Reitan Catheter Pump (RCP). Int J Cardiol 2019;275:53–58. [DOI] [PubMed] [Google Scholar]
- 130. Yau TM, Pagani FD, Mancini DM, Chang HL, Lala A, Woo YJ, Acker MA, Selzman CH, Soltesz EG, Kern JA, Maltais S, Charbonneau E, Pan S, Marks ME, Moquete EG, O’Sullivan KL, Taddei-Peters WC, McGowan LK, Green C, Rose EA, Jeffries N, Parides MK, Weisel RD, Miller MA, Hung J, O’Gara PT, Moskowitz AJ, Gelijns AC, Bagiella E, Milano CA; for the Cardiothoracic Surgical Trials Network. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA 2019; 321:1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, Ito K, Willcox JA, Arany Z, Li J, Owens AT, Govind R, Nunez B, Mazaika E, Bayes-Genis A, Walsh R, Finkelman B, Lupon J, Whiffin N, Serrano I, Midwinter W, Wilk A, Bardaji A, Ingold N, Buchan R, Tayal U, Pascual-Figal DA, de MA, Ahmad M, Garcia-Pinilla JM, Pantazis A, Dominguez F, John BA, O'Regan DP, Rosen SD, Prasad SK, Lara-Pezzi E, Provencio M, Lyon AR, Alonso-Pulpon L, Cook SA, DePalma SR, Barton PJR, Aplenc R, Seidman JG, Ky B, Ware JS, Seidman CE.. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation 2019;140:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yu AF, Yadav NU, Lung BY, Eaton AA, Thaler HT, Hudis CA, Dang CT, Steingart RM.. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat 2015;149:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, Gallagher C, Pohlmann PR, Nunes R, Herbolsheimer P, Warren R, Srichai MB, Hofmeyer M, Cunningham A, Timothee P, Asch FM, Shajahan-Haq A, Tan MT, Isaacs C, Swain SM.. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat 2019;175:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, Carver JR, Cohen AD, Engelhardt BG, Garfall AL, Goodman SA, Harrell SL, Kassim AA, Jadhav T, Jagasia M, Moslehi J, O’Quinn R, Savona MR, Slosky D, Smith A, Stadtmauer EA, Vogl DT, Waxman A, Lenihan D.. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abuosa AM, Elshiekh AH, Qureshi K, Abrar MB, Kholeif MA, Kinsara AJ, Andejani A, Ahmed AH, Cleland J.. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J 2018;70(Suppl. 3):S96–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li X, Li Y, Zhang T, Xiong X, Liu N, Pang B, Ruan Y, Gao Y, Shang H, Xing Y.. Role of cardioprotective agents on chemotherapy-induced heart failure: a systematic review and network meta-analysis of randomized controlled trials. Pharmacol Res 2020;151:104577.. [DOI] [PubMed] [Google Scholar]
- 137. Kapelios CJ, Lainscak M, Savarese G, Laroche C, Seferovic P, Ruschitzka F, Coats A, Anker SD, Crespo-Leiro MG, Filippatos G, Piepoli MF, Rosano G, Zanolla L, Aguiar C, Murin J, Leszek P, McDonagh T, Maggioni AP, Lund LH.. Sacubitril/valsartan eligibility and outcomes in the ESC-EORP-HFA Heart Failure Long-Term Registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM-HF trial, ESC guidelines, and real world. Eur J Heart Fail 2019;21:1383–1397. [DOI] [PubMed] [Google Scholar]
- 138. Loop MS, van Dyke MK, Chen L, Safford MM, Kilgore ML, Brown TM, Durant RW, Levitan EB.. Low utilization of beta-blockers among medicare beneficiaries hospitalized for heart failure with reduced ejection fraction. J Card Fail 2019;25:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Van Spall HGC, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, Maingi M, Tjandrawidjaja MC, Heffernan M, Zia MI, Porepa L, Panju M, Thabane L, Graham ID, Haynes RB, Haughton D, Simek KD, Ko DT, Connolly SJ.. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 2019;321:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schulz M, Griese‐Mammen N, Anker SD, Koehler F, Ihle P, Ruckes C, Schumacher PM, Trenk D, Böhm M, Laufs U; for the PHARM‐CHF Investigators. Pharmacy-based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM-CHF randomized controlled trial. Eur J Heart Fail 2019;21:1012–1021. [DOI] [PubMed] [Google Scholar]
- 141. Wagenaar KP, Broekhuizen BDL, Jaarsma T, Kok I, Mosterd A, Willems FF, Linssen GCM, Agema WRP, Anneveldt S, Lucas C, Mannaerts HFJ, Wajon E, Dickstein K, Cramer MJ, Landman MAJ, Hoes AW, Rutten FH.. Effectiveness of the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’ and an e-health adjusted care pathway in patients with stable heart failure: results of the ‘e-Vita HF’ randomized controlled trial. Eur J Heart Fail 2019;21:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shanbhag D, Graham ID, Harlos K, Haynes RB, Gabizon I, Connolly SJ, Van Spall H.. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: a systematic review. BMJ Open 2018;8:e017765.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Taylor RS, Long L, Mordi IR, Madsen MT, Davies EJ, Dalal H, Rees K, Singh SJ, Gluud C, Zwisler AD.. Exercise-based rehabilitation for heart failure: cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail 2019;7:691–705. [DOI] [PubMed] [Google Scholar]
- 144. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, Whellan D, O’Connor C, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista LS, Jolly K, Myers J, Nilsson BB, Passino C, Witham MD, Yeh GY.. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol 2019;73:1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, O'Connor C, Whellan D, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista L, Jolly K, Myers J, McKelvie RS, Nilsson BB, Passino C, Witham MD, Yeh GY, Zwisler AO; on behalf of the ExTraMATCH II Collaboration. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail 2018;20:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS.. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:CD003331.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Takeda A, Martin N, Taylor RS, Taylor SJ.. Disease management interventions for heart failure. Cochrane Database Syst Rev 2019;1:CD002752.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Taylor RS, Sadler S, Dalal HM, Warren FC, Jolly K, Davis RC, Doherty P, Miles J, Greaves C, Wingham J, Hillsdon M, Abraham C, Frost J, Singh S, Hayward C, Eyre V, Paul K, Lang CC, Smith K.. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur J Prev Cardiol 2019;26:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wingham J, Frost J, Britten N, Greaves C, Abraham C, Warren FC, Jolly K, Miles J, Paul K, Doherty PJ, Singh S, Davies R, Noonan M, Dalal H, Taylor RS.. Caregiver outcomes of the REACH-HF multicentre randomized controlled trial of home-based rehabilitation for heart failure with reduced ejection fraction. Eur J Cardiovasc Nurs 2019;18:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Johnson MJ, Cockayne S, Currow DC, Bell K, Hicks K, Fairhurst C, Gabe R, Torgerson D, Jefferson L, Oxberry S, Ghosh J, Hogg KJ, Murphy J, Allgar V, Cleland JGF, Clark AL.. Oral modified release morphine for breathlessness in chronic heart failure: a randomized placebo-controlled trial. ESC Heart Fail 2019;doi: 10.1002/ehf2.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK.. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ghimire A, Fine N, Ezekowitz JA, Howlett J, Youngson E, McAlister FA.. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram-based registry study. Eur Heart J 2019;40:2110–2117. [DOI] [PubMed] [Google Scholar]
- 153. Aguirre DL, Weber K, Bavendiek U, Bauersachs J, Wittes J, Yusuf S, Koch A.. Digoxin-mortality: randomized vs. observational comparison in the DIG trial. Eur Heart J 2019;40:3336–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simoes MV, Silvestre OM, Ribeiro ALP, Moura LZ, Beck-da-Silva L, Prado D, Sant'Anna RT, Bridi LH, Zimerman A, Raupp da RP, Biolo A.. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J 2019;40:3605–3612. [DOI] [PubMed] [Google Scholar]
- 155. Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland J.. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail 2017;19:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]