Abstract
Plastic products are inexpensive, convenient, and are have many applications in daily life. We overuse plastic-related products and ineffectively recycle plastic that is difficult to degrade. Plastic debris can be fragmented into smaller pieces by many physical and chemical processes. Plastic debris that is fragmented into microplastics or nanoplastics has unclear effects on organismal systems. Recently, this debris was shown to affect biota and to be gradually spreading through the food chain. In addition, studies have indicated that workers in plastic-related industries develop many kinds of cancer because of chronic exposure to high levels of airborne microplastics. Microplastics and nanoplastics are everywhere now, contaminating our water, air, and food chain. In this review, we introduce a classification of plastic polymers, define microplastics and nanoplastics, identify plastics that contaminate food, describe the damage and diseases caused by microplastics and nanoplastics, and the molecular and cellular mechanisms of this damage and disease as well as solutions for their amelioration. Thus, we expect to contribute to the understanding of the effects of microplastics and nanoplastics on cellular and molecular mechanisms and the ways that the uptake of microplastics and nanoplastics are potentially dangerous to our biota. After understanding the issues, we can focus on how to handle the problems caused by plastic overuse.
Keywords: plastic products, food chain, microplastics, nanoplastics
1. Introduction
Recently, plastic products have become inexpensive and convenient and are used in all aspects of daily life, such as food and product packaging, clothing, construction and car materials, household goods, medical devices, personal care products, and, toys. [1,2]. Although plastic products are relatively convenient, the negative influences of the “plastic era” caused by these inexpensive and convenient products include high levels of plastic production coupled with a slow biodegradation rate, uncontrolled use, and ineffective and irresponsible waste recycling, leading to plastic accumulation in our global environment, particularly in freshwater and marine environments [3,4,5,6,7,8,9,10]. The use of plastic products has increased rapidly, and 33 billion tons of plastic will likely be produced by 2050 [11], making the Pacific Ocean a giant garbage dump [12]. The plastic debris in aquatic environments is fragmented into smaller pieces by ultraviolet light and biodegraded plastic forms microplastics and nanoplastics [13,14,15]. However, the largest proportion of microplastics and nanoplastics is generated from the laundering of textiles with mixed synthetic fibers [16] and the friction of the tires of moving cars [17,18]. These microplastics and nanoplastics have unclear effects on organismal systems. Recently, evidence has been presented indicating that plastics significantly affect the growth and oxygen production of Prochlorococcus and microalgae. Prochlorococcus, especially, is the ocean’s most abundant photosynthetic bacteria and produces 10% of global oxygen. [19,20]. However, the growth of earthworms is meaningfully different in soil ecosystems, particularly agricultural land, contaminated with microplastics [21]. In addition, it is noteworthy that microplastics are widespread in naturally-occurring Arctic deep-sea sediments [22] and in snow ranging from the Alps to the Arctic [23]. Therefore, microplastic and nanoplastic contamination is everywhere [24]. Microplastics have been found in human stool [25] and humans can consume microplastics and nanoplastics through seafood [26,27,28,29,30] and water [31,32,33,34,35,36], etc. Whether plastics will harm our health is unclear; however, the potential consequences may affect the ecological functioning of the globe and future generations of organisms (Figure 1). In this review, we briefly introduce a classification of plastic materials and describe the origin of microplastics and nanoplastics, the food contaminated by microplastics and nanoplastics, the damage and diseases caused by microplastics and nanoplastics, the molecular and cellular mechanisms of the damage and diseases caused by microplastics and nanoplastics, and solutions to mediate the problems caused by plastic overuse.
Figure 1.
Some sources and deposits of microplastic and nanoplastic are the result of human needs. The potential impacts of these plastics on air, water, and many foods finally returns to affect humans. All pictures come from Pixabay (https://pixabay.com/).
2. Classification of Plastic Materials and Related Product Applications
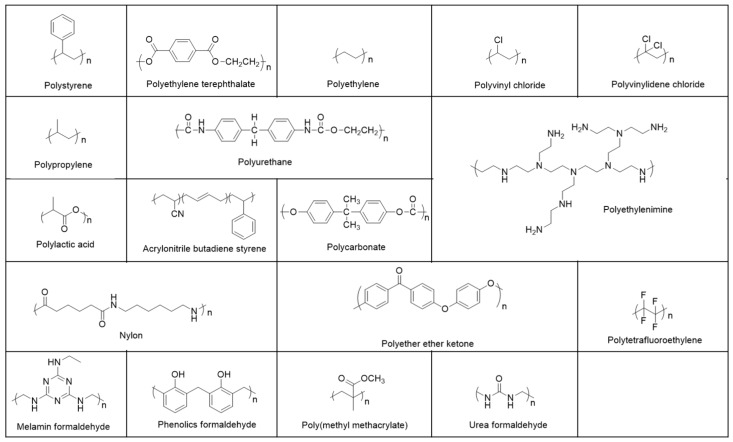
Plastic production has gradually increased every year, from 1.5 million tons in the 1950s [37] to an estimated 33 billion tons in 2050 [11]. Plastics are specifically derived from synthetic polymers generated by the polymerization of many monomers and mixtures of a range of materials [38]. Therefore, plastic is predominantly generated into polyethylene (PE), polyester (PES), polyethylene terephthalate (PET), polyetherimide (PEI) (Ultem), polystyrene (PS), polypropylene (PP), low-density polyethylene (LDPE) high-density polyethylene (HDPE), polyvinyl chloride (PVC), polyvinylidene chloride (PVDC) (Saran), polycarbonate (PC), polycarbonate/acrylonitrile butadiene styrene (PC/ABS), high-impact polystyrene (HIPS), polyamides (PA) (nylon), acrylonitrile butadiene styrene (ABS), polyurethanes (PU), urea–formaldehyde (UF), melamine formaldehyde (MF), polymethyl methacrylate (PMMA), polytetrafluoroethylene (PTFE), and polylactic acid (PLA), etc. [39] (Figure 2). The highest percentages of plastics produced worldwide meet the definition of thermoplastic: PP (21%), LDPE (18%), PVC (17%), and HDPE (15%) [40]. Plastic polymers have numerous applications in daily life [41]. PP is usually used in pots for plants, bags, industrial fibers, netting, medical masks, bottle caps, ropes, straws, containers, tanks and jugs, appliances, car fenders, plastic pressure pipe systems, and centrifuge tubes. LDPE is usually used in outdoor furniture, siding, wire cable, floor tiles, plastic bags, shower curtains, buckets, clamshell packaging, and soap dispenser bottles. PVC is usually used in plumbing pipes and guttering, siding, shower curtains, blood bags, window frames, and flooring. HDPE is usually used in detergent bottles, plastic bottles, plastic bags, bottle caps, and milk jugs [37,39,42].
Figure 2.
Typical polymer types and their chemical structures. Chemical structures are shown for polyethylene (PE), polyester (PES), polyethylene terephthalate (PET), polyethylenimine (PEI) (Ultem), polystyrene (PS), polylactic acid (PLA), polypropylene (PP), polyvinyl chloride (PVC), polyvinylidene chloride (PVDC) (Saran), polycarbonate (PC), polycarbonate/acrylonitrile butadiene styrene (PC/ABS), polyamides (PA) (nylon), acrylonitrile butadiene styrene (ABS), polyurethanes (PU), urea–formaldehyde (UF), melamine formaldehyde (MF), polymethyl methacrylate (PMMA), and polytetrafluoroethylene (PTFE).
3. Routes of Plastic Micromaterial and Nanomaterial Pollution
Plastic fragments can be generally divided into several types: macroplastics and mesoplastics are greater than 5 mm in size [43], microplastic particles are smaller than 5 mm [44], and nanoplastics are less than 1000 nm or 100 nm [45]. Currently, microplastics are found worldwide in freshwater and marine systems [46], in sediment [47], in soil [48], and within biota [49]. Nanoplastics are generated by the abiotic and biotic degradation of microplastics. For example, UV degradation of microplastics has been shown to generate nanoplastics [13,50], and digestive fragmentation has been proposed as a means by which nanoplastics can be generated from microplastics [51]. However, two types of microplastics, primary and secondary microplastics, are categorized by the form in which they are released. Primary microplastics are directly released into the environment as small particles. Secondary microplastics are derived from large plastic items being degraded into small plastic fragments upon exposure to the environment [52]. The largest proportion of these particles is derived from laundering textiles with mixed synthetic fibers [16] and the friction of car tires [17,18]. Secondary microplastics from synthetic textiles in garments are the major type of microplastics [53,54]. Each textile may release approximately 1900 fibers per washing [55]. Other sources of microplastics and nanoplastics are urban dust [56], road markings [17], and personal care products [57].
4. Presence of Plastic Micromaterials and Nanomaterials in Food and Food Products
Microplastics and nanoplastics are currently everywhere. In marine environments, seabirds and marine mammals ingest microplastics at low trophic levels [52,58]. Microplastics and nanoplastics have been detected at the base of the food web, specifically, in zooplankton, such as chaetognaths [59]. Crustaceans, such as the Japanese shore crab [60] and North Pacific krill [61], contain microplastics and nanoplastics. Sea fish, such as the northeastern Pacific Ocean forage fishes [62], areolate grouper and goldbanded jobfish, are contaminated with microplastics [63]. Oysters ingest polystyrene microplastics, which affect their reproduction [64]. The mussel Mytilus edulis ingests microplastics that translocate to the circulatory system [65]. Many kinds of mussels are contaminated, including blue mussels [26,37,66], Mediterranean mussels [61,67], brown mussels [66], and northern horse mussels [68]. Plastic additives and hydrophobic organic compounds (HOCs) are also found in mussels [69]. In summary, many kinds of seafood are potentially contaminated by microplastics and/or nanoplastics [30]. In addition, in our daily life, many consumables, such as tap water [70], bottled water [34,71], beer [70,72], sea salt [70,73], sugar [74], honey [74] and plastic teabags [75] have also been found to contain microplastics or nanoplastics. Even air [76,77] and unprocessed water [78] have been contaminated with microplastics. Sooner or later, the entire food chain will be contaminated with plastic. Some statistics from studies published by PubMed on animals contaminated by microplastics and/or nanoplastics are presented in Table 1.
Table 1.
Published studies from the National Center for Biotechnology Information (NCBI) on microplastic and/or nanoplastic contamination in different animal species.
| Animal Species | Number of Published Studies from NCBI |
|---|---|
| Human | 2 |
| Bear Mouse |
1 5 |
| Birds | 5 |
| Seabirds | 8 |
| Crustaceans | 68 |
| Bivalves | 79 |
| Fish and sea mammals | 161 |
| Insects | 5 |
| Turtles | 5 |
| Amphipods | 2 |
| Seaplants | 3 |
5. Damage and Diseases Caused by Plastic Micromaterials and Nanomaterials
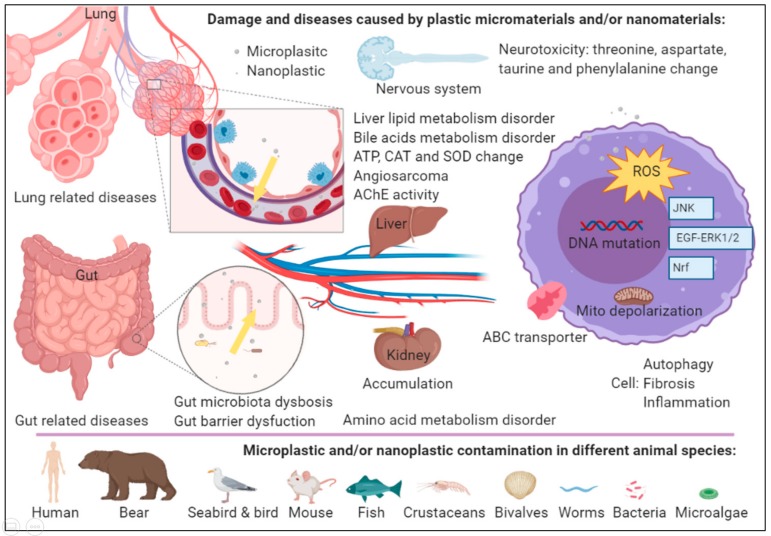
Many marine animals suffer from ingesting high amounts of plastic debris [79,80]. Fragments of this plastic debris, such as microplastics, accumulate in the gut and cause obstruction and inflammation in many organs in a wide range of living creatures [52,81]. Microplastics reduce photosynthesis in microalgae [20] and have a negative influence on the feeding behavior of zooplankton [58] and lugworms [82]. They also accumulate in and probably negatively influence the gills, stomach, and hepatopancreas of crabs [83], and they change the biomarkers and histology of fish tissues [84]. Evidence indicates that PS microplastics decrease the number of eggs and larvae produced and sperm velocity of oysters [64]. PS microplastics may also transport contaminants to microorganisms [81]. Studies have described the influence of microplastics on the digestive system [85]. The aquatic ecosystem has accommodated the ingestion of contaminated organisms [86]. Finally, this leads to the uptake of microplastics in the human intestine [26]. Several studies have indicated that PS microplastics can cause metabolic disorders of amino acids, bile acids [87], and liver lipids [88] in mice. Microplastics change gut microbiota dysbiosis and decrease gut mucin secretion in mice [87,88]. However, these microplastics or nanoplastics are also released to the atmosphere, becoming airborne contaminants [55,86,89]. Indeed, a study shows contamination in working environments [33]. Workers in the synthetic textiles, flock and vinyl chloride (VC), or polyvinyl chloride (PVC) industries are potentially exposed to high concentrations of microplastics in the air during work [76]. Synthetic textile workers potentially suffer higher rates of lung-cancer-related mortality [90] or stomach and esophageal cancers [91]. Flock workers have a high incidence of interstitial and lung diseases [92,93]. VC has been considered a carcinogenic factor and mostly causes angiosarcoma of the liver [94,95,96]. Microplastics or nanoplastics disrupt the endocrine system [97], induce neurotoxicity [98], and produce reproductive abnormities with trans-generational effects [99,100,101,102,103]. In addition, food and drink are a major vehicles of microplastic and nanoplastic exposure through which polymer elements and additives are potentially transported [104]. Risk assessments on using food packaging nanomaterials with antimicrobial activity, including titanium dioxide [105] and carbon nanotubes [106], have shown that they present risks comparable to those of using nanopolymers. The complex mixtures of plastic additives can dissolve in the polymer and leak into the surrounding environment [107]. The physical–chemical characteristics of these particles, such as the size, external charge, length:width ratio, porosity, surface corona, and hydrophilicity, cause different circulation times [108]. In addition, microplastics can absorb persistent organic pollutants (POPs) such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pesticides, including dichlorodiphenyltrichloroethane (DDT) and hexachlorobenzene (HCB), in the ocean [109,110]. These compounds have a higher affinity for plastic than for water [110,111]. Microplastics and/or nanoplastics are taken up into the gut and lungs, and enter many organs, where they potentially cause damage and result in disease.
6. Molecular and Cellular Mechanisms of Plastic Micromaterial and Nanomaterial Damage and Disease
Microplastics and/or nanoplastics can enter the circulation from the gut via trophic transfer [30] and from air [76,77]. Microplastics or nanoplastics inhibit the efflux pump and induce cytotoxicity in human intestinal cells [112]. The cytotoxicity induced by microplastics and/or nanoplastics stimulates oxidative stress via free radical generation originating from reactive oxygen species (ROS) [98,101,103,113,114,115]. Several studies have shown this connection in monogonont rotifer [116], Caenorhabditis elegans [117], Danio rerio [118], mouse liver [119], and human intestine cell lines [112]. Overproduced ROS can alter the homeostasis of cells by mediating antioxidant systems. ROS overwhelm the antioxidants produced in response to damage to cellular components, including DNA, carbohydrates, lipids, and proteins. This damage is associated with gene instability, physiological alterations, and carcinogenesis [120,121]. For example, scleractinian coral, Pocillopora damicornis, exposed to microplastics have increased superoxide dismutase (SOD) and catalase (CAT) activity and glutathione S-transferase (GST) and alkaline phosphatase (ALP) loss of function. SOD and CAT are antioxidant enzymes, GST is a detoxifying enzyme and ALP is an immune enzyme in coral. In addition, in coral, microplastics regulate genes that are related to the stress response, zymogen granules, c-Jun N-terminal kinase (JNK) signaling pathways, sterol transport, and the epidermal growth factor–extracellular signal-regulated kinase 1/2 (EGF-ERK1/2) pathway (Figure 3) [115]. In addition, PS nanoplastics increase oxidative stress, activate the expression of genes in the nuclear factor E2-related factor (Nrf) signaling pathway (Figure 3) [114], and increase expression of glutathione S-transferase 4 (GST-4) enzyme in Caenorhabditis elegans [117]. Additionally, a previous report showed that PS microplastics also induce inflammation and activate SOD and CAT activity in the livers of Danio rerio [118] and mice [119,122]. These findings indicate that microplastics induce oxidative stress as the main mechanism of toxicity induction in these organisms. PS microplastics can affect amino acid metabolism by increasing arginine and tyrosine and affect bile acid metabolism by mediating the levels of taurocholic acid (TCA), β-muricholic acid (βMCA), adenosine triphosphate (ATP)-binding cassette, subfamily B, member 11 (Abcb11) and cholesterol 7a-hydroxylase (Cyp7a1) [87]. They also affect liver lipid metabolism by changing the triglyceride (TG), total cholesterol (TCH), and pyruvate levels (Figure 3) [88]. PS microplastics also increased the acetylcholinesterase (AChE) activity and the related neurotransmitters such as threonine, aspartate, and taurine in a mouse model [122]. In addition, microplastics and nanoplastics elicit immunological responses [115,123,124], alter gene expression [88,103,113,114,125] and induce genotoxicity [113,126]. In kidney cells, VC stimulates the expression of fibrosis-related proteins, such as CTGF, PAI-1, and collagen 1, and autophagy-related proteins, such as Beclin 1 and LC3-II [127]. VC is also a carcinogenic factor and results in angiosarcoma of the liver [94,95,96]. Studies have indicated that VC causes several DNA mutations, such as Ras mutations [128], K-ras-2 mutations [129], p53 mutations [130,131], and p21 mutations [132].
Figure 3.
Impact of plastic micromaterials and nanomaterials in organisms. Microplastics and/or nanoplastics can enter the circulation from the gut and lungs and accumulate in the gut, liver, and kidney resulting in several diseases. At the cell level, microplastics or nanoplastics can inhibit the efflux pump and mitochondria depolarization, induce reactive oxygen species (ROS). They also affect several signaling pathways, cause fibrosis, autophagy, and even DNA mutations. Many animal species have been contaminated by microplastics and/or nanoplastics. The figure was created with BioRender.com.
7. Solutions for Reducing Plastic Micromaterials and Nanomaterials
A large area of accumulated garbage is adrift in the ocean [133]. Prevention and clean-up proposals have been made by political bodies around the world [134], such as the plastic reduction policy of Africa, which ranks first in the world [135]. A Netherlands-based organization, the Ocean Cleanup, uses massive drift nets to reduce the size of the Great Pacific Garbage Patch [136]. Wastewater treatment plants (WWTPs) in several countries have found microplastic particles [49,137,138,139]. Australia uses filters in large drains to stop garbage from entering the ocean [140]. Single-use plastic items are one of the components in this large area of plastic waste. In India, single-use plastic items, such as plastic bags, plastic spoons, plastic cups, plastic drinking straws, plastic jars, and plastic bottles, have been banned since October 2, 2019. The European Union has set a target to eliminate some single-use plastic items by 2021 [141]. Single-use plastic items, such as plastic straws, are being replaced—a Vietnamese company has developed a reed pipe to replace plastic straws [142], and a Taiwanese company has developed a straw with sugar cane [143]. In addition, facial cleansers containing plastic particles [57] have been banned in many countries [144]. On the one hand, plastic waste has been turned into resources. For example, a company in the Netherlands uses plastic to replace traditional road materials [145], and it is better than asphalt, with 60% greater strength [146]. In India, abandoned fishing nets have been turned into surfboards [147], in UK, students have successfully used fish skin and red algae as raw materials to develop plastic substitutes [148], and in Mexico, scientists have used cactus fruit to make nontoxic edible plastic [149]. On the other hand, due to physical and chemical changes, plastics become microplastics and nanoplastics. Therefore, some microbial biodegradation can be used to depolymerize those polymers into smaller monomers. Biodegradation is ultimately successful when plastics degrade monomers into CO2 and water. Marine bacteria are potential candidates for use in the biodegradation of plastic wastes [150]. PS is known to biodegrade in the gut of yellow mealworms [151]. Many fungal strains can also degrade several plastics, such as PVC, PHB, and PLA [39]. Recently, several enzymes have been identified as capable of degrading PET plastics [152].
8. Conclusions
We over-use plastics because they are inexpensive and convenient, and worldwide, ecosystems are suffering, and contaminating-levels of plastic debris is a concern that has been reported. Plastic must be managed (especially in single-use items) and recycled such that it is finally fragmented to small plastics. Most of the contamination by microplastics and nanoplastics is derived from laundering synthetic textiles and the friction from the tires of driven cars. Currently, there is no effective way to reduce the amount of microplastics and nanoplastics in the food chain. Furthermore, it is unclear how the mixture of different sized groups and material types interacts with living creatures. Previous studies have indicated that workers in plastic-related industries suffer many kinds of cancer by being exposed to high levels of airborne microplastics over many years. In addition, it is important to characterize the microplastics and nanoplastics that have accumulated in the food chain and to gain a clearer understanding of their negative impact on our bodies. Finally, the degradation of microplastics and nanoparticles from environmental bacteria and fungi remains a challenge for the scientific community.
Author Contributions
Conceptualization, Y.-L.W., Y.-F.L., and H.-W.C.; writing—original draft preparation, Y.-L.W., Y.-F.L., and H.-W.C.; writing—review and editing, Y.-L.W., Y.-H.L., I.-J.C., Y.-F.L., and H.-W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-038-069-MY3, MOST 108-2314-B-039-061-MY3 and MOST 108-2314-B-038-044) and China Medical University, Taichung, Taiwan (CMU108-N-09).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes C.J. Plastic pollution and potential solutions. Sci. Prog. 2018;101:207–260. doi: 10.3184/003685018X15294876706211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Torres E.R., Ortega-Ortiz C.D., Silva-Iniguez L., Nene-Preciado A., Orozco E.T. Floating marine debris in waters of the Mexican Central Pacific. Mar. Pollut. Bull. 2017;115:225–232. doi: 10.1016/j.marpolbul.2016.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Ryan P.G., Musker S., Rink A. Low densities of drifting litter in the African sector of the Southern Ocean. Mar. Pollut. Bull. 2014;89:16–19. doi: 10.1016/j.marpolbul.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Ryan P.G. Litter survey detects the South Atlantic ‘garbage patch’. Mar. Pollut. Bull. 2014;79:220–224. doi: 10.1016/j.marpolbul.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen M., Maximenko N., Thiel M., Cummins A., Lattin G., Wilson S., Hafner J., Zellers A., Rifman S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013;68:71–76. doi: 10.1016/j.marpolbul.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Cozar A., Echevarria F., Gonzalez-Gordillo J.I., Irigoien X., Ubeda B., Hernandez-Leon S., Palma A.T., Navarro S., Garcia-de-Lomas J., Ruiz A., et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA. 2014;111:10239–10244. doi: 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derraik J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002;44:842–852. doi: 10.1016/S0025-326X(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 9.Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 10.Lamb J.B., Willis B.L., Fiorenza E.A., Couch C.S., Howard R., Rader D.N., True J.D., Kelly L.A., Ahmad A., Jompa J., et al. Plastic waste associated with disease on coral reefs. Science. 2018;359:460–462. doi: 10.1126/science.aar3320. [DOI] [PubMed] [Google Scholar]
- 11.Rochman C.M., Browne M.A., Halpern B.S., Hentschel B.T., Hoh E., Karapanagioti H.K., Rios-Mendoza L.M., Takada H., Teh S., Thompson R.C. Policy: Classify plastic waste as hazardous. Nature. 2013;494:169–171. doi: 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- 12.Lebreton L., Slat B., Ferrari F., Sainte-Rose B., Aitken J., Marthouse R., Hajbane S., Cunsolo S., Schwarz A., Levivier A., et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018;8:4666. doi: 10.1038/s41598-018-22939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousif E., Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus. 2013;2:398. doi: 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewert B., Plassmann M.M., MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts. 2015;17:1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- 15.Song Y.K., Hong S.H., Jang M., Han G.M., Jung S.W., Shim W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017;51:4368–4376. doi: 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- 16.Carney Almroth B.M., Astrom L., Roslund S., Petersson H., Johansson M., Persson N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. Int. 2018;25:1191–1199. doi: 10.1007/s11356-017-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kole P.J., Lohr A.J., Van Belleghem F., Ragas A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health. 2017;14:1265. doi: 10.3390/ijerph14101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redondo-Hasselerharm P.E., De Ruijter V.N., Mintenig S.M., Verschoor A., Koelmans A.A. Ingestion and chronic effects of car tire tread particles on freshwater benthic macroinvertebrates. Environ. Sci. Technol. 2018;52:13986–13994. doi: 10.1021/acs.est.8b05035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetu S.G., Sarker I., Schrameyer V., Pickford R., Elbourne L.D.H., Moore L.R., Paulsen I.T. Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean’s most abundant photosynthetic bacteria. Commun. Biol. 2019;2:184. doi: 10.1038/s42003-019-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjollema S.B., Redondo-Hasselerharm P., Leslie H.A., Kraak M.H.S., Vethaak A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016;170:259–261. doi: 10.1016/j.aquatox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Boots B., Russell C.W., Green D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019 doi: 10.1021/acs.est.9b03304. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann M., Wirzberger V., Krumpen T., Lorenz C., Primpke S., Tekman M.B., Gerdts G. High quantities of microplastic in arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 2017;51:11000–11010. doi: 10.1021/acs.est.7b03331. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann M., Mutzel S., Primpke S., Tekman M.B., Trachsel J., Gerdts G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahul Hamid F., Bhatti M.S., Anuar N., Anuar N., Mohan P., Periathamby A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018;36:873–897. doi: 10.1177/0734242X18785730. [DOI] [PubMed] [Google Scholar]
- 25.Schwabl P., Koppel S., Konigshofer P., Bucsics T., Trauner M., Reiberger T., Liebmann B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019 doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- 26.Van Cauwenberghe L., Janssen C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Cho Y., Shim W.J., Jang M., Han G.M., Hong S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019;245:1107–1116. doi: 10.1016/j.envpol.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Qu X., Su L., Zhang W., Yang D., Kolandhasamy P., Li D., Shi H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016;214:177–184. doi: 10.1016/j.envpol.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Green C., Reynolds A., Shi H., Rotchell J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018;241:35–44. doi: 10.1016/j.envpol.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Smith M., Love D.C., Rochman C.M., Neff R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018;5:375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox K.D., Covernton G.A., Davies H.L., Dower J.F., Juanes F., Dudas S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 32.Kniggendorf A.K., Wetzel C., Roth B. Microplastics detection in streaming tap water with raman spectroscopy. Sensors. 2019;19:1839. doi: 10.3390/s19081839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panno S.V., Kelly W.R., Scott J., Zheng W., McNeish R.E., Holm N., Hoellein T.J., Baranski E.L. Microplastic contamination in karst groundwater systems. Ground Water. 2019;57:189–196. doi: 10.1111/gwat.12862. [DOI] [PubMed] [Google Scholar]
- 34.Welle F., Franz R. Microplastic in bottled natural mineral water—Literature review and considerations on exposure and risk assessment. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:2482–2492. doi: 10.1080/19440049.2018.1543957. [DOI] [PubMed] [Google Scholar]
- 35.Mason S.A., Welch V.G., Neratko J. Synthetic polymer contamination in bottled water. Front. Chem. 2018;6:407. doi: 10.3389/fchem.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koelmans A.A., Mohamed Nor N.H., Hermsen E., Kooi M., Mintenig S.M., De France J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019;155:410–422. doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W.C., Tse H.F., Fok L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016;566–567:333–349. doi: 10.1016/j.scitotenv.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 38.Thompson R.C., Swan S.H., Moore C.J., Vom Saal F.S. Our plastic age. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1973–1976. doi: 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S.K., Pal S., Ray S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. Int. 2013;20:4339–4355. doi: 10.1007/s11356-013-1706-x. [DOI] [PubMed] [Google Scholar]
- 40.Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Andrady A.L., Neal M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1977–1984. doi: 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J.T., Huang V.I. Evaluation of the efficiency of medical masks and the creation of new medical masks. J. Int. Med. Res. 2007;35:213–223. doi: 10.1177/147323000703500205. [DOI] [PubMed] [Google Scholar]
- 43.Alimba C.G., Faggio C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019;68:61–74. doi: 10.1016/j.etap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt C., Krauth T., Wagner S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017;51:12246–12253. doi: 10.1021/acs.est.7b02368. [DOI] [PubMed] [Google Scholar]
- 45.Gigault J., Halle A.T., Baudrimont M., Pascal P.Y., Gauffre F., Phi T.L., El Hadri H., Grassl B., Reynaud S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Barnes D.K., Galgani F., Thompson R.C., Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graca B., Szewc K., Zakrzewska D., Dolega A., Szczerbowska-Boruchowska M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea-a preliminary study. Environ. Sci. Pollut. Res. Int. 2017;24:7650–7661. doi: 10.1007/s11356-017-8419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheurer M., Bigalke M. Microplastics in swiss floodplain soils. Environ. Sci. Technol. 2018;52:3591–3598. doi: 10.1021/acs.est.7b06003. [DOI] [PubMed] [Google Scholar]
- 49.Leslie H.A., Brandsma S.H., Van Velzen M.J., Vethaak A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017;101:133–142. doi: 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Lambert S., Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016;145:265–268. doi: 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson A.L., Kawaguchi S., King C.K., Townsend K.A., King R., Huston W.M., Bengtson Nash S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018;9:1001. doi: 10.1038/s41467-018-03465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 53.Salvador Cesa F., Turra A., Baruque-Ramos J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017;598:1116–1129. doi: 10.1016/j.scitotenv.2017.04.172. [DOI] [PubMed] [Google Scholar]
- 54.Lares M., Ncibi M.C., Sillanpaa M., Sillanpaa M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–246. doi: 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 55.Browne M.A., Crump P., Niven S.J., Teuten E., Tonkin A., Galloway T., Thompson R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- 56.Dehghani S., Moore F., Akhbarizadeh R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. Int. 2017;24:20360–20371. doi: 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- 57.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28:2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Setala O., Fleming-Lehtinen V., Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014;185:77–83. doi: 10.1016/j.envpol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Von Moos N., Burkhardt-Holm P., Kohler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. After an experimental exposure. Environ. Sci. Technol. 2012;46:11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 60.Karlsson T.M., Vethaak A.D., Almroth B.C., Ariese F., Van Velzen M., Hassellov M., Leslie H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017;122:403–408. doi: 10.1016/j.marpolbul.2017.06.081. [DOI] [PubMed] [Google Scholar]
- 61.Wesch C., Bredimus K., Paulus M., Klein R. Towards the suitable monitoring of ingestion of microplastics by marine biota: A review. Environ. Pollut. 2016;218:1200–1208. doi: 10.1016/j.envpol.2016.08.076. [DOI] [PubMed] [Google Scholar]
- 62.Hipfner J.M., Galbraith M., Tucker S., Studholme K.R., Domalik A.D., Pearson S.F., Good T.P., Ross P.S., Hodum P. Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs. Environ. Pollut. 2018;239:215–222. doi: 10.1016/j.envpol.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Baalkhuyur F.M., Bin Dohaish E.A., Elhalwagy M.E.A., Alikunhi N.M., AlSuwailem A.M., Rostad A., Coker D.J., Berumen M.L., Duarte C.M. Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 2018;131:407–415. doi: 10.1016/j.marpolbul.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 64.Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M.E., Le Goic N., Quillien V., Mingant C., Epelboin Y., et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA. 2016;113:2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Browne M.A., Dissanayake A., Galloway T.S., Lowe D.M., Thompson R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L) Environ. Sci. Technol. 2008;42:5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 66.De Witte B., Devriese L., Bekaert K., Hoffman S., Vandermeersch G., Cooreman K., Robbens J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014;85:146–155. doi: 10.1016/j.marpolbul.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Li J., Yang D., Li L., Jabeen K., Shi H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015;207:190–195. doi: 10.1016/j.envpol.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Catarino A.I., Macchia V., Sanderson W.G., Thompson R.C., Henry T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018;237:675–684. doi: 10.1016/j.envpol.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 69.Hermabessiere L., Paul-Pont I., Cassone A.L., Himber C., Receveur J., Jezequel R., El Rakwe M., Rinnert E., Riviere G., Lambert C., et al. Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ. Pollut. 2019;250:807–819. doi: 10.1016/j.envpol.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 70.Kosuth M., Mason S.A., Wattenberg E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE. 2018;13:e0194970. doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schymanski D., Goldbeck C., Humpf H.U., Furst P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Liebezeit G., Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31:1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- 73.Iniguez M.E., Conesa J.A., Fullana A. Microplastics in Spanish table salt. Sci. Rep. 2017;7:8620. doi: 10.1038/s41598-017-09128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liebezeit G., Liebezeit E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013;30:2136–2140. doi: 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez L.M., Xu E.G., Larsson H.C.E., Tahara R., Maisuria V.B., Tufenkji N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019 doi: 10.1021/acs.est.9b02540. [DOI] [PubMed] [Google Scholar]
- 76.Prata J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 77.Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 78.Pivokonsky M., Cermakova L., Novotna K., Peer P., Cajthaml T., Janda V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 79.Wilcox C., Puckridge M., Schuyler Q.A., Townsend K., Hardesty B.D. A quantitative analysis linking sea turtle mortality and plastic debris ingestion. Sci. Rep. 2018;8:12536. doi: 10.1038/s41598-018-30038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Franeker J.A., Bravo Rebolledo E.L., Hesse E., LL I.J., Kuhn S., Leopold M., Mielke L. Plastic ingestion by harbour porpoises Phocoena phocoena in the Netherlands: Establishing a standardised method. Ambio. 2018;47:387–397. doi: 10.1007/s13280-017-1002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J., Tan Z., Peng J., Qiu Q., Li M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016;113:7–17. doi: 10.1016/j.marenvres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Besseling E., Wegner A., Foekema E.M., Van den Heuvel-Greve M.J., Koelmans A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.) Environ. Sci. Technol. 2013;47:593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- 83.Brennecke D., Ferreira E.C., Costa T.M., Appel D., Da Gama B.A., Lenz M. Ingested microplastics (>100 mum) are translocated to organs of the tropical fiddler crab Uca rapax. Mar. Pollut. Bull. 2015;96:491–495. doi: 10.1016/j.marpolbul.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Karami A., Romano N., Galloway T., Hamzah H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus) Environ. Res. 2016;151:58–70. doi: 10.1016/j.envres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 85.McGoran A.R., Clark P.F., Morritt D. Presence of microplastic in the digestive tracts of European flounder, Platichthys flesus, and European smelt, Osmerus eperlanus, from the River Thames. Environ. Pollut. 2017;220:744–751. doi: 10.1016/j.envpol.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 86.Vandermeersch G., Van Cauwenberghe L., Janssen C.R., Marques A., Granby K., Fait G., Kotterman M.J., Diogene J., Bekaert K., Robbens J., et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015;143:46–55. doi: 10.1016/j.envres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 87.Jin Y., Lu L., Tu W., Luo T., Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 88.Lu L., Wan Z., Luo T., Fu Z., Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 89.Frias J.P., Gago J., Otero V., Sobral P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016;114:24–30. doi: 10.1016/j.marenvres.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Hours M., Fevotte J., Lafont S., Bergeret A. Cancer mortality in a synthetic spinning plant in Besancon, France. Occup. Environ. Med. 2007;64:575–581. doi: 10.1136/oem.2006.028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallagher L.G., Li W., Ray R.M., Romano M.E., Wernli K.J., Gao D.L., Thomas D.B., Checkoway H. Occupational exposures and risk of stomach and esophageal cancers: Update of a cohort of female textile workers in Shanghai, China. Am. J. Ind. Med. 2015;58:267–275. doi: 10.1002/ajim.22412. [DOI] [PubMed] [Google Scholar]
- 92.Kern D.G., Crausman R.S., Durand K.T., Nayer A., Kuhn C., III. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann. Intern. Med. 1998;129:261–272. doi: 10.7326/0003-4819-129-4-199808150-00001. [DOI] [PubMed] [Google Scholar]
- 93.Turcotte S.E., Chee A., Walsh R., Grant F.C., Liss G.M., Boag A., Forkert L., Munt P.W., Lougheed M.D. Flock worker’s lung disease: Natural history of cases and exposed workers in Kingston, Ontario. Chest. 2013;143:1642–1648. doi: 10.1378/chest.12-0920. [DOI] [PubMed] [Google Scholar]
- 94.Huang N.C., Wann S.R., Chang H.T., Lin S.L., Wang J.S., Guo H.R. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: A hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011;11:142. doi: 10.1186/1471-230X-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vianna N.J., Brady J., Harper P. Angiosarcoma of the liver: A signal lesion of vinyl chloride exposure. Environ. Health Perspect. 1981;41:207–210. doi: 10.1289/ehp.8141207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elliott P., Kleinschmidt I. Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup. Environ. Med. 1997;54:14–18. doi: 10.1136/oem.54.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rochman C.M., Kurobe T., Flores I., Teh S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014;493:656–661. doi: 10.1016/j.scitotenv.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 98.Barboza L.G.A., Vieira L.R., Branco V., Figueiredo N., Carvalho F., Carvalho C., Guilhermino L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758) Aquat. Toxicol. 2018;195:49–57. doi: 10.1016/j.aquatox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Gardon T., Reisser C., Soyez C., Quillien V., Le Moullac G. Microplastics affect energy balance and gametogenesis in the pearl oyster pinctada margaritifera. Environ. Sci. Technol. 2018;52:5277–5286. doi: 10.1021/acs.est.8b00168. [DOI] [PubMed] [Google Scholar]
- 100.Tallec K., Huvet A., Di Poi C., Gonzalez-Fernandez C., Lambert C., Petton B., Le Goic N., Berchel M., Soudant P., Paul-Pont I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018;242:1226–1235. doi: 10.1016/j.envpol.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 101.Pitt J.A., Trevisan R., Massarsky A., Kozal J.S., Levin E.D., Di Giulio R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018;643:324–334. doi: 10.1016/j.scitotenv.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martins A., Guilhermino L. Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci. Total Environ. 2018;631–632:421–428. doi: 10.1016/j.scitotenv.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 103.Liu Z., Yu P., Cai M., Wu D., Zhang M., Huang Y., Zhao Y. Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere. 2019;215:74–81. doi: 10.1016/j.chemosphere.2018.09.176. [DOI] [PubMed] [Google Scholar]
- 104.Haldimann M., Alt A., Blanc A., Brunner K., Sager F., Dudler V. Migration of antimony from PET trays into food simulant and food: Determination of Arrhenius parameters and comparison of predicted and measured migration data. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013;30:587–598. doi: 10.1080/19440049.2012.751631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J., Zhou G., Chen C., Yu H., Wang T., Ma Y., Jia G., Gao Y., Li B., Sun J., et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 106.Poland C.A., Duffin R., Kinloch I., Maynard A., Wallace W.A., Seaton A., Stone V., Brown S., Macnee W., Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 107.Engler R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012;46:12302–12315. doi: 10.1021/es3027105. [DOI] [PubMed] [Google Scholar]
- 108.Huang Y., Mei L., Chen X., Wang Q. Recent developments in food packaging based on nanomaterials. Nanomaterials. 2018;8:830. doi: 10.3390/nano8100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mato Y., Isobe T., Takada H., Kanehiro H., Ohtake C., Kaminuma T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001;35:318–324. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- 110.Rochman C.M., Hoh E., Hentschel B.T., Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013;47:1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- 111.Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 112.Wu B., Wu X., Liu S., Wang Z., Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2cells. Chemosphere. 2019;221:333–341. doi: 10.1016/j.chemosphere.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 113.Brandts I., Teles M., Goncalves A.P., Barreto A., Franco-Martinez L., Tvarijonaviciute A., Martins M.A., Soares A., Tort L., Oliveira M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018;643:775–784. doi: 10.1016/j.scitotenv.2018.06.257. [DOI] [PubMed] [Google Scholar]
- 114.Qu M., Xu K., Li Y., Wong G., Wang D. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci. Total Environ. 2018;643:119–126. doi: 10.1016/j.scitotenv.2018.06.173. [DOI] [PubMed] [Google Scholar]
- 115.Tang J., Ni X., Zhou Z., Wang L., Lin S. Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. Pollut. 2018;243:66–74. doi: 10.1016/j.envpol.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 116.Jeong C.B., Won E.J., Kang H.M., Lee M.C., Hwang D.S., Hwang U.K., Zhou B., Souissi S., Lee S.J., Lee J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (brachionus koreanus) Environ. Sci. Technol. 2016;50:8849–8857. doi: 10.1021/acs.est.6b01441. [DOI] [PubMed] [Google Scholar]
- 117.Lei L., Wu S., Lu S., Liu M., Song Y., Fu Z., Shi H., Raley-Susman K.M., He D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018;619–620:1–8. doi: 10.1016/j.scitotenv.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 118.Lu Y., Zhang Y., Deng Y., Jiang W., Zhao Y., Geng J., Ding L., Ren H. Uptake and accumulation of polystyrene microplastics in zebrafish (danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- 119.Yang Y.F., Chen C.Y., Lu T.H., Liao C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019;366:703–713. doi: 10.1016/j.jhazmat.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 120.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deng Y., Zhang Y., Lemos B., Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brandts I., Teles M., Tvarijonaviciute A., Pereira M.L., Martins M.A., Tort L., Oliveira M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics. 2018;110:435–441. doi: 10.1016/j.ygeno.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 124.Revel M., Yakovenko N., Caley T., Guillet C., Chatel A., Mouneyrac C. Accumulation and immunotoxicity of microplastics in the estuarine worm Hediste diversicolor in environmentally relevant conditions of exposure. Environ. Sci. Pollut. Res. Int. 2018 doi: 10.1007/s11356-018-3497-6. [DOI] [PubMed] [Google Scholar]
- 125.Sleight V.A., Bakir A., Thompson R.C., Henry T.B. Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar. Pollut. Bull. 2017;116:291–297. doi: 10.1016/j.marpolbul.2016.12.055. [DOI] [PubMed] [Google Scholar]
- 126.Jiang X., Chen H., Liao Y., Ye Z., Li M., Klobucar G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019;250:831–838. doi: 10.1016/j.envpol.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 127.Hsu Y.H., Chuang H.C., Lee Y.H., Lin Y.F., Chiu Y.J., Wang Y.L., Wu M.S., Chiu H.W. Induction of fibrosis and autophagy in kidney cells by Vinyl chloride. Cells. 2019;8:601. doi: 10.3390/cells8060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boivin-Angele S., Lefrancois L., Froment O., Spiethoff A., Bogdanffy M.S., Wegener K., Wesch H., Barbin A., Bancel B., Trepo C., et al. Ras gene mutations in vinyl chloride-induced liver tumours are carcinogen-specific but vary with cell type and species. Int. J. Cancer. 2000;85:223–227. doi: 10.1002/(SICI)1097-0215(20000115)85:2<223::AID-IJC12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 129.Weihrauch M., Benick M., Lehner G., Wittekind M., Bader M., Wrbitzk R., Tannapfel A. High prevalence of K-ras-2 mutations in hepatocellular carcinomas in workers exposed to vinyl chloride. Int. Arch. Occup. Environ. Health. 2001;74:405–410. doi: 10.1007/s004200100244. [DOI] [PubMed] [Google Scholar]
- 130.Barbin A., Froment O., Boivin S., Marion M.J., Belpoggi F., Maltoni C., Montesano R. P53 gene mutation pattern in rat liver tumors induced by vinyl chloride. Cancer Res. 1997;57:1695–1698. [PubMed] [Google Scholar]
- 131.Hollstein M., Marion M.J., Lehman T., Welsh J., Harris C.C., Martel-Planche G., Kusters I., Montesano R. P53 mutations at A:T base pairs in angiosarcomas of vinyl chloride-exposed factory workers. Carcinogenesis. 1994;15:1–3. doi: 10.1093/carcin/15.1.1. [DOI] [PubMed] [Google Scholar]
- 132.De Vivo I., Marion M.J., Smith S.J., Carney W.P., Brandt-Rauf P.W. Mutant c-Ki-ras p21 protein in chemical carcinogenesis in humans exposed to vinyl chloride. Cancer Causes Control. 1994;5:273–278. doi: 10.1007/BF01830248. [DOI] [PubMed] [Google Scholar]
- 133.Loulad S., Houssa R., Rhinane H., Boumaaz A., Benazzouz A. Spatial distribution of marine debris on the seafloor of Moroccan waters. Mar. Pollut. Bull. 2017;124:303–313. doi: 10.1016/j.marpolbul.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 134.Xanthos D., Walker T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017;118:17–26. doi: 10.1016/j.marpolbul.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 135.Africa Is on the Right Path to Eradicate Plastics. [(accessed on 4 February 2020)]; Available online: https://www.unenvironment.org/news-and-stories/story/africa-right-path-eradicate-plastics.
- 136.A Floating Device Created to Clean Up Plastic from the Ocean Is Finally Doing Its Job, Organizers Say. [(accessed on 4 February 2020)]; Available online: https://edition.cnn.com/2019/10/02/tech/ocean-cleanup-catching-plastic-scn-trnd/index.html.
- 137.Ziajahromi S., Neale P.A., Rintoul L., Leusch F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017;112:93–99. doi: 10.1016/j.watres.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 138.Mason S.A., Garneau D., Sutton R., Chu Y., Ehmann K., Barnes J., Fink P., Papazissimos D., Rogers D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016;218:1045–1054. doi: 10.1016/j.envpol.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 139.Murphy F., Ewins C., Carbonnier F., Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;50:5800–5808. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- 140.Cheap Drainage Nets Keep Water Pollution at Bay in Australia. [(accessed on 4 February 2020)]; Available online: https://inhabitat.com/cheap-drainage-nets-keep-water-pollution-at-bay-in-australia/
- 141.Single-Use Plastic Ban: What Is Single-Use Plastic, Which Plastic Items Will Be Banned on October 2? [(accessed on 4 February 2020)]; Available online: https://www.jagranjosh.com/current-affairs/current-affairs-august-2019-what-is-singleuse-plastic-and-why-is-it-being-banned-1567074897-1.
- 142.Vietnam’s Wild Grass Straws. [(accessed on 4 February 2020)]; Available online: https://theaseanpost.com/article/vietnams-wild-grass-straws.
- 143.Taiwanese Entrepreneurs Patent Sugarcane Straws. [(accessed on 4 February 2020)]; Available online: https://www.taiwannews.com.tw/en/news/3477607.
- 144.Microbeads Are Officially Banned in Cosmetics from Today. Here’s What to Use Instead. [(accessed on 4 February 2020)]; Available online: https://www.telegraph.co.uk/beauty/skin/the-best-face-and-body-scrubs-without-plastic-microbeads/
- 145.A Road Full of Bottlenecks: Dutch Cycle Path Is Made of Plastic Waste. [(accessed on 4 February 2020)]; Available online: https://www.theguardian.com/environment/2018/sep/13/a-road-full-of-bottlenecks-dutch-cycle-path-is-made-of-plastic-waste.
- 146.Lockerbie Plastic Roads Firm MacRebur Opens First Factory. [(accessed on 4 February 2020)]; Available online: https://www.bbc.com/news/uk-scotland-south-scotland-47454719.
- 147.Project in India Is Turning Discarded Fishing Nets into Surfboards to Reduce Ocean Plastic. [(accessed on 4 February 2020)]; Available online: https://www.mentalfloss.com/article/595467/project-india-fishing-nets-surfboards-reduce-ocean-plastic.
- 148.Student Designs Sustainable Plastic Alternative Made of Fish Skin and Algae. [(accessed on 4 February 2020)]; Available online: http://www.ladbible.com/news/uk-student-designs-sustainable-plastic-alternative-made-using-fish-skin-20190605.
- 149.Scientist in Mexico Creates Biodegradable Plastic from Prickly Pear Cactus. [(accessed on 4 February 2020)]; Available online: https://www.forbes.com/sites/scottsnowden/2019/07/14/scientist-in-mexico-creates-biodegradable-plastic-from-prickly-pear-cactus/#2dca0dc76c49.
- 150.Urbanek A.K., Rymowicz W., Mironczuk A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018;102:7669–7678. doi: 10.1007/s00253-018-9195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brandon A.M., Gao S.H., Tian R., Ning D., Yang S.S., Zhou J., Wu W.M., Criddle C.S. Biodegradation of polyethylene and plastic mixtures in mealworms (Larvae of tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018;52:6526–6533. doi: 10.1021/acs.est.8b02301. [DOI] [PubMed] [Google Scholar]
- 152.Papadopoulou A., Hecht K., Buller R. Enzymatic PET degradation. Chimia (Aarau) 2019;73:743–749. doi: 10.2533/chimia.2019.743. [DOI] [PubMed] [Google Scholar]