Abstract
Background
Adult T-cell leukemia-lymphoma (ATL) is an aggressive mature lymphoid proliferation associated with poor prognosis. Standard of care includes chemotherapy and/or the combination of zidovudine and interferon-alpha. However, most patients experience relapse less than 6 months after diagnosis. Allogeneic stem cell transplantation is the only curative treatment, but is only feasible in a minority of cases. We previously showed in a mouse model that Arsenic trioxide (As2O3) targets ATL leukemia initiating cells.
Results
As2O3 consolidation was given in 9 patients with ATL (lymphoma n = 4; acute n = 2; and indolent n = 3), who were in complete (n = 4) and partial (n = 3) remission, in stable (n = 1) and in progressive (n = 1) disease. Patients received up to 8 weeks of As2O3 at the dose of 0.15 mg/kg/day intravenously in combination with zidovudine and interferon-alpha. One patient in progression died rapidly. Of the remaining eight patients, three with indolent ATL subtype showed overall survivals of 48, 53 and 97 months, and duration of response to As2O3 of 22, 25 and 73 months. The other 5 patients with aggressive ATL subtype had median OS of 36 months and a median duration of response of 10 months. Side effects were mostly hematological and cutaneous (one grade 3) and reversible with dose reduction of AZT/IFN and/or As2O3 discontinuation. The virus integration analysis revealed the regression of the predominant malignant clone in one patient with a chronic subtype.
Conclusion
These results suggest that consolidation with As2O3 could be an option for patients with ATL in response after induction therapy and who are not eligible for allogeneic stem cell transplantation.
Keywords: Arsenic trioxide, ATL
Background
Adult T-cell leukemia-lymphoma (ATL) is an aggressive and mature lymphoid proliferation associated with the human T cell leukemia virus type 1 (HTLV-1) [1, 2]. ATL patients have a heterogeneous presentation and the Shimoyama classification divides the disease into four major subtypes, from indolent, slowly progressive disease (smouldering and chronic) to aggressive and life-threatening disease (lymphoma and acute) [3]. All are characterized by a dismal long-term prognosis and a low median survival rate, of 8 months, 10 months, 31 months and 55 months for the acute, lymphoma, chronic and smouldering subtypes respectively [4].
Although chemotherapy combinations improve the response rate in aggressive ATL subtype, the overall survival remains poor. Patients with indolent ATL subtype have a better prognosis but long-term survival is also poor with a watchful-waiting policy or with chemotherapy [4]. The combination of zidovudine (AZT) and interferon-alpha (IFN) may induce long term response in indolent and a small proportion of acute type [5]. Allogeneic stem cell transplantation is the only curative treatment in responding patients but its use is limited to a minority of patients [6]. The anti-CCR4 antibody mogamulizumab showed interesting results in relapsed patients, but a randomized trial in newly diagnosed ATL showed no benefit of its addition to chemotherapy in term of progression-free survival (PFS) and overall survival (OS), despite an increase response rate [7, 8].
In prior ex vivo studies, we showed that arsenic trioxide synergizes with IFN to selectively induce ATL cell apoptosis through the degradation by the proteasome of the oncoprotein Tax [9, 10]. This combination showed some signals of efficacy but a low rate of response in relapsed or refractory ATL patients [11]. A pilot study reported 100% response rate including 70% complete remission in newly diagnosed chronic ATL patients treated with the combination of arsenic, IFN and AZT [12]. We recently showed that this combination cures ATL developed in Tax-transgenic mice through selective targeting of leukemia-initiating cell (LIC) activity [13], suggesting that the best use of arsenic and IFN may be as a consolidation or maintenance therapy. Thus, we performed a retrospective study analyzing the outcome of patients treated with the combination of arsenic trioxide and AZT/IFN as consolidation after induction therapy with chemotherapy or antiviral therapy.
Patients and methods
Patients and diagnosis criteria
This retrospective study included nine newly diagnosed, previously untreated ATL patients. Patients’ characteristics are described in Table 1. This study was approved by the local ethic committee (CNIL: number 1692254 and CPP IRB registration number 000001072). TP53 status was evaluated in seven patients by a functional assay as previously described [14].
Table 1.
Patient’s characteristics
| Patient | Sex | Age at diagnosis | Clinical subtype | p53 activity | First line treatment | Interval between induction therapy and As2O3 consolidation (months) | Disease status at time/after of A2So3 | Treatment duration (weeks) | OS since diagnosis (months) | Death | OS since arsenic (months) | Duration of response since As2O3 (months) | Progression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATL 6 | M | 51.6 | Chronic | F | AZT-IFN-VP16 | 24.4 | SD/SD | 4 | 53 | Yes | 28 | 22 | Yes |
| ATL 9 | M | 50.6 | Chronic | F | CHOP like | 20.1 | CR/CR | 6 | 48 | Yes | 28 | 25 | Yes |
| ATL 11 | M | 27.7 | Chronic | F | LSG 15 + AZT-IFN | 13.2 | VGPR/CR | 6 | 97 | Yes | 83 | 73 | Yesa |
| ATL 7 | F | 59.8 | Acute | F | CHOP like | 6.2 | PD/PD | 4 | 8 | Yes | 1 | NA | Yes |
| ATL 14 | F | 35.1 | Acute | NF | Alemtuzumab-CHOP | 2.2 | CR/CR | 4 | 12 | Yes | 10 | 5 | Yes |
| ATL 43 | F | 66.3 | Lymphoma | F | CHOP like | 6.3 | CR/CR | 8 | 19 | Yes | 12 | 5 | Yes |
| ATL 44 | F | 54.8 | Lymphoma | F | CHOP like | 3.8 | VGPR/CR | 8 | 36 | Yes | 32 | 29 | Yes |
| ATL 64 | M | 63.1 | Lymphoma | ND | CHOP/DHAOx | 13 | CR/CR | 8 | 65 | No | 51 | 51 | No |
| ATL 65 | M | 54.4 | Lymphoma | ND | CHOP | 4 | PR/PR | 16 | 41 | Yes | 36 | 10 | Yes |
M, male; F, female; CHOP, cyclcophosphamide, doxorubicine, oncovin, prednisone; DHAOx, dexamethasone, aracytine high dose, oxaliplatine; CR, complete response; PR, partial response; VGPR, very good partial response; SD, stable disease; PD, progressive disease
F, functional; NF, non functional (this patient was found to have a p.V274A variant of TP53)
aClone switch
Treatment schedule
After induction with chemotherapy (mainly anthracyclin-based regimen including LSG and CHOP like regimen) and/or AZT/IFN, patients received up to 8 weeks of arsenic at the dose of 0.15 mg/kg/day intravenously (Table 1) in combination with oral zidovudine (AZT; 600 mg/day) and subcutaneous recombinant IFN (Roferon, ROCHE® 3 millions/day) or pegylated IFN (PEG-IFN Viraferon MSD® 1.5 µg/kg/week). During the consolidation phase, patients could receive only IFN/AZT combination between the arsenic infusions.
In case of hematological toxicity, growth factors such as GCSF or EPO were used, or the antiviral therapy dose was reduced as per physician choice.
Response criteria and overall survival
Response evaluation was performed according to consensus published criteria [15]. Overall survival (OS) was defined as the period between initiation of treatment and the date of death or last follow-up.
HTLV-I proviral load and clonality assay
DNA extraction was done using Invitrogen kit (QiAmp or blood and cell’s DNA extraction kit) and performed according to manufacturer’s instructions. HTLV DNA was quantified by real-time PCR in the pX region as previously described [16]. High-throughput sequencing for the genome-wide identification and quantification of proviral integration sites was performed as previously described [17].
Results and discussion
At time of As2O3 initiation, four patients were in CR, two in VGPR, one in PR, one had stable disease and one progressive disease. The consolidation therapy with As2O3, IFN and AZT was administered for a median period of 6 months (2–24 months) after completion of first-line induction treatment (13 months when ATL7 is excluded). Side effects were mostly hematologic and manageable with discontinuation of AZT/IFN or addition of growth factors. Four patients experienced cutaneous side effects, including one grade 3, which were all reversible on discontinuation of As2O3 (Additional file 1: Table S1). In addition to these objective toxicities, most patients experienced severe fatigue especially during the last weeks of As2O3 therapy, which was rapidly reversible after As2O3 discontinuation.
The patient with progressive disease (acute type) at time of arsenic initiation did not respond to As2O3/AZT/IFN consolidation and died rapidly. The median duration of response in the other 8 patients was 24 months (5–73 months) and 39 months (7–86 months) from initiation of As2O3/AZT/IFN consolidation and from initiation of first line treatment, respectively. Median OS was 28 months (1–70 months) and 42 months (8–97 months) from As2O3/AZT/IFN consolidation start and initial diagnosis, respectively (Table 1). Two patients experienced a prolonged survival (Chronic n = 1, Lymphoma n = 1). One patient with a lymphoma subtype remained in remission 51 months from arsenic initiation and 64 months from diagnosis. The other patient with a chronic subtype remained in remission 86 months after Arsenic initiation and 97 months after diagnosis before relapsing with a new tumoral clone as described below. The three indolent ATLs had an OS of 48, 53 and 97 months and duration of response to As2O3 of 22, 25 and 73 months. The six aggressive ATLs had a median OS of 27,5 months (range 8–65 months) and a median duration of response to As2O3 of 10 months (5–55 months).
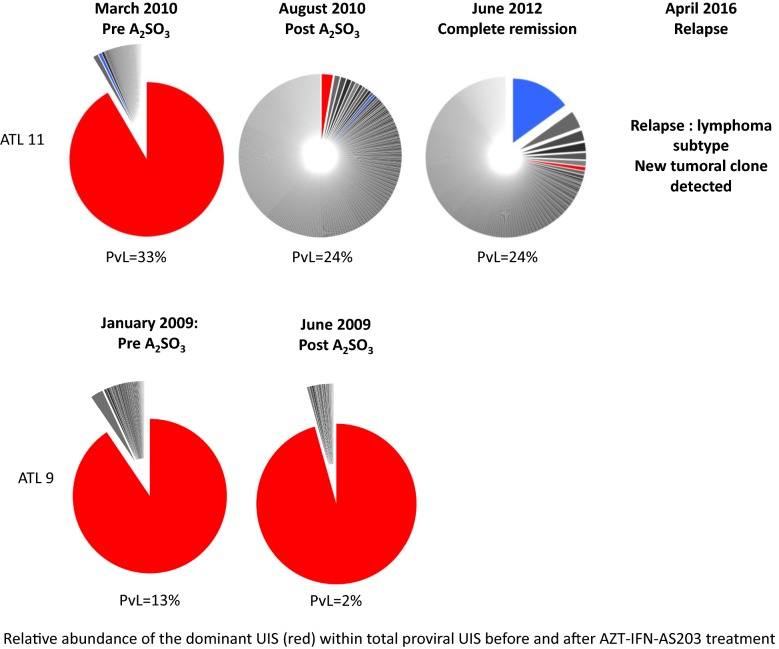
Longitudinal analysis of the HTLV-I proviral load (PvL) revealed no difference during treatment except in one patient (ATL 14) with acute ATL, who showed a dramatic decrease of proviral load after chemotherapy. Viral integration clonality analysis was assessed in 2 patients. One patient (ATL 11) who had a normal lymphocyte count but with an excess of phenotypically abnormal T-cells and one dominant clone representing 92% of infected cells exhibited 1 month after As2O3 treatment a regression of the predominant malignant clone and restoration of an oligoclonal architecture, both in proportion and in absolute count, while the proviral load remained stable. Interestingly, this patient remained in remission 86 months after initiation of arsenic and 97 months from diagnosis but finally relapsed with a different clone, as demonstrated by the finding of a different TCR rearrangement as previously published [18]. In contrast, another patient (ATL 9), with a chronic subtype initially treated with chemotherapy had a normal lymphocyte count with an excess of abnormal phenotype T cells with one dominant clone that represented 91% of infected cells, which remained unchanged after completion of As2O3 treatment. This patient progressed to an acute subtype 2 years later and died (Fig. 1).
Fig. 1.
Virus clonality architecture timeline. Responding (ATL 11) and resistant patients (ATL 9)
Taken together, as predicted by our mice model and our previous clinical study with the triple induction with As2O3/AZT/IFN in chronic ATL, this retrospective clinical analysis shows that As2O3 consolidation in combination with low-dose AZT/IFN maintenance may enhance long-term disease control also in ATL lymphoma with moderate side effects [12, 13]. In addition, although based on a small number, our data suggest that sequential analysis of proviral load and architecture of the virus clonality could serve as a good surrogate marker of long-term response rather than the viral load and lymphocyte count.
However, despite prolonged responses in some cases, most patients ultimately relapsed, suggesting that one cycle of arsenic consolidation may not be sufficient. Future trials are warranted to investigate whether or not multiple cycles of arsenic consolidation are needed in ATL to prevent relapses.
Supplementary information
Acknowledgements
Not applicable.
Abbreviations
- ATL
Adult T-cell leukemia-lymphoma
- HTLV-1
Human T cell leukemia virus type 1
- AZT
Zidovudine
- INF
Interferon-alpha
- PFS
Progression free survival
- OS
Overall survival
- LIC
Leukemic initiating cells
- WT
Wild type
- PEG-INF
Pegylated interferon
- TCR
T-cell receptor
Authors’ contributions
AM, AB, HdT, FS and OH designed research, AM, LC, AW, VA, VAF, HdT, CB, OH, FS performed research and analyzed data, AM, RD, MC, DS, LF, OH, FS treated patients and provided data. AM, AB, OH and FS wrote the first draft of the paper. All authors read and approved the final manuscript.
Funding
This study was supported by Institut national du cancer (INCA) and cancéropole d’Ile de France grants.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The local ethic committee approved this study (CNIL: number 1692254 and CPP IRB registration number 000001072) and all surviving patients gave their consent.
Consent for publication
All authors contributed to paper correction and editing.
Competing interests
The authors declare no conflict of interests related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ali Bazarbachi, Olivier Hermine and Felipe Suarez contributed equally to this work
Contributor Information
Ambroise Marçais, Email: ambroise.marcais@aphp.fr.
Olivier Hermine, Email: ohermine@gmail.com.
Felipe Suarez, Email: felipe.suarez@aphp.fr.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12977-020-0513-y.
References
- 1.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe T. Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood. 2017;129(9):1071–1081. doi: 10.1182/blood-2016-09-692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–1987) Br J Haematol. 1991;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 4.Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 5.Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 6.Kanda J, Hishizawa M, Utsunomiya A, et al. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood. 2012;119(9):2141–2148. doi: 10.1182/blood-2011-07-368233. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(9):1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 8.Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol. 2015;169(5):672–682. doi: 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazarbachi A, El-Sabban ME, Nasr R, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999;93(1):278–283. doi: 10.1182/blood.V93.1.278. [DOI] [PubMed] [Google Scholar]
- 10.El-Sabban ME, Nasr R, Dbaibo G, et al. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 2000;96(8):2849–2855. [PubMed] [Google Scholar]
- 11.Hermine O, Dombret H, Poupon J, et al. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J Off J Eur Haematol Assoc EHA. 2004;5(2):130–134. doi: 10.1038/sj.thj.6200374. [DOI] [PubMed] [Google Scholar]
- 12.Kchour G, Tarhini M, Kooshyar M-M, et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL) Blood. 2009;113(26):6528–6532. doi: 10.1182/blood-2009-03-211821. [DOI] [PubMed] [Google Scholar]
- 13.El Hajj H, El-Sabban M, Hasegawa H, et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J Exp Med. 2010;207(13):2785–2792. doi: 10.1084/jem.20101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaman JM, Frebourg T, Moreau V, et al. A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci USA. 1995;92(9):3963–3967. doi: 10.1073/pnas.92.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(3):453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marçais A, Waast L, Bruneau J, et al. Adult T cell leukemia aggressivenness correlates with loss of both 5-hydroxymethylcytosine and TET2 expression. Oncotarget. 2017;8(32):52256–52268. doi: 10.18632/oncotarget.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillet NA, Malani N, Melamed A, et al. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood. 2011;117(11):3113–3122. doi: 10.1182/blood-2010-10-312926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artesi M, Marçais A, Durkin K, et al. Monitoring molecular response in adult T-cell leukemia by high-throughput sequencing analysis of HTLV-1 clonality. Leukemia. 2017;31(11):2532–2535. doi: 10.1038/leu.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.