Abstract
Podocytes are visceral epithelial cells covering the outer surface of glomerular capillaries in the kidney. Blood is filtered through the slit diaphragm of podocytes to form urine. The functional and structural integrity of podocytes is essential for the normal function of the kidney. As a membrane-bound organelle, lysosomes are responsible for the degradation of molecules via hydrolytic enzymes. In addition to its degradative properties, recent studies have revealed that lysosomes may serve as a platform mediating cellular signaling in different types of cells. In the last decade, increasing evidence has revealed that the normal function of the lysosome is important for the maintenance of podocyte homeostasis. Podocytes have no ability to proliferate under most pathological conditions; therefore, lysosome-dependent autophagic flux is critical for podocyte survival. In addition, new insights into the pathogenic role of lysosome and associated signaling in podocyte injury and chronic kidney disease have recently emerged. Targeting lysosomal functions or signaling pathways are considered potential therapeutic strategies for some chronic glomerular diseases. This review briefly summarizes current evidence demonstrating the regulation of lysosomal function and signaling mechanisms as well as the canonical and noncanonical roles of podocyte lysosome dysfunction in the development of chronic glomerular diseases and associated therapeutic strategies.
Keywords: podocyte, lysosome, sphingolipids, exosome, chronic glomerular diseases
1. Introduction
In the early 1950s, Christian de Duve discovered “sac-like structures” that contained lytic enzymes while investigating the mechanism of action of insulin [1,2]. Following de Duve’s work, Alex Novikoff characterized the ultrastructure of these compartments, which led de Duve to rename them lysosomes [3]. In another pioneering study, Werner Strauss discovered that proteins localized in the lysosomes were fragmented by tracing the fate of radiolabeled extracellular proteins [4]. As the cell’s degradative organelle, the lysosome has gained notoriety as the “recycling center” of the cell.
Podocytes are terminally differentiated epithelial cells covering the outer surface of glomerular capillaries. They typically do not proliferate. Most glomerular diseases in which the podocyte is the target of injury are not associated with podocyte proliferation [5,6]. As the major degradative components of cells, the normal function of lysosomes is necessary to renew cellular activity and maintain the structural and functional integrity of podocytes. Over the last decade, the canonical notion of the lysosome as a simple recycling center has undergone a dramatic revolution. The mechanistic target of rapamycin complex 1 (mTORC1), the master regulator of cell growth, was found to be localized on the surface of the lysosome. As a result of this discovery, the lysosome is now recognized as a metabolic signaling hub. Recent studies have revealed the essential role of lysosome-dependent sphingolipid metabolism in glomerular disorders of genetic and non-genetic origin [7]. In this review, we focus on the lysosome as a platform for physiological and pathological signaling that controls the podocyte homeostasis and how the dysregulation of lysosome function leads to podocyte injury and glomerular diseases.
2. Different Types of Lysosomes
As a membrane-bound organelle in eukaryotic cells, lysosome originates from the Golgi apparatus and stays in the cytoplasm. Many acid hydrolases are responsible for intracellular digestion by lysosomes. Lysosomes are divided into two types according to their different functions: conventional lysosome and secretory lysosome. Conventional lysosome acts as a waste disposal machinery to control phagocytosis and autophagy [8,9]. Secretory lysosome can move towards and fuse to the plasma membrane, leading to the release of its contents into the extracellular space. Recently, a new type of lysosome has been found to repair the damaged plasma membrane via its fusion to plasma membrane [10,11,12,13]. This type of lysosome may also play an essential role in receptor-mediated endocytosis, leading to the recycling of receptors [14]. More recently, it has been found that exocytosis of non-secretory cells is dependent on lysosomes [11]. After lysosomes fuse to the plasma membrane, their contents are released to the extracellular space. Meanwhile, the components in lysosomal membrane are incorporated into the plasma membrane [11].
3. Cellular Functions Regulated by Lysosomes
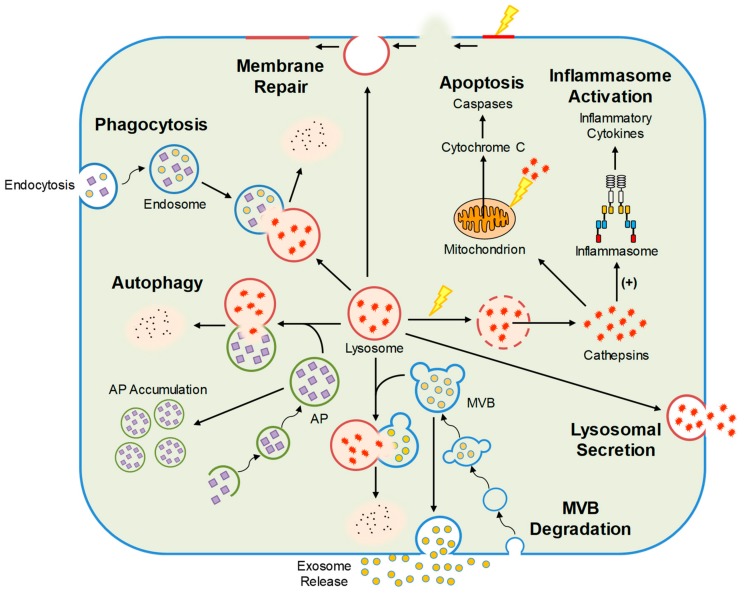
Intracellular destruction of endocytic, phagocytic, and autophagic materials is dependent on lysosomes which serve as the major degradative compartments with their digestive function [15,16,17]. Beyond intracellular digestion, lysosomes may mediate cellular signaling in different cells [18,19,20] such as their role in receptor recycling through an endocytic pathway [21] and as a Ca2+ store importantly participating in the physiological regulation of cell functions or activities in many tissues or cells [19,22,23,24,25], including podocytes [26,27,28]. In podocytes, normally functioning lysosomes are particularly important for the protection of this terminally differentiated cell type from the challenges of various danger factors [27,29,30,31]. Recent clinical and experimental studies have indicated that the deficiency or loss of lysosome function in podocytes results in proteinuria, glomerular sclerosis, and dramatically increased susceptibility to different pathological stimuli [32,33,34,35]. As shown in Figure 1, lysosomes regulate various important cellular functions. Here, we highlighted some lysosome functions which may be essential for the maintenance of podocyte homeostasis or implicated in the onset or development of podocyte injury and glomerular diseases.
Figure 1.
Cellular functions regulated by lysosomes including autophagy, MVB degradation, lysosomal secretion, phagocytosis, inflammasome activation, membrane repair, and apoptosis. AP, autophagosome; MVB, multivesicular body.
3.1. Autophagic Flux
The acidification of lysosomal compartment facilitates the function of lysosomal hydrolases, which is dependent on H+-ATPases on the lysosomal membrane. The Ca2+ enters the lysosomal compartment by H+/Ca2+ exchange under resting conditions and exits in response to various stimuli [23,36]. The regular influx and efflux of Ca2+ are essential for the normal functions of lysosomes. By destroying various aged organelles within the cell such as dysfunctional mitochondria, lysosomes maintain cell function and integrity. In this regard, lysosome-dependent autophagy has been found to control the degradation of damaged or aged materials in podocytes. This autophagic flux plays an important role in the clearance of waste in podocytes under normal condition and recovery of podocytes from damage to the glomeruli upon pathological stimuli. The degradation of defective or damaged mitochondria and consequent inhibition of pro-apoptotic protein release may be the mechanisms by which lysosomes attenuate cell apoptosis [37]. Furthermore, it has been found that the differentiation and maturation of podocytes are dependent on autophagy, indicating the importance of lysosome function in maintaining the epithelial phenotype of podocytes [26,38,39]. Various important cellular functions such as receptor-mediated endocytosis, receptor recycling, and cell membrane repair [14,40,41] requires normal function of lysosomes.
The localization of the lysosome as a dynamic organelle has been reported to change in response to a variety of treatments [42]. The kinesin and dynein motors mediate the lysosome movement along the microtubule in anterograde and retrograde directions, respectively [43,44]. Moreover, intracellular pH is one of the best-known regulators of lysosomal positioning. The lysosomes redistribute from their predominantly perinuclear location toward the cell periphery in response to acidification of lysosomal compartment [45]. Furthermore, nutrients and growth factors can drive lysosomes toward the plasma membrane, whereas starvation results in lysosomes moving toward perinuclear localization [46,47]. Vacuolar-type proton pumping ATPase (V-ATPase) is a ubiquitous enzyme responsible for H+ transport across membranes and acidification of cellular compartments in animals [48]. The phosphate bond energy of ATP is thus converted to a proton gradient across the membrane through the mechanical rotation of subunits. The proton pumping action of lysosomal V-ATPases results in the generations of an acidic lumen and proton gradient of lysosomes, which are essential for normal functions of lysosomes [48]. A recent study in our lab has revealed that inhibition of V-ATPase activity remarkably attenuated lysosome function, leading to autophagic deficiency and podocyte dedifferentiation [26].
As a master growth regulator, mTORC1 becomes activated at the cytosolic side of lysosomes in response to nutrients, which increases the peripheral appearance of lysosomes and inhibits fusion of autophagosome and lysosome. On the contrary, starvation causes the perinuclear clustering of lysosomes and enhances autophagic flux via inhibition of mTORC1 activity [49,50]. In HeLa cells, HIV-1 counteracts metabolic and environmental stress-induced intracellular repositioning of lysosomes through enhancement of mTORC1 activity [51]. In another study, knockdown of FUT1 has been found to elevate the peripheral distribution of lysosomes by inhibition of mTORC1 activity, leading to the enhancement of autophagic flux [52]. All these previous findings demonstrate that the mTOR signaling pathway plays an important role in the regulation of lysosome positioning and trafficking. A recent study in our lab has confirmed that mTORC1 inhibition by rapamycin may increase the fusion of autophagosome and lysosome by enhancing lysosome trafficking. Rapamycin-induced enhancement of autophagic flux may be substantially blocked by nicotinamide, CD38 shRNA, and bafilomycin [28]. Furthermore, palmitate, a saturated free fatty acid, has been found to activate mTORC1, leading to the endoplasmic reticulum (ER) stress-dependent apoptosis in podocytes. Inhibition of mTORC1 activity by rapamycin or siRNA for Raptor, a component of mTORC1, ameliorated palmitate-induced ER stress and apoptosis in podocytes [53]. Together, these previous studies have demonstrated that the regulation of lysosome trafficking and autophagic flux by targeting mTORC1 is a potential therapeutic strategy against podocyte injury and glomerular diseases. However, previous studies have revealed that the basal level of mTORC1 activity is required for maintaining the normal function of podocytes. Genetic deletion of mTORC1 in podocytes may cause the disruption of autophagic flux, leading to podocyte dysfunction and proteinuria [54,55]. In addition, patients with certain renal problems may suffer progressive proteinuria by taking currently available mTORC1 inhibitors [56,57,58]. Their side effects may be due to the inhibition of the basal level of mTORC1 activity and the mTORC2-Akt pathway, which are important for maintaining the integrity of podocytes [59,60].
3.2. Lipid Metabolism
As a digestive organelle, the lysosome is responsible for lipid metabolism. The deprivation of nutrient may result in association of lipid droplets and autophagic membrane components [61]. The storage of triglyceride in lipid droplets is elevated after inhibition of autophagy [61]. In macrophages, the lysosome is responsible for the metabolism and transport of cholesterol and low-density lipoprotein. Under normal conditions, lysosomal acid lipase hydrolyzes cholesteryl esters to free cholesterol. This product is then actively exported out of lysosomal compartment through Niemann–Pick-type C1 protein. Several lysosomal proteins such as acid sphingomyelinase (ASM), mucolipin-1, and H+-ATPase regulate metabolism and transport of cholesterol in lysosomes [62].
As components of the external layer of plasma membranes, sphingolipids are transported by the endocytic vesicular flow through the early and late endosomal compartment and degraded in lysosomes [63]. In the lysosomal compartment, sphingolipids are catabolized to simple compounds, such as sphingosine, fatty acids, and sugars [63,64]. These products are exported out of the lysosomes through specific membrane proteins and then partially recycled for the biosynthesis of new sphingolipids [65,66,67]. In addition, it has been reported that many cellular activities, such as proliferation, apoptosis, differentiation, and migration, are influenced by sphingolipids [68,69]. Furthermore, sphingolipids have been found to interact with extracellular matrix, growth factor receptors, and neighboring cells [70].
3.3. Inflammasome Activation
As a cytosolic multiprotein platform, inflammasome assembles in response to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [71]. As a vital intracellular homeostatic recycling process, autophagy has been shown to negatively regulate inflammasome activation in previous studies [72,73]. Damaged organelles such as mitochondria are removed by autophagy, leading to reduced release of mitochondrial-derived DAMPs and subsequent suppression of inflammasome activation [74,75,76]. Autophagic deficiency results in ROS-producing mitochondria accumulation, leading to enhancement of NLRP3 inflammasome activation in response to ATP, monosodium urate crystals, palmitic acid, and influenza A virus [74,75,77,78]. Autophagy also regulates inflammasome activation through the lysosome-dependent degradation of inflammasome complexes. It has been reported that pharmacological inhibition of autophagy can greatly increase activation of caspase-1 and inflammatory cytokine production in response to the induction of AIM2 in monocytes [79]. Moreover, it has been found that pro-IL-1β can be sequestered into autophagosomes for degradation by lysosomes after treatment of macrophages with rapamycin, a pharmacological inducer of autophagy that inhibits mTOR [80].
3.4. Exosome Release
The exosomes, one of the extracellular vesicles (EVs), are released by the fusion of the multivesicular body (MVB) to plasma membrane [81]. These EVs may regulate cell-to-cell communications. After long-time challenging and debating about the characterization and classification of EVs, EVs are now classified as three distinct populations including apoptotic bodies, microvesicles, and exosomes. Among them, exosomes are the smallest EVs with approximately 50–140 nm in diameter despite no set clear cut-off size to separate them from microvesicles. Different from other EVs, exosomes are formed through the endocytic process and released from intracellular MVBs through an active process. EVs or exosomes have been extensively studied for their biogenesis and related function in cell communication and in the pathogenesis of different diseases including renal diseases [82,83,84]. In recent studies, lysosome dysfunction induced by alkaline agents and lysosomal V-ATPase inhibitor leads to increase in exosome release of various cells such as neurons, epithelial cells, and vascular cells [85,86,87]. In addition, MVBs were found to fuse with autophagosomes (APs) to form amphisomes and subsequently fuse with lysosomes to terminate MVB fate and thereby reduce exosome release [88,89,90].
As an important biomarker indicating kidney function or disease, exosomes mediate intra-renal cell-to-cell communication and contribute to the development of various renal diseases [83]. There is evidence that exosomes containing podocalyxin, a glycoconjugate on the podocyte apical surface, are increased in diabetic mice even before the onset of albuminuria [91]. In some patients with focal segmental glomerulosclerosis (FSGS) and nephrotic syndrome (NS), podocyte-derived exosomes increased in concert with albuminuria and glomerular degeneration [83,92,93,94,95,96]. Increased exosomes may serve as a signaling vesicle to trigger phenotypic changes in neighboring cells [97] and they also participate in the development of albuminuria [98].
There is evidence that the lysosome-mediated regulation can actively respond to microenvironment changes such as increased autophagosomes, MVBs or other stress signals, which occurs in podocytes and other cells. Such active regulation of lysosome function determines the disposal of different intracellular vesicles such as phagosomes, autophagosomes, and MVBs [28,99,100,101]. Previous studies have shown that regular lysosome trafficking controls the fusion of lysosomes and MVBs. The lysosomal Ca2+ release determines the active movement of lysosomes [25,100]. As a ubiquitously expressed protein, transient receptor potential-mucolipin-1 (TRPML1) channel is an ion channel expressed in intracellular endosomes and lysosomes [102]. The Ca2+ enters the lysosomal compartment by H+/Ca2+ exchange and exits through TRPML1 channels in response to endogenously produced NAADP [23,103,104,105] or other factors like PIPs (PI(3,5)P2) and ions [19,106,107].
4. Podocyte Lysosome Dysfunction in Chronic Glomerular Diseases
Growing evidence reveals that pathological progression of podocyte injury in glomerular diseases originates from the disarrangement of lysosome function. The resolution of podocyte injury involves alteration of autophagic flux as well as targeting lysosome-dependent sphingolipid metabolism by pharmacological intervention or enzyme replacement therapy. All the mechanisms or regulatory pathways mentioned above that control or modulate lysosome function are important for the maintenance of podocyte activity and glomerular function. If they fail to function properly, podocyte dysfunction and glomerular injury will occur. Below, we highlight the role of lysosome dysfunction and associated dysregulation in several common nephropathies associated with podocyte injury.
4.1. Diabetic Nephropathy
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease (ESRD) worldwide [108]. Pathologic changes in the diabetic kidney including thickening of the glomerular basement membrane, mesangial expansion, proteinuria, inflammation, and fibrosis. Podocyte damage and loss contribute to the impairment of renal function in DN. In recent years, increasing evidence indicates that the disarrangement of lysosomal function is attributed to the initiation of podocyte injury and the development of ESRD during DN.
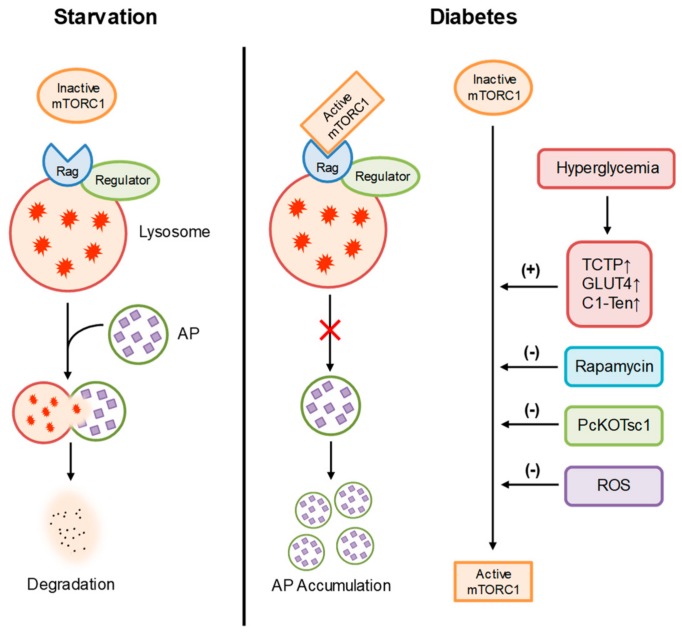
Previous studies have demonstrated that autophagy is renoprotective in various renal diseases including DN [109]. As an anaphase cell with a restricted capacity in differentiation and proliferation, podocyte is very dependent on autophagy because of its self-repaired feature [110]. Strong evidence has confirmed that activation of mTOR suppresses autophagy. By this mechanism, nutrient deficiency activates autophagy by suppressing the expression of mTOR. On the contrary, mTOR is activated by both growth factors and nutrients, such as glucose and amino acids [111,112,113]. During diabetes, hyperglycemia has been found to induce the overactivation of mTOR signaling in lysosomes [114]. The use of rapamycin as a specific mTORC1 inhibitor to effectively attenuate the development of glomerular injury in the animal models of DN has established that mTOR signaling and related lysosome function have an important role in the pathophysiology of DN. In this regard, translationally controlled tumor protein (TCTP), GLUT4, and C1-Ten have been found to regulate mTOR complex 1 (mTORC1) signaling in glomeruli and the podocyte is the principal cell responsible for the regulation of mTORC1 by these proteins [115,116,117]. During diabetes, elevation of these regulators may explain for overactivation of lysosomal mTORC1 in podocytes, leading to podocyte injury and glomerular diseases. Genetic deletions of these regulators have been shown to prevent the development of diabetic nephropathy, as indicated by the amelioration of proteinuria, mesangial expansion, podocyte loss and glomerular sclerosis. Reactive oxygen species (ROS) produced by mitochondria has also been reported to prevent mTOR activation in podocytes exposed to high glucose for 24 h, leading to enhancement of lysosome-dependent autophagy which may limit the augmentation of ROS produced by damaged mitochondria [118,119]. However, chronic exposure to high glucose leads to autophagy insufficiency and subsequently causes lysosomal dysfunction, leading to podocyte apoptosis [29]. Furthermore, genetically reducing mTOR levels by eliminating the Raptor allele dramatically prevents podocyte injury and ameliorates the progression of glomerular dysfunction during diabetes [54]. In addition, podocyte-specific mTORC1 activation induced by the ablation of an upstream negative regulator (PcKOTsc1) has been reported to recapitulate many features of DN, including podocyte loss, glomerular basement membrane thickening, mesangial expansion, and proteinuria in nondiabetic young and adult mice [120]. However, it has been reported that podocyte-specific deletion of the mTORC1 gene induces proteinuria and progressive glomerulosclerosis [54]. These findings suggest that excess of both activation and inhibition of mTOR signaling may cause podocyte injury. Given the important role of mTOR signaling in autophagy, lysosome dysfunction and autophagic deficiency due to overactivation of mTOR signaling may be involved in the pathogenesis of podocyte injury and glomerular damage in patients with diabetes. Figure 2 summarizes the regulation of mTORC1 in podocytes during DN.
Figure 2.
Regulation of mTORC1 in podocytes. During starvation, inactive mTORC1 has no effects on lysosome function and autophagic flux in podocytes. During diabetic nephropathy (DN), hyperglycemia upregulates TCTP, GLUT3, and C1-Ten, leading to overactivation of mTORC1 and inhibition of autophagy. As a specific mTORC1 inhibitor, rapamycin can prevent lysosome dysfunction, autophagic deficiency, and podocyte injury during diabetes. Reactive oxygen species (ROS) produced by mitochondria has also been reported to prevent mTORC1 activation in podocytes during diabetes.
In addition to autophagy, lysosome-dependent lipid metabolism plays an important role in podocyte injury and glomerular diseases during DN. A previous study has confirmed that rapamycin effectively attenuates STZ-induced DN which is shown by inhibition of glomerular enlargement and proteinuria [121]. Mechanically, they have found that ceramide and sphingomyelin are both remarkably elevated in the renal cortex of rats with DN while rapamycin significantly inhibited the elevations of ceramide and sphingomyelin. These results indicate that suppression of abnormal sphingolipid metabolism contributes to the therapeutic effect of rapamycin on DN [121]. Moreover, the activation of adiponectin receptors has been found to regulate the expression of lysosomal acid ceramidase (AC) which converts ceramide to sphingosine [122]. The reductions of both adiponectin receptor and lysosomal AC were detected in diabetic mice, which were significantly attenuated by AdipoRon, an adiponectin receptor agonist. Furthermore, AdipoRon-induced enhancement of lysosomal AC activity lowered cellular ceramide levels, which may contribute to the therapeutic effects of AdipoRon [122]. More recently, a clinical study has found that the elevation of urinary ceramide is associated with DN [123]. According to results from other studies, the abnormal sphingolipid metabolism by lysosomal enzymes may contribute to the elevation of urinary ceramide in DN patients [123]. These results further confirmed that abnormality of lysosome-dependent sphingolipid metabolism might be a molecular mechanism leading to podocyte injury and glomerular damage during diabetes.
Lysosome membrane permeabilization (LMP) has been found to initiate lysosome-dependent cell death [124]. Lysosome contents including cathepsins as the main stimulator of the cell death are released during the destabilization of lysosomal membrane [125]. Recent studies have shown the important role of LMP in podocyte injury under pathological conditions such as idiopathic membranous nephropathy [27] and diabetic nephropathy (DN) [126]. As a dangerous factor elevated during DN, advanced glycation end products (AGEs) have been found to induce LMP in murine podocytes [126]. Correspondingly, cathepsins B, D, and L were released into the cytosol of these cells. The LMP initiates the development of autophagic deficiency, apoptosis, and Rac-1-dependent actin-cytoskeletal disorganization in these cells. Interestingly, exposures to both C5b-9 and AGEs have been reported to enhance the production of reactive oxygen species (ROS) [127,128], while the occurrence of LMP is frequently associated with the overproduction of ROS [129,130,131]. As an intracellular cysteine protease, cathepsin L is upregulated in podocytes and glomeruli under different pathological conditions [132,133]. A recent study targeting cathepsin L has confirmed that cathepsin L-knockout mice do not develop podocyte injury and glomerular damage during diabetes [132]. Mechanistically, CD2-associated protein (Cd2ap) and synaptopodin proteolysis are promoted by cathepsin L, leading to podocyte damage and massive foot process effacement [134]. Cathepsin abrogation is protective against a variety of external stimuli. Furthermore, cathepsin L possesses the function of cleaving dynamin, which affects the regulation of the actin cytoskeleton in podocyte foot processes [135].
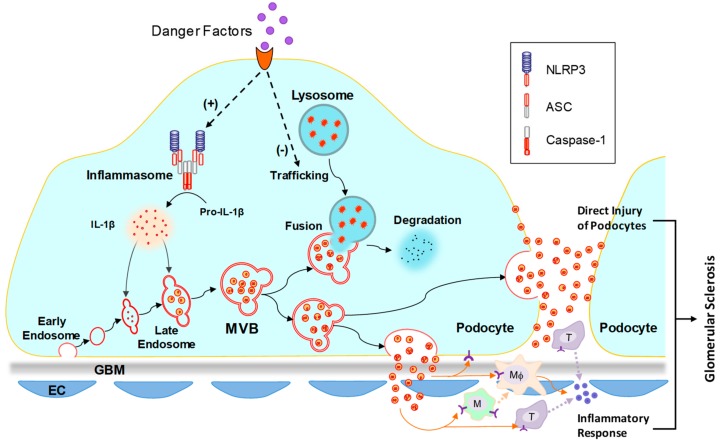
Recently, it has been found that D-ribose, an overlooked risk factor in the development of type II diabetes mellitus, produces AGEs much more rapidly than D-glucose [136,137]. A recent study in our lab has demonstrated that D-ribose induces formation and activation of NLRP3 inflammasome in podocytes via AGE/RAGE signaling pathway, which may contribute to the initiation of podocyte injury and glomerular damage during diabetes [138]. After NLRP3 inflammasome is activated, sphingolipid-mediated regulation of lysosome function significantly affects the release of inflammasome-derived products into extracellular space via exosomes [139]. Both inhibition of lysosomal ASM and activation of lysosomal AC attenuated inflammatory exosome release through enhancement of lysosome-dependent degradation of MVBs [139]. In conclusion, lysosomal sphingolipid metabolism as a regulator of inflammatory exosome release may be a novel target for the development of therapeutic strategy for prevention and treatment of DN. Figure 3 summarizes the mechanism of inflammatory exosome release in podocytes.
Figure 3.
Mechanism of inflammatory exosome release in podocytes. It has been reported that in podocytes, pathological stimuli may induce inflammasome activation and lysosome dysfunction. Activated inflammasome produces proinflammatory cytokines, such as IL-1β and IL-18. Lysosome dysfunction leads to reduced MVB degradation and increased exosome release. The proinflammatory cytokines in podocytes may be released through exosome release. The released inflammatory exosomes may induce direct injury of podocytes and inflammatory response, leading to the development of glomerular sclerosis. NLRP3, nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3; ASC, adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain; IL-1β, interleukin-1β; GBM, glomerular basement membrane; EC, endothelial cell.
4.2. Glomerular Injury during Fabry Disease
The mutation of the gene encoding alpha-galactosidase A (α-GalA), a lysosomal enzyme that hydrolyzes the terminal alpha-galactosyl moieties from glycolipids and glycoproteins, results in the systemic accumulation of globotriaoslyceramide (Gb3), which is so-called Fabry disease [140]. Elevation of urinary Gb3 is detected in patients with Fabry disease [141,142]. In the kidney, Gb3 accumulation occurred mainly within the lysosome, ER, and nuclear markers of renal cells [143], which may be reversed by enzyme replacement therapy using recombinant α-GalA [142]. In patients with Fabry disease, the pathological changes observed in podocytes included hypertrophy, lysosome enlargement, and characteristic inclusion bodies of glycolipids, which led to mesangial cell expansion [144]. A recent study has demonstrated that the intracellular Gb3 accumulates in response to the lentiviral knockdown of α-GalA in human podocytes [145]. Mechanically, the loss of mTOR kinase activity and dysregulated autophagy were associated with the intracellular Gb3 accumulation [145]. The link between α-GalA and mTOR signaling pathway has further confirmed the importance of normal Gb3 metabolism in the maintenance of podocyte homeostasis. Enzyme replacement therapy using recombinant human α-GalA has been found to attenuate Gb3 accumulation in different types of renal cells including vascular endothelial cells, vascular smooth muscle cells, mesangial cells, interstitial cells, distal tubular epithelial cells, and podocytes, which is associated with suspension of the progression of renal pathology and prevention of renal failure in patients with Fabry disease [146]. However, the clearance of Gb3 by enzyme replacement therapy in podocytes and distal tubular epithelial cells is more limited than the clearance observed in other cell types [146]. In this regard, a previous study has unveiled a strategy for specific delivery of recombinant α-GalA to podocytes. Mannose 6-phosphate/insulin-like growth II receptor, megalin, and sortilin have been identified in human podocytes [147]. It has been demonstrated these receptors possess delivery capabilities for enzyme replacement therapy. This study has identified potential pathways for potential non-carbohydrate-based drug delivery to podocytes for treatment of Fabry disease-associated podocyte injury and glomerular diseases.
4.3. Hyperhomocysteinemic Nephropathy
A homocysteine level that exceeds 15 μmol/L in the plasma of patients is characterized as hyperhomocysteinemia (hHcy). It has been reported that the progression of many chronic metabolic diseases including hypertension, peripheral vascular disease, Alzheimer’s disease, diabetes and atherosclerosis is attributed to elevated homocysteine (Hcy) [148]. Accumulation of Hcy or hHcy in the blood induces pathological alterations in the glomeruli including extracellular matrix accumulation and podocyte injury. The inability of this toxic compound to be properly cleared or degraded from the body eventually leads to compromised renal function and glomerulosclerosis [149,150]. Although it remains unclear how hHcy causes cellular injury and sclerotic changes in many organs and tissues, some studies have revealed that ceramide production is upregulated during hHcy, suggesting this sphingolipid may play a crucial role in these processes [151,152,153]. In this regard, a recent study in our lab has demonstrated that hHcy-induced NADPH oxidase-dependent superoxide production may be attributed to overexpression of lysosomal ASM and consequent ceramide accumulation in the glomeruli of hHcy mice [154]. A further study has confirmed that hHcy-induced glomerular ceramide accumulation occurs mainly in the podocytes [155]. In vivo evidence shows that ASM gene deletion blocks glomerular superoxide production and podocyte injury in mice with hHcy. Pharmacological inhibition of ASM by amitriptyline has been found to prevent ceramide accumulation, superoxide production, and cellular injury in cultured murine podocytes [155]. However, it remains unclear how the overproduction of ceramide by ASM leads to podocyte injury and glomerular diseases during hHcy.
Since superoxide production can induce inflammasome activation, we investigated whether inflammasome is involved in podocyte injury during hHcy. Strong evidence has confirmed that NLRP3 inflammasome formation and activation are important molecular mechanisms triggering podocyte injury and ultimately resulting in glomerular sclerosis during hHcy [156,157]. Moreover, endogenously produced reactive oxygen species has been found to contribute to the activation of NLRP3 inflammasome in podocytes during hHcy [158]. Recently, we have confirmed that the overproduction of ceramide by lysosomal ASM induces NLRP3 inflammasome activation in podocytes, leading to proteinuria and glomerulosclerosis [159]. The assembly and activation of the NLRP3 inflammasome may be attributed to superoxide production in glomeruli during ceramide accumulation. These results indicate that an imbalance of lysosome-dependent sphingolipid metabolism may be the molecular mechanism initiating NLRP3 inflammasome activation in podocytes, leading to podocyte injury and glomerular sclerosis during hHcy.
4.4. Obesity-Induced Podocyte Injury and Glomerular Disease
Previous studies have revealed that obesity is a risk factor of chronic kidney disease (CKD) and end-stage renal disease (ESRD) [160,161]. Adipose tissue, especially visceral fat, generates bioactive substances which contribute to pathological changes of renal hemodynamics and structure, leading to glomerular injury [162]. These bioactive substances derived from adipose tissue include the adipokines visfatin, leptin, and adiponectin as well as various cytokines such as tumor necrosis factor-α (TNF-α), resistin, and interleukin-6 (IL-6) [162]. As an anti-inflammatory adipokine, adiponectin inhibits pro-inflammatory cytokine release, enhances anti-inflammatory cytokine release, and restores intracellular ATP levels [163,164,165]. It has been reported that adiponectin can inhibit the development of various obesity-related diseases [163,166,167]. Recently, we have demonstrated that adiponectin inhibits pannexin-1 (Panx1) channel activity in podocytes via enhancement of lysosomal AC activity [168]. Since the Panx1 channel mediates ATP release, inhibition of the Panx1 channel may contribute to the anti-inflammatory property of adiponectin. Furthermore, the expression of AC has been found to be upregulated by activation of adiponectin receptors [122]. Therefore, hypoadiponectinemia may lead to enhancement of Panx1 channel activity during obesity, leading to increased ATP release, inflammasome activation, and consequent inflammatory response in local tissues such as glomeruli. Moreover, the high-fat diet (HFD) has been found to significantly increase glomerular ceramide production, NADPH oxidase-dependent superoxide production, and NLRP3 inflammasome formation in glomeruli of wild type mice [159]. The activation of NLRP3 inflammasome mainly occurs in podocytes. However, the deletion of the ASM gene totally blocked NLRP3 inflammasome activation and glomerulosclerosis during obesity [159]. These results indicate that the normal function of lysosomal ASM is essential for the maintenance of podocyte homeostasis. The imbalance of lysosome-dependent sphingolipid metabolism may induce NLRP3 inflammasome activation, leading to podocyte injury and glomerular sclerosis during obesity.
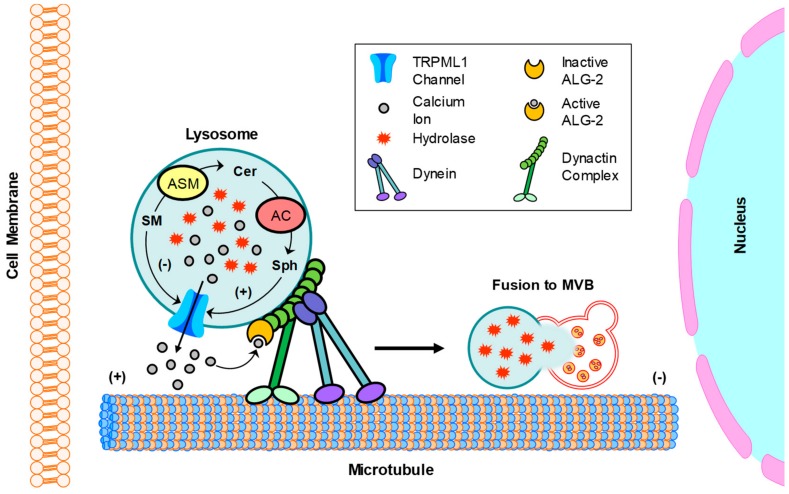
Recently, a clinical study has confirmed that urinary excretion of sphingolipids occurs in adolescents with severe obesity despite the absence of microalbuminuria. Therefore, urinary sphingolipids may be used as a parameter to detect early glomerular injury in adolescents with severe obesity [169]. The elevated urinary sphingolipids include ceramides, sphingomyelin, and glycosphingolipids. Given the recently discovered role of ceramide-enriched exosome as a passenger of danger signal and a stimulator of further damages [170,171,172], it is possible that the exosome plays a significant role in the development of glomerular diseases during obesity. In this regard, a recent study in our lab has revealed that TRPML1 channel-mediated Ca2+ release controls lysosome trafficking and lysosome-MVB interaction in podocytes [173]. Blockade of TRPML1 channel due to lysosomal AC dysfunction inhibits the fusion of lysosomes and MVBs, leading to enhanced exosome release from podocytes. Under pathological conditions, deficiency of lysosomal AC function may inhibit TRPML1 channel activity, leading to enhancement of podocyte-derived exosome release and consequent podocyte injury and glomerular damage. Figure 4 summarizes the regulation of lysosome trafficking by sphingolipids in podocytes.
Figure 4.
Regulation of lysosome trafficking by sphingolipids in podocytes. Dynein-mediated retrograde transport of lysosomes promotes their fusion with MVB. This transport is dependent on the TRPML1 channel-mediated Ca2+ release. In the lysosome, ASM converts SM into CER and AC converts CER to Sph. These sphingolipids, SM, Cer, and Sph had different effects on TRPML1 channel activity in podocytes, with inhibition by SM, no effect from Cer, but enhancement by Sph. SM, sphingomyelin; Cer; ceramide; Sph, sphingosine.
Although autophagy has been confirmed to be essential for the maintenance of podocyte differentiation, its role in obesity-induced podocyte injury may be different. Recently, it has been found that inhibition of GLUT4 translocation to the plasma membrane induces excessive autophagy in podocytes, which contributes to the development of obesity-related glomerulopathy [174]. The glucagon-like peptide-1 analog inhibits excess of autophagy in podocytes to prevent obesity-related glomerulopathy [174]. On the contrary, rapamycin, an autophagy inducer, was found to worsen podocyte injury induced by palmitic acid [174]. These results indicate that both inhibition and excess of lysosome-dependent autophagy can cause podocyte injury and glomerular diseases under pathological conditions.
4.5. APOL1-Associated Nephropathy
As a minor apoprotein component of high-density lipoprotein, apolipoprotein L1 (APOL1) is expressed in different tissues, such as kidney, liver, pancreas, and brain [175,176]. Recently, increasing evidence has indicated that a major disparity in renal health is strongly associated with two coding sequence variants in APOL1 [177,178,179,180]. Compared with European Americans, African Americans have higher possibility of suffering progressive nephropathy, including FSGS, human immunodeficiency virus (HIV)-associated nephropathy (HIVAN), and hypertension-associated ESRD [181,182]. Many African Americans possess risk variants of APOL1. On the contrary, coding sequence variants of APOL1 occur infrequently in European Americans [181,183,184]. This remarkable population disparity has confirmed that abnormality of APOL1 contributes to the development of progressive nephropathy.
A clinical study has shown that patients with FSGS and HIVAN have lower expression of APOL1 in podocytes compared to other kinds of cells [185]. In a recent study, enhanced lysosomal swelling and necrosis have been found in human podocytes expressing APOL1 variants [186]. The enhanced lysosomal swelling may be due to increased lysosomal membrane permeability in podocytes with APOL1 variants. As a secreted protein, APOL1 can induce lysosomal membrane depolarization and continuous chloride influx [187]. Correspondingly, inhibition of chloride channel and blockade of interaction between APOL1 and lysosome have been found to attenuate lysosomal swelling and podocyte injury due to APOL1 variants [186]. In this regard, an in vivo study has shown that transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice [188]. Podocyte foot process effacement, albuminuria, and glomerular sclerosis were found in mice with podocyte-specific inducible expression of the APOL1 risk variants. Mechanically, podocyte-specific inducible expression of the APOL1 risk variants may inhibit autophagic flux and induce inflammatory cell death, leading to autophagosome accumulation and podocyte loss [188]. More recently, it has been found that the interaction between APOL1 and microRNA-193a is involved in high glucose-induced podocyte dedifferentiation [189]. High glucose induced both reduction of APOL1 and upregulation of microRNA-193a which contributed to podocyte dedifferentiation. Moreover, APOL1 siRNA upregulated microRNA-193a and overexpression of microRNA-193a downregulated APOL1 in podocytes [189]. A further study has demonstrated that disruption of APOL1-microRNA-193a axis induces podocyte dedifferentiation through blockade of autophagic flux [190]. The expression of APOL1 risk variants has also been found to dedifferentiate podocytes through autophagic deficiency. In conclusion, APOL1 as a regulator of lysosome function may be a novel target for the development of therapeutic strategy against podocyte injury and glomerular diseases.
5. Therapeutic Potential of Chronic Glomerular Diseases by Restoring Lysosome Function
The prolonged clinical silence in chronic glomerular diseases leads to irreversible damage including accumulation of extracellular matrix proteins, proteinuria, glomerular hypertrophy, glomerular basement membrane thickening, mesangial expansion and glomerular fibrosis. Early detection of these pathological changes is necessary to facilitate therapies that can improve clinical outcomes. Management of risk factors such as hypertension, hyperglycemia, and albuminuria is essential for slowing progression to ESRD. Although beneficial effects have been confirmed in the usage of traditional therapeutics such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and statins [191], the morbidity and mortality of patients with CKD remain high. The identification of new therapeutic targets and the development of new strategies for the treatment of chronic glomerular diseases are imperative and there is a need to search for new pathways.
The abnormality of mTOR pathway signaling has been found to participate in all the key steps of DN progression, including damage and loss of podocytes, an early event in DN that further causes glomerular sclerosis. Increasing evidence has confirmed the efficacy of mTOR inhibitors in treating DN [192,193,194,195,196], although they may cause hyperglycemia due to the combination of impaired insulin secretion and insulin resistance [197]. Rapamycin is a well-known mTOR inhibitor, but its limited bioavailability led to the development of semisynthetic analogs, named rapalogues, which possesses improved pharmacokinetic properties and superior aqueous solubility. There are clinical evidences showing the serious disadvantage of rapalogues in terms of its desired therapeutic effects, and its cytostatic effect may partially inhibit its efficacy [198]. Furthermore, mTORC1 is the only target of rapamycin and rapalogues. The long-term treatments with these molecules may initiate aberrant feedback loops in mTOR network, leading to abnormal activation of compensatory pro-survival signaling pathways. The therapeutic effects of these treatments on glomerular diseases during diabetes can be severely compromised by these side effects. Another drawback of this approach was the ablation of raptor expression in podocytes and consequent development of proteinuria, which is consistent with the side effects of rapamycin in both animal models and humans [199,200,201,202,203]. In conclusion, increasing evidences have confirmed the significant contribution of mTOR activation in the development of DN by acting on different types of renal cells. However, this theoretically attractive therapeutic target is still lack of clinical trials using mTOR inhibitors against DN. The side effects of mTOR inhibitors in transplanted patients, such as dyslipidemia, hyperglycemia, and insulin resistance, may explain the scarceness of progression in clinical studies. Notably, pharmacological inhibition of mTORC1 with rapamycin is the only interfering method in most of the previous studies on the role of mTOR in the development of DN. A recent study has indicated that activation of mTORC2 also contributes to podocyte apoptosis and albuminuria in diabetic mice, which provides a novel therapeutic strategy for further exploration [175]. More recently, rapamycin has been found to regulate microRNA expression in kidney [204]. Furthermore, inhibition of microRNA-21 has been shown to inhibit abnormal activation of the PI3K/Akt/mTOR pathway and thereby enhance autophagic flux, leading to attenuation of high glucose-induced podocyte injury [205]. Correspondingly, it has been demonstrated that inhibition of microRNA-217 can block high glucose-induced podocyte injury by restoring autophagic flux [206]. These findings indicate that targeting microRNA may be a novel approach to regulate mTORC1 activity. However, it remains unknown whether long-term modification of microRNA produces side effects during treatment of DN. More experiments need to be performed to enhance our understanding of this therapeutic strategy.
EVs such as exosomes are found in almost all biofluids and the cargo of EVs change with disease states. This property positions EV as a potential source for the discovery of novel biomarkers of different diseases. In this regard, the urinary exosome has been reported to be a novel biomarker for renal disease [207,208,209]. Moreover, the stability and enrichment of miRNA in the exosome make it a promising candidate as a biomarker for different glomerular diseases [210,211,212,213,214,215]. However, recent studies have indicated that exosomes derived from glomerular cells such as podocytes may not be regarded only as an indicator of glomerular status. Furthermore, the biogenesis, degradation, and release of exosomes could be a novel target for the development of therapies against CKD. For example, D-ribose has been shown to induce NLRP3 inflammasome activation and inflammatory exosome release in podocytes both in vitro and in vivo [139]. In addition, TRPML1 channel-mediated Ca2+ release has been demonstrated to control lysosome trafficking, MVB degradation, and exosome release in murine podocytes [173]. Convincing evidence has shown that an imbalance of sphingolipid metabolism in lysosomes may block lysosome function and thereby results in the enhancement of exosome release. These released exosomes may induce or worsen the pathological changes in podocytes and glomeruli. Both inhibitions of lysosomal ASM by amitriptyline and activation of lysosomal AC by genistein have been confirmed to attenuate ceramide accumulation and podocyte injury [139,173]. However, there are still many steps before the therapeutic strategy of targeting exosome release being tested in preclinical studies. To our knowledge, amitriptyline has been demonstrated to act as an agonist for TrkA and TrkB receptors [216]. In addition, amitriptyline is a PARP1 inhibitor [217]. Furthermore, Amitriptyline acts as an antagonist or inverse agonist of serotonin receptors, α1-adrenergic receptor, histamine receptors, and muscarinic acetylcholine receptors, and as an agonist of sigma σ1 receptor [218,219,220]. Many side effects have been observed while taking amitriptyline. Therefore, new functional inhibitors of ASM with high efficacy and selectivity are required for clinical usage. As a well-known medicine for the treatment of mental illnesses, amitriptyline may affect the central nervous system if taking it for a long time. To overcome this obstacle, podocyte-specific aptamer-mediated targeted drug delivery may be a potential solution.
It is expected that more therapeutic strategies will be forthcoming, which include the use of ASM inhibitor, AC inducer, AdipoR agonist, TRPML1 channel agonist, mTOR inhibitor, lysosome stabilizer, and cathepsin inhibitor. These potential therapeutics may target different components of lysosome function, which may be selected for the use in the prevention or treatment of ESRD and associated glomerular diseases.
6. Concluding Remarks
This review briefly summarizes current evidence about molecular mechanisms by which the lysosome affects podocyte function and integrity under pathological conditions, including the involvement of mTORC1, microRNA, APOL1, cathepsin, α-GalA, ASM, AC, and TRPML1 channel. All these studies have provided innovative insights into ways to prevent aberrant lysosome function with the goal of preventing the development of podocyte damage and progressive renal insufficiency. The crosstalk between lysosomes and podocytes may be activated by pathological stimuli such as hyperglycemia, hHcy, obesity, cytokines, adipokines, and genetic defects. Therefore, lysosomes have been implicated in the development of a variety of renal diseases due to their induction of podocyte dysfunction and injury, which results in glomerulosclerosis, ultimately leading to ESRD. A deeper investigation is of the utmost importance to understand how these various signaling pathways interact to regulate lysosome function in podocytes, which may promote the development of more effective therapies for the prevention or treatment of glomerular diseases and consequent ESRD.
Funding
This study was supported by grants DK054927 and DK120491 from National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Bainton D.F. The discovery of lysosomes. J. Cell Biol. 1981;91:66s–76s. doi: 10.1083/jcb.91.3.66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 3.Essner E., Novikoff A.B. Localization of acid phosphatase activity in hepatic lysosomes by means of electron microscopy. J. Biophys Biochem. Cytol. 1961;9:773–784. doi: 10.1083/jcb.9.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straus W. Isolation and biochemical properties of droplets from the cells of rat kidney. J. Biol. Chem. 1954;207:745–755. [PubMed] [Google Scholar]
- 5.Mundel P., Shankland S.J. Podocyte biology and response to injury. J. Am. Soc. Nephrol. 2002;13:3005–3015. doi: 10.1097/01.ASN.0000039661.06947.FD. [DOI] [PubMed] [Google Scholar]
- 6.Pavenstadt H., Kriz W., Kretzler M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 7.Merscher S., Fornoni A. Podocyte pathology and nephropathy - sphingolipids in glomerular diseases. Front. Endocrinol. (Lausanne) 2014;5:127. doi: 10.3389/fendo.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky D.J., Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart L.M., Ezekowitz R.A. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Huynh C., Roth D., Ward D.M., Kaplan J., Andrews N.W. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc. Natl. Acad. Sci. USA. 2004;101:16795–16800. doi: 10.1073/pnas.0405905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal J.K., Andrews N.W., Simon S.M. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil P.L., Kirchhausen T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 13.Reddy A., Caler E.V., Andrews N.W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 14.Bennett E.M., Lin S.X., Towler M.C., Maxfield F.R., Brodsky F.M. Clathrin hub expression affects early endosome distribution with minimal impact on receptor sorting and recycling. Mol. Biol. Cell. 2001;12:2790–2799. doi: 10.1091/mbc.12.9.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn W.A., Jr. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 16.Luzio J.P., Pryor P.R., Bright N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 17.Mullins C., Bonifacino J.S. The molecular machinery for lysosome biogenesis. Bioessays. 2001;23:333–343. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- 18.Bao J.X., Jin S., Zhang F., Wang Z.C., Li N., Li P.L. Activation of membrane NADPH oxidase associated with lysosome-targeted acid sphingomyelinase in coronary endothelial cells. Antioxid Redox Signal. 2010;12:703–712. doi: 10.1089/ars.2009.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X.P., Shen D., Wang X., Dawson T., Li X., Zhang Q., Cheng X., Zhang Y., Weisman L.S., Delling M., et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen D., Wang X., Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays. 2011;33:448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward D.M., Pevsner J., Scullion M.A., Vaughn M., Kaplan J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol. Biol. Cell. 2000;11:2327–2333. doi: 10.1091/mbc.11.7.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell W.B., Deeter C., Gauthier K.M., Ingraham R.H., Falck J.R., Li P.L. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1656–H1664. doi: 10.1152/ajpheart.00597.2001. [DOI] [PubMed] [Google Scholar]
- 23.Churchill G.C., Okada Y., Thomas J.M., Genazzani A.A., Patel S., Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/S0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 24.Dai J., Kuo K.H., Leo J.M., van Breemen C., Lee C.H. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium. 2005;37:333–340. doi: 10.1016/j.ceca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Shen D., Wang X., Li X., Zhang X., Yao Z., Dibble S., Dong X.P., Yu T., Lieberman A.P., Showalter H.D., et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G., Li C.X., Xia M., Ritter J.K., Gehr T.W., Boini K., Li P.L. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: role of p62/Sequestosome 1 as a signaling hub. Cell Physiol. Biochem. 2015;35:1773–1786. doi: 10.1159/000373989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W.J., Li Z.H., Chen X.C., Zhao X.L., Zhong Z., Yang C., Wu H.L., An N., Li W.Y., Liu H.F. Blockage of the lysosome-dependent autophagic pathway contributes to complement membrane attack complex-induced podocyte injury in idiopathic membranous nephropathy. Sci. Rep. 2017;7:8643. doi: 10.1038/s41598-017-07889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong J., Xia M., Xu M., Zhang Y., Abais J.M., Li G., Riebling C.R., Ritter J.K., Boini K.M., Li P.L. Autophagy maturation associated with CD38-mediated regulation of lysosome function in mouse glomerular podocytes. J. Cell Mol. Med. 2013;17:1598–1607. doi: 10.1111/jcmm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang L., Zhou Y., Cao H., Wen P., Jiang L., He W., Dai C., Yang J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE. 2013;8:e60546. doi: 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang Y.L., Saleem M.A., Chan K.W., Yung B.Y., Law H.K. The cytoprotective role of autophagy in puromycin aminonucleoside treated human podocytes. Biochem. Biophys Res. Commun. 2014;443:628–634. doi: 10.1016/j.bbrc.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Yi M., Zhang L., Liu Y., Livingston M.J., Chen J.K., Nahman N.S., Jr., Liu F., Dong Z. Autophagy is activated to protect against podocyte injury in adriamycin-induced nephropathy. Am. J. Physiol. Renal. Physiol. 2017;313:F74–F84. doi: 10.1152/ajprenal.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akchurin O., Reidy K.J. Genetic causes of proteinuria and nephrotic syndrome: impact on podocyte pathobiology. Pediatr. Nephrol. 2015;30:221–233. doi: 10.1007/s00467-014-2753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartleben B., Godel M., Meyer-Schwesinger C., Liu S., Ulrich T., Kobler S., Wiech T., Grahammer F., Arnold S.J., Lindenmeyer M.T., et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., Li Q.X., Wang X.J., Zhang C., Duan Y.Q., Wang Z.Y., Zhang Y., Yu X., Li N.J., Sun J.P., et al. Beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 2016;7:e2183. doi: 10.1038/cddis.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tagawa A., Yasuda M., Kume S., Yamahara K., Nakazawa J., Chin-Kanasaki M., Araki H., Araki S., Koya D., Asanuma K., et al. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes. 2016;65:755–767. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 36.Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 37.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W., Jimenez-Sanchez M., Korolchuk V.I., Lichtenberg M., Luo S., et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C., Li W., Wen J., Yang Z. Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling. J. Cell Mol. Med. 2017;21:1315–1328. doi: 10.1111/jcmm.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Gao Y., Tian N., Wang T., Shi Y., Xu J., Wu B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-kappaB p65 axis. Sci. Rep. 2019;9:323. doi: 10.1038/s41598-018-36911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinnear N.P., Boittin F.X., Thomas J.M., Galione A., Evans A.M. Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J. Biol. Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki M., Masgrau R., Morgan A.J., Churchill G.C., Patel S., Ashcroft S.J., Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J. Biol. Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 42.Korolchuk V.I., Rubinsztein D.C. Regulation of autophagy by lysosomal positioning. Autophagy. 2011;7:927–928. doi: 10.4161/auto.7.8.15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollenbeck P.J., Swanson J.A. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990;346:864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- 44.Harada A., Takei Y., Kanai Y., Tanaka Y., Nonaka S., Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 1989;108:855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pu J., Keren-Kaplan T., Bonifacino J.S. A Ragulator-BORC interaction controls lysosome positioning in response to amino acid availability. J. Cell Biol. 2017;216:4183–4197. doi: 10.1083/jcb.201703094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pu J., Guardia C.M., Keren-Kaplan T., Bonifacino J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016;129:4329–4339. doi: 10.1242/jcs.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Futai M., Sun-Wada G.H., Wada Y., Matsumoto N., Nakanishi-Matsui M. Vacuolar-type ATPase: A proton pump to lysosomal trafficking. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2019;95:261–277. doi: 10.2183/pjab.95.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korolchuk V.I., Saiki S., Lichtenberg M., Siddiqi F.H., Roberts E.A., Imarisio S., Jahreiss L., Sarkar S., Futter M., Menzies F.M., et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong Z., Pedersen N.M., Wang L., Torgersen M.L., Stenmark H., Raiborg C. PtdIns3P controls mTORC1 signaling through lysosomal positioning. J. Cell Biol. 2017;216:4217–4233. doi: 10.1083/jcb.201611073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinti A., Le Sage V., Milev M.P., Valiente-Echeverria F., Crossie C., Miron M.J., Pante N., Olivier M., Mouland A.J. HIV-1 enhances mTORC1 activity and repositions lysosomes to the periphery by co-opting Rag GTPases. Sci. Rep. 2017;7:5515. doi: 10.1038/s41598-017-05410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan K.P., Ho M.Y., Cho H.C., Yu J., Hung J.T., Yu A.L. Fucosylation of LAMP-1 and LAMP-2 by FUT1 correlates with lysosomal positioning and autophagic flux of breast cancer cells. Cell Death Dis. 2016;7:e2347. doi: 10.1038/cddis.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasuda M., Tanaka Y., Kume S., Morita Y., Chin-Kanasaki M., Araki H., Isshiki K., Araki S., Koya D., Haneda M., et al. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim. Biophys Acta. 2014;1842:1097–1108. doi: 10.1016/j.bbadis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Godel M., Hartleben B., Herbach N., Liu S., Zschiedrich S., Lu S., Debreczeni-Mor A., Lindenmeyer M.T., Rastaldi M.P., Hartleben G., et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cina D.P., Onay T., Paltoo A., Li C., Maezawa Y., De Arteaga J., Jurisicova A., Quaggin S.E. Inhibition of MTOR disrupts autophagic flux in podocytes. J. Am. Soc. Nephrol. 2012;23:412–420. doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bumbea V., Kamar N., Ribes D., Esposito L., Modesto A., Guitard J., Nasou G., Durand D., Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol. Dial. Transplant. 2005;20:2517–2523. doi: 10.1093/ndt/gfh957. [DOI] [PubMed] [Google Scholar]
- 57.Straathof-Galema L., Wetzels J.F., Dijkman H.B., Steenbergen E.J., Hilbrands L.B. Sirolimus-associated heavy proteinuria in a renal transplant recipient: evidence for a tubular mechanism. Am. J. Transplant. 2006;6:429–433. doi: 10.1111/j.1600-6143.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 58.Fervenza F.C., Fitzpatrick P.M., Mertz J., Erickson S.B., Liggett S., Popham S., Wochos D.N., Synhavsky A., Hippler S., Larson T.S., et al. Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrol. Dial. Transplant. 2004;19:1288–1292. doi: 10.1093/ndt/gfh079. [DOI] [PubMed] [Google Scholar]
- 59.Canaud G., Bienaime F., Viau A., Treins C., Baron W., Nguyen C., Burtin M., Berissi S., Giannakakis K., Muda A.O., et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat. Med. 2013;19:1288–1296. doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 60.Reiser J. Akt2 relaxes podocytes in chronic kidney disease. Nat. Med. 2013;19:1212–1213. doi: 10.1038/nm.3357. [DOI] [PubMed] [Google Scholar]
- 61.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Evans T.D., Jeong S.J., Razani B. Classical and alternative roles for autophagy in lipid metabolism. Curr. Opin. Lipidol. 2018;29:203–211. doi: 10.1097/MOL.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolter T., Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 64.Kolter T., Sandhoff K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010;584:1700–1712. doi: 10.1016/j.febslet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Sonderfeld S., Conzelmann E., Schwarzmann G., Burg J., Hinrichs U., Sandhoff K. Incorporation and metabolism of ganglioside GM2 in skin fibroblasts from normal and GM2 gangliosidosis subjects. Eur. J. Biochem. 1985;149:247–255. doi: 10.1111/j.1432-1033.1985.tb08919.x. [DOI] [PubMed] [Google Scholar]
- 66.Riboni L., Bassi R., Prinetti A., Tettamanti G. Salvage of catabolic products in ganglioside metabolism: a study on rat cerebellar granule cells in culture. FEBS Lett. 1996;391:336–340. doi: 10.1016/0014-5793(96)00772-7. [DOI] [PubMed] [Google Scholar]
- 67.Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj. J. 2004;20:301–317. doi: 10.1023/B:GLYC.0000033627.02765.cc. [DOI] [PubMed] [Google Scholar]
- 68.Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 69.Kaipia A., Chun S.Y., Eisenhauer K., Hsueh A.J. Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology. 1996;137:4864–4870. doi: 10.1210/endo.137.11.8895358. [DOI] [PubMed] [Google Scholar]
- 70.Merrill A.H. Ceramide: a new lipid “second messenger”? Nutr. Rev. 1992;50:78–80. doi: 10.1111/j.1753-4887.1992.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 71.Vanaja S.K., Rathinam V.A., Fitzgerald K.A. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015;25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saitoh T., Fujita N., Jang M.H., Uematsu S., Yang B.G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 73.Shibutani S.T., Saitoh T., Nowag H., Munz C., Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 74.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 75.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lupfer C., Thomas P.G., Anand P.K., Vogel P., Milasta S., Martinez J., Huang G., Green M., Kundu M., Chi H., et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi C.S., Shenderov K., Huang N.N., Kabat J., Abu-Asab M., Fitzgerald K.A., Sher A., Kehrl J.H. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harris J., Hartman M., Roche C., Zeng S.G., O’Shea A., Sharp F.A., Lambe E.M., Creagh E.M., Golenbock D.T., Tschopp J., et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruno S., Porta S., Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur. J. Pharmacol. 2016;790:83–91. doi: 10.1016/j.ejphar.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 83.Erdbrugger U., Le T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016;27:12–26. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pomatto M.A.C., Gai C., Bussolati B., Camussi G. Extracellular Vesicles in Renal Pathophysiology. Front. Mol. Biosci. 2017;4:37. doi: 10.3389/fmolb.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvarez-Erviti L., Seow Y., Schapira A.H., Gardiner C., Sargent I.L., Wood M.J., Cooper J.M. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vingtdeux V., Hamdane M., Loyens A., Gele P., Drobeck H., Begard S., Galas M.C., Delacourte A., Beauvillain J.C., Buee L., et al. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 2007;282:18197–18205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- 87.Wei Y.M., Li X., Xu M., Abais J.M., Chen Y., Riebling C.R., Boini K.M., Li P.L., Zhang Y. Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol. Biochem. 2013;31:925–937. doi: 10.1159/000350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baixauli F., Lopez-Otin C., Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fader C.M., Sanchez D., Furlan M., Colombo M.I. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 2008;9:230–250. doi: 10.1111/j.1600-0854.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 90.Murrow L., Debnath J. ATG12-ATG3 connects basal autophagy and late endosome function. Autophagy. 2015;11:961–962. doi: 10.1080/15548627.2015.1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hara M., Yamagata K., Tomino Y., Saito A., Hirayama Y., Ogasawara S., Kurosawa H., Sekine S., Yan K. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia. 2012;55:2913–2919. doi: 10.1007/s00125-012-2661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stahl A.L., Johansson K., Mossberg M., Kahn R., Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019;34:11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hara M., Yanagihara T., Kihara I., Higashi K., Fujimoto K., Kajita T. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J. Am. Soc. Nephrol. 2005;16:408–416. doi: 10.1681/ASN.2004070564. [DOI] [PubMed] [Google Scholar]
- 94.Lee H., Han K.H., Lee S.E., Kim S.H., Kang H.G., Cheong H.I. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr. Nephrol. 2012;27:317–320. doi: 10.1007/s00467-011-2035-2. [DOI] [PubMed] [Google Scholar]
- 95.Lytvyn Y., Xiao F., Kennedy C.R., Perkins B.A., Reich H.N., Scholey J.W., Cherney D.Z., Burger D. Assessment of urinary microparticles in normotensive patients with type 1 diabetes. Diabetologia. 2017;60:581–584. doi: 10.1007/s00125-016-4190-2. [DOI] [PubMed] [Google Scholar]
- 96.Tkaczyk M., Baj Z. Surface markers of platelet function in idiopathic nephrotic syndrome in children. Pediatr. Nephrol. 2002;17:673–677. doi: 10.1007/s00467-002-0865-7. [DOI] [PubMed] [Google Scholar]
- 97.Wu X., Gao Y., Xu L., Dang W., Yan H., Zou D., Zhu Z., Luo L., Tian N., Wang X., et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017;7:9371. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castrop H., Schiessl I.M. Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol. (Oxf) 2017;219:544–553. doi: 10.1111/apha.12760. [DOI] [PubMed] [Google Scholar]
- 99.Bao J.X., Zhang Q.F., Wang M., Xia M., Boini K.M., Gulbins E., Zhang Y., Li P.L. Implication of CD38 gene in autophagic degradation of collagen I in mouse coronary arterial myocytes. Front. Biosci. (Landmark Ed.) 2017;22:558–569. doi: 10.2741/4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu M., Li X., Walsh S.W., Zhang Y., Abais J.M., Boini K.M., Li P.L. Intracellular two-phase Ca2+ release and apoptosis controlled by TRP-ML1 channel activity in coronary arterial myocytes. Am. J. Physiol. Cell Physiol. 2013;304:C458–C466. doi: 10.1152/ajpcell.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang F., Xu M., Han W.Q., Li P.L. Reconstitution of lysosomal NAADP-TRP-ML1 signaling pathway and its function in TRP-ML1(-/-) cells. Am. J. Physiol. Cell Physiol. 2011;301:C421–C430. doi: 10.1152/ajpcell.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun M., Goldin E., Stahl S., Falardeau J.L., Kennedy J.C., Acierno J.S., Jr., Bove C., Kaneski C.R., Nagle J., Bromley M.C., et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 103.Galione A. NAADP, a new intracellular messenger that mobilizes Ca2+ from acidic stores. Biochem. Soc. Trans. 2006;34:922–926. doi: 10.1042/BST0340922. [DOI] [PubMed] [Google Scholar]
- 104.Zhang F., Xia M., Li P.L. Lysosome-dependent Ca(2+) release response to Fas activation in coronary arterial myocytes through NAADP: evidence from CD38 gene knockouts. Am. J. Physiol. Cell Physiol. 2010;298:C1209–C1216. doi: 10.1152/ajpcell.00533.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang F., Zhang G., Zhang A.Y., Koeberl M.J., Wallander E., Li P.L. Production of NAADP and its role in Ca2+ mobilization associated with lysosomes in coronary arterial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H274–H282. doi: 10.1152/ajpheart.01064.2005. [DOI] [PubMed] [Google Scholar]
- 106.Dong X.P., Cheng X., Mills E., Delling M., Wang F., Kurz T., Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X., Rydzewski N., Hider A., Zhang X., Yang J., Wang W., Gao Q., Cheng X., Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang G., Ouyang J., Li S., Wang H., Lian B., Liu Z., Xie L. The analysis of risk factors for diabetic nephropathy progression and the construction of a prognostic database for chronic kidney diseases. J. Transl. Med. 2019;17:264. doi: 10.1186/s12967-019-2016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim W.Y., Nam S.A., Song H.C., Ko J.S., Park S.H., Kim H.L., Choi E.J., Kim Y.S., Kim J., Kim Y.K. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 2012;17:148–159. doi: 10.1111/j.1440-1797.2011.01541.x. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z., Choi M.E. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20:519–537. doi: 10.1089/ars.2013.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 112.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., Sabatini D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee M.J., Feliers D., Mariappan M.M., Sataranatarajan K., Mahimainathan L., Musi N., Foretz M., Viollet B., Weinberg J.M., Choudhury G.G., et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 114.Inoki K. Role of TSC-mTOR pathway in diabetic nephropathy. Diabetes Res. Clin. Pract. 2008;82(Suppl. 1):S59–S62. doi: 10.1016/j.diabres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 115.Kim D.K., Nam B.Y., Li J.J., Park J.T., Lee S.H., Kim D.H., Kim J.Y., Kang H.Y., Han S.H., Yoo T.H., et al. Translationally controlled tumour protein is associated with podocyte hypertrophy in a mouse model of type 1 diabetes. Diabetologia. 2012;55:1205–1217. doi: 10.1007/s00125-012-2467-7. [DOI] [PubMed] [Google Scholar]
- 116.Guzman J., Jauregui A.N., Merscher-Gomez S., Maiguel D., Muresan C., Mitrofanova A., Diez-Sampedro A., Szust J., Yoo T.H., Villarreal R., et al. Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes. 2014;63:701–714. doi: 10.2337/db13-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee J., Koh A., Jeong H., Kim E., Ha T.S., Saleem M.A., Ryu S.H. C1-Ten is a PTPase of nephrin, regulating podocyte hypertrophy through mTORC1 activation. Sci. Rep. 2017;7:12346. doi: 10.1038/s41598-017-12382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma T., Zhu J., Chen X., Zha D., Singhal P.C., Ding G. High glucose induces autophagy in podocytes. Exp. Cell Res. 2013;319:779–789. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Azad M.B., Chen Y., Gibson S.B. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 120.Inoki K., Mori H., Wang J., Suzuki T., Hong S., Yoshida S., Blattner S.M., Ikenoue T., Ruegg M.A., Hall M.N., et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu G., Han F., Yang Y., Xie Y., Jiang H., Mao Y., Wang H., Wang M., Chen R., Yang J., et al. Evaluation of sphingolipid metabolism in renal cortex of rats with streptozotocin-induced diabetes and the effects of rapamycin. Nephrol. Dial. Transplant. 2011;26:1493–1502. doi: 10.1093/ndt/gfq633. [DOI] [PubMed] [Google Scholar]
- 122.Choi S.R., Lim J.H., Kim M.Y., Kim E.N., Kim Y., Choi B.S., Kim Y.S., Kim H.W., Lim K.M., Kim M.J., et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism. 2018;85:348–360. doi: 10.1016/j.metabol.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 123.Morita Y., Kurano M., Sakai E., Nishikawa T., Nishikawa M., Sawabe M., Aoki J., Yatomi Y. Analysis of urinary sphingolipids using liquid chromatography-tandem mass spectrometry in diabetic nephropathy. J. Diabetes Investig. 2019 doi: 10.1111/jdi.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang F., Gomez-Sintes R., Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19:918–931. doi: 10.1111/tra.12613. [DOI] [PubMed] [Google Scholar]
- 125.Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal. 2012;17:766–774. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- 126.Liu W.J., Gan Y., Huang W.F., Wu H.L., Zhang X.Q., Zheng H.J., Liu H.F. Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis. 2019;10:806. doi: 10.1038/s41419-019-2002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adler S., Baker P.J., Johnson R.J., Ochi R.F., Pritzl P., Couser W.G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J. Clin. Invest. 1986;77:762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou L.L., Cao W., Xie C., Tian J., Zhou Z., Zhou Q., Zhu P., Li A., Liu Y., Miyata T., et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- 129.Liu W.J., Shen T.T., Chen R.H., Wu H.L., Wang Y.J., Deng J.K., Chen Q.H., Pan Q., Huang Fu C.M., Tao J.L., et al. Autophagy-Lysosome Pathway in Renal Tubular Epithelial Cells Is Disrupted by Advanced Glycation End Products in Diabetic Nephropathy. J. Biol. Chem. 2015;290:20499–20510. doi: 10.1074/jbc.M115.666354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu W.J., Xu B.H., Ye L., Liang D., Wu H.L., Zheng Y.Y., Deng J.K., Li B., Liu H.F. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 2015;308:F639–F649. doi: 10.1152/ajprenal.00383.2014. [DOI] [PubMed] [Google Scholar]
- 131.Johansson A.C., Appelqvist H., Nilsson C., Kagedal K., Roberg K., Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15:527–540. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garsen M., Rops A.L., Dijkman H., Willemsen B., van Kuppevelt T.H., Russel F.G., Rabelink T.J., Berden J.H., Reinheckel T., van der Vlag J. Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int. 2016;90:1012–1022. doi: 10.1016/j.kint.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 133.Sugimoto K., Miyazawa T., Enya T., Miyazaki K., Okada M., Takemura T. Cyclosporine A induced histological changes of Cathepsin L and CD2AP expression in renal glomeruli and tubules. Clin. Exp. Nephrol. 2017;21:83–91. doi: 10.1007/s10157-016-1257-9. [DOI] [PubMed] [Google Scholar]
- 134.Yaddanapudi S., Altintas M.M., Kistler A.D., Fernandez I., Moller C.C., Wei C., Peev V., Flesche J.B., Forst A.L., Li J., et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J. Clin. Invest. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sever S., Altintas M.M., Nankoe S.R., Moller C.C., Ko D., Wei C., Henderson J., del Re E.C., Hsing L., Erickson A., et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J. Clin. Invest. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L., Wei Y., Wang X., He R. D-Ribosylated Tau forms globular aggregates with high cytotoxicity. Cell Mol. Life Sci. 2009;66:2559–2571. doi: 10.1007/s00018-009-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei Y., Chen L., Chen J., Ge L., He R.Q. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009;10:10. doi: 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hong J., Li G., Zhang Q., Ritter J., Li W., Li P.L. D-Ribose Induces Podocyte NLRP3 Inflammasome Activation and Glomerular Injury via AGEs/RAGE Pathway. Front. Cell Dev. Biol. 2019;7:259. doi: 10.3389/fcell.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hong J., Bhat O.M., Li G., Dempsey S.K., Zhang Q., Ritter J.K., Li W., Li P.L. Lysosomal regulation of extracellular vesicle excretion during d-ribose-induced NLRP3 inflammasome activation in podocytes. Biochim. Biophys Acta Mol. Cell Res. 2019;1866:849–860. doi: 10.1016/j.bbamcr.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nance C.S., Klein C.J., Banikazemi M., Dikman S.H., Phelps R.G., McArthur J.C., Rodriguez M., Desnick R.J. Later-onset Fabry disease: an adult variant presenting with the cramp-fasciculation syndrome. Arch. Neurol. 2006;63:453–457. doi: 10.1001/archneur.63.3.453. [DOI] [PubMed] [Google Scholar]
- 141.Kruger R., Bruns K., Grunhage S., Rossmann H., Reinke J., Beck M., Lackner K.J. Determination of globotriaosylceramide in plasma and urine by mass spectrometry. Clin. Chem. Lab. Med. 2010;48:189–198. doi: 10.1515/CCLM.2010.048. [DOI] [PubMed] [Google Scholar]
- 142.Young E., Mills K., Morris P., Vellodi A., Lee P., Waldek S., Winchester B. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr. Suppl. 2005;94:51–54. doi: 10.1080/08035320510028111. discussion 37–58. [DOI] [PubMed] [Google Scholar]
- 143.Askari H., Kaneski C.R., Semino-Mora C., Desai P., Ang A., Kleiner D.E., Perlee L.T., Quezado M., Spollen L.E., Wustman B.A., et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007;451:823–834. doi: 10.1007/s00428-007-0468-6. [DOI] [PubMed] [Google Scholar]
- 144.Alroy J., Sabnis S., Kopp J.B. Renal pathology in Fabry disease. J. Am. Soc. Nephrol. 2002;13(Suppl. 2):S134–S138. doi: 10.1097/01.ASN.0000016684.07368.75. [DOI] [PubMed] [Google Scholar]
- 145.Liebau M.C., Braun F., Hopker K., Weitbrecht C., Bartels V., Muller R.U., Brodesser S., Saleem M.A., Benzing T., Schermer B., et al. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS ONE. 2013;8:e63506. doi: 10.1371/journal.pone.0063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thurberg B.L., Rennke H., Colvin R.B., Dikman S., Gordon R.E., Collins A.B., Desnick R.J., O’Callaghan M. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 147.Prabakaran T., Nielsen R., Larsen J.V., Sorensen S.S., Feldt-Rasmussen U., Saleem M.A., Petersen C.M., Verroust P.J., Christensen E.I. Receptor-mediated endocytosis of alpha-galactosidase A in human podocytes in Fabry disease. PLoS ONE. 2011;6:e25065. doi: 10.1371/journal.pone.0025065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reidy K., Kang H.M., Hostetter T., Susztak K. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ingram A.J., Krepinsky J.C., James L., Austin R.C., Tang D., Salapatek A.M., Thai K., Scholey J.W. Activation of mesangial cell MAPK in response to homocysteine. Kidney Int. 2004;66:733–745. doi: 10.1111/j.1523-1755.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 150.Yamasaki K., Muto J., Taylor K.R., Cogen A.L., Audish D., Bertin J., Grant E.P., Coyle A.J., Misaghi A., Hoffman H.M., et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yi F., Zhang A.Y., Janscha J.L., Li P.L., Zou A.P. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 152.Yi F., Zhang A.Y., Li N., Muh R.W., Fillet M., Renert A.F., Li P.L. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int. 2006;70:88–96. doi: 10.1038/sj.ki.5001517. [DOI] [PubMed] [Google Scholar]
- 153.Yi F., Chen Q.Z., Jin S., Li P.L. Mechanism of homocysteine-induced Rac1/NADPH oxidase activation in mesangial cells: role of guanine nucleotide exchange factor Vav2. Cell Physiol. Biochem. 2007;20:909–918. doi: 10.1159/000110451. [DOI] [PubMed] [Google Scholar]
- 154.Boini K.M., Xia M., Li C., Zhang C., Payne L.P., Abais J.M., Poklis J.L., Hylemon P.B., Li P.L. Acid sphingomyelinase gene deficiency ameliorates the hyperhomocysteinemia-induced glomerular injury in mice. Am. J. Pathol. 2011;179:2210–2219. doi: 10.1016/j.ajpath.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boini K.M., Xia M., Abais J.M., Xu M., Li C.X., Li P.L. Acid sphingomyelinase gene knockout ameliorates hyperhomocysteinemic glomerular injury in mice lacking cystathionine-beta-synthase. PLoS ONE. 2012;7:e45020. doi: 10.1371/journal.pone.0045020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang C., Boini K.M., Xia M., Abais J.M., Li X., Liu Q., Li P.L. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abais J.M., Zhang C., Xia M., Liu Q., Gehr T.W., Boini K.M., Li P.L. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abais J.M., Xia M., Li G., Gehr T.W., Boini K.M., Li P.L. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic. Biol. Med. 2014;67:211–220. doi: 10.1016/j.freeradbiomed.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boini K.M., Xia M., Koka S., Gehr T.W., Li P.L. Instigation of NLRP3 inflammasome activation and glomerular injury in mice on the high fat diet: role of acid sphingomyelinase gene. Oncotarget. 2016;7:19031–19044. doi: 10.18632/oncotarget.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boini K.M., Zhang C., Xia M., Han W.Q., Brimson C., Poklis J.L., Li P.L. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim. Biophys Acta. 2010;1801:1294–1304. doi: 10.1016/j.bbalip.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hall J.E., Henegar J.R., Dwyer T.M., Liu J., Da Silva A.A., Kuo J.J., Tallam L. Is obesity a major cause of chronic kidney disease? Adv. Ren Replace Ther. 2004;11:41–54. doi: 10.1053/j.arrt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 162.Axelsson J., Heimburger O., Stenvinkel P. Adipose tissue and inflammation in chronic kidney disease. Contrib. Nephrol. 2006;151:165–174. doi: 10.1159/000095327. [DOI] [PubMed] [Google Scholar]
- 163.Ouchi N., Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H., Yan W.J., Zhang J.L., Zhang F.Y., Gao C., Wang Y.J., Bond Law W., Tao L. Adiponectin partially rescues high glucose/high fat-induced impairment of mitochondrial biogenesis and function in a PGC-1alpha dependent manner. Eur Rev. Med. Pharmacol. Sci. 2017;21:590–599. [PubMed] [Google Scholar]
- 165.He Y., Zou L., Zhou Y., Hu H., Yao R., Jiang Y., Lau W.B., Yuan T., Huang W., Zeng Z., et al. Adiponectin ameliorates the apoptotic effects of paraquat on alveolar type cells via improvements in mitochondrial function. Mol. Med. Rep. 2016;14:746–752. doi: 10.3892/mmr.2016.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lara-Castro C., Fu Y., Chung B.H., Garvey W.T. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 167.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J. Allergy Clin. Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 168.Li G., Zhang Q., Hong J., Ritter J.K., Li P.L. Inhibition of pannexin-1 channel activity by adiponectin in podocytes: Role of acid ceramidase activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:1246–1256. doi: 10.1016/j.bbalip.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Davis S., Nehus E., Inge T., Zhang W., Setchell K., Mitsnefes M. Effect of bariatric surgery on urinary sphingolipids in adolescents with severe obesity. Surg. Obes. Relat Dis. 2018;14:446–451. doi: 10.1016/j.soard.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 170.Wang G., Dinkins M., He Q., Zhu G., Poirier C., Campbell A., Mayer-Proschel M., Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J. Biol. Chem. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kakazu E., Mauer A.S., Yin M., Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J. Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Podbielska M., Szulc Z.M., Kurowska E., Hogan E.L., Bielawski J., Bielawska A., Bhat N.R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016;57:2028–2039. doi: 10.1194/jlr.M070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li G., Huang D., Hong J., Bhat O.M., Yuan X., Li P.L. Control of Lysosomal TRPML1 Channel Activity and Exosome Release by Acid Ceramidase in Mouse Podocytes. Am. J. Physiol. Cell Physiol. 2019 doi: 10.1152/ajpcell.00150.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo H., Wang B., Li H., Ling L., Niu J., Gu Y. Glucagon-like peptide-1 analog prevents obesity-related glomerulopathy by inhibiting excessive autophagy in podocytes. Am. J. Physiol. Renal. Physiol. 2018;314:F181–F189. doi: 10.1152/ajprenal.00302.2017. [DOI] [PubMed] [Google Scholar]
- 175.Eid S., Boutary S., Braych K., Sabra R., Massaad C., Hamdy A., Rashid A., Moodad S., Block K., Gorin Y., et al. mTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes. Antioxid Redox. Signal. 2016;25:703–719. doi: 10.1089/ars.2015.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dunham I., Shimizu N., Roe B.A., Chissoe S., Hunt A.R., Collins J.E., Bruskiewich R., Beare D.M., Clamp M., Smink L.J., et al. The DNA sequence of human chromosome 22. Nature. 1999;402:489–495. doi: 10.1038/990031. [DOI] [PubMed] [Google Scholar]
- 177.Foster M.C., Coresh J., Fornage M., Astor B.C., Grams M., Franceschini N., Boerwinkle E., Parekh R.S., Kao W.H. APOL1 variants associate with increased risk of CKD among African Americans. J. Am. Soc. Nephrol. 2013;24:1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Friedman D.J., Kozlitina J., Genovese G., Jog P., Pollak M.R. Population-based risk assessment of APOL1 on renal disease. J. Am. Soc. Nephrol. 2011;22:2098–2105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Genovese G., Friedman D.J., Ross M.D., Lecordier L., Uzureau P., Freedman B.I., Bowden D.W., Langefeld C.D., Oleksyk T.K., Uscinski Knob A.L., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tzur S., Rosset S., Shemer R., Yudkovsky G., Selig S., Tarekegn A., Bekele E., Bradman N., Wasser W.G., Behar D.M., et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Human Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kopp J.B., Nelson G.W., Sampath K., Johnson R.C., Genovese G., An P., Friedman D., Briggs W., Dart R., Korbet S., et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Quaggin S.E., George A.L., Jr. Apolipoprotein l1 and the genetic basis for racial disparity in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:1955–1958. doi: 10.1681/ASN.2011090932. [DOI] [PubMed] [Google Scholar]
- 183.Genovese G., Friedman D.J., Pollak M.R. APOL1 variants and kidney disease in people of recent African ancestry. Nat. Rev. Nephrol. 2013;9:240–244. doi: 10.1038/nrneph.2013.34. [DOI] [PubMed] [Google Scholar]
- 184.Wasser W.G., Tzur S., Wolday D., Adu D., Baumstein D., Rosset S., Skorecki K. Population genetics of chronic kidney disease: the evolving story of APOL1. J. Nephrol. 2012;25:603–618. doi: 10.5301/jn.5000179. [DOI] [PubMed] [Google Scholar]
- 185.Madhavan S.M., O’Toole J.F., Konieczkowski M., Ganesan S., Bruggeman L.A., Sedor J.R. APOL1 localization in normal kidney and nondiabetic kidney disease. J. Am. Soc. Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lan X., Jhaveri A., Cheng K., Wen H., Saleem M.A., Mathieson P.W., Mikulak J., Aviram S., Malhotra A., Skorecki K., et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am. J. Physiol. Renal. Physiol. 2014;307:F326–F336. doi: 10.1152/ajprenal.00647.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perez-Morga D., Vanhollebeke B., Paturiaux-Hanocq F., Nolan D.P., Lins L., Homble F., Vanhamme L., Tebabi P., Pays A., Poelvoorde P., et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science. 2005;309:469–472. doi: 10.1126/science.1114566. [DOI] [PubMed] [Google Scholar]
- 188.Beckerman P., Bi-Karchin J., Park A.S., Qiu C., Dummer P.D., Soomro I., Boustany-Kari C.M., Pullen S.S., Miner J.H., Hu C.A., et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med. 2017;23:429–438. doi: 10.1038/nm.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mishra A., Ayasolla K., Kumar V., Lan X., Vashistha H., Aslam R., Hussain A., Chowdhary S., Marashi Shoshtari S., Paliwal N., et al. Modulation of apolipoprotein L1-microRNA-193a axis prevents podocyte dedifferentiation in high-glucose milieu. Am. J. Physiol. Renal. Physiol. 2018;314:F832–F843. doi: 10.1152/ajprenal.00541.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kumar V., Ayasolla K., Jha A., Mishra A., Vashistha H., Lan X., Qayyum M., Chinnapaka S., Purohit R., Mikulak J., et al. Disrupted apolipoprotein L1-miR193a axis dedifferentiates podocytes through autophagy blockade in an APOL1 risk milieu. Am. J. Physiol. Cell Physiol. 2019;317:C209–C225. doi: 10.1152/ajpcell.00538.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lambers Heerspink H.J., de Zeeuw D. Novel drugs and intervention strategies for the treatment of chronic kidney disease. Br. J. Clin. Pharmacol. 2013;76:536–550. doi: 10.1111/bcp.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu R., Bo H., Villani V., Spencer P.J., Fu P. The Inhibitory Effect of Rapamycin on Toll Like Receptor 4 and Interleukin 17 in the Early Stage of Rat Diabetic Nephropathy. Kidney Blood Press Res. 2016;41:55–69. doi: 10.1159/000368547. [DOI] [PubMed] [Google Scholar]
- 193.Velagapudi C., Bhandari B.S., Abboud-Werner S., Simone S., Abboud H.E., Habib S.L. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J. Am. Soc. Nephrol. 2011;22:262–273. doi: 10.1681/ASN.2010040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rosner M., Freilinger A., Hengstschlager M. Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene. 2007;26:521–531. doi: 10.1038/sj.onc.1209812. [DOI] [PubMed] [Google Scholar]
- 195.Allen D.A., Harwood S., Varagunam M., Raftery M.J., Yaqoob M.M. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17:908–910. doi: 10.1096/fj.02-0130fje. [DOI] [PubMed] [Google Scholar]
- 196.Mori H., Inoki K., Masutani K., Wakabayashi Y., Komai K., Nakagawa R., Guan K.L., Yoshimura A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophys Res. Commun. 2009;384:471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 197.Verges B., Cariou B. mTOR inhibitors and diabetes. Diabetes Res. Clin. Pract. 2015;110:101–108. doi: 10.1016/j.diabres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 198.Viana S.D., Reis F., Alves R. Therapeutic Use of mTOR Inhibitors in Renal Diseases: Advances, Drawbacks, and Challenges. Oxid Med. Cell Longev. 2018;2018:3693625. doi: 10.1155/2018/3693625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Amer H., Cosio F.G. Significance and management of proteinuria in kidney transplant recipients. J. Am. Soc. Nephrol. 2009;20:2490–2492. doi: 10.1681/ASN.2008091005. [DOI] [PubMed] [Google Scholar]
- 200.Torras J., Herrero-Fresneda I., Gulias O., Flaquer M., Vidal A., Cruzado J.M., Lloberas N., Franquesa M., Grinyo J.M. Rapamycin has dual opposing effects on proteinuric experimental nephropathies: is it a matter of podocyte damage? Nephrol. Dial. Transplant. 2009;24:3632–3640. doi: 10.1093/ndt/gfp367. [DOI] [PubMed] [Google Scholar]
- 201.Sereno J., Parada B., Rodrigues-Santos P., Lopes P.C., Carvalho E., Vala H., Teixeira-Lemos E., Alves R., Figueiredo A., Mota A., et al. Serum and renal tissue markers of nephropathy in rats under immunosuppressive therapy: cyclosporine versus sirolimus. Transplant. Proc. 2013;45:1149–1156. doi: 10.1016/j.transproceed.2013.02.085. [DOI] [PubMed] [Google Scholar]
- 202.Sereno J., Nunes S., Rodrigues-Santos P., Vala H., Rocha-Pereira P., Fernandes J., Santos-Silva A., Teixeira F., Reis F. Conversion to sirolimus ameliorates cyclosporine-induced nephropathy in the rat: focus on serum, urine, gene, and protein renal expression biomarkers. Biomed. Res. Int. 2014;2014:576929. doi: 10.1155/2014/576929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sereno J., Vala H., Nunes S., Rocha-Pereira P., Carvalho E., Alves R., Teixeira F., Reis F. Cyclosporine A-induced nephrotoxicity is ameliorated by dose reduction and conversion to sirolimus in the rat. J. Physiol. Pharmacol. 2015;66:285–299. [PubMed] [Google Scholar]
- 204.Cheng K., Rai P., Plagov A., Lan X., Mathieson P.W., Saleem M.A., Husain M., Malhotra A., Singhal P.C. Rapamycin-induced modulation of miRNA expression is associated with amelioration of HIV-associated nephropathy (HIVAN) Exp. Cell Res. 2013;319:2073–2080. doi: 10.1016/j.yexcr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu L., Fan Q., Wang X., Li L., Lu X., Yue Y., Cao X., Liu J., Zhao X., Wang L. Ursolic acid improves podocyte injury caused by high glucose. Nephrol. Dial. Transplant. 2017;32:1285–1293. doi: 10.1093/ndt/gfv382. [DOI] [PubMed] [Google Scholar]
- 206.Sun J., Li Z.P., Zhang R.Q., Zhang H.M. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem. Biophys. Res. Commun. 2017;483:318–324. doi: 10.1016/j.bbrc.2016.12.145. [DOI] [PubMed] [Google Scholar]
- 207.Yamamoto C.M., Murakami T., Oakes M.L., Mitsuhashi M., Kelly C., Henry R.R., Sharma K. Uromodulin mRNA from Urinary Extracellular Vesicles Correlate to Kidney Function Decline in Type 2 Diabetes Mellitus. Am. J. Nephrol. 2018;47:283–291. doi: 10.1159/000489129. [DOI] [PubMed] [Google Scholar]
- 208.Lv L.L., Cao Y.H., Pan M.M., Liu H., Tang R.N., Ma K.L., Chen P.S., Liu B.C. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin. Chim. Acta. 2014;428:26–31. doi: 10.1016/j.cca.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 209.Feng Y., Lv L.L., Wu W.J., Li Z.L., Chen J., Ni H.F., Zhou L.T., Tang T.T., Wang F.M., Wang B., et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. Am. J. Pathol. 2018;188:2542–2552. doi: 10.1016/j.ajpath.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 210.Lv L.L., Cao Y., Liu D., Xu M., Liu H., Tang R.N., Ma K.L., Liu B.C. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int. J. Biol. Sci. 2013;9:1021–1031. doi: 10.7150/ijbs.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Perez-Hernandez J., Olivares D., Forner M.J., Ortega A., Solaz E., Martinez F., Chaves F.J., Redon J., Cortes R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J. Transl. Med. 2018;16:228. doi: 10.1186/s12967-018-1604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lv L.L., Cao Y.H., Ni H.F., Xu M., Liu D., Liu H., Chen P.S., Liu B.C. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Renal. Physiol. 2013;305:F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 213.Ben-Dov I.Z., Tan Y.C., Morozov P., Wilson P.D., Rennert H., Blumenfeld J.D., Tuschl T. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS ONE. 2014;9:e86856. doi: 10.1371/journal.pone.0086856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mohan A., Singh R.S., Kumari M., Garg D., Upadhyay A., Ecelbarger C.M., Tripathy S., Tiwari S. Urinary Exosomal microRNA-451-5p Is a Potential Early Biomarker of Diabetic Nephropathy in Rats. PLoS ONE. 2016;11:e0154055. doi: 10.1371/journal.pone.0154055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ramezani A., Devaney J.M., Cohen S., Wing M.R., Scott R., Knoblach S., Singhal R., Howard L., Kopp J.B., Raj D.S. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur. J. Clin. Invest. 2015;45:394–404. doi: 10.1111/eci.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jang S.W., Liu X., Chan C.B., Weinshenker D., Hall R.A., Xiao G., Ye K. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem. Biol. 2009;16:644–656. doi: 10.1016/j.chembiol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fu L., Wang S., Wang X., Wang P., Zheng Y., Yao D., Guo M., Zhang L., Ouyang L. Crystal structure-based discovery of a novel synthesized PARP1 inhibitor (OL-1) with apoptosis-inducing mechanisms in triple-negative breast cancer. Sci. Rep. 2016;6:3. doi: 10.1038/s41598-016-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Owens M.J., Morgan W.N., Plott S.J., Nemeroff C.B. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J. Pharmacol. Exp. Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- 219.Rauser L., Savage J.E., Meltzer H.Y., Roth B.L. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor. J. Pharmacol. Exp. Ther. 2001;299:83–89. [PubMed] [Google Scholar]
- 220.Werling L.L., Keller A., Frank J.G., Nuwayhid S.J. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp. Neurol. 2007;207:248–257. doi: 10.1016/j.expneurol.2007.06.013. [DOI] [PubMed] [Google Scholar]