Abstract
Genetic studies of patients with neonatal progeroid syndrome led to the discovery of the novel fasting-induced, glucogenic, and orexigenic hormone named asprosin, the C-terminal cleavage product of profibrillin. Upon secretion, asprosin travels to the liver, where it exerts a glucogenic effect through OR4M1, an olfactory G-protein–coupled receptor. It also crosses the blood-brain barrier to stimulate appetite-modulating neurons in the arcuate nucleus of the hypothalamus, exerting an orexigenic effect via an as yet unidentified receptor. Specifically, it stimulates appetite by activating orexigenic AgRP neurons and inhibiting anorexigenic POMC neurons. Studies have also focused on the therapeutic potential of inhibiting asprosin for treatment of obesity and type 2 diabetes, both of which are characterized by high levels of circulating asprosin. It has been shown that anti-asprosin monoclonal antibodies reduce blood glucose, appetite, and body weight, validating asprosin as a therapeutic target. Current work aims to uncover key features of the asprosin biology such as the identification of its neuronal receptor, identification of the secretion mechanism from adipose tissue, and development of anti-asprosin monoclonal antibodies as diabetes and obesity therapies.
Discovery of Asprosin
The recent discovery of the glucogenic and orexigenic hormone asprosin has led to the unraveling of an energy regulation pathway that may be therapeutically beneficial for patients with obesity, diabetes, and metabolic syndrome. Asprosin was first identified in a group of patients with a rare genetic condition called neonatal progeroid syndrome (NPS) or marfanoid-progeroid-lipodystrophy (1). Patients with NPS have a unique phenotype characterized by extreme leanness, low appetite/calorie consumption, low energy expenditure, and partial lipodystrophy affecting the face and extremities (1–7). These physical characteristics are coupled with a metabolic signature consisting of reduced plasma insulin while maintaining euglycemia, demonstrating robust insulin sensitivity, which is unique for a lipodystrophy disorder (1). Romere et al. (1) elucidated the cause of this condition as a 3′ truncating mutation in the FBN1 gene. In all seven patients reported in the literature, the FBN1 mutation occurs 3′ to the last 50 nucleotides of the penultimate exon, leading to a predicted escape from nonsense mediated decay (NMD) and generation of a truncated profibrillin protein (1–7). Profibrillin is a 2,871–amino acid proprotein cleaved by the protease furin into a 140–amino acid C-terminal cleavage product and mature fibrillin-1 (1). In NPS, truncating mutations are clustered around the C-terminal cleavage site (1–7). This results in mutant fibrillin-1, which has been previously associated with Marfan syndrome (1–7), and the ablation of the C-terminal product. Besides serving as a “cap” that protects fibrillin-1 from premature processing, the C-terminal product, named asprosin (1), was not known to possess any independent activity. Despite heterozygosity, NPS patients display very little plasma asprosin (1). When the truncated version of profibrillin associated with NPS was overexpressed in wild-type cells, the cells displayed reduced secretion of asprosin into the media, suggesting that mutant profibrillin, which is predicted to escape mRNA NMD due to the truncation being 3′ to the NMD threshold, exerts a dominant negative effect on the secretion of asprosin from the wild-type allele (1). Given that NPS patients are 3–5 standard deviations leaner than patients with Marfan syndrome (8) and display robust insulin sensitivity despite partial lipodystrophy, Romere et al. hypothesized that reduction in circulating asprosin could account for these phenotypes.
The NPS phenotype has since been modeled in both mice and rabbits. Fbn1NPS/+ mice were created using CRISPR/Cas9 to induce a frame shift leading to the deletion of the asprosin-coding region (9). A similar approach has recently been undertaken in rabbits (10). In both species the result is a phenocopy of the human disorder, with significantly reduced body weight and adiposity (9,10). Notably, Fbn1NPS/+ mice display dramatic protection from diet-induced obesity and diabetes (9). To our knowledge, no other Fbn1 mutation in mice has been associated with reductions in body weight and adiposity or with improvement in glucose homeostasis. Thus, across species, strength of the genotype-phenotype correlation is astounding. This underpins asprosin’s role in maintenance of body weight, adiposity, and glucose homeostasis.
Profibrillin Cleavage and Asprosin Secretion
The asprosin mechanism begins with the cleavage of profibrillin. Profibrillin is cleaved by the calcium dependent furin/PACE (paired basic amino acid cleaving enzyme) serine endoprotease, which is part of the subtilisin family (11,12). While the specific cellular location of profibrillin cleavage is largely unknown, it is speculated to occur between the trans-Golgi network and the cell surface, or upon fibrillin-1 secretion (13,14). Furin cleaves asprosin at the R-C-K/R-R motif in the C-terminal domain (11,12). This cleavage event is important because it is required for the incorporation of fibrillin-1 into the extracellular matrix. Since furin is expressed in a plethora of cell lines and tissues (12), the presence or lack of this enzyme does not narrow down the possible locations of asprosin secretion.
Evidence suggests that asprosin is secreted from white adipose tissue, which accounts for 5–50% of human body weight and is already known to secrete adipokines such as leptin and adiponectin (15). While FBN1 is expressed in many tissues, its highest expression in both humans and mice is in white adipose (1). In vitro, differentiated adipocytes show a dramatic ability to generate and secrete asprosin compared with undifferentiated preadipocytes (1).
Since FBN1 (and thus, asprosin) is widely expressed in many human tissues, it is likely that white adipose is not the only source of plasma asprosin. Romere et al. (1) have reported asprosin secretion from wild-type human dermal fibroblasts suggesting that it may be secreted from skin. Lee et al. (16) found that MIN6 pancreatic β-cells and human primary islets containing β-cells secrete asprosin and that secretion is induced by palmitate in a dose-dependent manner. These studies show that skin and pancreatic β-cells may also secrete asprosin.
More recently, Ugur and Aydin (17) were able to detect asprosin from human saliva samples. Upon further investigation, asprosin was found in the supernatant of the submandibular gland at 14.4 ng/mg tissue and in the supernatant of parotid glands at 0.88 ng/mg tissue (17). Immunohistochemistry showed the presence of asprosin in the interlobular striated ducts and interlobular ducts of submandibular and parotid glands (17).
While how these multiple sites of asprosin secretion interact with each other remains unknown, some clues have emerged. Plasma asprosin follows a circadian oscillation where levels drop with the onset of feeding and increase after overnight fasting, suggesting that asprosin secretion is nutritionally regulated (1). Furthermore, conditions of overnutrition such as obesity, type 2 diabetes, and polycystic ovary syndrome (PCOS) have been associated with high circulating asprosin concentration in humans and rodents (9,17,18–21). Currently, mechanisms of asprosin secretion and its interaction with the environment are the focus of active investigation.
Asprosin Stimulates Hepatic Glucose Release Through OR4M1
Once in the circulation, asprosin targets the liver and the brain. Bacterially generated His-tagged recombinant asprosin has a murine half-life of ∼20 min, while its glycosylated mammalian counterpart has a murine half-life of ∼145 min (9). Recombinant asprosin labeled with 125I and injected intravenously in mice traffics to the liver (1). In the liver, asprosin’s main function is to stimulate hepatic glucose release. Increased plasma asprosin through both ectopic overexpression of full-length FBN1 and subcutaneous injection of bacterially expressed asprosin in fasted mice resulted in elevated glucose and insulin levels, coincident with a documented increase in hepatic glucose production (1).
Asprosin’s impact on glucose homeostasis is limited to hepatic glucose production with no direct impact on insulin levels or sensitivity. Romere et al. (1) performed two hyperinsulinemic-euglycemic clamp studies, one with asprosin gain of function and the other with asprosin loss of function. Overexpression of asprosin with an FBN1 adenovirus caused hepatic glucose production to increase (1). In FBN1 hypomorphic mice (homozygous MgR mice), which express about ∼20% of the FBN1 gene transcript and have a 70% reduction in circulating asprosin, there was a deficit in hepatic glucose production compared with wild-type mice (1). Neither clamp study showed a change in insulin sensitivity (1). Additionally, asprosin gain of function (recombinant asprosin injection or adenovirus-mediated overexpression) was associated with increased glucose and insulin, while immunologic loss of function (anti-asprosin antibody) or genetic loss of function (NPS mice and patients) was associated with decreased glucose and insulin (1,9). To observe the effect of asprosin without the compensatory effect of insulin, primary hepatocytes were exposed to increasing amounts of asprosin in cell culture and this resulted in a dose-dependent increase in glucose (1). By every indication, it would seem that asprosin-mediated change in plasma insulin is compensatory and secondary to its impact on plasma glucose.
Asprosin executes its glucogenic effect by using the cAMP second messenger system to activate PKA signaling. Treatment with recombinant asprosin in vitro or in vivo showed increased cAMP levels and PKA activity (1). However, when either G-protein function or cAMP-PKA signaling was inhibited pharmacologically, asprosin-mediated hepatocyte glucose release was blocked (1).
The use of the cAMP second messenger system implicates a G-protein–coupled receptor (GPCR) as the receptor for asprosin in the liver. Li et al. (22) recently discovered that asprosin promotes hepatic glucose release through a GPCR in the rhodopsin family called OR4M1. OR4M1 and its mouse ortholog, Olfr734, are previously identified olfactory receptors (ORs) expressed in the olfactory epithelium, olfactory bulb, liver, kidney, and testis (22). ORs in the olfactory epithelium detect the external environment, while ORs in peripheral tissues are known to be involved in skeletal muscle development, sperm chemotaxis, proliferation of prostate cancer cells, metabolite sensing in the gut, and glucose handling in the kidney (22). GST-asprosin was used to demonstrate high-affinity binding of asprosin to Olfr734 with a Kd of 18 nmol/L (22). Mutations were introduced to further test what amino acids are critical for the binding of asprosin to Olfr734 (22). Genetic elimination of Olfr734 in mice was able to blunt asprosin’s signal and reverse the metabolic effects of asprosin. Olfr734−/− mice created using a CRISPR-Cas9 system had lower body weight, food intake, fat mass, and plasma lipids compared with control mice (22). They also had reduced cAMP levels, decreased hepatic glucose release, and improved insulin sensitivity (as measured by insulin tolerance test, hyperinsulimic-euglycemic clamp, and glucose tolerance test) compared with wild-type mice (22). Liver-specific knockdown of Olfr734 using RNAi reduced glucose production, improved insulin sensitivity, decreased gluconeogenic gene expression, and decreased blood glucose, similar to Olfr734−/− global knockout mice (22). These effects were present regardless of whether the mice were fed a high-fat diet or a regular diet but were more pronounced in mice challenged with the high-fat diet. A similar decrease in glucose release was seen in cultured Olfr734 null primary hepatocytes, indicating that this change in glucose metabolism is cell autonomous (22). This study provides evidence that Olfr734 serves as the liver receptor of asprosin.
Given that glucagon exerts similar effects as asprosin on hepatic glucose production, Li et al. (22) assessed the contribution of the glucagon receptor (Gcgr) to the observed phenotypes. Both Gcgr−/− and Olfr734−/− mice have lower blood glucose, decreased body weight, and enhanced insulin sensitivity. Asprosin/Olfr734 signaling alone has about 50% of the effect on glucose production, intracellular cAMP levels, and gluconeogenic gene expression that glucagon/Gcgr signaling does in primary hepatocytes (22). However, when asprosin and glucagon are incubated together, intracellular cAMP level, gluconeogenic gene expression, and glucose production all increase synergistically (22). Furthermore, knockout of Olfr734 eliminated the asprosin effect on hepatic glucose production without affecting glucagon-mediated stimulation, and vice versa (22). This supports the conclusion that asprosin and glucagon work independently but synergistically to promote hepatic glucose production via their respective receptors.
The study by Li et al. (22) serves as important third-party confirmation of the original study on the discovery and glucogenic function of asprosin (1). Li et al. confirmed that fasting and obesity induce plasma asprosin, that asprosin binds and stimulates primary hepatocytes (in vitro) and the liver (in vivo) to increase glucose production via the cAMP/PKA pathway, and that neutralizing monoclonal antibodies can inhibit asprosin-mediated hepatic glucose production. Li et al. also provided an explanation for the difficulty we and others (23) have experienced using His-tagged recombinant asprosin. In our accumulating experience, His-tagged recombinant asprosin has shown a penchant for variable activity from batch to batch. This is not altogether surprising given that recombinant proteins have a long history of displaying instability or conformational changes in the absence of appropriate formulation. Often, they are sensitive to changes in buffer conditions, temperature, the absence of cofactors/chaperones, etc. Since the crystal structure of asprosin’s active form remains unknown, the only way to measure the activity of recombinant asprosin is through physiological response in mice or cells. Li et al. tagged their preparations with GST instead of His, leading to improved solubility and activity of recombinant asprosin. Per Li et al., “quality of recombinant asprosin is critical to its activity.” These observations suggest that asprosin requires a chaperone for full stability/activity, and our laboratory has begun experimenting with various tags and formulations besides GST in order to improve the stability/activity of recombinant asprosin. This development effort is essential to realize the goal of clinical asprosin replacement for NPS patients to combat hypophagia, extreme leanness, and low hepatic glucose production. Other methods of experimental asprosin manipulation that bypass the recombinant protein approach altogether, such as viral vectors (gain of function), monoclonal antibodies (loss of function), and mouse and human genetic studies (loss of function), all validate asprosin activity in vivo (1,9). We encourage the use of these tools (available via request) to confirm our results and further elucidate asprosin biology.
Asprosin Controls Hunger Through Modulation of AgRP and POMC Neurons
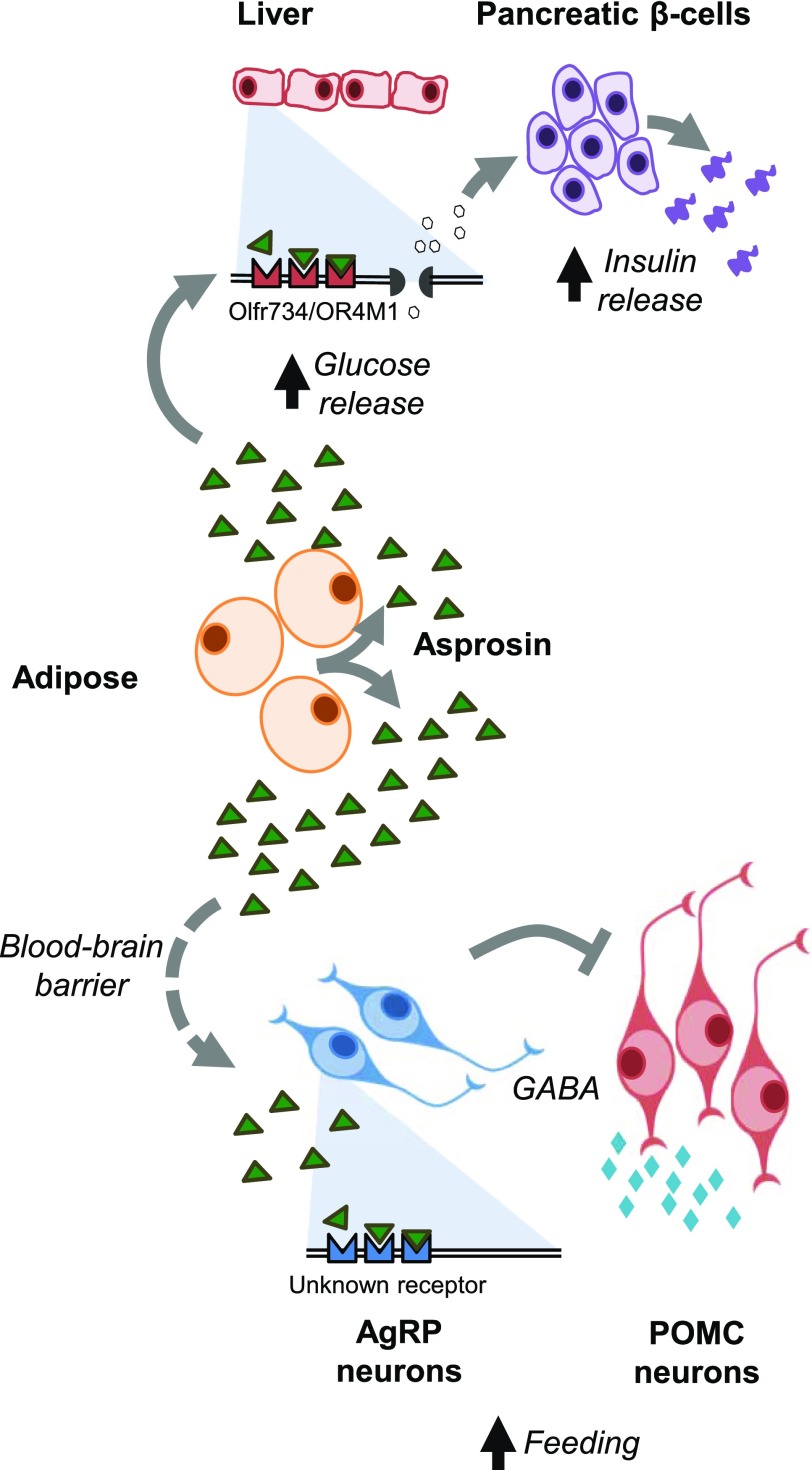
Asprosin can also exit the bloodstream and cross the blood-brain barrier to function in the brain (9) (Fig. 1). The first indication that asprosin was in fact a cerebrospinal fluid (CSF) protein, in addition to being a plasma protein, was the observation of asprosin in the CSF of rats at concentrations 5- to 10-fold lower than in the plasma (9). Similar to plasma, CSF asprosin was increased by fasting (9). Additionally, intravenously introduced asprosin showed a dramatic ability to cross the blood-brain barrier and enter the CSF (9).
Figure 1.
Asprosin mechanism of action. Upon secretion from white adipose tissue, asprosin travels to the liver and the brain. In the liver, asprosin exerts a glucogenic effect through the olfactory G-protein–coupled receptor OR4M1 (mouse ortholog Olfr734). In the brain, asprosin stimulates appetite by activating orexigenic AgRP neurons, through an unidentified receptor, and indirectly inhibiting anorexigenic POMC neurons.
A central mechanism of appetite regulation is via orexigenic AgRP neurons and anorexigenic POMC neurons in the arcuate nucleus of the hypothalamus (24). In Fbn1NPS/+ mice, AgRP neuron firing frequency and resting membrane potential were lower compared with wild-type but could be restored with central asprosin administration in vivo or incubation of hypothalamic slices with asprosin ex vivo (9). AgRP neuron ablation completely prevented asprosin-mediated appetite induction in chow-fed mice (9). However, AgRP neuron ablation was not sufficient to prevent asprosin-mediated appetite induction in mice on a high-fat diet, suggesting the use of other circuits in the context of a high-fat diet (9).
Asprosin directly activates orexigenic AgRP neurons and, using the neurotransmitter GABA, indirectly inhibits anorexigenic POMC neurons (9). Pretreatment of hypothalamic slices with inhibitors of G-protein, adenylate cyclase, or protein kinase A completely prevented asprosin-mediated AgRP neuron activation (9), implicating cAMP as a necessary component of asprosin-mediated AgRP neuron activation. The use of the cAMP second messenger system represents a commonality between asprosin’s molecular mechanisms in the brain and the liver.
However, despite the similar use of the cAMP second messenger system in both the liver and the brain, the liver asprosin receptor, Olfr734, may not be asprosin’s receptor in the AgRP neurons. Comprehensive gene expression profiles did not show Olfr734 expression in AgRP or POMC neurons in mice in either the fasted or fed state (25). It is important to note that GPCRs in general are expressed at low levels, particularly in the hypothalamus, so detection of Olfr734 expression in AgRP or POMC neurons may be difficult. Nevertheless, it remains unknown what receptor asprosin acts through in AgRP neurons, but promising candidates are currently being explored.
Asprosin appears to influence energy regulation by directly affecting energy intake and indirectly affecting energy expenditure as a compensatory measure. NPS patients show reduced energy expenditure as measured by indirect calorimetry and doubly labeled water, to go along with their hypophagia and leanness (9). Fbn1NPS/+ mice also display reduced energy expenditure; however, this normalized completely after mice were fed a high-fat diet (9). This suggests that the reduction in energy expenditure in NPS patients and NPS mice fed normal chow is a compensatory mechanism to prevent an unsustainable energy deficit. Mice subcutaneously injected with recombinant asprosin displayed greater food intake but no statistically significant change in energy expenditure (9). Hepatic overexpression of human FBN1 using adenovirus also increased plasma asprosin twofold with greater food intake but without statistically significant changes in energy expenditure (9). Lastly, asprosin-specific antibody treatment reduced daily food intake without changing energy expenditure in Leprdb/db mice and DIO mice (9). These observations suggest that asprosin exerts little direct effect on energy expenditure and that its impact on energy balance is largely due to its orexigenic activity.
When mice were given a single dose of recombinant asprosin, they displayed greater food intake over a 24-h period (9). Interestingly, it took several hours after injection before the increase in food intake occurred, differentiating asprosin from the acutely acting hormone ghrelin (26). Also, a single subcutaneous dose of recombinant asprosin rescued the hypophagia observed in Fbn1NPS/+ mice, confirming that hypophagia associated with NPS is due to a deficiency in asprosin (9).
Leptin, an anorexigenic hormone secreted from white adipose tissue, also regulates energy balance through modulation of AgRP and POMC neurons (27). Leptin levels are significantly lower in NPS patients and Fbn1NPS/+ mice (9), likely due to the observed paucity in adipose mass. Notably, leptin deficiency is unable to overcome the hypophagia and leanness associated with NPS. Leptin signaling also does not appear to be necessary for asprosin action. Leprdb/db mice show elevation in plasma asprosin (9), and immunologic asprosin neutralization in such mice resulted in reduced food intake and body weight despite the absence of leptin signaling (9). This indicates that asprosin works through a pathway that is independent of the leptin pathway to modulate appetite.
Despite ample evidence elucidating asprosin’s effect on AgRP neurons, there are many other mechanisms by which asprosin could control food intake. Food intake involves many specific behaviors such as olfaction, anticipation, locomotion, motor control of ingestion, etc. All of these behaviors are coordinated by a complex neuronal circuitry regulated by hormones and neurotransmitters. Food odors are also known to regulate AgRP neurons (22). AgRP and POMC neurons project into other parts of the hypothalamus including the paraventricular nucleus, lateral hypothalamic area, and the dorsomedial nucleus, which in turn project to intrahypothalamic and extrahypothalamic regions to regulate feeding and other processes (28). It would seem likely that asprosin-mediated modulation of food intake involves other cell types and neuronal circuits besides AgRP and POMC neurons and other behaviors besides orexigenic activity.
Remaining Questions Regarding Asprosin’s Mechanism
Despite advances in the understanding of asprosin function, there are many aspects of the asprosin mechanism that have yet to be understood. For example, little is known about the secretion mechanism of asprosin. How white adipose and other asprosin-producing tissues respond to environmental stimuli and various nutritional states at the cellular level to secrete asprosin is still largely a mystery. Additionally, how this process goes biochemically awry in NPS is an open question.
It is also unclear how asprosin’s function in the brain is related to its function in the liver. It has been speculated that asprosin could provide a connection between the feeding circuitry of the hypothalamus and hepatic glucose release. Potential ways this could occur is through modulation of sympathetic or parasympathetic drive at the level of the liver and also other organs known to be important for glucose homeostasis (29). Although asprosin treatment in mice had no effect on plasma catecholamine levels (1), this does not rule out other potential central mechanisms in addition to the demonstrated direct effect on hepatocytes. While the asprosin receptor in the liver has been identified, it is still unclear what its receptor in the brain is and whether/how this relates to the liver receptor. Identification of the receptor in both organs is crucial for further understanding how asprosin works and how its action in both of these organs is connected. It is also important to identify all the asprosin receptors given that they might be therapeutically beneficial considering that dysregulation of the asprosin-mediated energy cycle presents as a variety of disease phenotypes in humans.
Clinical Studies of Asprosin’s Role in Obesity, Diabetes, and PCOS
While patients with NPS have too little plasma asprosin, patients with conditions on the other end of the energy spectrum such as obesity, diabetes, and PCOS have too much (Table 1). Obesity is characterized by an overall increase in adiposity and, given that asprosin is secreted by adipose tissue, it is not surprising that both obese humans and mice (high-fat diet–fed, Lepob/ob and Lepdb/db mutation) show pathologically elevated levels of asprosin compared with control subjects (9). Asprosin-induced hyperphagia and hepatic glucose production could therefore be mechanisms that drive development of metabolic syndrome.
Table 1.
Clinical studies of asprosin
| Disease | Study | Conclusions |
|---|---|---|
| Obesity | Duerrschmid et al. Asprosin is a centrally acting orexigenic hormone (9) | Obese humans and mice have elevated levels of asprosin. |
| Obesity, bariatric surgery | Wang et al. Serum asprosin levels and bariatric surgery outcomes in obese adults (33) | Amount of circulating asprosin prebariatric surgery is directly related to amount of weight loss seen 6 months post-surgery. |
| Obesity | Wang et al. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity (32) | Serum asprosin levels are significantly elevated in obese children compared with lean controls. Serum asprosin is positively correlated with waist-to-hip ratio, diastolic blood pressure, HOMA insulin resistance, leptin to adiponectin ratio, and TNF-α independent of BMI and age. |
| Obesity | Ugur and Aydin. Saliva and blood asprosin hormone concentration associated with obesity (17) | Blood and saliva asprosin levels increase with an increase in BMI. Saliva asprosin levels were ∼threefold higher in class I obesity, ∼fivefold higher in class II obesity, and ∼sixfold higher in class III obesity. |
| Obesity | Long et al. Decreased circulating levels of asprosin in obese children (30) | Fasting asprosin levels were lower in obese children than in normal weight children. Asprosin levels were lower in boys than in girls in the obese group. This is not consistent with previous studies. |
| Obesity | Sunnetci Silistre and Hatipoglu. Increased serum circulating asprosin levels in children with obesity (31) | Asprosin levels are significantly higher in obese children compared to normal weight children. |
| Exercise | Wiecek et al. Acute anaerobic exercise affects the secretion of asprosin, irisin, and other cytokines – a comparison between sexes (37) | Acute anaerobic exercise induced an increase in asprosin secretion in women. |
| Type 2 diabetes | Zhang et al. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride (18) | Asprosin levels are significantly higher in patients with type 2 diabetes. |
| Type 2 diabetes | Wang et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion (19) | Plasma asprosin concentration is positively correlated with patient waist circumference, fasting plasma glucose, HbA1c, triglycerides, impaired glucose regulation, type 2 diabetes, and HOMA for insulin resistance. |
| PCOS | Alan et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome (20) | Patients with PCOS have higher levels of circulating asprosin, and plasma asprosin levels are positively correlated with insulin resistance, BMI, and free androgen index. |
| PCOS | Li et al. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic related diseases (21) | Asprosin is higher in patients with PCOS compared with healthy female patients but still lower than female patients with type 2 diabetes. Asprosin levels were positively correlated with fasting plasma glucose, HbA1c, HOMA for insulin resistance, LDL cholesterol/apoB, apoplipoprotein E, and testosterone. Asprosin is an independent risk factor for type 2 diabetes or PCOS. |
| Pathological pregnancies | Baykus et al. Asprosin in umbilical cord of newborns and maternal blood of gestational diabetes, preeclampsia, severe preeclampsia, intrauterine growth retardation and macrosemic fetus (38) | Pregnant women with gestational diabetes mellitus, preeclampsia, severe preeclampsia, and macrosemic fetuses have significantly higher asprosin levels compared with healthy pregnant women, while women with intrauterine growth retardation have significantly decreased levels of asprosin. |
| Acute coronary syndrome | Acara et al. A novel biochemical marker for predicting the severity of ACS with unstable angina pectoris: asprosin (39) | Asprosin is a marker for unstable angina pectoris and the severity of acute coronary syndrome. |
| Anorexia Nervosa | Hu et al. Increased plasma asprosin levels in patients with drug-naive anorexia nervosa (40) | Elevated plasma asprosin concentration in patients with anorexia nervosa in response to starvation is associated with an interoceptive deficit. Plasma asprosin levels are negatively correlated with the duration of the illness. High asprosin levels may also contribute to bulimia. |
It is important to note that one study by Long et al. (30) found that plasma asprosin concentrations were decreased in obese children relative to normal weight control subjects. However, two additional studies have shown that plasma asprosin levels are significantly elevated in obese children compared to normal weight subjects (31,32). Given the preponderance of evidence showing asprosin elevation in obese humans (adults and children) and mouse models, it is unclear as to the reason for this discrepancy in the study by Long et al. (30).
Currently one of the most effective treatments for obesity is bariatric surgery. Bariatric surgery leads to weight loss by modulating a variety of different processes including nutrient absorption, appetite regulation, stomach size, gut microbiota, vagal nerve response, and gastrointestinal hormone levels (33). Recently, it has been shown in a study of 117 obese patients that the amount of circulating asprosin pre–bariatric surgery is directly related to the amount of weight loss seen 6 months after surgery (33). While weight loss was not related to post-surgery asprosin levels, patients had reduced circulating asprosin 6 months after the surgery (33). The reduction in asprosin post-surgery could be due to a reduction in adipose tissue mass. However, given the correlation between the amount of asprosin pre–bariatric surgery and weight loss 6 months after surgery, this study indicates that asprosin may have an obesogenic function that is disrupted by bariatric surgery. While the full role of asprosin in bariatric surgery remains relatively unclear, it is an area that warrants further exploration with the hopes of understanding how the gastrointestinal tract is involved in asprosin function and identifying which patients may have the best response to bariatric surgery.
Obesity is often linked to other metabolic disorders such as diabetes and PCOS, and, unsurprisingly, asprosin may be important in both. In a study of 84 adults with type 2 diabetes and 86 control subjects, increased plasma asprosin concentrations were correlated with type 2 diabetes (18). While serum asprosin was positively associated with adiposity-related parameters such as BMI, waist circumference, and waist-to-hip ratio in patients with type 2 diabetes, these adiposity markers were not independently associated with asprosin concentrations (18). Another study of 143 participants confirmed this result by determining that plasma asprosin levels were higher in patients with impaired glucose regulation or type 2 diabetes than in patients with normal glucose regulation after adjusting for age, sex, and obesity parameters (19). The authors also showed that plasma asprosin concentration is positively correlated with patient waist circumference, fasting plasma glucose, HbA1c, triglycerides, impaired glucose regulation, type 2 diabetes, and HOMA for insulin resistance (19). Another metabolic condition characterized by obesity and insulin resistance is PCOS. Similar to patients with freestanding obesity or diabetes, patients with PCOS had higher levels of circulating asprosin compared with control subjects and plasma asprosin levels were positively correlated with insulin resistance, BMI, and free androgen index (20).
Therapeutic Benefit of Targeting Asprosin
Remarkably, when Fbn1NPS/+ mice, which have low asprosin, were put on a high-fat diet (60% calories from fat) for 6 months, they were completely protected from both obesity and diabetes compared with wild-type mice (9). This observation is in line with the leanness and robust insulin sensitivity displayed by NPS patients (a condition characterized by low plasma asprosin in humans) (1,9). These observations suggested that inhibiting asprosin could be therapeutic for patients with metabolic syndrome, a patient population in need of improvement in both body weight and insulin resistance. To test this theory, a mouse monoclonal antibody against asprosin was generated (9). Treatment with a single dose of the anti-asprosin antibody reduced fasted insulin levels in two independent mouse models of hyperinsulinemia (insulin resistance) (1). These immunologic effects were validated by genetic loss of function in FBN1 hypomorphic mice (homozygous MgR mice), which express about ∼20% of the FBN1 gene transcript and have a 70% reduction in circulating asprosin (1). A hyperinsulinemic-euglycemic clamp study verified that asprosin deficiency results in decreased hepatic glucose production. Such mice display low plasma insulin (fed) and low plasma glucose (fasted), and replenishing asprosin levels results in restoration of glucose homeostasis (1). These observations provide a novel therapeutic target and mechanism against insulin resistance and diabetes.
Neutralization of asprosin with the asprosin-specific antibody also had a prominent effect on the feeding behavior of mice. The antibody decreased AgRP neuron stimulation, which resulted in a reduction of daily food intake (9). In Leprdb/db and DIO mice, treatment with the asprosin antibody for 5 days showed an improvement in body weight (9). The asprosin antibody’s effect on weight loss indicates that inhibiting asprosin pharmacologically could also be beneficial for obese patients.
Although humans and mice with NPS serve as genetic validation of the antiobesity and antidiabetic efficacy, and indeed safety, of pharmacologic asprosin inhibition, the extent and magnitude of its protective effect remains unknown. Besides ghrelin, asprosin is one of the very few known orexigenic hormones, with the vast majority of known appetite-influencing hormones being anorexigenic (34). This provides a fresh approach toward obesity pharmacotherapy particularly given the human and murine genetic validation. Additionally, the vast majority of patients with obesity also manifest with insulin resistance (35). Thus, asprosin’s dual effect, via distinct spatio-temporal mechanisms on glucose and appetite regulation, make it a particularly attractive therapeutic target against metabolic syndrome. Validating such an approach, monoclonal antibodies targeting asprosin reduce plasma glucose and insulin independent of their effect on appetite and body weight reduction in different mouse models of obesity and diabetes (1).
In addition to the immunologic approach, continuing discovery and characterization of asprosin receptors will enable screening and identification of small molecule inhibitors. This may allow oral dosing and perhaps even combination therapy. The immunologic approach has two benefits: using a monoclonal antibody to inhibit asprosin has already displayed proof-of-concept in mice, and monoclonal antibodies in general tend to be more specific with fewer off-target effects than small molecule therapies (36). In any event, this is an exciting time in obesity and diabetes research due to the potential identification of a brand new therapeutic target.
It is becoming increasingly common to discover therapeutic targets by studying humans with rare genetic conditions, and this is yet another example of such an approach. While there is still a lot to learn about asprosin, it is clear that such knowledge will lead to a better understanding of human energy regulation and have implications on obesity and diabetes that are increasing in prevalence across human populations.
Article Information
Acknowledgments. The authors thank J. Stamler (Harrington Discovery Institute at University Hospitals, Cleveland Medical Center and Institute for Transformative Molecular Medicine, Case Western Reserve University, Cleveland, OH), C. Duerrschmid (Institute for Transformative Molecular Medicine, Case Western Reserve University, Cleveland, OH), I. Mishra (Institute for Transformative Molecular Medicine, Case Western Reserve University, Cleveland, OH), and M. Jain (Kennedy Krieger Institute, Johns Hopkins Medical Center, Baltimore, MD) for critical reading of the manuscript.
Duality of Interest. A.R.C. is the co-founder and director of Gracili Therapeutics, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. The manuscript was written by J.G.H. and W.X. and edited by A.R.C.
References
- 1.Romere C, Duerrschmid C, Bournat J, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 2016;165:566–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill B, Simha V, Kotha V, Garg A. Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am J Med Genet A 2007;143A:1421–1430 [DOI] [PubMed] [Google Scholar]
- 3.Takenouchi T, Hida M, Sakamoto Y, et al. Severe congenital lipodystrophy and a progeroid appearance: mutation in the penultimate exon of FBN1 causing a recognizable phenotype. Am J Med Genet A 2013;161A:3057–3062 [DOI] [PubMed] [Google Scholar]
- 4.Goldblatt J, Hyatt J, Edwards C, Walpole I. Further evidence for a Marfanoid syndrome with neonatal progeroid features and severe generalized lipodystrophy due to frameshift mutations near the 3′ end of the FBN1 gene. Am J Med Genet A 2011;155A:717–720 [DOI] [PubMed] [Google Scholar]
- 5.Graul-Neumann LM, Kienitz T, Robinson PN, et al. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am J Med Genet A 2010;152A:2749–2755 [DOI] [PubMed] [Google Scholar]
- 6.Horn D, Robinson PN. Progeroid facial features and lipodystrophy associated with a novel splice site mutation in the final intron of the FBN1 gene. Am J Med Genet A 2011;155A:721–724 [DOI] [PubMed] [Google Scholar]
- 7.Jacquinet A, Verloes A, Callewaert B, et al. Neonatal progeroid variant of Marfan syndrome with congenital lipodystrophy results from mutations at the 3′ end of FBN1 gene. Eur J Med Genet 2014;57:230–234 [DOI] [PubMed] [Google Scholar]
- 8.Erkula G, Jones KB, Sponseller PD, Dietz HC, Pyeritz RE. Growth and maturation in Marfan syndrome. Am J Med Genet 2002;109:100–115 [DOI] [PubMed] [Google Scholar]
- 9.Duerrschmid C, He Y, Wang C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med 2017;23:1444–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Yao B, Yang Q, et al. Truncated C-terminus of fibrillin-1 induces Marfanoid-progeroid-lipodystrophy (MPL) syndrome in rabbit. Dis Model Mech 2018;11:dmm031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghunath M, Putnam EA, Ritty T, et al. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci 1999;112:1093–1100 [DOI] [PubMed] [Google Scholar]
- 12.Lönnqvist L, Reinhardt D, Sakai L, Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet 1998;7:2039–2044 [DOI] [PubMed] [Google Scholar]
- 13.Ritty TM, Broekelmann T, Tisdale C, Milewicz DM, Mecham RP. Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem 1999;274:8933–8940 [DOI] [PubMed] [Google Scholar]
- 14.Wallis DD, Putnam EA, Cretoiu JS, et al. Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones. J Cell Biochem 2003;90:641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajimura S. Adipose tissue in 2016: advances in the understanding of adipose tissue biology. Nat Rev Endocrinol 2017;13:69–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T, Yun S, Jeong JH, Jung TW. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol 2019;486:96–104 [DOI] [PubMed] [Google Scholar]
- 17.Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol 2019;2019:2521096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Chen C, Zhou N, Fu Y, Cheng X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta 2019;489:183–188 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Qu H, Xiong X, et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediators Inflamm 2018;2018:9471583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alan M, Gurlek B, Yilmaz A, et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 2019;35:220–223 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Liao M, Shen R, et al. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators Inflamm 2018;2018:7375294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li E, Shan H, Chen L, et al. OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab 2019;30:319–328.e8 [DOI] [PubMed] [Google Scholar]
- 23.von Herrath M, Pagni PP, Grove K, et al. Case reports of pre-clinical replication studies in metabolism and diabetes. Cell Metab 2019;29:795–802 [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. BioEssays 2016;38:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 2015;4:09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194–198 [DOI] [PubMed] [Google Scholar]
- 27.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 2007;8:21–34 [DOI] [PubMed] [Google Scholar]
- 28.Bouret SG. Development of hypothalamic circuits that control food intake and energy balance. In Appetite and Food Intake: Central Control. 2nd ed., Chapter 7. Harris RBS, Ed. Boca Raton, FL, CRC Press/Taylor & Francis, 2017 [PubMed] [Google Scholar]
- 29.Mizuno K, Ueno Y. Autonomic nervous system and the liver. Hepatol Res 2017;47:160–165 [DOI] [PubMed] [Google Scholar]
- 30.Long W, Xie X, Du C, et al. Decreased circulating levels of asprosin in obese children. Horm Res Paediatr 2019;91:271–277 [DOI] [PubMed] [Google Scholar]
- 31.Sunnetci Sillistre E. and Hatipoglu HU. Increased serum circulating asprosin levels in children with obesity. Pediatr Int. 30 January 2020 [Epub ahead of print]. DOI: 10.1111/ped.1417. [DOI] [PubMed]
- 32.Wang M, Yin C, Wang L, et al. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab 2019;75:205–212 [DOI] [PubMed] [Google Scholar]
- 33.Wang C-Y, Lin TA, Liu KH, et al. Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes 2019;43:1019–1025 [DOI] [PubMed] [Google Scholar]
- 34.Beutler LR, Knight ZA. A spotlight on appetite. Neuron 2018;97:739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J. Mechanisms of insulin resistance in obesity. Front Med 2013;7:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan H. An overall comparison of small molecules and large biologics in ADME testing. ADMET DMPK 2016;4:1–22 [Google Scholar]
- 37.Wiecek M, Szymura J, Maciejczyk M, Kantorowicz M, Szygula Z. Acute anaerobic exercise affects the secretion of asprosin, irisin, and other cytokines – a comparison between sexes. Front Physiol 2018;9:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baykus Y, Yavuzkir S, Ustebay S, Ugur K, Deniz R, Aydin S. Asprosin in umbilical cord of newborns and maternal blood of gestational diabetes, preeclampsia, severe preeclampsia, intrauterine growth retardation and macrosemic fetus. Peptides 2019;120:170132. [DOI] [PubMed] [Google Scholar]
- 39.Acara AC, Bolatkale M, Kızıloglu İ, İbisxoglu E, Can Ç. A novel biochemical marker for predicting the severity of ACS with unstable angina pectoris: asprosin. Am J Emerg Med 2018;36:1504–1505 [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Xu Y, Zheng Y, et al. Increased plasma asprosin levels in patients with drug-naive anorexia nervosa. Eat Weight Disord. 5 February 2020 [Epub ahead of print]. DOI: 10.1007/s40519-020-00845-3. [DOI] [PubMed]