Abstract
Some nanomaterials including Fe0, Ag0, and ZnO are well known for their antibacterial effects. However, very few studies have examined antibacterial effects of nanohybrids. Given that metal oxides, mainly ZnO and TiO2, are known to increase mobility, surface area, and photocatalysis when combined with carbon-based nanomaterials, ZnO- and TiO2-conjugated carbon nanotube and graphene oxide nanohybrids were investigated for their antibacterial effects on Escherichia coli (DH5α, a multidrug-resistant coliform bacterium). Graphene-oxide (GO)-based nanohybrids (ZnO–GO and TiO2–GO) induced increased dispersion compared to carbon-nanotube (CNT)-based nanohybrids (ZnO–CNT and TiO2–CNT). Among the four types of nanohybrids, ZnO-conjugated nanohybrids exhibited a higher antibacterial property, resulting in the antibacterial effect (measured with growth inhibition of cells) in the order ZnO–GO > ZnO–CNT > TiO2–GO > TiO2–CNT. Among four possible antibacterial mechanisms (generation of reactive oxygen species (ROS), physicochemical characteristics, the steric effect, and release of metal ions), a primary mechanism—ROS generation—was identified; whereas, physicochemical characteristics and the steric effect were part of contributing mechanisms. The increasing dispersion of TiO2/ZnO on GO may have contributed to the antibacterial effects due to increasing surface areas. Similarly, significant damages to E. coli cell membranes were found by the GO sheet with its sharp edges. Our results suggest that applying GO-based ZnO or TiO2 could be an effective antibacterial method, especially for the treatment of multidrug-resistant bacteria in the water.
Keywords: Antibacterial activity, E. coli (DH5α), Nanohybrids, Carbon nanotube, Graphene oxide, Antibacterial-resistant bacteria
1. Introduction
Nanomaterials, due to their unique mechanical and catalytic properties and electrical conductivity, have been widely applied for manufacturing industrial products, with an exponential potential increase of nanomaterials, particularly ZnO, TiO2, carbonaceous nanomaterials, silver, and iron in the future (Klaine et al., 2008; Batley et al., 2012; Jeon et al., 2016). Metal oxides, particularly ZnO and TiO2, are commonly exploited in cosmetics, paints, coatings, and solar cells because of their photocatalytic properties (Wang et al., 2016a). Similarly, two commonly used nanomaterials graphene oxide (GO) and carbon nanotubes (CNTs) are found in various industrial applications. For instance, GO—a monolayer of carbon atoms that are packed into a twodimensional structure—is chemically modified with oxidizers on graphene with a large number of oxygen bonds to be used as a dispersant in water (Wang et al., 2014; Hu et al., 2010). Such unique properties enable GO to provide a large active site with other metals through both electrostatic and coordinate approaches (Wang et al., 2014). CNTs, primarily consisting of carbon, have been widely used, especially in plastics, catalysts, batteries, electronic components, aircrafts, and automotive industries, in photocatalytic applications because of their effectiveness in catalyzing materials (Akhavan et al., 2011). They have also been used to design semiconductor materials for the synergetic combination of their unique electrical and structural properties (Das et al., 2014).
Thus, there is an increasing concern about the release of nanomaterials into the environment. Very few materials are released as single-type nanoparticles; instead, materials exhibit a heterogeneous form, including nanohybrids in the environmental media (Klaine et al., 2008; Wang et al., 2016a; Weir et al., 2012). Several studies have found that nanoparticles affect aquatic organisms through toxicity causing mechanisms, including reactive oxygen species (ROS) generation, release of metal ions, and disruption of the cell membranes (Batley et al., 2012; Kumar et al., 2011; Lin et al., 2014). Despite a number of reports on toxicity effects of nanomaterials (Baek et al., 2017, 2018; Choi et al., 2018; Wang et al., 2016b), few studies have investigated the antibacterial effects of nanohybrids. Combined nanocomposites are found to not only offer a large surface area but also to increase the photocatalytic effect (Huang et al., 2014). Given recently emerging concerns about multidrug-resistant bacteria detected in water (Jerome, 2016) and drug-resistant contaminated surface water, especially after a hurricane (Guarino, 2017), it is imperative to explore antibacterial effects of nanohybrids and develop a cost-effective and safe treatment application of nanomaterials to minimize risks associated with such contaminants in water. This study aims to (1) characterize four types of synthesized nanohybrids (NHs) using a scanning electron microscope (SEM), a transmission electron microscope (TEM), Fourier-transform infrared spectroscopy (FT-IR), and thermal gravimetric analysis (TGA), thereby identifying antibacterial properties and changes in physicochemical characteristics of NHs, (2) identify the antibacterial effects of ZnO/TiO2-conjugated GO- and CNT-based nanohybrids, and (3) explore mechanisms attributable to antibacterial effects of NHs on Escherichia coli (DH5α, a multidrug-resistant coliform bacterium). E. coli (DH5α) was selected since it is the foundation of aquatic ecosystems (van Elsas et al., 2011) and one of the aquatic indicator organisms, yet it is classified as a multidrug-resistant coliform bacteria (Tetz et al., 2012; Molina-Aja et al., 2002; Kȩdzierska et al., 1999).
2. Materials and methods
2.1. Materials
A single-layer graphene oxide (GO; > 99% purity, bulk density of 0.26 g/cm3, diameter of 1–5 μm, and thickness of 0.8–1.2 nm; from Hummers’ method) was supplied by ACS Material (CA, USA); a multiwalled carbon nanotube (MWCNT; > 98% purity, 6–13 nm (O.D.) × 2.5–20 μm (L)) was purchased from Sigma-Aldrich (MO, USA). A zinc nitrate hexahydrate (Zn(NO3)2·6H2O; > 98% purity) and ZnCl2 (> 99.99% purity) for ZnO nanocomposite synthesis; and a titanium oxysulfate solution (TiOSO4; ~15 wt% and 99.99% purity) for TiO2 nanocomposite synthesis were purchased from Sigma-Aldrich (MO, USA).
2.2. Synthesis of nanohybrids
For the synthesis of four types of nanohybrids, a hydrothermal process was used following the literature (Fu et al., 2018). First, the ZnO–GO nanohybrid was synthesized by dispersing 50 mg GO into a 100-mL deionized (DI) water ultrasonic bath for 1 h, followed by centrifugation of the GO solvent at 4000 rpm and an addition of 5 mmol of Zn(NO3)2·6H2O to ammonia water at pH 8. After sonication, the mixture was autoclaved and heated at 160 °C for 10 h, followed by separation using centrifugation and washing with DI water. Afterward, the synthesized ZnO–GO was dried under vacuum at 60 °C for 24 h. The synthesis of ZnO–CNT adopted a method by Wang and coauthors’ study (2008). First, nitric acid (80%) was added to a MWCNT at 80 °C for 4 h and then washed with DI water several times. A NH3 H2O solution was slowly added into a ZnCl2 solution under stirring.
Afterward, the white precipitate disappeared, forming Zn(NH3)42+, followed by the soaking of the CNT solution into a Zn(NH3)42+ solution for 48 h and centrifugation. The resultant products were dried at 70 °C in a vacuum oven and calcined at 300 °C for 4 h in atmospheric nitrogen. For the synthesis of TiO2–GO, a literature method was used as follows (Stengl et al., 2013). First, an ammonium hydroxide solution (10%) was added slowly into 100 mL of 1.6 M TiOSO4 under constant stirring at 0 °C in an ice bath until a homogeneous mixture was formed at pH 8.0. The white precipitate was obtained by filtration and mixed with 100 mL of a 15% hydrogen peroxide solution. After a yellow solution of the titania peroxo complex was obtained, 50 mg GO was dispersed in water using a sonicator and added into the yellow solution of the titania peroxo complex. Subsequently, the mixture was annealed in a heated mantle in a round-bottom flask with a reflux cooler at 100 °C for 48 h. Finally, the resultant product was filtered off and dried at 105 °C for 10 h. In regard to the synthesis of TiO2–CNT, the following method was applied according to the literature (Huang et al., 2014). An MWCNT (50 mg) was added into 100 mL of the TiOSO4 (0.01 M) solution followed by addition of 1 mL of H2SO4 (0.2 M) under ultrasound sonication. Afterward, the suspension was refluxed in thermostatic water (80 °C) for 72 h and dispersed for 10 min every 6 h to avoid agglomeration of MWCNTs.
2.3. Characterization of nanohybrids
The morphologies, elemental analyses, and the nanostructures of the particles were characterized with Field Emission Scanning Electron Microscopy (SEM; Philips XL-30 FEG), Energy Dispersive Spectroscopy (EDS), and JEOL 1400 Transmission Electron Microscopy (TEM). For SEM preparation, a small sample of each particle was coated with a thin (20 nm) coating of Pd in a Cressington sputter coater. Samples were then placed in a FEI XL-30 Field Emission SEM and imaged at several magnifications. For SEM preparation of control and experimental E. coli cultures, each culture was preserved according to the following protocol. Cultures were initially fixed in 2% glutaraldehyde in a phosphatebuffered saline (PBS) buffer. Samples were then rinsed three times for 5 min each in the PBS buffer and dehydrated three times for 5 min each in a graded series of ethanol (20%, 50%, 70%, 95%, and 100%). Following dehydration, samples were dried in three changes for 5 min each in hexamethyldisilazane (HMDS). Samples were outgassed overnight, placed on stubs, and coated as outlined above. EDS in SEM particulate samples was conducted at various magnifications, and Xrays were collected using a scan speed of 2 at a working distance of 10 mm. The elemental spectra produced were then saved and subjected to semi-quantitative analysis using the EDS software to collect the relative elemental percentages present. An Oxford EDS system fitted on the SEM was used for this analysis.
Samples for TEM were diluted, and a solution drop was placed on a copper grid before air-drying. The samples were then imaged at several magnifications in a Philips CM-10 TEM (Eindhoven, Netherlands) at the Miller School of Medicine TEM Core at the University of Miami. Cell cultures were prepared as described above for SEM until after the final dehydration step. After dehydration in 100% ethanol, samples were placed in molds containing Spurr’s embedding resin and polymerized for 3 day at 60 °C. Following polymerization, the blocks were sectioned using a Porter Blum MT2 ultramicrotome fitted with a diamond knife, and sections were then floated onto grids and imaged. Fourier transform infrared (FT-IR) spectra were used to identify the different functional groups that are present in nanocomposites by measuring the vibrational frequencies of the chemical bonds. The sample powders were ground and compressed into a pellet whose spectra are in the 600–4000 cm−1 range and measured with PerkinElmer Frontier FTIR (MA, USA). The decomposition pattern of the NHs was evaluated by thermogravimetric analysis (TGA) using a TGA55 (DE, USA). Approximately 20–30 mg of the sample were loaded on a pan, and the temperature was ramped at the rate of 10 °C per minute from ambient to 1000 °C in an inert atmosphere that was purged with nitrogen.
2.4. E. coli cell cultivation and cell viability analysis
The antibacterial properties of the NHs were evaluated on E. coli bacteria. An E. coli colony was cultivated with MAX Efficiency DH5α Competent Cells (Invitrogen, CA). E. coli cells were incubated in a culture tube containing 4 mL of the LB broth at 37 °C under shaking at 225 rpm for 14 h. A 100 μL cell suspension was incubated in a 1 L flask containing the LB broth at 37 °C under shaking at 225 rpm for 14 h. The cultivated cells were exposed to four different types of nanohybrid suspensions (i.e. 10, 100, and 300 mg L−1) in 4 mL LB broth. E. coli exposed to nanohybrid suspensions were incubated at 37 °C under shaking at 225 rpm for 6 h. A sample was taken every 2 h into a 96-well plate, and the optical density (OD600) was measured at 600 nm with a microplate reader (Spectra MAX 190, Molecular Devices, CA). The ratio of E. coli cells’ growth inhibition (%) was calculated as a percentage of survived cells compared to control cells after 6 h of exposure. Samples were plated in a triplicate, and all experiments were performed under sterile conditions.
2.5. ROS detection
Cellular ROS generation was quantitated using DCFDA, a cellular ROS detection assay kit (Abcam Inc, Toronto, ON, Canada). The assay uses the cell reagent 2′,7′–dichlorofluorescin diacetate (DCFDA), a fluorogenic dye that measures hydroxyl, peroxyl, and other ROS activities within the cell. E. coli cells exposed to four different nanohybrid suspensions were collected in a conical tube and washed by centrifugation in PBS. The cells were resuspended in the diluted DCFDA solution and incubated at 37 °C for 30 min in the dark. The antioxidant N-acetyl cysteine (NAC) was used to investigate the effect of the ROS scavenger to confirm the toxicity mechanism. NAC has been commonly used as a ROS inhibitor by promoting the production of antioxidant enzymes or providing a precursor for glutathione synthesis (Ma et al., 2014). Thus, cell cultures were pretreated with and without NAC (0.5 and 2 mM) and exposed to 100 mg L−1 of NHs for 72 h. Then, cells in the presence and in the absence of NAC were transferred into a clearbottom 96-well microplate after being washed by centrifugation with the PBS buffer. DCF was detected by fluorescence spectroscopy with maximum excitation and emission spectra of 485 nm and 535 nm, respectively, in the presence of the buffer.
2.6. Statistical analysis
All experiments were performed at least in triplicate. Statistical analysis was performed using the statistical Student’s t-test with Graph Pad Prism 3.0 (Graph Pad Software Inc., San Diego, CA), and all data were examined at a statistical significance p < 0.05.
3. Results and discussion
3.1. Characterization of the synthesized nanohybrids
The four types of synthesized nanohybrids (ZnO–GO, ZnO–CNT, TiO2–GO and TiO2–CNT) through the hydrothermal process were confirmed for their components as shown in SEM and TEM images (Fig. 1). Fig. 1A illustrates ZnO–GO showing aggregates of ZnO formed with clusters that covered the surface of the GO sheet. Irregular agglomerate dispersions were observed around the GO sheet, and the TEM image of ZnO–GO (Fig. 1B) also revealed the presence of ZnO on the GO surface. Images C and D in Fig. 1 illustrate synthesized TiO2–GO, and, as indicated by the images, TiO2 clusters were formed along the side between GO sheets. Notably, it was observed that the aggregated size of TiO2 onto GO was larger with less dispersion compared to that of ZnO–GO. High surface area and morphology of GO sheets suggest promotion of effective dispersion of metal oxides on GO, thereby forming a more uniform distribution of ZnO on the GO sheet compared to that on TiO2–GO.
Fig. 1.
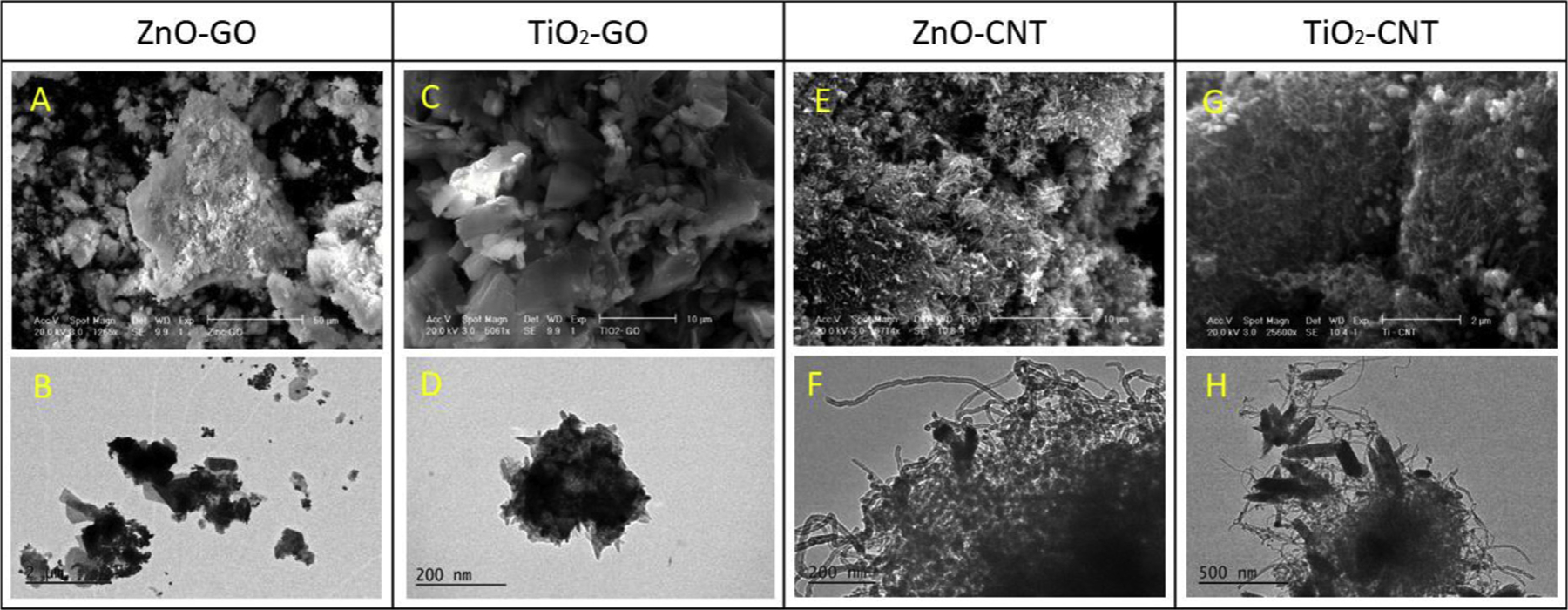
SEM and TEM micrographs of four types of NHs.
Fig. 1E and G (CNT coated with ZnO and TiO2, respectively) indicate well-dispersed ZnO and TiO2 on CNT, and CNT appears to serve as a substrate for ZnO and TiO2 NPs crystallites (Byrappa et al., 2008). However, unlike GO-based ZnO/TiO2, CNT-based ZnO/TiO2 formed more aggregates, as illustrated in Fig. 1H. As with the case of GO-based ZnO and TiO2, a larger aggregation cluster from TiO2–CNT was observed compared to ZnO–CNT. Fig. 1F and H are TEM images of CNT decorated by spherical ZnO NPs and rod-shaped TiO2 NPs, respectively, indicating that ZnO NPs are fixed in the aggregated structure of CNT (Upasani et al., 2017), and TiO2 aggregates are made through the selfassembly of rod-shaped TiO2 NPs. As stated previously, aggregation of GO-based ZnO and TiO2 had more reactive sites and uniform dispersion compared to CNT-based ZnO and TiO2, possibly because of GO’s flat shape, and a considerably larger portion of metal-oxide nanoparticles on GO as compared to those onto CNT.
Fig. 2A presents the Raman spectra of pure nanoparticles (GO, CNT, ZnO and TiO2) and nanohybrids (ZnO–GO, ZnO–CNT, TiO2–GO and TiO2–CNT). The vibration peaks of TiO2 are presented at 398 cm−1, 519 cm−1 and 641 cm−1, indicating the presence of the TiO2 anatase phase in all samples. The 430 cm−1 and 580 cm−1 peaks correspond to E2 and E1 (LO) modes of the wurtzite ZnO structure (Zamiri et al., 2014). The G band (related to the C–C vibrational mode) is the Raman spectrum of graphene-based materials, and it typically has a peak around 1580 cm−1. The D band peak around 1350 cm−1 corresponds to disordered carbon or a defective graphitic structure introduced to the crystalline structure (Perreault et al., 2015; Karaolia et al., 2018).
Fig. 2.

(A) Raman and (B) TGA analyses of four types of NHs.
The intensity of the D band in CNT based material is more significant or higher than that of the D band in the GO-based material. This suggests CNT-based nanocomposites (ZnO–CNT and TiO2–CNT) have more unstable and deficient structures than GO-based nanocomposites (ZnO–GO and TiO2–GO) (Karaolia et al., 2018).
Fig. 2B indicates the decomposition behavior of NHs by thermal gravimetric analysis (TGA). A major weight loss of ZnO–GO is observed at 800 °C, indicating 20% of weight loss caused by the combustion of carbon. According to a previous study, GO has a low-temperature stability and loses most of its mass when heated (Hsiao et al., 2013). Mass losses are due to decomposition of oxygen-containing function groups on GO to CO, CO2 and H2O and the thermal decomposition of the GO structure. A previous study analyzed GO-silica nanosheets and confirmed that a silica nanosheet acted as a protective layer that prevents oxidation degradation (Hsiao et al., 2013). The data suggest that ZnO and TiO2 protect GO because ZnO and TiO2 are far more thermally stable. Various forms of adsorbed water and partial degradation of GO resulted in the initial mass loss of ZnO–GO until 200 °C and further mass decrease.
However, since TiO2 is not as protective as the ZnO, the GO part of TiO2–GO degraded continuously until 600 °C. At 600 °C, GO was completely degraded, with only TiO2 left. TiO2–GO had approximately 50% mass loss, suggesting that 50% of the material was GO. According to a study by Bom et al. (2002), CNTs tend to decompose and lose most, if not all, of their mass at 500 °C, while our study indicates that TiO2 has a significant degree of stability compared to CNTs, as indicated by only a slight mass loss, most likely adsorption until 400 °C. Upon decomposition, the sample remains stable until 800 °C. More free water than adsorbed water is found in ZnO–CNT, as indicated by the sharp drop in a mass loss until 100 °C. Yet, the protecting effect of metal oxides on CNT is still apparent as indicated by slow degradation over a larger temperature range. Both ZnO–CNT and TiO2–CNT are likely to contain around 20% or less CNT based on the total mass loss.
3.2. Antibacterial effects of NHs on E. coli
Four mechanisms related to antibacterial effects of NHs are possible. First, metal oxide (e.g., ZnO and TiO2) nanoparticles are well known for their toxicity, especially as a photocatalyst under UV irradiation. Four types of NHs may have a significant generation of ROS, but the extent of ROS generation may be different depending on the degree of growth inhibition of the bacterial cells. Second, as previously observed in the characterization study, if GO serves as a platform for dispersion, steric effects could enhance the antibacterial effects of NHs on the bacteria. Third, if a significant release is observed from ZnO conjugated NHs, significant antibacterial effects may be found. Lastly, physicochemical properties may affect the antibacterial effects of NHs. To elaborate the aforementioned hypotheses, the growth inhibition of E. coli by NHs was quantified by estimating the number of cells using OD600, after the exposure of E. coli to NHs, whereas controls had NH free suspensions.
In nanohybrid-exposed cells, concentration–response relationships were drawn as increasing inhibition of bacterial growth over a 6 h time frame, except at the concentration of 20 mg L−1 and below 20 mg L−1 (Fig. 3A). The most significant antibacterial effect was observed from the exposure of ZnO–GO to E. coli (72% growth inhibition in 6 h exposure time). In a 2-h exposure of ZnO–GO at a 300 mg L−1 concentration to E. coli, the cell activity significantly decreased, and the cell growth curve indicated much slower cell growth than other cells exposed to 20 and 100 mg L−1 of ZnO–GO. The cell activity exposed to ZnO–CNT was also gradually reduced over time with approximately 35% growth inhibition after 6 h exposure, suggesting that over the exposure time, cell growth is significantly hindered, especially at higher concentrations of NHs. Comparing the growth inhibition of E. coli by NHs at a constant exposure time (6 h) (Fig. 3B), there was no growth inhibition of the bacteria from the four types of NHs at the lowest concentration (20 mg L−1). By contrast, the growth inhibition becomes significant as the concentrations of NHs increase.
Fig. 3.
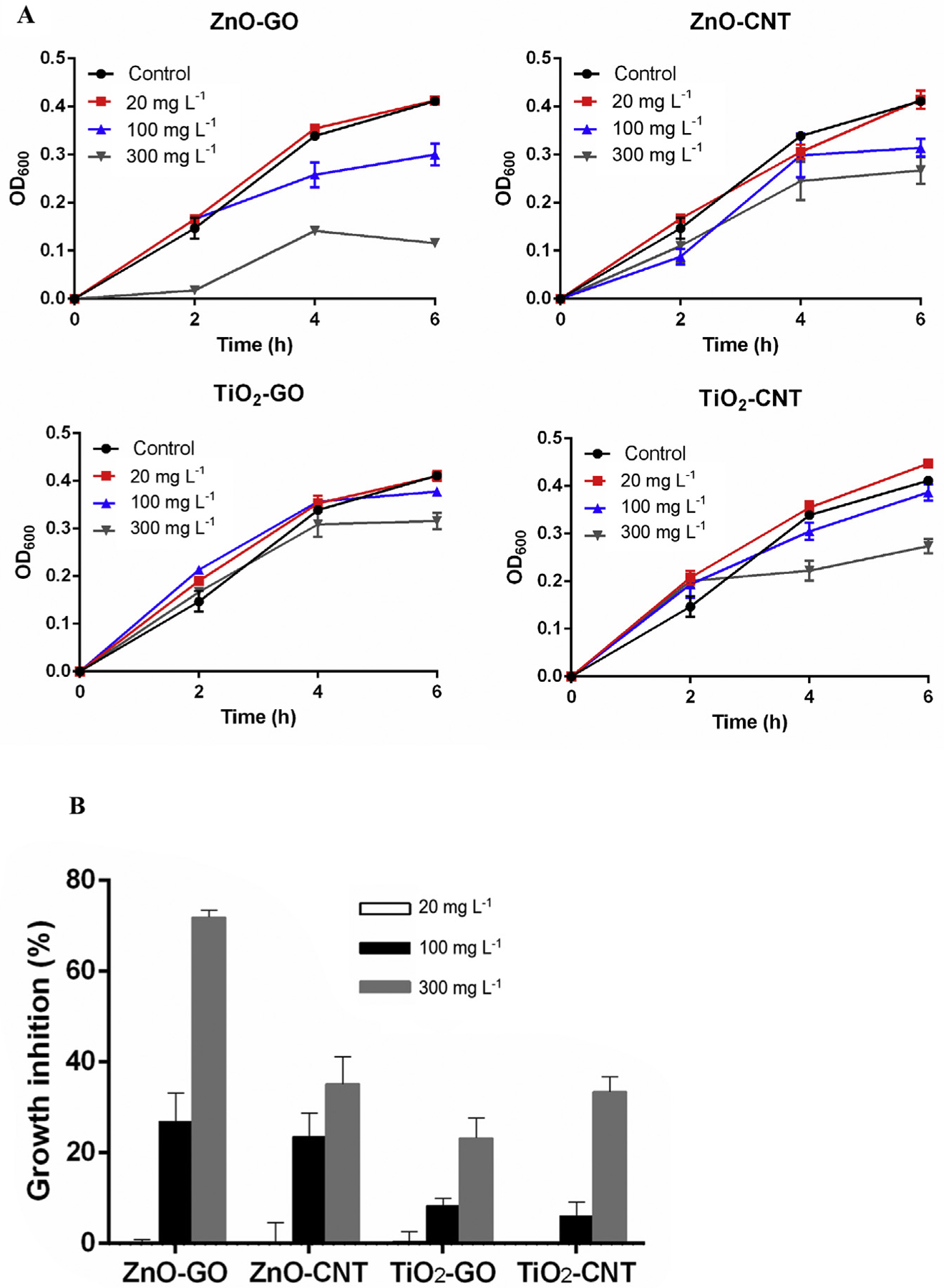
Antibacterial effect of four different types of NHs on E. coli (A) growth curve and (B) growth inhibition at 6 h.
Remarkably, ZnO-based NHs were more toxic than TiO2-based NHs, possibly because of higher solubility of ZnO NPs than that of TiO2 NPs; this is consistent with findings of other studies (Adams et al., 2006; Aruoja et al., 2009; Dasari et al., 2013). For instance, the growth inhibition rates of ZnO–GO and ZnO–CNT at a 100 mg L−1 concentration were around 27% and 24%, respectively, while 8.3% and 6.1% of growth inhibition were observed from TiO2–GO and TiO2–CNT correspondingly. As concentrations of NHs increased from 100 mg L−1 to 300 mg L−1, the most significant antibacterial effect was found from exposure of E. coli to ZnO–GO compared to that of ZnO–CNT, indicating that GO may act as a substrate responsible for the steric effect. Overall, the antibacterial effect was in the order ZnO–GO ≫ ZnO–CNT > TiO2–CNT ≈ TiO2–GO. The growth inhibition rates of TiO2–GO and TiO2–CNT were similar, at 8.3% and 6.1% at 100 mg L−1, and at 23.2, and 33.4% at 300 mg L−1, respectively, indicating that cell physiology, metabolism, degree of contact and agglomeration state might have contributed to the disparity in antibacterial effect (Adams et al., 2006; De Angelis et al., 2013).
A previous study (Adams et al., 2006) showed that the antibacterial activity of TiO2 NPs was significantly greater in the presence of light than in the dark, and under dark conditions, cell death after exposure to TiO2 NPs was diminished compared to that from exposure to ZnO NPs. Similarly, in this study, the generation of ROS might have contributed to the antibacterial effects of NHs. Nonetheless, less information is available as to what mechanisms contribute to the antibacterial effects of NHs and the discrepancy in antibacterial effects among NHs. Several studies (Li et al., 2014; Lin et al., 2014; Nam et al., 2014; Qi et al., 2017) revealed that the interaction between NPs and bacteria or the adsorption of NPs on bacteria or metal ions released from NPs can cause mechanical damages to cell walls, thereby disrupting the balance of charges on the surface of cells and resulting in bacterial death. In general, the antibacterial effects of NHs on E. coli suggest that the GO sheet serves as a platform for ZnO dispersion, so ZnO–GO NHs enhance the antibacterial property on bacterial cells due to ZnO-GO’s increased surface area (Chung et al., 2017; Rajeswari and Prabu, 2017). Several studies (Chen et al., 2013; Wang et al., 2014) demonstrated that the ZnO–GO nanocomposite has a better photocatalytic activity than the ZnO nanoparticle itself. Similarly, CNT is considered to have high efficiency in strengthening the photocatalytic activity of ZnO or TiO2 when ZnO or TiO2 is combined with a CNT (Qi et al., 2017; Sui et al., 2013; Nosrati et al., 2017).
3.3. Quantification of ROS generation
The production of ROS from NHs could be one of the critical factors influencing the antibacterial property. When light energy absorption induces charge separation, a nanocomposite produces pairs of electrons and holes. Then, excited electron and hole carriers move onto the surface of the nanocomposite and facilitate oxidation and reduction reactions that generate ROS including superoxide, hydrogen peroxide, and hydroxyl radicals, which are known to enhance the photocatalytic activity of nanocomposites, especially upon doping or coupling with other semiconductors such as carbon materials (Qi et al., 2017).
To explore one of the primary mechanisms, ROS generation from ZnO–GO, ZnO–CNT, TiO2–GO, and TiO2–CNT upon exposure to E. coli was quantified using oxidant-sensing fluorescent DCF-DA. The generation of ROS was enhanced with increasing concentrations (20–300 mg L−1) of four types of NHs and exposure times (Fig. 4). As an example, E. coli cells treated with ZnO–GO in the absence of NAC indicated that the ROS generation increased to 1.90 times for 20 mg L−1, 1.93 times for 100 mg L−1 and 2.32 times for 300 mg L−1. The increasing rate of ROS generation over exposure time and increasing concentration of NPs is also consistent with our recent study (Baek et al., 2017, 2018). To confirm the role of ROS in enhanced antibacterial effects by NHs, a scavenger of ROS, N-acetyl-L-cysteine (NAC), at concentrations of 0.5 and 2 mM, was used to pretreat cells. Compared to untreated cells with NAC, treated cells with NAC from ZnO–GO produced almost the same or even lower fluorescence intensity compared to cells exposed to 20 mg L−1 of ZnO–GO without NAC. Further, cells treated with a higher concentration of NAC (2 mM) produced significantly lower ROS compared to those with 0.5 mM NAC.
Fig. 4.

The amount of ROS release from E. coli exposed to four different types of NHs over time
Cells treated with 0.5 mM NAC produced slightly less ROS than those without NAC in ZnO–CNT. The ROS generation at 300 mg L−1 ZnO–CNT was significantly decreased at 2 mM NAC compared to that at 0.5 mM NAC. As NAC concentration increased from 0.5 mM to 2 mM, ROS generation from NAC-treated cells was also decreased, possibly due to the maximum capacity of NAC that is limited by an increase of accumulated ROS generation according to the amount of NAC (Ma et al., 2014). Similarly, the addition of NAC to TiO2–GO was also observed to reduce the fluorescence intensity at the concentration of 2 mM, while ROS generation from 0.5 mM of NAC had almost the same value as that from NAC-untreated cells.
In regard to TiO2–CNT, no significant difference in ROS generation was observed in the presence or in the absence of NAC. Given that NAC inhibits the generation of ROS, the primary antibacterial mechanism may not be due to ROS generation. Results on the quantification of ROS from NHs suggest that the extent of the antibacterial property of NHs is correlated with the amount of ROS generated from NHs upon exposure to E. coli, as ZnO–GO > ZnO–CNT > TiO2–GO > TiO2–CNT, suggesting that the primary antibacterial mechanism is ROS generation. Several studies (Adams et al., 2006; Kumar et al., 2011) indicated that the toxicity of ZnO is greater than that of TiO2 because of oxidative stress, particularly because there is more ROS generation from ZnO than from TiO2 NPs upon the interaction between bacterial cells and NPs. It was also reported that the difference in antibacterial effect was due to the agglomeration state of NPs from the interaction of NPs with bacterial cells (De Angelis et al., 2013). According to the study (Jassby et al., 2012), the agglomeration structure and size could affect quenching of both holes and free hydroxyl radicals, thereby influencing electrons or holes on NP surfaces. Cell deformation and rupture can be influenced by the generation of ROS since ROS reacts with organic biomolecules, including lipids, carbohydrates, proteins, and DNA (Dizaj et al., 2014).
A recent study (Huang et al., 2015) showed that GO can improve not only the dispersity of NPs but also active sites for functionalization, especially when GO is combined with metal oxides including ZnO and TiO2. Due to the increased surface area of GO, which enhances the dispersion of metal-oxide NPs, photocatalytic activity could also be enhanced (Liu et al., 2012b). The increasing adsorption of ZnO–GO nanocomposites causes electron transfer between ZnO and GO, thereby suppressing the recombination of electron–hole pairs by electron transfer (Zhang et al., 2011; Chen et al., 2013). Another study (Karaolia et al., 2018) found that E. coli exposed to TiO2–GO resulted in the reduction of a colony-forming unit (CFU) concentration of cells around 31% and suggested that ROS is the main influencing factor for inactivation of the damaged cells; this result was consistent with that of the present study. Under solar radiation, TiO2–GO composites indicated further removal of antibiotic-related microcontaminants in urban wastewater effluents (Karaolia et al., 2018).
According to this study (Qi et al., 2017), CNT enhanced the exposure of active sites and improved the dispersion of photocatalysts. Thus, the long distance of shutting electrons leads to blocking of the recombination of photo-generated e− and h+ pairs. While our experiments were carried out under preset room lights (for consistent conditions) in a microbiology laboratory, the photocatalytic effects by TiO2 and ZnO may also have contributed to the antibacterial property of NHs. Fig. 5 is a schematic of the proposed mechanism regarding the photocatalytic effect of NHs. For instance, when ZnO–CNT or TiO2–CNT absorbs light energy, photo-generated electrons are transferred from the valence band (VB) to the conduction band (CB) within metal-oxide NPs and leave holes in the VB as illustrated in Fig. 5.
Fig. 5.
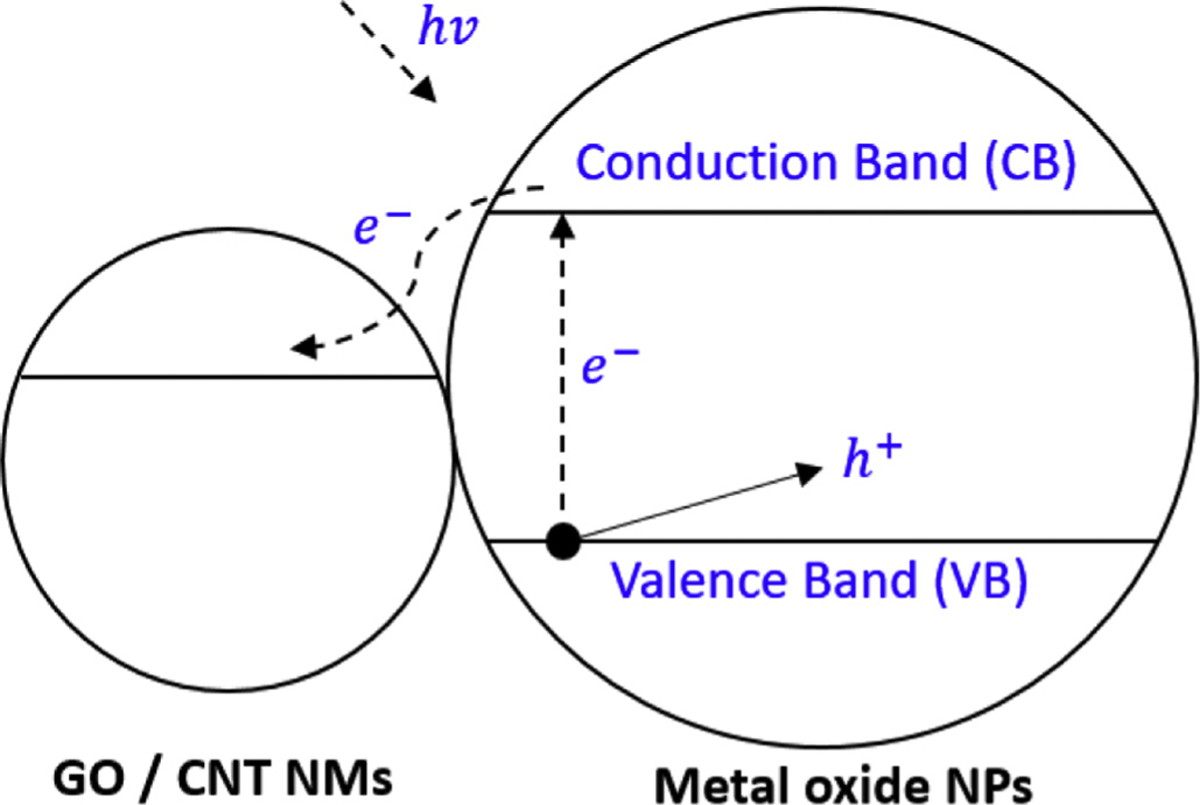
Schematic of the proposed mechanism for enhanced photocatalysis of NHs.
In the absence of the CNT, most of e− and h+ charges recombine quickly without any chemical activity (Yao et al., 2008). Therefore, this electron separation reduces recombination of electron–hole pairs and increases photocatalytic activity (Karaolia et al., 2018; Qi et al., 2017). A recent study illustrated that increasing the amount of TiO2 on a CNT surface produced more electron–hole pairs and thus more effective removal of tetracycline (TC) by using the TiO2–CNT nanocomposite (Ahmadi et al., 2017). Different sorption and photocatalytic activity may result from adjusting the nanohybrid ratio among nanomaterials. A study by Song et al. (2012) proved that a TiO2–CNT proportion over 1.5% caused low sorption and photocatalytic activity because of significant aggregation of TiO2 on the CNT surface. Increasing the content of TiO2 in nanohybrids may block the adsorption sites of CNT, thereby decreasing the overall adsorption capacity of TiO2 on the CNT surface (Das et al., 2014; Zhang et al., 2011).
3.4. SEM and TEM images of cells
As one of the antibacterial mechanisms, changes in physicochemical property may cause different degrees of the antibacterial property among different types of NHs. To better comprehend the interaction of NHs with E. coli cells, the surface morphologies of NHs after 6 h exposure of NHs to the cells were examined using SEM and TEM. The SEM results indicate that E. coli exhibited adsorption on or coverage of the surface of NHs (Fig. 6). In some of the bacteria, the membrane was collapsed and damaged (Fig. 6E), and some cell morphologies changed from a rod shape to a globular shape that appears in the pre-death stage. SEM images (Fig. 6 A, C, E, and G) indicate that the NHs changed the morphology of E. coli, inducing the growth inhibition and death of the cells. To further confirm the interaction between NHs and cells, TEM images (Fig. 6 B, D, F, and H) were taken through the cross-sectional examination of E. coli cells.
Fig. 6.
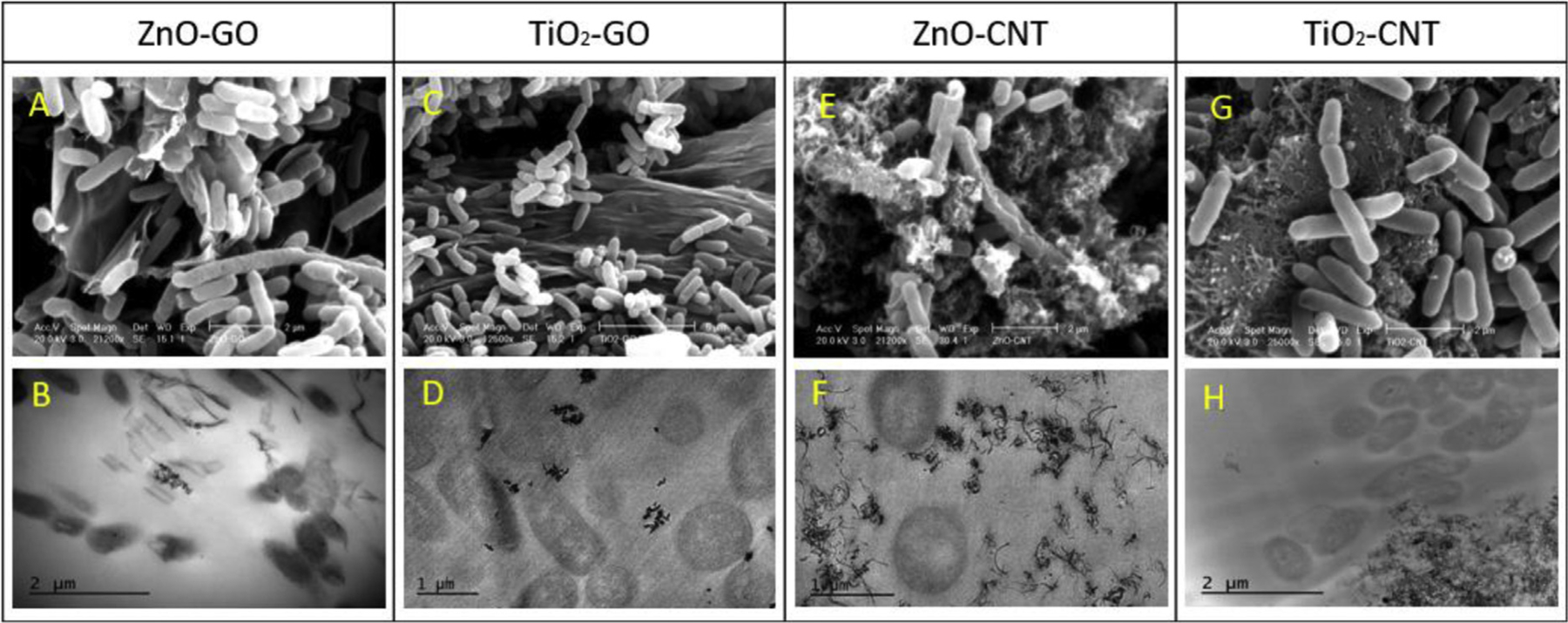
SEM and TEM images of four different types of NHs after their exposure to E. coli for 6 h.
Attachment of cells on the GO sheet resulted in cell-membrane damages, and a cut was observed upon exposure of E. coli to ZnO–GO (Fig. 6A). By contrast, the cells exposed to TiO2–GO were healthier than those exposed to ZnO-GO, and TiO2–GO NPs exhibited more aggregation than ZnO–GO did (Fig. 6 C and D). Fig. 6B indicates some physical interactions between GO sheets and bacterial cells. Bacterial cells may be involved in the GO edge, and ZnO NPs are attached on the surface of the cells. The TEM image of TiO2–GO indicates adhesion of nanocomposites to the cell surfaces, but without direct disruption of the bacterial membrane through penetration (Fig. 6D).
When ZnO NPs are attached to the surfaces of cells, bacterial death is initiated through disorganization of the cell membranes. Such electrostatic attachment and direct contact of ZnO NPs on bacterial cells cause damages to cell membranes, and wrapping of cell membranes with ZnO–GO composites may further prevent bacteria from being nourished through the medium (Stoimenov et al., 2002; Wang et al., 2014). While physical interaction between the GO sheet and cell layers could damage cells because of the membrane piercing into bacterial cells or GO slicing the cell, E. coli cells are found to have a repulsive interaction with GO (Mao et al., 2014; Romero-Vargas Castrillón et al., 2015; Tu et al., 2013). This indicates the influence of the GO sheet’s size on the antibacterial effect. This means more antibacterial effect from a smaller sheet size through oxidative mechanisms in which the higher defect density occurs as GO size decreases (Perreault et al., 2015).
Controversially, another study revealed that larger GO sheets induce a higher antibacterial effect by contributing to the capacity of larger sheets to cover bacterial cells and prevent the proliferation of cells (Liu et al., 2012a). Even if GO sheets are in contact with the bacterial cells, antibacterial properties of GO can still remain (Mangadlao et al., 2015). Layer types of GO were found to contain antimicrobial properties, rather than the physical interaction of GO sheets with cells (Hui et al., 2014). Such a study suggests the contact or direct piercing of the membrane by GO sheets to be an essential factor for such an antibacterial activity of GO.
Compared to GO-based NHs, the ZnO–CNT nanocomposite exhibits a mass of tangles around E. coli cells that are attached around cell membranes (Fig. 6E and F). The TEM images of E. coli after exposure to ZnO–CNT indicate that cell membranes were damaged by direct contact with CNT, but there is no penetration of CNT into the cells (Fig. 6F). TEM images of E. coli after exposure to TiO2–CNT for 6 h indicate that there is no direct interaction between cells and the TiO2–CNT nanocomposite (Fig. 6H). TiO2-CNT nanocomposites aggregated each other, but a single particle does not appear to stick to the cell surface. Such CNT clusters were observed in another study (Yang et al., 2010), which indicated that a short CNT (< 1 μm) does not interact with cells by their edge, whereas longer CNTs (1–5 μm) wrap on bacterial cells and individual CNTs can be found to interact with cells at the edge of a CNT. No internalization of a CNT into bacteria cells was reported, even if the bacteria wall was deeply associated with the CNT (Simon-Deckers et al., 2009).
3.5. FT-IR spectroscopy
The characteristic FT-IR spectra of GO, CNT, ZnO–GO, and TiO2–GO nanocomposites were examined to further investigate the interaction between NHs and E. coli (Fig. 7). As illustrated in Fig. 7A, the IR peaks of oxygen-containing functional groups at 1725 cm−1, 1150 cm−1, and 1180 cm−1 are assigned to C=O, C–OH, and C–O stretching vibrations, respectively. The peak at 1630 cm−1 is attributed to the C=C bond, and the IR spectrum of ZnO had a peak around 650 cm−1 associated with Zn–O. The intensity of the O–H peak for GO decreased significantly in ZnO–GO, and TiO2–GO composites associated with the oxygen functional groups were dramatically eliminated (Liu et al., 2017; Rajeswari and Prabu, 2017). Such decreased peaks in ZnO–GO indicate the further reduction of GO and attachment of ZnO NPs onto the GO nanosheet (Rajaura et al., 2017). The strong peaks around 500–900 cm−1 are attributed to the stretching vibration of Ti–O–Ti and possibly to Ti–O–C bonds (Karaolia et al., 2018). By contrast, as illustrated in Fig. 7B (IR spectra of CNT, ZnO–CNT, and TiO2–CNT nanocomposites), the CNT spectrum had no clear peaks at 500–4000 cm−1 (Nguyen and Shim, 2015). The spectrum from ZnO–CNT and TiO2–CNT also indicated O–H, C–H, C=O, C=C, and C–O peaks. The peaks corresponding to Ti–OH and Ti–O–Ti groups are at 1300 cm−1, and 550 cm−1, respectively (Yuan et al., 2017), and the peak around 500–550 cm−1 is attributed to the ZnO vibration (Upasani et al., 2017).
Fig. 7.
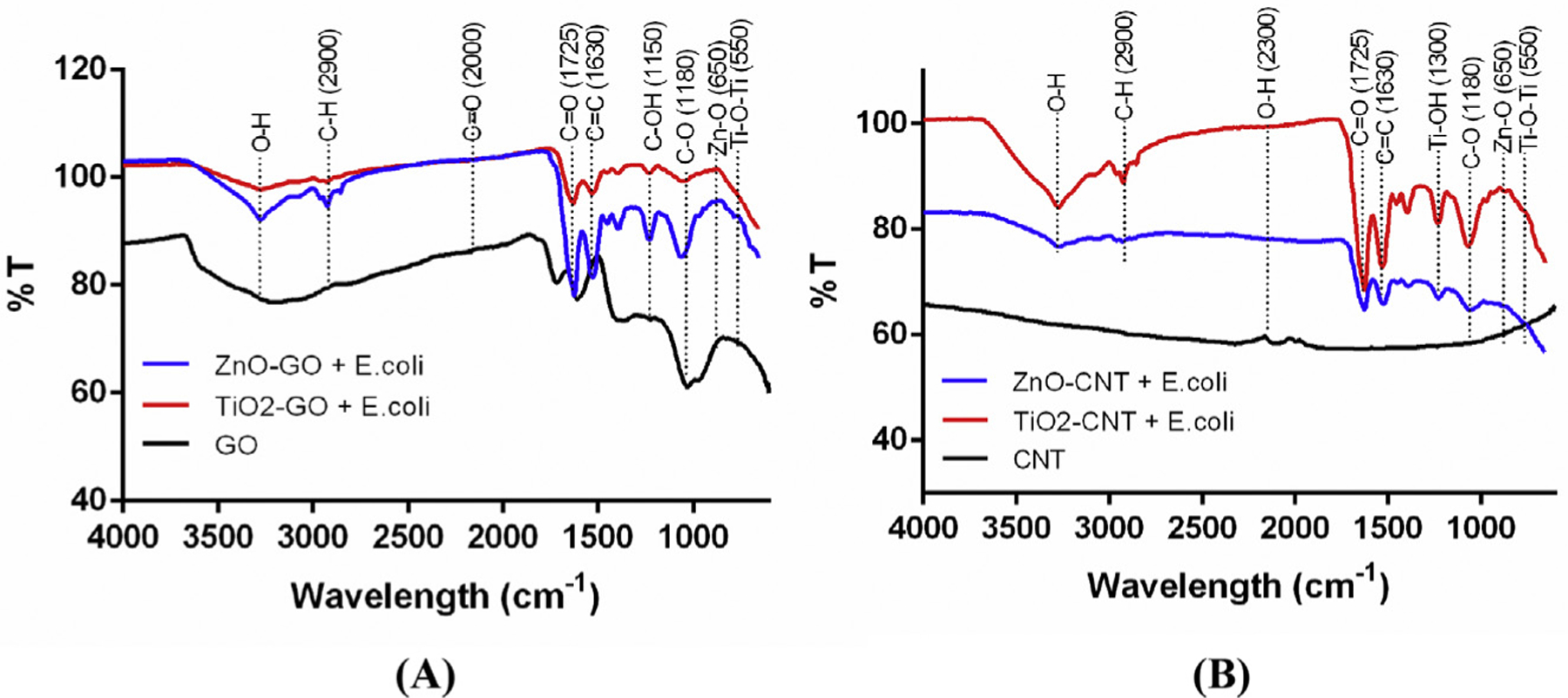
The FT-IR analysis of four different types of NHs with and without E. coli cells.
4. Conclusions
The antibacterial properties of four types of NHs (GO-based vs. CNT-based ZnO/TiO2) on E. coli were most significant in ZnO-GO, followed by those in ZnO–CNT and TiO2–CNT ≈ TiO2–GO. It was discovered that GO acts as a substrate to increase the dispersion of metal oxides (ZnO/TiO2) with a sheet-shaped morphology. Antibacterial activities of the NHs indicated both dose and exposure-time dependency, which was consistent with growth inhibitions of the bacterial cells and ROS generation. Among four NHs, NHs containing ZnO revealed higher toxicity to E. coli than those containing the TiO2 component. Both GO and CNT increased the dispersion of metal-oxide NPs through increasing ROS generation by suppressing the recombination of electron–hole pairs. Although the SEM and TEM images revealed that GO-based NHs had more attachment on the bacterial surface, CNT-based NHs significantly aggregated each other without interaction with cells. Further results indicate that GO-based NHs had a better interaction with ZnO through dispersion and significant enhancement of antibacterial effects in comparison to CNT-based NHs. The FT-IR analysis suggests the formation of considerable functional groups on the surfaces of NHs, as compared to the bare carbon materials. Among four hypothesized mechanisms, ROS generation and physicochemical property changes were found to be the primary antibacterial mechanisms, as confirmed by the quantification of ROS generation and sharp morphology changes that induced damages on cell membranes.
Although this study was primarily focused on antibacterial mechanisms among four types of NHs, the results are significant in terms of designing new antibacterial treatment methods, especially for multidrug-resistant bacteria detected in water to protect the environment and public health. The NHs could also be applied as an alternative method to conventional chlorine disinfection technology, especially for controlling regrowth of persistent microorganisms in water. Further studies would be necessary to develop point-of-use water treatment technology, especially under solar energy to facilitate photocatalytic properties of NHs for controlling emerging contaminants of concern, such as the recently emerging multidrug-resistant bacteria in the water.
Additionally, while conventional filtration technology could be applied for the removal of NHs, cost-effective and environmentally friendly methods, including a treatment system involving nanoparticleencapsulated alginate (Baek et al., 2019) should be developed and optimized for the best treatment efficiency.
Acknowledgments
This research was supported by the U.S. Environmental Protection Agency through the Office of Research and Development (AWARD: EP 17Z000237). The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. We thank Dr. Minseo Park and Mr. Min Khanal at Auburn University for Raman spectroscopy analysis as well as Dr. Suraneni Prannoy and Mr. Sivakumar Ramanathan for TGA analysis.
Abbreviations:
- CNT
Carbon nanotube
- E. coli
Escherichia coli
- GO
Graphene oxide
- NPs
Nanoparticles
- TiO2-CNT
Synthesized TiO2-CNT nanocomposite
- ZnO-CNT
Synthesized ZnO-CNT nanocomposite
- TiO2- GO
Synthesized TiO2-GO nanocomposite
- ZnO-GO
Synthesized ZnO-GO nanocomposite
- NHs
Nanohybrids
References
- Adams LK, Lyon DY, Alvarez PJ, 2006. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 40, 3527–3532. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Ramezani Motlagh H, Jaafarzadeh N, Mostoufi A, Saeedi R, Barzegar G, Jorfi S, 2017. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag 186, 55–63. [DOI] [PubMed] [Google Scholar]
- Akhavan O, Azimirad R, Safa S, 2011. Functionalized carbon nanotubes in ZnO thin films for photoinactivation of bacteria. Mater. Chem. Phys 130, 598–602. [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A, 2009. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ 407, 1461–1468. [DOI] [PubMed] [Google Scholar]
- Baek S, Joo SH, Blackwelder P, Toborek M, 2018. Effects of coating materials on antibacterial properties of industrial and sunscreen-derived titanium-dioxide nanoparticles on Escherichia coli. Chemosphere 208, 196–206. [DOI] [PubMed] [Google Scholar]
- Baek S, Joo SH, Kumar N, Toborek M, 2017. Antibacterial effect and toxicity pathways of industrial and sunscreen ZnO nanoparticles on Escherichia coli. J. Environ. Chem. Eng 5, 3024–3032. [Google Scholar]
- Baek S, Joo SH, Toborek M, 2019. Treatment of antibiotic resistant bacteria by encapsulation of ZnO nanoparticles in an alginate biopolymer: insights into treatment mechanisms. J. Hazard Mater 373, 122–130. [DOI] [PubMed] [Google Scholar]
- Batley GE, Kirby JK, McLaughlin MJ, 2012. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res 46, 854–862. [DOI] [PubMed] [Google Scholar]
- Bom D, Andrews R, Jacques D, Anthony J, Chen B, Meier MS, Selegue JP, 2002. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2, 615–619. [Google Scholar]
- Byrappa K, Dayananda AS, Sajan CP, Basavalingu B, Shayan MB, Soga K, Yoshimura M, 2008. Hydrothermal preparation of ZnO:CNT and TiO2:CNT composites and their photocatalytic applications. J. Mater. Sci 43, 2348–2355. [Google Scholar]
- Chen Y-L, Zhang C-E, Deng C, Fei P, Zhong M, Su B-T, 2013. Preparation of ZnO/GO composite material with highly photocatalytic performance via an improved two-step method. Chin. Chem. Lett 24, 518–520. [Google Scholar]
- Choi Y, Kim HA, Kim KW, Lee BT, 2018. Comparative toxicity of silver nanoparticles and silver ions to Escherichia coli. J. Environ. Sci. (China) 66, 50–60. [DOI] [PubMed] [Google Scholar]
- Chung YT, Mahmoudi E, Mohammad AW, Benamor A, Johnson D, Hilal N, 2017. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 402, 123–132. [Google Scholar]
- Das R, Abd Hamid SB, Ali ME, Ismail AF, Annuar MSM, Ramakrishna S, 2014. Multifunctional carbon nanotubes in water treatment: the present, past and future. Desalination 354, 160–179. [Google Scholar]
- Dasari TP, Pathakoti K, Hwang H-M, 2013. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J. Environ. Sci 25, 882–888. [DOI] [PubMed] [Google Scholar]
- De Angelis I, Barone F, Zijno A, Bizzarri L, Russo MT, Pozzi R, Franchini F, Giudetti G, Uboldi C, Ponti J, Rossi F, De Berardis B, 2013. Comparative study of ZnO and TiO(2) nanoparticles: physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 7, 1361–1372. [DOI] [PubMed] [Google Scholar]
- Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K, 2014. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl 44, 278–284. [DOI] [PubMed] [Google Scholar]
- Fu H, Jiang Y, Ding J, Zhang J, Zhang M, Zhu Y, Li H, 2018. Zinc oxide nanoparticle incorporated graphene oxide as sensing coating for interferometric optical microfiber for ammonia gas detection. Sensor. Actuator. B Chem 254, 239–247. [Google Scholar]
- Guarino B, 2017. The Health Dangers from Hurricane Harvey’s Floods and Houston’s Chemical Plants. https://www.washingtonpost.com/news/to-your-health/wp/2017/08/29/the-health-consequences-to-expect-from-hurricane-harveys-floods/?noredirect=on&utm_term=.5316de927b18, Accessed date: 12 March 2018.
- Hsiao MC, Ma CC, Chiang JC, Ho KK, Chou TY, Xie X, Tsai CH, Chang LH, Hsieh CK, 2013. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide-silica hybrid nanosheets. Nanoscale 5, 5863–5871. [DOI] [PubMed] [Google Scholar]
- Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C, 2010. Graphene-based antibacterial paper. ACS Nano 4, 4317–4323. [DOI] [PubMed] [Google Scholar]
- Huang J, Zang J, Zhao Y, Dong L, Wang Y, 2014. One-step synthesis of nanocrystalline TiO2-coated carbon nanotube support for Pt electrocatalyst in direct methanol fuel cell. Mater. Lett 137, 335–338. [Google Scholar]
- Huang Y, Wang T, Zhao X, Wang X, Zhou L, Yang Y, Liao F, Ju Y, 2015. Poly (lactic acid)/graphene oxide-ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J. Appl. Chem. Biotechnol 90, 1677–1684. [Google Scholar]
- Hui L, Piao JG, Auletta J, Hu K, Zhu Y, Meyer T, Liu H, Yang L, 2014. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces 6, 13183–13190. [DOI] [PubMed] [Google Scholar]
- Jassby D, Farner Budarz J, Wiesner M, 2012. Impact of aggregate size and structure on the photocatalytic properties of TiO2 and ZnO nanoparticles. Environ. Sci. Technol 46, 6934–6941. [DOI] [PubMed] [Google Scholar]
- Jeon S-K, Kim E-J, Lee J, Lee S, 2016. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard Mater 317, 312–318. [DOI] [PubMed] [Google Scholar]
- Jerome S, 2016. For First Time Ever, Superbugs Found in Drinking Water. https://www.wateronline.com/doc/for-first-time-ever-superbugs-found-in-drinking-water-0001, Accessed date: 12 March 2018.
- Karaolia P, Michael-Kordatou I, Hapeshi E, Drosou C, Bertakis Y, Christofilos D, Armatas GS, Sygellou L, Schwartz T, Xekoukoulotakis NP, Fatta-Kassinos D, 2018. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B 224, 810–824. [Google Scholar]
- Kȩdzierska S, Stainiszewska M, Potrykus J, Wegrzyn G, 1999. The effect of some antibiotic-resistance-conferring plasmids on the removal of the heat-aggregated proteins from Escherichia coli cells. FEMS Microbiol. Lett 176, 279–284. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR, 2008. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem 27, 1825–1851. [DOI] [PubMed] [Google Scholar]
- Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A, 2011. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med 51, 1872–1881. [DOI] [PubMed] [Google Scholar]
- Li Y, Niu J, Zhang W, Zhang L, Shang E, 2014. Influence of aqueous media on the ROS-mediated toxicity of ZnO nanoparticles toward green fluorescent protein-expressing Escherichia coli under UV-365 irradiation. Langmuir 30, 2852–2862. [DOI] [PubMed] [Google Scholar]
- Lin X, Li J, Ma S, Liu G, Yang K, Tong M, Lin D, 2014. Toxicity of TiO2 nanoparticles to Escherichia coli: effects of particle size, crystal phase and water chemistry. PLoS One 9, e110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LP, Yang XN, Ye L, Xue DD, Liu M, Jia SR, Hou Y, Chu LQ, Zhong C, 2017. Preparation and characterization of a photocatalytic antibacterial material: graphene oxide/TiO2/bacterial cellulose nanocomposite. Carbohydr. Polym 174, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, Wang L, Kong J, Chen Y, 2012a. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 28, 12364–12372. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hu Y, Zhou M, Qian H, Hu X, 2012b. Microwave-assisted non-aqueous route to deposit well-dispersed ZnO nanocrystals on reduced graphene oxide sheets with improved photoactivity for the decolorization of dyes under visible light. Appl. Catal. B 125, 425–431. [Google Scholar]
- Ma H, Wallis LK, Diamond S, Li S, Canas-Carrell J, Parra A, 2014. Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ. Pollut 193, 165–172. [DOI] [PubMed] [Google Scholar]
- Mangadlao JD, Santos CM, Felipe MJ, de Leon AC, Rodrigues DF, Advincula RC, 2015. On the antibacterial mechanism of graphene oxide (GO) LangmuirBlodgett films. Chem. Commun 51, 2886–2889. [DOI] [PubMed] [Google Scholar]
- Mao J, Guo R, Yan LT, 2014. Simulation and analysis of cellular internalization pathways and membrane perturbation for graphene nanosheets. Biomaterials 35, 6069–6077. [DOI] [PubMed] [Google Scholar]
- Molina-Aja A, Garcia-Gasca A, Abreu-Grobois A, Bolan-Mejia C, Roque A, GomezGil B, 2002. Plasmid profiling and antibiotic resistance of Vibrio strains isolated from cultured penaeid shrimp. FEMS Microbiol. Lett 213, 7–12. [DOI] [PubMed] [Google Scholar]
- Nam D-H, Lee B-C, Eom I-C, Kim P, Yeo M-K, 2014. Uptake and bioaccumulation of titanium- and silver-nanoparticles in aquatic ecosystems. Mol. Cell. Toxicol 10, 9–17. [Google Scholar]
- Nguyen VH, Shim J-J, 2015. Green synthesis and characterization of carbon nanotubes/polyaniline nanocomposites. J. Spectrosc 2015, 1–9. [Google Scholar]
- Nosrati R, Olad A, Shakoori S, 2017. Preparation of an antibacterial, hydrophilic and photocatalytically active polyacrylic coating using TiO2 nanoparticles sensitized by graphene oxide. Mater. Sci. Eng. C. Mater. Biol. Appl 80, 642–651. [DOI] [PubMed] [Google Scholar]
- Perreault F, de Faria AF, Nejati S, Elimelech M, 2015. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9 7726–7236. [DOI] [PubMed] [Google Scholar]
- Qi K, Cheng B, Yu J, Ho W, 2017. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Comp 727, 792–820. [Google Scholar]
- Rajaura RS, Sharma V, Ronin RS, Gupta DK, Srivastava S, Agrawal K, Vijay YK, 2017. Synthesis, characterization and enhanced antimicrobial activity of reduced graphene oxide–zinc oxide nanocomposite. Mater. Res. Express 4, 025401. [Google Scholar]
- Rajeswari R, Prabu HG, 2017. Synthesis characterization, antimicrobial, antioxidant, and cytotoxic activities of ZnO nanorods on reduced graphene oxide. J. Inorg. Organomet. Polym. Mater 28, 679–693. [Google Scholar]
- Romero-Vargas Castrillón S, Perreault F, de Faria AF, Elimelech M, 2015. Interaction of graphene oxide with bacterial cell membranes: insights from force spectroscopy. Environ. Sci. Technol. Lett 2, 112–117. [Google Scholar]
- Simon-Deckers A, Loo S, Mayne-L’hermite M, Herlin-Boime N, Menguy N, Reynaud C, Gouget B, Carrière M, 2009. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol 43, 8423–8429. [DOI] [PubMed] [Google Scholar]
- Song C, Chen P, Wang C, Zhu L, 2012. Photodegradation of perfluorooctanoic acid by synthesized TiO2-MWCNT composites under 365 nm UV irradiation. Chemosphere 86, 853–859. [DOI] [PubMed] [Google Scholar]
- Stengl V, Bakardjieva S, Grygar TM, Bludska J, Kormunda M, 2013. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ, 2002. Metal oxide nanoparticles as bactericidal agents. Langmuir 18, 6679–6686. [Google Scholar]
- Sui M, Zhang L, Sheng L, Huang S, She L, 2013. Synthesis of ZnO coated multiwalled carbon nanotubes and their antibacterial activities. Sci. Total Environ 452–453, 148–154. [DOI] [PubMed] [Google Scholar]
- Tetz G, Artemenko N, Zaslavskaya N, Sternin Y, Knorring G, Tetz V, 2012. Effect of nucleolytic, proteolytic, and lipolytic enzymes on transfer of antibiotic resistance genes in mixed bacterial communities. Univers. J. Med. Dent 1, 46–50. [Google Scholar]
- Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R, 2013. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol 8, 594–601. [DOI] [PubMed] [Google Scholar]
- Upasani P, Sreekumar TV, Gaikar VG, Jha N, 2017. Preparation of ZnO nanoribbon–MWCNT composite film and its application as antimicrobial bandage, antibacterial filter and thermal IR camouflage material. Bull. Mater. Sci 40, 865–876. [Google Scholar]
- van Elsas JD, Semenov AV, Costa R, Trevors JT, 2011. Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 5, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lin Z, Wang T, Yao Z, Qin M, Zheng S, Lu W, 2016a. Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J. Hazard Mater 308, 328–334. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia B, Zhu X, Chen J, Qiu S, Li J, 2008. Controlled modification of multiwalled carbon nanotubes with ZnO nanostructures. J. Solid State Chem 181, 822–827. [Google Scholar]
- Wang Y, Zhu X, Lao Y, Lv X, Tao Y, Huang B, Wang J, Zhou J, Cai Z, 2016b. TiO2 nanoparticles in the marine environment: physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ 565, 818–826. [DOI] [PubMed] [Google Scholar]
- Wang YW, Cao A, Jiang Y, Zhang X, Liu JH, Liu Y, Wang H, 2014. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 6, 2791–2798. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N, 2012. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol 46, 2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Mamouni J, Tang Y, Yang L, 2010. Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir 26, 16013–16019. [DOI] [PubMed] [Google Scholar]
- Yao Y, Li G, Ciston S, Lueptow RM, Gray KA, 2008. Photoreactive TiO2/carbon nanotube composites: synthesis and reactivity. Environ. Sci. Technol 42, 4952–4957. [DOI] [PubMed] [Google Scholar]
- Yuan C, Hung CH, Yuan CS, Li HW, 2017. Preparation and application of immobilized surfactant-modified PANi-CNT/TiO(2) under visible-light irradiation. Materials-Basel 10, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamiri R, Rebelo A, Zamiri G, Adnani A, Kuashal A, Belsley MS, Ferreira JMF, 2014. Far-infrared optical constants of ZnO and ZnO/Ag nanostructures. RSC Adv. 4, 20902–20908. [Google Scholar]
- Zhang K, Zhang FJ, Chen ML, Oh WC, 2011. Comparison of catalytic activities for photocatalytic and sonocatalytic degradation of methylene blue in present of anatase TiO2-CNT catalysts. Ultrason. Sonochem 18, 765–772. [DOI] [PubMed] [Google Scholar]


