Abstract
The stem cells of neurogenesis and carcinogenesis share many properties, including proliferative rate, an extensive replicative potential, the potential to generate different cell types of a given tissue, and an ability to independently migrate to a damaged area. This is also evidenced by the common molecular principles regulating key processes associated with cell division and apoptosis. Autosomal recessive primary microcephaly (MCPH) is a neurogenic mitotic disorder that is characterized by decreased brain size and mental retardation. Until now, a total of 25 genes have been identified that are known to be associated with MCPH. The inactivation (yin) of most MCPH genes leads to neurogenesis defects, while the upregulation (yang) of some MCPH genes is associated with different kinds of carcinogenesis. Here, we try to summarize the roles of MCPH genes in these two diseases and explore the underlying mechanisms, which will help us to explore new, attractive approaches to targeting tumor cells that are resistant to the current therapies.
Keywords: autosomal recessive primary microcephaly, neurogenesis, carcinogenesis, centrosome, cell cycle, cell apoptosis
1. Introduction
Embryonic stem cells are considered pluripotent, meaning that they are capable of differentiating into multiple cell types and maintaining the ability to self-renew to produce more of the same type of stem cells. Differentiated cells originating from stem cells make up the tissues and organs of animals and plants. Neurogenesis is the process of generating neurons by neural stem cell (NSC) proliferation, neuron migration, and differentiation. Proper neurogenesis is fundamental for normal brain development [1]. Carcinogenesis defines the initiation of a tumor, or the process of transforming normal cells into cancer cells, which is determined by some factors regulating cell growth and division [2]. Cancer stem cells (CSCs) are a subpopulation of stem-like-cell properties commonly shared with normal tissue stem cells, including extensive self-renewal ability (symmetrical and asymmetrical) and differentiation capacity [3]. CSCs exhibit characteristics of both stem cells and cancer cells [4].
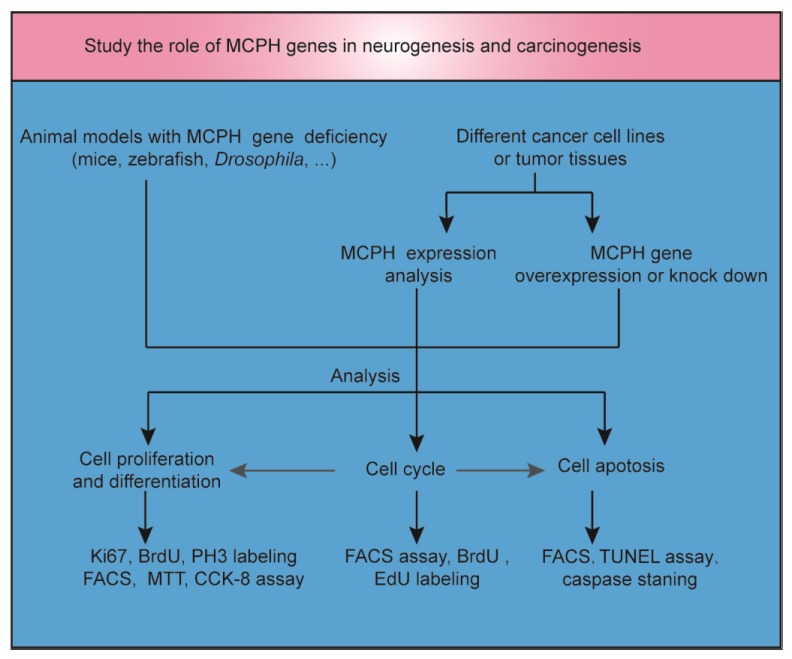
Microcephaly, often described as ‘small head’, is a feature of many clinical disorders and can have environmental, maternal, or genetic etiologies. Autosomal recessive primary microcephaly (MCPH) is a rare encephalopathy caused by a dysfunction in neurodevelopment. To study the role of MCPH genes in neurogenesis and carcinogenesis, animal models and cancer cell lines or tumor tissues with MCPH gene deficiency or overexpression were established (Figure 1). Many genes linked to MCPH encode proteins involved in DNA repair or cell cycle control [5]. DNA replication, DNA repair, cell cycle progression, and the maintenance of genome stability are fundamental physiological processes that need to be tightly balanced to achieve normal neurogenesis in the brain or other tissues without resulting in carcinogenesis. Both neurogenesis and carcinogenesis have a gene expression signature that includes DNA and histone modifications [6,7]. In addition, there are overlapping migratory mechanisms between neural progenitor cells (NPCs) and brain tumor stem cells [8]. In light of the critical role of MCPH genes in mitosis, cell cycle, and apoptosis regulation, it is possible that the inhibition of the function of these genes may specifically affect the proliferation and survival of tumor cells [9]. In this review, we focus on novel insights into the overlapping mechanisms of neurogenesis and carcinogenesis by exploring the role of MCPH genes in brain development and cancer occurrence. Furthermore, we try to tap potential strategies for regulating the signaling of MCPH genes in cancer.
Figure 1.
The role of MCPH genes in neurogenesis and carcinogenesis. Animal models with MCPH gene deficiency were established to study the role of MCPH genes in neurogenesis. MCPH gene overexpression or knockdown was induced in cancer cell lines or tumor tissues to study the role of MCPH genes in carcinogenesis. At the same time, MCPH gene/protein expression was analyzed by RNA sequencing, real-time PCR, or Western blot in carcinoma and precancerous tissue. Cell cycle, cell division, differentiation, and apoptosis were examined to study the mechanism of neurogenesis and carcinogenesis mediated by MCPH genes. BrdU, 5-bromo-2′-deoxyuridine; CCK-8, cell counting kit-8; EdU, 5-ethynyl-2′-deoxyuridine; FACS, fluorescent-activated cell sorting; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; TUNEL, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling.
2. MCPH Gene Deficiency Leads to Neurogenesis Defects
MCPH patients show reduced brain size, pachygyria, and loss of the gray–white junction. Head circumference (HC) is one of the most useful indirect measurements for diagnosing microcephaly. Its clinical criterion is a HC that is three standard deviations below the mean (−3 SD) [10]. MCPH causes intellectual disability, accompanied by seizures, motor disorders, and speech and language disabilities [11]. The incidence of MCPH is about 2–12 in 10,000 live births [10]. At present, 25 genes have been identified and found to be associated with MCPH [12,13]. The cerebral cortex is the region most affected in microcephaly patients. During cortical development, MCPH genes function in several important early developmental events: NPC proliferation, differentiation into neurons, the migration of neurons to the specific position of the cortical plate, and the formation of axons and dendrites from which functional synapses are produced [14]. When any of these processes go awry because of a genetic mutation, the consequences can lead to severe neurological diseases such as MCPH.
To study the function of MCPH genes, animal models with MCPH gene mutation/deficiency have been established in zebrafish, Drosophila, mice, and non-human primates (cynomolgus macaque) [12,15]. Mimicking human MCPH symptoms, the most striking phenotype of MCPH gene deficiency mice was their significantly small brain size [13]. NPC (Pax6, Sox2, and Tbr2) and neuron (Tuj1, NeuN, Tbr1, and so on) markers were applied to test the effect of MCPH gene disturbance on cortical development. Fewer cells being labeled with proliferation markers Ki67 and 5-bromo-2′-deoxyuridine (BrdU) in MCPH gene-deficient mice brains indicates less NPC proliferation compared with wild-type mice [16,17,18]. NPCs increase the progenitor cell pool through symmetrical division (maintaining the progenitor cell pool) and produce progenitor cells and nerve precursors through asymmetrical division. The latter eventually undergo migration and differentiation to form the brain [19]. Some MCPH gene knockout (KO) mice showed a disrupted balance between symmetric and asymmetric division in the neocortex [16,20]. The neuronal migration defect was observed after Mcph2 or Mcph5 knockdown in the developing neocortex [18,21]. In addition, neuronal connectivity and neuron myelination defects were detected in MCPH gene KO mice [22,23].
3. Some MCPH Genes Can Be Considered as Potential Cancer Biomarkers
Several MCPH genes, such as MCPH1, MCPH2, MCPH5, MCPH7, MCPH8, MCPH10, MCPH12, MCPH15, and MCPH17-21, have been reported to regulate both neurogenesis and carcinogenesis (Table 1). MCPH gene overexpression is always associated with centriole overduplication, multipolar spindles, anaphase-lagging chromosomes, and micronuclei. MCPH4, MCPH7, MCPH8, MCPH10, MCPH13, MCPH17, MCPH19, and MCPH23 are highly expressed in different tumor tissues or cancer cell lines, indicating that some MCPH genes can be considered as oncogenes or potential cancer biomarkers [24,25,26,27,28,29,30,31]. The knockdown or inhibited expression of these genes may be a potential therapeutic method for cancer treatment [31,32,33]. In addition, the overexpression of MCPH2, MCPH5, MCPH7, MCPH14, MCPH20, MCPH21, MCPH23, and MCPH24 is associated with aggressiveness and poor outcome in patients with cancer [28,34,35,36,37,38,39]. However, MCPH1 and MCPH15 are downregulated in tumor tissues or cancer cell lines and are considered as novel tumor suppressor genes [40,41].
Table 1.
MCPH genes regulate both neurogenesis and carcinogenesis.
| Gene | Role in/Effect on Neurogenesis | Ref. | Role in/Effect on Carcinogenesis | Ref. |
|---|---|---|---|---|
| MCPH1 (BRIT1) | Premature neurogenic production | [16] | Cell cycle and apoptosis (cervical cancer) | [40] |
| Reductions in head circumference, premature chromosome condensation, and hypoplasia of the corpus callosum | [15] | Migration and invasion (lung cancer) | [42] | |
|
MCPH2
(WDR62) |
Mitotic spindle orientation | [43] | Cell cycle (gastric cancer, GC) | [44] |
| Regulating intermediate neural and glial progenitors | [45] | Centrosome amplification (ovarian cancer, OC) | [46] | |
| Cell cycle, centriole biogenesis, and mitotic spindle orientation | [17] | Cell growth (lung adenocarcinoma) | [34] | |
| Regulating neural stem cell division | [47,48] | |||
| MCPH3 (CDK5RAP2) | Reduced neurons and neural progenitors, premature cell cycle exit, and increased cell death | [49] | N/A (Leukemia) | [50] |
| Premature neuronal differentiation | [51] | |||
| MCPH4 (CASC5) | N/A | Cell proliferation and apoptosis (colorectal cancers, CRCs) | [52] | |
| Nuclear division of cells (GC) | [27] | |||
|
MCPH5
(ASPM) |
Defects in cortical layers IV, V, and VI formation and imbalance of horizontal and vertical neurites | [53] | Differentiation and metastasis (bladder cancer) | [35] |
| Abnormal proliferation and differentiation of nerve stem/progenitor cells | [54] | Cell cycle progression (pancreatic cancer prognosis) | [55] | |
|
MCPH6
(CENPJ) |
Cell proliferation, cell apoptosis | [56] | N/A | |
| Centrosome generation and microtubule stability, progenitor division, and neuronal migration | [57] | |||
|
MCPH7
(STIL) |
Centriole duplication | [58,59] | Cell proliferation, cell cycle G2/M phase and apoptosis (GC) | [32] |
| Centromere assembly | [60] | Cell proliferation and apoptosis (prostate cancer, PCa) | [28] | |
| Cell cycle and chromosomal segregation | [61] | DNA repair (OC) | [62] | |
| MCPH8 (CEP135) | Assembly of centrosome and microtubule | [63] | Centriole duplication (breast cancer) | [29] |
| Establishment of centrosome asymmetry | [64] | |||
| MCPH10 (ZNF335) | Neural progenitor self-renewal, neurogenesis, and neuronal differentiation | [65] | tumor progression (Bladder cancer) | [24] |
|
MCPH12
(CDK6) |
Regulating radial glial cells G1 and S phases | [66] | DNA damage response, apoptosis (epithelial ovarian cancer) | [67] |
| Positively regulates the proliferation of hippocampal progenitors | [68] | Cell cycle (glioblastoma) | [69] | |
| Proliferation of neural stem cells | [70] | Cell cycle, cell proliferation and angiogenesis (hematopoietic malignancies) | [71] | |
| Cell cycle and apoptosis (T-cell acute lymphoblastic leukemia) | [72] | |||
| MCPH13 (CENPE) | N/A | Cell G2/M phase and proliferation (lung cancer) | [25] | |
| MCPH14 (SAS6) | N/A | Centrosome amplification, mitotic abnormality (CRCs) | [73] | |
| MCPH15 (MFSD2A) | Blood–brain barrier disruption | [74] | Cell cycle (G1 phase) and matrix attachment (lung cancer) | [41] |
|
MCPH16
(ANKLE2) |
Reduced cell proliferation | [75] | N/A | |
|
MCPH17
(CIT) |
Apoptosis of neuronal progenitors [76,77] | Cell cycle and apoptosis (colon cancer, CC) | [26] | |
| DNA damage, proliferation, cell senescence and apoptosis (medulloblastoma) | [33] | |||
|
MCPH18
(WDFY3) |
Perinatal lethality, telencephalic junction, axonal connectivity defect, and localization of glial guidepost cells | [23] | Cell proliferation, migration, invasion, and epithelial-to-mesenchymal transition (OC) | [78] |
| Neurodevelopmental delay, intellectual disability, macrocephaly, and psychiatric disorders | [79] | |||
| Distribution in neuronal projection and axon guidance | [80] | |||
|
MCPH19
(COPB2) |
Increased apoptosis in the brain and slow growth of neurospheres | [81] | Cell cycle, apoptosis and proliferation (PCa) | [30,82] |
| Cell proliferation and apoptosis (lung adenocarcinoma) | [83] | |||
| Cell G1 phase, proliferation and apoptosis (cholangiocellular cancers) | [84] | |||
| Suppresses cell proliferation and induces cell G0/G1 or S phase arrest (CC) | [85] | |||
| Cell proliferation and apoptosis (GC) | [86] | |||
|
MCPH20
(KIF14) |
Flat head, motor impairment, growth retardation, decreased cell proliferation, and cell death | [22] | Induced cell cycle arrest and apoptosis (PCa) | [36] |
| Regulation of the expression of transcription factors (SP1, YY1) (ovarian cancers) | [87] | |||
| Cell cycle and proliferation (hepatocellular carcinoma) | [88] | |||
|
MCPH21
(NCAPD2) |
Reduced cortex | [89] | Promote cell cycle and enhance [39] invasion (Triple-negative breast cancer) |
|
|
MCPH23
(NCAPH) |
N/A | Cell cycle (G2/M phase), cells proliferation, migration and apoptosis (CC) | [31] | |
| Cell cycle (PCa) | [38] | |||
|
MCPH24
(NUP37) |
N/A | Cell growth, migration and invasion (hepatocellular carcinoma) | [37] | |
|
MCPH25
(MAP11) |
Microcephaly, decreased neuronal proliferation, a reduction in white matter, and hypoplasia of corpus callosum | [90] | N/A | |
CC, colon cancer; CRCs, colorectal cancers; GC, gastric cancer; N/A, not applicable/reported; OC, ovarian cancer; PCa, prostate cancer; Ref., reference.
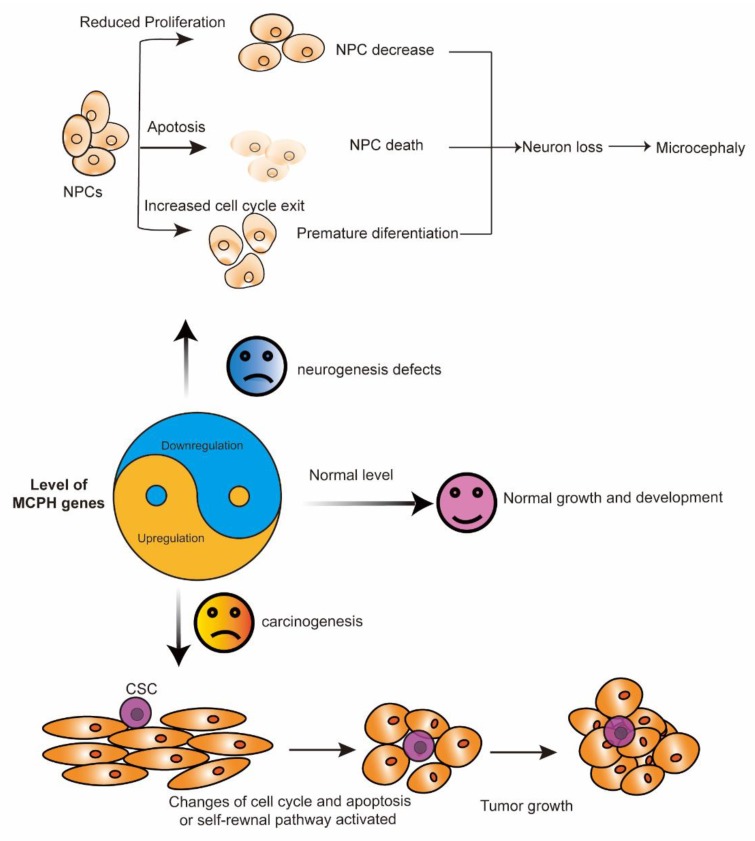
In summary, the expression level of the MCPH gene is important for normal brain development and that of tissues without cancer. In cortical NPCs, the loss of the MCPH gene results in cell division failure, a reduction in cell proliferation, and increased apoptosis. The most common phenotypes representing defects in neurogenesis include decreased neural progenitors, premature differentiation, and cell death. These processes finally lead to neuron loss and reduced brain size, which is also called microcephaly (Figure 2). The development of cancer is a complicated process in which a large number of factors interact to disrupt normal cell growth and division. Some MCPH gene upregulation is associated with aggressiveness and a poor outcome in patients with cancer, as indicated by the role of MCPH genes in carcinogenesis (Figure 2).
Figure 2.
The yin and yang function of MCPH genes. MCPH gene deficiency (yin) leads to neurogenesis defects, while its overexpression (yang) is associated with carcinogenesis. In cortical NPCs, the loss of the MCPH gene results in premature differentiation, a reduction in cell proliferation, and increased apoptosis. These processes finally lead to neuron loss and microcephaly. During carcinogenesis, the overexpression of some MCPH genes, except for MCPH1 and MCPH15, change the cell cycle and the cell apoptosis regulation of the cells and finally lead to tumor growth. CSC, cancer stem cell; MCPH, autosomal recessive primary microcephaly; NPCs, neural progenitor cells.
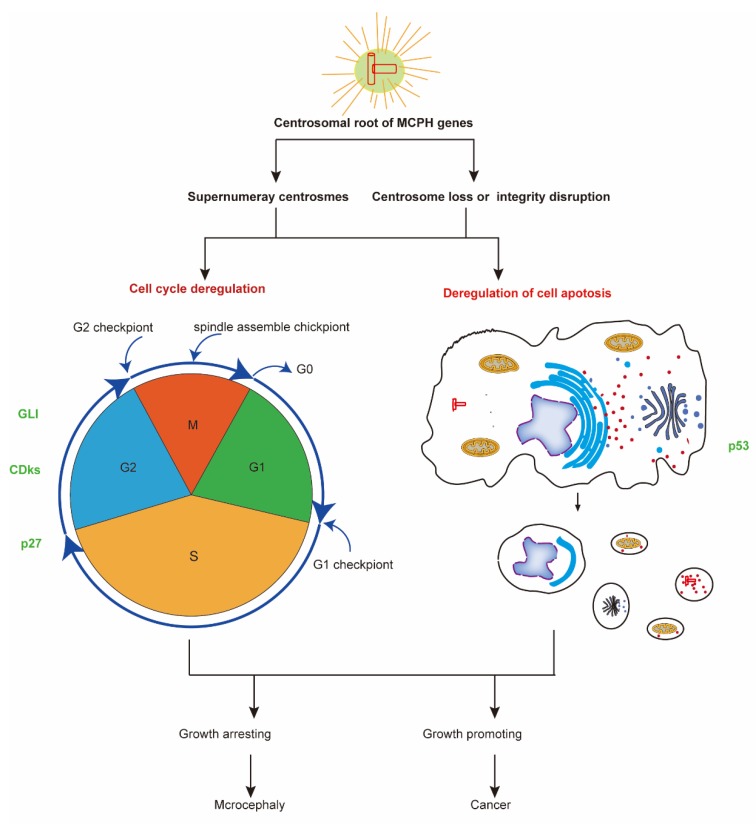
4. The Centrosomal Root of MCPH Genes
The centrosome, which acts as the main microtubule-organizing center in animal cells as well as a regulator of cell cycle progression, is a non-membrane-bound organelle composed of two centrioles surrounded by pericentriolar material [91]. Centrosomes are closely linked to neurodevelopment, not only due to their key role in cell division but also because of their participation in cell polarization and migration in the developing brain [92]. Abnormal centrosomes are one cause of human tumors, whereas mutations in centrosome proteins such as MCPH genes have recently been genetically linked to microcephaly and dwarfism [93].
MCPH1 (BRIT1), the first gene found to cause microcephaly, plays an important role in controlling mitosis, centrosome separation, and DNA damage repair [94]. MCPH2 (WDR62), the next most common gene causative of microcephaly, showed cell cycle-dependent expression and functions in centriole biogenesis and mitotic spindle orientation [17]. MCPH3 (CDK5RAP2) protein products are important centrosome materials required for centrosome assembly and cell division [95]. MCPH5 (ASPM) participates in spindle localization during neurogenesis [96,97], while MCPH6 (CENPJ) serves as a centromere protein required for the spindle checkpoint [57,98]. In addition, MCPH6 regulates ciliary decomposition and neurogenesis through the KIF2A terminal directed motor protein [56]. It has been found that the TCP domain of MCPH7 (STIL), essential for the replication of the centriole, constitutes a proline recognition domain, forming a 1:1 complex with the short and highly conserved target motifs in MCPH7 [59]. This regulates the replication of centrioles in vivo [58]. Other studies have shown that the human microcephalic malformation protein rotatin (RTTN) directly interacts with STIL and acts downstream of STIL-mediated centrosome assembly [60], thereby affecting the development of the brain. MCPH8 (CEP135) is particularly important in the assembly, amplification, and microtubule binding of the centriole [99]. Mutations in this gene cause microtubule and centrosome assembly defects, which have a serious impact on the normal occurrence of nerves and cause primary microcephaly [63]. In the Drosophila nervous system, the central protein BLD10/MCPH8 is vital to the establishment of centrosome asymmetry in Drosophila neuroblasts [64]. In breast cancer, mutations in MCPH8 promote centriole overduplication, leading to chromosome segregation errors in breast cancer cells [29]. MCPH9 (CEP152) acts as a scaffold and is essential for centriole expansion and spindle formation [100]. MCPH12 (CDK6), which encodes cyclin-dependent kinase 6, is associated with the centrosome during mitosis [101], and MCPH14 (SAS6) is a central component of centrioles and is necessary for their duplication and function [59,102].
Neurogenesis and carcinogenesis are both associated with abnormal centrosome duplications [61]. It is difficult to rationalize how centrosome anomalies lead to neurogenesis defects or tumors. Supernumerary centrosomes are sufficient to drive aneuploidy and the development of spontaneous tumors in multiple tissues [103]. Most MCPH genes, except MCPH1 and MCPH15, are highly expressed in tumors, but there is a conspicuous absence of direct genetic evidence linking the level of the MCPH gene to supernumerary centrosomes in human carcinogenesis. Aneuploidy generated during development by centrosome amplification is not always compatible with tumor initiation. Supernumerary centrosomes are also reported in patients or mouse models with mutations in MCPH genes such as MCPH3, MCPH7, and MCPH8 [63,104,105]. One explanation is that mammalian NPCs, compared with other cells, may be particularly vulnerable to centrosome amplification because they have to divide asymmetrically and, therefore, lead to premature differentiation or death [93]. Future work should study whether MCPH gene mutations result in tumors in other tissues apart from the brain. Another explanation of this discrepancy is that the mutations in the studied MCPH genes indicate that they are not null alleles. This is well studied in MCPH7/STIL. No expression or residual expression of MCPH7 causes an absence of centrosome or reduced centrosome, and the upregulation of STIL leads to centrosome amplification [61]. However, STIL mutations in patients leads to STIL stabilization and triggers centriole amplification [104]. Moreover, this discrepancy may be dependent on p53 expression. p53 is a tumor suppressor whose mutation is most commonly found in cancer cells. p53Plk4OE brains cause the accumulation of extra centrosomes and aneuploidy leads to premature neuronal differentiation, resulting in the generation of a microcephalic brain [92]. In contrast, supernumerary centrosomes in PLK4OE/p53cKO mice were sufficient to generate aneuploidy in the adult epidermis and accelerate skin tumorigenesis [106].
Both excessively high and overly weak expression of MCPH7 lead to microcephaly, through either reduced or amplified centrosome duplication [61]. Centrosome depletion or the disruption of its integrity also leads to neurogenesis defects or carcinogenesis. MCPH6 deletion leads to a progressive loss of centrioles and centrosomes, which results in a substantial loss of neurons and microcephaly in the developing mouse brain [107]. The loss of the microcephaly proteins MCPH2 or MCPH5, or both, impairs centriole duplication and leads to centrosome and cilia loss [17]. In addition, centrosome loss also results in an unstable genome and malignant prostate tumors [108].
5. MCPH Gene Regulate Neurogenesis and Carcinogenesis through Regulation of Cell Cycle and Cell Division
The centrosome is required for several cell cycle transitions, including G1 to S phase, G2 to mitosis, and metaphase to anaphase [109]. The centrosomal root of MCPH decides the role of MCPH genes in the regulation of cell cycle and cell division (Figure 3). Most MCPH gene deficiencies lead to reduced NPC proliferation in developing brains. This is evidenced by animal models with MCPH gene depletions. The Chk1–Cdc25–Cdk1 pathway is disrupted in Mcph1-deficient mice, which further distorts mitotic spindle alignment and shifts the division plane of neuroprogenitors [16]. Studies on Mcph2 deficiency mice have indicated that MCPH2 mainly functions in neuron proliferation and differentiation by regulating neural stem cell division in the central nervous system [17,18,47,48]. The total number of cells and the thickness of the cortical plate were significantly decreased in Mcph5 KO mice compared with wild-type mice. Mcph5 deficiency leads to the abnormal proliferation and differentiation of nerve stem or progenitor cells and affects the development of the cerebral cortex [54]. Specifically, knockdown of Mcph6 in mice brains by in utero electroporation suggests that MCPH6 regulates progenitor cell division by mediating the Ascl1-regulated generation of the central body and the stability of microtubules during neuronal mitosis [57]. As MCPH12 is a key gene in cell cycle regulation, it can also regulate neuronal output and cortical size [66]. In addition, during the development of the hippocampus, p27 can negatively regulate the expression of MCPH12, thereby regulating the proliferation of hippocampal cells [68]. During the development of the neocortex, the loss of MCPH17 mainly affects neurogenic divisions [76]. In Mcph20 mutant mice, BrdU labeling indicates decreased cell proliferation during the development of the cerebral cortex [22]. MCPH25 depletion in a zebrafish model caused microcephaly and reduced neuron proliferation. At the end of cell division, MCPH25 and PLK1 partially co-localized to regulate cell division and control cell exfoliation [90].
Figure 3.
The common mechanism of neurogenesis and carcinogenesis. Most MCPH genes have a centrosomal root and take part in regulating the cell cycle and cell apoptosis. The deregulation of cell cycle and cell apoptosis processes leads to diseases including microcephaly and cancer. Some molecular factors, such as p53, CDK, and GlI family proteins, function in both MCPH-related neurogenesis and carcinogenesis processes. CDKs, cyclin-dependent kinases; GLI, human glioblastoma protein; MCPH, autosomal recessive primary microcephaly.
Some MCPH-associated genes, such as MCPH1, MCPH2, MCPH12, MCPH17, and MCPH20, regulate both neuroprogenitor and cancer cell proliferation through regulation of the cell cycle and cell division. MCPH1 functions as a tumor suppressor. Overexpression of MCPH1 inhibits human cervical cancer cell growth through regulating cell cycle-related proteins, such as cyclinA2/CDK2 and CDC25C-cyclinB/CDC2 [40]. Ectopic expression of MCPH1 through genetic approaches effectively suppressed breast cancer cell proliferation and colony formation in vitro and tumor growth in vivo [110]. The knockdown of MCPH2 induces G2/M phase arrest in gastric cancer (GC) [44]. In glioblastoma, SUMO1 modification stabilizes MCPH12 drives the cell cycle, resulting in the development and progression of cancer [69]. MCPH17 is overexpressed in human colon cancer tissues and cell lines, and MCPH17 knockdown represses cellular proliferation and colony formation [26]. MCPH20 knockdown is known to suppress prostate cancer proliferation [36].
Though their role in neurogenesis has not yet been reported, the abnormal expression of most MCPH genes affects the cell cycle or cell proliferation in cancers. The MCPH4 gene encodes a kinetochore protein that plays an important role in mitosis. The abnormal expression of MCPH4 in colorectal tumors affects cell proliferation and apoptosis [52]. In GC cells, miR-193b-3p might contribute to the mitotic nuclear division of GC cells by mediating the upregulation of MCPH4 [27]. MCPH7 has a role to play in both primary microcephaly and cancer by its involvement in cell cycle perturbations and chromosomal segregation [61]. MCPH7 regulates the proliferation of GC cells through the IGF-1/PI3K/Akt pathway. The deletion of this gene induces cell cycle arrest in the G2/M phase and induces GC cell apoptosis [32]. In prostate cancer (PCa), MCPH7 can affect the MAPK/ERK, PI3K/AKT, and AMPK signaling pathways, consequently promoting the proliferation of PCa cells through colony formation and inhibition of cell apoptosis [28]. MCPH13 is a human kinetochore protein. The overexpression of forkhead box M1 (FOXM1) promotes MCPH13 expression and proliferation in lung cancer cells [25]. In colorectal cancers, MCPH14 gene mutations cause mitotic abnormalities and lead to cancer [73]. In non-small cell lung cancer cell lines and primary lung adenocarcinomas in different tissue types, the expression of MCPH15 was strongly downregulated, affecting cell adhesion, migration, and regulation of the G1 cell cycle phase [41]. The MCPH18 gene regulates ovarian cancer cell proliferation, migration, invasion, and epithelial mesenchymal transformation [78]. The MCPH19 gene is associated with the occurrence of PCa and cholangiocarcinoma by regulating the G1 cell cycle phase and apoptotic pathway [82,84]. In colon cancer, the downregulation of MCPH19 can induce cell cycle arrest in the G0/G1 or S phase [85], subsequently inhibiting the occurrence of cancer. In lung adenocarcinoma, the upregulation of Yap1 upregulates MCPH19 expression, which inhibits cell apoptosis and promotes cell growth and tumorigenesis [83]. MCPH20 can be used as a potential oncogene. In vivo human studies have found that MCPH20 knockdown by small interfering RNA leads to G2 arrest and reduced proliferation in PCa cells. MCPH20 knockdown also affects the cell cycle and regulates the expression of GAD45A, GAD45B, p21, PIDD, and SHISA5, all of which contribute to growth arrest and apoptosis induction [36]. In human colon cancer (CC), the KO of MCPH23 can inhibit the proliferation, in vitro migration, and xenograft tumorigenesis of CC cells by inhibiting the cell cycle during G2/M phase transition, and inducing cell apoptosis [31]. In PCa, MCPH23 interacts with a variety of proteins during the regulation of PCa-associated cell cycles. These proteins include Aurora kinase A, Aurora kinase B, and cyclin-dependent kinase 1 [38]. Consistently, the deletion of low-density lipoprotein-related receptor 5 inhibits liver cancer cell proliferation via destabilizing MCPH24 in a β-catenin-independent way [111].
6. MCPH Gene Regulate Neurogenesis and Carcinogenesis via Cell Apoptosis Regulation
Apoptosis (or programmed cell death) is one of the central cellular processes in brain development and carcinogenesis. MCPH genes regulate proper centrosome formation, including the complex comprising the centrosome, centrioles, and connecting filaments that is required for apoptosis [112] (Figure 3). Caspase staining indicates more apoptosis in the Mcph1-null neocortex before and after γ-irradiation compared to wild-type samples [113]. Mcph5 deficient inhibits postnatal cerebellar neurogenesis through apoptosis in mice brains [114]. In mammals, the loss of MCPH17 leads to substantial cytokine depletion and apoptosis in neuronal progenitor and male germline cells, leading to severe microcephaly and testicular dysplasia [76]. In mammalian and Drosophila models, the loss of MCPH17 can additionally cause DNA damage accumulation and chromosomal instability. This leads to a failure of cell division and apoptosis of neural progenitor cells [77]. Used to mark apoptotic cells and associated with a missense variant, caspase 3 expression was increased in the cortex of Mcph19 mutant mice during brain development [81]. Mcph20 mutant mice showed increased numbers of apoptotic cells, as revealed by TUNEL staining in the cerebral cortex [22].
The downregulation of most MCPH genes leads to apoptosis, indicating that most of them can be considered as potential therapeutic targets for cancer treatment. Genetic deletion of Mcph5 in mice reduces the growth of medulloblastoma and increases DNA damage [114]. Inducible knockdown of MCPH7 in cancer cells in vitro decreased CDK1/CYCLIN B activity and induced apoptosis [115]. In medulloblastoma cell lines and mouse models, the absence or deletion of MCPH17 increased apoptosis, thus suggesting its potential role in medulloblastoma treatment [33]. In addition, the apoptosis rate was increased in MCPH19 knockdown PCa cells [82]. MCPH20 was consequently considered to be a novel candidate oncogene. The knockdown of MCPH20 in DU145 and PC3 PCa cells induced cell apoptosis [36]. Consistently, high expression of MCPH12 affects cell apoptosis by altering the cell cycle process, leading to the occurrence of various cancers [67,116]. In contrast, MCPH1 is an early DNA damage response protein [117]. In the process of carcinogenesis, the overexpression of MCPH1 inhibits uncontrolled cell growth by regulating several apoptosis-related proteins and activating cell apoptosis. These include p53, Bcl-2, Bax, cytochrome c, caspase-3, and PARP-1 [40,118].
7. The Molecular Regulators of MCPH Genes during Neurogenesis and Carcinogenesis
Cell division and cell apoptosis regulation by MCPH genes indicate that there are common molecular mechanisms underlying the development of the brain and cancer during neurogenesis and carcinogenesis, respectively. Several cell signaling pathways regulated by MCPH genes have been studied. Some molecular factors, such as p53, CDK, and GlI family proteins, function in both neurogenesis and carcinogenesis processes (Table 2).
Table 2.
The molecular mechanism of MCPH-associated genes relative to neurogenesis and carcinogenesis.
| Gene | Neurogenesis | Ref | Carcinogenesis | Ref. |
|---|---|---|---|---|
| MCPH1 (BRIT1) | CHK1, CDC25, CDK1 | [16] | SLUG, CDK1, p53, CDH1, MDM2, SNAIL | [42] |
| p53 | [110] | |||
| CyclinA2, CDK2, CDC25C-cyclinB, CDC2, p53, BCL-2, Bax, Cytochrome c, Caspase-3, PARP-1 | [40] | |||
|
MCPH2
(WDR62) |
JNK | [18] | N/A | |
| AURKA | [119] | |||
| PLK1 | [43] | |||
| MCPH3 (CDK5RAP2) | p35 | [120] | N/A | |
| MST1, TAZ (Hippo) | [121] | |||
| MCPH4 (CASC5) | BUB1, BUBR, ZWINT-1 | [122] | BUB1 | [52] |
| miR-193b-3p | [27] | |||
| p53 | [123] | |||
|
MCPH5 (ASPM) |
Wnt p53 |
[21] [114] |
Wnt | [55,124,125] |
|
MCPH6
(CENPJ) |
KIF2A | [56] | N/A | |
| ASCL1 | [57] | |||
|
MCPH7
(STIL) |
CPAP | [58] | Casepase-3/7, MAPK/ERK, PI3K/AKT, AMPK | [28] |
| RTTN | [60] | PD-L1 | [126] | |
| CDK1 | [104] | IGF-1/PI3K/Akt | [32] | |
| GLI1(Shh) | [127] | GLI1(Shh) | [128] | |
| MCPH10 (ZNF335) | REST/NRSF | [65] | N/A | |
|
MCPH12
(CDK6) |
GLI3 (Shh) | [66] | FOXO3 | [67] |
| Neuroglobin | [70] | GLI2 (Shh) | [129] | |
| p27 | [68] | p16INK4a, VEGF-A | [71] | |
| CD25, Notch | [72] | |||
| SUMO1 | [69] | |||
|
MCPH13
(CENPE) |
N/A | FOXM1 | [25] | |
|
MCPH17
(CIT) |
Tubulin β-III | [76] | p53 | [26] |
| Trp53 | [77] | Tp53, Tp73 | [33] | |
|
MCPH18
(WDFY3) |
Wnt | [79] | miR-18a, RORA | [78] |
|
MCPH19
(COPB2) |
Camk1γb | [130] | p21, WAF1/CIP1, P27 KIP1, CDK2, CDK4, Cyclin D1 | [82] |
| YAP1 (Hippo) | [83] | |||
| RTK | [86] | |||
|
MCPH20
(KIF14) |
N/A | p27 (KIP1), SKP2, CKS1 | [88] | |
| GADD45A, GADD45B, P21, PIDD, SHISA5, p53, Hippo | [36] | |||
| AKT | [131] | |||
| SP1, YY1 | [87] | |||
|
MCPH21
(NCAPD2) |
N/A | p53 | [39] | |
|
MCPH24
(NUP37) |
N/A | YAP (Hippo) | [37] | |
|
MCPH25
(MAP11) |
PLK1 | [90] | N/A | |
N/A, not applicable/reported.
The p53 gene (TP53) is classified as a tumor suppressor gene, and upregulation of its activity led to neural progenitor apoptosis in MCPH gene-deficient mice. Deletion of Mcph5 increases DNA damage and induces postnatal cerebellar progenitor apoptosis that depends on p53 [114]. Mcph17 KO led to TP53 activation and the TP53-dependent apoptosis of NPCs and neurons in developing brains. NPC death was dramatically reduced in Citk and Trp53 double KO mice [77]. During carcinogenesis, the overexpression of MCPH1 inhibits the migration and invasion of lung cancer cells through the accumulation of p53 [42]. MCPH1 regulates p53 activity and protein stability through blocking MDM2-mediated p53 ubiquitination in breast cancer cells [110]. Conversely, some MCPH gene overexpression promotes cancer cell progression, sometimes through the regulation of p53 activity. MCPH12 contributes to tumor formation by inducing a complex transcriptional program to block p53 in hematopoietic cells [132]. Microarray and bioinformatics analyses indicate that the overexpression of the MCPH17 gene in human colon cancer tissue can affect the occurrence of cancer through the p53 signaling pathway [26]. Consistently, inactivation of some MCPH genes typically inhibits cancer cell progression, sometimes by p53 activity regulation. MCPH7 depletion induces cancer cell apoptosis in a p53-independent manner [115]. Transcriptome analysis by RNA sequencing demonstrated that MCPH20 suppression led to transcriptional changes of genes involved in the p53 signaling pathway, which may lead to apoptosis in prostate cancer [36]. Thus, these results suggest that the p53 status must be considered when designing combinatorial or sequential approaches in precision medicine.
Emerging evidence suggests that tumor cells require specific interphase cyclin-dependent kinases (CDKs) for proliferation. Mutant MCPH1 cells have low levels of Tyr 15-phosphorylated Cdk1 (pY15-Cdk1) in S and G2 phases, while Cdk1 triggers the translocation of MCPH7 from centrosomes to the cytoplasm [104,133]. In addition, MCPH19 knockdown in human prostate carcinoma cells decreases CDK2 and CDK4 expression [82]. This indicates the role of CDKs in microcephaly and carcinoma. Cdkn1b (cyclin-dependent kinase inhibitor 1B), also known as p27, is involved in the regulation of the cell cycle and functions in both neurogenesis and carcinogenesis. During hippocampal neurogenesis, the proliferation of progenitor cells specifically relies on the p27-dependent regulation of MCPH12 kinase activity. CDK2, CDK4, and cyclin D1 were downregulated, whereas p21 (Waf1/Cip1) and p27 (Kip1) were upregulated in MCPH19 knockdown cells compared with in control cells [68]. The role of p27 in carcinogenesis was revealed through studies involving MCPH19 [82]. In MCPH20-silenced hepatocellular carcinoma cells, the levels of cyclins E1, D1, and B1 were profoundly decreased, while the p27 protein level specifically increased [88].
The glioblastoma (GLI) zinc-finger transcription factors, acting as effectors of Sonic hedgehog (Shh) signaling, belong to the C2H2-type zinc finger protein subclass, which is critical for normal embryo development and cancer progression [134,135]. In dopaminergic neurons, MCPH7 functions through the Shh pathway by releasing the inhibition of tumor suppressor protein suppressor of fused (SUFU) to GLI1, and thereby enhances the Shh target gene transcription that is required for neural proliferation, protection, and regeneration [127]. During pancreatic carcinogenesis, MCPH7 is responsible for the depression of GLI1, which is a crucial step in activating Hh signaling in cancer cells [128]. GLI3 controls the onset of cortical neurogenesis by determining the level of MCPH12 expression [66]. Similarly, GLI2 binds to the MCPH12 promoter and activates MCPH12 expression, thereby promoting uncontrolled medulloblastoma cell proliferation [129].
In addition, some MCPH genes function in pathways known to be important for stem cell self-renewal, such as the Wnt (MCPH5 and MCPH18), Notch (MCPH12), Hippo (MCPH3, MCPH19, MCPH20 and MCPH24), and Shh (MCPH7 and MCPH12), all of which were previously identified as being relevant to cancer (Table 2). Hence, a clinical trial using inhibitors to block these pathways can be set for cancer treatment.
8. Conclusions and Perspectives
During brain development, mitosis, the cell cycle, and genomic stability maintenance are particularly important. Disturbance of these processes will cause neurological disorders such as MCPH. Meanwhile, MCPH genes and related pathways play important roles in the process of carcinogenesis or the maintenance of CSCs. Since oncogenesis has always been a difficult problem to address, exploring the molecular mechanism of oncogenesis and finding solutions for it are currently an important research direction. Most importantly, the depletion of some MCPH genes typically suppresses cell division and tumor growth, indicating that the MCPH genes can be considered suitable targets in cancer treatment. Recently, because of its association with microcephaly and other severe neurological diseases, the Zika virus became an emerging flavivirus [136]. Interestingly, several studies have applied this virus to the treatment of glioblastoma—the most aggressive form of brain cancer [137,138,139]. Here, we explored the common role of MCPH gene in neurodevelopment and carcinogenesis. This not only helped in understanding the pathogenesis of microcephaly and cancer but also facilitated investigations into therapeutic strategies against each of these elements.
Traditional cancer therapies have focused on shrinking tumors, but with only moderate improvements. Recently, researchers have focused on studying how cancers arise and developing drugs that kill CSCs. The targeting of cancer by inactivating MCPH genes has the potential to be very attractive. Our study on MCPH genes provides many of the same features and factors shared by neurogenesis and carcinogenesis. However, the common mechanisms that underlie NSCs and CSCs still need to be explored. There are some studies relating MCPH genes with Wnt, Notch, Hippo, and Hedgehog signaling pathways, which are critical for stem cell self-renewal. In the future, substantial effort should be focused on determining whether MCPH genes regulate tumor growth through the regulation of CSC self-renewal.
Acknowledgments
We apologize to the colleagues whose contributions were not discussed and cited due to space limitations.
Abbreviations
| MCPH | MCPH Autosomal recessive primary microcephaly |
| NSC | Neural stem cell |
| CSCs | Cancer stem cells |
| NPCs | Neural progenitor cells |
| KO | Knockout |
| BrdU | 5-bromo-2′-deoxyuridine |
| CCK-8 | Cell counting kit-8 |
| EdU | 5-ethynyl-2′-deoxyuridine |
| FACS | Fluorescent-activated cell sorting |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling |
| HC | Head circumference |
| GC | Gastric cancer |
| OC | Ovarian cancer |
| CRCs | Colorectal cancers |
| CC | Colon cancer |
| PCa | Prostate cancer |
| CDKs | Cyclin-dependent kinases |
| GLI | Glioblastoma protein |
| FOXM1 | Forkhead box M1 |
| TP53 | The p53 gene |
| pY15-Cdk1 | Tyr 15-phosphorylated Cdk1 |
| Cdk1b | Cyclin-dependent kinase inhibitor 1B |
| Shh | Sonic hedgehog |
| SUFU | Suppressor of fused |
Author Contributions
X.Z. and D.X. conceptualized the paper; X.Z., Y.Z., and J.Y. wrote the paper and prepared the figures; and D.X. revised the paper and provided supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the authors’ laboratories was supported by grants from by the National Natural Science Foundation (NSFC) of China (31970920, 31600841) and funding to Dr. Dan Xu by the Science Foundation of the Fujian Province (No. 2018J01730).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Taverna E., Gotz M., Huttner W.B. The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell. Dev. Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 2.Land H., Parada L.F., Weinberg R.A. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 3.Toh T.B., Lim J.J., Chow E.K. Epigenetics in cancer stem cells. Mol. Cancer. 2017;16:29. doi: 10.1186/s12943-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen J.M., Jordan C.T. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayaraman D., Bae B.I., Walsh C.A. The Genetics of Primary Microcephaly. Annu. Rev. Genom. Hum. Genet. 2018;19:177–200. doi: 10.1146/annurev-genom-083117-021441. [DOI] [PubMed] [Google Scholar]
- 6.Yao B., Christian K.M., He C., Jin P., Ming G.L., Song H. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016;17:537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylin S.B. StemBook. Harvard Stem Cell Insititute; Cambridge, MA, USA: 2009. Stem cells, cancer, and epigenetics. [DOI] [PubMed] [Google Scholar]
- 8.Zarco N., Norton E., Quinones-Hinojosa A., Guerrero-Cazares H. Overlapping migratory mechanisms between neural progenitor cells and brain tumor stem cells. Cell. Mol. Life Sci. 2019;76:3553–3570. doi: 10.1007/s00018-019-03149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallavicini G., Berto G.E., Di Cunto F. Precision Revisited: Targeting Microcephaly Kinases in Brain Tumors. Int. J. Mol. Sci. 2019;20:2098. doi: 10.3390/ijms20092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmood S., Ahmad W., Hassan M.J. Autosomal Recessive Primary Microcephaly (MCPH): Clinical manifestations, genetic heterogeneity and mutation continuum. Orphanet J. Rare Dis. 2011;6:39. doi: 10.1186/1750-1172-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J., Eyaid W., Mochida G.H., Al-Moayyad F., Bodell A., Woods C.G., Walsh C.A. ASPM mutations identified in patients with primary microcephaly and seizures. J. Med. Genet. 2005;42:725–729. doi: 10.1136/jmg.2004.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faheem M., Naseer M.I., Rasool M., Chaudhary A.G., Kumosani T.A., Ilyas A.M., Pushparaj P., Ahmed F., Algahtani H.A., Al-Qahtani M.H., et al. Molecular genetics of human primary microcephaly: An overview. BMC Med. Genom. 2015;8:S4. doi: 10.1186/1755-8794-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.J., Zhou X.K., Xu D. Update on autosomal recessive primary microcephaly (MCPH)-associated proteins. Yi Chuan Hered. 2019;41:905–918. doi: 10.16288/j.yczz.19-070. [DOI] [PubMed] [Google Scholar]
- 14.Manzini M.C., Walsh C.A. What disorders of cortical development tell us about the cortex: One plus one does not always make two. Curr. Opin. Genet. Dev. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke Q., Li W., Lai X., Chen H., Huang L., Kang Z., Li K., Ren J., Lin X., Zheng H., et al. TALEN-based generation of a cynomolgus monkey disease model for human microcephaly. Cell Res. 2016;26:1048–1061. doi: 10.1038/cr.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber R., Zhou Z.W., Sukchev M., Joerss T., Frappart P.O., Wang Z.Q. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat. Cell Biol. 2011;13:1325–1334. doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman D., Kodani A., Gonzalez D.M., Mancias J.D., Mochida G.H., Vagnoni C., Johnson J., Krogan N., Harper J.W., Reiter J.F., et al. Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron. 2016;92:813–828. doi: 10.1016/j.neuron.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D., Zhang F., Wang Y., Sun Y., Xu Z. Microcephaly-associated protein WDR62 regulates neurogenesis through JNK1 in the developing neocortex. Cell Rep. 2014;6:104–116. doi: 10.1016/j.celrep.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Florio M., Huttner W.B. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141:2182–2194. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 20.Fish J.L., Kosodo Y., Enard W., Paabo S., Huttner W.B. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman J.J., Durak O., Tsai L.H. ASPM regulates Wnt signaling pathway activity in the developing brain. Genes Dev. 2011;25:1909–1914. doi: 10.1101/gad.16830211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujikura K., Setsu T., Tanigaki K., Abe T., Kiyonari H., Terashima T., Sakisaka T. Kif14 mutation causes severe brain malformation and hypomyelination. PLoS ONE. 2013;8:e53490. doi: 10.1371/journal.pone.0053490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragich J.M., Kuwajima T., Hirose-Ikeda M., Yoon M.S., Eenjes E., Bosco J.R., Fox L.M., Lystad A.H., Oo T.F., Yarygina O., et al. Autophagy linked FYVE (Alfy/WDFY3) is required for establishing neuronal connectivity in the mammalian brain. eLife. 2016;5:e14810. doi: 10.7554/eLife.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frantzi M., Zoidakis J., Papadopoulos T., Zurbig P., Katafigiotis I., Stravodimos K., Lazaris A., Giannopoulou I., Ploumidis A., Mischak H., et al. IMAC fractionation in combination with LC-MS reveals H2B and NIF-1 peptides as potential bladder cancer biomarkers. J. Proteome Res. 2013;12:3969–3979. doi: 10.1021/pr400255h. [DOI] [PubMed] [Google Scholar]
- 25.Shan L., Zhao M., Lu Y., Ning H., Yang S., Song Y., Chai W., Shi X. CENPE promotes lung adenocarcinoma proliferation and is directly regulated by FOXM1. Int. J. Oncol. 2019;55:257–266. doi: 10.3892/ijo.2019.4805. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z., Zhu X., Xu W., Zhang Y., Chen L., Qiu F., Zhang B., Wu L., Peng Z., Tang H. Up-regulation of CIT promotes the growth of colon cancer cells. Oncotarget. 2017;8:71954–71964. doi: 10.18632/oncotarget.18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song B., Du J., Song D.F., Ren J.C., Feng Y. Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and miR-1179 may contribute to the progression of gastric cancer. Biol. Res. 2018;51:44. doi: 10.1186/s40659-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X.C., Xiao Y., Yan W.G., Ji Z.G., Zheng G.Y. The human oncogene SCL/TAL1 interrupting locus (STIL) promotes tumor growth through MAPK/ERK, PI3K/Akt and AMPK pathways in prostate cancer. Gene. 2019;686:220–227. doi: 10.1016/j.gene.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Ganapathi Sankaran D., Stemm-Wolf A.J., Pearson C.G. CEP135 isoform dysregulation promotes centrosome amplification in breast cancer cells. Mol. Biol. Cell. 2019;30:1230–1244. doi: 10.1091/mbc.E18-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mi Y., Yu M., Zhang L., Sun C., Wei B., Ding W., Zhu Y., Tang J., Xia G., Zhu L. COPB2 is upregulated in prostate cancer and regulates PC-3 cell proliferation, Cell Cycle, and Apoptosis. Arch. Med. Res. 2016;47:411–418. doi: 10.1016/j.arcmed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Yin L., Jiang L.P., Shen Q.S., Xiong Q.X., Zhuo X., Zhang L.L., Yu H.J., Guo X., Luo Y., Dong J., et al. NCAPH plays important roles in human colon cancer. Cell Death Dis. 2017;8:e2680. doi: 10.1038/cddis.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Zhang Y., Dou Z., Jiang H., Wang Y., Gao X., Xin X. Knockdown of STIL suppresses the progression of gastric cancer by down-regulating the IGF-1/PI3K/AKT pathway. J. Cell. Mol. Med. 2019:23. doi: 10.1111/jcmm.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallavicini G., Sgro F., Garello F., Falcone M., Bitonto V., Berto G.E., Bianchi F.T., Gai M., Chiotto A.M.A., Filippi M., et al. Inactivation of citron kinase inhibits medulloblastoma progression by inducing apoptosis and cell senescence. Cancer Res. 2018;78:4599–4612. doi: 10.1158/0008-5472.CAN-17-4060. [DOI] [PubMed] [Google Scholar]
- 34.Shinmura K., Kato H., Kawanishi Y., Igarashi H., Inoue Y., Yoshimura K., Nakamura S., Fujita H., Funai K., Tanahashi M., et al. WDR62 overexpression is associated with a poor prognosis in patients with lung adenocarcinoma. Mol. Carcinog. 2017;56:1984–1991. doi: 10.1002/mc.22647. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z., Zhang Q., Luh F., Jin B., Liu X. Overexpression of the ASPM gene is associated with aggressiveness and poor outcome in bladder cancer. Oncol. Lett. 2019;17:1865–1876. doi: 10.3892/ol.2018.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Yuan Y., Liang P., Zhang Z., Guo X., Xia L., Zhao Y., Shu X.S., Sun S., Ying Y., et al. Overexpression of a novel candidate oncogene KIF14 correlates with tumor progression and poor prognosis in prostate cancer. Oncotarget. 2017;8:45459–45469. doi: 10.18632/oncotarget.17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo X., Liu Y., Feng W., Lei L., Du Y., Wu J., Wang S. NUP37, a positive regulator of YAP/TEAD signaling, promotes the progression of hepatocellular carcinoma. Oncotarget. 2017;8:98004–98013. doi: 10.18632/oncotarget.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui F., Hu J., Xu Z., Tan J., Tang H. Overexpression of NCAPH is upregulated and predicts a poor prognosis in prostate cancer. Oncol. Lett. 2019;17:5768–5776. doi: 10.3892/ol.2019.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Liu F., Zhang C., Ren M., Kuang M., Xiao T., Di X., Feng L., Fu L., Cheng S. Non-SMC condensin I complex subunit D2 is a prognostic factor in triple-negative breast cancer for the ability to promote cell cycle and enhance invasion. Am. J. Pathol. 2020;190:37–47. doi: 10.1016/j.ajpath.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Mai L., Yi F., Gou X., Zhang J., Wang C., Liu G., Bu Y., Yuan C., Deng L., Song F. The overexpression of MCPH1 inhibits cell growth through regulating cell cycle-related proteins and activating cytochrome c-caspase 3 signaling in cervical cancer. Mol. Cell. Biochem. 2014;392:95–107. doi: 10.1007/s11010-014-2022-6. [DOI] [PubMed] [Google Scholar]
- 41.Spinola M., Falvella F.S., Colombo F., Sullivan J.P., Shames D.S., Girard L., Spessotto P., Minna J.D., Dragani T.A. MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Mol. Cancer. 2010;9:62. doi: 10.1186/1476-4598-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X., Liu W., Liu X., Ai Q., Yu J. Overexpression of MCPH1 inhibits the migration and invasion of lung cancer cells. Oncotargets Ther. 2018;11:3111–3117. doi: 10.2147/OTT.S156102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto T., Akutsu S.N., Fukumitsu A., Morino H., Masatsuna Y., Hosoba K., Kawakami H., Yamamoto T., Shimizu K., Ohashi H., et al. PLK1-mediated phosphorylation of WDR62/MCPH2 ensures proper mitotic spindle orientation. Hum. Mol. Genet. 2017;26:4429–4440. doi: 10.1093/hmg/ddx330. [DOI] [PubMed] [Google Scholar]
- 44.Zeng S., Tao Y., Huang J., Zhang S., Shen L., Yang H., Pei H., Zhong M., Zhang G., Liu T., et al. WD40 repeat-containing 62 overexpression as a novel indicator of poor prognosis for human gastric cancer. Eur. J. Cancer. 2013;49:3752–3762. doi: 10.1016/j.ejca.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Alshawaf A.J., Antonic A., Skafidas E., Ng D.C., Dottori M. WDR62 regulates early neural and glial progenitor specification of human pluripotent stem cells. Stem Cells Int. 2017;2017:9. doi: 10.1155/2017/7848932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Tian Y., Yu J.J., He J., Luo J., Zhang S., Tang C.E., Tao Y.M. Overexpression of WDR62 is associated with centrosome amplification in human ovarian cancer. J. Ovarian Res. 2013;6:55. doi: 10.1186/1757-2215-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J.F., Zhang Y., Wilde J., Hansen K.C., Lai F., Niswander L. Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 2014;5:3885. doi: 10.1038/ncomms4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu D., Yao M., Wang Y., Yuan L., Hoeck J.D., Yu J., Liu L., Yeap Y.Y.C., Zhang W., Zhang F., et al. MEKK3 coordinates with FBW7 to regulate WDR62 stability and neurogenesis. PLoS Biol. 2018;16:e2006613. doi: 10.1371/journal.pbio.2006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lizarraga S.B., Margossian S.P., Harris M.H., Campagna D.R., Han A.P., Blevins S., Mudbhary R., Barker J.E., Walsh C.A., Fleming M.D. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walz C., Curtis C., Schnittger S., Schultheis B., Metzgeroth G., Schoch C., Lengfelder E., Erben P., Muller M.C., Haferlach T., et al. Transient response to imatinib in a chronic eosinophilic leukemia associated with ins(9;4)(q33;q12q25) and a CDK5RAP2-PDGFRA fusion gene. Genes Chromosomes Cancer. 2006;45:950–956. doi: 10.1002/gcc.20359. [DOI] [PubMed] [Google Scholar]
- 51.Buchman J.J., Tseng H.C., Zhou Y., Frank C.L., Xie Z., Tsai L.H. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 2010;66:386–402. doi: 10.1016/j.neuron.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Bai T., Zhao Y., Liu Y., Cai B., Dong N., Li B. Effect of KNL1 on the proliferation and apoptosis of colorectal cancer cells. Technol. Cancer Res. Treat. 2019:18. doi: 10.1177/1533033819858668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogi H., Nitta N., Tando S., Fujimori A., Aoki I., Fushiki S., Itoh K. Longitudinal diffusion tensor imaging revealed nerve fiber alterations in Aspm mutated microcephaly model mice. Neuroscience. 2018;371:325–336. doi: 10.1016/j.neuroscience.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Fujimori A., Itoh K., Goto S., Hirakawa H., Wang B., Kokubo T., Kito S., Tsukamoto S., Fushiki S. Disruption of Aspm causes microcephaly with abnormal neuronal differentiation. Brain Dev. 2014;36:661–669. doi: 10.1016/j.braindev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Hsu C.C., Liao W.Y., Chan T.S., Chen W.Y., Lee C.T., Shan Y.S., Huang P.J., Hou Y.C., Li C.R., Tsai K.K. The differential distributions of ASPM isoforms and their roles in Wnt signaling, cell cycle progression, and pancreatic cancer prognosis. J. Pathol. 2019 doi: 10.1002/path.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding W., Wu Q., Sun L., Pan N.C., Wang X. Cenpj regulates cilia disassembly and neurogenesis in the developing mouse cortex. J. Neurosci. Off. J. Soc. Neurosci. 2019;39:1994–2010. doi: 10.1523/JNEUROSCI.1849-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcez P.P., Diaz-Alonso J., Crespo-Enriquez I., Castro D., Bell D., Guillemot F. Cenpj/CPAP regulates progenitor divisions and neuronal migration in the cerebral cortex downstream of Ascl1. Nat. Commun. 2015;6:6474. doi: 10.1038/ncomms7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cottee M.A., Muschalik N., Wong Y.L., Johnson C.M., Johnson S., Andreeva A., Oegema K., Lea S.M., Raff J.W., van Breugel M. Crystal structures of the CPAP/STIL complex reveal its role in centriole assembly and human microcephaly. eLife. 2013;2:e01071. doi: 10.7554/eLife.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arquint C., Nigg E.A. The PLK4-STIL-SAS-6 module at the core of centriole duplication. Biochem. Soc. Trans. 2016;44:1253–1263. doi: 10.1042/BST20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H.Y., Wu C.T., Tang C.C., Lin Y.N., Wang W.J., Tang T.K. Human microcephaly protein RTTN interacts with STIL and is required to build full-length centrioles. Nat. Commun. 2017;8:247. doi: 10.1038/s41467-017-00305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patwardhan D., Mani S., Passemard S., Gressens P., El Ghouzzi V. STIL balancing primary microcephaly and cancer. Cell Death Dis. 2018;9:65. doi: 10.1038/s41419-017-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabinowicz N., Mangala L.S., Brown K.R., Checa-Rodriguez C., Castiel A., Moskovich O., Zarfati G., Trakhtenbrot L., Levy-Barda A., Jiang D., et al. Targeting the centriolar replication factor STIL synergizes with DNA damaging agents for treatment of ovarian cancer. Oncotarget. 2017;8:27380–27392. doi: 10.18632/oncotarget.16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussain M.S., Baig S.M., Neumann S., Nurnberg G., Farooq M., Ahmad I., Alef T., Hennies H.C., Technau M., Altmuller J., et al. A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am. J. Hum. Genet. 2012;90:871–878. doi: 10.1016/j.ajhg.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh P., Ramdas Nair A., Cabernard C. The centriolar protein Bld10/Cep135 is required to establish centrosome asymmetry in Drosophila neuroblasts. Curr. Biol. CB. 2014;24:1548–1555. doi: 10.1016/j.cub.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y.J., Baltus A.E., Mathew R.S., Murphy E.A., Evrony G.D., Gonzalez D.M., Wang E.P., Marshall-Walker C.A., Barry B.J., Murn J., et al. Microcephaly gene links trithorax and REST/NRSF to control neural stem cell proliferation and differentiation. Cell. 2012;151:1097–1112. doi: 10.1016/j.cell.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasenpusch-Theil K., West S., Kelman A., Kozic Z., Horrocks S., McMahon A.P., Price D.J., Mason J.O., Theil T. Gli3 controls the onset of cortical neurogenesis by regulating the radial glial cell cycle through Cdk6 expression. Development. 2018;145:dev163147. doi: 10.1242/dev.163147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dall’Acqua A., Sonego M., Pellizzari I., Pellarin I., Canzonieri V., D’Andrea S., Benevol S., Sorio R., Giorda G., Califano D., et al. CDK6 protects epithelial ovarian cancer from platinum-induced death via FOXO3 regulation. EMBO Mol. Med. 2017;9:1415–1433. doi: 10.15252/emmm.201607012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caron N., Genin E.C., Marlier Q., Verteneuil S., Beukelaers P., Morel L., Hu M.G., Hinds P.W., Nguyen L., Vandenbosch R., et al. Proliferation of hippocampal progenitors relies on p27-dependent regulation of Cdk6 kinase activity. Cell. Mol. Life Sci. 2018;75:3817–3827. doi: 10.1007/s00018-018-2832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellail A.C., Olson J.J., Hao C. SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nat. Commun. 2014;5:4234. doi: 10.1038/ncomms5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luyckx E., Van Leuven W., Andre D., Quarta A., Reekmans K., Fransen E., Moens L., Hankeln T., Ponsaerts P., Dewilde S. Loss of neuroglobin expression alters Cdkn1a/Cdk6-expression resulting in increased proliferation of neural stem cells. Stem Cells Dev. 2018;27:378–390. doi: 10.1089/scd.2017.0097. [DOI] [PubMed] [Google Scholar]
- 71.Kollmann K., Heller G., Schneckenleithner C., Warsch W., Scheicher R., Ott R.G., Schafer M., Fajmann S., Schlederer M., Schiefer A.I., et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24:167–181. doi: 10.1016/j.ccr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jena N., Sheng J., Hu J.K., Li W., Zhou W., Lee G., Tsichlis N., Pathak A., Brown N., Deshpande A., et al. CDK6-mediated repression of CD25 is required for induction and maintenance of Notch1-induced T-cell acute lymphoblastic leukemia. Leukemia. 2016;30:1033–1043. doi: 10.1038/leu.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shinmura K., Kato H., Kawanishi Y., Nagura K., Kamo T., Okubo Y., Inoue Y., Kurabe N., Du C., Iwaizumi M., et al. SASS6 overexpression is associated with mitotic chromosomal abnormalities and a poor prognosis in patients with colorectal cancer. Oncol. Rep. 2015;34:727–738. doi: 10.3892/or.2015.4014. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H., Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto S., Jaiswal M., Charng W.L., Gambin T., Karaca E., Mirzaa G., Wiszniewski W., Sandoval H., Haelterman N.A., Xiong B., et al. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–214. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sgro F., Bianchi F.T., Falcone M., Pallavicini G., Gai M., Chiotto A.M., Berto G.E., Turco E., Chang Y.J., Huttner W.B., et al. Tissue-specific control of midbody microtubule stability by Citron kinase through modulation of TUBB3 phosphorylation. Cell Death Differ. 2016;23:801–813. doi: 10.1038/cdd.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianchi F.T., Tocco C., Pallavicini G., Liu Y., Verni F., Merigliano C., Bonaccorsi S., El-Assawy N., Priano L., Gai M., et al. Citron kinase deficiency leads to chromosomal instability and TP53-sensitive microcephaly. Cell Rep. 2017;18:1674–1686. doi: 10.1016/j.celrep.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li W., Ma S., Bai X., Pan W., Ai L., Tan W. Long noncoding RNA WDFY3-AS2 suppresses tumor progression by acting as a competing endogenous RNA of microRNA-18a in ovarian cancer. J. Cell. Physiol. 2019 doi: 10.1002/jcp.29028. [DOI] [PubMed] [Google Scholar]
- 79.Le Duc D., Giulivi C., Hiatt S.M., Napoli E., Panoutsopoulos A., Harlan De Crescenzo A., Kotzaeridou U., Syrbe S., Anagnostou E., Azage M., et al. Pathogenic WDFY3 variants cause neurodevelopmental disorders and opposing effects on brain size. Brain A J. Neurol. 2019;142:2617–2630. doi: 10.1093/brain/awz198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Napoli E., Song G., Panoutsopoulos A., Halmai J., Levenson R., Zarbalis K.S., Giulivi C., Riyadh M.A., Kaushik G. Beyond autophagy: A novel role for autism-linked Wdfy3 in brain mitophagy. Sci. Rep. 2018;8:11348. doi: 10.1038/s41598-018-29421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiStasio A., Driver A., Sund K., Donlin M., Muraleedharan R.M., Pooya S., Kline-Fath B., Kaufman K.M., Prows C.A., Schorry E., et al. Copb2 is essential for embryogenesis and hypomorphic mutations cause human microcephaly. Hum. Mol. Genet. 2017;26:4836–4848. doi: 10.1093/hmg/ddx362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mi Y., Sun C., Wei B., Sun F., Guo Y., Hu Q., Ding W., Zhu L., Xia G. Coatomer subunit beta 2 (COPB2), identified by label-free quantitative proteomics, regulates cell proliferation and apoptosis in human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2018;495:473–480. doi: 10.1016/j.bbrc.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 83.Pu X., Wang J., Li W., Fan W., Wang L., Mao Y., Yang S., Liu S., Xu J., Lv Z., et al. COPB2 promotes cell proliferation and tumorigenesis through up-regulating YAP1 expression in lung adenocarcinoma cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018;103:373–380. doi: 10.1016/j.biopha.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Li Z.S., Liu C.H., Liu Z., Zhu C.L., Huang Q. Downregulation of COPB2 by RNAi inhibits growth of human cholangiocellular carcinoma cells. Eur. Rev. Med Pharmacol. Sci. 2018;22:985–992. doi: 10.26355/eurrev_201802_14380. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Chai Z., Wang M., Jin Y., Yang A., Li M. COPB2 suppresses cell proliferation and induces cell cycle arrest in human colon cancer by regulating cell cycle-related proteins. Exp. Ther. Med. 2018;15:777–784. doi: 10.3892/etm.2017.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.An C., Li H., Zhang X., Wang J., Qiang Y., Ye X., Li Q., Guan Q., Zhou Y. Silencing of COPB2 inhibits the proliferation of gastric cancer cells and induces apoptosis via suppression of the RTK signaling pathway. Int. J. Oncol. 2019;54:1195–1208. doi: 10.3892/ijo.2019.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Theriault B.L., Basavarajappa H.D., Lim H., Pajovic S., Gallie B.L., Corson T.W. Transcriptional and epigenetic regulation of KIF14 overexpression in ovarian cancer. PLoS ONE. 2014;9:e91540. doi: 10.1371/journal.pone.0091540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu H., Choe C., Shin S.H., Park S.W., Kim H.S., Jung S.H., Yim S.H., Kim T.M., Chung Y.J. Silencing of KIF14 interferes with cell cycle progression and cytokinesis by blocking the p27(Kip1) ubiquitination pathway in hepatocellular carcinoma. Exp. Mol. Med. 2014;46:e97. doi: 10.1038/emm.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin C.A., Murray J.E., Carroll P., Leitch A., Mackenzie K.J., Halachev M., Fetit A.E., Keith C., Bicknell L.S., Fluteau A., et al. Mutations in genes encoding condensin complex proteins cause microcephaly through decatenation failure at mitosis. Genes Dev. 2016;30:2158–2172. doi: 10.1101/gad.286351.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perez Y., Bar-Yaacov R., Kadir R., Wormser O., Shelef I., Birk O.S., Flusser H., Birnbaum R.Y. Mutations in the microtubule-associated protein MAP11 (C7orf43) cause microcephaly in humans and zebrafish. Brain. 2019;142:574–585. doi: 10.1093/brain/awz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez A.D., Feldman J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017;44:93–101. doi: 10.1016/j.ceb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marthiens V., Rujano M.A., Pennetier C., Tessier S., Paul-Gilloteaux P., Basto R. Centrosome amplification causes microcephaly. Nat. Cell Biol. 2013;15:731–740. doi: 10.1038/ncb2746. [DOI] [PubMed] [Google Scholar]
- 93.Nigg E.A., Raff J.W. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 94.Liang Y., Gao H., Lin S.Y., Peng G., Huang X., Zhang P., Goss J.A., Brunicardi F.C., Multani A.S., Chang S., et al. BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet. 2010;6:e1000826. doi: 10.1371/journal.pgen.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barr A.R., Kilmartin J.V., Gergely F. CDK5RAP2 functions in centrosome to spindle pole attachment and DNA damage response. J. Cell Biol. 2010;189:23–39. doi: 10.1083/jcb.200912163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Letard P., Drunat S., Vial Y., Duerinckx S., Ernault A., Amram D., Arpin S., Bertoli M., Busa T., Ceulemans B., et al. Autosomal recessive primary microcephaly due to ASPM mutations: An update. Hum. Mutat. 2018;39:319–332. doi: 10.1002/humu.23381. [DOI] [PubMed] [Google Scholar]
- 97.Okamoto N., Kohmoto T., Naruto T., Masuda K., Imoto I. Primary microcephaly caused by novel compound heterozygous mutations in ASPM. Hum. Genome Var. 2018;5:18015. doi: 10.1038/hgv.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang X., Liu D., Lv S., Wang H., Zhong X., Liu B., Wang B., Liao J., Li J., Pfeifer G.P., et al. CDK5RAP2 is required for spindle checkpoint function. Cell Cycle. 2009;8:1206–1216. doi: 10.4161/cc.8.8.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Y.C., Chang C.W., Hsu W.B., Tang C.J., Lin Y.N., Chou E.J., Wu C.T., Tang T.K. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J. 2013;32:1141–1154. doi: 10.1038/emboj.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cizmecioglu O., Arnold M., Bahtz R., Settele F., Ehret L., Haselmann-Weiss U., Antony C., Hoffmann I. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J. Cell Biol. 2010;191:731–739. doi: 10.1083/jcb.201007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hussain M.S., Baig S.M., Neumann S., Peche V.S., Szczepanski S., Nurnberg G., Tariq M., Jameel M., Khan T.N., Fatima A., et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Hum. Mol. Genet. 2013;22:5199–5214. doi: 10.1093/hmg/ddt374. [DOI] [PubMed] [Google Scholar]
- 102.Strnad P., Leidel S., Vinogradova T., Euteneuer U., Khodjakov A., Gonczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levine M.S., Bakker B., Boeckx B., Moyett J., Lu J., Vitre B., Spierings D.C., Lansdorp P.M., Cleveland D.W., Lambrechts D., et al. Centrosome amplification is sufficient to promote spontaneous tumorigenesis in mammals. Dev. Cell. 2017;40:313–322. doi: 10.1016/j.devcel.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arquint C., Nigg E.A. STIL microcephaly mutations interfere with APC/C-mediated degradation and cause centriole amplification. Curr. Biol. 2014;24:351–360. doi: 10.1016/j.cub.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 105.Megraw T.L., Sharkey J.T., Nowakowski R.S. Cdk5rap2 exposes the centrosomal root of microcephaly syndromes. Trends Cell Biol. 2011;21:470–480. doi: 10.1016/j.tcb.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sercin O., Larsimont J.C., Karambelas A.E., Marthiens V., Moers V., Boeckx B., Le Mercier M., Lambrechts D., Basto R., Blanpain C. Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis. Nat. Cell Biol. 2016;18:100–110. doi: 10.1038/ncb3270. [DOI] [PubMed] [Google Scholar]
- 107.Insolera R., Bazzi H., Shao W., Anderson K.V., Shi S.H. Cortical neurogenesis in the absence of centrioles. Nat. Neurosci. 2014;17:1528–1535. doi: 10.1038/nn.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang M., Nagle R.B., Knudsen B.S., Cress A.E., Rogers G.C. Centrosome loss results in an unstable genome and malignant prostate tumors. Oncogene. 2020;39:399–413. doi: 10.1038/s41388-019-0995-z. [DOI] [PubMed] [Google Scholar]
- 109.Doxsey S., Zimmerman W., Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 110.Zhang B., Wang E., Dai H., Hu R., Liang Y., Li K., Wang G., Peng G., Lin S.Y. BRIT1 regulates p53 stability and functions as a tumor suppressor in breast cancer. Carcinogenesis. 2013;34:2271–2280. doi: 10.1093/carcin/bgt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen J., Wo D., Ma E., Yan H., Peng J., Zhu W., Fang Y., Ren D.N. Deletion of low-density lipoprotein-related receptor 5 inhibits liver Cancer cell proliferation via destabilizing Nucleoporin 37. Cell Commun. Signal. 2019;17:174. doi: 10.1186/s12964-019-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong Q. The centrosome-centered cell-brain in apoptosis. Med. Hypotheses. 2003;61:126–132. doi: 10.1016/S0306-9877(03)00144-0. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Z.W., Tapias A., Bruhn C., Gruber R., Sukchev M., Wang Z.Q. DNA damage response in microcephaly development of MCPH1 mouse model. DNA Repair. 2013;12:645–655. doi: 10.1016/j.dnarep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 114.Williams S.E., Garcia I., Crowther A.J., Li S., Stewart A., Liu H., Lough K.J., O’Neill S., Veleta K., Oyarzabal E.A., et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development. 2015;142:3921–3932. doi: 10.1242/dev.124271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Erez A., Castiel A., Trakhtenbrot L., Perelman M., Rosenthal E., Goldstein I., Stettner N., Harmelin A., Eldar-Finkelman H., Campaner S., et al. The SIL gene is essential for mitotic entry and survival of cancer cells. Cancer Res. 2007;67:4022–4027. doi: 10.1158/0008-5472.CAN-07-0064. [DOI] [PubMed] [Google Scholar]
- 116.Alves C.L., Elias D., Lyng M., Bak M., Kirkegaard T., Lykkesfeldt A.E., Ditzel H.J. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin. Cancer Res. 2016;22:5514–5526. doi: 10.1158/1078-0432.CCR-15-1984. [DOI] [PubMed] [Google Scholar]
- 117.Peng G., Yim E.K., Dai H., Jackson A.P., Burgt I., Pan M.R., Hu R., Li K., Lin S.Y. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat. Cell Biol. 2009;11:865–872. doi: 10.1038/ncb1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou L., Bai Y., Li Y., Liu X., Tan T., Meng S., He W., Wu X., Dong Z. Overexpression of MCPH1 inhibits uncontrolled cell growth by promoting cell apoptosis and arresting the cell cycle in S and G2/M phase in lung cancer cells. Oncol. Lett. 2016;11:365–372. doi: 10.3892/ol.2015.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lim N.R., Shohayeb B., Zaytseva O., Mitchell N., Millard S.S., Ng D.C.H., Quinn L.M. Glial-specific functions of microcephaly protein WDR62 and interaction with the mitotic kinase AURKA are essential for Drosophila brain growth. Stem Cell Rep. 2017;9:32–41. doi: 10.1016/j.stemcr.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosales J.L., Rattner J.B., Lee K.Y. The primary microcephaly 3 (MCPH3) interacting protein, p35 and its catalytic subunit, Cdk5, are centrosomal proteins. Cell Cycle. 2010;9:618–620. doi: 10.4161/cc.9.3.10597. [DOI] [PubMed] [Google Scholar]
- 121.Sukumaran S.K., Stumpf M., Salamon S., Ahmad I., Bhattacharya K., Fischer S., Muller R., Altmuller J., Budde B., Thiele H., et al. CDK5RAP2 interaction with components of the Hippo signaling pathway may play a role in primary microcephaly. Mol. Genet. Genom. 2017;292:365–383. doi: 10.1007/s00438-016-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Genin A., Desir J., Lambert N., Biervliet M., Van Der Aa N., Pierquin G., Killian A., Tosi M., Urbina M., Lefort A., et al. Kinetochore KMN network gene CASC5 mutated in primary microcephaly. Hum. Mol. Genet. 2012;21:5306–5317. doi: 10.1093/hmg/dds386. [DOI] [PubMed] [Google Scholar]
- 123.Urata Y.N., Takeshita F., Tanaka H., Ochiya T., Takimoto M. Targeted Knockdown of the kinetochore protein D40/Knl-1 inhibits human cancer in a p53 status-independent manner. Sci. Rep. 2015;5:13676. doi: 10.1038/srep13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang W.Y., Hsu C.C., Wang T.Y., Li C.R., Hou Y.C., Chu J.M., Lee C.T., Liu M.S., Su J.J., Jian K.Y., et al. A gene expression signature of epithelial tubulogenesis and a role for ASPM in pancreatic tumor progression. Gastroenterology. 2013;145:1110–1120. doi: 10.1053/j.gastro.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 125.Pai V.C., Hsu C.C., Chan T.S., Liao W.Y., Chuu C.P., Chen W.Y., Li C.R., Lin C.Y., Huang S.P., Chen L.T., et al. ASPM promotes prostate cancer stemness and progression by augmenting Wnt-Dvl-3-beta-catenin signaling. Oncogene. 2019;38:1340–1353. doi: 10.1038/s41388-018-0497-4. [DOI] [PubMed] [Google Scholar]
- 126.Pan B.J., Xu C., Ping G.Q., Song G.X., Zhang W.M., Wang C., Zhang Z.H. Correlation analysis of PD-L1 expression and prognosis in triple-negative breast cancers. Zhonghua Bing Li Xue Za Zhi Chin. J. Pathol. 2017;46:822–826. doi: 10.3760/cma.j.issn.0529-5807.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 127.Izraeli S., Lowe L.A., Bertness V.L., Good D.J., Dorward D.W., Kirsch I.R., Kuehn M.R. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399:691–694. doi: 10.1038/21429. [DOI] [PubMed] [Google Scholar]
- 128.Kasai K., Inaguma S., Yoneyama A., Yoshikawa K., Ikeda H. SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res. 2008;68:7723–7729. doi: 10.1158/0008-5472.CAN-07-6661. [DOI] [PubMed] [Google Scholar]
- 129.Raleigh D.R., Choksi P.K., Krup A.L., Mayer W., Santos N., Reiter J.F. Hedgehog signaling drives medulloblastoma growth via CDK6. J. Clin. Investig. 2018;128:120–124. doi: 10.1172/JCI92710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herrero-Turrion M.J., Rodriguez-Martin I., Lopez-Bellido R., Rodriguez R.E. Whole-genome expression profile in zebrafish embryos after chronic exposure to morphine: Identification of new genes associated with neuronal function and mu opioid receptor expression. BMC Genom. 2014;15:874. doi: 10.1186/1471-2164-15-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singel S.M., Cornelius C., Zaganjor E., Batten K., Sarode V.R., Buckley D.L., Peng Y., John G.B., Li H.C., Sadeghi N., et al. KIF14 promotes AKT phosphorylation and contributes to chemoresistance in triple-negative breast cancer. Neoplasia. 2014;16:247–256. doi: 10.1016/j.neo.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellutti F., Tigan A.S., Nebenfuehr S., Dolezal M., Zojer M., Grausenburger R., Hartenberger S., Kollmann S., Doma E., Prchal-Murphy M., et al. CDK6 antagonizes p53-induced responses during tumorigenesis. Cancer Discov. 2018;8:884–897. doi: 10.1158/2159-8290.CD-17-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arroyo M., Kuriyama R., Trimborn M., Keifenheim D., Canuelo A., Sanchez A., Clarke D.J., Marchal J.A. MCPH1, mutated in primary microcephaly, is required for efficient chromosome alignment during mitosis. Sci. Rep. 2017;7:13019. doi: 10.1038/s41598-017-12793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villavicencio E.H., Walterhouse D.O., Iannaccone P.M. The sonic hedgehog-patched-gli pathway in human development and disease. Am. J. Hum. Genet. 2000;67:1047–1054. doi: 10.1016/S0002-9297(07)62934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Armas-Lopez L., Zuniga J., Arrieta O., Avila-Moreno F. The Hedgehog-GLI pathway in embryonic development and cancer: Implications for pulmonary oncology therapy. Oncotarget. 2017;8:60684–60703. doi: 10.18632/oncotarget.19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu D., Li C., Qin C.F., Xu Z. Update on the animal Models and underlying mechanisms for ZIKV-induced microcephaly. Annu. Rev. Virol. 2019;6:459–479. doi: 10.1146/annurev-virology-092818-015740. [DOI] [PubMed] [Google Scholar]
- 137.Chen Q., Wu J., Ye Q., Ma F., Zhu Q., Wu Y., Shan C., Xie X., Li D., Zhan X., et al. Treatment of human glioblastoma with a live attenuated Zika virus vaccine candidate. MBio. 2018:9. doi: 10.1128/mBio.01683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu Z., Gorman M.J., McKenzie L.D., Chai J.N., Hubert C.G., Prager B.C., Fernandez E., Richner J.M., Zhang R., Shan C., et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017;214:2843–2857. doi: 10.1084/jem.20171093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lubin J.A., Zhang R.R., Kuo J.S. Zika virus has oncolytic activity against glioblastoma stem cells. Neurosurgery. 2018;82:E113–E114. doi: 10.1093/neuros/nyy047. [DOI] [PMC free article] [PubMed] [Google Scholar]