Abstract
Maternal obesity during pregnancy is a now a public health burden that may be the culprit underlying the ever-increasing rates of adult obesity worldwide. Understanding the association between maternal obesity and adult offspring’s obesity would inform policy and practice regarding offspring health through available resources and interventions. This review first summarizes the programming effects of maternal obesity and discusses the possible underlying mechanisms. We then summarize the current evidence suggesting that maternal consumption of resveratrol is helpful in maternal obesity and alleviates its consequences. In conclusion, maternal obesity can program offspring development in an adverse way. Maternal resveratrol could be considered as a potential regimen in reprogramming adverse outcomes in the context of maternal obesity.
Keywords: maternal obesity, resveratrol, programming, reprogramming
1. Introduction
Obesity is a common metabolic disorder and is prevalent worldwide [1]. According to the World Health Organization, nearly 39% of adults aged 18 years and over were overweight in 2016, and 13% were obese [2]. In the United States, the prevalence of obese women aged 20–39 years old tripled between 1960 and 2000 [3]. Likewise, in the UK, between 1990 and 2004, the body mass index (BMI) of women with singleton pregnancies at their first prenatal booking increased by an average of 1.37 kg/m2 and the prevalence of obesity tripled [4]. Based on the US Institute of Medicine guidelines, approximately 40% of women gain an excessive amount of weight during pregnancy in Western countries [5]. Maternal obesity is also linked with individual’s socioeconomic position and environmental determinants of health [6]. Maternal obesity during pregnancy includes pre-pregnancy obesity and excessive gestational weight gain. Both pre-pregnancy maternal obesity and excessive gestational weight gain increase the placental transfer of nutrients to the developing fetus and affect fetal development. Maternal obesity during pregnancy represents significant health risks to both the mother and the baby, and is associated with an increased risk of miscarriage, gestational diabetes, stillbirth, preeclampsia leading to preterm birth, and Cesarean delivery [7]. The works cited in this review were identified eligible studies and reviews through manual searches related to maternal obesity. It is well-known that pre-pregnancy maternal obesity and excessive gestational weight gain have different mechanisms and clinical implications. In this manuscript, we use the term maternal obesity to mean maternal obesity during pregnancy.
2. Long-Term Public Health Issues of Maternal Obesity
Epidemiological and experimental studies provide evidence of a persistent and deleterious effect of maternal obesity on their offspring, supporting the hypothesis of intrauterine programming [8]. Clinical and animal studies have shown a maternal obesogenic environment, i.e., during the periods of periconception, pregnancy, or lactation, increasing the risk for the development of obesity and related cardiometabolic [9,10] and neurodevelopmental disorders [11] in offspring in adulthood. The rise in obesity prevalence is partly due to increase in pre-existing type 2 diabetes among pregnant women. Besides, maternal obesity is strongly linked with gestational diabetes [12]. Maternal obesity is a growing problem in both the developed and developing world because it can lead to a cyclical transgenerational transmission of obesity. Understanding the associations between maternal obesity and offspring health would inform public health policy, practice, and early intervention.
3. Specific Mechanisms Underlying How Maternal Obesity Programs Offspring
Growing evidence suggests that developmental programming is noted in obese mothers’ offspring [13]. Below are some major underlying mechanisms of how maternal obesity exerts its effects on the placenta, fetus, and offspring development.
3.1. Epigenome
Epigenetics is defined as mechanisms of long-term stable regulation of gene expression that do not involve a change in the DNA sequence. Epigenetic mechanisms include DNA methylation, histone modification such as acetylation, sumoylation, ubiquitination, and methylation, and non-coding RNAs.
In mammals, early embryogenesis is a critical period for the establishment of the epigenome [14]. Maternal obesity may influence the placenta and offspring epigenetic landscape [15,16]. Ge et al. found that DNA methylation in differential methylation regions of Peg3 is altered in spermatozoa of offspring from obese mothers, but is not affected in spermatozoa of offspring from diabetic mothers [17]. Epigenetic changes in the fetus as a consequence of in utero exposure to maternal obesity-related factors could have persistent effects and cause metabolic abnormalities at later ages.
3.2. Gut-Brain Axis
The bidirectional communication system, mediated by hormonal, immunological, and neural signals, between the gut and the brain constitutes the gut-brain axis. Pregnant mothers’ gut microbiota undergo dynamic compositional changes during gestation [18]. Inadequate nutrition during pregnancy has been related to altered maternal microbiota [19]. A human study showed that shifts in the gut microbiota during pregnancy are sensitive to the maternal pre-pregnancy BMI, as well as to weight gain during pregnancy [20]. In a prospective follow-up study of 256 women, infants’ fecal microbial composition was related to the weight and weight gain of their mothers during pregnancy. Infants of obese and high gestational weight gain women demonstrate lower concentrations of the genus Bifidobacterium, considered protective bacteria, and higher concentrations of proinflammatory bacteria, including Bacterioides, Clostridium, and Staphylococcus [21]. Similarly, in rodents, consumption of a high-fat diet (HFD) or a Western diet prior to and during pregnancy could alter the trajectory of maternal and offspring microbiota [22]. Putative mechanisms of dysbiosis that could lead to obesity might be due to increased energy extraction and storage from ingested nutrients [23].
3.3. Inflammation
Pregnancy itself involves a state of mild maternal systemic inflammation, with the placenta also actively produces a variety of immunomodulatory hormones and cytokines [24,25]. In humans, maternal systemic and placental inflammation has been observed in pregnancies complicated by obesity [26]. Maternal obesity leads to placental inflammation and increased cytokine production in human [27,28], rodent [29], and ovine models [30], including elevation of interleukin 6, interleukin 1β, and tumor necrosis factor-α production [28,30], as well as an increase in infiltrating monocytes and activated macrophages [27,28]. It has been shown that maternal inflammatory mediators during pregnancy correlate with fetal adiposity and neonatal fat mass [31]. Maternal obesity-mediated inflammation and placental-mediated inflammation may interact with each other, thus altering fetal development [32].
3.4. Mitochondrial Function
Mitochondria play an important role in metabolism and provide the principal energy source of the cell. Mitochondrial dysfunction has been identified in early embryogenesis in obese mothers. Igosheva et al. showed that maternal obesity prior to conception is associated with altered mitochondria in mouse oocytes and zygotes. Mouse oocytes and embryos of obese dams have increased mitochondrial membrane potential, higher levels of oxidative phosphorylation, and increased reactive oxygen species production compared to those of lean mothers. mtDNA copy number is also increased in response to oxidative damage [33].
3.5. Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) is a target gene of miR-210. Maternal obesity could lead to increased levels of miRNA-210 and decreased levels of BDNF mRNA in placentas from female fetuses, and decreased proBDNF in placentas from male fetuses. In addition, maternal obesity adversely affects BDNF/tropomyosin receptor kinase B (TRKB) signaling in the placenta in a sexually dimorphic manner [34]. Recently, Fusco et al. showed maternal HFD-dependent insulin resistance downregulates BDNF and insulin signaling in maternal tissues and inhibits BDNF expression in both the germline and hippocampus of progeny [35]. The role of BDNF in developmental programming is highlighted by Briana et al. [36].
4. Programming Effects of Maternal Obesity Across Life-Span
4.1. Placental Alterations
The placenta is an important organ vital for fetal growth; compromised function is associated with abnormal fetus development. The link between in utero and later adult cardiovascular disease was first noticed by Barker et al. in an epidemiological study [37].
The placenta is both a target and producer of inflammatory mediators in the context of maternal obesity [25]. Maternal obesity leads to altered nutrient handling [38], energy modulation [39], and reduced angiogenesis. In humans, maternal obesity causes reduced chorionic plate artery function, which may reduce blood supply to the fetus and place the fetus at risk [40]. Rodent models of maternal obesity report decreased layer thickness, reduced trophoblast proliferation, increased macrophage activation, elevated cytokine gene expression, altered vascular development, and resultant hypoxia in the labyrinth [27,41].
4.2. Fetal Alterations
Fetuses of obese mothers are at increased risk of macrosomia and intrauterine growth retardation [38]. A previous study examined the fetal brain of term fetuses of obese rats fed a HFD. This study points to increased inflammation and oxidative stress in fetal brains of obese dams and dysregulation of monoamine neurotransmitter and hypothalamic orexigenic signaling. In immunohistochemical analysis, developmental alterations in the hypothalamic and extra-hypothalamic regions in the fetal brain from HFD dams are suggestive of a predisposition for the development of obesity and possibly neurodevelopmental abnormalities in offspring [42]. In a sheep study, fetal hearts from dams with maternal obesity display a normal cardiac contractile function during basal perfusion. However, they develop an impaired heart-rate-left-ventricular-developed pressure product following high workload stress [43]. Maternal obesity also affects adipose tissue development and differentiation during the fetal period [44,45].
4.3. Young Offspring Alterations
Infants born to overweight and obese mothers are more likely to be large for their gestational age and macrosomic [46]. Recently, a meta-analysis revealed a 264% increase in the odds of child obesity when mothers have obesity before conception [47]. In a rodent model, offspring from rats fed a diet with 50% fat calories from embryonic day 6 to postnatal day 15 are obese at 70 days of age [48]. During lactation, on postnatal day 15, the pups show an increase in the expression of 5 orexigenic peptides, both in the paraventricular nucleus (PVN) and in the parafornical lateral hypothalamus. Conversely, the expression of these orexigenic peptides is downregulated in the arcuate nucleus (ARC) [48]. The authors showed that perinatal HFD exposure reveals changes in the offspring hypothalamus that became apparent at preweaning [48]. Of note, other factors such as maternal education, socioeconomic position, early childhood environment and nutrition are also crucial determinants of early childhood development [49].
4.4. Adult Offspring Alterations
Epidemiological and experimental studies show that maternal obesity is associated with increased susceptibility to hepatic steatosis and inflammation [50], hypertension and cardiovascular disorders [51], kidney disease [52], neurodevelopmental disorders [42] as well as obesity and insulin resistance [53] in offspring at different stages of development. Below, we summarize the programming effects in a few main organs in the context of maternal obesity.
4.4.1. Brain
Mounting evidence from both prenatal and lactational human epidemiologic and animal studies suggests that exposure to maternal obesity and a HFD are associated with neurodevelopmental and psychiatric disorders in offspring. These disorders include cognitive impairment, autism spectrum disorders, attention deficit hyperactivity disorder, mood disorders, schizophrenia, and eating disorders [11].
The hypothalamus plays a critical role in energy homeostasis by regulating both appetite and energy expenditure. Hypothalamic ARC is a putative site of appetite regulation and translates peripheral signals to orexigenic agouti-related peptide (AgRP) or anorexigenic pro-opiomelanocortin (POMC)-expressing neurons. Desai et al. showed that adult rat offspring born to high-fat fed dams are obese at six months of age. They demonstrated that maternal obesity programs the offspring hypothalamic ARC, resulting in enhanced activity in AgRP neurons relative to POMC (pro-opiomelanocortin) neurons and hyperphagia [54]. Prior studies also showed maternal obesity/overnutrition programs offspring hyperphagia via appetite/satiety gene expression [48,55].
The hippocampus is critical to learning and memory and is especially susceptible to early-life nutritional insults. Tozuka et al. reported that mouse pups from dams fed with a HFD show peroxidized lipid accumulation in many brain regions, impaired adult neurogenesis in the hippocampus, and deficits in spatial learning performance [56,57]. Impaired hippocampal BDNF expression in the context of maternal obesity has been linked to deficits in spatial learning and memory in juvenile and adult offspring [57,58]. It is conceivable that the deleterious effects of maternal obesity on offspring learning and memory may be mediated by alterations of BDNF-mediated synaptic plasticity.
4.4.2. Liver
Animal models have demonstrated that offspring of diet-induced obese dams develop metabolic complications, including nonalcoholic-fatty liver disease (NAFLD) [50]. Offspring of maternal obesity have increased liver weight and lipid accumulation. Additionally, upregulated sterol regulatory element-binding protein 1 and 20 downstream genes involved in hepatic lipid biosynthesis and reduced expression of peroxisome proliferator-activated receptor alpha (PPARα) and related genes involved in fatty acid oxidation are involved in the pathogenesis of NAFLD in the context of maternal obesity [59].
In a mouse model of maternal obesity, offspring develop NAFLD prior to any differences in body weight or body composition. Offspring of obese dams have higher liver lipid content, as well as increased levels of peroxisome proliferator-activated receptor gamma (PPARγ) and reduced levels of triglyceride lipase. Increased liver glycogen and protein content are also observed in offspring of obese dams [50].
4.4.3. Kidney
Armitage et al. intended to obtain better insight into hypertension in offspring from maternally obese dams. They found renal stereology showed no differences in kidney weight, glomerular number, or volume in adult rat offspring from dams fed a lard-rich diet compared with dams fed a control diet. However, renal renin and Na+, K+-ATPase activity are significantly reduced in adult offspring of maternally obese dams compared with controls [60].
In a mouse model of maternal obesity, the renal function of offspring from obese mothers was considerably worse compared to offspring from non-obese mothers, especially when they developed diabetes. Glastras et al. found that adult male mice offspring of obese mothers have increased renal fibrosis, inflammation, and oxidative stress. Furthermore, offspring exposed to maternal obesity have increased susceptibility to renal damage when a second hit of streptozotocin is imposed. The authors proposed that maternal obesity should be considered a risk factor for chronic kidney disease [52].
4.4.4. Cardiovascular System
The increased rates of hypertension in modern society may have resulted from the nutritional environment during early life. A population-based birth cohort of 2432 Australians showed that greater maternal gestational weight gain is independent from maternal pre-pregnancy body mass index, associates with a higher body mass index and tends to be associated with a higher systolic blood pressure in the offspring at age 21 of years [61]. Armitage et al. studied hypertension in offspring of maternally obese mothers. Using aorta stereology, they show increased aortic stiffness and reduced endothelium-dependent relaxation in adult offspring of maternally obese dams compared with controls [60]. Recently, a study using birth records from 37,709 participants showed that a higher maternal body mass index at the first antenatal visit is associated with an increased risk of premature death and hospital admissions for cardiovascular events in adult offspring. The offspring of overweight mothers also carried a higher risk of adverse outcomes compared to offspring of mothers with a normal BMI [62].
Maternal obesity and/or HFD exposure may predispose offspring to a higher prevalence of postnatal fat diet-induced metabolic diseases including cardiovascular complications later on in adult life [9].
4.4.5. Adipose Tissue
Maternal obesity affects adipocyte development and alters functional properties of adipocytes from the fetal period [44] to adulthood [63], resulting in higher white adipose tissue (WAT) mass and larger adipocytes [64]. Upregulated PPARγ, whose activity is enhanced under limited or excess nutrient availability, is one of the important features of enhanced adipogenesis and fat expansion in programmed offspring of obese dams [44]. This is associated with downregulation of PPARγ corepressors [65].
5. Reprogramming the Adversities Encountered in Maternal Obesity
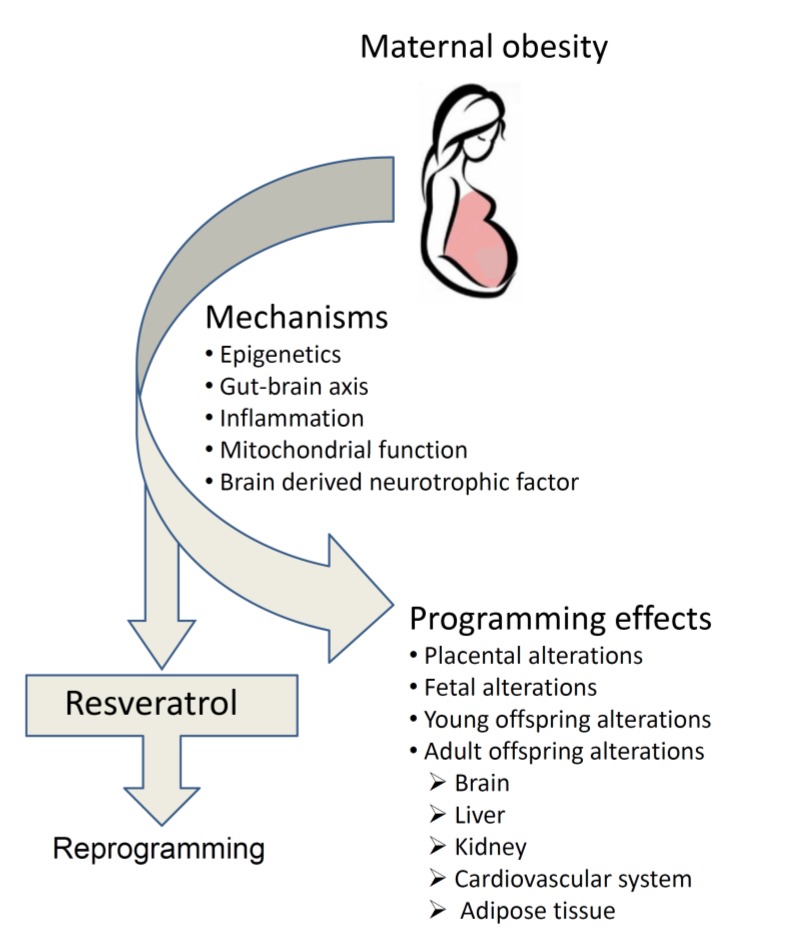
Reprogramming strategies refers to maneuvers to reverse malprogrammed development to resume normal development [66,67]. These strategies could be nutritional interventions, lifestyle modifications, or pharmacological interventions [68]. A mechanistic understanding of how the maternal intrauterine and lactational environment may be mediating offspring common morbidities is critical, because early life may represent an important window for targeted intervention to ameliorate fetal and offspring risk [66]. A previous review suggests that early-life resveratrol supplementation could be considered as a reprogramming strategy against the development of metabolic syndrome-related disorders [67]. Supplementing with certain classes of nutrients in gestation is helpful in reprogramming of maternal adversities. Resveratrol has powerful anti-oxidant and anti-inflammatory activities. Resveratrol can cross placenta and seems to have no obvious toxicity in humans. Below, we discuss the safety profile of resveratrol taken during pregnancy and its possible reprogramming potential in the context of maternal obesity. Figure 1 depicts the programming effects of maternal obesity and the reprogramming effects of maternal resveratrol intake.
Figure 1.
Programming effects and underlying mechanisms of maternal obesity and reprogramming effects of maternal resveratrol intake.
6. Resveratrol Safety Profiles
Resveratrol (trans-3,5,4’-trihydroxystilbene) is a phenolic compound with powerful anti-oxidant activity, which is found in various plants such as grapes and berries [69]. In humans, resveratrol has been reported to be safe at doses up to 5 g per day [70]; however, trans-resveratrol at a dose of 2000 mg twice daily resulted in diarrhea and clinically irrelevant changes of serum potassium and total bilirubin concentrations in healthy volunteers [71].
In a comprehensive study of the safety profile of resveratrol, a 28-day study of resveratrol causes no adverse effects in rats at 50, 150, and 500 mg/kg/day after 28 days of gavage intake. In addition, resveratrol did not cause any adverse effects in rats at up to 700 mg/kg/day after 90 days. In terms of reproductive toxicity assays, 750 mg/kg/day of resveratrol did not induce any adverse reproductive effects in an embryo-fetal study [72].
7. Maternal Resveratrol Administration in the Context of Maternal Adversities
Maternal resveratrol intake has been shown to alleviate impaired hippocampal neurogenesis and increase hippocampal BDNF expression in offspring of prenatally stressed dams [73]. Similarly, maternal resveratrol supplementation prevents prenatal stress-induced cognitive spatial deficits accompanied by enhanced hippocampal mitochondrial function and reduced oxidative stress [74]. Care et al. showed that maternal resveratrol supplementation during pregnancy and lactation ameliorated the development of hypertension in adult offspring [75]. Likewise, Chen et al. reported that maternal resveratrol intake during pregnancy and lactation could alleviate programmed hypertension induced by combined maternal NG-nitro-L-arginine-methyl ester treatment and postnatal HFD [76]. In preeclampsia patients, both the time and dose of blood pressure control are significantly reduced in those who received resveratrol supplementation [77]. Recently, Ding et al. scrutinized the potential use of resveratrol in adverse human pregnancies [78].
8. Possible Beneficial Mechanisms of Maternal Resveratrol Administration in the Context of Maternal Obesity
The beneficial effects of maternal intake of resveratrol on reprogramming might be via its actions on the pregnant mother, placenta, or fetus. Resveratrol could influence placental function through its anti-inflammatory [79] and anti-oxidant activities [80]. From the perspective of the fetus, resveratrol can cross the placenta and affect the fetus directly [81]. From the maternal perspective, resveratrol has been shown to protect against HFD-induced obesity in animal studies [82,83] and exerts health benefits in obese individuals [84].
It was reported that resveratrol is able to modulate methylation and acetylation of lysine 9 of histone H3 in zygotic pronuclei [85]. These changes in zygotes may lead to more successful preimplantation embryo development. Likewise, pregnant mice supplemented with quercetin, a flavonoid, show significantly enhanced expression of Cyp1a1, Cyp1b1, Nqo1, and Ugt1a6 in mouse fetal liver tissues at gestational day 14.5, which persisted into adulthood in a tissue- and gender-dependent manner [86].
Resveratrol could affect gut microbiota and their metabolic products, such as short chain fatty acids and intraluminal lipids, and hence is helpful in the context of obesity and its associated conditions [87,88].
Anti-obesity activity of resveratrol has been reported in laboratory animal models, including mice [89], rats [90], primates [91], and humans [92]. The attributed pathways occur in the WAT and include adipogenesis, lipolysis, and lipogenesis [93]. Resveratrol may act as a fat browning activator, leading to enhanced glucose homeostasis and weight loss, and involvement in the secretion of many myokines and adipokines [82]. It is conceivable that resveratrol might reduce maternal obesity-mediated inflammation.
Resveratrol can modulate mitochondrial ultra-structure, function, and dynamics, and cause alterations in mitochondrial physiology [94]. Resveratrol has beneficial effects in mitochondrial function in an ex vivo study performed in muscle fibers from HFD mice. Resveratrol induces PGC-1α activity by facilitating sirtuin 1-mediated deacetylation [89]. It is cautioned that resveratrol may cause mitotoxicity in the human placental explant [95].
Resveratrol may have antidepressant-like effects in stressed rats, mediated in part by the up-regulation of BDNF levels in the hippocampus and amygdala [96]. Maternal BDNF may reach the fetal brain through the utero-placental barrier [97]. To the best of our knowledge, there are no reports in the literature on the effects of resveratrol on the embryonic expression of BDNF.
Current trends include improved management during pregnancy, such as approaches to reduce BMI before conception in women of reproductive age and increased attention to postpartum weight management and vigorous maternal glycemic control. A behavioral intervention with diet and physical activity in obese mothers is insufficient to reduce the incidence of fetal macrosomia or to prevent the occurrence of gestational diabetes mellitus [98]. Since maternal obesity can induce negative influences in offspring beginning from the embryo through development, the potential implication of maternal resveratrol administration in reprogramming is worth investigation [99].
In Grove’s laboratory, they studied pregnant Japanese macaques fed a Western-style diet (36% fat) supplemented with 0.37% resveratrol throughout pregnancy. Resveratrol use during pregnancy yields improvements in the maternal and placental phenotype with beneficial effects in the fetal liver. However, the fetal pancreatic mass was enlarged by 42%, with a 12-fold increase in Ki67 immunohistochemistry in the exocrine compartment of the pancreas. Thus, the authors cautioned against the use of resveratrol by pregnant women [100]. Additionally, the same authors found that consumption of a Western-style diet resulted in impaired fetal islet capillary density and sympathetic islet innervation, suggesting a novel mechanism by which maternal Western-style diet consumption leads to increased susceptibility to type 2 diabetes in offspring. Resveratrol mitigated maternal diet-induced harmful effects in the pancreas in the fetus and juvenile offspring. However, resveratrol supplementation caused pancreas hypervascularization compared with controls. Thus, the authors again stated that the use of resveratrol in pregnancy required caution [101].
In a mouse model, Zou et al. found that maternal HFD intake during pregnancy and lactation impairs the development of offspring brown adipose tissue (BAT) and beige adipocytes at weaning and has persistent effects on the metabolic health of adult offspring. Importantly, administration of 0.2% (W/W) maternal resveratrol promotes a thermogenic program in BAT and WAT during early offspring development, and induces beige adipocyte development of WAT in adult male offspring. In addition, maternal resveratrol intervention protects offspring against HFD-induced obesity [102].
Ros et al. administered resveratrol in the drinking water (50 mg/L) of Wistar rats fed a high-fat diet (fat; 61.6%) during pregnancy and lactation. Resveratrol did not affect the body weight, visceral fat mass, or serum leptin levels in pregnant/lactating dams. Resveratrol intake during pregnancy and lactation decreases body weight, adipose tissue, lipid profiles, and serum leptin levels at weaning in offspring from dams on a HFD [103].
One clinical study performed in overweight pregnant women found that 80 mg of resveratrol shows significantly improved the lipid and glucose parameters compared to controls after 30 or 60 days of treatment [104]. Table 1 summarizes the current available clinical and animal studies performed with maternal administration of resveratrol for the intervention of maternal obesity/overweight.
Table 1.
Clinical and animal studies of maternal obesity/overweight treated with maternal resveratrol.
| Gender/Species | Diet Module Obesity | Dose and Period of Resveratrol Supplementation | Maternal Obesity/Over-Weight Offspring Obesity Group Size | Age at Evaluation Group Size | Major Beneficial Findings in Offspring | Reference |
|---|---|---|---|---|---|---|
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/−N = 6 | Gestational day 130 | Restored the loss of fetal islet vascularization | [101] |
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/−N = 6 | Gestational day 130 | 30% maternal weight loss, increased uterine artery blood flow, decreased placenta inflammation, reduced fetal liver triglyceride deposition | [100] |
| Male and female Wistar rats | Maternal high-fat diet (61.6%) | Resveratrol (50 mg/L) in drinking water during pregnancy and lactation | +/+N = 4–6 | 3 weeks | Attenuated hyperglycemia, obesity and hyperlipidemia. Resveratrol reduced body weight, leptin, visceral adipose tissue, and subcutaneous adipose tissue, with females being more affected | [102] |
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/− N = 6 | Gestational day 130 N = 5–10 | Resveratrol stimulated placental DHA uptake activity, AMPK activation, and transporter expression | [95] |
| Male C57BL/6 J mice | Maternal plus postnatal high-fat diet for 11 weeks | 0.2% w/w (~200 mg/kg/day) resveratrol in diet during pregnancy and lactation | +/+ N = 10 | 14 weeks | Promotes beige adipocyte development in offspring white adipose tissue. Protects offspring against high-fat diet-induced obesity | [101] |
| Italy | Human | Resveratrol 80mg/day | +/N 110 pregnant women | 30 days and 60 days | Supplementation of resveratrol to DCI/MI improves mother glucose and lipid control | [103] |
9. Conclusions
Public health approaches to reduce obesity epidemic is a top priority [105]. The transmission of the altered phenotypes to the subsequent generations in the context of maternal obesity should not be overlooked. Hence, this seems to be one of the very important tasks of preventive medicine in the current practice.
The future tasks will be to translate this mechanistic understanding into prevention and directed interventions aimed at reducing the burden of maternal obesity. Approaches to reduce BMI before conception in women of reproductive age and increased attention to postpartum weight management and vigorous maternal glycemic control are encouraged. Resveratrol has a lower intestinal absorption rate and poor solubility. Therefore, the future task is the development of a resveratrol formulation with better pharmacokinetic and pharmacodynamic properties. Clinical trials aiming to determine the ideal dosage and therapeutic window of resveratrol as the reprogramming therapy are needed. In addition, the potential role of resveratrol in reprogramming the adversities of maternal obesity worth further research.
Acknowledgments
This work was funded by Chang Gung Memorial Hospital, Kaohsiung, Taiwan (CMRPG8J0861) to Dr. L.-T. Huang and (CMRPG8K0561) to Mei-Hsin Hsu.
Author Contributions
M.-H.H., Y.-C.C., and J.-M.S. wrote the draft manuscript. L.-T.H. edited and wrote the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Afshin A., Reitsma M.B., Murray C.J.L. Health effects of overweight and obesity in 195 countries. N. Engl. J. Med. 2017;377:1496–1497. doi: 10.1056/NEJMc1710026. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Obesity and Overweight: Media Center. WHO; Geneva, Switzerland: 2013. [Google Scholar]
- 3.Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L. Prevalence and trends in obesity among US adults 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Kanagalingam M.G., Forouhi N.G., Greer I.A., Sattar N. Changes in booking body mass index over a decade: Retrospective analysis from a Glasgow Maternity Hospital. BJOG. 2005;112:1431–1433. doi: 10.1111/j.1471-0528.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 5.Yaktine K.M., Editors A.L. Weight Gain during Pregnancy: Reexamining the Guidelines. National Academies Press; Cambridge, MA, USA: 2009. [PubMed] [Google Scholar]
- 6.Bann D., Cooper R., Wills A.K., Adams J., Kuh D. NSHD scientific and data collection team. Socioeconomic position across life and body composition in early old age: Findings from a British birth cohort study. J. Epidemiol. Community Health. 2014;68:516–523. doi: 10.1136/jech-2013-203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galtier-Dereure F., Boegner C., Bringer J. Obesity and pregnancy: Complications and cost. Am. J. Clin. Nutr. 2000;71:1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Twinn D.S., Hjort L., Novakovic B., Ozanne S.E., Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62:1789–1801. doi: 10.1007/s00125-019-4951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong M., Zheng Q., Ford S.P., Nathanielsz P.W., Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J. Mol. Cell Cardiol. 2013;55:111–116. doi: 10.1016/j.yjmcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015;30:1141–1152. doi: 10.1007/s10654-015-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlow A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017;37:95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchi J., Berg M., Dencker A., Olander E.K., Begley C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015;16:621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 13.Iozzo P., Holmes M., Schmidt M.V., Cirulli F., Guzzardi M.A., Berry A., Balsevich G., Andreassi M.G., Wesselink J.J., Liistro T., et al. Developmental ORIgins of healthy and unhealthy ageiNg: The role of maternal obesity-introduction to DORIAN. Obes. Facts. 2014;7:130–151. doi: 10.1159/000362656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiménez-Chillarón J.C., Díaz R., Martínez D., Pentinat T., Ramón-Krauel M., Ribó S. The role of nutrition on epigenetic modifications and their implications on health. Biochimie. 2012;94:2242–2263. doi: 10.1016/j.biochi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Martin C.L., Jima D., Sharp G.C., McCullough L.E., Park S.S., Gowdy K.M., Skaar D., Cowley M., Maguire R.L., Fuemmeler B., et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: An epigenome-wide association study. Epigenetics. 2019;14:325–340. doi: 10.1080/15592294.2019.1581594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogues P., Dos Santos E., Jammes H., Berveiller P., Arnould L., Vialard F., Dieudonné M.N. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin. Epigenetics. 2019;11:1–18. doi: 10.1186/s13148-019-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge Z.J., Liang Q.X., Hou Y., Han Z.M., Schatten H., Sun Q.Y., Zhang C.L. Maternal obesity and diabetes may cause DNA methylation alteration in the spermatozoa of offspring in mice. Reprod. Biol. Endocrinol. 2014;12:29. doi: 10.1186/1477-7827-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koren O., Goodrich J.K., Cullende R.T.C., Spor A., Laitinen K., Bäckhed H.K. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zacarías M.F., Collado M.C., Gómez-Gallego C., Flinck H., Aittoniemi J., Isolauri E. Pregestational overweight and obesity are associated with differences in gut microbiota composition and systemic inflammation in the third trimester. PLoS ONE. 2018;13:e0200305. doi: 10.1371/journal.pone.0200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santacruz A. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 21.Collado M.C., Isolauri E., Laitinen K., Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010;92:1023–1030. doi: 10.3945/ajcn.2010.29877. [DOI] [PubMed] [Google Scholar]
- 22.Steegenga W.T., Mischke M., Lute C., Boekschoten M.V., Lendvai A., Pruis M.G. Maternal exposure to a Western-style diet causes differences in intestinal microbiota composition and gene expression of suckling mouse pups. Mol. Nutr. Food Res. 2017;61:1. doi: 10.1002/mnfr.201600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohir W., Ratcliffe E.M., Sloboda D.M. Of the bugs that shape us: Maternal obesity, the gut microbiome, and long-term disease risk. Pediatr. Res. 2015;77:196–204. doi: 10.1038/pr.2014.169. [DOI] [PubMed] [Google Scholar]
- 24.Rusterholz C., Hahn S., Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 25.Pantham P., Aye I.L., Powell T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36:709–715. doi: 10.1016/j.placenta.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aye I.L., Lager S., Ramirez V.I., Gaccioli F., Dudley D.J., Jansson T. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol. Reprod. 2014;90:1–9. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challier J.C., Basu S., Bintein T., Minium J., Hotmire K., Catalano P.M. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts K.A., Riley S.C., Reynolds R.M., Barr S., Evans M., Statham A. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Kim D.W., Young S.L., Grattan D.R., Jasoni C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014;90:1–11. doi: 10.1095/biolreprod.113.117259. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M.J., Du M., Nathanielsz P.W., Ford S.P. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta. 2010;31:387–391. doi: 10.1016/j.placenta.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Radaelli T., Uvena-Celebrezze J., Minium J., Huston-Presley L., Catalano P., Hauguel-de Mouzon S. Maternal interleukin-6: Marker of fetal growth and adiposity. J. Soc. Gynecol. Investig. 2006;13:53–57. doi: 10.1016/j.jsgi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Denison F.C., Roberts K.A., Barr S.M., Norman J.E. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140:373–385. doi: 10.1530/REP-10-0074. [DOI] [PubMed] [Google Scholar]
- 33.Igosheva N., Abramov A.Y., Poston L., Eckert J.J., Fleming T.P., Duchen M.R., McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS. ONE. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prince C.S., Maloyan A., Myat T.L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta. 2017;49:55–63. doi: 10.1016/j.placenta.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusco S., Spinelli M., Cocco S., Ripoli C., Mastrodonato A., Natale F., Rinaudo M., Livrizzi G., Grassi C. Maternal insulin resistance multigenerationally impairs synaptic plasticity and memory via gametic mechanisms. Nat. Commun. 2019;10:1–17. doi: 10.1038/s41467-019-12793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briana D.D., Malamitsi-Puchner A. Developmental origins of adult health and disease: The metabolic role of BDNF from early life to adulthood. Metabolism. 2018;81:45–51. doi: 10.1016/j.metabol.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Barker D.J., Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 38.Lewis R.M., Desoye G. Placental lipid and fatty acid transfer in maternal overnutrition. Ann. Nutr. Metab. 2017;70:228–231. doi: 10.1159/000463397. [DOI] [PubMed] [Google Scholar]
- 39.Myatt L., Maloyan A. Obesity and placental function. Semin. Reprod. 2016;34:42–49. doi: 10.1055/s-0035-1570027. [DOI] [PubMed] [Google Scholar]
- 40.Hayward C.E., Higgins L., Cowley E.J., Greenwood S.L., Mills T.A., Sibley C.P. Chorionic plate arterial function is altered in maternal obesity. Placenta. 2013;34:281–287. doi: 10.1016/j.placenta.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes E.K., Lechowicz A., Petrik J.J., Storozhuk Y., Paez-Parent S., Dai Q. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: Role of altered development of the placental vasculature. PLoS. ONE. 2012;7:e33370. doi: 10.1371/journal.pone.0033370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stachowiak E.K., Srinivasan M., Stachowiak M.K., Patel M.S. Maternal obesity induced by a high fat diet causes altered cellular development in fetal brains suggestive of a predisposition of offspring to neurological disorders in later life. Metab. Brain Dis. 2013;28:721–725. doi: 10.1007/s11011-013-9437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Ma H., Tong C., Zhang H., Lawlis G.B., Li Y., Zang M., Ren J., Nijland M.J., Ford S.P., et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 2010;24:2066–2076. doi: 10.1096/fj.09-142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhlhausler B., Smith S.R. Early-life origins of metabolic dysfunction: Role of the adipocyte. Trends Endocrinol. Metab. 2009;2:51–57. doi: 10.1016/j.tem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Borengasser S.J., Zhong Y., Kang P., Lindsey F., Ronis M.J., Badger T.M. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154:4113–4125. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Z., Han S., Zhu J., Sun X., Ji C., Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heslehurst N., Vieira R., Akhter Z., Bailey H., Slack E., Ngongalah L., Pemu A., Rankin J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019;16:e1002817. doi: 10.1371/journal.pmed.1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang G.Q., Gaysinskaya V., Karatayev O., Leibowitz S.F. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Britto P.R., Lye S.J., Proulx K., Yousafzai A.K., Matthews S.G., Vaivada T., Perez-Escamilla R., Rao N., Ip P., Fernald L.C.H., et al. Early Childhood Development Interventions Review Group, for the Lancet Early Childhood Development Series Steering Committee. Lancet. 2017;389:91–102. doi: 10.1016/S0140-6736(16)31390-3. [DOI] [PubMed] [Google Scholar]
- 50.Alfaradhi M.Z., Fernandez-Twinn D.S., Martin-Gronert M.S., Musial B., Fowden A., Ozanne S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R26–R34. doi: 10.1152/ajpregu.00049.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henry S.L., Barzel B., Wood-Bradley R.J., Burke S.L., Head G.A., Armitage J.A. Developmental origins of obesity-related hypertension. Clin. Exp. Pharmacol. Physiol. 2012;39:799–806. doi: 10.1111/j.1440-1681.2011.05579.x. [DOI] [PubMed] [Google Scholar]
- 52.Glastras S.J., Tsang M., Teh R., Chen H., McGrath R.T., Zaky A.A., Pollock C.A., Saad S. Maternal obesity promotes diabetic nephropathy in rodent offspring. Sci. Rep. 2016;6:27769. doi: 10.1038/srep27769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuelsson A.M., Matthews P.A., Argenton M., Christie M.R., McConnell J.M., Jansen E.H., Piersma A.H., Ozanne S.E., Twinn D.F., Remacle C., et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 54.Desai M., Ross M.G. Fetal programming of adipose tissue: Effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin. Reprod. Med. 2011;29:237–245. doi: 10.1055/s-0031-1275517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris M.J., Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int. J. Obes. 2009;33:115–122. doi: 10.1038/ijo.2008.213. [DOI] [PubMed] [Google Scholar]
- 56.Tozuka Y., Wada E., Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009;23:1920–1934. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- 57.Tozuka Y., Kumon M., Wada E. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010;57:235–247. doi: 10.1016/j.neuint.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Page K.C., Jones E.K., Anday E.K. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R527–R537. doi: 10.1152/ajpregu.00319.2013. [DOI] [PubMed] [Google Scholar]
- 59.Shankar K., Kang P., Harrell A., Zhong Y., Marecki J.C., Ronis M.J.J., Badger T.M. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology. 2010;151:2577–2589. doi: 10.1210/en.2010-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armitage J.A., Lakasing L., Taylor P.D., Balachandran A.A., Jensen R.I., Dekou V., Ashton N., Nyengaard J.R., Poston L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J. Physiol. 2005;565:171–184. doi: 10.1113/jphysiol.2005.084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mamun A.A., O’Callaghan M., Callaway L., Williams G., Najman J., Lawlor D.A. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: Evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds R.M., Allan K.M., Raja E.A., Bhattacharya S., McNeill G., Hannaford P.C., Sarwar N., Lee A.J., Bhattacharya S., Norman J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murabayashi N., Sugiyama T., Zhang L., Kamimoto Y., Umekawa T., Ma N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;169:39–44. doi: 10.1016/j.ejogrb.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Lecoutre S., Breton C. The cellularity of offspring’s adipose tissue is programmed by maternal nutritional manipulations. Adipocyte. 2014;3:256–262. doi: 10.4161/adip.29806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desai M., Han G., Ross M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite. 2016;99:193–199. doi: 10.1016/j.appet.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tain Y.L., Huang L.T., Hsu C.N. Developmental programming of adult disease: Reprogramming by melatonin? Int. J. Mol. Sci. 2017;18:426. doi: 10.3390/ijms18020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tain Y.L., Hsu C.N. Developmental programming of the metabolic syndrome: Can we reprogram with resveratrol? Int. J. Mol. Sci. 2018;19:2584. doi: 10.3390/ijms19092584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu C.N., Tain Y.L. The good, the bad, and the ugly of pregnancy nutrients and developmental programming of adult disease. Nutrients. 2019;11:894. doi: 10.3390/nu11040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 70.Boocock D.J., Faust G.E., Patel K.R., Schinas A.M., Brown V.A., Ducharme M.P., Booth T.D., Crowell J.A., Perloff M., Gescher A.J., et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer. Epidemiol. Biomark. Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 71.la Porte C., Voduc N., Zhang G., Seguin I., Tardiff D., Singhal N., Cameron D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharm. 2010;49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Williams L.D., Burdock G.A., Edwards J.A., Beck M., Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Madhyastha S., Sekhar S., Rao G. Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int. J. Dev. Neurosci. 2013;31:580–585. doi: 10.1016/j.ijdevneu.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Cao K., Zheng A., Xu J., Li H., Liu J., Peng Y., Long J., Zou X., Li Y., Chen C., et al. AMPK activation prevents prenatal stress-induced cognitive impairment: Modulation of mitochondrial content and oxidative stress. Free Radic. Biol. Med. 2014;75:156–166. doi: 10.1016/j.freeradbiomed.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 75.Care A.S., Sung M.M., Panahi S., Gragasin F.S., Dyck J.R., Davidge S.T., Bourque S.L. Perinatal resveratrol supplementation to spontaneously hypertensive rat dams mitigates the development of hypertension in adult offspring. Hypertension. 2016;67:1038–1044. doi: 10.1161/HYPERTENSIONAHA.115.06793. [DOI] [PubMed] [Google Scholar]
- 76.Chen H.E., Lin Y.J., Lin I.C., Yu H.R., Sheen J.M., Tsai C.C., Huang L.T., Tain Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019;70:28–37. doi: 10.1016/j.jnutbio.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Ding J., Kang Y., Fan Y., Chen Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017;6:595–600. doi: 10.1530/EC-17-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darby J.R.T., Mohd Dollah M.H.B., Regnault T.R.H., Williams M.T., Morrison J.L. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications-Are we ready for the clinic? Pharmacol. Res. 2019;144:264–278. doi: 10.1016/j.phrs.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Oliveira A.L.B., Monteiro V.V.S., Navegantes-Lima K.C., Reis J.F., Gomes R.S., Rodrigues D.V.S. Resveratrol role in autoimmune disease-a mini-review. Nutrients. 2017;9:1306. doi: 10.3390/nu9121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Truong V.L., Jun M., Jeong W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2017;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 81.Bourque S.L., Dolinsky V.W., Dyck J.R., Davidge S.T. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33:449–452. doi: 10.1016/j.placenta.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Kim O.Y., Chung J.Y., Song J. Effect of resveratrol on adipokines and myokines involved in fat browning: Perspectives in healthy weight against obesity. Pharmacol. Res. 2019;148:104411. doi: 10.1016/j.phrs.2019.104411. [DOI] [PubMed] [Google Scholar]
- 83.Jeon B.T., Jeong E.A., Shin H.J., Lee Y., Lee D.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Timmers S., Konings E., Bilet L., Houtkooper R.H., van de Weijer T., Goossens G.H., Hoeks J., van der Krieken S., Ryu D., Kersten S., et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adamkova K., Yi Y.J., Petr J., Zalmanova T., Hoskova K., Jelinkova P. SIRT1-dependent modulation of methylation and acetylation of histone H3 on lysine 9 (H3K9) in the zygotic pronuclei improves porcine embryo development. J. Anim. Sci. Biotechnol. 2017;8:83. doi: 10.1186/s40104-017-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanhees K., van Schooten F.J., Moonen E.J., Maas L.M., van Waalwijk van Doorn-Khosrovani S.B., Godschalk R.W. Maternal intake of quercetin during gestation alters ex vivo benzo[a]pyrene metabolism and DNA adduct formation in adult offspring. Mutagenesis. 2012;27:445–451. doi: 10.1093/mutage/ges002. [DOI] [PubMed] [Google Scholar]
- 87.Sheen J.M., Yu H.R., Tain Y.L., Tsai W.L., Tiao M.M., Lin I.C., Tsai C.C., Lin Y.J., Huang L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018;8:5607. doi: 10.1038/s41598-018-24010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaplin A., Carpene C., Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10:1651. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Rivera L., Morón R., Sánchez M., Zarzuelo A., Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 91.Dal-Pan A., Blanc S., Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010;10:11.:11. doi: 10.1186/1472-6793-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh A.P., Singh R., Verma S.S., Rai V., Kaschula C.H., Maiti P., Gupta S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- 93.Xie H., Lim B., Lodish H.F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Oliveira M.R., Nabavi S.F., Manayi A., Daglia M., Hajheydari Z., Nabavi S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta. 2016;1860:727–745. doi: 10.1016/j.bbagen.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Landau D., Haghiac M., Minium J., Skomorovska-Prokvolit Y., Calabuig-Navarro V., O’Tierney-Ginn P. Activation of AMPK in Human Placental Explants Impairs Mitochondrial Function and Cellular Metabolism. Reprod. Sci. 2019;26:487–495. doi: 10.1177/1933719118776803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu D., Xie K., Yang X., Gu J., Ge L., Wang X., Wang Z. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav. Brain Res. 2014;264:9–16. doi: 10.1016/j.bbr.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 97.Kodomari I., Wada E., Nakamura S., Wada K. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 2009;54:95–98. doi: 10.1016/j.neuint.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Poston L., Bell R., Croker H., Flynn A.C., Godfrey K.M., Goff L. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 99.Zheng S., Feng Q., Cheng J., Zheng J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018;38:BSR20171741. doi: 10.1042/BSR20171741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts V.H., Pound L.D., Thorn S.R., Gillingham M.B., Thornburg K.L., Friedman J.E., Frias A.E., Grove K.L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28:2466–2477. doi: 10.1096/fj.13-245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pound L.D., Comstock S.M., Grove K.L. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am. J. Physiol. Endocrinol. Metab. 2014;307:E115–E123. doi: 10.1152/ajpendo.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou T., Chen D., Yang Q., Wang B., Zhu M.J., Nathanielsz P.W., Du M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017;595:1547–1562. doi: 10.1113/JP273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ros P., Díaz F., Freire-Regatillo A., Argente-Arizón P., Barrios V., Argente J., Chowen J.A. Resveratrol intake during pregnancy and lactation modulates the early metabolic effects of maternal nutrition differently in male and female offspring. Endocrinology. 2018;159:810–825. doi: 10.1210/en.2017-00610. [DOI] [PubMed] [Google Scholar]
- 104.Malvasi A., Kosmas I., Mynbaev O.A., Sparic R., Gustapane S., Guido M. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. Ther. 2017;168:e240–e247. doi: 10.7417/T.2017.2013. [DOI] [PubMed] [Google Scholar]
- 105.Hanson M., Barker M., Dodd J.M., Kumanyika S., Norris S., Steegers E., Stephenson J., Thangaratinam S., Yang H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76. doi: 10.1016/S2213-8587(16)30108-5. [DOI] [PubMed] [Google Scholar]