Abstract
Spätzle family proteins activate the Toll pathway and induce antimicrobial peptide (AMP) production against microbial infections. However, the functional importance of Tmspätzle4 (TmSpz4) in the immune response of Tenebrio molitor has not been reported. Therefore, here, we have identified and functionally characterized the role of TmSpz4 against bacterial and fungal infections. We showed that TmSpz4 expression was significantly induced in hemocytes at 6 h post-injection with Escherichia coli, Staphylococcus aureus, and Candida albicans. TmSpz4 knock-down significantly reduced larval survival against E. coli and C. albicans. To understand the reason for the survivability difference, the role of TmSpz4 in AMP production was examined in TmSpz4-silenced larvae following microbe injection. The AMPs that are active against Gram-negative bacteria, including TmTenecin-2, TmTenecin-4, TmAttacin-1a, TmDefensin-2, and TmCecropin-2, were significantly downregulated in response to E. coli in TmSpz4-silenced larvae. Similarly, the expression of TmTenecin-1, TmTenecin-3, TmThaumatin-like protein-1 and -2, TmDefensin-1, TmDefensin-2, and TmCecropin-2 were downregulated in response to C. albicans in TmSpz4-silenced larvae. In addition, the transcription factor NF-κB (TmDorX1 and TmDorX2) expression was significantly suppression in TmSpz4-silenced larvae. In conclusion, these results suggest that TmSpz4 plays a key role in regulating immune responses of T. molitor against to E. coli and C. albicans.
Keywords: AMP expression, defense response, mealworm, Spätzle, Toll receptor
1. Introduction
Insects are the largest and most diverse group of animals on Earth, and have highly adaptable defenses against different environmental threats, including microorganisms (bacteria, fungi, and viruses) and parasites. To survive against microorganisms, insects have developed a potent defense mechanism, known as innate immunity, which can recognize and eliminate microbes [1]. This well-developed system includes physical defenses, as well as cellular and humoral immunity. The humoral immune system principally relies on antimicrobial peptides (AMPs), lectins, lysozyme, and protease inhibitors [2]. The innate immune system depends on pattern recognition receptors (PRRs) that recognize conserved molecules on pathogens, called pathogen-associated molecular patterns [3]. Recognition of invading pathogens activates signal transduction pathways [Toll or immune deficiency (IMD)], leading to the expression of AMPs that attack the invading pathogens [4,5].
Since Toll was first identified as a regulator of dorsoventral axis establishment during embryonic development [6], scientists have made great progress in developing genetic and molecular biological methodologies to demonstrate the functional role of the Toll pathway in the Drosophila immune system [7,8,9]. Specifically, the Toll pathway is activated when peptidoglycan recognition proteins (PGRPs) or Gram-negative bacteria binding proteins (GNBPs) recognize microbe-derived peptidoglycan or β-1,3 glucan [10] and the signal generated by this recognition is conveyed to a proteolytic cascade that leads to cleavage of the cytokine-like protein pro-spätzle. The resulting mature spätzle functions as a ligand that activates the Toll receptor [11,12]. Spätzle is an extracellular cytokine-like protein and a Toll receptor ligand. Its inactive form, pro-spätzle, contains a signal peptide, a regulatory N-terminal pro-domain, and a signaling precursor consisting of a C-terminal active fragment of 106 amino acids [13,14]. Active spätzle is generated during development and/or during the immune response to microbial infection. More specifically, during an immune response, a proteolytic cascade cleaves pro-spätzle, releasing active spätzle [11].
In Drosophila, six spätzle homologues (Spz1–6) have been identified, including the first identified spätzle gene, spz-1, which encodes a protein containing neurotrophin-like cystine-knot domains [15]. The study of the interaction of spätzle family proteins with each other and with Toll receptors during the activation of AMP gene expression showed that the binding of Spz-1, -2, and -5 to the Toll-1 and Toll-7 ectodomains promotes the activation of drosomycin and several other AMP genes [16,17,18].
The functions of the spätzle family proteins have been investigated in Anopheles gambiae and Aedes aegypti in response to fungal challenge [19,20], in Bombyx mori in response to microbial infection [21], and in Manduca sexta in response to Gram-positive bacterial, Gram-negative bacterial, and fungal infections [22]. In B. mori, spätzle4 has been shown to play an important role against Gram-negative bacteria, Gram-positive bacteria, and fungi, specifically in the integument [23].
In shrimp (Litopenaeus vannamei), a spätzle gene (LvSpz4) was functionally characterized and shown to be involved in innate immunity; more specifically, it was shown to be involved in the cross talk between the TLR-NF-κB pathway and unfolded protein response (UPR) [24]. A spätzle-like protein was identified in Chinese shrimp (Fenneropenaeus chinensis) and shown to function in the innate immune responses to bacteria and viruses [25].
In the last decade understanding of T. molitor proteolytic cascade has increased greatly due to the intensive biochemical studies on modular serine protease (MSP), spätzle-processing enzyme (SPE)-activating enzyme (SAE), and SPE which results in pro-spätzle cleavage [11,26]. The importance of spätzle genes in innate immune responses against microbial infections extends from insects to shrimp. However, despite their biochemical characterization, the functions of spätzle genes in the T. molitor immune response to microbial challenge have remained elusive. In order to further investigate spätzle genes, we have conducted RNA-seq and genome sequencing, and thus identified nine spätzle genes (TmSpz-like, -1b, -3, -4, -5, -6, -7, -7a, and -7b). In the current study, we focused on the identification and functional characterization of TmSpätzle4 in immune responses against Gram-positive and Gram-negative bacteria and fungi.
2. Results
2.1. Sequence Identification and Phylogenetic Analysis Of TmSpz4
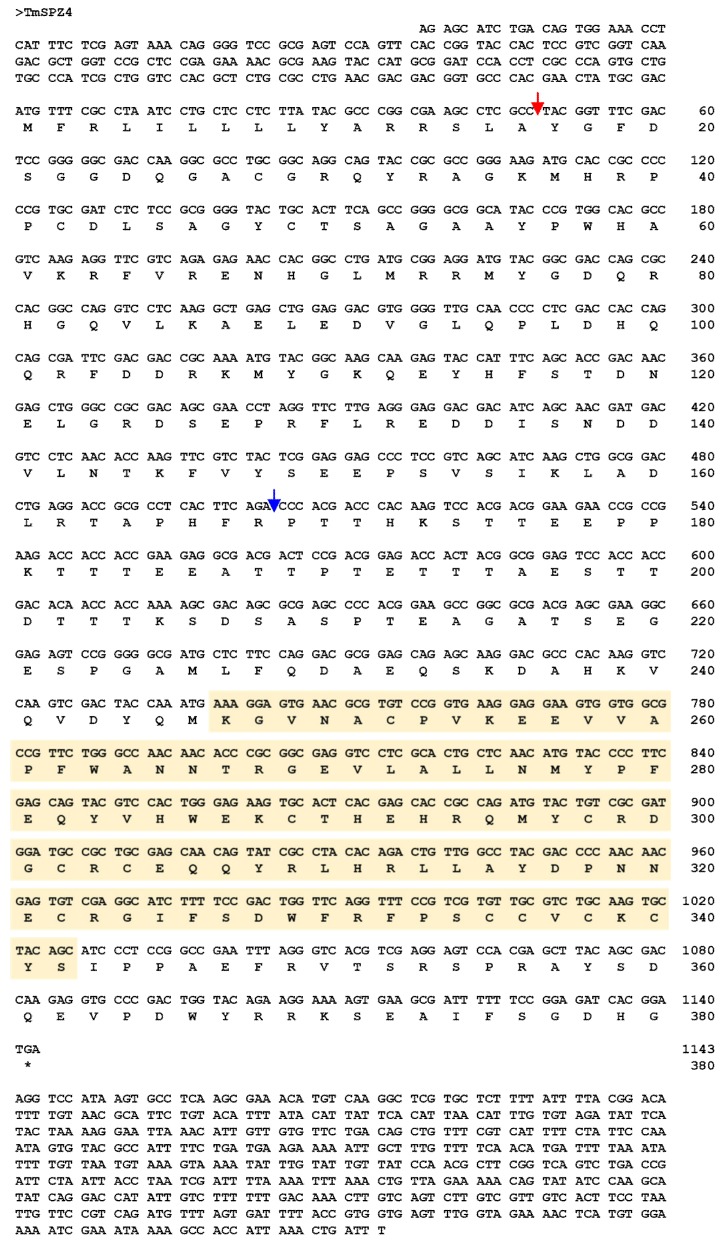
The full-length cDNA sequence of TmSpz4 was obtained from the T. molitor RNAseq database by a local tblastn search of the T. molitor nucleotide database using the T. castaneum spätzle4 protein sequence as the query. The TmSpz4 open reading frame (ORF) is 1143 bp long, and it encodes a 380 amino acid long protein (Figure 1). The 5′- and 3′-untranslated regions (UTR) of TmSpz4 were 203 and 820 bp in length, respectively. Domain analysis suggested that TmSpz4 contains one cystine-knot domain at the C-terminus, which is a ligand of the Toll receptor; one cleavage site that is predicted to be processed by SPE (Figure 1); and a predicted signal peptide. Phylogenetic analysis revealed that the Spz4 sequences of the Coleopteran insects (including Tribolium castaneum spätzle 4) were grouped together (Figure S2).
Figure 1.
Nucleotide and deduced amino acid sequence of TmSpz4. TmSpz4 contains a 1143 bp open reading frame encoding a predicted polypeptide of 380 amino acid residues. Domain analysis showed that TmSpz4 includes one cystine-knot domain (yellow box), one signal peptide region (red arrow), and one cleavage site (blue arrow).
2.2. Developmental and Tissue-Specific Expression Patterns of TmSpz4
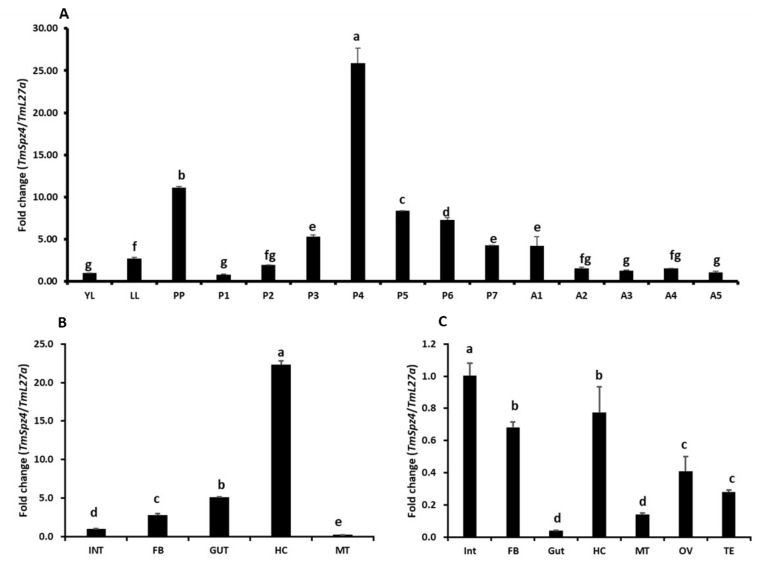
The expression patterns of TmSpz4 mRNA transcripts in mealworm across developmental stages and tissues were examined by RT-qPCR. TmSpz4 transcript expression was observed in all analyzed developmental stages and tissues, and the highest expression was observed at the prepupal and 4-day-old pupal stages. The mRNA levels increased from the young larval stage to the prepupal stage and from the 1-day-old pupal stage to the 4-day-old pupal stage (Figure 2A). In pupae, once expression peaked, it gradually decreased through the rest of the pupal stages. In adults, TmSpz4 expression was constantly low, except in 1-day-old adults, in which it was slightly higher.
Figure 2.
The expression patterns of TmSpz4 gene in developmental and tissue of T. molitor. The developmental expression patterns of TmSpz4 in mealworm at the young larval (YL), late larval (LL), pre-pupal (PP), 1–7-day-old pupal (P1–7), and 1–5-day-old adult (A1–5) stages were examined (A). In each experiment, RNA extracted from 20 individuals was used to synthesize cDNA. In larvae, TmSpz4 expression gradually increased from the YL to the PP stage. In the pupae, the highest expression was observed at the 4-day-old pupal stage. In adults, there was no difference in TmSpz4 expression from day 2 to day 5. Tissue-specific expression patterns of TmSpz4 were also investigated in late larvae (B) and five-day-old adults (C). Hemocytes (HC), gut, fat body (FB), Malpighian tubules (MT), integument (INT) (for late instar larvae and adults), and testes (TE) and ovaries (OV) (for adults) were dissected and collected from 20 late larvae and 5-day-old adults. T. molitor 60S ribosomal protein L27a (TmL27a) was included as an endogenous control to normalize RNA levels among samples. The data are the means of three biological replicates. One-way ANOVA and Tukey’s multiple range test at 95% confidence level (p < 0.05) were performed and used to determine the level of significance of differences. The graph indicated by the same letter (a, b, c, d, e, f, g, fg) are not significantly different by Tukey’s multiple range (p < 0.05).
Examination of expression levels in different tissues revealed that TmSpz4 was highly expressed in the hemocytes of late larvae (Figure 2B), while in adults, TmSpz4 expression was highest in the integument, followed by the hemocytes, fat body, ovaries, and testes (Figure 2C). Conversely, TmSpz4 expression levels were low in the integument and Malpighian tubules of late larvae and in the gut and Malpighian tubules of adults.
2.3. Temporal Induction Pattern of TmSpz4
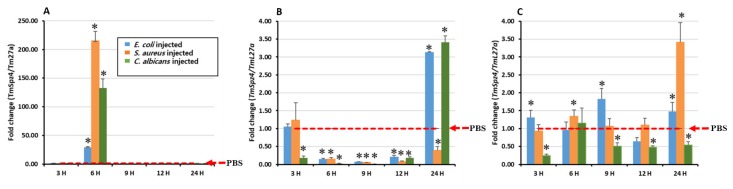
To determine whether TmSpz4 expression is induced by immune challenge, the expression of TmSpz4 in T. molitor larvae was examined over time after injecting E. coli, S. aureus, or C. albicans. PBS (pH 7) was injected as a control. Then, three immune tissues (hemocytes, fat body, and gut) were collected at 3, 6, 9, 12, and 24 h post-injection to isolate total RNA, and TmSpz4 expression in them was analyzed by RT-qPCR. Microbial challenge time-dependently induced the transcription of TmSpz4 in all tested tissues. The highest expression was observed in hemocytes at 6 h post-infection of all test microorganisms (Figure 3A). In the gut, injection of E. coli and S. aureus highly induced TmSpz4 expression at 9 and 24 h post-injection, respectively (Figure 3C), whereas in the fat body, the highest expression was detected at 24 h post-injection of E. coli and C. albicans (Figure 3B).
Figure 3.
Induction patterns of TmSpz4 in different T. molitor larval tissues. Temporal expression was analyzed in the hemocytes (A), fat body (B), and gut (C) of young larvae at 3, 6, 9, 12, and 24 h post-injection with E. coli (106 cells/μL), S. aureus (106 cells/μL), or C. albicans (5 × 104 cells/μL). Twenty young mealworm larvae were used for each time point. TmSpz4 expression levels were normalized to those in PBS-injected controls. T. molitor 60S ribosomal protein L27a (TmL27a) was used as an internal control. The dotted red line indicates PBS injection control. Asterisks indicate significant differences between infected and PBS injected larval group by Student’s t-test (p < 0.05). The vertical bars indicate Mean ± SD (n = 20).
2.4. Effect of TmSpz4 RNAi on T. Molitor Survivability
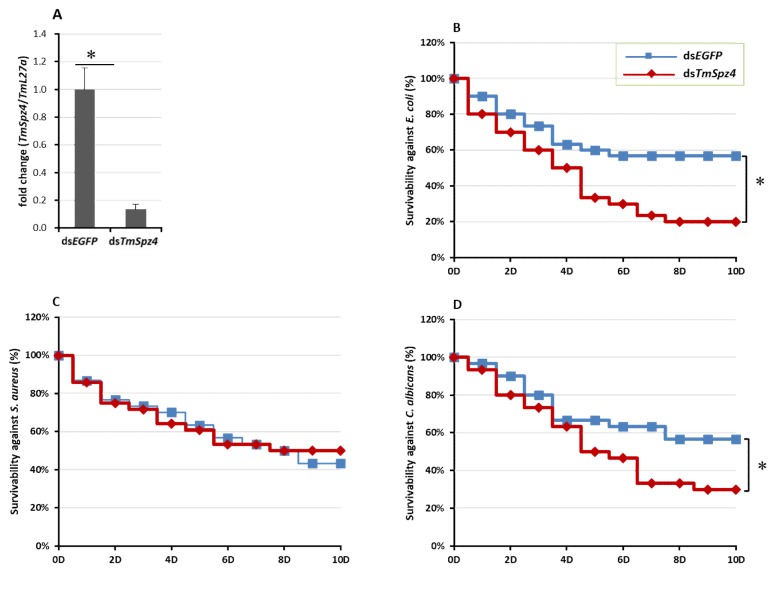
Based on the observed temporal induction of TmSpz4 following microorganism injection, we sought to determine the role of TmSpz4 in resistance to bacteria and fungi by silencing TmSpz4 transcript levels in T. molitor larvae through RNAi. TmSpz4 mRNA levels were decreased by 90% 5 days after dsTmSpz4 injection (Figure 4A).
Figure 4.
Effect of dsTmSpz4 on the survival of T. molitor larvae. The silencing efficiency of dmTmSpz4 was measured by qRT-PCR at 5 days post-injection (A). Then, the TmSpz4-silenced larvae were injected with E. coli (B), S. aureus (C), or C. albicans (D) and survival was monitored. dsEGFP-injected larvae were included as a negative control. The data are an average of three biologically independent replicate experiments. Asterisks indicate significant differences between dsTmSpz4- and dsEGFP-injected groups (p < 0.05). Statistical analysis of survival analysis was carried out based on Kaplan-Meier plots (log-rank chi-square test; * p < 0.05).
After confirming the efficient knock-down of TmSpz4 in larvae, they were challenged with bacteria or fungi. The survival of TmSpz4-silenced T. molitor larvae following microbial injection was monitored for 10 days. Injection of dsTmSpz4 and/or dsEGFP did not affect the survival of PBS-injected T. molitor larvae. However, dsTmSpz4-injected larvae were significantly more susceptible to E. coli (64.7%) (Figure 4B) and C. albicans (47%; Figure 4D). In contrast, the survival rates of dsTmSpz4-injected larvae did not differ significantly from that of the control after infection with S. aureus (Figure 4C).
2.5. Effects of TmSpz4 Gene Silencing on the Expression of AMPs
The survival study showed that TmSpz4 knock-down reduced the survival of T. molitor larvae following challenge with E. coli and C. albicans, suggesting the importance of TmSpz4 in the immune defense against Gram-negative bacteria and fungi. Thus, to characterize the function of TmSpz4 in the production of AMPs in response to microbial infection, TmSpz4 expression was silenced in T. molitor larvae, and the larvae were challenged with E. coli, S. aureus, or C. albicans. Then, the expression levels of 14 different AMP genes were assessed at 24 h post-infection.
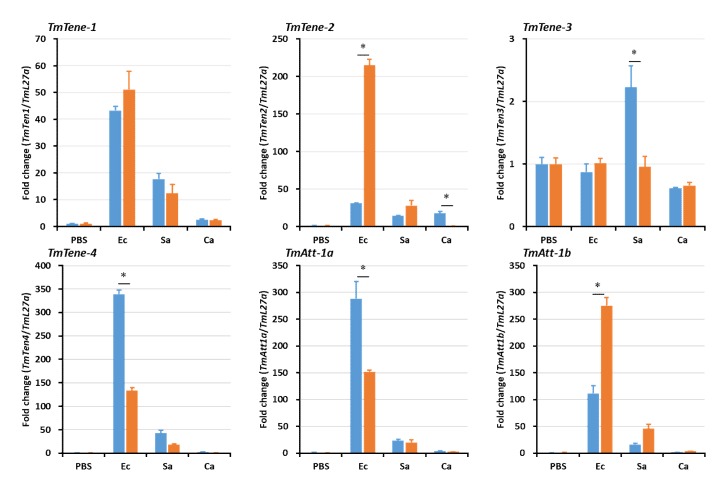
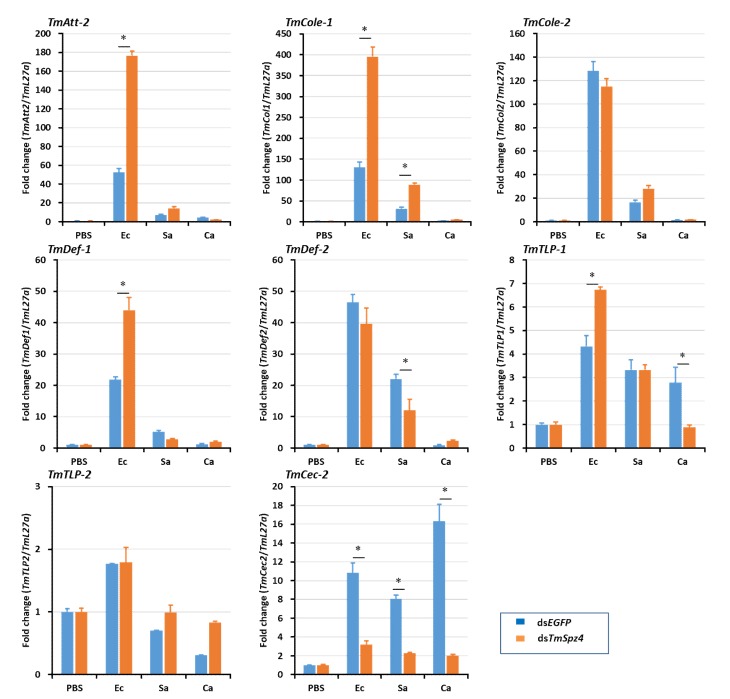
In hemocytes (Figure 5), the expression levels of the AMP genes were significantly reduced in TmSpz4-silenced larvae following microbial challenge, including that of TmTen-2 by E. coli; those of TmTen-2, -3, -4, and TmCec-2 by S. aureus; and those of TmTen-1, -2, -3, TmAtt-2, TmCol-2, TmTLP-1, and TmTLP-2 by C. albicans. In the fat body, the expression levels of TmTen-2, -3, -4, TmAtt-1a, TmDef-2, TmTLP-2, and TmCec-2 were reduced by E. coli; those of TmTLP-1 and TmCec-2 were reduced by S. aureus; and those of TmTen-1, -2, -4, TmAtt-1a, TmAtt-1b, TmAtt-2, TmDef-1, TmDef-2, TmTLP-1, and TmTLP-2 were reduced by C. albicans in TmSpz4-silenced larvae (Figure 6). In the gut of TmSpz4-silenced larvae, the expression levels of TmTen-4, TmAtt-1a, and TmCec-2 were reduced by E. coli; those of TmTen-3, -4, TmDef-2, and TmCec-2 were reduced by S. aureus; and those of TmTen-2, -4, TmAtt-2, TmDef-1, TmTLP-1, and TmCol-2 were reduced by C. albicans when compared with the levels in dsEGFP-injected larvae (Figure 7).
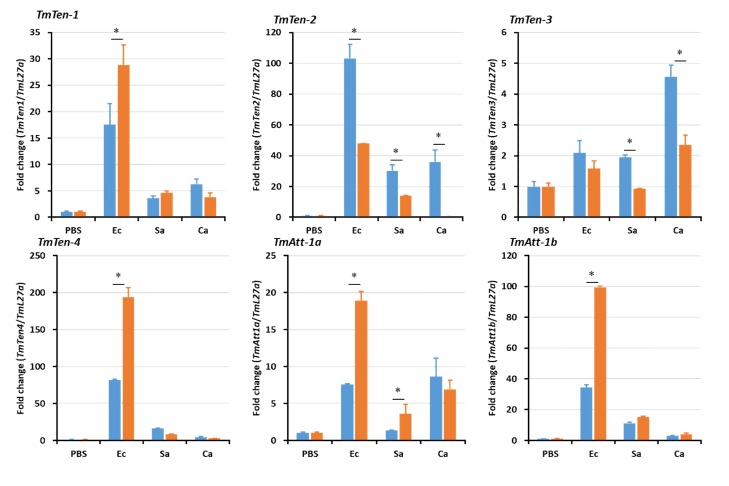
Figure 5.
Antimicrobial peptide (AMP) induction patterns in hemocytes of TmSpz4-silenced larvae. AMP gene expression levels in the hemocytes of TmSpz4-knock-down T. molitor larvae were assessed after injection with E. coli (Ec), S. aureus (Sa), or C. albicans (Ca). PBS was injected as a control 5 days post-TmSpz4 silencing. At 24 h post-microbial challenge, the expression levels of several AMP genes, including those of TmTen-1, TmTen-2, TmTen-3, TmTen-4, TmAtt-1a, TmAtt-1b, TmAtt-2, TmDef-1, TmDef-2, TmCol-1, TmCol-2, TmCec-2, TmTLP-1, and TmTLP-2, were measured by qRT-PCR. dsEGFP was injected as a negative control, and TmL27a expression was measured as an internal control. All experiments were performed in triplicate. Asterisks indicate significant differences between dsTmSpz4- and dsEGFP-treated groups when compared by Student’s t-test (p < 0.05).
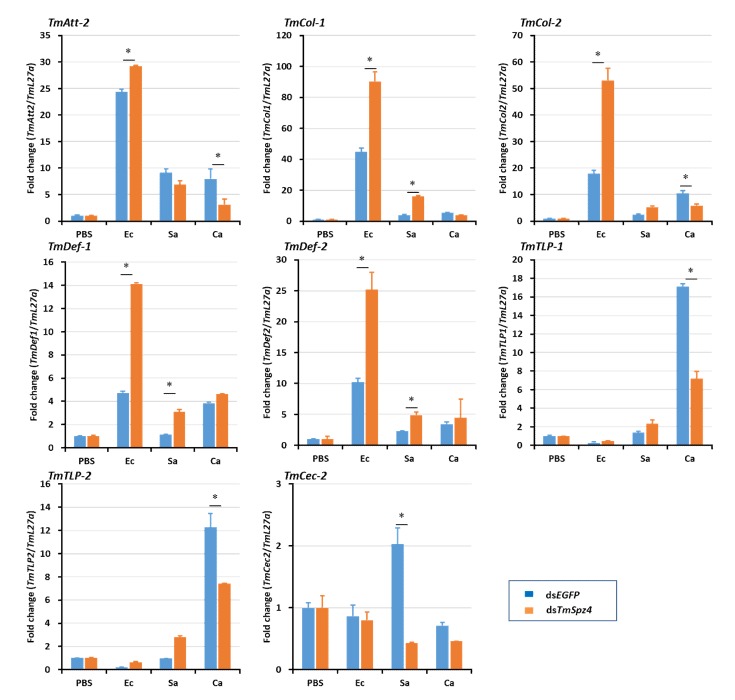
Figure 6.
Antimicrobial peptide (AMP) induction patterns in fat body of TmSpz4-silenced larvae. AMP gene expression levels in the fat body of TmSpz4-knock-down T. molitor larvae were assessed after injection with E. coli (Ec), S. aureus (Sa), or C. albicans (Ca). PBS was injected as a control 5 days post-TmSpz4 silencing. At 24 h post-microbial challenge, the expression levels of several AMP genes, including those of TmTen-1, TmTen-2, TmTen-3, TmTen-4, TmAtt-1a, TmAtt-1b, TmAtt-2, TmDef-1, TmDef-2, TmCol-1, TmCol-2, TmCec-2, TmTLP-1, and TmTLP-2, were measured by qRT-PCR. dsEGFP was injected as a negative control, and TmL27a expression was measured as an internal control. All experiments were performed in triplicate. Asterisks indicate significant differences between dsTmSpz4- and dsEGFP-treated groups when compared by Student’s t-test (p < 0.05).
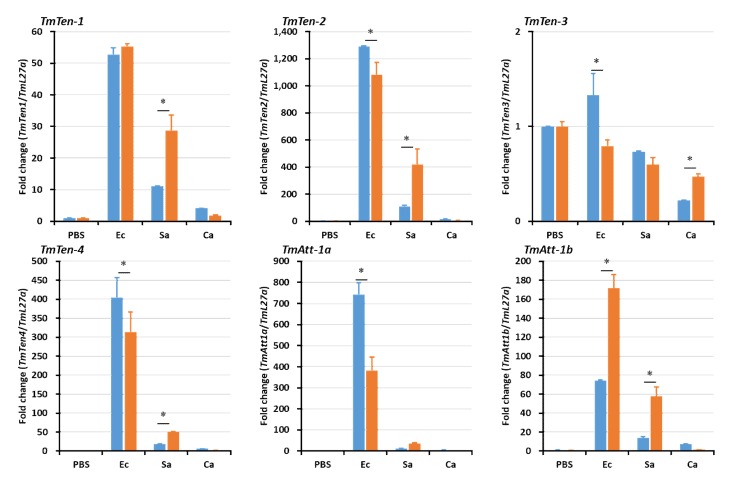
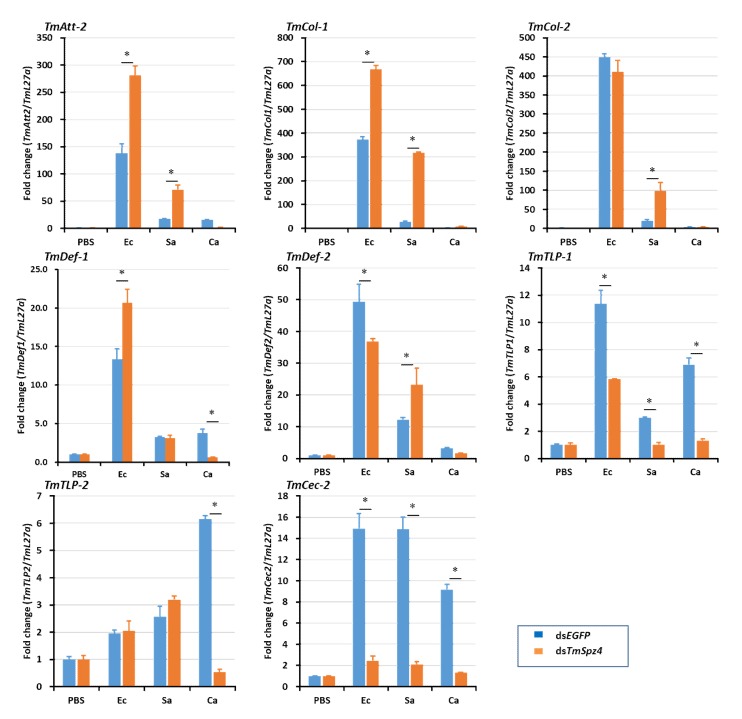
Figure 7.
Antimicrobial peptide (AMP) induction patterns in gut of TmSpz4-silenced larvae. The antimicrobial peptide expression levels in TmSpz4-knock-down of T. molitor larval gut were performed by injecting either E. coli (Ec), S. aureus (Sa), or C. albicans (Ca). PBS was injected as a control 5 days post-TmSpz4 silencing. At 24 h post-microbial challenge, the expression levels of several AMP genes, including those of TmTen-1, TmTen-2, TmTen-3, TmTen-4, TmAtt-1a, TmAtt-1b, TmAtt-2, TmDef-1, TmDef-2, TmCol-1, TmCol-2, TmCec-2, TmTLP-1, and TmTLP-2, were measured by qRT-PCR. dsEGFP was injected as a negative control, and TmL27a expression was measured as an internal control. All experiments were performed in triplicate. Asterisks indicate significant differences between dsTmSpz4- and dsEGFP-treated groups when compared by Student’s t-test (p < 0.05). Effects of TmSpz4 on the expression patterns of NF-κB genes.
Interestingly, in contrast, TmSpz4 knock-down increased the mRNA levels of TmTen-4, TmAtt-1a, TmAtt-1b, TmCol-1, TmCol-2, and TmDef-2 in the hemocytes of E. coli-challenged larvae (Figure 6). Similarly, the expression levels of TmAtt-1b, TmAtt-2, and TmCol-1 in the fat body (Figure 6) and those of TmTen-2, TmAtt-1b, TmAtt-2, TmCol-1, and TmDef-2 in the gut (Figure 7) were increased in TmSpz4-silenced larvae after E. coli injection.
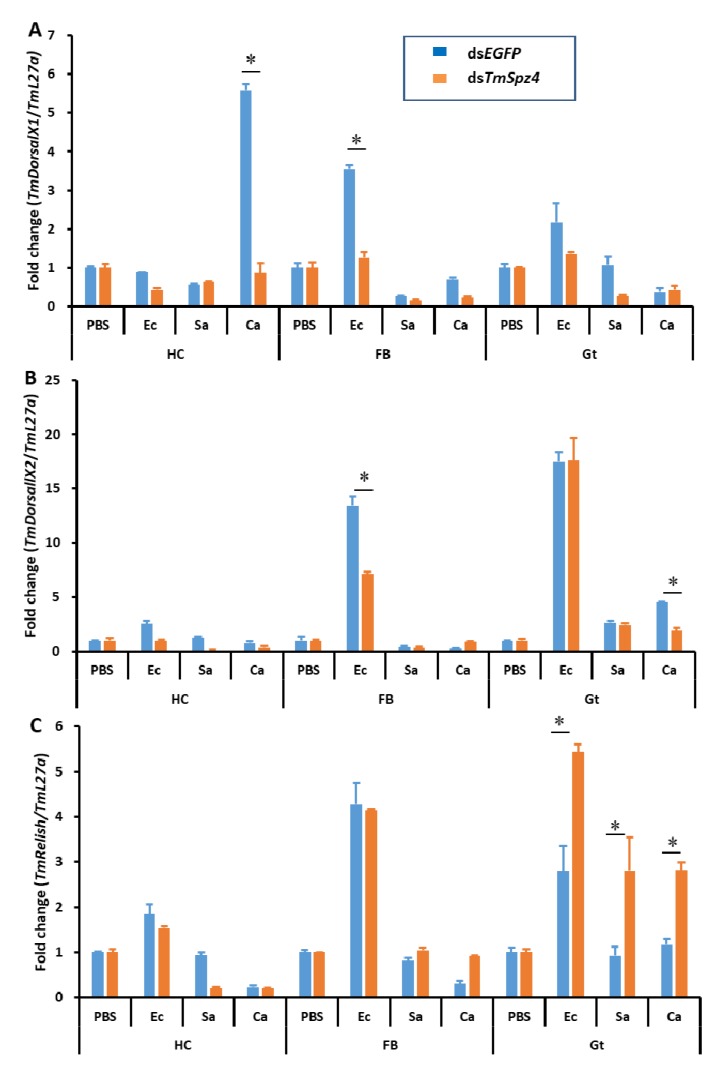
The expression of NF-κB genes, TmDorX1, TmDorX2, and TmRelish, were investigated under the same conditions as those used in the AMP expression experiment. dsTmSpz4 injection significantly decreased the expression levels of TmDorX1 and TmDorX2 in the fat body following challenge with E. coli (Figure 8A,B). Similarly, TmSpz4 knock-down significantly reduced the expression of TmDorX1 in hemocytes and TmDorX2 in the gut following C. albicans challenge (Figure 8A,B). In the gut, injection of TmSpz4 RNAi upregulated the expression of TmRelish following challenge with all test microorganisms (Figure 8C).
Figure 8.
Effect of Tmspz4 gene silencing on expression of NF-κB genes. Three different pathogens, E. coli, S. aureus, and C. albicans, were injected into TmSpz4-silenced T. molitor young larvae, and the expression of the NF-κB genes TmDorX1 (A), TmDorX2 (B), and TmRel (C) were then investigated by RT-qPCR. Larvae were injected with dsEGFP as a negative control, and TmL27a expression was assessed as an internal control. All experiments were performed in triplicate. Asterisks indicate significant differences in NF-κB gene expression between the dsTmSpz4- and dsEGFP-treated groups when compared by Student’s t-test (p < 0.05).
3. Discussion
The Toll receptor, which plays important roles in the production of AMPs in response to infection in insects, is activated by the endogenous cytokine ligand spätzle [14,27,28]. Thus, the molecular functions of spätzle proteins in Toll receptor activation and the subsequent activation of AMPs have been well studied in various insects. For example, in Drosophila, Spz-1, -2, and -5 bind to Toll-1 and Toll-7 to produce drosomycin and several other AMPs [16,29]. Additionally, in B. mori, spätzle-1 binds to the Toll receptor to activate the production of attacin-1, cecropin-6, and moricin [30].
In current study, TmSpz4 of T. molitor was identified and functionally characterized. The identified TmSpz4 protein contains one cystine-knot domain at the C-terminus, which is a Toll receptor ligand; one cleavage site, which is predicted to be processed by SPE; and one signal peptide, which enables its transport through cell membranes. Spätzle is synthesized in an inactive form (pro-spätzle), the N-terminus signal peptide of which is removed to allow the secretion of mature spätzle [27].
A previous study on Drosophila demonstrated a cross talk between a steroid hormone (ecdysone) or juvenile hormone and immune-related genes. Briefly, the ecdysone hormone activated a nuclear receptor to generate a heterodimer with ultraspiracle, promoting the transcription of immune-related genes [31]. It is well known that ecdysone is critical for the activation of AMP gene expression and phagocytosis [32]. Investigation of the expression of ecdysone during the development of Drosophila by radioimmune assay showed that ecdysone activity was highest during the pupal, prepupal, and late larval stages (in descending order) [33]. Accordingly, in our study as well, developmental stage and tissue-specific expression analysis of TmSpz4 revealed the highest expression during the prepupal and pupal stages, in the hemocytes of larvae, and in the integument, hemocytes, and fat body of adults. Given that hemocytes play important roles in immunity, nutrient transportation, and growth hormone synthesis [34,35]. Taken together, these data shows that the highest TmSpz4 expression is during transitional stages and in growth hormone-synthesizing tissues (hemocytes) under normal conditions. The molecular relationship between developmental hormone and TmSpz4 during normal developmental condition needs to be investigated to further understand the role of developmental hormones in the expression of TmSpz4. The Toll receptor ligand spätzle was activated when PGRPs or GNBP recognized peptidoglycan (PGN) or β-1,3 glucan from Gram-positive bacteria or fungi [10,14]. Pattern recognition proteins (e.g., PGRPs and GNBP) are found on the plasma membrane of fat body and hemocytes cells [36]. Consequently, in the current study, the highest and earliest induction of TmSpz4 in T. molitor larvae following challenge with S. aureus, C. albicans, and E. coli was observed in hemocytes. During infection in Drosophila, hemocytes synthesized and secreted signals that could be detected by the fat body [37], suggesting early recognition of infection by hemocytes. The induction of TmSpz4 expression by E. coli suggests the presence of a signaling cross talk between the Toll and IMD pathways in T. molitor. Similarly, the previous in vitro experiments showed that E. coli induced the activation of spätzle in T. molitor larvae, suggesting that the polymeric DAP-type PGN forms a complex with Tenebrio PGRP-SA to activate the Toll receptor ligand, spätzle [30]. Similarly, in our recent publication we have reported TmSpz6 is important in regulating the AMPs production in response to E. coli [38] The survival results in our study support the induction analysis results, implying the importance of TmSpz4 in the defense response of T. molitor larvae against C. albicans and E. coli by regulating different AMPs production. Specifically, TmSpz4 silencing resulted in increased susceptibility of the larvae to E. coli and C. albicans infections. Similarly, TmSpz4 silencing suppressed the induction of several AMP genes following challenge with E. coli (TmTen-2, TmTen-4, TmAtt-1a, TmDef-2, and TmCec-2) and C. albicans (TmTen-1, TmTen-3, TmTLP-1, TmTLP-2, TmDef-1, TmDef-2, and TmCec-2). These suppressed AMPs have been shown to have antibacterial activity against both gram-negative bacteria and fungi [39,40,41,42]. In particular, glycine-rich AMPs, such as attacins, in Hyalophora cecropia [43] and T. molitor [44], as well as tenecin-2 and -4 in T. molitor [30,45] have been shown to be particularly effective against Gram-negative bacteria. The effectiveness of tenecin-3 [46], tenecin-1, tenecin-2 [47], and defensins [48] against fungal infections has also been reported.
Activation of either the Toll pathway by Lys-type PGN or β-1,3 glucan or the IMD pathway by DAP-type PGN leads to translocation of the NF-κB family transcription factors Dorsal and Relish [49]. The extracellular protein spätzle, which is generated during development and/or in response to microbial infection during the insect immune response, activates the Toll pathway [11]. Therefore, we wanted to determine if TmSpz4 affects the expression of Dorsal and subsequent AMP production in T. molitor in response to microbial challenge. Thus, the transcription levels of Dorsal and Relish were quantified in TmSpz4-silenced T. molitor larvae challenged with different microbes. In agreement with the AMP expression results in this study, TmDorX1 and TmDorX2 were significantly suppressed in the hemocytes and fat body of TmSpz4-silenced larvae following challenge with E. coli or C. albicans, indicating that TmSpz4 is important in the expression of TmDorX2. Active spätzle binds to Toll receptor to activate the Toll pathway; then, the MyD88-Tube-Pelle complex leads to the phosphorylation and degradation of Cactus, an inhibitor of NF-κB. Thus, after cactus is degraded, both Dorsal and Dif are translocated to the nucleus and bind to the κB-related sequences in AMP genes [50]. Taken together, the results of this study suggest the importance of TmSp4 in the humoral immunity of T. molitor through the activation of the Toll pathway, subsequent activation of the NF-κB transcription factor (TmDorX1 and TmDorX2), and ultimate production of AMPs against E. coli and C. albicans infection.
4. Materials and Methods
4.1. Insect Culture
The coleopteran insect T. molitor (commonly known as mealworm) was maintained at 27 ± 1 °C and 60 ± 5% relative humidity in the dark on an artificial diet containing 170 g whole-wheat flour, 20 g fried bean powder, 10 g soy protein, 100 g of wheat bran, 200 mL sterile water, 0.5 g chloramphenicol, 0.5 g sorbic acid, and 0.5 mL propionic acid. For the experiments, 10th–12th instar larvae were used. To ensure uniformity in size, the larvae were separated according to physical size using a set of laboratory test sieves (Pascall Eng. Co., Ltd., Crawley, UK).
4.2. Preparation of Microorganisms
A Gram-negative bacterial strain (Escherichia coli K12), a Gram-positive bacterial strain (Staphylococcus aureus RN4220), and a fungus (Candida albicans AUMC 13529) were used to study the function of TmSpz4 in the innate immune response of mealworms against microbial infections. These microorganisms were cultured in Luria-Bertani (LB; E. coli and S. aureus) and Sabouraud dextrose (C. albicans) broths at 37 °C overnight and then subcultured at 37 °C for 3 h. Then, the microorganisms were harvested and washed twice with phosphate-buffered saline (PBS; pH 7.0) by centrifugation at 3500 rpm for 10 min. The microbes were suspended in PBS, and the cell density was determined by measuring the OD600. Finally, 106 cells/μL of E. coli and S. aureus and 5 × 104 cells/μL of C. albicans were separately prepared for use in the subsequent challenge experiments.
4.3. Identification and Cloning of Full-Length cDNA Sequence of TmSpz4
The T. molitor TmSpz4 gene was identified by a local BLAST analysis using the amino acid sequence of the T. castaneum spz4 gene (EFA09263.2) as the query. The partial cDNA sequence of Tmspz4 was obtained from the T. molitor RNAseq database, and the full-length cDNA sequence of TmSpz4 (MT075617) was identified by 5′- and 3′-rapid amplification of cDNA end (RACE) PCR using the SMARTer RACE cDNA amplification kit (Clontech, Mountain View, CA, USA), according to the manufacturer’s instructions. PCR was performed using the AccuPower® PyroHotStart Taq PCR PreMix (Bioneer, Daejeon, Korea) and TmSpz4-specific primers (RACE primers: TmAtg4-cloning_Fw and TmSpz4-cloning_Rv; Table 1) under the following cycling conditions: pre-denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 2 min, with a final extension step at 72 °C for 5 min on a MyGenie96 Thermal Block (Bioneer). The PCR products were purified using the AccuPrep® PCR Purification Kit (Bioneer), immediately ligated into T-Blunt vectors (Solgent, Daejeon, Korea), and transformed into E. coli DH5α competent cells, according to the manufacturer’s instructions. Plasmid DNA was extracted from the transformed cells using the AccuPrep® Nano-Plus Plasmid Extraction Kit (Bioneer) and then sequenced and analyzed. Finally, the full-length cDNA sequence of TmSpz4 was obtained (Figure S1).
Table 1.
Sequences of the primers used in this study.
| Primer Name | Sequence (5′-3′) |
|---|---|
| TmSpz4-qPCR-Fw | GGCGATGCTCTTCCAGGAC |
| TmSpz4-qPCR-Rv | CGCGTTCACTCCTTTCATTTGG |
| TmSpz4-T7-Fw | TAATACGACTCACTATAGGGTCCAGATGTACTGTCGCGATG |
| TmSpz4-T7-Rv | TAATACGACTCACTATAGGGTTTCCTTCTGTACCAGTCGGG |
| TmSpz4-cloning-Fw | ACCGACACAACCACCAAAAG |
| TmSpz4-cloning-Rv | ATCCGTGATCTCCGGAAAAA |
| TmSpz4-cloning-FullORF-Fw | GACGGTGCCCACGAACTAT |
| TmSpz4-cloning-FullORF-Rv | AAGAGCACGAGCCTTGACAT |
| TmL27a_qPCR_Fw | TCATCCTGAAGGCAAAGCTCCAGT |
| TmL27a_qPCR_Rv | AGGTTGGTTAGGCAGGCACCTTTA |
| dsEGFP_Fw | TAATACGACTCACTATAGGGTCGTAAACGGCCACAAGTTC |
| dsEGFP_Rv | TAATACGACTCACTATAGGGT TGCTCAGGTAGTGTTGTCG |
| TmTenecin-1_Fw | CAGCTGAAGAAATCGAACAAGG |
| TmTenecin-1_Rv | CAGACCCTCTTTCCGTTACAGT |
| TmTenecin-2_Fw | CAGCAAAACGGAGGATGGTC |
| TmTenecin-2_Rv | CGTTGAAATCGTGATCTTGTCC |
| TmTenecin-3_Fw | GATTTGCTTGATTCTGGTGGTC |
| TmTenecin-3_Rv | CTGATGGCCTCCTAAATGTCC |
| TmTenecin-4_Fw | GGACATTGAAGATCCAGGAAAG |
| TmTenecin-4_Rv | CGGTGTTCCTTATGTAGAGCTG |
| TmDefensin-1_Fw | AAATCGAACAAGGCCAACAC |
| TmDefencin-1_Rv | GCAAATGCAGACCCTCTTTC |
| TmDefencin-2_Fw | GGGATGCCTCATGAAGATGTAG |
| TmDefencin-2_Rv | CCAATGCAAACACATTCGTC |
| TmColoptericin-1_Fw | GGACAGAATGGTGGATGGTC |
| TmColoptericin-1_Rv | CTCCAACATTCCAGGTAGGC |
| TmColoptericin-2_Fw | GGACGGTTCTGATCTTCTTGAT |
| TmColoptericin-2_Rv | CAGCTGTTTGTTTGTTCTCGTC |
| TmAttacin-1a_Fw | GAAACGAAATGGAAGGTGGA |
| TmAttacin-1a_Rv | TGCTTCGGCAGACAATACAG |
| TmAttacin-1b_Fw | GAGCTGTGAATGCAGGACAA |
| TmAttacin-1b_Rv | CCCTCTGATGAAACCTCCAA |
| TmAttacin-2_Fw | AACTGGGATATTCGCACGTC |
| TmAttacin-2_Rv | CCCTCCGAAATGTCTGTTGT |
| TmCecropin-2_Fw | TACTAGCAGCGCCAAAACCT |
| TmCecropin-2_Rv | CTGGAACATTAGGCGGAGAA |
| TmThaumatin-likeprotein-1_Fw | CTCAAAGGACACGCAGGACT |
| TmThaumatin-like protein-1_Rv | ACTTTGAGCTTCTCGGGACA |
| TmThaumatin-like protein-2_Fw | CCGTCTGGCTAGGAGTTCTG |
| TmThaumatin-like protein-2_Rv | ACTCCTCCAGCTCCGTTACA |
| TmDorsal-X1_qPCR_Fw | AGCGTTGAGGTTTCGGTATG |
| TmDorsal-X1_qPCR_Rv | TCTTTGGTGACGCAAGACAC |
| TmDorsal-X2_qPCR_Fw | ACACCCCCGAAATCACAAAC |
| TmDorsal-X2_qPCR_Rv | TTTCAGAGCGCCAGGTTTTG |
| TmRelish_qPCR_Fw | AGCGTCAAGTTGGAGCAGAT |
| TmRelish_qPCR_Rv | GTCCGGACCTCAAGTGT |
Underline indicates T7 promoter sequences.
4.4. Domain and Phylogenetic Analysis
The domains of TmSpz4 were analyzed using the InterProScan 5 and BLAST programs. A multiple sequence alignment was performed with representative Spz4 protein sequences from other insects obtained from GenBank using ClustalX2. Phylogenetic analyses of TmSpz4 homologues protein were performed using the Clustal X2 and the phylogenic tree was constructed by MEGA7 programs using the maximum likelihood and bootstrapped of 1000 replications. The following Protein sequences were used to construct the phylogenetic tree. DmSpz4; Drosophila melanogaster spatzle 4 (AAF53100.2), DmSpz6; Drosophila melanogaster spatzle 6 (AAF47261.1), DmSpz5; Drosophila melanogaster spatzle 5 (AAF47694.1), DmSpz; Drosophila melanogaster spatzle (AAF82745.1), DmSpz3; Drosophila melanogaster spatzle 3 (AAF52574.2), DmNTP1-H; Drosophila melanogaster neurotrophin 1, isoform H (AGB94113.1), DmNTP1-E; Drosophila melanogaster neurotrophin 1, isoform E (ACZ94621.1), DmNTP1-D; Drosophila melanogaster neurotrophin 1, isoform D (NP_001163348.1), TcSpz7; Tribolium castaneum spatzle 7 (EEZ99267.2), TcSpz4; Tribolium castaneum spatzle 4 (EFA09263.2), TcSpz5; Tribolium castaneum spatzle 5 (EEZ97725.1), TcSpz3; Tribolium castaneum spaetzle 3 (NP_001153625.1), TcSpz6; Tribolium castaneum spatzle 6 precursor (NP_001164082.1), TcSpz; Tribolium castaneum spaetzle (EEZ99207.1), TcP-Spz; Tribolium castaneum PREDICTED: protein spaetzle (XP_008201191.1), TcSpz-like; Tribolium castaneum spaetzle-like protein (EEZ99268.2).
4.5. TmSpz4 Expression and Temporal Induction Pattern Analysis
Total RNA was extracted from whole T. molitor (n = 20) at various developmental stages, including egg (EG), young instar larval (YL; 10th–12th instar larvae), late instar larval (LL; 19th–20th instar larvae), prepupal (PP), 1- to 7-day-old pupal (P1–7), and 1- to 5-day-old adult (A1–5) stages. To investigate tissue-specific TmSpz4 expression, RNA was extracted from various tissues (n = 20), including the gut, hemocytes, integument, Malpighian tubules, and fat body of late instar larvae and the ovaries and testes of adults. To study the induction patterns of TmSpz4 in different T. molitor larval tissues in response to microbial challenge, E. coli (Gram-negative bacteria), S. aureus (Gram-positive bacteria), or C. albicans (fungi) were injected into young instar larvae. Three immune-response related tissues, hemocytes, fat body, and gut, were collected at 3, 6, 9, 12, and 24 h post-injection into 500 μL of guanidine thiocyanate RNA lysis buffer (2 mL 0.5 M EDTA, 1 mL 1 M MES Buffer, 17.72 g guanidine thiocyanate, 0.58 g sodium chloride, 0.7 mg phenol red, 25 μL Tween-80, 250 μL acetic acid glacial, and 500 μL isoamyl alcohol) and homogenized using a homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) at 7500 rpm for 20 s.
Total RNA was extracted from the collected samples using the modified LogSpin RNA isolation method [51]. Briefly, homogenized samples were centrifuged for 5 min at 13,000 rpm and 4 °C. The diluted supernatant (300 μL) was transferred into a new 1.5 mL epitube, mixed with one volume of pure ethanol, transferred into a silica spin column (KA-0133-1; Bioneer, Daejeon, Korea), and centrifuged for 30 s at 13,000 rpm and 4 °C. The silica spin column was treated with DNase (M6101; Promega, WI, USA) at 25 °C for 15 min and washed with 3 M sodium acetate buffer and 80% ethanol. After drying by centrifugation at 13,000 rpm and 4 °C for 2 min, total RNA was eluted with 30 μL of distilled water (W4502-1L; Sigma-aldrich, MO, USA). The eluted RNA (2 μg) was used to generate cDNA using the AccuPower® RT PreMix (Bioneer) and Oligo (dT) 12–18 primers on a MyGenie96 Thermal Block (Bioneer), according to the manufacturer’s instructions.
Quantitative PCR (qPCR) was performed using gene-specific primers, under the following cycling conditions: an initial denaturation step at 94 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 30 s. The 2−ΔΔCt method [52] was used to analyze TmSpz4 expression levels. The T. molitor gene encoding ribosomal protein L27a (TmL27a) was used as an internal control for the normalization of differences in template concentration between samples.
4.6. Effect of TmSpz4 Gene Silencing in Response to Microorganisms
To synthesize the double-stranded RNA of the TmSpz4 gene, forward and reverse primers containing the T7 promoter sequence at their 5′ ends were designed using the SnapDragon-Long dsRNA design software (https://www.flyrnai.org/cgibin/RNAi_find_primers.pl) (Table 1 and Figure S1). The PCR product was amplified using AccuPower® Pfu PCR PreMix with TmSpz4_Fw and TmSpz4_Rv (Table 1) under the following cycling conditions: an initial denaturation step at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 30 s, with a final extension step at 72 °C for 5 min. The PCR products were purified using the AccuPrep PCR Purification Kit (Bioneer), and dsRNA was synthesized using the AmpliscribeTM T7-FlashTM Transcription Kit (Epicentre Biotechnologies, Madison, WI, USA), according to the manufacturer’s instructions. After synthesis, the dsRNA was purified by precipitation with 5 M ammonium acetate and 80% ethanol. Subsequently, it was quantified using an Epoch spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). As a control, the dsRNA of enhanced green fluorescent protein (dsEGFP) was synthesized, and all samples were stored at −20 °C until use.
Synthesized dsTmSpz4 was diluted to a final concentration of 1 µg/µL and then injected into young-instar larvae (10th–12th instars; n = 30) using disposable needles mounted on a micro-applicator (Picospiritzer III Micro Dispense System; Parker Hannifin, Hollis, NH, USA). Another set of young-instar larvae (n = 30) were injected with equal amounts of dsEGFP as a negative control. Injected larvae were maintained on an artificial diet under standard rearing conditions. Evaluation of TmSpz4 knock-down showed a greater than 90% reduction at 5 days post-injection.
To study the importance of TmSpz4 in T. molitor immune responses, TmSpz4-silenced and dsEGFP-injected larvae were challenged with E. coli (106 cells/μL), S. aureus (106 cells/μL), or C. albicans (5 × 104 cells/μL) in triplicate experiments. The challenged larvae were maintained for 10 days, and the number of living larvae were recorded during this time period. The survival rates of the TmSpz4-silenced group were compared to those of the control groups. Statistical analysis was conducted using the SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA), and cumulative survival was analyzed by Tukey’s multiple test, at a significance level of p < 0.05.
4.7. Effect of TmSpz4 RNAi on AMP Expression against Microbial Challenge
To characterize the function of TmSpz4 in the regulation of AMP gene expression in response to microbial infection, TmSpz4 expression in larvae was first silenced using RNAi; then, these larvae were injected with E. coli, S. aureus, and C. albicans. Larvae injected with dsEGFP and PBS were used as the negative and injection controls, respectively. The hemocytes, fat body, and guts were dissected at 24 h post-microorganism injection. Total RNA was extracted and cDNA was synthesized as described above. Then, qRT-PCR was conducted using specific primers (Table 1) to analyze the temporal expression patterns of the fourteen AMP genes TmTenecin-1 (TmTen-1), TmTenecin-2 (TmTen-2), TmTenecin-3 (TmTen-3), TmTenecin-4 (TmTen-4), TmAttacin-1a (TmAtt-1a), TmAttacin-1b (TmAtt-1b), TmAttacin-2 (TmAtt-2), TmDefensin-1 (TmDef-1), TmDefensin-2 (TmDef-2), TmColptericin-1 (TmCol-1), TmColptericin-2 (TmCol-2), TmCecropin-2 (TmCec-2), TmThaumatin-like protein-1 (TmTLP-1), and TmThaumatin-like protein-2 (TmTLP-2).
4.8. Effects of dsTmSpz4 on the Expression Patterns of NF-κB Genes
To understand the effect of TmSpz4-RNAi on the expression of the NF-κB genes such as TmDorsal-X1 (TmDor-X1), TmDorsal-X2 (TmDor-X2), and TmRelish (TmRel), TmSpz4 gene was silenced in the young instars larvae of T. molitor and other larval group were injected with dsEGFP as a negative control. Subsequently, E. coli, S. aureus, and C. albicans, were injected into TmSpz4-silenced and control larval group. After 24 h post microbial challenge, hemocytes, fat body and gut were dissected. Total RNA was extracted and cDNA was synthesized as described above. Then, qRT-PCR was conducted using TmDorsal and TmRelish specific primers (Table 1). All experiments were performed in triplicate.
4.9. Data Analysis
All experiments were triplicated. The mean expression of TmSpz4 in the developmental stage were subjected to analysis of variance (ANOVA) using SAS 9.4 and means were compared by Tukey’s multiple range test (p < 0.05). Statistical analysis of survival analysis was carried out based on Kaplan-Meier plots (log-rank chi-square test; * p < 0.05). Comparative AMP gene expression was calculated using the delta delta Ct method (ΔΔCt). The fold change compared to the internal (TmL27a) and external (PBS) controls was calculated by the 2–(ΔΔCt) method.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/5/1878/s1.
Author Contributions
Y.S.H. and Y.H.J. conceived and designed the experiments; T.T.E., M.K., Y.M.B. and D.H.K. performed the experiments; T.T.E. analyzed the data; Y.S.H., and Y.H.J. contributed reagents/materials/analysis tools; T.T.E., wrote the manuscript; Y.S.H., Y.H.J. and Y.S.L. and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and future Planning (Grant No. 2018R1A2A2A05023367).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Rosales C., Vonnie S. Insect Physiology and Ecology. InTeach Publication CCBY; London, UK: 2017. Cellular and molecular mechanisms of insect immunity; pp. 179–212. [Google Scholar]
- 2.Jiang Y.-Y. A review of researches on agglutinins of Lepidoptera insect. Entomol. J. E. China. 2006;15:25–29. [Google Scholar]
- 3.Janeway C.A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. doi: 10.1101/SQB.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Imler J.-L., Bulet P. Mechanisms of Epithelial Defense. Volume 86. Karger Publishers; Basel, Switzerland: 2005. Antimicrobial peptides in Drosophila: Structures, activities and gene regulation; pp. 1–21. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Zhang Y., Zhang R., Zhang J. The diversity of pattern recognition receptors (PRRs) involved with insect defence against pathogens. Curr. Opin. Insect Sci. 2019;33:105–110. doi: 10.1016/j.cois.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Anderson K.V., Bokla L., Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 7.Levitin A., Whiteway M. Drosophila innate immunity and response to fungal infections. Cell. Microbiol. 2008;10:1021–1026. doi: 10.1111/j.1462-5822.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrandon D., Imler J.-L., Hetru C., Hoffmann J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007;7:862. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 9.De Gregorio E., Spellman P.T., Tzou P., Rubin G.M., Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Chamy L., Leclerc V., Caldelari I., Reichhart J.-M. Sensing of’danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways’ upstream’of Toll. Nat. Immunol. 2008;9:1165. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh K.-B., Kim C.-H., Lee H., Kwon H.-M., Park J.-W., Ryu J.-H., Kurokawa K., Ha N.-C., Lee W.-J., Lemaitre B. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J. Biol. Chem. 2009;284:19474–19481. doi: 10.1074/jbc.M109.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobert V., Gottar M., Matskevich A.A., Rutschmann S., Royet J., Belvin M., Hoffmann J.A., Ferrandon D. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- 13.DeLotto Y., Smith C., DeLotto R. Multiple isoforms of the Drosophila Spätzle protein are encoded by alternatively spliced maternal mRNAs in the precellular blastoderm embryo. Mol. Gen. Genet. MGG. 2001;264:643–652. doi: 10.1007/s004380000350. [DOI] [PubMed] [Google Scholar]
- 14.Arnot C.J., Gay N.J., Gangloff M. Molecular mechanism that induces activation of Spätzle, the ligand for the Drosophila Toll receptor. J. Biol. Chem. 2010;285:19502–19509. doi: 10.1074/jbc.M109.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker J.S., Mizuguchi K., Gay N.J. A family of proteins related to Spätzle, the toll receptor ligand, are encoded in the Drosophila genome. Proteins Struct. Funct. Bioinform. 2001;45:71–80. doi: 10.1002/prot.1125. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury M., Li C.-F., He Z., Lu Y., Liu X.-S., Wang Y.-F., Ip Y.T., Strand M.R., Yu X.-Q. Toll family members bind multiple Spätzle proteins and activate antimicrobial peptide gene expression in Drosophila. J. Biol. Chem. 2019;294:10172–10181. doi: 10.1074/jbc.RA118.006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury M., He Z., Lu Y., Liu X., Wang Y., Yu X.-Q. Multiple Toll-Spätzle Pathways in Drosophila melanogaster Immunity. bioRxiv. 2018:420679. [Google Scholar]
- 18.Nonaka S., Kawamura K., Hori A., Salim E., Fukushima K., Nakanishi Y., Kuraishi T. Characterization of Spz5 as a novel ligand for Drosophila Toll-1 receptor. Biochem. Biophys. Res. Commun. 2018;506:510–515. doi: 10.1016/j.bbrc.2018.10.096. [DOI] [PubMed] [Google Scholar]
- 19.Christophides G.K., Zdobnov E., Barillas-Mury C., Birney E., Blandin S., Blass C., Brey P.T., Collins F.H., Danielli A., Dimopoulos G. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 20.Shin S.W., Bian G., Raikhel A.S. A toll receptor and a cytokine, Toll5A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J. Biol. Chem. 2006;281:39388–39395. doi: 10.1074/jbc.M608912200. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Cheng T., Rayaprolu S., Zou Z., Xia Q., Xiang Z., Jiang H. Proteolytic activation of pro-spätzle is required for the induced transcription of antimicrobial peptide genes in lepidopteran insects. Dev. Comp. Immunol. 2007;31:1002–1012. doi: 10.1016/j.dci.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An C., Jiang H., Kanost M.R. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y., Liu J., Li F., Wang X., Li X., Shen Z., Wu J. Immune-Related Gene Spatzle4 and Its Differential Immune Responses against Microbes in the Silkworm, Bombyx Mori. Am. J. Clin. Exp. Med. 2015;4:344–349. doi: 10.11648/j.ajcem.20150306.14. [DOI] [Google Scholar]
- 24.Yuan K., Yuan F.-H., Weng S.-P., He J.-G., Chen Y.-H. Identification and functional characterization of a novel Spätzle gene in Litopenaeus vannamei. Dev. Comp. Immunol. 2017;68:46–57. doi: 10.1016/j.dci.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Shi X.-Z., Zhang R.-R., Jia Y.-P., Zhao X.-F., Yu X.-Q., Wang J.-X. Identification and molecular characterization of a Spätzle-like protein from Chinese shrimp (Fenneropenaeus chinensis) Fish Shellfish. Immunol. 2009;27:610–617. doi: 10.1016/j.fsi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim C.-H., Kim S.-J., Kan H., Kwon H.-M., Roh K.-B., Jiang R., Yang Y., Park J.-W., Lee H.-H., Ha N.-C. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J. Biol. Chem. 2008;283:7599–7607. doi: 10.1074/jbc.M710216200. [DOI] [PubMed] [Google Scholar]
- 27.Weber A.N., Gangloff M., Moncrieffe M.C., Hyvert Y., Imler J.-L., Gay N.J. Role of the Spätzle pro-domain in the generation of an active Toll receptor ligand. J. Biol. Chem. 2007;282:13522–13531. doi: 10.1074/jbc.M700068200. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Zhu S. Evolutionary and functional epitopes of the Spatzle protein: New insights into activation of the Toll receptor. Cell. Mol. Life Sci. 2009;66:1595–1602. doi: 10.1007/s00018-009-9028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imler J.-L., Hoffmann J.A. Signaling mechanisms in the antimicrobial host defense of Drosophila. Curr. Opin. Microbiol. 2000;3:16–22. doi: 10.1016/S1369-5274(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y., Park J.-W., Kwon H.-M., Hwang H.-O., Jang I.-H., Masuda A., Kurokawa K., Nakayama H., Lee W.-J., Dohmae N. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J. Biol. Chem. 2010;285:32937–32945. doi: 10.1074/jbc.M110.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thummel C.S. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev. Cell. 2001;1:453–465. doi: 10.1016/S1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 32.Dimarcq J.-L., Imler J.-L., Lanot R., Ezekowitz R.A.B., Hoffmann J.A., Janeway C.A., Lagueux M. Treatment of l (2) mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem. Mol. Biol. 1997;27:877–886. doi: 10.1016/S0965-1748(97)00072-6. [DOI] [PubMed] [Google Scholar]
- 33.Borst D.W., Bollenbacher W.E., O’Connor J.D., King D.S., Fristrom J.W. Ecdysone levels during metamorphosis ofDrosophila melanogaster. Dev. Biol. 1974;39:308–316. doi: 10.1016/0012-1606(74)90242-5. [DOI] [PubMed] [Google Scholar]
- 34.Ling E., Shirai K., Kanekatsu R., Kiguchi K. Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: Prohemocytes have the function of phagocytosis. Cell Tissue Res. 2005;320:535–543. doi: 10.1007/s00441-004-1038-8. [DOI] [PubMed] [Google Scholar]
- 35.Merchant D., Ertl R.L., Rennard S.I., Stanley D.W., Miller J.S. Eicosanoids mediate insect hemocyte migration. J. Insect Physiol. 2008;54:215–221. doi: 10.1016/j.jinsphys.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Tsakas S., Marmaras V. Insect immunity and its signalling: An overview. Invertebr. Surviv. J. 2010;7:228–238. [Google Scholar]
- 37.Agaisse H., Petersen U.-M., Boutros M., Mathey-Prevot B., Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 2003;5:441–450. doi: 10.1016/S1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 38.Edosa T.T., Jo Y.H., Keshavarz M., Bae Y.M., Kim D.H., Lee Y.S., Han Y.S. TmSpz6 Is Essential for Regulating the Immune Response to Escherichia Coli and Staphylococcus Aureus Infection in Tenebrio Molitor. Insects. 2020;11:105. doi: 10.3390/insects11020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulet P., Cociancich S., Dimarcq J.-L., Lambert J., Reichhart J.-M., Hoffmann D., Hetru C., Hoffmann J. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J. Biol. Chem. 1991;266:24520–24525. [PubMed] [Google Scholar]
- 40.Carlsson A., Nyström T., de Cock H., Bennich H. Attacin-an insect immune protein-binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology. 1998;144:2179–2188. doi: 10.1099/00221287-144-8-2179. [DOI] [PubMed] [Google Scholar]
- 41.Bechinger B., Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta BBA Biomembr. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang L., Xu X., Freed S., Gao Y., Yu J., Wang S., Ju W., Zhang Y., Jin F. Cecropins from Plutella xylostella and Their Interaction with Metarhizium anisopliae. PLoS ONE. 2015;10:e0142451. doi: 10.1371/journal.pone.0142451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlsson A., Engström P., Palva E.T., Bennich H. Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect. Immun. 1991;59:3040–3045. doi: 10.1128/IAI.59.9.3040-3045.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jo Y.H., Park S., Park K.B., Noh M.Y., Cho J.H., Ko H.J., Kim C.E., Patnaik B.B., Kim J., Won R. In silico identification, characterization and expression analysis of attacin gene family in response to bacterial and fungal pathogens in Tenebrio molitor. Entomol. Res. 2018;48:45–54. doi: 10.1111/1748-5967.12287. [DOI] [Google Scholar]
- 45.Chae J.-H., Kurokawa K., So Y.-I., Hwang H.O., Kim M.-S., Park J.-W., Jo Y.-H., Lee Y.S., Lee B.L. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Dev. Comp. Immunol. 2012;36:540–546. doi: 10.1016/j.dci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Maistrou S., Paris V., Jensen A.B., Rolff J., Meyling N.V., Zanchi C. A constitutively expressed antifungal peptide protects Tenebrio molitor during a natural infection by the entomopathogenic fungus Beauveria bassiana. Dev. Comp. Immunol. 2018;86:26–33. doi: 10.1016/j.dci.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y.T., Lee M.R., Lee S.J., Kim S., Nai Y.S., Kim J.S. Tenebrio molitor Gram-negative-binding protein 3 (TmGNBP3) is essential for inducing downstream antifungal Tenecin 1 gene expression against infection with Beauveria bassiana JEF-007. Insect Sci. 2018;25:969–977. doi: 10.1111/1744-7917.12482. [DOI] [PubMed] [Google Scholar]
- 48.Thevissen K., Warnecke D.C., Francois I.E., Leipelt M., Heinz E., Ott C., Zahringer U., Thomma B.P., Ferket K.K., Cammue B.P. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2004;279:3900–3905. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- 49.Ganesan S., Aggarwal K., Paquette N., Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valanne S., Wang J.-H., Rämet M. The Drosophila toll signaling pathway. J. Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 51.Yaffe H., Buxdorf K., Shapira I., Ein-Gedi S., Moyal-Ben Zvi M., Fridman E., Moshelion M., Levy M. LogSpin: A simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res. Notes. 2012;5:45. doi: 10.1186/1756-0500-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.