Abstract
Flavodoxins are small soluble electron transfer proteins widely present in bacteria and absent in vertebrates. Flavodoxins participate in different metabolic pathways and, in some bacteria, they have been shown to be essential proteins representing promising therapeutic targets to fight bacterial infections. Using purified flavodoxin and chemical libraries, leads can be identified that block flavodoxin function and act as bactericidal molecules, as it has been demonstrated for Helicobacter pylori (Hp), the most prevalent human gastric pathogen. Increasing antimicrobial resistance by this bacterium has led current therapies to lose effectiveness, so alternative treatments are urgently required. Here, we summarize, with a focus on flavodoxin, opportunities for pharmacological intervention offered by the potential protein targets described for this bacterium and provide information on other gastrointestinal pathogens and also on bacteria from the gut microbiota that contain flavodoxin. The process of discovery and development of novel antimicrobials specific for Hp flavodoxin that is being carried out in our group is explained, as it can be extrapolated to the discovery of inhibitors specific for other gastric pathogens. The high specificity for Hp of the antimicrobials developed may be of help to reduce damage to the gut microbiota and to slow down the development of resistant Hp mutants.
Keywords: flavodoxin, drug discovery, therapeutic target, Helicobacter pylori (Hp), antimicrobial resistance, gastric pathogens, gastric microbiota
1. Introduction
Helicobacter pylori (Hp) is a Gram-negative, spiral-shaped bacterium that colonizes the gastric mucosa of over 4 billion people worldwide [1,2,3]. The prevalence of this infection increases with age and varies depending on the world region, being higher in developing countries (up to 88%) than in developed ones [2,3,4]. It is suggested that the HLA-DQA1 gene influences the human susceptibility to Hp infection, the development of related diseases, and the host’s response against this bacterium [5]. The ways in which Hp is acquired are proposed to include intake of contaminated water and direct human–human contact [3]. Diet, hygiene, and lifestyle play an important role in Hp transmission [2], and unless antimicrobial therapy is administered, humans can remain infected for life [3]. Although most infected people are asymptomatic [3], Hp colonization of the gastric epithelial cells can cause an inflammatory response in the mucosa. The initial gastritis can progress to chronic non-atrophic, active or atrophic gastritis and lead to duodenal and gastric ulcers or even to intestinal metaplasia and dysplasia, occasionally causing gastric mucosa-associated lymphoid tissue (MALT) lymphoma or gastric adenocarcinoma [3,6]. In fact, Hp is the only bacterium classified as a Class I carcinogen by the International Agency for Research on Cancer [3,6,7] and, as shown by epidemiological studies, it seems to be the most common infectious agent related to cancers, 6.2% of all cancer cases worldwide being attributable to Hp [6,8]. The risk of developing Hp-related cancer has been suggested to depend on the Hp strain, the host traits, and the interactions between bacterium and host [9]. Besides, Hp has been reported to be involved in extragastric pathologies such as neurological, dermatological, hematologic, ocular, cardiovascular, metabolic, allergic, liver, and biliary diseases [10,11]. The eradication of Hp has been recommended in order to decrease gastric mucosa inflammation and to prevent its progression to preneoplasic lesions and the development of gastric cancer and/or other extragastric diseases [12,13].
Conventional treatment of Hp infection has relied on two or three broad-spectrum antimicrobials plus a proton-pump inhibitor (PPI) such as omeprazole, esomeprazole or rabeprazole. Although standard triple therapy, which is based on clarithromycin, amoxicillin, or metronidazole and a PPI, has been prescribed for decades, nowadays it does not accomplish acceptable eradication rates because of Hp resistance, especially to metronidazole and clarithromycin. In areas of high (>15%) resistance to the latter antibiotic, bismuth or non-bismuth quadruple regimens must be followed. They consist of a PPI plus three antimicrobials: metronidazole, tetracycline, and bismuth in the first therapy and metronidazole, amoxicillin, and clarithromycin in the second one [14,15]. These last regimens seem the most effective ones to overcome antibiotic resistance, the main proposed reason of treatment failure together with low patient compliance to therapy, high gastric bacterial load, cytochrome P450 polymorphism (CYP2C19), and high gastric acidity [16]. Antibiotic resistance to Hp has been suggested to arise from point mutations, drug inactivation, the activation of drug efflux pumps, altered membrane permeability, biofilm formation or the presence of bacterial dormant forms [17]. The high genetic diversity of Hp allows the bacterium to evade the immune response and to adapt to environment challenges such as antimicrobials [18,19]. The annual Hp reinfection rate is up to 8.7% and depends on world region, age, education level, proportion of household members infected, and socioeconomic status of the patients [12]. While the reported prevalences of amoxicillin (0–21.4%) and tetracycline (0–32.4%) resistance are moderate, those of metronidazole (2.1–99.5%), clarithromycin (7.9–52.6%), and levofloxacin (0–55.6%) are quite high [12]. In fact, clarithromycin-resistant Hp strains were included by the World Health Organization in the high-priority group of pathogens that urgently require novel treatments [20]. Additional therapeutic regimens have been proposed that include the use of vonoprazan, furazolidone, rifabutin, fluoroquinolones, and probiotics-containing treatments [12,13,15,21,22]. Recent works suggest that therapies against Hp should be adapted to local antibiotic resistances, and the Maastrich V/Florence consensus report recommended, after failure of second-line treatment, culture with susceptibility testing or molecular determination of genotype resistance [13,15,21,22,23]. While prophylactic or therapeutic vaccines for Hp have been investigated, no vaccine has been developed yet, probably because of high Hp genetic variability together with the fact that the infection downregulates the host’s immune response which highlights the importance of selecting Hp antigens and adjuvants capable of triggering a strong host immune reaction [24,25]. Several novel therapeutic strategies for the treatment of Hp infection have been suggested including phototherapy and the use of antimicrobial peptides, gastric mucins, polysaccharides or bioactive compounds [24]. Related to the use of novel bioactive compounds, key Hp gene products have been proposed for directed therapies [26]. One of them is flavodoxin [27,28], a small electron transfer protein involved in an essential Hp metabolic pathway. Flavodoxin is also expressed in other gastrointestinal pathogens and also in human gut commensal bacteria. As it is essential for some commensal bacteria [29], it is important to develop flavodoxin-based therapies that are not harmful to these microorganisms in order to avoid side effects on the gastrointestinal microbiota. On the other hand, as flavodoxin is also essential for several gastrointestinal pathogens, this protein constitutes a useful target for developing specific treatments against them.
In this review, we compile and discuss Hp proteins that may act as potential targets with a special focus on the properties of flavodoxin that make it a promising therapeutic target for treating this infection. We then summarize ongoing efforts to develop Hp-specific flavodoxin inhibitors, and, finally, we discuss the possibility of extrapolating them to target the flavodoxins of other gastric pathogens for the treatment of the corresponding infections.
2. Targets for Hp Infection
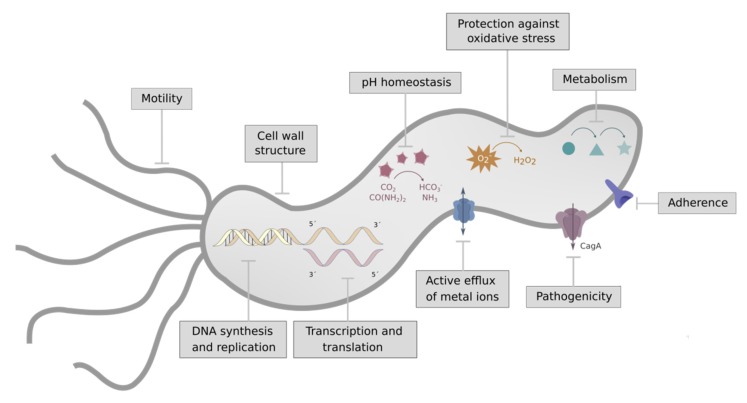
Because resistance to currently used antibiotics in Hp infection is widespread, new antimicrobials targeting bacterial functions different from the classically targeted ones (e.g., cell wall integrity, nucleic acid synthesis and replication, or transcription and translation) are required. The new targets must be essential for bacterial survival or important factors for colonization or virulence, and they should be absent in humans so that toxicity risk is minimized. Complying with those requisites, several Hp pathways (Figure 1) have been proposed for the development of new drugs. Some of them are detailed in Table 1, and the more relevant ones are described below.
Figure 1.
Pathways providing therapeutic targets to fight Helicobacter pylori infection.
Table 1.
Targets for Hp infection treatment.
| Target | Pathway | Reference |
|---|---|---|
| Metabolism | ||
| Type II 3-dehydroquinase dehydratase (DHQ2) | Shikimate pathway | [30,31,32,33] |
| Shikimate 5-dehydrogenase (SDHase) | Shikimate pathway | [30,33] |
| Shikimate kinase (SK) | Shikimate pathway | [30,33,34] |
| Chorismate synthase | Shikimate pathway | [30,33] |
| Phosphopantetheine adenylyltransferase (PPAT) | Coenzyme A biosynthesis | [35,36] |
| Carbon starvation protein A | Starvation response, utilization of peptides, and host–pathogen interactions | [37] |
| Methylthiotransferase (MiaB) | Protein synthesis | [37] |
| Ribosomal RNA small subunit methyltransferase E | Protein synthesis | [37] |
| Ribosomal protein L11 methyltransferase | Protein synthesis | [37] |
| Tetrapyrrole (Corrin-Porphyrin) methylase family protein | Protein synthesis | [37] |
| Peptide chain release factor 1 | Protein synthesis | [37] |
| Fumarate reductase (FrdA, FrdB, and FrdC) | Krebs cycle and anaerobic respiration | [33,38] |
| Glu-tRNAGln amidotransferase, subunits A (GatA), B (GatB), and C (GatC) | Protein synthesis | [26,33] |
| Helicase-nuclease DNA Repair Enzymes (AddAB) | DNA damage reparation | [39,40] |
| Cytochrome C-type biogenesis protein CcdA | Cytochrome C synthesis | [37] |
| Cytochrome C oxidase, subunits CcoN, CcoO, CcoP and CcoQ | ATP synthesis | [37] |
| Flavodoxin (Fld) | Oxidative decarboxylation of pyruvate | [28,41,42,43] |
| Pyruvate:ferredoxin oxidoreductase (POR), subunit α (porA), β (porB), ϒ (porC or porG) and δ (porD) | Oxidative decarboxylation of pyruvate | [26,33,44,45,46] |
| Flavodoxin:quinone reductase (FqrB) | Oxidative decarboxylation of pyruvate | [33,42,46] |
| 2-oxoglutarate:acceptor oxidoreductase, subunits A (OorA), B (OorB), C (OorC) and D (OorD) | Succinyl-CoA production | [26,33,45] |
| Cell Wall Structure | ||
| N-succinyl-L,L-diaminopimelic acid desuccinylase, SDAP-deacylase (DapE) | Succinylase pathway (lysine biosynthesis) | [17,47,48] |
| Glutamate racemase MurI | Peptidoglycan biosynthesis | [49,50] |
| Multi-drug resistance protein MsbA | Lipopolysaccharide biosynthesis | [51] |
| UDP-galactose 4-epimerase (GalE) | Lipopolysaccharide biosynthesis | [52] |
| pH Homeostasis | ||
| Urease, subunits α (UreA), and β (UreB) | Acclimation to low pH | [33,53,54,55,56,57,58] |
| Nickel-responsive regulator (NikR) | Urease expression and nickel uptake regulator | [53,54] |
| Nickel–cobalt transporter (NixA) | Nickel/cobalt transport | [54,59,60] |
| Urease accessory protein UreE | Urease maturation | [33,54,59,61,62,63] |
| Urease accessory protein UreF | Urease maturation | [54,61,62] |
| Urease accessory protein UreG | Urease maturation | [33,54,62,63] |
| Urease accessory protein UreH | Urease maturation | [54,61] |
| Hydrogenase/urease maturation factor (HypA) | Urease maturation | [61,63] |
| Hydrogenase/urease maturation factor (HypB) | Urease maturation | [61,63] |
| Heat Shock Protein A (HspA) | Nickel homeostasis | [59,64,65] |
| Hpn | Nickel homeostasis and storage | [59,61,64] |
| Acid-activated urea channel (UreI) | Urea permeability | [33,66] |
| α-carbonic anhydrase | Acclimation to low pH | [33,67,68,69,70,71] |
| β-carbonic anhydrase | Acclimation to low pH | [33,67,68,69,71,72] |
| Virulence (Adherence, Motility and Pathogenicity) | ||
| Spore coat polysaccharide biosynthesis protein C (PseC) | Pseudaminic acid biosynthesis pathway (Pse): flagellin glycosylation | [33,73,74] |
| Heat-inducible transcription repressor (HrcA) | Flagella biosynthesis | [37] |
| Transcriptional repressor of DnaK operon (HspR) | Flagella biosynthesis | [37] |
| Major flagellin FlaA | Flagellar filament composition | [33,53,75,76,77] |
| Minor flagellin FlaB | Flagellar filament composition | [53,75,76,77] |
| Flagellar hook-associated protein 2 (FliD) | Flagellum assembly (filament capping) | [33,76,77] |
| Flagellar hook-associated protein 1 (FlgK) | Flagellum assembly (hook-filament junction formation) | [33,76,77,78] |
| ATP-binding protein (YlxH) | Flagella biosynthesis | [33,79] |
| Flagellar basal body L-ring protein (FlgH) | Flagellum assembly (L-ring composition) | [33,77] |
| Flagellar basal body P-ring protein (FlgI) | Flagellum assembly (P-ring composition) | [33,77] |
| Flagellar basal body M-ring protein (FliF) | Flagellum assembly (MS ring composition) | [33,77] |
| Flagellar biosynthetic protein (FliP) | Flagellum assembly (Flagellar export component) | [33,60,77] |
| Flagellar biosynthetic protein (FliQ) | Flagellum assembly (Flagellar export component) | [33,77] |
| Flagellar motor switch protein (FliY) | Flagellum assembly (C-ring composition; Flagellar export component) | [33,77] |
| Flagellum-specific ATP synthase (FliI) | Flagellum assembly (Flagellar export component) | [33,77] |
| Flagella-specific σ factor (FliA) | Flagellum assembly (regulatory protein) | [53,77] |
| FlgM (putative antagonist of FliA) | Flagellum assembly (regulatory protein) | [53,77] |
| Cytotoxin-associated gene A (CagA) | cag pathogenicity island (host cell metabolism modulation, inflammation, metaplasia and precancerous transformations) | [80,81,82,83] |
| cag-Type IV secretion system (T4SS) | cag pathogenicity island (translocation of bacterial factors (e.g., Cag A and peptidoglycan) into host cells) | [80,83] |
| HopQ adhesin (outer membrane protein) | Adhesion to host cells and translocation of CagA into host cells | [84] |
| Vacuolating cytotoxin (VacA) | Cellular vacuolation, apoptosis and inhibition of cell cycle progression and host immune response | [81,82,85,86] |
| Blood group antigen binding adhesin (BabA) | Adhesion to host cells | [80,81,82] |
| High temperature requirement A (HtrA) | Chaperone and proteolytic activities (intercellular adhesion cleavage) | [37,85,87] |
| Sialic acid-binding adhesin (SabA) (outer membrane protein) | Bacterial migration to epithelium surface | [76,88] |
| HopZ adhesin (outer membrane protein) | Adhesion to host cells | [76,80,85] |
| OipA adhesin (outer membrane protein) | Adhesion to host cells | [76,80,85] |
| AlpA/B adhesin (outer membrane protein) | Adhesion to host cells | [76,80,85] |
| Active Efflux of Metal Ions | ||
| Cation efflux system protein CusA | Efflux of cobalt/zinc/cadmium | [37] |
| Cobalt/Zinc/Cadmium efflux system membrane fusion protein | Efflux of cobalt/zinc/cadmium | [37] |
| Cobalt/Zinc/Cadmium resistance protein (CzcA, CzcB and CzcC) | Efflux of cobalt/zinc/cadmium | [37,89] |
| CznABC metal efflux pump | Efflux of cadmium/zinc/nickel | [89] |
| Ferrix siderophore transport system TonB periplasmic binding protein | Iron transport | [37] |
| Ferric siderophore transport system ExbB biopolymer transport protein | Iron transport | [37,60] |
| Haemin uptake system ATP binding protein | Iron transport | [37] |
| Protection Against Stress | ||
| Glutathionyl spermidine synthetase | Intracellular thiol redox balance regulation | [37] |
| Iron-binding ferritin-like antioxidant protein | Prevention of toxic reactive species formation | [37] |
| DNA-binding protein Dps | DNA breaking protection | [37] |
| Superoxide dismutase | Superoxide dismutation | [37] |
| Thioredoxin reductase | Prevention of toxic reactive species formation | [37] |
| RNA polymerase σ54 factor | Survival under stress conditions | [33,90] |
| Multi-drug resistance protein MsbA | Efflux of hydrophobic drugs | [33,51] |
| Exodeoxyribonuclease (LexA) | SOS response activation | [33,91] |
| Homeostatic stress regulator (HsrA) | Regulation of gene expression | [92,93,94,95] |
2.1. Metabolism
The shikimic acid pathway uses erythrose-4-phosphate and phosphoenol pyruvate to produce chorismic acid, the precursor of aromatic amino acids, folate cofactors, ubiquinone, and vitamins E and K. This biosynthetic route involves four Hp essential enzymes that are absent in mammals: 3-dehydroquinate dehydratase, shikimate dehydrogenase, shikimate kinase, and chorismate synthase [30,31,32,34]. The biosynthesis of coenzyme A (CoA), an essential bacterial cofactor, is achieved with participation of phosphopantetheine adenylyltransferase (PPAT), the inactivation of which prevents bacterial viability [35,36]. Fumarate reductase is a key enzyme for aerobic and anaerobic respiration which contains three subunits: FrdA, FrdB, and FrdC. Some fumarate reductase inhibitors used to treat helmintic infection have also shown inhibitory and bactericidal properties against Hp [33,38]. Several other Hp enzymes have been associated with bacterial respiration, rendering them critical for bacterial survival. Some of them: cytochrome c-type biogenesis protein CcdA and cytochrome c oxidase subunits CcoN, CcoO, CcoP, and CcoQ have been described as potential drug targets for Hp infection [37]. Enzymes and electron carrier proteins that take part in pyruvate decarboxylation (such as flavodoxin (Fld), pyruvate:flavodoxin oxidoreductase (POR), and flavodoxin:quinone reductase B (FqrB)) have also been identified as essential proteins for Hp survival [26,28,41,42,43,44,45,46].
2.2. Cell Wall Structure
Peptidoglycan, synthesized by Mur enzymes in a multistep pathway, is an essential component of the bacterial cell wall. MurA, acting on the first step, is targeted by Fosfomycin [50]. For glutamate racemase MurI, transforming L-Glu into D-Glu, several inhibitors have been described [49,50]. The widely used β-lactam antibiotics inhibit peptidoglycan cross-linking which is carried out in the last steps of the pathway [17,49,50]. On the other hand, the succinylase pathway is the only route in Hp for the synthesis of lysine, a required element of the bacterial peptidoglycan cell wall. N-succinyl-L,L-diaminopimelic acid desuccinylase (SDAP-deacylase; DapE), an enzyme of this route, has been identified as essential for Hp survival [47,48].
2.3. pH Homeostasis
Helicobacter pylori is able to survive the acidic pH in the stomach thanks to, at least, two enzymes playing a fundamental role in acid acclimation. Urease and carbonic anhydrase maintain neutral pH in the bacterial cytoplasm and periplasm by converting urea and carbon dioxide into ammonia and bicarbonate [54,55,67]. Urease, which is absent in humans, is a critical enzyme for Hp colonization of the host stomach. It is composed of α (UreA) and β (UreB) subunits and its activity requires Ni (II) ions [53,54,56]. The putative nickel-responsive regulator (NikR) regulates urease expression and nickel uptake [54]. This metal goes into the cytoplasm, where urease is localized, through the nickel–cobalt transporter NixA [54,59], and its incorporation to the active sites of urease taking place during enzyme maturation depending on UreD, UreE, UreF, UreG, and UreH accessory proteins [54,59,61,62,63] and on the HypA and HypB hydrogenase/urease maturation factors [61,63]. Conversely, HspA and Hpn proteins are related to nickel homeostasis, storage, and protection from higher concentrations of metal ions [59,61,64]. The access of urea to urease is restricted by an H+-gated pore (UreI) which regulates the urea entry into the cytoplasmic space [66]. The activity of these proteins is needed for urease function and, thus, for Hp colonization, and some of them (specifically, urease and HspA) can be inhibited by bismuth, an antimicrobial currently used in therapy [59]. On the other hand, two different types of carbonic anhydrase, α- and β-, have been identified in Hp periplasm and cytoplasm, respectively. Both metalloenzymes have been described to be essential for acid acclimation, biosynthetic reactions, bacterial survival, and colonization of the stomach and duodenum [67,68,70,71,72]. They are targeted by sulphonamide antimicrobial agents and phenol-derivatives [68,69,71].
2.4. Virulence (Adherence, Motility, and Pathogenicity)
Helicobacter pylori has developed structures and mechanisms contributing to bacterial virulence. Among them are adhesins, pili, flagella, and extracellular polymeric matrix materials such as DNA, polysaccharides, proteins, and lipids [96]. Biofilms are seen as virulence factors, and several mucoactive and antibiofilm substances, such as N-acetylcysteine and erdosteine, have been proposed as new adjuvant agents for therapy [96,97,98]. Motility, a crucial virulence factor needed for persistent Hp infection, is provided by flagella which allow bacteria to travel through the mucus layer from the gastric lumen to the epithelial surface, its site of infection. Flagellar filaments consist of the major (FlaA) and the minor (FlaB) flagellin proteins [53,75,76,78]. Additional related genes, fliD and flgK, are required for flagellar filaments assembly and flagella formation [76,77,78]. For flagellar assembly and motility, flagellin needs to be O-glycosylated with pseudaminic acid (Pse). Thus, the Pse biosynthesis pathway and, in particular, the aminotransferase enzyme PseC have potential as targets [73,74]. Severe complications of bacterial infection have been related to Hp adherence to host cells. Secretion of virulence factors, such as those encoded in the cytotoxin-associated gene pathogenicity island cagPAI (CagA: the cytotoxin-associated antigen, and T4SS: the cag-type IV secretion system), the vacuolating cytotoxin VacA, or the blood group antigen-binding adhesin BabA, have been related to increased epithelial damage and predisposition to gastric carcinogenesis [82]. In particular, BabA enables bacterial contact with the stomach mucosa. Then, VacA delivery and T4SS signaling are induced: VacA leads to membrane pore formation, cellular vacuolation, apoptosis, and inhibition of immune cells [85,86], while the T4SS pathway translocates CagA into host epithelial cells, where it modulates aspects of the host metabolism and provokes inflammation, metaplasia, and neoplastic transformations [80,81,82,83,86]. The adhesin BabA has been associated to disease-related strains and cagPAI and VacA have been linked to increased gastric cancer risk [81,86]. On the other hand, the T4SS route is favored by interaction between the adhesin HopQ and the human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) [84]. In addition to those indicated above, other virulence factors have been identified in Hp which include adhesins, such as HopZ, OipA, SabA, and AlpA/B [76,80,85], and the HtrA serine protease, an essential periplasmic protein with chaperone and proteolytic activities involved in quality control and stress responses [85]. Specifically, HtrA is involved in the cleavage of the tumor suppressor E-cadherin and so in the disruption of intercellular adhesion and access of bacteria to intracellular spaces [85,87,99,100].
2.5. Active Efflux of Metal Ions
The levels of cobalt, zinc, cadmium, and iron need to be regulated in Hp as both too low and too high concentrations can be detrimental for bacterial life. Thus, enzymes that control metal levels (such as cation efflux system protein CusA, cobalt/zinc/cadmium efflux system membrane fusion protein, cobalt/zinc/cadmium resistance proteins CzcA, CzcB, and CzcC, CznABC metal efflux pump, ferrix siderophore transport system TonB periplasmic binding protein, ferric siderophore transport system ExbB biopolymer transport protein, and Haemin uptake system ATP binding protein) are crucial for Hp virulence and adaptation to gastric environment and, therefore, for bacterial survival [37,89].
2.6. Protection against Oxidative Stress
Toxic reactive species can cause oxidative stress to Hp, leading to cell death. For this reason, bacterial gene products involved in protection against reactive oxygen species, superoxide, and free radicals have been proposed as therapeutic targets. They include glutathionyl spermidine synthetase, iron-binding ferritin-like antioxidant protein, DNA-binding protein Dps, and superoxide dismutase [37]. Homeostatic stress regulator (HsrA) is an orphan response regulator unique among epsilonproteobacteria. It syncs metabolic functions and virulence with availability of nutrients and cell division. This protein regulates its own expression and that of a large number of genes involved in transcription, translation, energy, and nitrogen metabolism as well as redox homeostasis and oxidative stress defense. Due to the fact of its essentiality, its absence in humans and the availability of an X-ray structure, it has been proposed as a promising therapeutic target against Hp [92,93,94,95].
As can be seen, several Hp gene products have been described that may constitute appropriate targets for the development of novel therapies against this bacterial infection. Among them, flavodoxin stands out as a promising candidate due to the fact of its essentiality for Hp, its absence in humans, and because of structural features that will be discussed below. Stimulated by these facts, our laboratory has undergone several studies aimed at understanding the biophysical properties of Hp flavodoxin and to identify and perfect molecules that could bind to it and interfere with its vital function. The following sections include a detailed description of this flavodoxin, focusing on the properties that make it a fine drug target.
3. An Overview of Flavodoxins and of the Flavodoxin from Hp
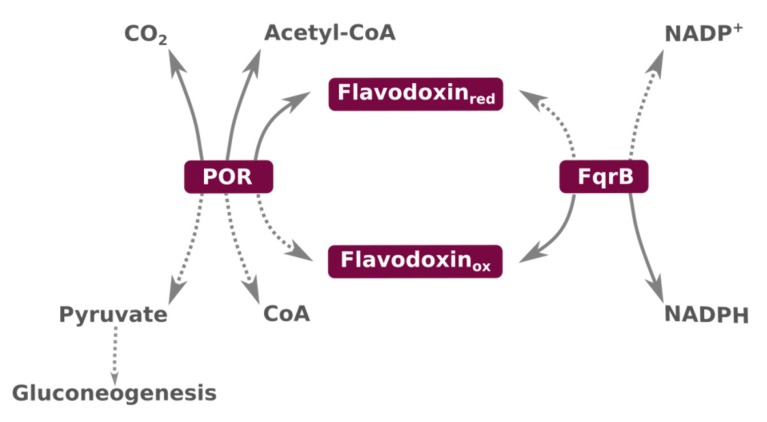
Flavodoxins are acidic proteins that contain a flavin cofactor (flavin mononucleotide, FMN) acting as an electron transfer center [27]. They are small (14.5–23 kDa) α/β proteins with five α-helices packing against a central five-stranded β-sheet, thus forming an αβα sandwich [27,101]. In some bacteria (e.g., Escherichia coli, Azotobacter vinelandii, or Desulfovibrio vulgaris), flavodoxins are constitutive proteins, while in others, such as in several Anabaena strains, flavodoxin synthesis is induced in low iron conditions, where it replaces ferredoxin [102,103,104,105,106,107], a constitutive sulfoferric protein that transfers electrons one by one. Although the FMN in flavodoxin only participates in two-electron transfer reactions when it is free in solution, apoflavodoxins modify the redox potentials of FMN molecules bound to them so that they can accept and donate electrons one by one [27]. Flavodoxins transfer electrons among different partner proteins. In some photosynthetic and/or N2-fixing bacteria, flavodoxin shuttles electrons from PSI to NADP+ or N2 via FNR and nitrogenase, respectively [27,108,109]. In an analogous fashion, flavodoxin donates electrons to a variety of partner proteins in different bacteria to perform biosynthetic reactions. For example, flavodoxin activates cobalamin-dependent methionine synthase, pyruvate formate-lyase, and anaerobic ribonucleotide reductase in Escherichia coli [110,111,112,113], as well as biotin synthase in Escherichia coli [111,112] and Bacillus subtilis [114]. Flavodoxin is also involved in nitrate reduction in Azotobacter vinelandii [115] and in the activation of pyruvate formate-lyase by free radicals formation [116]. On the other hand, flavodoxin has been found to function as an electron acceptor of the pyruvate oxidoreductase enzyme complex (POR) which catalyzes the oxidative decarboxylation of pyruvate in Hp [44] (Figure 2).
Figure 2.
The POR:FldA:FqrB pathway in H. pylori. Flavodoxin (Fld) shuttles electrons between pyruvate oxidoreductase complex (POR) and flavodoxin:quinone reductase (FqrB) in a reversible pathway which plays a central role in the bacterial metabolism, as it represents an essential route for CO2 fixation and pyruvate metabolism. The pyruvate decarboxylation pathway is represented by solid lines, whereas the pyruvate synthesis pathway (contributing to gluconeogenesis) is indicated by dotted lines. Adapted from Reference [46].
The FMN cofactor in flavodoxin is made of an isoalloxazine aromatic ring system connected to a phosphate group by a ribityl chain, and it appears tightly bound at the carboxy-terminal end of the flavodoxin β-sheet. The isoalloxazine moiety usually interacts with aromatic residues, while the phosphate group forms hydrogen bonds with mainly threonine side chains and several main chain NH groups of the protein [27,41]. Those interactions provide high stability to the apoflavodoxin FMN complex, which has a formation that begins by interaction of the isoalloxazin group with the folded apoprotein, followed by docking of the phosphate group [27,117]. The phosphate binding site has been described to be pre-formed in Anabaena PCC7119, Hp [118], and Streptococcus pneumoniae [119] apoflavodoxins, probably due to the presence of bound ions that mimic the phosphate group (Figure 3). From sequence alignment and structural considerations, flavodoxins can be divided into two groups: long-chain (18–23 kDa) (e.g., those in Anabaena PCC 7119 or Hp) and short-chain flavodoxins (14.5–17 kDa) (e.g., those in Clostridium beijerincki or Desulfovibrio vulgaris). Long-chain flavodoxins contain an extra 20 residue loop intercalated in the β5-strand of the β-sheet [27,120,121] that does not seem to be relevant for protein stability or folding. Its sequence conservation suggests it may play a functional role [120], and it has been suggested to be responsible for the recognition of FNR and methionine synthase in E. coli [122]. Despite their differences, long- and short-chain flavodoxins share a similar three-dimensional structure. In both families, sequence conservation is high at the isoalloxazine binding loops (often referred to as the Y- and W-loops), the phosphate binding loop (P-loop) and, in the long-chain ones, their characteristic long loop. In particular, the phosphate binding site is highly conserved, the consensus sequence being T/S-X-T-G-X-T [27,41,120]. On the other hand, the W- and Y-loops usually contain, respectively, Trp and Tyr residues that are involved in the binding of the isoalloxacin moiety in FMN. Some flavodoxins, nevertheless, contain other residues in those key FMN binding positions (e.g., the tryptophan residue of the W-loop appears replaced by methionine in Clostridium beijerinckii flavodoxin, by leucine in Azobacter vinelandii or by alanine in Hp) [28,41,123].
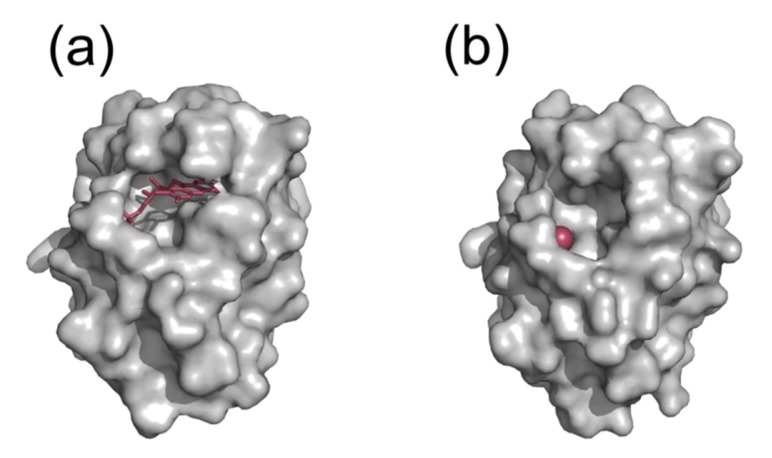
Figure 3.
Molecular surface representation of holo (a) and apo (b) flavodoxin from Hp. FMN cofactor and a chloride ion bound at the FMN phosphate site are shown as red sticks and a sphere, respectively. The two structures are similar and exhibit an unusual pocket close to the cofactor binding site. Most other (apo)flavodoxins lack such surface pocket.
The flavodoxin from Hp (Hp-Fld) is involved in a metabolic pathway essential for Hp viability: the oxidative decarboxylation of pyruvate by the pyruvate oxidoreductase complex (POR) [27,44,45,124]. Flavodoxin synthesis in Hp is constitutive and detectable even in dormant forms of Hp which have a significantly reduced metabolic activity [125]. Hp flavodoxin has been related to low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma pathogenesis, and antibodies against this flavodoxin have been found in patients [126]. In anaerobic conditions, flavodoxin is able to activate imidazole antimicrobials (such as metronidazole) [124,127] and transform them into reactive intermediates that cause DNA lethal damage [124,127,128]. Mutations in ferredoxin (FdxA), ferredoxin-like protein (FdxB), NAD(P)H flavinnitroreductase (FrxA), oxygen-insensitive NAD(P)H nitroreductase (RdxA), flavodoxin (FldA), the γ-subunit of 2-oxoglutarate oxidoreductase (OorD) or the γ-subunit of pyruvate ferredoxin oxidoreductase (PorD) have been related to metronidazole resistance in Hp [129,130]. Hp flavodoxin is encoded by the fldA gene [41,131], and its 164 residue amino acid sequence is similar to that of other flavodoxins, especially long-chain ones (sequence identities approximately 40%). Sequence differences are noticed at the cofactor binding site, specifically at the phosphate loop which, in Hp, is slightly different (T-D-S-G-N-A) from the general flavodoxin motif (T/S-X-T-G-X-T) [41]. The Hp-Fld structure is similar to that of other known flavodoxins, although the presence of some shorter loops and of an elevated percentage of small side-chain residues makes it slightly more compact [41]. Interestingly, in Hp flavodoxin, a bulky residue located in the W-loop of most flavodoxins (typically a Trp residue), appears replaced by an alanine residue (position 55). This substitution, which lowers the affinity for FMN [41], opens a pocket at the protein surface, near the bound cofactor [41,132], where small organic compounds could bind and inhibit complex formation or electron transfer reactions with partner proteins.
As Hp lacks several essential genes of the glycolysis pathway, pyruvate formation through carbon dioxide fixation may be physiologically favored, because it is the single gluconeogenic pathway in this bacterium [46]. On the other hand, oxidative decarboxylation of pyruvate is a fundamental reaction catalyzed by the pyruvate dehydrogenase complex in most aerobic organisms or by POR in anaerobic ones [133,134]. Thus, Hp POR catalyzes the last step of carbohydrates fermentation as well as the inverse pyruvate oxidative decarboxylation [135]. In this bidirectional electron transfer pathway, another essential enzyme, flavodoxin quinone reductase B (Figure 2), is involved [46].
4. Flavodoxins in other Pathogenic Bacteria and in the Gut Microbiota
As flavodoxin is present in gastrointestinal pathogens other than Hp, the potential of flavodoxin inhibitor-based therapies against those additional pathogens should be explored, especially in the cases of pathogens for which an essential flavodoxin has been described. On the other hand, flavodoxin is also present in a variety of gut commensal bacteria and the possible negative side effects of Hp flavodoxin inhibitors on the human microbiota should be evaluated too.
Indeed, flavodoxin genes are present in many bacteria (mainly Gram-negative ones), especially in Proteobacteria, Cyanobacteria, Aquificae, Firmicutes, Bacteroidetes, Fusobacteria, and Spirochaetes. We have collected and combined in Table 2 flavodoxin information available in the Uniprot database [136] (searching for “flavodoxin” and refining by “reviewed”), in the NCBI database [137] (searching for “flavodoxin” on the “Protein” tab, then refining by “Bacteria” on the species tag, selecting on the source databases’ tag “PDB” and “UniProtKB/Swiss-Prot”, and specifying on the sequence length’s tag from 130 to 199 residues), and in the flavodoxin-related literature available in PubMed [137].
Table 2.
Some flavodoxin-containing bacteria a.
| Microorganism | Protein Name | Gene Name | Seq. Length | Long/short Chain | Phylum | Gram Stain |
|---|---|---|---|---|---|---|
| Anabaena (Nostoc) sp. | Flavodoxin | isiB | 170 | Long | Cyanobacteria | Negative |
| Aquifex aeolicus | Flavodoxin | fldA | 185 | Long | Aquificae | Negative |
| Azotobacter vinelandii | Flavodoxin 1 | Avin45950 | 174 | Long | Proteobacteria | Negative |
| Azotobacter vinelandii | Flavodoxin 2 | nifF | 180 | Long | Proteobacteria | Negative |
| Azotobacter chroococcum | Flavodoxin B | nifF | 180 | Long | Proteobacteria | Negative |
| Bacillus cereus | Flavodoxin | BC_1376 | 148 | Short | Firmicutes | Positive |
| Bacillus cereus | Flavodoxin | BC_3541 | 154 | Short | Firmicutes | Positive |
| Bacillus subtilis | Probable flavodoxin 2 | ykuP | 151 | Short | Firmicutes | Positive |
| Bacillus subtilis | Probable flavodoxin 1 | ykuN | 158 | Short | Firmicutes | Positive |
| Bacteroides uniformis | Flavodoxin | BACUNI_04544 | 178 | Long | Bacteroidetes | Negative |
| Buchnera aphidicola | Flavodoxin | fldA BUsg_289_ | 154 | Long b | Proteobacteria | Negative |
| Buchnera aphidicola | Flavodoxin | fldA BU299 | 171 | Long | Proteobacteria | Negative |
| Buchnera aphidicola | Flavodoxin | fldA bbp_277 | 174 | Long | Proteobacteria | Negative |
| Campylobacter jejuni | Flavodoxin | fldA | 163 | Long | Proteobacteria | Negative |
| Clostridium beijerinckii c | Flavodoxin | 138 | Short | Firmicutes | Positive | |
| Clostridium pasteurianum | Flavodoxin | CLPA_c13840 d | 140 | Short | Firmicutes | Positive |
| Clostridium saccharobutylicum | Flavodoxin | floX | 160 | Long | Firmicutes | Positive |
| Desulfovibrio desulfuricans | Flavodoxin | Ddes_1951 | 148 | Short | Proteobacteria | Negative |
| Desulfovibrio gigas c | Flavodoxin | 146 | Short | Proteobacteria | Negative | |
| Desulfovibrio gigas c | Flavodoxin | 147 | Short | Proteobacteria | Negative | |
| Desulfovibrio salexigens | Flavodoxin | Desal_0805 | 146 | Short | Proteobacteria | Negative |
| Desulfovibrio vulgaris | Flavodoxin | DVU_2680/DvMF_1143 | 148 | Short | Proteobacteria | Negative |
| Escherichia coli | Protein MioC | mioC | 147 | Short | Proteobacteria | Negative |
| Escherichia coli | Uncharacterized protein YqcA | yqcA | 149 | Short | Proteobacteria | Negative |
| Escherichia coli | Flavodoxin 2 | fldB | 173 | Long | Proteobacteria | Negative |
| Escherichia coli | Flavodoxin 1 | fldA | 176 | Long | Proteobacteria | Negative |
| Fusobacterium nucleatum | Flavodoxin | FN0724 | 167 | Long | Fusobacteria | Negative |
| Haemophilus influenzae | Protein MioC homolog | mioC | 146 | Short | Proteobacteria | Negative |
| Haemophilus influenzae | Flavodoxin | fldA | 174 | Long | Proteobacteria | Negative |
| Helicobacter pylori | Flavodoxin | fldA | 164 | Long | Proteobacteria | Negative |
| Klebsiella pneumoniae | Flavodoxin | fldA/nifF | 176 | Long | Proteobacteria | Negative |
| Lactobacillus reuteri | Flavodoxin/nitric oxide synthase | Lreu_1727 | 149 | Short | Firmicutes | Positive |
| Listeria monocytogenes | Lmo2153 protein | lmo2153 | 145 | Short | Firmicutes | Positive |
| Megasphaera elsdenii(Peptostreptococcus elsdenii) c | Flavodoxin | 137 | Short | Firmicutes | Negative | |
| Pantoea agglomerans (Enterobacter agglomerans) | Flavodoxin | nifF | 177 | Long | Proteobacteria | Negative |
| Pasteurella multocida | Protein mioC homolog | mioC | 147 | Short | Proteobacteria | Negative |
| Pectobacterium carotovorum c | Exoenzyme regulation regulon ORF2 | 151 | Short | Proteobacteria | Negative | |
| Pseudomonas aeruginosa | Uncharacterized protein PA3435 | PA3435 | 150 | Short | Proteobacteria | Negative |
| Pseudomonas aeruginosa | Flavodoxin FldP | fldP | 184 | Long | Proteobacteria | Negative |
| Pseudomonas putida | Flavodoxin | mioC | 151 | Short | Proteobacteria | Negative |
| Rhodobacter capsulatus | Flavodoxin | nifF | 182 | Long | Proteobacteria | Negative |
| Salmonella Typhi | Flavodoxin 2 | fldB | 173 | Long | Proteobacteria | Negative |
| Salmonella Typhi | Flavodoxin | fldA | 176 | Long | Proteobacteria | Negative |
| Salmonella Typhimurium | Flavodoxin 2 | fldB | 173 | Long | Proteobacteria | Negative |
| Salmonella Typhimurium | Flavodoxin 1 | fldA | 176 | Long | Proteobacteria | Negative |
| Shewanella oneidensis | Flavodoxin Protein MioC | mioC | 146 | Short | Proteobacteria | Negative |
| Shewanella oneidensis | tRNA pseudouridine synthase C-associated flavoprotein YqcA | yqcA | 154 | Short | Proteobacteria | Negative |
| Shewanella oneidensis | Flavodoxin | fldA | 175 | Long | Proteobacteria | Negative |
| Shigella flexneri | Uncharacterized protein YqcA | yqcA | 149 | Short | Proteobacteria | Negative |
| Shigella flexneri | Flavodoxin 1 | fldA | 176 | Long | Proteobacteria | Negative |
| Streptococcus agalactiae e | Flavodoxin | mioC | 147 | Short | Firmicutes | Positive |
| Streptococcus pneumoniae | Flavodoxin | flaV | 147 | Short | Firmicutes | Positive |
| Synechococcus sp. | Flavodoxin | isiB | 170 | Long | Cyanobacteria | Negative |
| Synechocystis sp. | Flavodoxin | isiB | 170 | Long | Cyanobacteria | Negative |
| Treponema pallidum | Flavodoxin | fldA | 146 | Short | Spirochaetes | Negative f |
| Trichodesmium erythraeum | Flavodoxin | fld | 171 | Long | Cyanobacteria | Negative |
| Vibrio cholerae | Protein MioC homolog | mioC | 144 | Short | Proteobacteria | Negative |
| Vibrio cholerae | Flavodoxin | fld1 | 175 | Long | Proteobacteria | Negative |
| Vibrio cholerae | Flavodoxin | fld2 | 198 | Long | Proteobacteria | Negative |
| Wolinella succinogenes | Flavodoxin | fldA | 171 | Long | Proteobacteria | Negative |
a Extracted from Uniprot by searching for “flavodoxin” and refining by “reviewed”, from NCBI by searching for “flavodoxin” in the “Protein” tab and refining by “Bacteria” (in the species tag), “PDB and UniProtKB/Swiss-Prot” (in the source databases’ tag) and “from 130 to 199 residues” (in the sequence length’s tag) and from References [114,119,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159]. Despite the fact that there is a great deal of unreviewed flavodoxin sequences in Uniprot, we chose to include only those that we found as described, which were flavodoxins with an existence that appeared to be firmly established. b Although the length of this sequence is more typical of short-chain flavodoxins, we classified it here as long-chain due to the absence, in sequence alignment with long chain-flavodoxins, of the characteristic 20 residue gap formed in so-aligned short-chain sequences. c Unnamed gene (gene name not reported yet. Alternative sequences may be reported elsewhere). d Although the isolation of a 148 residue flavodoxin from Clostridium pasteurianum has been reported [157,159], we did not find any sequences of such length in Uniprot. On the other hand, the sequence reported in those papers was not complete. Among the sequences in Uniprot, the one which is 140 residues in length (gene name CLPA_c13840) has the highest identity in sequence with the partial sequences reported. e Extracted from the DEG database [160]. This sequence was not identified by following the search pathway used for the rest of the sequences reported in the table. f Its Gram stain classification has been controversial [161,162].
Most of these flavodoxins appear in Proteobacteria, and some of them are known to be essential for bacterial viability. Essential flavodoxins annotated in the DEG database [160] and retrieved by searching for “flavodoxin” by function in the bacteria’s tab are shown in Table 3.
Table 3.
Bacteria with flavodoxins that are essential for viability a.
| Microorganism | Sequence Length | Long/Short Chain | Phylum | Gram Stain |
|---|---|---|---|---|
| Campylobacter jejuni | 163 | Long | Proteobacteria | Negative |
| Escherichia coli | 176 | Long | Proteobacteria | Negative |
| Haemophilus influenzae | 174 | Long | Proteobacteria | Negative |
| Helicobacter pylori | 164 | Long | Proteobacteria | Negative |
| Salmonella Typhi | 176 | Long | Proteobacteria | Negative |
| Salmonella Typhimurium | 176 | Long | Proteobacteria | Negative |
| Shewanella oneidensis | 175 | Long | Proteobacteria | Negative |
| Streptococcus agalactiae | 147 | Short | Firmicutes | Positive |
| Vibrio cholerae | 175 | Long | Proteobacteria | Negative |
a Obtained from DEG database [160].
To anticipate the potential side effects on the human gastric microbiota associated to the use of flavodoxin-targeting therapies, we have revised the presence of flavodoxin in these commensal bacteria. Some of the key organisms present in the human gastric microbiota are shown in Table 4 classified by genus, phylum, gram staining, oxygen requirement, and (non)presence of flavodoxin(s). Essential flavodoxins are indicated, where appropriate. As shown in the table, a variety of bacterial genera from the human gastric microbiota have been identified as flavodoxin-expressing organisms. Particular care should be taken with these microorganisms in flavodoxin-based treatments, especially with Escherichiacoli, Haemophilus influenzae, and Streptococcus agalactiae which have flavodoxins that are essential for bacterial viability. Besides, the flavodoxins from the first two ones share a high percentage of identity (above 40%) with Hp-Fld (Figure A1, Appendix A). As sequence identity levels higher than 35–40% usually involve substantial structural similarity [163], it is important to ensure that compounds developed against Hp flavodoxin are not able to kill these microorganisms at the minimal inhibitory concentrations (MIC) determined for Hp. On the other hand, it has been proposed that flavodoxin-inhibitors could interact with the protein near its cofactor binding site and then suppress flavodoxin function by modification of the cofactor redox potential or by steric blockage of the interaction between the flavodoxin and its redox partners [28]. Therefore, if Hp-Fld inhibitors bind the protein at the pocket created by the presence of an alanine at position 55, no side effects on the human microbiota would be expected because E. coli and H. influenzae flavodoxins have a bulky tryptophan residue at this position and that of S. agalactiae contains an also bulky tyrosine residue. (Figure A1, Appendix A)
Table 4.
Flavodoxin in the main bacterial genera of the human gut microbiota a.
| Genus | Flavodoxin | Phylum | Gram Stain | Oxygen Requirement |
|---|---|---|---|---|
| Akkermansia | Unreviewed | Verrucomicrobia | Negative | Anaerobe |
| Alistipes | Unreviewed | Bacteroidetes | Negative | Anaerobe |
| Bacteriodes | Yes | Bacteroidetes | Negative | Anaerobe |
| Bifidobacterium | Unreviewed | Actinobacteria | Positive | Anaerobe |
| Clostridium | Yes | Firmicutes | Positive | Anaerobe |
| Eggerthella | Unreviewed | Actinobacteria | Positive | Anaerobe |
| Enterococcus | Unreviewed | Firmicutes | Positive | Facultative anaerobe |
| Escherichia | Yes b | Proteobacteria | Negative | Facultative anaerobe |
| Eubacterium | Unreviewed | Firmicutes | Positive | Anaerobe |
| Fusobacterium | Yes | Fusobacteria | Negative | Anaerobe |
| Haemophilus | Yes b | Proteobacteria | Negative | Facultative anaerobe |
| Lactobacillus | Yes | Firmicutes | Positive | Microaerophile |
| Neisseria | No | Proteobacteria | Negative | Aerobe |
| Odoribacter | Unreviewed | Bacteroidetes | Negative | Anaerobe |
| Parabacteroides | Unreviewed | Bacteroidetes | Negative | Anaerobe |
| Peptococcus | Unreviewed | Firmicutes | Positive | Anaerobe |
| Peptostreptococcus | Yes | Firmicutes | Positive | Anaerobe |
| Porphyromonas | Unreviewed | Bacteroidetes | Negative | Anaerobe |
| Prevotella | Unreviewed | Bacteroidetes | Negative | Anaerobe |
| Propionibacterium | Unreviewed | Actinobacteria | Positive | Anaerobe |
| Pseudomonas | Yes | Proteobacteria | Negative | Aerobe |
| Roseburia | Unreviewed | Firmicutes | Positive | Anaerobe |
| Rothia | Unreviewed | Actinobacteria | Positive | Anaerobe |
| Ruminococcus | Unreviewed | Firmicutes | Positive | Anaerobe |
| Staphylococcus | Unreviewed | Firmicutes | Positive | Facultative anaerobe |
| Streptococcus | Yes b | Firmicutes | Positive | Facultative anaerobe |
| Veillonella | Unreviewed | Firmicutes | Negative | Anaerobe |
a The information related to the bacterial composition of the human gut microbiota was extracted from References [164,165,166,167,168,169,170]. Unreviewed indicates the existence of flavodoxin sequences reported as such in Uniprot. No scientific literature about them has been found. b Essential flavodoxin according to the DEG database [160].
On the other hand, flavodoxin is present in gastrointestinal pathogens, for some of which it is an essential protein. In Table 5, major human stomach pathogens are classified by genus, phylum, gram staining, oxygen requirement, and flavodoxin expression (Yes/No). If flavodoxin is essential for their viability, it is indicated. While different bacteria included in this table (Firmicutes, Bacteroidetes, and Proteobacteria) express flavodoxin, it has been described so far as essential only for some Proteobacteria: Campylobacter, Escherichia, Helicobacter, Salmonella, and Vibrio. As shown in Figure A2 (Appendix A), sequence identities between Hp (strain J99) flavodoxin and those from Campylobacter jejuni (strain ATCC 700819), Vibrio cholerae (strain ATCC 39541), Escherichia coli (strain K12), Salmonella enterica subsp. enterica serovar Typhi (strain Ty2), and Salmonellaenterica subsp. enterica serovar Typhimurium (strain ATCC 700720) are around 40% which is high enough to assume these proteins will show a high structural similarity [163]. Nevertheless, the amino acid sequences of these five flavodoxins, having a tryptophan residue where Hp-Fld carries an alanine one, suggest their tridimensional structures will not display a pocket near the FMN cofactor, and they may not be affected by Hp-Fld inhibitors.
Table 5.
Flavodoxin in human gastrointestinal pathogens a.
| Genus | Flavodoxin | Phylum | Gram Stain | Oxygen Requirement |
|---|---|---|---|---|
| Bacillus | Yes | Firmicutes | Positive | Aerobe |
| Bacteroides | Yes | Bacteroidetes | Negative | Anaerobe |
| Campylobacter | Yes b | Proteobacteria | Negative | Microaerophile |
| Clostridium | Yes | Firmicutes | Positive | Anaerobe |
| Escherichia | Yes b | Proteobacteria | Negative | Facultative anaerobe |
| Helicobacter | Yes b | Proteobacteria | Negative | Microaerophile |
| Listeria | Yes | Firmicutes | Positive | Facultative anaerobe |
| Peptostreptococcus | Yes | Firmicutes | Positive | Anaerobe |
| Salmonella | Yes b | Proteobacteria | Negative | Facultative anaerobe |
| Shigella | Yes | Proteobacteria | Negative | Facultative anaerobe |
| Staphylococcus | Unreviewed | Firmicutes | Positive | Facultative anaerobe |
| Vibrio | Yes b | Proteobacteria | Negative | Facultative anaerobe |
| Yersinia | Unreviewed | Proteobacteria | Negative | Facultative anaerobe |
a The information related to the bacterial genera which cause gastrointestinal diseases was extracted from References [171,172,173,174,175,176,177,178,179,180,181,182,183]. Unreviewed indicates flavodoxin sequences reported as such in Uniprot. No scientific literature about them was found. b Essential flavodoxin according to the DEG database [160].
5. Discovery of Specific Inhibitors of Hp Flavodoxin Using an Approach that Can Be Transferred to Other Pathogens
The eradication rates achieved by the conventional triple and quadruple therapies used for treating Hp infection have been decreasing, so alternative treatments are needed to fight this prevailing infection. Among the different strategies available, development of inhibitors of essential Hp targets seems promising. Following this track, our group has focused on the identification and improvement of small molecule inhibitors to target the essential Hp flavodoxin. As explained above, Hp flavodoxin bears in its surface a peculiar pocket near the FMN binding site that could be exploited to identify molecules that bind there and block function by either modifying the redox potential of the protein or by sterically interfering with the recognition of partner proteins [28]. The Hp-Fld surface pocket is absent in other epsilonproteobacteria, such as Campylobacter jejuni or Wolinella succinogenes, that carry the characteristic Trp residue of the W-loop, or in other flavodoxins where the Trp residue has been replaced by leucine (e.g., in Azotobacter vinelandii [123]) or methionine (e.g., in Clostridium beijerinkii [41]). The pocket is not expected either to be present in several Helicobacter species such as Helicobacter hepaticus (unable to colonize the human gastric mucosa but associated with chronic hepatitis, liver adenocarcinoma in mice, cholecystitis and gallbladder human cancer) [184,185], where its flavodoxin carries a tyrosine replacing the Trp. However, the pocket probably appears in the flavodoxins from Helicobacter acinonychis (a bacteria establishing lifelong infections in the stomach of cheetah and other felines) [186] and Helicobacter felis (which has been related to the development of gastritis in humans) [187] as, similarly to Hp flavodoxin, they carry an alanine residue replacing the Trp. Thus, the pocket initially observed in Hp-Fld as formed by replacement of the bulky Trp residue at the W-loop by an Ala residue is an almost exclusive feature of Hp-Fld which can be used to develop highly selective inhibitors. A priori, such new inhibitors would not give rise to side effects in humans, because flavodoxin is not present in vertebrates. An alternative possibility to use flavodoxin as an antimicrobial target stems from the fact that redox proteins have been suggested to be capable of activating nitro-compounds, as it has been demonstrated for nitroimidazole drugs such as metronidazole. Reduction of nitro groups present in prodrugs could yield cytotoxic products which would be able to act as anti-Hp compounds [124,127,128,129,188,189,190,191,192].
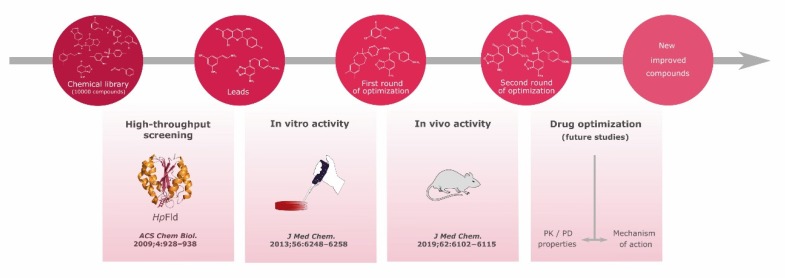
Our research group has been working on the identification and development of new compounds targeting Hp flavodoxin by following the alternative possibilities of blocking flavodoxin function using binding molecules and that of using compounds that could be activated by their conversion into reactive toxic species after reduction (Figure 4). First, small molecules, such as benzylamine, were identified as binders to Hp-Fld [28]. Due to the fact of their low binding affinity, they were poor inhibitors. A high-throughput screening method was subsequently implemented to identify them from a chemical library of other small organic molecules which could bind tighter to the protein. Thus, a 10,000 molecule chemical library was screened using pure recombinant Hp-Fld which led to the identification of 29 binding compounds that stabilized the protein as indicated by their capability to increase the temperature of mid-denaturation [42]. Four of those compounds (compounds I, II, III, and IV) were, in addition, able to inhibit the in vitro electron transfer between Fld and their recombinantly produced partner proteins POR and FqrB. Three of them (I, II, and IV) showed bactericidal activity against Hp cells and seemed to be selective for this bacterium. Inhibitors I and II exhibited therapeutic indexes (TI) of around 10, meaning their minimal cytotoxic concentrations (MCC) for eukaryotic cells were 10 times higher than the corresponding minimal inhibitory concentrations (MIC) for Hp. Nonetheless, compound IV showed lower TI due to the higher cytotoxicity and lower efficacy than molecules I and II. On the other hand, those three compounds (I, II, and IV) did not seem to be nephrotoxic or hepatotoxic when they were administered to mice at 10 mg/kg body weight, and they did not produce pathological changes in stomach, liver, heart, lung or kidney at 1 or 10 mg/kg body weight. In an attempt to obtain detailed structural information on the complexes formed by the inhibitors with the target, the interaction of compounds III (the only bacteriostatic compound of the four hits) and IV with Hp-Fld was studied by crystallography and NMR, respectively. Inhibitor III was able to replace FMN and establish hydrogen bonds and hydrophobic interactions with the protein through its nitro group and benzene ring, respectively. However, it was unclear whether the structure solved was showing the functional inhibitory interaction or rather the FMN replacement by the inhibitor occurred in a subsequent step. The NMR analysis indicated that compound IV also appeared to interact with flavodoxin through its nitro group which suggested a role for this functional group in the formation of the inhibitor–flavodoxin complex. Crystallization trials are underway to try to obtain the X-ray structure of the complex between flavodoxin and compound IV and derivatives of it.
Figure 4.
The steps followed and planned in the discovery of flavodoxin inhibitors as new therapies against Hp infection.
In a first round of optimization of the initial inhibitors, 102 new molecules related to inhibitors I, II, and IV were synthetized or acquired, and their toxicity and activity were tested [43]. Among them, 20 compounds were able to bind to flavodoxin with dissociation constants in the micromolar range, some of them with higher affinity than those of the initial hits. Most of these analogues inhibited bacterial growth in vitro and nine of them showed higher therapeutic indexes than those of their parent bactericidal compounds I, II, and IV. Large increases were observed in the therapeutic indexes of the new analogues of II and IV (up to 25 times for derivatives of II and up to 59 times for derivatives of IV). Six of these compounds were further tested, and it was confirmed that they kept a similar binding affinity to flavodoxin and that they also displayed bactericidal properties [43].
A second round of optimization was then carried out. To improve the therapeutic and pharmacokinetic properties of compounds I, II, and IV, new variants carrying modified redox forms of nitro, sulfur and vinyl groups of the lead-molecules were synthesized [193]. Derivatives that contain partially or fully reduced forms of the nitro and/or ethylene groups, or partially or fully oxidized forms of the sulfur atom displayed a considerably lower toxicity against HeLa cells and mice than the corresponding leads. While the therapeutic indexes of derivatives of I or II did not represent a significant improvement, some of the derivatives of IV were effective, according to EUCAST (The European Committee on Antimicrobial Susceptibility Testing) criteria, against Hp clinical isolates resistant to common antibiotics such as metronidazole, clarithromycin, and rifampicin. Furthermore, four of these new compound IV derivatives, used as sole agents, were able to significantly reduce Hp gastric colonization in the mouse model of infection and indeed to eradicate the infection in some mice. At present, we will continue the development of derivatives of compound IV through the design and testing of more soluble variants with optimized metabolic stability and bioavailability, and we are testing their effect on other bacteria including those present in the gastric microbiota.
Our preliminary results indicate the Hp-Fld inhibitors so far developed are highly specific for this bacterium, and that they do not show activity (or very low) against a representative panel of other bacteria from different phyla. This selectivity will be useful to minimize the generation of resistances and suggests these inhibitors will be less damaging to the gut microbiota than broad-spectrum antibiotics. Thus, this new family of selective Hp-inhibitors could provide an opportunity for the formulation of therapeutic alternatives to fight Hp-drug resistant strains [193]. As explained, due to the fact of their high selectivity, these Hp-inhibitors will not likely be effective against other pathogens. However, other bacterium-specific flavodoxin inhibitors can be identified anew, though specific screening of chemical libraries against other essential flavodoxins. Such bacterium-specific flavodoxin-inhibitors could also be developed into novel antimicrobials against gastrointestinal pathogens such as Bacillus, Campylobacter, Listeria, Salmonella, Shigella or Vibrio. To do so, the target-based approach followed to discover Hp-Fld inhibitors can be readily applied to identify specific antimicrobials against other flavodoxin-containing pathogens. Moreover, the steps carried out to improve the therapeutic and pharmacokinetic properties of the Hp-Fld inhibitors could also be followed in order to enhance the antimicrobial properties of these compounds against those bacteria.
Abbreviations
| Ala (A) | Alanine |
| ATCC | American Type Culture Collection |
| DNA | Deoxyribonucleic acid |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| Fld | Flavodoxin |
| FMN | Flavinmononucleotide |
| FqrB | Flavodoxin:quinone reductase |
| Hp | Helicobacter pylori |
| MALT | Gastric mucosa-associated lymphoid tissue lymphoma |
| MCC | Minimal cytotoxic concentration |
| MIC | Minimal inhibitory concentration |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NCBI | National Center for Biotechnology Information |
| NMR | Nuclear Magnetic Resonance |
| PD | Pharmacodynamics |
| PDB | Protein Data Bank |
| POR | Pyruvate oxidoreductase complex |
| PPI | Proton-pump inhibitor |
| PK | Pharmacokinetics |
| TI | Therapeutic index |
| Trp (W) | Tryptophan |
| Tyr (Y) | Tyrosine |
Appendix A
Figure A1.
(a) Multiple sequence alignment of flavodoxins from Streptococcus agalactiae (ATCC BAA-611), Hp (strain J99), Escherichia coli (strain K12), and Haemophilus influenzae (strain ATCC 51907). It has been performed with Clustal Omega [194]. Asterisks (*) indicate positions with a single residue; colons (:) indicate conservation between groups of strongly similar properties; dots (.) indicate conservation between groups of weakly similar properties. (b) Sequence identity between Hp (strain J99) flavodoxin and that from Streptococcus agalactiae (ATCC BAA-611), Escherichia coli (strain K12), and Haemophilus influenzae (strain ATCC 51907). It has been calculated with Clustal Omega [194].
Figure A2.
(a) Multiple sequence alignment of flavodoxins from Campylobacter jejuni (strain ATCC 700819), Hp (strain J99), Vibrio cholerae (strain ATCC 39541), Escherichia coli (strain K12), Salmonella enterica subsp. enterica serovar Typhi (strain Ty2) and Salmonella enterica subsp. enterica serovar Typhimurium (strain ATCC 700720). It has been performed with Clustal Omega [194]. (b) Sequence identity between Hp (strain J99) flavodoxin and that from Campylobacter jejuni (strain ATCC 700819), Vibrio cholerae (strain ATCC 39541), Escherichia coli (strain K12), Salmonella enterica subsp. enterica serovar Typhi (strain Ty2), and Salmonellaenterica subsp. enterica serovar Typhimurium (strain ATCC 700720). It has been calculated with Clustal Omega [194].
Author Contributions
Conceptualization, J.S.; investigation, S.S.; data curation, S.S. and J.S.; writing—original draft preparation, S.S. and J.S.; writing—review and editing, S.S. and J.S.; supervision, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINECO, Spain, grant BFU2016-78232-P, by the Joint Programming Initiative in Antimicrobial Resistance: JPIAMR, grant PCI2019-103369, and by Gobierno de Aragón, Spain, grant LMP30_18. S.S. is recipient of a pre-doctoral contract from the Aragonese Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Malfertheiner P., Venerito M., Schulz C. Helicobacter pylori Infection: New facts in clinical management. Curr. Treat. Options Gastroenterol. 2018;16:605–615. doi: 10.1007/s11938-018-0209-8. [DOI] [PubMed] [Google Scholar]
- 2.Sjomina O., Pavlova J., Niv Y., Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23:e12514. doi: 10.1111/hel.12514. [DOI] [PubMed] [Google Scholar]
- 3.Percival S.L., Williams D.W. Microbiology of Waterborne Diseases. Academic Press; Cambridge, MA, USA: 2014. Chapter 7—Helicobacter pylori; pp. 119–154. [Google Scholar]
- 4.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Azuma T., Konishi J., Tanaka Y., Hirai M., Ito S., Kato T., Kohli Y. Contribution of HLA-DQA gene to host’s response against Helicobacter pylori. Lancet. 1994;343:542–543. doi: 10.1016/S0140-6736(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X.-Y., Zhang P.-Y., Aboul-Soud M.A.M. From inflammation to gastric cancer: Role of Helicobacter pylori (review) Oncol. Lett. 2017;13:543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO IARC Agents Classified by the IARC Monographs, Volumes 1–124. [(accessed on 9 March 2020)]; Available online: https://monographs.iarc.fr/list-of-classifications.
- 8.Plummer M., Franceschi S., Vignat J., Forman D., De Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 9.Polk D.B., Peek R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravina A.G., Zagari R.M., De Musis C., Romano L., Loguercio C., Romano M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018;24:3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsay F.-W., Hsu P.-I. H pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018;26:65. doi: 10.1186/s12929-018-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y., Zhu Y., Lu N.-H. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front. Cell. Infect. Microbiol. 2017;7:168. doi: 10.3389/fcimb.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 14.Flores-Treviño S., Mendoza-Olazarán S., Bocanegra-Ibarias P., Maldonado-Garza H.J., Garza-González E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 2018;12:819–827. doi: 10.1080/17474124.2018.1496017. [DOI] [PubMed] [Google Scholar]
- 15.Graham D.Y., Dore M.P. Helicobacter pylori therapy: A paradigm shift. Expert Rev. Anti-Infect. Ther. 2016;14:577–585. doi: 10.1080/14787210.2016.1178065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Lv Z., Zhong Y., Liu D., Chen S., Xie Y. The internalization of Helicobacter pylori plays a role in the failure of H pylori eradication. Helicobacter. 2017;22:e12324. doi: 10.1111/hel.12324. [DOI] [PubMed] [Google Scholar]
- 17.Debraekeleer A., Remaut H. Future perspective for potential Helicobacter pylori eradication therapies. Future Microbiol. 2018;13:671–687. doi: 10.2217/fmb-2017-0115. [DOI] [PubMed] [Google Scholar]
- 18.Abadi A.T.B. Strategies used by Helicobacter pylori to establish persistent infection. World J. Gastroenterol. 2017;23:2870–2882. doi: 10.3748/wjg.v23.i16.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusters J.G., Van Vliet A.H.M., Kuipers E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 21.Goderska K., Agudo-Pena S., Alarcon T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018;102:1–7. doi: 10.1007/s00253-017-8535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Morain N.R., Dore M.P., O’Connor A.J.P., Gisbert J.P., O’Morain C.A. Treatment of Helicobacter pylori infection in 2018. Helicobacter. 2018;23:e12519. doi: 10.1111/hel.12519. [DOI] [PubMed] [Google Scholar]
- 23.Secka O., Berg D.E., Antonio M., Corrah T., Tapgun M., Walton R., Thomas V., Galano J.J., Sancho J., Adegbola R.A., et al. Antimicrobial susceptibility and resistance patterns among Helicobacter pylori strains from the gambia, West Africa. Antimicrob. Agents Chemother. 2013;57:1231–1237. doi: 10.1128/AAC.00517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala G., Escobedo-Hinojosa W.I., De la Cruz-Herrera C.F., Romero I. Exploring alternative treatments for Helicobacter pylori infection. World J. Gastroenterol. 2014;20:1450–1469. doi: 10.3748/wjg.v20.i6.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zawahir S., Czinn S.J., Nedrud J.G., Blanchard T.G. Vaccinating against Helicobacter pylori in the developing world. Gut Microbes. 2013;4:568–576. doi: 10.4161/gmic.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalker A.F., Minehart H.W., Hughes N.J., Koretke K.K., Lonetto M.A., Brinkman K.K., Warren P.V., Lupas A., Stanhope M.J., Brown J.R., et al. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 2001;183:1259–1268. doi: 10.1128/JB.183.4.1259-1268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancho J. Flavodoxins: Sequence, folding, binding, function and Beyond. Cell. Mol. Life Sci. 2006;63:855–864. doi: 10.1007/s00018-005-5514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremades N., Bueno M., Toja M., Sancho J. Towards a new therapeutic target: Helicobacter pylori flavodoxin. Biophys. Chem. 2005;115:267–276. doi: 10.1016/j.bpc.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 29.Puan K.J., Wang H., Dairi T., Kuzuyama T., Morita C.T. fldA is an essential gene required in the 2-C-methyl-D-erythritol 4-phosphate pathway for isoprenoid biosynthesis. FEBS Lett. 2005;579:3802–3806. doi: 10.1016/j.febslet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 30.González-Bello C. Inhibition of shikimate kinase and type II dehydroquinase for antibiotic discovery: Structure-based design and simulation studies. Curr. Top. Med. Chem. 2016;16:960–977. doi: 10.2174/1568026615666150825142527. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Sixto C., Prazeres V.F.V., Castedo L., Suh S.W., Lamb H., Hawkins A.R., Cañada F.J., Jiménez-Barbero J., González-Bello C. Competitive Inhibitors of Helicobacter pylori type II dehydroquinase: Synthesis, biological evaluation, and NMR studies. ChemMedChem. 2008;3:756–770. doi: 10.1002/cmdc.200700307. [DOI] [PubMed] [Google Scholar]
- 32.Prazeres V.F.V., Tizón L., Otero J.M., Guardado-Calvo P., Llamas-Saiz A.L., Van Raaij M.J., Castedo L., Lamb H., Hawkins A.R., González-Bello C. Synthesis and biological evaluation of new nanomolar competitive inhibitors of Helicobacter pylori type II dehydroquinase. Structural details of the role of the aromatic moieties with essential residues. J. Med. Chem. 2010;53:191–200. doi: 10.1021/jm9010466. [DOI] [PubMed] [Google Scholar]
- 33.Duckworth M.J., Okoli A.S., Mendz G.L. Novel Helicobacter pylori therapeutic targets: The unusual aspects. Expert Rev. Anti-Infect. Ther. 2009;7:835–867. doi: 10.1586/eri.09.61. [DOI] [PubMed] [Google Scholar]
- 34.Pernas M., Blanco B., Lence E., Thompson P., Hawkins A.R., González-Bello C. Synthesis of rigidified shikimic acid derivatives by ring-closing metathesis to imprint inhibitor efficacy against shikimate kinase enzyme. Org. Chem. Front. 2019;6:2514–2528. doi: 10.1039/C9QO00562E. [DOI] [Google Scholar]
- 35.Cheng C.-S., Chen C.-H., Luo Y.-C., Chen W.-T., Chang S.-Y., Lyu P.-C., Kao M.-C., Yin H.-S. Crystal structure and biophysical characterisation of Helicobacter pylori phosphopantetheine adenylyltransferase. Biochem. Biophys. Res. Commun. 2011;408:356–361. doi: 10.1016/j.bbrc.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 36.Cheng C.-S., Jia K.-F., Chen T., Chang S.-Y., Lin M.-S., Yin H.-S. Experimentally validated novel inhibitors of Helicobacter pylori phosphopantetheine adenylyltransferase discovered by virtual high-throughput screening. PLoS ONE. 2013;8:e74271. doi: 10.1371/journal.pone.0074271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neelapu N.R.R., Mutha N.V.R., Akula S. Identification of potential drug targets in Helicobacter pylori strain HPAG1 by in silico genome analysis. Infect. Disord. Drug Targets. 2015;15:106–117. doi: 10.2174/1871526515666150724111528. [DOI] [PubMed] [Google Scholar]
- 38.Ge Z. Potential of fumarate reductase as a novel therapeutic target in Helicobacter pylori infection. Expert Opin. Targets. 2002;6:135–146. doi: 10.1517/14728222.6.2.135. [DOI] [PubMed] [Google Scholar]
- 39.Amundsen S.K., Spicer T., Karabulut A.C., Londoño L.M., Eberhardt C., Fernandez Vega V., Bannister T.D., Hodder P., Smith G.R. Small-molecule inhibitors of bacterial AddAB and RecBCD Helicase-nuclease DNA repair enzymes. ACS Chem. Biol. 2012;7:879–891. doi: 10.1021/cb300018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shadrick W.R., Ndjomou J., Kolli R., Mukherjee S., Hanson A.M., Frick D.N. Discovering new medicines targeting helicases: Challenges and recent progress. J. Biomol. Screen. 2013;18:761–781. doi: 10.1177/1087057113482586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freigang J., Diederichs K., Schäfer K.P., Welte W., Paul R. Crystal structure of oxidized flavodoxin, an essential protein in Helicobacter pylori. Protein Sci. 2002;11:253–261. doi: 10.1110/ps.28602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cremades N., Velazquez-Campoy A., Martinez-Julvez M., Neira J.L., Perez-Dorado I., Hermoso J., Jimenez P., Lanas A., Hoffman P.S., Sancho J. Discovery of specific flavodoxin inhibitors as potential therapeutic agents against Helicobacter pylori infection. ACS Chem. Biol. 2009;4:928–938. doi: 10.1021/cb900166q. [DOI] [PubMed] [Google Scholar]
- 43.Galano J.J., Alías M., Pérez R., Velázquez-Campoy A., Hoffman P.S., Sancho J. Improved flavodoxin inhibitors with potential therapeutic effects against Helicobacter pylori infection. J. Med. Chem. 2013;56:6248–6258. doi: 10.1021/jm400786q. [DOI] [PubMed] [Google Scholar]
- 44.Hughes N.J., Chalk P.A., Clayton C.L., Kelly D.J. Identification of carboxylation enzymes and characterization of a novel four-subunit pyruvate: Flavodoxin oxidoreductase from Helicobacter pylori. J. Bacteriol. 1995;177:3953–3959. doi: 10.1128/JB.177.14.3953-3959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes N.J., Clayton C.L., Chalk P.A., Kelly D.J. Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate: Flavodoxin and 2-Oxoglutarate: Acceptor oxidoreductases which mediate electron transport to NADP. J. Bacteriol. 1998;180:1119–1128. doi: 10.1128/JB.180.5.1119-1128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurice M., Cremades N., Croxen M.A., Sisson G., Sancho J., Hoffman P.S. Flavodoxin: Quinone reductase (FqrB): A redox partner of pyruvate: Ferredoxin oxidoreductase that reversibly couples pyruvate oxidation to NADPH production in Helicobacter pylori and Campylobacter jejuni. J. Bacteriol. 2007;189:4764–4773. doi: 10.1128/JB.00287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandal R.S., Das S. In silico approach towards identification of potential inhibitors of Helicobacter pylori DapE. J. Biomol. Struct. Dyn. 2014;33:1460–1473. doi: 10.1080/07391102.2014.954272. [DOI] [PubMed] [Google Scholar]
- 48.Karita M., Etterbeek M.L., Forsyth M.H., Tummuru M.K.R., Blaser M.J. Characterization of Helicobacter pylori dapE and Construction of a Conditionally Lethal dapE Mutant. Infect. Immun. 1997;65:4158–4164. doi: 10.1128/IAI.65.10.4158-4164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basarab G.S., Hill P.J., Rastagar A., Webborn P.J.H. Design of Helicobacter pylori glutamate racemase inhibitors as selective antibacterial agents: A novel pro-drug approach to increase exposure. Bioorg. Med. Chem. Lett. 2008;18:4716–4722. doi: 10.1016/j.bmcl.2008.06.092. [DOI] [PubMed] [Google Scholar]
- 50.Keating T.A. Resistance mechanism to an uncompetitive inhibitor of a single-substrate, single-product enzyme: A study of Helicobacter pylori glutamate racemase. Future Med. Chem. 2013;5:1203–1214. doi: 10.4155/fmc.13.94. [DOI] [PubMed] [Google Scholar]
- 51.Chiu H.-C., Lin T.-L., Yang J.-C., Wang J.-T. Synergistic effect of imp/ostA and msbA in hydrophobic drug resistance of Helicobacter pylori. BMC Microbiol. 2009;9:136. doi: 10.1186/1471-2180-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon D.-H., Woo J.-S., Perng C.-L., Go M.F., Graham D.Y., El-Zaatari F.A.K. The effect of galE gene inactivation on lipopolysaccharide profile of Helicobacter pylori. Curr. Microbiol. 1998;37:144–148. doi: 10.1007/s002849900354. [DOI] [PubMed] [Google Scholar]
- 53.Loughlin M.F. Novel therapeutic targets in Helicobacter pylori. Expert Opin. Targets. 2003;7:725–735. doi: 10.1517/14728222.7.6.725. [DOI] [PubMed] [Google Scholar]
- 54.Van Vliet A.H.M., Ernst F.D., Kusters J.G. NikR-mediated regulation of Helicobacter pylori acid adaptation. Trends Microbiol. 2004;12:489–494. doi: 10.1016/j.tim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Saravanakumar K., Chellia R., Hu X., Kathiresan K., Oh D.-H., Wang M.-H. Eradication of Helicobacter pylori through the inhibition of urease and peptide deformylase: Computational and biological studies. Microb. Pathog. 2019;128:236–244. doi: 10.1016/j.micpath.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Carlini C.R., Ligabue-Braun R. Ureases as multifunctional toxic proteins: A review. Toxicon. 2016;110:90–109. doi: 10.1016/j.toxicon.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y.-S., Su M.-M., Zhang X.-P., Liu Q.-X., He Z.-X., Xu C., Zhu H.-L. Developing potential Helicobacter pylori urease inhibitors from novel oxoindoline derivatives: Synthesis, biological evaluation and in silico study. Bioorg. Med. Chem. Lett. 2018;28:3182–3186. doi: 10.1016/j.bmcl.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Kafarski P., Talma M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018;13:101–112. doi: 10.1016/j.jare.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowinska-Zyrek M., Witkowska D., Valensin D., Kamyszc W., Kozlowski H. The C terminus of HspA—A potential target for native Ni(II) and Bi(III) anti-ulcer drugs. Dalton Trans. 2010;39:5814–5826. doi: 10.1039/c0dt00013b. [DOI] [PubMed] [Google Scholar]
- 60.Nammi D., Srimath-Tirumala-Peddinti R.C.P.K., Neelapu N.R.R. Identification of Drug Targets in Helicobacter pylori by in silico Analysis: Possible Therapeutic Implications for Gastric cancer. Curr. Cancer Drug Targets. 2016;16:79–98. doi: 10.2174/1568009615666150602143239. [DOI] [PubMed] [Google Scholar]
- 61.Yang X., Li H., Cheng T., Xia W., Lai Y.-T., Sun H. Nickel translocation between metallochaperones HypA and UreE in Helicobacter pylori. Metallomics. 2014;6:1731–1736. doi: 10.1039/C4MT00134F. [DOI] [PubMed] [Google Scholar]
- 62.Tarsia C., Danielli A., Florini F., Cinelli P., Ciurli S., Zambelli B. Targeting Helicobacter pylori urease activity and maturation: In-cell highthroughput approach for drug discovery. BBA Gen. Subj. 2018;1862:2245–2253. doi: 10.1016/j.bbagen.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 63.Johnson R.C., Hu H.Q., Merrell D.S., Maroney M.J. Dynamic HypA zinc site is essential for acid viability and proper urease maturation in Helicobacter pylori. Metallomics. 2015;7:674–682. doi: 10.1039/C4MT00306C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowinska-Zyrek M., Witkowska D., Bielinska S., Kamyszb W., Kozlowski H. The -Cys–Cys- motif in Helicobacter pylori’s Hpn and HspA proteins is an essential anchoring site for metal ions. Dalton Trans. 2011;40:5604–5610. doi: 10.1039/c1dt10187k. [DOI] [PubMed] [Google Scholar]
- 65.Ge R., Sun X., Gu Q., Watt R.M., Tanner J.A., Wong B.C.Y., Xia H.H., Huang J.-D., He Q.-Y., Sun H. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. J. Biol. Inorg. Chem. 2007;12:831–843. doi: 10.1007/s00775-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 66.Graham D.Y., Miftahussurur M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018;13:51–57. doi: 10.1016/j.jare.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishimori I., Onishi S., Takeuchi H., Supuran C.T. The α and β classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr. Pharm. Des. 2008;14:622–630. doi: 10.2174/138161208783877875. [DOI] [PubMed] [Google Scholar]
- 68.Modakh J.K., Liu Y.C., Machuca M.A., Supuran C.T., Roujeinikova A. Structural basis for the inhibition of Helicobacter pylori α-carbonic anhydrase by sulfonamides. PLoS ONE. 2015;10:e0127149. doi: 10.1371/journal.pone.0127149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modak J.K., Liu Y.C., Supuran C.T., Roujeinikova A. Structure-activity relationship for sulfonamide inhibition of Helicobacter pylori α-carbonic anhydrase. J. Med. Chem. 2016;59:11098–11109. doi: 10.1021/acs.jmedchem.6b01333. [DOI] [PubMed] [Google Scholar]
- 70.Marcus E.A., Moshfegh A.P., Sachs G., Scott D.R. The Periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 2005;187:729–738. doi: 10.1128/JB.187.2.729-738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Capasso C., Supuran C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Targets. 2015;19:1689–1704. doi: 10.1517/14728222.2015.1067685. [DOI] [PubMed] [Google Scholar]
- 72.Nishimori I., Minakuch T., Kohsaki T., Onishi S., Takeuchi H., Vullo D., Scozzafavac A., Supuran C.T. Carbonic anhydrase inhibitors: The β-carbonic anhydrase from Helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. Bioorg. Med. Chem. Lett. 2007;17:3585–3594. doi: 10.1016/j.bmcl.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 73.Ménard R., Schoenhofen I.C., Tao L., Aubry A., Bouchard P., Reid C.W., Lachance P., Twine S.M., Fulton K.M., Cui Q., et al. Small-Molecule Inhibitors of the Pseudaminic Acid Biosynthetic Pathway: Targeting Motility as a Key Bacterial Virulence Factor. Antimicrob. Agents Chemother. 2014;58:7430–7440. doi: 10.1128/AAC.03858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schoenhofen I.C., Lunin V.V., Julien J.-P., Li Y., Ajamian E., Matte A., Cygler M., Brisson J.-R., Aubry A., Logan S.M., et al. Structural and functional characterization of PseC, an aminotransferase involved in the biosynthesis of pseudaminic acid, an essential flagellar modification in Helicobacter pylori. J. Biol. Chem. 2006;281:8907–8916. doi: 10.1074/jbc.M512987200. [DOI] [PubMed] [Google Scholar]
- 75.Legrain P., Strosberg D. Protein interaction domain mapping for the selection of validated targets and lead compounds in the anti-infectious area. Curr. Pharm. Des. 2002;8:1189–1198. doi: 10.2174/1381612023394872. [DOI] [PubMed] [Google Scholar]
- 76.Sheu B.-S., Yang H.-B., Yeh Y.-C., Wu J.-J. Helicobacter pylori colonization of the human gastric epithelium: A bug’s first step is a novel target for us. J. Gastroenterol. Hepatol. 2010;25:26–32. doi: 10.1111/j.1440-1746.2009.06141.x. [DOI] [PubMed] [Google Scholar]
- 77.Pulic I., Loconte V., Zanotti G. Structural Characterization at the atomic level of a molecular nano-machine: The state of the art of Helicobacter pylori flagellum organization. Am. J. Biochem. Biotechnol. 2014;10:143–161. [Google Scholar]
- 78.Wu J.-J., Sheu B.-S., Huang A.-H., Lin S.-T., Yang H.-B. Characterization of flgK gene and FlgK protein required for H pylori Colonization-from cloning to clinical relevance. World J. Gastroenterol. 2006;12:3989–3993. doi: 10.3748/wjg.v12.i25.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Amsterdam K., Van Der Ende A. Helicobacter pylori HP1034 (ylxH) is required for motility. Helicobacter. 2004;9:387–395. doi: 10.1111/j.1083-4389.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 80.Ishijima N., Suzuki M., Ashida H., Ichikawa Y., Kanegae Y., Saito I., Borén T., Haas R., Sasakawa C., Mimuro H. BabA-mediated adherence is a potentiator of the Helicobacter pylori Type IV secretion system activity. J. Biol. Chem. 2011;286:25256–25264. doi: 10.1074/jbc.M111.233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moonens K., Gideonsson P., Subedi S., Bugaytsova J., Romao E., Mendez M., Nordén J., Fallah M., Rakhimova L., Shevtsova A., et al. Structural insights into polymorphic ABO Glycan binding by Helicobacter pylori. Cell Host Microbe. 2016;19:55–66. doi: 10.1016/j.chom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prinz C., Schoniger M., Rad R., Becker I., Keiditsch E., Wagenpfeil S., Classen M., Rosch T., Schepp W., Gerhard M. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61:1903–1909. [PubMed] [Google Scholar]
- 83.Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microb. Pathog. 2016;93:44–55. doi: 10.1016/j.micpath.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Javaheri A., Kruse T., Moonens K., Mejías-Luque R., Debraekeleer A., Asche C.I., Tegtmeyer N., Kalali B., Bach N.C., Sieber S.A., et al. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat. Microbiol. 2016;2:16189. doi: 10.1038/nmicrobiol.2016.189. [DOI] [PubMed] [Google Scholar]
- 85.Tegtmeyer N., Moodley Y., Yamaoka Y., Pernitzsch S.R., Schmidt V., Rivas Traverso F., Schmidt T.P., Rad R., Yeoh K.G., Bow H., et al. Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Mol. Microbiol. 2016;99:925–944. doi: 10.1111/mmi.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee D.S., Moss S.F. Targeting Helicobacter pylori in gastric carcinogenesis. Expert Opin. Targets. 2007;11:757–769. doi: 10.1517/14728222.11.6.757. [DOI] [PubMed] [Google Scholar]
- 87.Hoy B., Lower M., Weydig C., Carra G., Tegtmeyer N., Geppert T., Schroder P., Sewald N., Backert S., Schneider G., et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahdavi J., Sondén B., Hurtig M., Olfat F.O., Forsberg L., Roche N., Ångström J., Larsson T., Teneberg S., Karlsson K.-A., et al. Helicobacter pylori SabA Adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stähler F.N., Odenbreit S., Haas R., Wilrich J., Van Vliet A.H.M., Kusters J.G., Kist M., Bereswill S. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 2006;74:3845–3852. doi: 10.1128/IAI.02025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Y., Liu S., Li W., Shan Y., Li X., Lu X., Li Y., Guo Q., Zhou Y., Jia J. Proteomic analysis of the function of sigma factor σ54 in Helicobacter pylori survival with nutrition deficiency stress in vitro. PLoS ONE. 2013;8:e72920. doi: 10.1371/journal.pone.0072920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorer M.S., Sessler T.H., Salama N.R. Recombination and DNA Repair in Helicobacter pylori. Annu. Rev. Microbiol. 2011;65:329–348. doi: 10.1146/annurev-micro-090110-102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olekhnovich I.N., Vitko S., Valliere M., Hoffmana P.S. Response to metronidazole and oxidative stress is mediated through homeostatic regulator HsrA (HP1043) in Helicobacter pylori. J. Bacteriol. 2014;196:729–739. doi: 10.1128/JB.01047-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pelliciari S., Pinatel E., Vannini A., Peano C., Puccio S., De Bellis G., Danielli A., Scarlato V., Roncarati D. Insight into the essential role of the Helicobacter pylori HP1043 orphan response regulator: Genome-wide identification and characterization of the DNA-binding sites. Sci. Rep. 2017;7:41063. doi: 10.1038/srep41063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.González A., Salillas S., Velázquez-Campoy A., Espinosa Angarica V., Fillat M.F., Sancho J., Lanas Á. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci. Rep. 2019;9:11294. doi: 10.1038/s41598-019-47746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.González A., Casado J., Chueca E., Salillas S., Velázquez-Campoy A., Angarica V.E., Bénejat L., Guignard J., Giese A., Sancho J., et al. Repurposing dihydropyridines for treatment of Helicobacter pylori infection. Pharmaceutics. 2019;11:681. doi: 10.3390/pharmaceutics11120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cammarota G., Sanguinetti M., Gallo A., Posteraro B. Review article: Biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment. Pharm. 2012;36:222–230. doi: 10.1111/j.1365-2036.2012.05165.x. [DOI] [PubMed] [Google Scholar]
- 97.Huynh H.Q., Couper R.T.L., Tran C.D., Moore L., Kelso R., Butler R.N. N-Acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig. Dis. Sci. 2004;49:1853–1861. doi: 10.1007/s10620-004-9583-2. [DOI] [PubMed] [Google Scholar]
- 98.Abut E., Yasar B., Güveli H., Bölükbas C., Bölükbas F.F., Dalay A.R., Kurdas O.Ö. Effect of the mucolytic erdosteine on the success rate of PPI-based first-line triple therapy for Helicobacter pylori eradication: A prospective, double-blind, randomized, placebo-controlled study. Scand. J. Gastroenterol. 2010;45:677–683. doi: 10.3109/00365521003702726. [DOI] [PubMed] [Google Scholar]
- 99.Backert S., Bernegger S., Skórko-Glonek J., Wessler S. Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis. Cell. Microbiol. 2018;20:e12845. doi: 10.1111/cmi.12845. [DOI] [PubMed] [Google Scholar]
- 100.Costa A.M., Leite M., Seruca R., Figueiredo C. Adherens junctions as targets of microorganisms: A focus on Helicobacter pylori. FEBS Lett. 2013;587:259–265. doi: 10.1016/j.febslet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Orengo C., Michie A., Jones S., Jones D., Swindells M., Thornton J. CATH—A hierarchic classification of protein domain structures. Structure. 1997;5:1093–1108. doi: 10.1016/S0969-2126(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 102.Fillat M.F., Sandmann G., Gomez-Moreno C. Flavodoxin from the nitrogen-fixing cyanobacterium Anabaena PCC 7119. Arch. Microbiol. 1988;150:160–164. doi: 10.1007/BF00425156. [DOI] [Google Scholar]
- 103.Sandmann G., Peleato M.L., Fillat M.F., Lázaro M.C., Gómez-Moreno C. Consequences of the iron-dependent formation of ferredoxin and flavodoxin on photosynthesis and nitrogen fixation on Anabaena strains. Photosynth. Res. 1990;26:119–125. doi: 10.1007/BF00047083. [DOI] [PubMed] [Google Scholar]
- 104.Ifuku O., Koga N., Haze S., Kishimoto J., Wachi Y. Flavodoxin is required for conversion of dethiobiotin to biotin in Escherichia coli. Eur. J. Biochem. 1994;224:173–178. doi: 10.1111/j.1432-1033.1994.tb20009.x. [DOI] [PubMed] [Google Scholar]
- 105.Vetter H., Knappe J. Flavodoxin and ferredoxin of Escherichia coli. Hoppe Seylers Z. Physiol. Chem. 1971;352:433–446. doi: 10.1515/bchm2.1971.352.1.433. [DOI] [PubMed] [Google Scholar]
- 106.Van Lin B., Bothe H. Flavodoxin from Azotobacter vinelandii. Arch. Microbiol. 1972;82:155–172. doi: 10.1007/BF01890407. [DOI] [PubMed] [Google Scholar]
- 107.Knauf M.A., Lohr F., Blumel M., Mayhew S.G., Ruterjans H. NMR investigation of the solution conformation of oxidized flavodoxin from Desulfovibrio vulgaris. Determination of the tertiary structure and detection of protein-bound water molecules. Eur. J. Biochem. 1996;238:423–434. doi: 10.1111/j.1432-1033.1996.0423z.x. [DOI] [PubMed] [Google Scholar]
- 108.Rascio N., La Rocca N. Reference Module in Earth Systems and Environmental Sciences. Elsevier; Amsterdam, The Netherlands: 2013. Biological Nitrogen Fixation. [DOI] [Google Scholar]
- 109.Simondsen R.P., Tollin G. Structure-function relations in flavodoxins. Mol. Cell. Biochem. 1980;33:13–24. doi: 10.1007/BF00224568. [DOI] [PubMed] [Google Scholar]
- 110.Osborne C., Chen L.-M., Matthews R.G. Isolation, cloning, mapping, and nucleotide sequencing of the gene encoding flavodoxin in Escherichia coli. J. Bacteriol. 1991;173:1729–1737. doi: 10.1128/JB.173.5.1729-1737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoover D.M., Ludwig M.L. A flavodoxin that is required for enzyme activation: The structure of oxidized flavodoxin from Escherichia coli at 1.8 A resolution. Protein Sci. 1997;6:2525–2537. doi: 10.1002/pro.5560061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanyal I., Gibson K.J., Flint D.H. Escherichia coli biotin synthase: An investigation into the factors required for its activity and its sulfur donor. Arch. Biochem. Biophys. 1996;326:48–56. doi: 10.1006/abbi.1996.0045. [DOI] [PubMed] [Google Scholar]
- 113.Bianchi V., Eliasson R., Fontecave M., Mulliez E., Hoover D.M., Matthews R.G., Reichard P. Flavodoxin is required for the activation of the anaerobic ribonucleotide reductase. Biochem. Biophys. Res. Commun. 1993;197:792–797. doi: 10.1006/bbrc.1993.2548. [DOI] [PubMed] [Google Scholar]
- 114.Lawson R.J., Von Wachenfeldt C., Haq I., Perkins J., Munro A.W. Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: Biophysical properties and interactions with cytochrome P450 bioI. Biochemistry. 2004;43:12390–12409. doi: 10.1021/bi049131t. [DOI] [PubMed] [Google Scholar]
- 115.Gangeswaran R., Eady R.R. Flavodoxin 1 of Azotobacter vinelandii : Characterization and role in electron donation to purified assimilatory nitrate reductase. Biochem. J. 1996;317:103–108. doi: 10.1042/bj3170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sawers G., Watson G. A glycyl radical solution: Oxygen-dependent interconversion of pyruvate formate-lyase. Mol. Microbiol. 1998;29:945–954. doi: 10.1046/j.1365-2958.1998.00941.x. [DOI] [PubMed] [Google Scholar]
- 117.Campos L.A., Sancho J. Native-Specific Stabilization of Flavodoxin by the FMN Cofactor: Structural and Thermodynamical Explanation. Proteins Struct. Funct. Bioinform. 2006;63:581–594. doi: 10.1002/prot.20855. [DOI] [PubMed] [Google Scholar]
- 118.Martıínez-Júlvez M., Cremades N., Bueno M., Pérez-Dorado I., Maya C., Cuesta-López S., Prada D., Falo F., Hermoso J.A., Sancho J. Common conformational changes in flavodoxins induced by FMN and anion binding: The structure of Helicobacter pylori apoflavodoxin. Proteins. 2007;69:581–594. doi: 10.1002/prot.21410. [DOI] [PubMed] [Google Scholar]
- 119.Rodríguez-Cárdenas Á., Rojas A.L., Conde-Giménez M., Velázquez-Campoy A., Hurtado-Guerrero R., Sancho J. Streptococcus pneumoniae TIGR4 flavodoxin: Structural and biophysical characterization of a novel drug target. PLoS ONE. 2016;11:e0161020. doi: 10.1371/journal.pone.0161020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.López-Llano J., Maldonado S., Bueno M., Lostao A., Jiménez M.Á., Lillo M.P., Sancho J. The long and short flavodoxins: I. The role of the differentiating loop in apoflavodoxin structure and FMN binding. J. Biol. Chem. 2004;279:47177–47183. doi: 10.1074/jbc.M405792200. [DOI] [PubMed] [Google Scholar]
- 121.López-Llano J., Maldonado S., Jain S., Lostao A., Godoy-Ruiz R., Sanchez-Ruiz J.M., Cortijo M., Fernández-Recio J., Sancho J. The long and short flavodoxins: II. The role of the differentiating loop in apoflavodoxin stability and folding mechanism. J. Biol. Chem. 2004;279:47184–47191. doi: 10.1074/jbc.M405791200. [DOI] [PubMed] [Google Scholar]
- 122.Hall D.A., Kooi C.W.V., Stasik C.N., Stevens S.Y., Zuiderweg E.R.P., Matthews R.G. Mapping the interactions between flavodoxin and its physiological partners flavodoxin reductase and cobalamin-dependent methionine synthase. Proc. Natl. Acad. Sci. USA. 2001;98:9521–9526. doi: 10.1073/pnas.171168898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alagaratnam S., Van Pouderoyen G., Pijning T., Dijkstra B.W., Cavazzini D., Rossi G.L., Van Dongen W.M.A.M., Van Mierlo C.P.M., Van Berkel W.J.H., Canters G.W. A crystallographic study of Cys69Ala flavodoxin II from Azotobacter vinelandii: Structural determinants of redox potential. Protein Sci. 2005;14:2284–2295. doi: 10.1110/ps.051582605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaihovaaraa P., Höök-Nikannea J., Uusi-Oukarib M., Kosunenc T.U., Salaspuro M. Flavodoxin-dependent pyruvate oxidation, acetate production and metronidazole reduction by Helicobacter pylori. J. Antimicrob. Chemother. 1998;41:171–177. doi: 10.1093/jac/41.2.171. [DOI] [PubMed] [Google Scholar]
- 125.Paul R., Bosch F.U., Schafer K.P. Overexpression and Purification of Helicobacter pylori Flavodoxin and Induction of a Specific Antiserum in Rabbits. Protein Expr. Purif. 2001;22:399–405. doi: 10.1006/prep.2001.1467. [DOI] [PubMed] [Google Scholar]
- 126.Chang C., Chen L., Yang J., Lin J., Chang K., Wang J. Isolation of a Helicobacter pylori Protein, FldA, Associated With Mucosa-Associated Lymphoid Tissue Lymphoma of the Stomach. Gastroenterology. 1999;117:82–88. doi: 10.1016/S0016-5085(99)70553-6. [DOI] [PubMed] [Google Scholar]
- 127.Gerrits M.M., Van Der Wouden E.J., Bax D.A., Van Zwet A.A., Van Vliet A.H.M., De Jong A., Kusters J.G., Thijs J.C., Kuipers E.J. Role of the rdxA and frxA genes in oxygen-dependent metronidazole resistance of Helicobacter pylori. J. Med. Microbiol. 2004;53:1123–1128. doi: 10.1099/jmm.0.45701-0. [DOI] [PubMed] [Google Scholar]
- 128.Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–171. [PubMed] [Google Scholar]
- 129.Kwon D.H., El-Zaatari F.A.K., Kato M., Osato M.S., Reddy R., Yamaoka Y., Graham D.Y. Analysis of rdxA and involvement of additional genes encoding NAD(P)H flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori. Antimicrob. Agents Chemother. 2000;44:2133–2142. doi: 10.1128/AAC.44.8.2133-2142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim S.Y., Joo Y.M., Lee H.S., Chung I.S., Yoo Y.J., Merrell D.S., Cha J.H. Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J. Antibiot. (Tokyo) 2009;62:43–50. doi: 10.1038/ja.2008.6. [DOI] [PubMed] [Google Scholar]
- 131.Tomb J.F., White O., Kerlavage A.R., Clayton R.A., Sutton G.G., Fleischmann R.D., Ketchum K.A., Klenk H.P., Gill S., Dougherty B.A., et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;389:412. doi: 10.1038/38792. [DOI] [PubMed] [Google Scholar]
- 132.Cremades N., Velazquez-Campoy A., Freire E., Sancho J. The flavodoxin from Helicobacter pylori: Structural Determinants of Thermostability and FMN Cofactor Binding. Biochemistry. 2008;47:627–639. doi: 10.1021/bi701365e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hutcherson J.A., Sinclair K.M., Belvin B.R., Gui Q., Hoffman P.S., Lewis J.P. Amixicile, a novel strategy for targeting oral anaerobic pathogens. Sci. Rep. 2017;7:10474. doi: 10.1038/s41598-017-09616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kletzin A., Adams M.W.W. Molecular and phylogenetic characterization of pyruvate and 2-ketoisovalerate ferredoxin oxidoreductases from Pyrococcus furiosus and pyruvate ferredoxin oxidoreductase from Thermotoga maritima. J. Bacteriol. 1996;178:248–257. doi: 10.1128/JB.178.1.248-257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018;46:D8–D13. doi: 10.1093/nar/gkx1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chazarreta-Cifre L., Martiarena L., de Mendoza D., Altabe S.G. Role of ferredoxin and flavodoxins in Bacillus subtilis fatty acid desaturation. J. Bacteriol. 2011;193:4043–4048. doi: 10.1128/JB.05103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Z.Q., Lawson R.J., Buddha M.R., Wei C.C., Crane B.R., Munro A.W., Stuehr D.J. Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J. Biol. Chem. 2007;282:2196–2202. doi: 10.1074/jbc.M608206200. [DOI] [PubMed] [Google Scholar]
- 140.Yeom J., Jeon C.O., Madsen E.L., Park W. In vitro and in vivo interactions of ferredoxin-NADP+ reductases in Pseudomonas putida. J. Biochem. 2009;145:481–491. doi: 10.1093/jb/mvn185. [DOI] [PubMed] [Google Scholar]
- 141.Yeom J.-K., Park W.-J. Biochemical characterization of ferredoxin-NADP+ reductase interaction with flavodoxin in Pseudomonas putida. BMB Rep. 2012;45:476–481. doi: 10.5483/BMBRep.2012.45.8.071. [DOI] [PubMed] [Google Scholar]
- 142.Yeom J., Park W. Pleiotropic effects of the mioC mutation on the physiology of Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 2012;335:47–57. doi: 10.1111/j.1574-6968.2012.02643.x. [DOI] [PubMed] [Google Scholar]
- 143.Moyano A.J., Tobares R.A., Rizzi Y.S., Krapp A.R., Mondotte J.A., Bocco J.L., Saleh M.C., Carrillo N., Smania A.M. A Long-Chain Flavodoxin Protects Pseudomonas aeruginosa from Oxidative Stress and Host Bacterial Clearance. PLoS Genet. 2014;10:e1004163. doi: 10.1371/journal.pgen.1004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodrigues C., Kapil A., Sharma A., Ragupathi N.K.D., Inbanathan F.Y., Veeraraghavan B., Kang G. Whole-Genome Shotgun Sequencing of Cephalosporin-Resistant Salmonella enterica Serovar Typhi. Genome Announc. 2017;5:e01639-16. doi: 10.1128/genomeA.01639-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kasai S. Freshwater bioluminescence in Vibrio albensis (Vibrio cholerae biovar albensis) NCIMB 41 is caused by a two-nucleotide deletion in luxO. J. Biochem. 2006;139:471–482. doi: 10.1093/jb/mvj048. [DOI] [PubMed] [Google Scholar]
- 146.Taylor A.J., Kelly D.J. The Function, Biogenesis and Regulation of the Electron Transport Chains in Campylobacter jejuni: New Insights into the Bioenergetics of a Major Food-Borne Pathogen. Volume 74. Elsevier; Amsterdam, The Netherlands: 2019. [DOI] [PubMed] [Google Scholar]
- 147.Weerakoon D.R., Olson J.W. The Campylobacter jejuni NADH: Ubiquinone oxidoreductase (complex I) utilizes flavodoxin rather than NADH. J. Bacteriol. 2008;190:915–925. doi: 10.1128/JB.01647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kendall J.J., Barrero-Tobon A.M., Hendrixson D.R., Kelly D.J. Hemerythrins in the microaerophilic bacterium Campylobacter jejuni help protect key iron-sulphur cluster enzymes from oxidative damage. Environ. Microbiol. 2014;16:1105–1121. doi: 10.1111/1462-2920.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kolker E., Picone A.F., Galperin M.Y., Romine M.F., Higdon R., Makarova K.S., Kolker N., Anderson G.A., Qiu X., Auberry K.J., et al. Global profiling of Shewanella oneidensis MR-1: Expression of hypothetical genes and improved functional annotations. Proc. Natl. Acad. Sci. USA. 2005;102:2099–2104. doi: 10.1073/pnas.0409111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Biel S., Klimmek O., Groß R., Kröger A. Flavodoxin from Wolinella succinogenes. Arch. Microbiol. 1996;166:122–127. doi: 10.1007/s002030050365. [DOI] [PubMed] [Google Scholar]
- 151.Tanaka M., Haniu M., Yasunobu K.T. Amind acid sequence of the Peptostreptococcus elsdenii flavodoxin. Biochem. Biophys. Res. Commun. 1971;44:886–892. doi: 10.1016/0006-291X(71)90794-7. [DOI] [PubMed] [Google Scholar]
- 152.Mayhew S.G., Massey V. Studies on the kinetics and mechanism of reduction of flavodoxin from Peptostreptococcus elsdenii by sodium dithionite. Biochim. Biophys. Acta. 1973;315:181–190. doi: 10.1016/0005-2744(73)90141-1. [DOI] [PubMed] [Google Scholar]
- 153.Mayhew S.G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J. Biol. Chem. 1969;244:794–802. [PubMed] [Google Scholar]
- 154.Fox E.M., Allnutt T., Bradbury M.I., Fanning S., Chandry P.S. Comparative genomics of the Listeria monocytogenes ST204 subgroup. Front. Microbiol. 2016;7:2057. doi: 10.3389/fmicb.2016.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo Q., Shang J., Feng X., Guo X., Zhang L., Zhou Q. PrfA led to reduced biofilm formation and contributed to altered gene expression patterns in biofilm-forming Listeria monocytogenes. Curr. Microbiol. 2013;67:372–378. doi: 10.1007/s00284-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 156.Ofer A., Kreft J., Logan D.T., Cohen G., Borovok I., Aharonowitz Y. Implications of the inability of Listeria monocytogenes EGD-e to grow anaerobically due to a deletion in the class III NrdD ribonucleotide reductase for its use as a model laboratory strain. J. Bacteriol. 2011;193:2931–2940. doi: 10.1128/JB.01405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Knight E., Jr., Hardy R.W.F. Flavodoxin of Clostridium pasteurianum. Methods Enzymol. 1971;18:592–598. [Google Scholar]
- 158.Plegaria J.S., Sutter M., Ferlez B., Aussignargues C., Niklas J., Poluektov O.G., Fromwiller C., Teravest M., Utschig L.M., Tiede D.M., et al. Structural and functional characterization of a short-chain flavodoxin associated with a noncanonical 1,2-propanediol utilization bacterial microcompartment. Biochemistry. 2017;56:5679–5690. doi: 10.1021/acs.biochem.7b00682. [DOI] [PubMed] [Google Scholar]
- 159.Fox J.L., Smith S.S., Brown J.R. Amino acid sequences of Clostridium pasteurianum flavodoxin. Z. Naturforsch. B. 1972;27:1096–1100. doi: 10.1515/znb-1972-0932. [DOI] [PubMed] [Google Scholar]
- 160.Luo H., Lin Y., Gao F., Zhang C.-T., Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014;42:D574–D580. doi: 10.1093/nar/gkt1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wilkes-Weiss D. The Gram-staining properties of Treponema pallidum. Transl. Res. J. Lab. Clin. Med. 1924;9:716–718. [Google Scholar]
- 162.Zhou X., Li Y. Atlas of Oral Microbiology: From Healthy Microflora to Disease. Elsevier; Amsterdam, The Netherlands: 2015. Chapter 4—Subgingival microbes; pp. 67–93. [Google Scholar]
- 163.Krissinel E. On the relationship between sequence and structure similarities in proteomics. Bioinformatics. 2007;23:717–723. doi: 10.1093/bioinformatics/btm006. [DOI] [PubMed] [Google Scholar]
- 164.Nardone G., Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015;3:255–260. doi: 10.1177/2050640614566846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vasile Petra C., Rus A., Dumitraşcu D.L. Gastric microbiota: Tracing the culprit. Clujul Med. 2017;90:369–376. doi: 10.15386/cjmed-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sebastián-Domingo J.J., Sánchez-Sánchez C. From the intestinal flora to the microbiome. Rev. Esp. Enferm. Dig. 2018;110:51–56. doi: 10.17235/reed.2017.4947/2017. [DOI] [PubMed] [Google Scholar]
- 167.Wang H., Wei C.X., Min L., Zhu L.Y. Good or bad: Gut bacteria in human health and diseases. Biotechnol. Biotechnol. Equip. 2018;32:1075–1080. doi: 10.1080/13102818.2018.1481350. [DOI] [Google Scholar]
- 168.Lagier J.-C., Million M., Hugon P., Armougom F., Raoult D. Human gut microbiota: Repertoire and variations. Front. Cell. Infect. Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sohn M.B., An L., Pookhao N., Li Q. Accurate genome relative abundance estimation for closely related species in a metagenomic sample. BMC Bioinform. 2014;15:242. doi: 10.1186/1471-2105-15-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dieterich W., Schink M., Zopf Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018;6:116. doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gorbach S.L. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. Chapter 95—Microbiology of the gastrointestinal tract. [PubMed] [Google Scholar]
- 172.World Health Organization . Readings on Diarrhoea: Student Manual. WHO; Geneva, Switzerland: 1992. pp. 1–143. [Google Scholar]
- 173.Ducarmon Q.R., Zwittink R.D., Hornung B.V.H., Van Schaik W., Young V.B., Kuijper E.J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019;83:e00007–e00019. doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chatterjee R., Shreenivas M.M., Sunil R., Chakravortty D. Enteropathogens: Tuning their gene expression for Hassle-Free survival. Front. Microbiol. 2019;9:3303. doi: 10.3389/fmicb.2018.03303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hone D., Hackett J. Vaccination against Enteric Bacterial Diseases. Rev. Infect. Dis. 1989;11:853–877. doi: 10.1093/clinids/11.6.853. [DOI] [PubMed] [Google Scholar]
- 177.Smith A.M. Review of molecular subtyping methodologies used to investigate outbreaks due to multidrug-resistant enteric bacterial pathogens in sub-Saharan Africa. Afr. J. Lab. Med. 2019;8:a760. doi: 10.4102/ajlm.v8i1.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Das S., Mohakud N.K., Suar M., Sahu B.R. Vaccine development for enteric bacterial pathogens: Where do we stand? Pathog. Dis. 2018;76:1–14. doi: 10.1093/femspd/fty057. [DOI] [PubMed] [Google Scholar]
- 179.Sistrunk J.R., Nickerson K.P., Chanin R.B., Rasko D.A., Faherty C.S. Survival of the fittest: How bacterial pathogens utilize bile to enhance infection. Clin. Microbiol. Rev. 2016;29:819–836. doi: 10.1128/CMR.00031-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wallace N., Zani A., Abrams E., Sun Y. Chapter 4—The Impact of Oxygen on Bacterial Enteric Pathogens. Volume 95. Elsevier; Amsterdam, The Netherlands: 2016. [DOI] [PubMed] [Google Scholar]
- 181.Riddle M.S., Chen W.H., Kirkwood C.D., MacLennan C.A. Update on vaccines for enteric pathogens. Clin. Microbiol. Infect. 2018;24:1039–1045. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 182.Croxen M.A., Law R.J., Scholz R., Keeney K.M., Wlodarska M., Finlay B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ishimaru K., Sasaki M., Narimatsu H., Arimizu Y., Gotoh Y., Nakamura K., Hayashi T., Ogura Y. Escherichia coli O8 : H8 carrying a novel variant of the heat-labile enterotoxin LT2 gene caused outbreaks of diarrhea. Open Forum Infect. Dis. 2020;7:ofaa021. doi: 10.1093/ofid/ofaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fox J.G., Dewhirst F.E., Tully J.G., Paster B.J., Yan L., Taylor N.S., Collins M.J., Gorelick P.L., Ward J.M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 1994;32:1238–1245. doi: 10.1128/JCM.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Falsafi T., Mahboubi M. Helicobacter hepaticus, a new pathogenic species of the Helicobacter genus: Similarities and differences with H. Pylori. Iran. J. Microbiol. 2013;5:185–194. [PMC free article] [PubMed] [Google Scholar]
- 186.Dailidiene D., Dailide G., Ogura K., Zhang M., Mukhopadhyay A.K., Eaton K.A., Cattoli G., Kusters J.G., Berg D.E. Helicobacter acinonychis: Genetic and rodent infection studies of a Helicobacter pylori-like gastric pathogen of cheetahs and other big cats. J. Bacteriol. 2004;186:356–365. doi: 10.1128/JB.186.2.356-365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lash R.H., Lauwers G.Y., Odze R.D., Genta R.M. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2009. Chapter 12—Inflammatory disorders of the stomach; pp. 269–320. [Google Scholar]
- 188.Edwards D.I. Nitroimidazole drugs—Action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 189.Edwards D.I. Nitroimidazole drugs—action and resistance mechanisms. II. Mechanisms of resistance. J. Antimicrob. Chemother. 1993;31:201–210. doi: 10.1093/jac/31.2.201. [DOI] [PubMed] [Google Scholar]
- 190.Ang C.W., Jarrad A.M., Cooper M.A., Blaskovich M.A.T. Nitroimidazoles: Molecular fireworks that combat a broad spectrum of infectious diseases. J. Med. Chem. 2017;60:7636–7657. doi: 10.1021/acs.jmedchem.7b00143. [DOI] [PubMed] [Google Scholar]
- 191.Edwards D.I. Mechanisms of selective toxicity of metronidazole and other nitroimidazole drugs. Br. J. Vener. Dis. 1980;56:285–290. doi: 10.1136/sti.56.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sisson G., Goodwin A., Raudonikiene A., Hughes N.J., Mukhopadhyay A.K., Berg D.E., Hoffman P.S. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 2002;46:2116–2123. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Salillas S., Alías M., Michel V., Mahía A., Lucía A., Rodrigues L., Bueno J., Galano-Frutos J.J., De Reuse H., Velázquez-Campoy A., et al. Design, synthesis, and efficacy testing of nitroethylene- and 7-nitrobenzoxadiazol-based flavodoxin inhibitors against Helicobacter pylori drug-resistant clinical strains and in Helicobacter pylori-infected mice. J. Med. Chem. 2019;62:6102–6115. doi: 10.1021/acs.jmedchem.9b00355. [DOI] [PubMed] [Google Scholar]
- 194.Madeira F., Park Y.M., Lee J., Buso N., Gur T., Madhusoodanan N., Basutkar P., Tivey A.R.N., Potter S.C., Finn R.D., et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]