Abstract
Glucagon and its partner insulin are dually linked in both their secretion from islet cells and their action in the liver. Glucagon signaling increases hepatic glucose output, and hyperglucagonemia is partly responsible for the hyperglycemia in diabetes, making glucagon an attractive target for therapeutic intervention. Interrupting glucagon signaling lowers blood glucose but also results in hyperglucagonemia and α-cell hyperplasia. Investigation of the mechanism for α-cell proliferation led to the description of a conserved liver–α-cell axis where glucagon is a critical regulator of amino acid homeostasis. In return, amino acids regulate α-cell function and proliferation. New evidence suggests that dysfunction of the axis in humans may result in the hyperglucagonemia observed in diabetes. This discussion outlines important but often overlooked roles for glucagon that extend beyond glycemia and supports a new role for α-cells as amino acid sensors.
Introduction
Glucagon, a 29–amino acid peptide derived from the preproglucagon gene, is produced by α-cells in the pancreatic islet. Together with insulin secreted from β-cells, these two hormones are major regulators of nutrient homeostasis. Relative to our understanding of β-cells, α-cells remain less understood. However, new research into factors that regulate α-cell biology is illuminating its role. This article will discuss the relationship between glucagon and amino acids while simultaneously suggesting that glucagon’s role of being simply a counterregulatory hormone to raise glucose levels is too simplistic (1).
Interrupting Liver Glucagon Signaling Improves Glycemia but Results in Hyperglucagonemia and α-Cell Hyperplasia
Given its history, it is understandable how glucagon received its name (short for GLUCose+AGONist). Glucagon was first described independently in 1923 as a “toxic fraction” and “hyperglycemic substance” in attempts to purify insulin from pancreatic extracts (2,3). Glucagon was further characterized 25 years later by Earl Sutherland and R.D.H. Heard et al. independently as a “hyperglycemic glycogenolytic” contaminant of insulin-containing pancreatic extracts and was purified by Sutherland a year later (2–6). Therefore, the history of glucagon is intimately intertwined with that of insulin and their respective roles in blood glucose. While glucagon receptors (Gcgr) are also expressed in kidney, brain, skin, and pancreas, glucagon’s major site of action is the liver, where increased signaling stimulates hepatic glucose output. Exogenous glucagon administration rescues hypoglycemia in individuals with insulin-dependent diabetes. Conversely, hyperglucagonemia contributes to hyperglycemia in diabetes through increased hepatic glucose output. Our understanding of glucagon has been aided greatly by studies where glucagon action is neutralized. In 1982, a Gcgr antagonist was reported to dramatically lower blood glucose in streptozocin-treated diabetic rats (7) but did not result in severe hypoglycemia. Similarly, Gcgr antagonism is effective at lowering blood glucose in humans with type 1 or type 2 diabetes (8). Together, these and other studies demonstrate a link between glucagon action and glycemia supporting glucagon antagonism as a potential therapeutic avenue for treating diabetes. However, further studies revealed other unexpected consequences to interrupting glucagon signaling, including dyslipidemia, hyperglucagonemia, and α-cell hyperplasia. This article will explore the latter two.
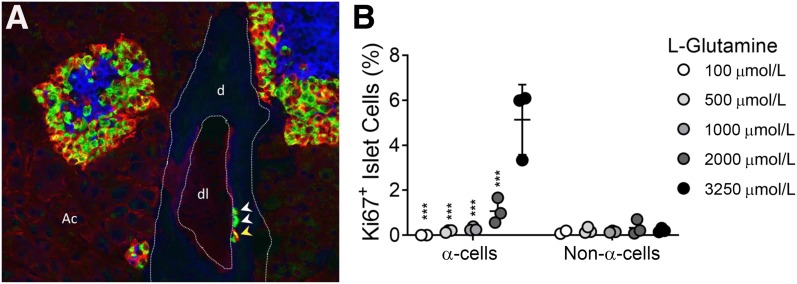
The first clear examples linking loss of glucagon signaling to α-cell hyperplasia came from efforts to generate glucagon antibodies in 1984 in rabbits (9). Rabbits immunized with glucagon peptides developed α-cell hyperplasia as a result of glucagon neutralization in the circulation. Global Gcgr knockout mice (Gcgr−/−) have lower blood glucose than wild-type littermates but also develop hyperglucagonemia and α-cell hyperplasia (10). Several other rodent models have α-cell hyperplasia including mice with liver-specific deletion of either Gcgr or Gsα protein (11,12). In addition, loss of preproglucagon gene expression or prohormone convertase 2 (PC2), the enzyme that liberates the mature glucagon peptide, results in α-cell hyperplasia (13). Acute interruption of glucagon signaling using small-molecule antagonists, antibodies, or antisense oligos in rodents also results in α-cell hyperplasia (14,15). α-Cell proliferation in response to interrupted glucagon signaling is conserved across vertebrate species including zebrafish and humans (16–18). α-Cell hyperplasia is driven at least in part by α-cell proliferation, although transdifferentiation of other pancreatic cell types has not been excluded. Additionally, single α-cells being frequently observed in the ductal linings of Gcgr−/− mice raises the possibility of neogenesis (18) (Fig. 1A). The commonality of these models is a loss of glucagon signaling in the liver, underscoring the primacy of liver as the target of glucagon.
Figure 1.
Interrupted glucagon signaling and hyperaminoacidemia result in α-cell proliferation and hyperplasia. A: Immunohistochemical analyses of Gcgr monoclonal antibody–treated mouse pancreas shows α-cell hyperplasia and single α-cells present in the ductal lining, similar to findings in ref. 10. Insulin, blue; glucagon, green; and SLC38A5, red. White arrowheads indicate single glucagon+ α-cells. Yellow arrowhead indicates SLC38A5+ glucagon+ α-cell. SLC38A5 is expressed in both α-cells and acinar cells of pancreas from mice with interrupted glucagon signaling. d, ductal tissue; dl, ductal lumen; Ac, acinar tissue. B: α-Cell proliferation and non–α-cell proliferation in mouse islets treated with high levels of amino acids and different doses of glutamine, similar to findings in ref. 16. Normal and Gcgr−/− serum levels of glutamine are 500 μmol/L and 3,250 μmol/L, respectively. (***P < 0.001 vs. 3,250 μmol/L glutamine α-cell proliferation; mean ± SD, n = 2–3 individual experiments.)
How then does loss of glucagon signaling in liver result in α-cell proliferation? One hypothesis was that a signal from the liver (a circulating factor) initiates events leading to α-cell proliferation. This was first tested by islet transplantation studies (11). After isolation and removal of α-cells from the pancreatic environment and transplantation of them under the kidney of mice with interrupted glucagon signaling, α-cells in normal mouse islets proliferated, indicating no requirement of local pancreatic signaling. Importantly, human islets transplanted into immunodeficient mice treated with Gcgr monoclonal antibody also have increased α-cell proliferation (16,19). Gcgr−/− islets transplanted into Gcgr+/+ (wild-type) mice had reduced α-cell proliferation and glucagon content, suggesting that a factor present in the environment of Gcgr−/− mouse, but absent or reduced in the Gcgr+/+ recipient, is necessary to maintain the expanded α-cell mass (11). Similarly, studies where either treatment with Gcgr inhibitors is withdrawn or where glucagon levels in proglucagon gene–null mice are restored also show that α-cell mass returns to normal once glucagon signaling is restored (14,20). Since islets are revascularized within 1 week after transplantation, circulating factors from the liver of mice with interrupted glucagon signaling likely stimulated the observed α-cell growth (11).
The Liver–α-Cell Axis
Islet culture studies supported the presence of an α-cell mitogen in serum from mice with interrupted glucagon signaling (16). For identification of these circulating factors, a variety of approaches, including liver and adipose transcriptomics, serum proteomics/metabolomics, and islet culture assays to screen candidates for effects on α-cell proliferation were used. Liver transcriptomics from both Gcgr−/− and Gcgr monoclonal antibody–treated mice revealed a few secreted factors (e.g., defensin B1, hepcidin, activin A, bile acids) that were upregulated in mice with interrupted glucagon signaling, and these were tested as candidates for the α-cell factors (16,21). Levels of IL-6, a factor previously implicated in the regulation of α-cell mass, were not increased in mice with interrupted glucagon signaling (11,16,22). Only one of these secreted protein candidates, an adipose tissue–induced factor, ANGPTL-4, was shown to stimulate α-cell proliferation (23). However, ANGPTL-4 did not stimulate α-cell proliferation in other studies (16,24).
By further transcriptomic analyses in multiple mouse models with interrupted glucagon signaling, alterations in gene expression related to amino acid catabolism including genes involved in glutamine (Gls2, Slc1a4), serine (Sds, Sdsl), and arginine (Ass1, Asl, Arg1, Slc7a2) metabolism and transport were identified. Consequently, three- to sixfold increases in most serum amino acids were observed in mice with interrupted glucagon signaling by multiple mechanisms (16,17,21,25,26). Small-molecule screening in a zebrafish model of interrupted glucagon signaling also showed that α-cell numbers were sensitive to mTOR inactivation, further supporting a role for amino acids (16,18). Using mouse islet cultures, media containing high levels of amino acids mimicking serum levels observed in mice with interrupted glucagon signaling selectively stimulated α-cell proliferation but not β- or δ-cell proliferation (16) (Fig. 1B). Additionally, of these high levels of amino acids, high levels of l-glutamine are required to stimulate mTOR-dependent α-cell proliferation. These studies demonstrate from fish to man a conserved liver–α-cell axis endocrine loop where loss of glucagon signaling impairs hepatic amino acid catabolism leading to increased amino acids in circulation (16,17,21,27). The resultant hyperaminoacidemia, particularly high levels of glutamine, feeds back to pancreatic islet α-cells and stimulates glucagon secretion and proliferation (Fig. 2).
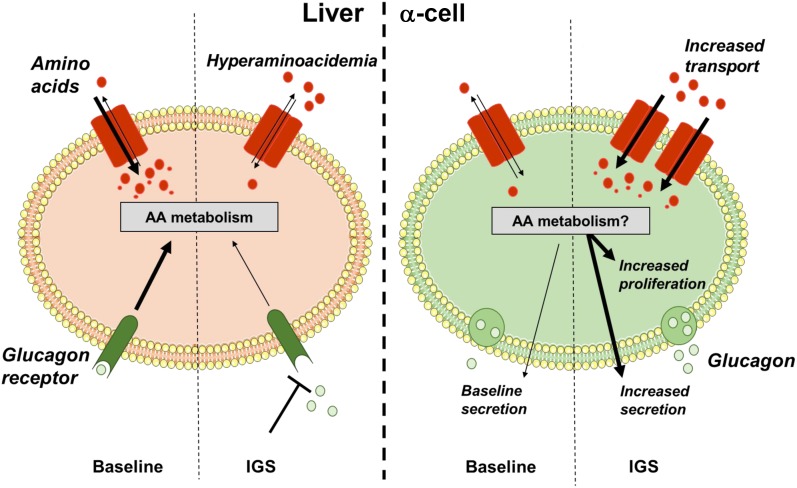
Figure 2.
A liver–α-cell axis that reciprocally regulates amino acid homeostasis and α-cell function and proliferation. Under baseline conditions, glucagon is secreted from pancreatic α-cells, where it acts on liver Gcgr. Glucagon signaling promotes amino acid transport and catabolism, maintaining blood amino acid levels. When glucagon signaling is interrupted (e.g., genetic knockdown, small molecule), genes involved in amino acid transport and catabolism in the liver are downregulated. Impaired uptake and catabolism potentially result in the observed hyperaminoacidemia. Through an endocrine feedback loop, amino acids stimulate glucagon secretion acutely and under sustained stimulation promote α-cell proliferation and hyperplasia. AA, amino acid; IGS, interrupted glucagon signaling.
In investigations into how amino acids could have such potent and selective mitogenic effects on α-cells, we and others identified that mouse α-cells upregulate the expression of a glutamine transporter, SLC38A5 (Fig. 1A), and that SLC38A5 expression is partially required for α-cell expansion in response to hyperaminoacidemia (16,17). SLC38A5 expression is restricted to acinar cells under normal conditions in adult mice (16), consistent with RNA sequencing studies that found that Slc38a5 expression restricted to α-cells during development in the mouse (28). However, few α-cells (∼1%) express SLC38A5 in the adult mouse pancreas. When glucagon signaling is interrupted in mice, SLC38A5 expression is reinitiated in the adult mouse α-cell within 4 days (16). Therefore, hyperaminoacidemia may be related to fetal programming (discussed further below) and hyperaminoacidemia in the adult state reinitiates an immature state in α-cells that facilitates proliferation. A direct comparison of mouse gene expression in α-cells during development versus adulthood under hyperaminoacidemic conditions has not been performed, but the issue merits further study.
While Slc38a5 expression is upregulated in mouse α-cells under conditions of interrupted glucagon signaling, it is unclear what influence Slc38a5 expression in the α-cell has on α-cell proliferation directly. Studies on SLC38A5 have been performed in global Slc38a5 knockout animals (both zebrafish and mouse). Despite global Slc38a5−/− mice developing hyperaminoaciduria, it cannot be excluded that Slc38a5 expression in some other tissue (e.g., liver) plays a role in α-cell proliferation. Both Slc38a5 knockout fish and Slc38a5−/− mice with interrupted glucagon signaling have reduced but not complete loss of α-cell proliferation (16,17). Interestingly, only 1% of α-cells express SLC38A5 when mouse islets are cultured in media containing high levels of amino acid despite proliferation levels exceeding 4% after 3 days (16). Surprisingly, SLC38A5 expression was not detected in proliferating human α-cells (16,17). These data suggest that other transporters may play a role in both mouse and human α-cell proliferation. Recently, Okamoto and colleagues searched for common features in the expression of amino acid transporter genes in human islets transplanted into Gcgr antibody–treated mice (29). They found that the neutral amino acid transport SLC38A4, the cationic amino acid transporter SLC7A2, and the Q-dependent amino acid exchanger SLC7A8 with its heavy-chain SLC3A2 (CD98) are the most highly expressed amino acid transporters in human islets. Similar to previous reports, SLC38A5 expression was not observed. However, gene expression for the glutamine transporter SLC38A4 is upregulated in human α-cells from mice treated with Gcgr antibody. Therefore, it is possible that SLC38A4, which also transports glutamine, in lieu of SLC38A5, promotes human α-cell proliferation in response to hyperaminoacidemia, and this merits further investigation.
l-glutamine is required for α-cell proliferation when glucagon signaling is interrupted, but high levels of glutamine cannot stimulate α-cell proliferation alone (16). It is important to understand which other amino acids play a major role in signaling in the α-cell and which play a supportive role. It is possible that some amino acids play a major role in secretion while having little influence on proliferation and vice versa. Enhanced amino acid transport capacity and catabolism by α-cells lead to α-cell proliferation, but this may also be the driver of enhanced glucagon secretion. Together, these studies support a role for α-cells as critical amino acid sensors in the body.
Amino Acids as Secretagogues
Amino acids are potent stimulators of both glucagon and insulin secretion, although not all amino acids stimulate with similar potency. While the recent observations from our group and others were the first to link amino acids to regulation of α-cell proliferation and mass, the influence of amino acids on glucagon secretion has been known since the 1960s (30). Amino acid levels increase in the circulation postprandially after protein consumption, and this is exacerbated by a chronic high-protein diet (31). In humans consuming a whey protein drink, peripheral plasma levels of most amino acids rose by two- to fourfold within 2 h, leading to increased glucagon secretion (32). Diet type can influence amino acid absorption as well. Importantly, the source of dietary protein also affects the bioavailability of individual amino acids and thus their portal concentrations. Therefore, certain protein-rich foods may be more likely than others to affect postprandial glucagon and insulin release.
Presumably, amino acids work by convergent mechanisms to promote secretion. However, the exact mechanisms are poorly defined. For the most commonly studied amino acid, arginine, this occurs via membrane transport where intracellular accumulation of the cationic amino acid alters membrane polarization (33). However, this cannot explain the very potent secretagogue action of either glutamine (polar and uncharged) or alanine (small and hydrophobic). The large repertoire of amino acid transporters expressed on α-cells favors enhanced glucagon secretion. Studies aimed at defining the transport and metabolism of amino acids in α-cells and whether those mechanisms are shared by β-cells will be useful to understand how amino acids have unique effects on islet cells. Cancer cells also have a unique repertoire of amino acid transporters to drive their growth (34), and applying what has been learned by the cancer field and new techniques (e.g., isotopomer analyses, metabolomics, MALDI imaging) represents an exciting opportunity to advance our understanding of the biology of islets, especially in the context of overnutrition and metabolic disease. These future efforts will aid the understanding of an important role of the liver–α-cell axis to regulate glucagon secretion.
Amino Acid Receptors in Islets
An alternative to transport would be receptor signaling such as G-protein–coupled receptors or ionotropic receptors. Glutamate is cosecreted with glucagon from α-cells (35). Metabotropic glutamate receptors and AMPA and kainate-dependent ionotropic glutamate receptors on α-cells mediate an autocrine positive feedback to stimulate glucagon secretion (36). Additionally, α-cells have high levels of glutaminase activity that could support glutamate production to drive this feedback process (37).
Another candidate expressed in pancreatic islets is the calcium sensing receptor (CasR), which has affinity for a broad number of amino acids. However, it is preferentially responsive to phenylalanine and tryptophan—the two amino acids seemingly unaffected by Gcgr antagonism. CASR, which is also stimulated by amino acids, has been reported in pancreatic islets and specifically in human β-cells (38,39). Mice expressing a germline gain-of-function form of CASR have impaired glucose-stimulated insulin secretion and glucagon suppression with increased α-proliferation, suggesting that CASR signaling plays a role in determining both islet cell function and mass (40). Similar to CASR, GPR142 is expressed on β-cells and a subset of α-cells (41). GPR142 small-molecule agonists and its endogenous ligands tryptophan and phenylalanine stimulate insulin secretion and β-cell proliferation partially through α-cell–derived paracrine glucagon-like peptide 1 (GLP-1) stimulation of β-cells (41–43).
How Do Amino Acids Stimulate Both Glucagon and Insulin Secretion?
It is likely that paracrine stimulation of β-cells through either α-cell–derived glucagon, GLP-1, or both is a potential mechanism to explain as least some of the effects of amino acids on the stimulation of insulin secretion. In 1974, studies with perfused rat pancreata demonstrated that in the absence of glucose, arginine-stimulated glucagon secretion is robust, biphasic, and dose dependent, while insulin secretion is a tonic, single-phase stimulation that requires very high concentrations of arginine (44). Importantly, arginine-stimulated glucagon secretion is only partially attenuated by glucose, supporting a primary role for α-cells as amino acid sensors. However, in the presence of glucose, arginine-stimulated insulin secretion is robust, biphasic, and requires much less arginine. This is very similar to studies with incretins like GLP-1 or gastric inhibitory peptide (GIP) where these proteins have little impact on insulin secretion alone but, rather, potentiate glucose-stimulated secretion. Recently, Campbell and colleagues demonstrated that the amino acids glutamine, arginine, and alanine fail to stimulate insulin secretion in the absence of glucagon and GLP-1 signaling (45). Therefore, the reduced amino acid transporter expression on β-cells coupled with glucose-dependent responses suggests that the mechanisms by which amino acids stimulate insulin secretion may be distinct from those stimulating glucagon secretion and perhaps are paracrine in nature.
Neural Factors Regulate Amino Acid–Stimulated Glucagon Secretion
In addition to direct amino acid signaling on pancreatic islet cells, amino acid tone is modified by neural circuits. Rodents with hepatic-vagal denervation have stronger responses to amino acids like arginine, leucine, and alanine, secreting more glucagon (46). This negative feedback may help avoid an exaggerated glucagon response and prevent unwanted rises in hepatic glucose output in response to excessive protein consumption, although it is unclear how vagal afferents sense changes in blood amino acid levels.
What Is the Influence of Glucose on the Liver–α-Cell Axis?
If the major role of glucagon is to stimulate hepatic glucose output, then why did the liver–α-cell axis evolve independent of glucose levels? Conventionally, glucagon secretion is highest under low glucose conditions, while high glucose inhibits glucagon secretion. However, glucagon secretion is stimulated by high glucose when α-cells are dispersed, suggesting that a large proportion of glucose inhibition of glucagon secretion is indirectly mediated by paracrine factors (e.g., insulin, somatostatin, GABA) while only a small proportion may be due to direct glucose sensing (47,48). However, amino acids promote glucagon secretion independent of glucose levels (45,49). Rather, glucagon secretion magnitude in response to amino acids can be modified by glucose levels. It is for these reasons that I propose that amino acids are the primary drivers of glucagon secretion.
Importantly, hypoglycemia in the absence of hyperaminoacidemia (e.g., congenital hyperinsulinemia) does not increase α-cell mass. Furthermore, if glucose were the primary regulator of α-cell activity, then one might expect α-cell proliferation to be reduced in models of diabetes when glucagon signaling is interrupted. However, diabetic mice treated with Gcgr inhibitors display reductions in blood glucose (but not euglycemia), hyperglucagonemia, and increased α cell mass (14,50,51).
Is There Evidence for the Liver–α-Cell Axis in Humans in Health and Disease?
α-Cell hyperplasia in both types 1 and 2 diabetes remains controversial, and reports in favor are likely the result of decreased β-cell–to–α-cell ratios or mild increases in α-cell mass at best (52–57). Similarly, studies in animal models of diabetes are not consistently in favor of the presence of increased α-cell mass. However, obesity and type 2 diabetes are consistently associated with hyperaminoacidemia, including increases in branched-chain amino acids, glutamate, and aromatic amino acids (tryptophan and phenylalanine) (58–60). Recently, Wewer and colleagues reported that glucagon levels inversely related to amino acid levels such as alanine and insulin resistance may drive glucagon resistance in a fatty liver disease state (61). We found that elevated glutamine levels were required for α-cell proliferation in models of interrupted glucagon signaling. Conversely, individuals with diabetes have decreased serum glutamine levels (60). If glutamine is the major driver of α-cell proliferation, then it is possible that changes in amino acid levels associated with obesity and diabetes may not affect α-cell proliferation but still promote hyperglucagonemia. Further investigation is required in this area.
Glucagonoma and Mahvash Disease
Glucagonoma syndrome is a rare malignancy characterized by hyperglucagonemia and pancreatic α-cell–rich adenomas. Although sometimes misdiagnosed as type 2 diabetes due to occasional hyperglycemia secondary to severe hyperglucagonemia, nearly all individuals experience hypoaminoacidemia. Conversely, a few rare reported cases of homozygotic inactivation of the Gcgr resulting in pancreatic neuroendocrine tumors enriched for α-cells have been reported in man (62). Mahvash disease, first described in 2008, is a distinct disorder from glucagonoma syndrome, with an estimated incidence of four in one million. Typically, these individuals present as adults with abdominal pain, normal to mild hypoglycemia, and severe hyperglucagonemia. More recently, a pediatric case of Mahvash was described where the individual was identified on newborn screen with hyperargininemia and diagnosed with arginase deficiency (63). Though misdiagnosed, importantly, this individual’s symptoms were well controlled with a low-protein diet and an ammonia scavenger. When the individual was removed from a low-protein diet after arginase deficiency was ruled out, she developed hyperglucagonemia and hyperaminoacidemia, which her physicians found to resemble the phenotype of Gcgr−/− mice. This case further supports a role of amino acids in Mahvash disease and α-cell hyperactivity, although it is unclear whether α-cell hyperplasia is present in younger individuals. If these syndromes are rare, what was the selective pressure to evolve such a robust secretory and proliferative response to hyperaminoacidemia in α-cells? Are there normal physiological processes linked to hyperaminoacidemia?
Hyperaminoacidemia Is a Hallmark of Fetal Development
Growing a new organism is a tremendous undertaking. Pregnancy is a physiological condition associated with positive nitrogen balance, although maternal amino acid levels are slightly lower than in normal conditions. This is because the mammalian placenta has evolved a robust expression network of nutrient transporters to deliver nutrients including amino acids to the developing offspring while maintaining a protective barrier. Consequently, fetal amino acid levels are more than threefold higher than the maternal circulation (64). Impairment of the nutrient uptake system is linked to intrauterine growth restriction, which can have both immediate consequences for the offspring, including small for gestational age and hypoglycemia, and long-term consequences, such as increased risk of future obesity, impaired glucose tolerance, and diabetes. Recent studies have linked intrauterine growth restriction to decreased pancreatic size, islet mass, and poor insulin secretion after birth. Interestingly, infusion of amino acids alone into the fetal circulation in an intrauterine growth restriction model can restore islet mass and function after birth (65,66). Similarly, Solloway et al. (21) showed that postnatal amino acid levels rose and fell in Gcgr−/− mice in correlation with α-cell proliferation and changes in α-cell mass. Therefore, amino acid delivery during development may play a critical role in determining both α- and β-cell mass at birth, and impaired delivery of amino acids during development may predict future development of diabetes. If this is true, then arguably the relationship between amino acids and glucagon is founded in development and would be paramount to produce viable offspring, meriting future study.
Heresy—the Primary Goal of Glucagon Is Not to Regulate Blood Glucose
One goal of this article has been to explore a growing appreciation for the relationship between glucagon and amino acids—a relationship that is often an afterthought. Evidence for this at the time of preparation for this manuscript includes the authority Wikipedia in its opening paragraph description of glucagon (en.wikipedia.org/wiki/Glucagon): “It works to raise the concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body.” This is a reference to Voet and Voet’s biochemistry textbook (67). While describing glucagon as “the main catabolic hormone” is an allusion to glucagon’s role in amino acid catabolism, unsurprisingly, a link between glucagon and amino acids is not explicitly mentioned. Glucagon raises blood glucose during fasting and is associated with glycemia during exercise. From an evolutionary standpoint, this would be an important adaptation to handle food scarcity or possibly maintain glucose to tissues to escape a predator. From the point of view of modern humans, this adaptation is less advantageous with caloric excess pervasive in modern society. Perhaps glucagon evolved to provide some protection against hypoglycemia. However, an additional selective pressure to respond to amino acids was also present, as indicated by the liver–α-cell axis.
So why such a strong link between glucagon and amino acids? Cellular protein turnover is estimated at 1–2% of total every day. Approximately 75% of liberated amino acids are recycled to make new proteins, while the remainder are catabolized to make new cellular macromolecules or released into the circulation. The daily recommended intake of protein is 46 g for women and 56 g for men each day (0.8 g protein g/kg body wt). However, according to a recent review of data from the National Health and Nutrition Examination Survey (NHANES) study, Americans consume on average two times this amount, thereby creating an excess of amino acids available from the diet (68). This positive nitrogen balance drives cell growth, including skeletal muscle. With the exception of those used as neurotransmitters in synaptic vesicles (e.g., glutamate), free amino acids are not stored. Therefore, what is not used for protein synthesis must be disposed of by other mechanisms.
In order to maintain nutrient availability during feeding, fasting, and resting, nutrients (e.g., glucose, amino acids, and lipids) are coordinately mobilized in and out of blood and from storage depots to meet the changing demands of the organism. Importantly, nutrient concentrations in the blood are normally maintained within a narrow range (e.g., 5–6 mmol/L glucose, ∼400–600 μmol/L glutamine, ∼300–500 μmol/L alanine). The role of glucagon in increasing hepatic glucose output via glycogenolysis and to a lesser extent gluconeogenesis to maintain euglycemia has overshadowed the role of glucagon in ureagenesis. Glucagon stimulates uptake and metabolism of amino acids in the liver (69). Ureagenesis (urea cycle) generates one cytosolic fumarate that together with the product of mitochondrial pyruvate metabolism produces two phosphoenolpyruvate (PEP) molecules. These PEP molecules then can create glucose, linking carbon flux from ureagenesis to gluconeogenesis. The nitrogenous urea generated from the catabolism of arginine to ornithine is then excreted though urine. Therefore, the process of gluconeogenesis and ureagenesis appears to be intrinsically linked. However, the process of removing excess ammonia in the form of urea is of equal importance biologically to maintaining glycemia. Inborn errors of metabolism include disorders of the urea cycle from disruption of one of six enzymes. As a result of impaired enzymatic functions, toxic levels of ammonia and/or arginine develop in blood, resulting in neurological symptoms, seizure, and anorexia. Left untreated, these disorders can be fatal. Therefore, it is also conceivable that glucagon stimulation of ureagenesis evolved as a means to dispose of large excesses of ammonia resulting from consumption of high protein volumes—a common occurrence in carnivorous and omnivorous species.
Concluding Remarks
The liver–α-cell axis is conserved across vertebrate species. Interrupted glucagon signaling impairs liver catabolism of amino acids, leading to hyperaminoacidemia. Remarkably, high levels of amino acid stimulate glucagon secretion and under sustained hyperaminoacidemia can promote α-cell hyperplasia. However, many unanswered questions remain surrounding the mechanism of amino acid regulation of α-cell function. While you may remain unconvinced that the primary role of the α-cell and glucagon is to sense, communicate, and regulate amino acid status, it is the hope that this article has provided a different perspective on glucagon biology beyond its role in glycemia.
Article Information
Funding. This work was generously supported by grants from JDRF (PDF-2014-189 and SRA-2016-149-Q-R) and the National Institutes of Health, including the Vanderbilt Diabetes Research and Training Center DK20593, DK117147, and K01DK117969.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this work were presented at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Dean ED, Vuguin PM, Charron MJ. Glucagon: the name says it all, or not! Endocrinology 2019;160:1359–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher NF. Preparation of insulin. Am J Physiol 1923;67:57–64 [Google Scholar]
- 3.Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem 1923;58:337–346 [Google Scholar]
- 4.Heard RDE, Lozinski E, et al. . An alpha-cell hormone of the islets of Langerhans. J Biol Chem 1948;172:857. [PubMed] [Google Scholar]
- 5.Sutherland EW, De Duve C. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem 1948;175:663–674 [PubMed] [Google Scholar]
- 6.Sutherland EW, De Duve C. A glycogenolytic factor from pancreas. Fed Proc 1948;7:195. [PubMed] [Google Scholar]
- 7.Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science 1982;215:1115–1116 [DOI] [PubMed] [Google Scholar]
- 8.Scheen AJ, Paquot N, Lefèbvre PJ. Investigational glucagon receptor antagonists in phase I and II clinical trials for diabetes. Expert Opin Investig Drugs 2017;26:1373–1389 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs U, Hahn von Dorsche H, Ziegler M. Glucagon antibodies alter Langerhans’ islets. Biomed Biochim Acta 1984;43:691–693 [PubMed] [Google Scholar]
- 10.Gelling RW, Du XQ, Dichmann DS, et al. . Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longuet C, Robledo AM, Dean ED, et al. . Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 2013;62:1196–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Gavrilova O, Zhao WQ, et al. . Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest 2005;115:3217–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuta M, Yano H, Zhou A, et al. . Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A 1997;94:6646–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu W, Yan H, Winters KA, et al. . Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 2009;331:871–881 [DOI] [PubMed] [Google Scholar]
- 15.Sloop KW, Cao JX, Siesky AM, et al. . Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 2004;113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean ED, Li M, Prasad N, et al. . Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab 2017;25:1362–1373.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Okamoto H1, Huang Z, et al. . Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab 2017; 25:1348–1361.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Dean ED, Zhao L, Nicholson WE, Powers AC, Chen W. Glucagon receptor inactivation leads to α-cell hyperplasia in zebrafish. J Endocrinol 2015;227:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ban K, Kim KH, Cho CK, et al. . Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology 2010;151:1520–1531 [DOI] [PubMed] [Google Scholar]
- 20.Webb GC, Akbar MS, Zhao C, Swift HH, Steiner DF. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes 2002;51:398–405 [DOI] [PubMed] [Google Scholar]
- 21.Solloway MJ, Madjidi A, Gu C, et al. . Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Reports 2015;12:495–510 [DOI] [PubMed] [Google Scholar]
- 22.Ellingsgaard H, Ehses JA, Hammar EB, et al. . Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A 2008;105:13163–13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Zvi D, Barrandon O, Hadley S, Blum B, Peterson QP, Melton DA. Angptl4 links α-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci U S A 2015;112:15498–15503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto H, Cavino K, Na E, et al. . Angptl4 does not control hyperglucagonemia or α-cell hyperplasia following glucagon receptor inhibition. Proc Natl Acad Sci U S A 2017;114:2747–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, MacDougall ML, McDowell MT, et al. . Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor (GCGR) knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genomics 2011;12:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe C, Seino Y, Miyahira H, et al. . Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 2012;61:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holst JJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes 2017;66:235–240 [DOI] [PubMed] [Google Scholar]
- 28.Stanescu DE, Yu R, Won KJ, Stoffers DA. Single cell transcriptomic profiling of mouse pancreatic progenitors. Physiol Genomics 2017;49:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Dominguez Gutierrez G, Xin Y, et al. . Increased SLC38A4 amino acid transporter expression in human pancreatic α-cells following glucagon receptor inhibition. Endocrinology 2019;160:979–988 [DOI] [PubMed] [Google Scholar]
- 30.Ohneda A, Parada E, Eisentraut AM, Unger RH. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest 1968;47:2305–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azzout-Marniche D, Gaudichon C, Blouet C, et al. . Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol 2007;292:R1400–R1407 [DOI] [PubMed] [Google Scholar]
- 32.Ang T, Bruce CR, Kowalski GM. Postprandial aminogenic insulin and glucagon secretion can stimulate glucose flux in humans. Diabetes 2019;68:939–946 [DOI] [PubMed] [Google Scholar]
- 33.Thams P, Capito K. L-arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur J Endocrinol 1999;140:87–93 [DOI] [PubMed] [Google Scholar]
- 34.Bröer S, Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J 2017;474:1935–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi M, Yamada H, Uehara S, et al. . Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 2003;278:1966–1974 [DOI] [PubMed] [Google Scholar]
- 36.Cabrera O, Jacques-Silva MC, Speier S, et al. . Glutamate is a positive autocrine signal for glucagon release. Cell Metab 2008;7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montero F, Baglietto-Vargas D, Moreno-González I, et al. . Glutaminase activity is confined to the mantle of the islets of Langerhans. Biochimie 2007;89:1366–1371 [DOI] [PubMed] [Google Scholar]
- 38.Saunders DC, Brissova M, Phillips N, et al. . Ectonucleoside triphosphate diphosphohydrolase-3 antibody targets adult human pancreatic β cells for in vitro and in vivo analysis. Cell Metab 2019;29:745–754.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 2008;135:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babinsky VN, Hannan FM, Ramracheya RD, et al. . Mutant mice with calcium-sensing receptor activation have hyperglycemia that is rectified by calcilytic therapy. Endocrinology 2017;158:2486–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin HV, Wang J, Wang J, et al. . GPR142 prompts glucagon-like peptide-1 release from islets to improve β cell function. Mol Metab 2018;11:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toda N, Hao X, Ogawa Y, et al. . Potent and orally bioavailable GPR142 agonists as novel insulin secretagogues for the treatment of type 2 diabetes. ACS Med Chem Lett 2013;4:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Carrillo JJ, Lin HV. GPR142 agonists stimulate glucose-dependent insulin secretion via Gq-dependent signaling. PLoS One 2016;11:e0154452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerich JE, Charles MA, Grodsky GM. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 1974;54:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capozzi ME, Svendsen B, Encisco SE, et al. . β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K, Inoue S, Saito S, Nagase H, Takamura Y. Hepatic vagal amino acid sensors modulate amino acid induced insulin and glucagon secretion in the rat. J Auton Nerv Syst 1993;42:225–231 [DOI] [PubMed] [Google Scholar]
- 47.Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology 2005;146:4861–4870 [DOI] [PubMed] [Google Scholar]
- 48.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 49.Zhu L, Dattaroy D, Pham J, et al. . Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;4:e127994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damond N, Thorel F, Moyers JS, et al. . Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife 2016;5:e13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conarello SL, Jiang G, Mu J, et al. . Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 2007;50:142–150 [DOI] [PubMed] [Google Scholar]
- 52.Orci L, Baetens D, Rufener C, et al. . Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A 1976;73:1338–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 2011;54:1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujita Y, Kozawa J, Iwahashi H, et al. . Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. J Diabetes Investig 2018;9:1270–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inaishi J, Saisho Y, Sato S, et al. . Effects of obesity and diabetes on α- and β-cell mass in surgically resected human pancreas. J Clin Endocrinol Metab 2016;101:2874–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilimnik G, Zhao B, Jo J, et al. . Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One 2011;6:e27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnet-Serrano F, Diedisheim M, Mallone R, Larger E. Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: an analysis based on the nPOD repository. PLoS One 2018;13:e0191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016;8:E405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guasch-Ferré M, Hruby A, Toledo E, et al. . Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wewer Albrechtsen NJ, Færch K, Jensen TM, et al. . Evidence of a liver-alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 2018;61:671–680 [DOI] [PubMed] [Google Scholar]
- 62.Yu R. Mahvash disease: 10 years after discovery. Pancreas 2018;47:511–515 [DOI] [PubMed] [Google Scholar]
- 63.Li H, Zhao L, Singh R, et al. . The first pediatric case of glucagon receptor defect due to biallelic mutations in GCGR is identified by newborn screening of elevated arginine. Mol Genet Metab Rep 2018;17:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young M, Prenton MA. Maternal and fetal plasma amino acid concentrations during gestation and in retarded fetal growth. J Obstet Gynaecol Br Commonw 1969;76:333–334 [DOI] [PubMed] [Google Scholar]
- 65.Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 2011;3:428–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown LD, Davis M, Wai S, et al. . Chronically increased amino acids improve insulin secretion, pancreatic vascularity, and islet size in growth-restricted fetal sheep. Endocrinology 2016;157:3788–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voet D, Voet JG. Biochemistry 4th ed Hoboken, NJ, John Wiley & Sons, 2011, xxv, 1428, 53 p [Google Scholar]
- 68.Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL 3rd. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007-2010. Nutrients 2015;7:7058–7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallette LE, Exton JH, Park. Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem 1969;244:5724–5728 [PubMed] [Google Scholar]