Abstract
Honey is both a complex food and medicine as well as a healthy alternative to refined sugar. Besides a complex mixture of carbohydrates, honey contains other minor substances which may threaten human health in excess concentrations. Several environmental conditions can affect the quality of honey. This research paper aims to measure the degree of heavy metals (Lead (Pb), Cadmium (Cd), Zinc (Zn), and Copper (Cu)) in some polyfloral honey from an industrial area of Romania, considered to be one of the most polluted regions in Eastern Europe. The samples were collected from six stationary apiaries and analysed using the atomic absorption spectrometry method. The content of Pb was higher in the sampling areas exposed directly to the polluted air masses. Cd concentration decreases exponentially while Cu concentration increases as the distance from the source of pollution increases. The checking of the quality of polyfloral honey from local producers is imperative because this product is intended to be consumed by the beekeeper’s family or the local community without being sold to an authorised processor. The results of the study can help to set a threshold for the concentration of Pb and Cd in honey marketed in the European Union.
Keywords: health risk, AAS (atomic absorbtion spectrometry), historical polluted area
1. Introduction
Food safety has become an essential food quality attribute, not only because of the major role played by foodstuffs within a human healthy diet, but also the issue of public concern [1]. The presence of heavy metals in foodstuffs is becoming increasingly more obvious and food consumption represents the main way of access to the human body [1,2] with potentially negative effects on human health, especially because heavy metals de-regulate the immune system [3] and lead to severe diseases, including cancer, cardiovascular diseases, and neurological disorders [4,5,6]. Heavy metals persist and biomagnify in trophic chains, and when in concentrations above the maximum permissible limits, they become toxic [7]. High concentrations of heavy metals pose a risk to consumer health, while prolonged consumption of honey containing Cu and Fe causes gastrointestinal disorders [8]. Heavy metals can affect the quality of life when these are accumulated in the body at a toxic level, and may threaten the health of consumers [9,10].
Honey represents an important element of the human diet because of its positive nutritional and health effects [11]. Honey is a bee product from the nectar or excretions of plants, composed of a complex mixture of carbohydrates [12]. The main nutritional value of honey is due to the presence of simple, inverted sugars, such as glucose and fructose, which are an immediate and prompt source of energy for the human body—for example, 100 g of honey generates 300 kcal of energy [13]. According to Bilsel et al. [14], honey displays its curative properties in the treatment of diabetic ulcer and external wounds in the case of allergies, inflammation of the pharynx, and coughs, and it also has visible antimicrobial properties [15], can prevent gastroesophageal reflux [16], and its components have shown apoptotic effects in colon cancer cells [17]. Honey contains minor constituents, such as enzymes, proteins, amino and organic acids, vitamins, lipids, volatile chemicals, flavonoids, phenolic acids, and minerals [18,19,20]. The quality and biochemical proprieties of honey depend on the nectar source, climatic conditions, maturation period of the honey, production methods, and processing and storage conditions. Honey also contains macro- and microelements that are the minor constituents of honey present in the range of 0.02%–1.03%. The heavy metal concentration in honey depends on the geographical origin of the flower composition [21] and its presence indicates that the hives were close to contamination sources. The concentration of heavy metals is correlated with the honey’s spatial distribution from the main pollution source, but also with the air dispersion conditions from the area [22].
Hernandez et al. [23] list K, P, Mg, Al, Ca, Na, Fe, Mn, Cu, Zn, Cl, S, and Si as common mineral components in honey, while Batista et al. [24] note that potentially toxic metals, such as Pb, Hg, and Cd appear at lower concentrations than other heavy metals.
Porrini et al. [25] showed the concentrating role of bees regarding heavy metal pollutant accumulation in bee products, highlighting that the hair on the bee’s body retained atmospheric particles containing heavy metals. Anthropogenic sources can also contaminate honey [26].Historical pollution of the environment contributes to the more pronounced internalization of pollutants in the hive [27,28]. High levels of Cd in honey were detected in agroecosystems due to the use of mineral fertilisers and pesticides [29]. Regardless of the polluting source, both Pb and Cd are heavy metals that contaminate bee products [30].
Setting up the apiary in the vicinity of a point source or of diffuse pollution sources, the use of agrochemicals, fertilisers containing heavy metals, and the fumigation of bees causes the contamination of honey and bee products and the intake of pollutants into the hive [31]. Gonzalez et al. [32] explained that high Cd content in honey could arise by improper storage or equipment-related contamination. Contamination of honey and other bee products may be due to the environment or improper storage, handling, or processing. Storage in containers and the use of galvanised tools can also contaminate honey with Zn [33].
Bees collect pollutants from the environment, such as radioactive substances, heavy metals, and inorganic compounds, with all of these being indicators of local pollution. Perugini et al. [34] considered honey to be a time and spatial Pb contamination detector. By visiting several flowers, beesbioconcentrate metal pollutants from the environment, which can have potentially harmful effects on human health. According to Celli and Porrini [35], the flying range of bees covers an area of about 7 km2, and as the bee gets in direct contact with heavy metals deposited on plants, the honey and other bee products become a good indicator of the degree of environmental contamination [30,36].
According to European Committee (EC) [37], Romania is one of many honey-producing countries, alongside Spain, Hungary, Germany, Italy, Greece, France, and Poland which are all countries with a favourable climate for beekeeping. In this context, studies were conducted in order to determine the quality and volatile compounds of Romanian honey [38,39,40]. In 2015, honey production in Romania was 35,000 tons. Roman [26] showed that the honey market was imposing ever-higher quality standards, where consumers are asking for quality honey with no additional waste nor loads of potentially toxic metal pollutants. Due to an increase in the anthropological footprint of ecosystems and the degree of pollution thereof, the quality of honey is an obvious and stringent issue which concerns both honey producers and processors, where appropriate, and especially consumers who consume unprocessed honey directly from producers.
The purpose of this research is to determine heavy metal content (Pb, Cd, Cu, and Zn) in honey from private apiaries located in the most well-known historical polluted area in Romania. The analytical values obtained can provide information on the regional dynamics of pollution and allow for the adoption of acceptable thresholds in terms of the heavy metal content of natural honey, which are currently not in place.
2. Materials and Methods
2.1. Sample Collection Area
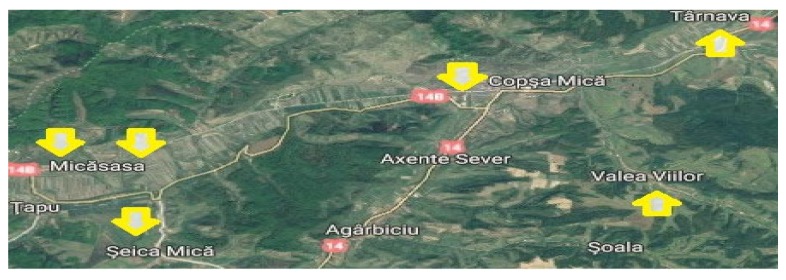
The polyfloral honey samples were collected directly from local beekeepers at six stationary apiaries (four samples from each apiary) from the town of Copșa Mică, Romania and the neighboring villages of Micăsasa, Târnava, Valea Viilor, and Şeica Mică (Table 1, Figure 1).
Table 1.
Location of the apiaries.
| No. | Plot Identification | Site Description in Relation with Pollution Edge | Distance from the Main Pollution Source (km) |
|---|---|---|---|
| 1 | 46°03′27.97″ N, 24°04′28.43″ E, 312 m.a.s.l |
Secondary valley channeling the atmospheric circulation of pollutants from the main valley | 9.90 |
| 2 | 46°05′10.60″ N, 24°05′29.97″ E, 283 m.a.s.l |
Site frontal exposure to thepollution source | 10.54 |
| 3 | 46°05′24.03″ N, 24°06′52.86″ E, 280 m.a.s.l |
Site frontal exposure to thepollution source | 8.72 |
| 4 | 46°08′17.88″ N, 24°17′26.16″ E, 287 m.a.s.l |
Site frontal exposure to thepollution source | 5.91 |
| 5 | 46°05′03.91″ N, 24°16′30.08″ E, 340 m.a.s.l |
Secondary valley channeling the atmospheric circulation of pollutants from the main valley | 5.49 |
| 6 | 46°06′23.92″ N, 24°13′27.14″ E, 310 m.a.s.l |
Site frontal exposure to thepollution source | 1.12 |
Figure 1.
Map of the honey sampling localities.
This paper aims to identify the presence of potentially toxic heavy metals in the honey collected from an environment polluted for over 60 years by industrial activity with non-ferrous metals on the industrial platform of Copșa Mică. The dispersion of pollutants emitted by the main polluter of the Copșa Mică area is governed by the local climate. The wind regime of the area is influenced by the land orography, especially the presence of the Târnava Mare valley, the main air masses being channeled into the corridor of the Târnava Mare river (northeastern and southwestern air circulation) and the corridor of the Visa river (South–North air circulation).
The source of the pollution whose effects are analyzed in this article is an industrial park in the town of Copșa Mică (46°06′59.10″ N and 24°13′15.43″ E) in the center of Romania.
2.2. Method of Analysis
The collected samples were stored in hermetically sealed plastic containers and kept in a cool and dark space at temperatures ranging between 4–5 °C until analytical determinations were carried out. Before analysing the polyfloral honey samples without visible granules, the samples were shaken for homogenization purposes, and those containing crystallised sugar (four samples) were heated to 65 °C in a water bath for 30 minutes to homogenise and solubilise the crystals. From the homogenised samples, 1 g of honey was weighed into polypropylene tubes, which were dissolved in 100 ml of heated, deionised, ultra-pure water(18.2 MΩ-cm resistivity from the water purification system Direct Q3UV Smart, Milipore, SAS, Molsheim, France). The solutions thus obtained were subjected to wet mineralisation, using 0.5 ml of analytically pure HNO3 (Merck, Darmstadt, Germany) concentrate (65% v/v) as an oxidising agent, and the Top Wave Analytic Jena AG (Germany) microwave system was also used, designed for pressure digestion at temperatures up to 230 °C and pressures up to a maximum of 100 bars (1450 psi). The digester could contain a maximum of 24 digestion vessels made of modified polytetrafluorethylene (TFM-PTFE). Vessels were cleaned with 50 ml HNO3 before each mineralization. The glassware used was left overnight in a 10% HNO3 solution and rinsed prior to use with purified water to prevent possible contamination with Pb, Cd, Cu, and Zn. The extract was then filtered, ransferred to 25 mL volumetric flasks, and made to volume with deionised water. Mineralization was carried out in four steps, at temperatures of 145, 170,190, and 100 °C (Table 2).
Table 2.
Temperature program of digestion.
| Step | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Temp. [°C] | 145 | 170 | 190 | 100 |
| Ramp [min] | 2 | 5 | 2 | 1 |
| Hold [min] | 5 | 10 | 15 | 10 |
Digestions of polyfloral honey samples were carried out in triplicate. A blank digest was carried out.
For the quantitative analysis of Pb, Cd, Zn, and Cu in the obtained extracts, we used the atomic absorption spectrometry method (AAS) because the harvested honey originated from a medium historically affected with a significant load of metal pollutants [41]. Quantitative determination of Cu and Zn was realized by FAAS (flame atomic absorbtion spectrometry) [42] while the concentration of non-essential trace metals Pb and Cd was performed using GFAAS (graphite furnace atomic absorption spectrometry) [43]. All samples were analysed in triplicate, and mean values were obtained. We used the Shimadzu AA-6300 Spectrometer equipped with a graphite furnace and single-element hollow cathode lamps adapted to eachanalysed metal (Cu was measured using the multi-element hollow method lamp), and the D2 lamp for background correction (BGC-D2). In FAAS for determination of Zn and Cu, an air-acetylene flame was used (Acetylene purity 98%, flow rate 1.8–2.0 L/min.) and a slot burner head from titan which was 10 cm long. For graphite furnace determination, argon (Ar) was used (flow rate 0–1.5L/min). The instrument parameters were optimised following the manufacturer’s recommendations (Table 3). Merck standard solutions (1000 mgL−1) of Pb, Cd, Zn, and Cu were used to prepare the working standards [44]
Table 3.
Instrumental analytical values of investigated trace elements in FAAS.
| Element | Flame Type | Fuel FlowL/min | Lamp Current mA |
Wavelength nm |
Slit Width nm |
Air Flow L/min |
|---|---|---|---|---|---|---|
| Zn | Air-Acetylene | 2.00 | 3 | 213.9 | 0.7 | 17.00 |
| Cu | Air-Acetylene | 2.00 | 3 | 324.8 | 0.7 | 17.00 |
During the analyses, the argon flow rate through the graphite tube was 250 mL/min. Settings for graphite furnace atomic absorption spectrometry (GFAAS) are summarized in Table 4.
Table 4.
GFAAS program for non-essential trace elements Pb and Cd.
| Non-Essential Trace Metal | Pb | Cd |
|---|---|---|
| Wavelength (nm) | 283.3 | 228.8 |
| Argon flow (Ml/min) | 250 | 250 |
| Sample volume (µL) | 20 | 20 |
| Heating Program Temperature (°C; ramp time (s), hold time (s). | ||
| Drying 1 | 100 (5.20) | 100 (5.20) |
| Drying 2 | 140 (15.5) | 140 (15.15) |
| Ashing | 700 (10.20) | 700 (10.20) |
| Atomization | 1800 (0.5) | 1650 (0.5) |
| Cleaning | 2600 (1.3) | 2600 (1.3) |
3. Results
All samples subjected to analytical determinations had detectable Pb, Cd, Cu, and Zn content. Concentration values show a significant dispersion; the differences between the apiaries were statistically less reliable for copper (Table 5). Statistical analyses was performed using STATISTICA 8.0 (Stat Soft, Tulsa, OK, USA).
Table 5.
Statistical data on heavy metal content in polyfloral honey.
| Metal | Range | Median | Coefficient of Variation (%) | The Significance of the Differences between Plots | |
|---|---|---|---|---|---|
| t | p | ||||
| Pb (mg/kg) | 0.76–3.41 | 1.49 | 56.27 | 4.35 | 0.007 |
| Cd (mg/kg) | 0.05–3.81 | 2.20 | 81.90 | 2.99 | 0.030 |
| Zn (mg/kg) | 15.00–36.40 | 20.40 | 36.74 | 6.67 | 0.001 |
| Cu (mg/kg) | 2.00–33.00 | 3.70 | 122.63 | 2.00 | 0.100 |
The order of the accumulation degree expressed in median concentration values mg/kg for the elements surveyed is Zn > Cu > Cd > Pb.
The differences in terms of frontal exposure versus secondary valley exposure are statistically significant only for the Pb content (Table 6), which is, on average, 1 mg/kg higher in the apiaries located on the main valley, which is the driver for the pollutants of the apiaries on the side valleys.
Table 6.
Stratification of heavy metal concentrations in polyfloral honey.
| Dependent Variable | ||||
|---|---|---|---|---|
| Grouping variable | Pb | Cd | Zn | Cu |
| p from Mann–Whitney U test (0.05 is the threshold value for statistical significance) | ||||
| Exposure to air circulation | 0.05 | 0.64 | 0.64 | 0.90 |
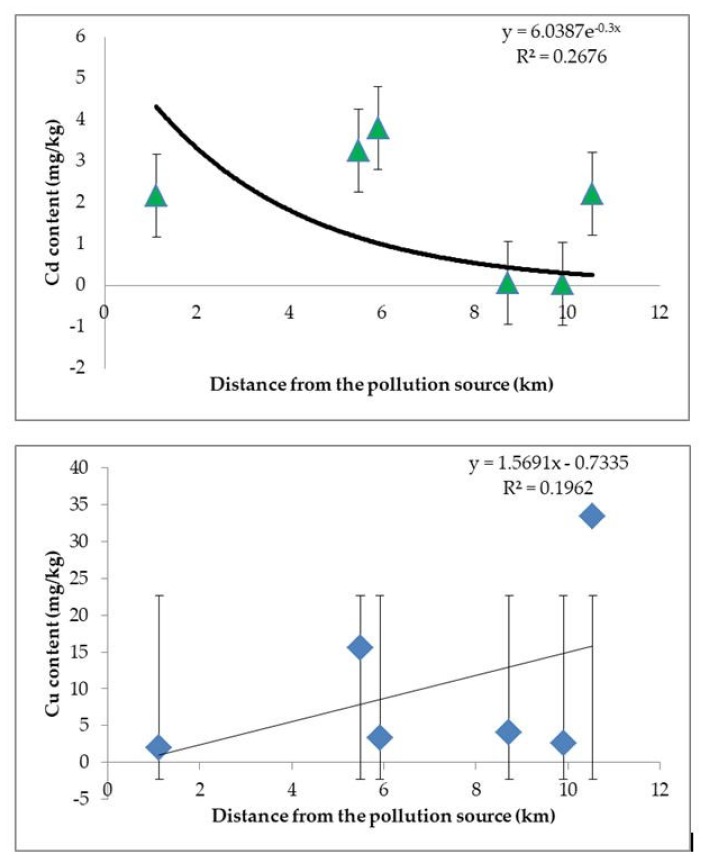
From the Figure, concentrations of Cd in honey show a decreasing trend while moving away from the source of pollution, while Cu concentrations are increasing (Figure 2). Levels of Pb and Zn vary independently of the distance from the source of pollution (Spearman rank-order correlation = −0.029, p = 0.96).
Figure 2.
Cd (up) and Cu (down) content in honey against the distance from the main source of pollution.
4. Discussion
The polluters on the industrial platform were present mainly in the valley of the Târnava Mare river being scattered to the West (village of Micăsasa and city of Blaj), the East (city of Mediaş and town of Dumbrăveni), or South in the direction of the Șomârd-Şoala villages [45]. The preferential circulation of the air masses guided by the orography of the land resulted in the accumulation of significant amounts of Pb and Cd in the honey sampled from the localities of Târnava (located east of the main polluter) and Valea Viilor (southeast of Copşa Mică). The analytical values of Zn and Cu are above those determined in the honey sampled in the town of Copşa Mică, which supports the model of the remote dispersion of pollutants and the importance of the role of orography.
The accumulation of Pb in the environment is mainly due to mining, the melting and production of metals, and the battery industry, as Pb is a non-essential metal that has no physiological role in the metabolism of plants or animals. The exposure to Pb occurs through contact with soil, air, water, and contaminated food [46], and in time it causes anemia, neurological effects, nephropathy, renal tubular dysfunction, as well as impairment of reproductive function in both genders [47].
Although it is easily surpassed by Cd in terms of average and maximum levels, the honey sampled contained significant amounts of concentrated Pb (Table 2). Golob et al. [48] determined a maximum Pb concentration of 79.1 mg/kg in honey sampled in Slovenia, and Yilmaz and Yavuz [49], discovered high values of 4.2–6.3 mg/kg Pb in honey sampled in the Southeast Anatolia Region, Turkey. Concentrations of up to 80.37 mg/kg were detected in honey sampled in the polluted areas of Italy by Dambrosio and Marchesini [50]. Frias et al. [51] determined high Pb concentrations of 31.50 mg/kg in the honey sampled in Tenerife, Spain. The maximum Pb concentrations are lower than those obtained between 2005–2011 by Berinde and Michnea [52], who also determined Pbconcentration values ranging between 0.18–20.34 mg/kg in the area of the city of Baia Mare, which is also a historical polluted area of Romania. Bogdanov [30] reviewed the results presented in various papers in the literature and noted concentrations of Pb in honey ranging between 0.01–1.8 mg/kg and concentrations of Cd ranging between 0.03–2.1 mg/kg.
As far as the Pb content in Polish honey goes, Pb concentration values above 1 mg/kg were also obtained by Roman [27] from the honey sampled in the Legnica and Glogow industrial regions (Pb median values ranging between 0.465–1.097 mg/kg) and between 0.17–1.90 mg/kg in the Wroclaw region (Lower Silesia), [53]. Oddi and Bertani [54], Conti et al. [55], and Porrini et al. [28] determined maximum Pb concentration values ranging between 1.10–1.74 mg/kg in polyfloral honey sampled in Italy. Singh et al. [56], reported Pb concentration values ranging between 0.2–4.2 mg/kg in the case of 13 samples of monofloral and polyfloral honey collected from different regions of Karnataka, India. An analysis of some honey batches collected on the Czech market in 1999 from different honey producers indicated Pb concentration values ranging between 0.0184–1.0003 mg/kg [57].
Low concentrations of Pb which are below the values presented in our research were discovered in honey samples studied by Tuzen and Soylak [26] in Central Anatolia, with values ranging between 0.0176–0.0321 mg/kg; the same goes for Matusevicius et al. [58] in samples from different areas of Lithuania, being 0.0032–0.0241 mg/kg; Derebasi et al. [59] determined very low concentrations of Pb (6.68–7.30 ppb/kg) in honey sampled directly from producers in the Black Sea Region of Turkey in 2007, and Dzugan et al. [60] mentioned in their research maximum levels of Pb of 0.18 mg/kg in polyfloral honey sampled from Podkarpackie in Southeast Poland. In Romania, Simedru et al. [61] also determined low Pb concentration values (0.06–0.19 mg/kg) in honey sampled in Cluj County.
With regard to Cd concentrations, similar or lower levels in comparison with those determined in our research were reported by Dzugan et al. [60] (0.03 ppm) in Podcapacia, Southeast Poland; Derebasi et al. [59] (0.07 mg/kg) in Turkey; Tuzen and Soylak [26] (between 0.010–0.022 mg/kg) in Central Anatolia-Turkey; and Celechovska and Vorlova [57] (between 0.0005–0.0774 mg/kg) and Singh et al. [56] (0.005–0.76 mg/kg) in Karnataka India. Matusevicius et al. [58] reported maximum Cd concentrations (between 0.0039–0.0165 mg/kg) in different regions of Lithuania. Cd concentration values in polyfloral honey in the sample plot of Târnava, Micăsasa II, CopşaMică, and ValeaViilor surpassed the ones obtained by Bratu and Georgescu [62] in CopşaMică at a distance of 8–25 km from the main source of pollution. Polyfloral honey from the sample plot of ŞeicaMică and Micăsasa I was found to have a lower Cd content, as against the one analysed by Bratua nd Georgescu [62] (0.015–0.032 mg/kg).
The maximum concentration of Cd of 3.809 mg/kg that was determined from the polyfloral honey sampled from Târnava apiary was below the values observed by Frias et al. [51] (46.32 mg/kg Cd) in Tenerife, Spain. Simedru et al. [61] showed that the level of Cd in honey samples in Cluj County was below the detection limit of the used equipment. Devillers et al. [63] studied 150 samples of acacia honey from polluted areas (50%) and “virtually unpolluted” areas in the territory of France, and noticed the absence of Cd and Pb from the analysed samples, although other metals such as Ag, Zn, and Cr were detected as consequences of anthropogenic pollution.
Although zinc is an essential element, excessive bio-accumulated concentrations are the effect of anthropogenic intake. Acute poisoning with Zn occurs through inhalation at the workplace with symptoms such as nausea, vomiting, diarrhea, lethargy, and fever [64].
The honey examined by us contains the essential Zn and Cu trace elements in concentrations ranging between 15.00–36.40 mg/kg in the case of Zn, the median value being 20.400 mg/kg, and between 2.00–33.00 mg/kg in the case of Cu with a median value of 3.70 mg/kg. The maximum values close to the Zn values determined by us are those reported by Celechovska and Vorlova [57], as well as by Tuzen and Soylak [26] (Table 7).
Table 7.
Comparative results on honey contamination with Zn and Cu in different research areas.
| Literature Source | Zn Concentration (mg/kg) | Cu Concentration (mg/kg) | Honey Samples Source |
|---|---|---|---|
| Dzugan et al., 2017 [60] | 12.57 | 0.77 | Polyfloral honey/Podkarpackie southeastern Poland |
| Derebasi et al., 2014 [59] | 0.15–0.17 | 0.17–0.19 | Turkey, Black Sea Region |
| Tuzen and Soylak, 2005 [26] | 1.1–24.2 | 0.25–1.10 | Turkey, Central Anatolia Region |
| Berinde and Michnea, 2013 [52] | 1.09–1.39 | 0.24–0.32 | Romania, city of Baia Mare |
| Bratu and Georgescu, 2005 [62] | 2.3 | Honey samples provided by Bee Breeders Association/unpolluted area | |
| 1.8–5.6 | Romania, CopșaMică, polluted area 8–25 km away from the source of pollution | ||
| Ciobanu and Rădulescu, 2016 [65] | 0.987 | 18.89 | Polyfloral honey/Romania, Timiș County, in the vicinity of sources of pollution |
| Celechovska and Vorlova, 2001 [57] | 0.190–22.9 | 0.057–1.55 | Honey samples from the Czech market |
| Devillers et al., 2002 [63] | 0.04–5.96 | 0.03–2.30 | Acacia honey/Polluted and unpolluted areas of France |
| Roman et al., 2011 [53] | 0.51–7.85 | 0.45–2.43 | Poland, Wroclaw |
| Matusevicius et al., 2010 [58] | 0.564–5.008 | 0.1106–0.3894 | Honey from various areas of Lithuania |
The level of heavy metals in honey can often be increased by poor harvesting and storage conditions, and contamination during the fumigation, extraction, and storage of honey which are non-compliant with hygiene standards recommended or required by law. This is why honey monitoring is required at both the producer and processor levels. The high values in terms of non-essential heavy metals concentration measured in sampled honey are a warning for the local population, as in terms of the provisional tolerable weekly intake (PTWI) for Pb and Cd, other food coming from a polluted environment also participates, and the potentially toxic heavy metal concentration in the human body subsequently increases.
According to EC 2000a, Regulation (EC) No. 396/2005 [66], Food Standard Agency, 2003: The Honey Regulations 2003 [67], the current legislation does not mention maximum permitted limits of heavy metals in honey. However, according to Byrne [68], the European Commission recommends an acceptable maximum level of 1 mg/kg in the case of Pb and 0.1 mg/kg in the case of Cd.
Commission Regulation (EC) No. 1881/2006 of 19 December 2006, in setting maximum levels of contaminants in foodstuffs [69] (as amended by the Commission Regulation (EU) 2015/1005 of 25 June 2015 [70] sets the maximum level of Pb at 0.10 mg/kg and of Cd at 0.05 mg/kg. Thus, as to the median value of Pb, the content of honey harvested in Copşa Mică and its surroundings exceeds 14.9 times, and the median value of Cd exceeds 44 times the maximum permissible limits imposed by the European Commission in foodstuffs. Two samples of honey were from Şeica Mică and Valea Viilor, respectively; the villages of Şeica Mică and Micăsasa I had concentrations of Pb and Cd below the maximum permissible limits. Both maximum and median concentrations of Zn and Cu pose no risk of toxicity to the human body.
5. Conclusions
Historical pollution in the surveyed area, totaling over six decades of permanent contact with pollutants, marked the main bee product—honey. The results of this study offers valuable information regarding the effects of the environment on the quality of polyfloral honey produced in the Copșa Mică area. Analytical determinations of the content of potentially toxic metal pollutants in the polyfloral honey collected from the local bee producers with stationary hives in the closing year of industrial production activity indicated high concentrations of accumulated heavy metals, posing a risk to the health of consumers. The apiaries located on the valley that channel the pollutants from the industrial platform are prone to higher Pb bioaccumulations than the apiaries situated on the side valleys. Concentrations of Cd were exponentially diminishing while moving away from the source of pollution, while Cu concentrations increased linearly.
The results are useful for improving the quality of the honey value chain. An integrated program on quality assurance should be conducted. Beekeepers should pay attention to the location of the apiaries since there still are high accumulations of heavy metals in the area. Because of the interrelation between environmental pollution and food, honey and bee products need to be carefully monitored to eliminate even suspected contamination, especially in a historically polluted area from appointed source pollution, such as the town of Copșa Mică and its neighborhood. This study also has several limitations related to the limited number of analyzed apiaries and research areas. For future studies, the research area should be extended, and comparative analysis should be conducted.
Author Contributions
Conceptualization and methodology and writing—original manuscript, S.B. and G.G.; writing—review and editing, I.T.; formal analysis, software I.A.V., F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liang G., Li W.G.B., Zuo J., Pan L., Liu X. Analysis of Heavy Metals in Foodstuffs and an Assessment of the Health Risks to the General Public via Consumption in Beijing, China. Int. J. Environ. Res. Public Health. 2019;16:909. doi: 10.3390/ijerph16060909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan S.M., Wang W.X., Ni I.H. The uptake of Cd, Cr, and Zn by the macroalgaenteromorphacrinita and subsequent transfer to the marine herbivorous rabbitfish, siganus. Arch. Environ. Contam. Toxicol. 2003;44:298–306. doi: 10.1007/s00244-002-2077-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.J., Lim H.S., Lee K.R., Choi M.H., Kang N.M., Lee C.H., Oh E.J., Park H.K. Determination of Trace Metal Levels in the General Population of Korea. Int. J. Environ. Res. Public Health. 2017;14:702. doi: 10.3390/ijerph14070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy Metal Toxicity and the Environment. In: Luch A., editor. Molecular, Clinical and Environmental Toxicology. Volume 101. Springer; Basel, Switzerland: 2012. pp. 134–164. Experientia Supplementum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matés J.M., Segura J.A., Alonso F.J., Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010;49:1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Lamas G.A., Navas-Acien A., Mark D.B., Lee K.L. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016;67:2411–2418. doi: 10.1016/j.jacc.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mejías E., Garrido T. Analytical Procedures for Determining Heavy Metal Contents in Honey: A Bioindicator of Environmental Pollution. In: de Alencar V., de Toledo A., editors. Honey Analysis, Chapter:14. INTECH; London, UK: 2007. pp. 311–324. [DOI] [Google Scholar]
- 8.Salem S.N. 1982. Honey regimen in gastrointestinal disorders. Bulletin of Islam. Med. 1982;2:422–425. [Google Scholar]
- 9.Ru Q.M., Feng Q., He J.Z. Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chem. Toxicol. 2013;53:256–262. doi: 10.1016/j.fct.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Munoz O.R., Camara C. Speciation related to human health. In: Ebdon L., Pitts L., Cornelis R., Crew H., Donard O.F.X., Quevauviller P., editors. Trace Element Speciation for Environment, Food and Health. UK. The Royal Society of Chemistry; London, UK: 2001. pp. 331–353. [Google Scholar]
- 11.Alvarez-Suarez J.M., Tulipani S., Romandini S., Bertoli E., Battino M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010;3:15–23. doi: 10.1007/s12349-009-0051-6. [DOI] [Google Scholar]
- 12.Saxena S., Gautam S., Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118:391–397. doi: 10.1016/j.foodchem.2009.05.001. [DOI] [Google Scholar]
- 13.Baglio E. Chemistry and Technology of Honey Production. Molecular Science. Chem. Foods. 2018;59:12. [Google Scholar]
- 14.Bilsel Y., Bugra D., Yamaner S., Bulut T., Cevikbas U., Turkoglu U. Could Honey Have a Place in Colitis Therapy. Dig. Surg. 2002;29:306–312. doi: 10.1159/000064580. [DOI] [PubMed] [Google Scholar]
- 15.White W.J., Subers M.H., Schepartz A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta. 1963;73:57–70. doi: 10.1016/0926-6569(63)90108-1. [DOI] [PubMed] [Google Scholar]
- 16.Math M.V., Balasubramaniam P. Viscosity and flow of honey. Indian J. Physiol. Pharmacol. 2001;45:supplement Abstract-Phy-76. [Google Scholar]
- 17.Jaganathan S.K., Mandal M. Honey constituents and their apoptotic effect in colon cancer cells. J. ApiProduct ApiMedical Sci. 2009;1:29–36. doi: 10.3896/IBRA.4.01.2.02. [DOI] [Google Scholar]
- 18.Blasa M., Candiracci M., Accorsi A., Piacentini M.P., Albertini M.C., Piatti E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006;97:217–222. doi: 10.1016/j.foodchem.2005.03.039. [DOI] [Google Scholar]
- 19.Bilandzic N., Dokic M., Sedak M., Kolanovic B.S., Varenina I., Koncurat A., Rudan N. Determination of trace elements in Croatian floral honey originating from different regions. Food Chem. 2011;128:1160–1164. doi: 10.1016/j.foodchem.2011.04.023. [DOI] [Google Scholar]
- 20.Osés S.M., Pascual-Maté A., Fernández-Muiño M.A., López-Díaz T.M., Sancho M.T. Bioactive properties of honey with propolis. Food Chem. 2016;196:1215–1223. doi: 10.1016/j.foodchem.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Rashed M.N., Soltan M.E. Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J. Food Compos. Anal. 2004;17:725–735. doi: 10.1016/j.jfca.2003.10.004. [DOI] [Google Scholar]
- 22.Mihaieascu T., Odagiu A., Goji G., Mihaiescu R., Oroian I. Heavy metals contamination of common blackberry in an area with a historical pollution—Copsa Mica (Romania); Proceedings of the 17th International Multidisciplinary Scientific GeoConference SGEM; Albena, Bulgaria. 29 June–5 July 2017; 2017. pp. 845–852. [DOI] [Google Scholar]
- 23.Hernandez O.M., Fraga J.M.G., Jimenez A.I., Jimenez F., Arias J.J. Characterization of honey from the Canary Islands: Determinationof the mineral content by atomic absorption spectrophotometry. Food Chem. 2005;93:449–458. doi: 10.1016/j.foodchem.2004.10.036. [DOI] [Google Scholar]
- 24.Batista B.L., Da Silva L.R.S., Rocha B.A., Rodrigues J.L., Berretta-Silva A.A., Bonates T.O., Gomes V.S.D., Barbosa R.M., Barbosa F. Multi-element determination in Brazilian honey samples by inductively coupled plasma mass spectrometry and estimation of geographic origin with data mining techniques. Food Res. Int. 2012;49:209–215. doi: 10.1016/j.foodres.2012.07.015. [DOI] [Google Scholar]
- 25.Porrini C., Sabatini A.G., Girotti S., Ghini S., Medrzycki P., Grillenzoni F., Bortolotti L., Gattavecchia E., Celli G. Honey bees and bee products as monitors of the environmental contamination. Apiacta. 2003;38:63–70. [Google Scholar]
- 26.Tuzen M., Soylak M. Trace heavy metal levels in microwave digested honey samples from middle Anatolia, Turkey. J. Food Drug Anal. 2005;13:343–347. [Google Scholar]
- 27.Roman A. Bees and their products as pollution bio-indicator in the copper (LGOM) andlime-cement (Opole) industry areas. Zesz. Nauk. Akademii Rolniczej we Wrocławiu. 1997;323:175–193. [Google Scholar]
- 28.Porrini C., Ghini S., Girotti S., Sabatini A.G., Gattavecchia E., Celli G. Use of honey bees as bioindicators of environmental pollution in Italy. In: Devillers J., Pham-Delègue M.-H., editors. Honey Bees: Estimating the Environmental Impact of Chemicals. Taylor&Francis; Oxfordshire, UK: 2002. pp. 186–247. [Google Scholar]
- 29.Roman A., Bartkowiak A., Reginia M. The accumulation of selected chemical elements of toxic properties in bee honey originating from the industrial and rural-forest areas. ISAH-Tartu. 2007;2:877–881. [Google Scholar]
- 30.Bogdanov S. Contaminants of Bee Products. Apidologie. 2006;37:1–18. doi: 10.1051/apido:2005043. [DOI] [Google Scholar]
- 31.Montenegro G., Mejías E. Biological applications of honeys produced by Apismellifera. Biol. Res. 2013;46:341–345. doi: 10.4067/S0716-97602013000400005. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez Paramas A.M., Barez A.G., Garcia-Villanova R.J., Pala T.R., Albajar A.R., Sanchez J.S. Geographical discrimination of honeys by using mineral composition and common chemical quality parameters. J. Sci. Food Agric. 2000;80:157–165. doi: 10.1002/(SICI)1097-0010(20000101)80:1<157::AID-JSFA506>3.0.CO;2-B. [DOI] [Google Scholar]
- 33.Darmati D., Boskovic L., Darmati S. Trace elements in honey from Sumadija region. Hrana-i-Ishrana. 1985;26:129–131. [Google Scholar]
- 34.Perugini M., Manera M., Grotta L., Abete M.C., Tarasco R., Amorena M. Heavy metals (Hg, Cr, Cd and Pb) contamination in urban areas and wildlife reserves: Honeybees as bio-indicators. Biol. Trace Elem. Res. 2011;140:170–176. doi: 10.1007/s12011-010-8688-z. [DOI] [PubMed] [Google Scholar]
- 35.Celli G., Porrini C. L’ape, unefficacebioindicatoredeipesticidi. Le Scienze. 1991;274:42–54. [Google Scholar]
- 36.Fredes C., Montenegro G. Heavy metals and other trace elements contents in Chilean honey. Cien. Inv. Agric. 2006;33:50–58. doi: 10.7764/rcia.v33i1.328. [DOI] [Google Scholar]
- 37.European Commission . Honey Market Presentation. Agriculture and Rural Development. European Commission; Brussels, Belgium: 2017. p. 27. [Google Scholar]
- 38.Pérez R.A., Sánchez-Brunete C., Calvo R.M., Tadeo J.L. Analysis of Volatiles from Spain Honeys by Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2002;50:2633–2637. doi: 10.1021/jf011551r. [DOI] [PubMed] [Google Scholar]
- 39.Cimpoiu C., Hosu A., Miclăuş V., Puşcaş A. Determination of floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;100:149–154. doi: 10.1016/j.saa.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Mădaş N.M., Mărghitaş L.A., Dezmirean D.S., Bonta V., Bobiş O., Fauconnier M.L., Francis F., Haubruge E., Nguyen K.B. Volatile Profile and Physico-Chemical Analysis of Acacia Honey for Geographical Origin and Nutritional Value Determination. Foods. 2019;8:445. doi: 10.3390/foods8100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert O., Piroux M., Puyo S., Thorin C., Larhantec M., Delbac F., Pouliquen H. Bees, honey and pollen as sentinels for lead environmental contamination. Environ. Pollut. 2012;170:254–259. doi: 10.1016/j.envpol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Tuzen M., Silici S., Mendil D., Soylak M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007;103:325–330. doi: 10.1016/j.foodchem.2006.07.053. [DOI] [Google Scholar]
- 43.Santos D., Barbosa F., Tomazelli A.C., Krug F.J., Nobrega J.A., Arruda M.A.Z. Determination of Cd and Pb in food slurries by GF AAS using cryogenic grinding forsample preparation. Analy. Bioanal. Chem. 2002;373:183–189. doi: 10.1007/s00216-002-1296-9. [DOI] [PubMed] [Google Scholar]
- 44.AOAC . Official Methods for Heavy Metal Determination in Human and Pests. 18th ed. AOAC; Virginia, MD, USA: 2006. pp. 63–65. [Google Scholar]
- 45.Alexa B., Cotarlea I., Barbatei R. Poluarea Padurilor din Ocolul Silvic Medias si Lucrarile de Reconstructie Realizate. Editura Constant; Sibiu, Romania: 2014. [Google Scholar]
- 46.Kabata-Pendias A. Trace Elements in Soils and Plants. 4th ed. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 47.HPA . Chemical Hazards Compendium and Health Emergency Planning. Lead: Health Effects, Incident Management and Toxicology. Lead: Toxicological Overview. GOV.UK; London, UK: 2016. p. 14. [Google Scholar]
- 48.Golob T., Dobersek U., Kump P., Necemer M. Determination of trace and minor elements in Slovenian honey by total reflection X-ray fluorescence spectroscopy. Food Chem. 2005;91:593–600. doi: 10.1016/j.foodchem.2004.04.043. [DOI] [Google Scholar]
- 49.Yilmaz H., Yavuz O. Content of some trace metals in honey from South Eastern Anatolia. Food Chem. 1999;65:475–476. doi: 10.1016/S0308-8146(98)00205-2. [DOI] [Google Scholar]
- 50.Dambrosio M., Marchesihi A. Research on contamination by heavy metals in honey sample. Atti Della Soc. Ital. Sci. Nat. 2002;123:342–348. [Google Scholar]
- 51.Frias I., Rubio C., Gonzalez-Iglesias T., Gutierrez A.J., Gonzalez-Weller D., Hardisson A. Metals in fresh honeys from Tenerife Island, Spain. Bull. Environ. Contam. Toxicol. 2008;80:30–33. doi: 10.1007/s00128-007-9301-9. [DOI] [PubMed] [Google Scholar]
- 52.Berinde Z.M., Michnea A.M. A Comparative Study of Environmental and Honey pollution with heavy metals. J. Sci. Arts Year. 2013;2:173–180. [Google Scholar]
- 53.Roman A., Majewska B.M., Popiela-Pleban E. Comparative study of selected toxic elements in propolis and honey. J. Apic. Sci. 2011;55:97–105. [Google Scholar]
- 54.Oddi P., Bertani P. Contaminanti nel miele: Nota 1. Residui di Pb e Cd. Atti S.I.S. Vet. 1987;XLI:998–1000. [Google Scholar]
- 55.Conti M.E., Saccares S., Cubadda F., Cavallina R., Tenoglio C.A., Ciprotti L. Il mielenellazio: Indaginesulcontenuto in metallitracce e radionuclidi. Riv. Sci. Aliment. 1998;2:107–119. [Google Scholar]
- 56.Singh C., Shubharani R., Sivaram V. Assessment of heavy metals in honey by atomic absorption spectrometer. World J. Pharm. Pharm. Sci. 2014;3:509–515. [Google Scholar]
- 57.Celechovska O., Vorlova L. Groups of Honey-Physicochemical Properties and Heavy Metals. Acta. Vet. Brno. 2001;70:91–95. doi: 10.2754/avb200170010091. [DOI] [Google Scholar]
- 58.Matusevicius P., Staniskiene B., Budreckiene R. Metals and organochlorine compounds in Lithuanian honey. Polish J. Food Nutr. Sci. 2010;60:159–163. [Google Scholar]
- 59.Derebaşı E., Bulut G., Col M., Güney F., Yaşar N., Ertürk O. Physicochemical and residue analysis of honey from Black Sea Region of Turkey. Fresenius Environ. Bull. 2014;23:10–17. [Google Scholar]
- 60.Dżugan M., Zaguła G., Wesołowska M., Sowa P., Puchalski C.Z. Levels of toxic and essential metals in varietal honeys from Podkarpacie. J. Elem. 2017;22:1039–1048. doi: 10.5601/jelem.2016.21.4.1298. [DOI] [Google Scholar]
- 61.Simedru D., Becze A., Cadar O., Roman M., Tanaselia C. Polycyclic Aromatic Hydrocarbons and heavy metals contamination in honey from Cluj County, Romania. Agricultura. 2017;1–2:101–102. [Google Scholar]
- 62.Bratu I., Georgescu C. Chemical contamination of bee honey-identifying sensor of the environment pollution. J. Cent. Eur. Agric. 2005;6:95–98. [Google Scholar]
- 63.Devillers J., Doré J.C., VielMarenco C.M., Poirier-Duchêne F., Galand N., Subirana N. Typology of French acacia honeys based on their concentrations in metallic and nonmetallic elements. In: Devillers J., Pham-Delègue M.-H., editors. Honey Bees: Estimating the Environmental Impact of Chemicals. Taylor & Francis; Oxfordshire, UK: 2002. pp. 248–269. [Google Scholar]
- 64.World Health Organization . Environmental Health Criteria 221-Zinc. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 65.Ciobanu O., Rădulescu H. Monitoring of Heavy Metals residues in honey. Res. J. Agric. Sci. 2016;48:13. [Google Scholar]
- 66.Europe Union . Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Europe Union; Luxembourg, France: 2005. [Google Scholar]
- 67.Food Standards Agency . Honey Regulations 2003 Guidance Notes. Food Standards Agency; London, UK: 2003. [Google Scholar]
- 68.Byrne D. EC Commission Decision (draft) Amending Annex II to Council Directive. Europe Union; Luxembourg, France: 2000. [Google Scholar]
- 69.Europe Union . Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Europe Union; Luxembourg, France: 2006. [Google Scholar]
- 70.Europe Union . Commission Regulation (EU) 2015/1005 of 25 June 2015. Europe Union; Luxembourg, France: 2015. [Google Scholar]