Abstract
Roots anchor plants and take up water and nutrients from the soil; therefore, root development strongly affects plant growth and productivity. Moreover, increasing evidence indicates that root development is deeply involved in plant tolerance to abiotic stresses such as drought and salinity. These findings suggest that modulating root growth and development provides a potentially useful approach to improve plant abiotic stress tolerance. Such targeted approaches may avoid the yield penalties that result from growth–defense trade-offs produced by global induction of defenses against abiotic stresses. This review summarizes the developmental mechanisms underlying root development and discusses recent studies about modulation of root growth and stress tolerance in rice.
Keywords: root, auxin, abiotic stress, tolerance, rice
1. Introduction
Abiotic stress, including drought, low or high temperature, and salinity, seriously affects growth and productivity of plants [1]. Furthermore, global climate change may be increasing the frequency and severity of abiotic stress, thus threatening food production around the world [2]. In nature, plants are continuously exposed to different abiotic stresses and have evolved a variety of defense mechanisms to survive these abiotic stresses. Therefore, manipulation of these defenses may allow us to produce crop plants with improved tolerance to abiotic stress [3]. To understand the mechanisms of these stress responses and enable approaches to improve stress tolerance, extensive studies have identified many genetic components that mediate plant responses to abiotic stresses. These studies further revealed that abscisic acid (ABA) and jasmonic acid (JA) are key signaling molecules that mediate plant responses to abiotic stresses [4,5,6,7].
The essential roles of ABA and JA in plant stress responses and stress tolerance provide important clues for the development of stress-tolerant plants, and many successful studies reported stress-tolerant crops generated by modulation of ABA or JA responses. For example, activation or overexpression of OsPYL10, an ABA receptor increased ABA responses, leading to improved tolerance to drought [8], and overexpression of OsbZIP42 or heterologous expression of ZmbZIP4 (encoding positive regulators of ABA signaling) conferred drought tolerance in rice [9,10]. Additionally, a knock-out of the JA signaling repressor OsJAZ1 increased JA sensitivity and drought tolerance; conversely, overexpression of OsJAZ1 reduced JA sensitivity and drought tolerance [11]. These studies suggested that modulation of ABA or JA responses is a key strategy to enhance tolerance to abiotic stress. However, because of growth–defense trade-offs, activation of defense systems frequently reduces growth and productivity in plants [12]. Indeed, a growing number of studies reported that enhancing ABA or JA responses negatively affects plant growth and productivity under normal growth conditions [13,14,15].
Targeting specific tissues or organs, rather than altering responses on a whole-plant basis, has emerged as an alternative strategy for the development of crops with improved abiotic stress tolerance and growth. The root system is a key target for this strategy, as roots are responsible for the uptake of water and nutrients, as well as plant anchorage in soil; therefore, plant fitness and productivity largely depend on root development. Based on the potential for root development to influence crop production, optimization of root development is expected to be crucial for enabling the next Green Revolution [16]. In addition, root development affects the plant response to environmental conditions, and developmental plasticity of roots may help plants to survive abiotic stress conditions [17,18]. More importantly, several studies reported that modulating root development improved stress tolerance in crops and increased yield [19,20,21,22]. These observations suggested that modulation of root development could be a key strategy for development of crops with improved stress tolerance and yield, minimizing or avoiding the penalties of growth–defense trade-offs.
Rice is an important staple crop supporting approximately two-thirds of the world’s population [23]. The rice root system is composed of seminal, crown, and lateral roots. The seminal root develops from the seed during embryogenesis, and the crown roots that constitute the major root system of rice develop from the stem during post-embryogenesis. Lateral roots develop from lateral root primordia that originate from pericycle cells of seminal and crown roots, and the formation of lateral roots is responsible for extensive increase in the surface area of the root system for an uptake of water and nutrient from the soil [24,25]. The molecular and genetic mechanisms underlying root development in rice remain less well-studied than those of Arabidopsis. However, increasing evidence suggests that rice and Arabidopsis share many mechanisms of root development, including the function of auxin as a key regulator, and modulation of root development is a good strategy to improve stress tolerance without yield penalties in rice. In this review, we briefly describe the mechanisms underlying root development in Arabidopsis and rice, focusing on auxin. Additionally, we discuss recent studies reporting enhanced abiotic stress tolerance by modulating root development in rice.
2. Root Development in Arabidopsis
2.1. Root Formation and Growth
Roots initially form during embryogenesis through cell division of the zygote. The zygote asymmetrically divides to form two cells: a small cell in the apical position and a large cell in the basal position. The small cell divides vertically to form the proembryo and the large cell divides horizontally to form the suspensor, which connects the embryo to the maternal tissue. The proembryo contributes the stem cells for root epidermis, ground, and vascular tissues and the uppermost cell of the suspensor develops into the hypophysis, a progenitor of the quiescent center (QC), which is responsible for determination of cell identity and meristematic activity of root stem cells by preventing differentiation of the stem cells [26,27,28]. The embryonic root continues to grow in the apical direction, and the growth is promoted by the division of stem cells in the root apical meristem (RAM). The RAM is composed of undifferentiated cells and plays a central role in the growth of roots by protecting the stem cell niche and maintaining the cell division activity of the undifferentiated cells. Therefore, RAM activity required for root development is regulated by balancing cell division and differentiation, and auxin mediates this process [29,30].
2.2. Auxin and Root Development
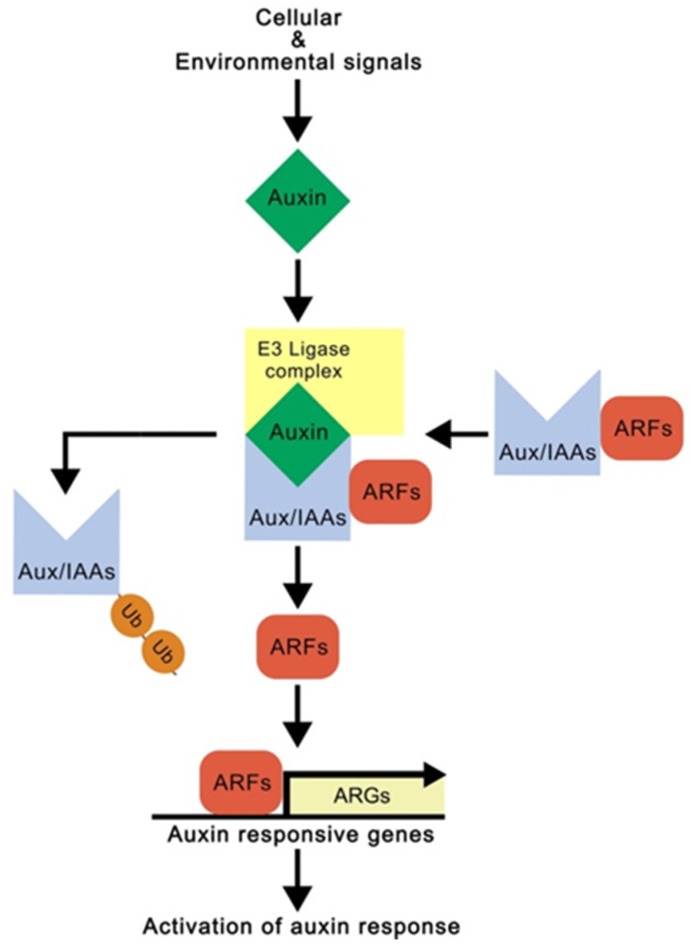
Auxin is a key regulator of plant development and growth, determining cell identity and regulating cell division and differentiation. Previously many studies demonstrated that regulation of auxin response is a key process controlling root development [31,32,33]. Auxin biosynthesis and signaling pathway are deeply involved in the auxin-mediated root development, and the results showing that root development is strongly inhibited in auxin signaling or deficiency mutants such as axr3-1 and trp2-12 support this [34,35]. In plants, indole-3-acetic acid is the predominant natural auxin and is biosynthesized through tryptophan-independent and -dependent pathways [36,37]. The tryptophan-dependent pathway is mediated by tryptophan aminotransferase, which converts tryptophan to indole-3-pyruvate, and by flavin monooxygenase, which converts indole-3-pyruvate to auxin. This tryptophan-dependent pathway is currently the best understood auxin biosynthetic pathway in plants. The biosynthesis of auxin induces auxin responses through the auxin signaling pathway, which mediates E3 ligase complex-mediated proteolysis of Aux/IAA auxin signaling repressor proteins (Figure 1). The proteolysis of Aux/IAAs leads to the release of AUXIN RESPONSE FACTORs (ARFs), which activate transcription of auxin-responsive genes [38,39]. ARFs such as MONOPTEROS (MP/ARF5) and NONPHOTOTROPIC HYPOCOTYL4 (NPH4/ARF4) initially mediate root responses to auxin, and these ARFs, in turn, activate transcriptional expression of auxin-responsive transcription factor PLETHORAs (PLTs), which play a pivotal role in maintenance of the stem cell niche and cell proliferation in a dosage-dependent manner [40,41,42].
Figure 1.
A schematic of the auxin signaling pathway. ARFs and Aux/IAAs function as positive and negative regulators in the auxin signaling pathway. In response to cellular and environmental signals, auxin is biosynthesized and the auxin promotes E3 ligase complex-mediated proteolysis of Aux/IAAs. The degradation leads to the release of ARFs and the activation of auxin response. Ub and ARGs indicate ubiquitin and auxin-responsive genes, respectively.
In roots, auxin accumulates at the root tip, specifically in the root stem cells. These cells specifically express auxin biosynthesis genes [43,44]. In addition to the root tip-specific expression of auxin biosynthesis genes, polar auxin transport is largely responsible for root tip-specific accumulation of auxin. This transport is mediated by polar auxin transporters such as PIN-FORMED PROTEINs (PINs) and AUXIN TRANSPORTER PROTEINs (AUXs) P-GLYCOPROTEINs (PGPs) [45,46]. Polar auxin transport generates an auxin gradient in the RAM, with high auxin at the proximal position and low auxin at the distal position of the RAM [47]. The auxin gradient is essential for maintenance of RAM activity and root development, and previous studies showing that mutation of genes involved in polar auxin transport inhibits root development by disrupting proper distribution of auxin in roots, suggest that polar auxin transport is another key process controlling the tissue-specific auxin required for root development [48,49,50].
Root growth is strongly affected by abiotic stresses such as drought and salinity, and auxin is deeply involved in this process. Root growth is suppressed in response to the stress hormone JA, and a study by Chen et al. (2011) suggested that auxin-responsive PLTs mediate this suppression [51]. Indeed, exogenous JA treatment reduces the expression of PLTs in wild-type plants but not in JA-signaling mutants such as coi1-1 and myc2. Furthermore, the JA response factor MYC2, which induces expression of JA-responsive genes, directly binds to the promoter of PLTs, and suppresses the expression of PLTs. These findings indicated that MYC2 mediates JA-induced inhibition of apical root growth by directly suppressing the expression of auxin-responsive PLTs. This also suggested that auxin and its downstream regulators mediate the stress-induced inhibition of root growth, and the hypothesis is supported by an increasing number of studies [52,53,54].
Root growth is also regulated by other developmental processes including cell wall development. Plant cells are encased by cell walls mostly made of three types of polysaccharides: cellulose, hemicelluloses, and pectins, and cortical microtubules regulate development of cell walls by guiding cellulose deposition [55,56]. Plant cell walls are flexible and diverse, and cell wall development is involved in the growth of roots because roots are composed of a variety type of cells with different functions and developmental stages [55]. Indeed, bul, bot1, and prc1 mutants, which have defects in microtubule organization and cellulose synthesis, exhibit short-root phenotypes [57,58,59]. The relationship between auxin and cell wall development remains largely unknown in root development, but a growing number of studies propose that auxin is possibly involved in the cell wall-mediated root development [60,61].
3. Rice Root Development
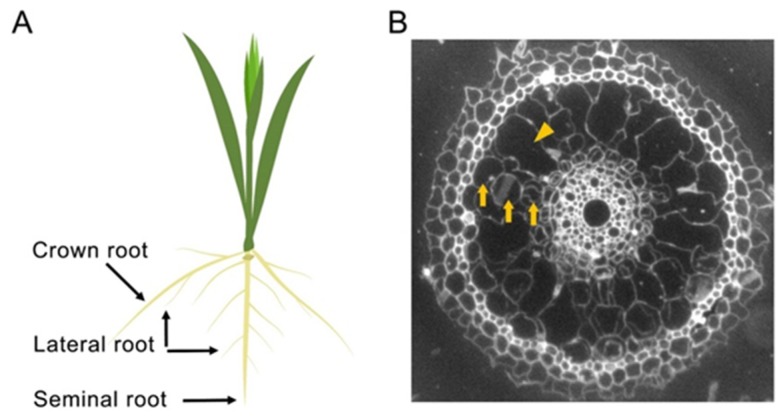
Rice roots have several distinct characteristics compared to Arabidopsis roots. In dicots, including Arabidopsis, roots can grow laterally via cell proliferation in the cambium layer, which increases the diameter of the root [62]. Cambium develops from procambium, and has higher meristematic activity. In contrast to dicot plants, the procambium of monocots differentiates into vascular tissues and consequently, cambium does not form and lateral growth does not occur [62]. In addition, Arabidopsis roots form a single layer of cortex between epidermis and endodermis, but rice roots form a multi-layer cortex by periclinal division of endodermal cells [63,64] (Figure 2). During maturation, the multi-layer cortex is destroyed by programmed cell death and develops into aerenchyma, a gas space that facilitates internal oxygen transport [65]. The precise developmental and molecular mechanisms that are specific to rice root development largely remain unknown. However, ongoing studies revealed that overall, the rice root system shares similar mechanisms to those of Arabidopsis, and that auxin is a key regulator of rice root development.
Figure 2.
Anatomy of rice roots. (A) A schematic of rice root system composed of seminal, crown and lateral roots. (B) A radial anatomy of rice roots was visualized by transverse sectioning of 12-day-old seminal roots. Arrow and arrowhead indicate cortex and aerenchyma, respectively.
3.1. Formation of Crown Roots in Rice
Although the seminal root is the first to emerge from the seed, crown roots constitute the major root system of rice. Crown roots develop from a meristem on the lowest node of the stem; this radial meristem shares common characteristics with the root pericycle tissues responsible for the formation of lateral roots [66,67]. The first identified gene related to crown root formation was CROWN ROOTLESS1/ADVENTITIOUS ROOTLESS1 (CRL1) [68]. CRL1 is specifically expressed in the tissues where crown roots are initiated and auxin promotes CRL1 expression. Indeed, CRL1 has auxin response elements in its promoter and is a direct target of ARF. In the crl1 mutant, crown root formation is strongly inhibited. Together, these observations indicated that CRL1 regulates the formation of crown roots, and auxin signaling is deeply involved in this process. Studies using auxin biosynthesis and signaling mutants further demonstrated the role of auxin in crown root formation. Transgenic rice overexpressing the auxin biosynthetic gene OsYUCCA1 formed more crown roots, whereas a gain-of-function mutant of OsIAA23 developed fewer crown roots [69,70]. Polar auxin transport also mediates the auxin-dependent crown root formation. OsPIN1 responsible for auxin distribution is specifically expressed in lateral root primordia of crown roots. A knock-down of OsPIN1 by RNA interference suppressed crown root formation, and treatment of NPA (N-1-naphthylphalamic acid, an auxin-transport inhibitor) mimicked the knock-down effect of OsPIN1 on crown root formation [71]. A study of OsGNOM1 also supports the essential role of polar auxin transport in crown root formation. GNOM encodes a large guanine nucleotide exchange factor for the ADP-ribosylation factor, and regulates auxin transport in Arabidopsis [72]. In rice, OsGNOM1 regulates expression of auxin efflux genes such as OsPIN2, OsPIN5b, and OsPIN9, and the osgnom1 mutant has defects in crown root formation [73]. In addition, identification and characterization of regulators such as CRL6 and WOX11 further support the role of auxin in crown root formation in rice. Auxin rescued the defective crown root formation in crl6 mutant, and endogenous auxin accumulation by OsYUCCA1 overexpression or exogenous auxin treatment promoted expression of WOX11, a key positive regulator of crown root formation [74,75,76]. A recent study using OsSPL3 revealed that microRNA signaling is involved in formation of crown roots in rice, and suggested that OsmiR156-OsSPL3 module regulates crown root formation by controlling auxin signaling [77].
3.2. Formation of Lateral Roots in Rice
Similar to the formation of crown roots, the formation of lateral roots largely depends on auxin and auxin-related genetic components. Formation of lateral roots extensively increases the surface area of root systems for resource acquisition from the soil [24,25]. Lateral roots originate primarily from pericycle tissue of roots, and early studies using altered lateral root formation 1 (alf1) and lateral rootless 1 (lrt1) suggested that auxin is involved in lateral root formation in rice [78,79]. A mutant rice arm1 isolated by screening for resistance to 2,4-dichlorophenoxyacetic acid (2,4-D) was defective in lateral root formation [78]. lrt1 mutant rice has defects in the formation of lateral roots, and the lateral root-less phenotype of lrt1 mutants was rescued by exogenous auxin [79], suggesting that auxin is involved in this process.
The involvement of auxin in lateral root formation has been further demonstrated by several studies. For example, mutation of OsAUX1 (encoding an auxin influx carrier) suppressed the lateral root initiation, while overexpression of OsAUX1 promoted lateral root formation [80]. RNAi silencing of OsMADS25 (encoding a transcription factor that directly represses OsIAA14 expression) reduced lateral root formation as well as root elongation by controlling auxin responses [81,82]. Although accumulating evidence indicates that auxin is a key regulator of lateral root formation, the precise molecular mechanism underlying this process remains largely unelucidated in rice.
3.3. Root Growth in Rice
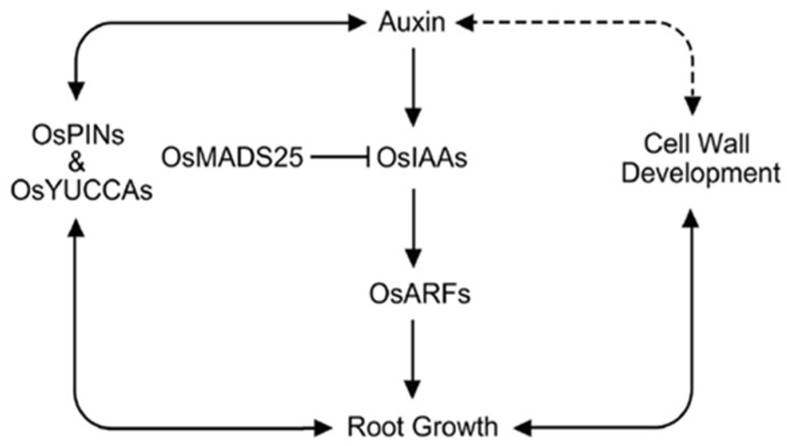
Since meristematic activity of the RAM is essential for the growth of roots in rice, mutant rice with defects in division and survival of RAM cells show severe defects in root growth [83]. Similar to the root system of Arabidopsis, auxin responses controlled by auxin biosynthesis, polar auxin transport, and signaling pathways are key factors determining RAM activity and the growth of roots (Figure 3). ARFs play an essential role in the regulation of auxin responses by governing transcription of auxin-responsive genes. In rice, OsARF12 controls apical root growth by regulating the expression of OsYUCCAs for auxin biosynthesis and OsPINs for polar auxin transport [84]. Consequently, mutant plants that lack expression of OsARF12 showed lower auxin levels in their roots and formed shorter roots, compared to wild-type plants. This indicates that, in addition to affecting root initiation, auxin affects root growth in rice.
Figure 3.
A schematic of auxin-mediated root growth in rice. Auxin response regulates root growth in rice, and the auxin response is controlled by auxin-related genes such as OsYUCCAs for auxin biosynthesis, OsPINs for transport, and OsARFs for signaling. OsMADS25 is not a direct component of the auxin signaling pathway, but regulates root growth by controlling the expression of OsIAAs. Cell wall development also regulates root growth in rice, and it is likely that auxin is involved in this process. Dashed arrow indicates hypothetical regulation.
The essential role of auxin in the growth of rice roots is further supported by recent studies using OsPIN1b and OsMADS25 [81,82]. A study of OsPIN1b (encoding an auxin efflux carrier) showed that OsPIN1b is specifically expressed in the rice root tip and mutant plants that lack expression of OsPIN1b developed short roots [81]. Unlike ARFs and IAAs, the OsMAD25 transcription factor is not a direct component of the auxin signaling pathway. However, OsMAD25 represses transcription of OsIAA14 (encoding a repressor of auxin signaling) by directly binding to the CArG-box in the promoter of OsIAA14. As expected, overexpression of OsMAD25 promoted rice root growth, whereas RNAi-mediated silencing of OsMAD25 reduced root growth, supporting the essential role of auxin in growth of rice roots [82].
Iron homeostasis is involved in the plant response to stress [85] and a study of OsARF12 suggested a relationship between root growth and stress responses [84]. This study showed that osarf12 mutant rice with defects in auxin signaling formed short roots compared with wild-type plants and affected iron homeostasis by reducing the expression of MITOCHONDRIAL IRON-REGULATED (OsMIR) and inducing expression of SHORT POSTEMBRYONIC ROOT1 (OsSPR1). Previously, it was shown that OsMIR and OsSPR1 positively and negatively regulate iron homeostasis, respectively, and consequently, knockout of OsMIR and OsSPR1 affects iron homeostasis [86,87]. These results support that OsARF12 responsible for auxin-mediated root growth also regulates iron homeostasis by controlling the expression of OsMIR and OsSPR1. Although the precise relationship between root growth and iron homeostasis remains elusive, this finding suggests that auxin-mediated root growth might be involved in iron homeostasis and plant stress responses.
Similar to the development of Arabidopsis roots, development of cell walls is involved in the growth of rice roots. In rice, OsGLU3 encodes a membrane-bound endo-1,4-β-glucanase acting on β-glucans, a major polysaccharide component of rice cell walls [88,89]. OsGLU3 is predominately expressed in roots, and OsGLU3 proteins localize at the plasma membrane. Osglu3 mutant rice exhibited lower crystalline cellulose contents in root cell walls. The RAM of the Osglu3 mutant is smaller than that of wild-type plants, leading to a short-root phenotype. This suggested that development of root cell walls modulates the growth of roots, and a study showing that knock-down of the OsEXPA8 (encoding an enzyme responsible for cell wall development) induced short root phenotype supported this [90]. The smaller size of the RAM in the cell wall mutants indicated that the short-root phenotype was caused by the reduced RAM activity. Since auxin is a key regulator of RAM activity, these observations suggested that auxin is involved in cell wall-mediated root growth (Figure 3), and a study using OsMOGS (encoding mannosyl-oligosaccharide glucosidase for cell wall formation) partially supported this [91]. In this study, Osmogs mutant rice with low auxin contents had impaired root growth and reduced cell wall thickness due to decreased cellulose synthase.
3.4. Complexity of Auxin-Mediated Root Growth and Development
Auxin is a key regulator promoting root formation and growth in rice. However, root phenotypes of several mutants suggest that the developmental mechanisms controlling root formation and growth downstream auxin are not simple. For example, rice auxin resistant mutant 1 (arm1) does not respond to auxin, and forms longer roots. Interestingly, the mutant develops fewer lateral roots than wild-type plants [78]. Overexpression of OsYUCCA1 (encoding an enzyme responsible for auxin biosynthesis) promotes formation of crown roots, but suppresses root growth [69]. In addition, suppression of OsESG1 (encoding an S-domain receptor-like kinase) promotes root elongation, but reduces the formation of crown roots by regulating auxin distribution [92]. Unlike ARM1, OsYUCCA1, and OsESG1, OsMADS25 promotes root growth and lateral root formation at the same time. Both root growth and lateral root formation was suppressed by RNAi silencing of OsMADS25, but was promoted by overexpression of OsMADS25 [82]. These findings suggest that auxin pathways responsible for root formation and growth interact in a complex manner, and the complexity may be involved in developmental flexibility of root development in response to ever-changing environmental conditions, including abiotic stress.
4. Root Development and Stress Tolerance in Rice
Previous studies of the relationship between root development and stress tolerance suggested that root development affects plant tolerance to abiotic stresses, including drought [93,94,95]. Several studies of OsNAC transcription factors have convincingly shown the role of roots in stress tolerance. NAC transcription factors are plant-specific transcription factors involved in development and stress responses [96,97]. Whole-plant expression of drought-responsive OsNAC5, OsNAC6, OsNAC9, and OsNAC10 increased drought tolerance by promoting root growth in width [19,20,21,22]. More importantly, root-specific overexpression of these transcription factors promoted the root diameter and stress tolerance more effectively than whole-body overexpression. The improved tolerance led to an increase in rice yield under drought conditions. For example, transgenic rice with root-specific overexpression of OsNAC5, OsNAC6, OsNAC9, and OsNAC10 showed 22–63%, 27–74%, 28–72%, and 25–42% increased grain yield compared to wild-type controls under stress conditions, and the grain yield of the root-specific overexpressors tended to be higher than that of the whole-plant overexpressor. Additionally, the transgenic rice showed similar or higher grain yields even in normal growth conditions compared to wild-type control plants. Molecular mechanisms underlying the NAC-dependent root growth and stress tolerance remain unknown, but it is expected that the increased root diameter improves drought tolerance by affecting root penetration through soil [20]. These findings indicated that promoting root growth improves tolerance to abiotic stress and rice productivity. This finding is supported by studies using OsMADS25 that regulates root elongation and formation in rice by controlling the auxin response [82,98]. In this study, the knock-down mutant of OsMADS25 with reduced root growth exhibited high sensitivity to salinity and oxidative stress, while transgenic rice overexpressing OsMADS25 displayed enhanced tolerance to the abiotic stress [98].
In addition, a study of DEEPER ROOTING 1 (DRO1) showed that modulation of the root system architecture affects rice yield under drought conditions [99]. In this study, they identified DRO1 that regulates root architecture in rice. Since expression of DRO1 is dramatically regulated by auxin, it is likely that auxin is deeply involved in the regulation of root architecture in rice. The angle of root growth was increased by DRO1, and consequently, roots of the rice with higher expression of DRO1 grew in a more downward direction [99]. Furthermore, introduction of DRO1 into the rice with a shallow-rooting system improved drought tolerance by promoting downward root growth, leading to enhanced drought tolerance and high grain yield under drought stress compared to the control plants. Additionally, the modulation of root system architecture by DRO1 did not affect grain yield under normal growth conditions. Collectively, these suggest that modulation of root growth or architecture is a good strategy to develop stress-tolerant rice with no or minimal growth–defense trade-offs.
Root-specific lignification also affects stress tolerance in rice [100]. Lignin is hydrophobic and a key component of plant secondary cell walls, where it inhibits water loss from plant tissues [101]. Lignin biosynthesis is deeply involved in stress tolerance; for example, under drought conditions, maize drought-tolerant inbred lines exhibited increased accumulation of lignin compared with drought-sensitive lines [102]. Lee et al. (2016) revealed that OsERF71 promoted lignin biosynthesis in rice by directly activating the expression of lignin biosynthesis genes such as CINNAMOYL-COENZYME A REDUCTASE 1 (OsCCR1), and root-specific lignification by OsERF71 promoted drought tolerance and increased grain yield up to 23–32% under drought conditions [100]. The role of lignin in rice stress tolerance is also supported by a recent study of OsTF1L, a rice HD-Zip transcription factor that regulates lignin biosynthesis [103].
5. Future Perspectives
Evolution of roots allowed land plants to survive and colonize the terrestrial environment [104]. Now, modulation of root growth could improve plant growth and tolerance to abiotic stress, and optimization or modulation of root development may enable the next Green Revolution [16]. The function of roots relies on the development of a variety of root tissues with specialized functions. For example, root hairs take up water and nutrients, the endodermis selectively transports nutrients, the vasculature is for long-distance transport of water, nutrient and carbon assimilates, and the aerenchyma functions in gas exchange. This suggests that modulation of root tissue development expands the possible approaches for the development of rice with improved yield and tolerance. However, despite significant progress in our understanding of rice root development, most developmental mechanisms underlying these processes remain largely unknown. Further molecular and genetic studies for the identification and characterization of genetic components involved in rice root development will provide important clues for the development of rice with higher yield and stress tolerance.
Author Contributions
G.J. and Y.D.C. designed the review; D.H.S., S.S. and G.J. access information; D.H.S., S.S. and G.J. wrote the article with contributions of Y.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01323901 and PJ01364301) Rural Development Administration, Republic of Korea, and the National Research Foundation of Korea Grant funded by the Korean Government (MOE) [NRF-2019R1A2C1007103].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.He M., He C.-Q., Ding N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018;9:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raza A., Razzaq A., Mehmood S.S., Zou X., Zhang X., Lv Y., Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants. 2019;8:34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechtold U., Field B. Molecular Mechanisms Controlling Plant Growth during Abiotic Stress. Volume 69. Oxford University Press; Oxford, UK: 2018. pp. 2753–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sah S.K., Reddy K.R., Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016;7:571. doi: 10.3389/fpls.2016.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He R., Zhuang Y., Cai Y., Agüero C.B., Liu S., Wu J., Deng S., Walker M.A., Lu J., Zhang Y. Overexpression of 9-cis-epoxycarotenoid dioxygenase cisgene in grapevine increases drought tolerance and results in pleiotropic effects. Front. Plant Sci. 2018;9:970. doi: 10.3389/fpls.2018.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo J.S., Joo J., Kim M.J., Kim Y.K., Nahm B.H., Song S.I., Cheong J.J., Lee J.S., Kim J.K., Choi Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011;65:907–921. doi: 10.1111/j.1365-313X.2010.04477.x. [DOI] [PubMed] [Google Scholar]
- 7.Jang G., Yoon Y., Choi Y.D. Crosstalk with Jasmonic Acid Integrates Multiple Responses in Plant Development. Int. J. Mol. Sci. 2020;21:305. doi: 10.3390/ijms21010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma R.K., Santosh Kumar V., Yadav S.K., Pushkar S., Rao M.V., Chinnusamy V. Overexpression of ABA Receptor PYL10 gene confers drought and cold tolerance to indica rice. Front. Plant Sci. 2019;10:1488. doi: 10.3389/fpls.2019.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo J., Lee Y.H., Song S.I. OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice. Planta. 2019;249:1521–1533. doi: 10.1007/s00425-019-03104-7. [DOI] [PubMed] [Google Scholar]
- 10.Ma H., Liu C., Li Z., Ran Q., Xie G., Wang B., Fang S., Chu J., Zhang J. ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol. 2018;178:753–770. doi: 10.1104/pp.18.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J., Wu H., Ma S., Xiang D., Liu R., Xiong L. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017;8:2108. doi: 10.3389/fpls.2017.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karasov T.L., Chae E., Herman J.J., Bergelson J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell. 2017;29:666–680. doi: 10.1105/tpc.16.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H., Lee K., Hwang H., Bhatnagar N., Kim D.-Y., Yoon I.S., Byun M.-O., Kim S.T., Jung K.-H., Kim B.-G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014;65:453–464. doi: 10.1093/jxb/ert397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E.H., Kim Y.S., Park S.-H., Koo Y.J., Do Choi Y., Chung Y.-Y., Lee I.-J., Kim J.-K. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol. 2009;149:1751–1760. doi: 10.1104/pp.108.134684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arend M., Schnitzler J.-P., Ehlting B., Hänsch R., Lange T., Rennenberg H., Himmelbach A., Grill E., Fromm J. Expression of the Arabidopsis mutant ABI1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiol. 2009;151:2110–2119. doi: 10.1104/pp.109.144956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villordon A.Q., Ginzberg I., Firon N. Root architecture and root and tuber crop productivity. Trends Plant Sci. 2014;19:419–425. doi: 10.1016/j.tplants.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Suralta R.R., Kano-Nakata M., Niones J.M., Inukai Y., Kameoka E., Tran T.T., Menge D., Mitsuya S., Yamauchi A. Root plasticity for maintenance of productivity under abiotic stressed soil environments in rice: Progress and prospects. Field Crop. Res. 2018;220:57–66. doi: 10.1016/j.fcr.2016.06.023. [DOI] [Google Scholar]
- 18.Grossman J.D., Rice K.J. Evolution of root plasticity responses to variation in soil nutrient distribution and concentration. Evol. Appl. 2012;5:850–857. doi: 10.1111/j.1752-4571.2012.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong J.S., Kim Y.S., Redillas M.C., Jang G., Jung H., Bang S.W., Choi Y.D., Ha S.H., Reuzeau C., Kim J.K. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2013;11:101–114. doi: 10.1111/pbi.12011. [DOI] [PubMed] [Google Scholar]
- 20.Redillas M.C., Jeong J.S., Kim Y.S., Jung H., Bang S.W., Choi Y.D., Ha S.H., Reuzeau C., Kim J.K. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 2012;10:792–805. doi: 10.1111/j.1467-7652.2012.00697.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.K., Chung P.J., Jeong J.S., Jang G., Bang S.W., Jung H., Kim Y.S., Ha S.H., Choi Y.D., Kim J.K. The rice OsNAC6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol. J. 2017;15:754–764. doi: 10.1111/pbi.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong J.S., Kim Y.S., Baek K.H., Jung H., Ha S.-H., Do Choi Y., Kim M., Reuzeau C., Kim J.-K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010;153:185–197. doi: 10.1104/pp.110.154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C., Liu B., Piao S., Wang X., Lobell D.B., Huang Y., Huang M., Yao Y., Bassu S., Ciais P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA. 2017;114:9326–9331. doi: 10.1073/pnas.1701762114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banda J., Bellande K., Von Wangenheim D., Goh T., Guyomarc’h S., Laplaze L., Bennett M.J. Lateral root formation in Arabidopsis: A well-ordered LRexit. Trends Plant Sci. 2019;114:9326–9331. doi: 10.1016/j.tplants.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz Rosquete M., Waidmann S., Kleine-Vehn J. PIN7 auxin carrier has a preferential role in terminating radial root expansion in Arabidopsis thaliana. Int. J. Mol. Sci. 2018;19:1238. doi: 10.3390/ijms19041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheres B., Wolkenfelt H., Willemsen V., Terlouw M., Lawson E., Dean C., Weisbeek P. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- 27.Möller B., Weijers D. Auxin control of embryo patterning. Cold Spring Harb. Perspect. Biol. 2009;1:a001545. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/S0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 29.Kerk N.M., Jiang K., Feldman L.J. Auxin metabolism in the root apical meristem. Plant Physiol. 2000;122:925–932. doi: 10.1104/pp.122.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyashima S., Sebastian J., Lee J.Y., Helariutta Y. Stem cell function during plant vascular development. Embo J. 2013;32:178–193. doi: 10.1038/emboj.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueiredo D.D., Köhler C. Auxin: A molecular trigger of seed development. Genes Dev. 2018;32:479–490. doi: 10.1101/gad.312546.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J., Zhou J.-J., Zhang J.-Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018;19:259. doi: 10.3390/ijms19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao G., Zhang Y. Adaptive Growth: Shaping Auxin-Mediated Root System Architecture. Trends Plant Sci. 2019;21:121–123. doi: 10.1016/j.tplants.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Ursache R., Miyashima S., Chen Q., Vatén A., Nakajima K., Carlsbecker A., Zhao Y., Helariutta Y., Dettmer J. Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development. 2014;141:1250–1259. doi: 10.1242/dev.103473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., He P., Ma X., Yang Z., Pang C., Yu J., Wang G., Friml J., Xiao G. Auxin-mediated statolith production for root gravitropism. New Phytol. 2019;224:761–774. doi: 10.1111/nph.15932. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Yang W., Zuo Y., Zhu L., Hastwell A.H., Chen L., Tian Y., Su C., Ferguson B.J., Li X. GmYUC2a mediates auxin biosynthesis during root development and nodulation in soybean. J. Exp. Bot. 2019;70:3165–3176. doi: 10.1093/jxb/erz144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B., Chu J., Yu T., Xu Q., Sun X., Yuan J., Xiong G., Wang G., Wang Y., Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:4821–4826. doi: 10.1073/pnas.1503998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 39.Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479. doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harper R.M., Stowe-Evans E.L., Luesse D.R., Muto H., Tatematsu K., Watahiki M.K., Yamamoto K., Liscum E. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell. 2000;12:757–770. doi: 10.1105/tpc.12.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krogan N.T., Marcos D., Weiner A.I., Berleth T. The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol. 2016;212:42–50. doi: 10.1111/nph.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mähönen A.P., Ten Tusscher K., Siligato R., Smetana O., Díaz-Triviño S., Salojärvi J., Wachsman G., Prasad K., Heidstra R., Scheres B. PLETHORA gradient formation mechanism separates auxin responses. Nature. 2014;515:125–129. doi: 10.1038/nature13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersson S.V., Johansson A.I., Kowalczyk M., Makoveychuk A., Wang J.Y., Moritz T., Grebe M., Benfey P.N., Sandberg G., Ljung K. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell. 2009;21:1659–1668. doi: 10.1105/tpc.109.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brumos J., Robles L.M., Yun J., Vu T.C., Jackson S., Alonso J.M., Stepanova A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell. 2018;47:306–318.e5. doi: 10.1016/j.devcel.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Petrášek J., Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 46.Olatunji D., Geelen D., Verstraeten I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017;18:2587. doi: 10.3390/ijms18122587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petricka J.J., Winter C.M., Benfey P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012;63:563–590. doi: 10.1146/annurev-arplant-042811-105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 49.Giehl R.F., Lima J.E., Von Wirén N. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell. 2012;24:33–49. doi: 10.1105/tpc.111.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J.-J., Luo J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018;19:2759. doi: 10.3390/ijms19092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q., Sun J., Zhai Q., Zhou W., Qi L., Xu L., Wang B., Chen R., Jiang H., Qi J. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell. 2011;23:3335–3352. doi: 10.1105/tpc.111.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouzroud S., Gouiaa S., Hu N., Bernadac A., Mila I., Bendaou N., Smouni A., Bouzayen M., Zouine M. Auxin Response Factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum) PLoS ONE. 2018;13:e0193517. doi: 10.1371/journal.pone.0193517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H., Feng F., Liu J., Zhao Q. Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front. Plant Sci. 2018;9:659. doi: 10.3389/fpls.2018.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korver R.A., Koevoets I.T., Testerink C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018;23:783–793. doi: 10.1016/j.tplants.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somssich M., Khan G.A., Persson S. Cell Wall heterogeneity in root development of Arabidopsis. Front. Plant Sci. 2016;7:1242. doi: 10.3389/fpls.2016.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molines A.T., Marion J., Chabout S., Besse L., Dompierre J.P., Mouille G., Coquelle F.M. EB1 contributes to microtubule bundling and organization, along with root growth, in Arabidopsis thaliana. Biol. Open. 2018;7:bio030510. doi: 10.1242/bio.030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catterou M., Dubois F., Schaller H., Aubanelle L., Vilcot B., Sangwan-Norreel B.S., Sangwan R.S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. II. Effects of brassinosteroids on microtubules and cell elongation in the bul1 mutant. Planta. 2001;212:673–683. doi: 10.1007/s004250000467. [DOI] [PubMed] [Google Scholar]
- 58.Bichet A., Desnos T., Turner S., Grandjean O., Höfte H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001;25:137–148. doi: 10.1046/j.1365-313x.2001.00946.x. [DOI] [PubMed] [Google Scholar]
- 59.Paredez A.R., Persson S., Ehrhardt D.W., Somerville C.R. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol. 2008;147:1723–1734. doi: 10.1104/pp.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoenaers S., Balcerowicz D., Breen G., Hill K., Zdanio M., Mouille G., Holman T.J., Oh J., Wilson M.H., Nikonorova N. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018;28:722–732. doi: 10.1016/j.cub.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 61.Huang J.-B., Zou Y., Zhang X., Wang M., Dong Q., Tao L.-Z. RIBOSE PHOSPHATE ISOMERSASE 1 influences root development by acting on cell wall biosynthesis, actin organization and auxin transport in Arabidopsis. Front. Plant Sci. 2019;10:1641. doi: 10.3389/fpls.2019.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esau K. Vascular Differentiation in Plants. Holt, Rinehart and Winston; New York, NY, USA: 1965. [Google Scholar]
- 63.Cui H., Levesque M.P., Vernoux T., Jung J.W., Paquette A.J., Gallagher K.L., Wang J.Y., Blilou I., Scheres B., Benfey P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 64.Miyashima S., Nakajima K. The root endodermis: A hub of developmental signals and nutrient flow. Plant Signal. Behav. 2011;6:1954–1958. doi: 10.4161/psb.6.12.18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamauchi T., Tanaka A., Inahashi H., Nishizawa N.K., Tsutsumi N., Inukai Y., Nakazono M. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA. 2019;116:20770–20775. doi: 10.1073/pnas.1907181116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coudert P., Van Anh Le T., Gantet P. Rice: A model plant to decipher the hidden origin of adventitious roots. In: Beeckman T., editor. Plant Roots: The Hidden Half. CRC Press; Boca Raton, FL, USA: 2013. [Google Scholar]
- 67.Mai C.D., Phung N.T., To H.T., Gonin M., Hoang G.T., Nguyen K.L., Do V.N., Courtois B., Gantet P. Genes controlling root development in rice. Rice. 2014;7:30. doi: 10.1186/s12284-014-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inukai Y., Sakamoto T., Ueguchi-Tanaka M., Shibata Y., Gomi K., Umemura I., Hasegawa Y., Ashikari M., Kitano H., Matsuoka M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell. 2005;17:1387–1396. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto Y., Kamiya N., Morinaka Y., Matsuoka M., Sazuka T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007;143:1362–1371. doi: 10.1104/pp.106.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jun N., Gaohang W., Zhenxing Z., Huanhuan Z., Yunrong W., Ping W. OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J. 2011;68:433–442. doi: 10.1111/j.1365-313X.2011.04698.x. [DOI] [PubMed] [Google Scholar]
- 71.Xu M., Zhu L., Shou H., Wu P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005;46:1674–1681. doi: 10.1093/pcp/pci183. [DOI] [PubMed] [Google Scholar]
- 72.Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- 73.Liu S., Wang J., Wang L., Wang X., Xue Y., Wu P., Shou H. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009;19:1110–1119. doi: 10.1038/cr.2009.70. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Wang D., Gan T., Liu L., Long W., Wang Y., Niu M., Li X., Zheng M., Jiang L. CRL6, a member of the CHD protein family, is required for crown root development in rice. Plant Physiol. Biochem. 2016;105:185–194. doi: 10.1016/j.plaphy.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 75.Zhang T., Li R., Xing J., Yan L., Wang R., Zhao Y. The YUCCA-auxin-WOX11 module controls crown root development in rice. Front. Plant Sci. 2018;9:523. doi: 10.3389/fpls.2018.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., Hu Y., Dai M., Huang L., Zhou D.-X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009;21:736–748. doi: 10.1105/tpc.108.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shao Y., Zhou H.-Z., Wu Y., Zhang H., Lin J., Jiang X., He Q., Zhu J., Li Y., Yu H. OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell. 2019;31:1257–1275. doi: 10.1105/tpc.19.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chhun T., Taketa S., Tsurumi S., Ichii M. Interaction between two auxin-resistant mutants and their effects on lateral root formation in rice (Oryza sativa L.) J. Exp. Bot. 2003;54:2701–2708. doi: 10.1093/jxb/erg306. [DOI] [PubMed] [Google Scholar]
- 79.Chhun T., Taketa S., Tsurumi S., Ichii M. The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice (Oryza sativa L.) Plant Growth Regul. 2003;39:161–170. doi: 10.1023/A:1022592511387. [DOI] [Google Scholar]
- 80.Zhao H., Ma T., Wang X., Deng Y., Ma H., Zhang R., Zhao J. OsAUX1 controls lateral root initiation in rice (Oryza sativa L.) Plant Cell Environ. 2015;38:2208–2222. doi: 10.1111/pce.12467. [DOI] [PubMed] [Google Scholar]
- 81.Sun H., Tao J., Bi Y., Hou M., Lou J., Chen X., Zhang X., Luo L., Xie X., Yoneyama K. OsPIN1b is involved in rice seminal root elongation by regulating root apical meristem activity in response to low nitrogen and phosphate. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-29784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang G., Xu N., Chen H., Wang G., Huang J. OsMADS25 regulates root system development via auxin signalling in rice. Plant J. 2018;95:1004–1022. doi: 10.1111/tpj.14007. [DOI] [PubMed] [Google Scholar]
- 83.Li J., Zhu S., Song X., Shen Y., Chen H., Yu J., Yi K., Liu Y., Karplus V.J., Wu P. A rice glutamate receptor–like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell. 2006;18:340–349. doi: 10.1105/tpc.105.037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi Y., Wang S., Shen C., Zhang S., Chen Y., Xu Y., Liu Y., Wu Y., Jiang D. OsARF12, a transcription activator on auxin response gene, regulates root elongation and affects iron accumulation in rice (Oryza sativa) New Phytol. 2012;193:109–120. doi: 10.1111/j.1469-8137.2011.03910.x. [DOI] [PubMed] [Google Scholar]
- 85.Tripathi D.K., Singh S., Gaur S., Singh S., Yadav V., Liu S., Singh V.P., Sharma S., Srivastava P., Prasad S.M. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Environ. Sci. 2018;5:86. doi: 10.3389/fenvs.2017.00086. [DOI] [Google Scholar]
- 86.Ishimaru Y., Bashir K., Fujimoto M., An G., Itai R.N., Tsutsumi N., Nakanishi H., Nishizawa N.K. Rice-specific mitochondrial iron-regulated gene (MIR) plays an important role in iron homeostasis. Mol. Plant. 2009;2:1059–1066. doi: 10.1093/mp/ssp051. [DOI] [PubMed] [Google Scholar]
- 87.Jia L., Wu Z., Hao X., Carrie C., Zheng L., Whelan J., Wu Y., Wang S., Wu P., Mao C. Identification of a novel mitochondrial protein, short postembryonic roots 1 (SPR1), involved in root development and iron homeostasis in Oryza sativa. New Phytol. 2011;189:843–855. doi: 10.1111/j.1469-8137.2010.03513.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J.-W., Xu L., Wu Y.-R., Chen X.-A., Liu Y., Zhu S.-H., Ding W.-N., Wu P., Yi K.-K. OsGLU3, a putative membrane-bound endo-1, 4-beta-glucanase, is required for root cell elongation and division in rice (Oryza sativa L.) Mol. Plant. 2012;5:176–186. doi: 10.1093/mp/ssr084. [DOI] [PubMed] [Google Scholar]
- 89.Glass M., Barkwill S., Unda F., Mansfield S.D. Endo-β-1, 4-glucanases impact plant cell wall development by influencing cellulose crystallization. J. Integr. Plant Biol. 2015;57:396–410. doi: 10.1111/jipb.12353. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Ma N., Qiu S., Zou H., Zang G., Kang Z., Wang G., Huang J. Regulation of the α-expansin gene OsEXPA8 expression affects root system architecture in transgenic rice plants. Mol. Breed. 2014;34:47–57. doi: 10.1007/s11032-014-0016-4. [DOI] [Google Scholar]
- 91.Wang S., Xu Y., Li Z., Zhang S., Lim J.M., Lee K.O., Li C., Qian Q., Jiang D.A., Qi Y. OsMOGS is required for N-glycan formation and auxin-mediated root development in rice (Oryza sativa L.) Plant J. 2014;78:632–645. doi: 10.1111/tpj.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan J., Li Z., Wang Q., Yang L., Yao F., Liu W. An S-domain receptor-like kinase, OsESG1, regulates early crown root development and drought resistance in rice. Plant Sci. 2020;290:110318. doi: 10.1016/j.plantsci.2019.110318. [DOI] [PubMed] [Google Scholar]
- 93.Loresto G.C., ZHANG W.-X., Chaudhary D., Chang T. Aeroponic technique for screening the drought avoidance mechanism of rice genotypes by the root characters. Garcia De Orta. Série De Estud. Agronómicos. 1983;10:77–82. [Google Scholar]
- 94.Ekanayake I., O’toole J., Garrity D., Masajo T. Inheritance of root characters and their relations to drought resistance in rice 1. Crop Sci. 1985;25:927–933. doi: 10.2135/cropsci1985.0011183X002500060007x. [DOI] [Google Scholar]
- 95.Pushpam R., Manonmani S., Varthini N.V., Robin S. Studies on yield, root characters related to drought tolerance and their association in upland rice genotypes. Electron. J. Plant Breed. 2018;9:856–862. doi: 10.5958/0975-928X.2018.00106.0. [DOI] [Google Scholar]
- 96.Huysmans M., Buono R.A., Skorzinski N., Radio M.C., De Winter F., Parizot B., Mertens J., Karimi M., Fendrych M., Nowack M.K. NAC transcription factors ANAC087 and ANAC046 control distinct aspects of programmed cell death in the Arabidopsis columella and lateral root cap. Plant Cell. 2018;30:2197–2213. doi: 10.1105/tpc.18.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diao W., Snyder J.C., Wang S., Liu J., Pan B., Guo G., Ge W., Dawood M.H.S.A. Genome-wide analyses of the NAC transcription factor gene family in pepper (Capsicum annuum L.): Chromosome location, phylogeny, structure, expression patterns, cis-elements in the promoter, and interaction network. Int. J. Mol. Sci. 2018;19:1028. doi: 10.3390/ijms19041028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu N., Chu Y., Chen H., Li X., Wu Q., Jin L., Wang G., Huang J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA–mediated regulatory pathway and ROS scavenging. Plos Genet. 2018;14:e1007662. doi: 10.1371/journal.pgen.1007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uga Y., Sugimoto K., Ogawa S., Rane J., Ishitani M., Hara N., Kitomi Y., Inukai Y., Ono K., Kanno N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013;45:1097. doi: 10.1038/ng.2725. [DOI] [PubMed] [Google Scholar]
- 100.Lee D.-K., Jung H., Jang G., Jeong J.S., Kim Y.S., Ha S.-H., Do Choi Y., Kim J.-K. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016;172:575–588. doi: 10.1104/pp.16.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barros J., Serk H., Granlund I., Pesquet E. The cell biology of lignification in higher plants. Ann. Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Y., Li W.C., Xu Y., Li G., Liao Y., Fu F.-L. Differential expression of candidate genes for lignin biosynthesis under drought stress in maize leaves. J. Appl. Genet. 2009;50:213–223. doi: 10.1007/BF03195675. [DOI] [PubMed] [Google Scholar]
- 103.Bang S.W., Lee D.K., Jung H., Chung P.J., Kim Y.S., Choi Y.D., Suh J.W., Kim J.K. Overexpression of OsTF1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant Biotechnol. J. 2019;17:118–131. doi: 10.1111/pbi.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Du Y., Scheres B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018;69:155–167. doi: 10.1093/jxb/erx223. [DOI] [PubMed] [Google Scholar]