Abstract
Secretion of glucagon from the pancreatic α-cells is conventionally seen as the first and most important defense against hypoglycemia. Recent findings, however, show that α-cell signals stimulate insulin secretion from the neighboring β-cell. This article focuses on these seemingly counterintuitive local actions of α-cells and describes how they impact islet biology and glucose metabolism. It is mostly based on studies published in the last decade on the physiology of α-cells in human islets and incorporates results from rodents where appropriate. As this and the accompanying articles show, the emerging picture of α-cell function is one of increased complexity that needs to be considered when developing new therapies aimed at promoting islet function in the context of diabetes.
Paracrinological Considerations
α- and β-Cells: From This Day Forward, for Better, for Worse, for Richer, for Poorer, in Sickness and in Health, to Love and to Cherish
Hormone secretion from the pancreatic islet is central to the regulation of glucose metabolism. Responsible for most of the islet’s hormonal output are the insulin-secreting β-cells and the glucagon-secreting α cells. Because these hormones have opposite effects on plasma glucose levels, α- and β-cells have long been considered functional antagonists. The islet also contains other endocrine cells that secrete hormones, but their actions may be more local. Recent genome-wide association studies point out that variants of genes expressed in the islet are associated with diabetes. This, combined with the increased availability of human islets for research (1), has revitalized the field of diabetes research and led to a renaissance in islet biology studies. Even the basic anatomy of the islet is being revisited, with studies showing that there are vast species differences in islet cytoarchitecture (2–6). A remarkable feature in all vertebrate species is that, despite their variety, islets always contain α- and β-cells. In our research group, we always wonder why it makes evolutionary sense to have these two cells packed together. The most common explanation is that it is important for β-cells to inhibit α-cell activity so that release of hormones with antagonistic effects does not overlap (7). Using findings on human α-cells published over the last decade, this article will try to show that this is probably not the whole story.
Most, if Not All, Islet Cells Release Paracrine Signals
A common feature of islet endocrine cells is that they release molecules that serve as paracrine signals. In many cases, even the hormones they secrete have local effects. This paracrine function may not be exclusive to endocrine cells. Indeed, secretions from islet pericytes and local macrophages have been shown to exert trophic effects on β-cells (8–10). The effects of paracrine signals are being investigated intensely because they will help us understand how hormone secretion is orchestrated in the islet. The paracrine signaling pathways could in principle also be targeted for therapeutic intervention. Before we delve deeper into the subject, however, we need some terminological and conceptual definitions.
In the pancreatic islet, paracrine signals can influence other cells within the same islet by diffusing through the interstitial space or circulating in local blood vessels. In autocrine signaling, by contrast, a cell secretes a signaling molecule that binds to receptors on the same cell or other cells of the same cell type (e.g., β-cell to β-cell communication). The same signaling molecule can be used in different contexts such as endocrine, paracrine, autocrine, or synaptic signaling. What then are the criteria to consider that a molecule is a paracrine signal? By adopting standards used for decades in the neurosciences (11), we propose that to be a bona fide paracrine signal 1) the molecule must be present or produced in the islet cell, 2) the molecule must be released in response to cell stimulation, and 3) specific receptors for the molecule must be present on target cells in the islet. These criteria seem obvious, but for many signaling candidates they have not been met.
A first step is to detect the signaling molecules in islet cells, but often it is easier to show that the cells express components of the synthetic machinery producing the molecule. Because many of the signaling molecules also participate in general cellular metabolism (e.g., GABA or glutamate), demonstrating the presence of a candidate molecule or its biosynthetic pathway may not be sufficient. Perhaps the most rigorous approach is to show that secretory granules or vesicles contain the molecule, which requires challenging methodologies such as postembedding immunoelectron microscopy. A cell’s secretory phenotype can also be defined by its expression of vesicular transporters for transmitters. A cell expressing the vesicular acetylcholine transporter (vAChT) can thus be considered cholinergic (12,13). This approach, however, may overlook cells that use different mechanisms to secrete paracrine molecules (e.g., ATP release through pannexins) (14).
A second, more challenging criterion is to demonstrate that the paracrine signal is released from the islet cell. This is difficult because the molecule is very likely secreted at low concentrations, degraded by enzymes, or taken up by membrane transporters. Another challenge is to selectively stimulate the islet cell in question and to locate the source for the paracrine signal in the intact islet. This may require measuring molecules secreted from single, dispersed islet cells, at a great loss of detectability. Here, islet cells of a given type could be enriched using cell sorting, with such protocols being recently established for human islets. For several candidate molecules, these technical limitations have prevented demonstrating that they are bona fide paracrine signals in the pancreatic islet.
A third criterion is that the target cell must express receptors for the signaling molecule. The problem here is that many receptor genes are expressed at very low levels and may not be detected, and there are few reliable antibodies for immunohistochemical visualization. A common approach is to stimulate islets pharmacologically and examine the effects on hormone release, Ca2+ responses, or changes in membrane potential. Most of the candidate paracrine signals have been proposed based on pharmacological stimulation of receptors on islet cells. However, meeting this criterion alone is not enough to identify a bona fide paracrine signal because the receptors may be receptors for humoral or neural communication.
Islet Endocrine Cells Are Great Targets for Paracrine Signals
Islet endocrine cells are notable glucose sensors. In a process termed stimulus-secretion coupling, these cells use a series of metabolic reactions to convert glucose stimulation into changes in membrane potential: glucose transport into the cells via low-affinity glucose transporters, glucose phosphorylation by the enzyme glucokinase, and the subsequent metabolism of glucose that increases the intracellular ATP-to-ADP ratio. This elevated ratio closes KATP channels, depolarizes the membrane, and opens voltage-gated Ca2+ channels, triggering hormone secretion (15). The complex stimulus-secretion coupling in islet cells not only ensures that glucose metabolism is coupled to membrane electrical activity but also provides multiple points where external signals can modulate hormone secretion. Because islet endocrine cells display complex electrical activity with bursts of action potentials, many signals impinging on islet endocrine cells can change the secretory behavior of the cells by altering membrane electrical activity. Function of endocrine cells can also be regulated at the level of the secretory pathway. Many processes related to granule exocytosis are dependent on second messenger cascades (e.g., cAMP, Ca2+) that can be activated by ligands binding to membrane receptors. Paracrine or autocrine signals can therefore target receptors to manipulate different intracellular signaling cascades and thus regulate islet hormone secretion.
The Particular Case of the Human Islet
α- and δ-cells are not segregated to the periphery in human islets as they are in rodent islets (4–6). Most β-cells in the human islet are close to α-cells, δ-cells, or both. The association of β-cells with α-cells in human islets is so close that, even after dispersion of islets into single cells, most β-cells remain attached to an α-cell (16). This has profound implications for islet function. The close apposition allows endocrine cells to interact using membrane-bound molecules, an interaction that promotes function and survival (17). Because of these close contacts, electrical coupling between α- and β-cells is also conceivable (18). Although this remains to be established in the human islet, this direct communication could contribute to control the biosynthesis and release of secretory products, as well as to cell survival (19). Because many signaling molecules are rapidly degraded in the interstitial space and in the bloodstream, the proximity of β-cells with α-cells also promotes paracrine interactions.
There are additional players that could play a role in α-cell to β-cell communication. Molecules released by α-cells can be degraded by enzymes or bound by molecules residing in the extracellular matrix (e.g., cholinesterases [see below]), which likely shapes the duration and magnitude of the effects on the target cell. Of course, we cannot rule out the contribution of a third party such as the somatostatin-secreting δ-cell. In the human islet, δ-cells are strategically positioned to play this role (20). Thus, in addition to a direct effect on β-cells, signaling molecules released from α-cells can recruit δ-cells to modulate the net effect on β-cells (e.g., acetylcholine [21]).
At this point, it is important to note that until recently it was thought that the impact of α-cells on β-cell function is negligible (22–25). This can be explained by the smaller proportion and spatial segregation of α-cells in mouse and rat islets, the most common research models. It is also possible that the fluid dynamics in the rodent islet hinder paracrine signals from reaching β-cells. Indeed, a study using the perfused rat pancreas model showed that glucose-induced insulin secretion is not affected by signals from neighboring α-cells (23). In the human islet, by contrast, the percentage of α-cells is higher, α-cells and β-cells are aligned randomly along blood vessels, and most β-cells face α-cells (>70%) (4), making it likely for β-cells to be directly exposed to α-cell secretion. As discussed below, α-cell–derived signals can indeed alter β-cell function in the human islet and, according to new studies, also in the rodent islet.
The Role of Glucagon as a Paracrine Signal
The In Vivo Effects of Intraislet Glucagon
Glucagon is a major hyperglycemic hormone in the organism that counters decreases in plasma glucose levels. Glucagon secretion is thought to provide the first line of defense in glucose counterregulation (26). Glucagon, however, has also been known for decades as a strong amplifier of insulin secretion (27). We recently confirmed that glucagon input increases insulin secretion from human β-cells (28). In in vitro perifusion studies of hormone secretion, we found that the glucagon receptor antagonists L-168,49 and des-His1-[Glu9]-glucagon (1-29) amide decreased insulin secretion stimulated by increases in glucose concentration (see also 29). It is well established that human β-cells express glucagon and glucagon-like peptide 1 (GLP-1) receptors (30,31), but the in vivo role of paracrine glucagon signaling had remained elusive until recently.
Determining local glucagon concentration in the islet in vivo is beyond what current methods can detect, but there is nevertheless a strong case to be made that local glucagon amplifies β-cell activity in the living organism. We found that local glucagon affects insulin secretion from human islets using an in vivo model that reproduces blood flow, capillarity, and ultrastructural features of the islet vasculature in the pancreas (28). In mice, glycemic levels are lower when the percentages of α-cells are higher, suggesting that if glucagon input is increased it may lead to similar effects (28). The strong insulinotropic effects of glucagon (27–29), the increased insulin secretion from β-cells overexpressing glucagon receptors (32,33), and the association of glucagon receptor mutations with reduced insulin secretion and type 2 diabetes (34,35) all support the notion that intraislet glucagon influences insulin secretion.
A series of recent studies in rodents also point out that intraislet glucagon is needed for adequate insulin secretion in vivo (36–38). These studies found that paracrine glucagon stimulates insulin secretion by activating glucagon and GLP-1 receptors on β-cells. These findings contrast with previous reports that ruled out an influence of α-cells on β-cells based on the theory of core-to-mantle blood flow (39,40) but are consistent with studies that revised these older notions about perfusion of the mouse islet (41–43). It is now clear from the various sophisticated mouse models used in the different studies that local glucagon signaling is required for appropriate insulin secretion, to preserve glucose tolerance during the metabolic stress induced by high-fat feeding, and to maintain proper glucose homeostasis in vivo (36–38). That previous studies using ablation of α-cells failed to show this important intraislet cross talk is probably due to the deployment of compensatory mechanisms or to an incomplete reduction in α-cells (44).
Homeostatic Considerations
How can the stimulatory effects of glucagon on the hypoglycemic hormone insulin be reconciled with the hyperglycemic function of glucagon? One answer to this conundrum is to consider glucagon as a hormone that participates in different regulatory circuits. In one circuit, activated during normoglycemia, glucagon secretion reaches concentrations large enough to amplify insulin secretion from neighboring β-cells. As we recently showed, this local secretion is needed to maintain the human glycemic set point (28). Glucagon secretion under these circumstances is probably not strong enough to reach plasma levels that produce systemic responses. By contrast, when glycemia drops, a second circuit is activated in which glucagon secretion becomes strong enough to produce systemic, hyperglycemic effects. Under these hypoglycemic conditions, glucagon cannot stimulate β-cells because glucose levels are no longer permissive for insulin secretion.
The function of paracrine glucagon under normoglycemic conditions can be characterized in engineering terms. To maintain glucose homeostasis, the regulatory system must include sensors, disturbance detectors, an integrator, and effectors. Both α- and β-cells are specialized glucose detectors endowed with mechanisms to sense glucose. Any change in the regulated variable, glucose concentration, produces changes in α- and β-cell physiology that can be considered disturbance signals (e.g., cell membrane depolarization or hyperpolarization, changes in intracellular Ca2+ concentration). These error signals eventually converge on insulin granule exocytosis, which is the integrator (controller) that uses the disturbance signals to send out the control signal insulin to the effector organs (liver, muscles, and fat). By increasing cAMP concentration in β-cells (37), glucagon secretion produces a disturbance signal that is one of the input signals for insulin exocytosis. When activated during glucose counterregulation, by contrast, α-cells become integrators (controllers) themselves, and glucagon acts as a control signal that directly instructs effector organs to produce and release glucose.
It is likely that the glycemic set point results from the dynamic interactions between α- and β-cells. Theoretical models of glucose homeostasis estimate that interactions between α- and β-cells provide a better control of glycemia (45,46). These models posit that the interactions between α- and β-cells need to be asymmetric to provide a negative feedback loop. In fact, all known secretory products of β-cells inhibit glucagon secretion, whereas glucagon and acetylcholine, both secreted from α-cells in human islets, stimulate insulin secretion (47). This arrangement attenuates exacerbated responses and works best with the prevailing small fluctuations in plasma glucose levels. Interrupting this feedback loop by inhibiting glucagon receptors on β-cells acutely destabilizes the glycemic set point (28), confirming the predictions made by the mathematical models.
That the human glucostat depends not solely on the β-cell but on the functional cooperation between α- and β-cells has implications for therapies aimed at reconstituting the β-cell population to treat diabetes. It is likely that the glycemia levels set by β-cells without glucagon input would be characteristic of prediabetes. Because inhibiting glucagon receptors systemically may also eliminate this crucial local input to the β-cell, new approaches to inhibit the contribution of glucagon to hyperglycemia need to be reexamined.
The α-Cell as a Local Source of Acetylcholine
Acetylcholine: A Shifting Story
Acetylcholine is a major amplifier of β-cell secretion: it stimulates insulin release by increasing the cytoplasmic free Ca2+ concentration, [Ca2+]i, via inositol phosphate production and enhancing the effects of Ca2+ on exocytosis via protein kinase C in β-cells (48). Metabotropic receptors for acetylcholine, called muscarinic receptors, expressed in pancreatic β-cells are essential for maintaining proper insulin secretion and glucose homeostasis in mice (49,50). Cholinergic agonists have been reported to restore defective glucose-stimulated insulin secretion (51,52). In human islets, several endocrine cells express muscarinic receptors (31). Variations in the gene that encodes the muscarinic receptor M3 are associated with increased risk for early-onset type 2 diabetes (53). It is generally believed that acetylcholine is released during the cephalic phase of food ingestion from parasympathetic nerve endings in the pancreatic islet to prepare the β-cell for the upcoming rise in nutrient levels (48,49,54). The consensus is that the endocrine pancreas is richly innervated by the autonomic nervous system (54,55), with studies based on the cholinesterase technique revealing dense parasympathetic innervation in cat, rat, rabbit, and human islets (56–59).
A decade ago we started examining human pancreatic islets for the presence of prototypical cholinergic markers and found to our surprise that vAChT and choline acetyltransferase (ChAT) were expressed in α-cells (60). We further determined that α-cells release acetylcholine using a biosensor cell approach (60,61). These results have been replicated using analytical methods and in monkeys (Fig. 1). While acetylcholine also has paracrine effects on other cells within the human islet (e.g., δ-cells) (21), we propose that acetylcholine serves as a feed-forward signal to keep the β-cell responsive to future challenges, thus limiting plasma glucose fluctuations. In this sense, its role may be similar to that of glucagon but using different second messenger systems (cAMP versus Ca2+ release from intracellular stores). Moreover, the intracellular signaling pathways activated by acetylcholine may promote long-term survival of β-cells (62).
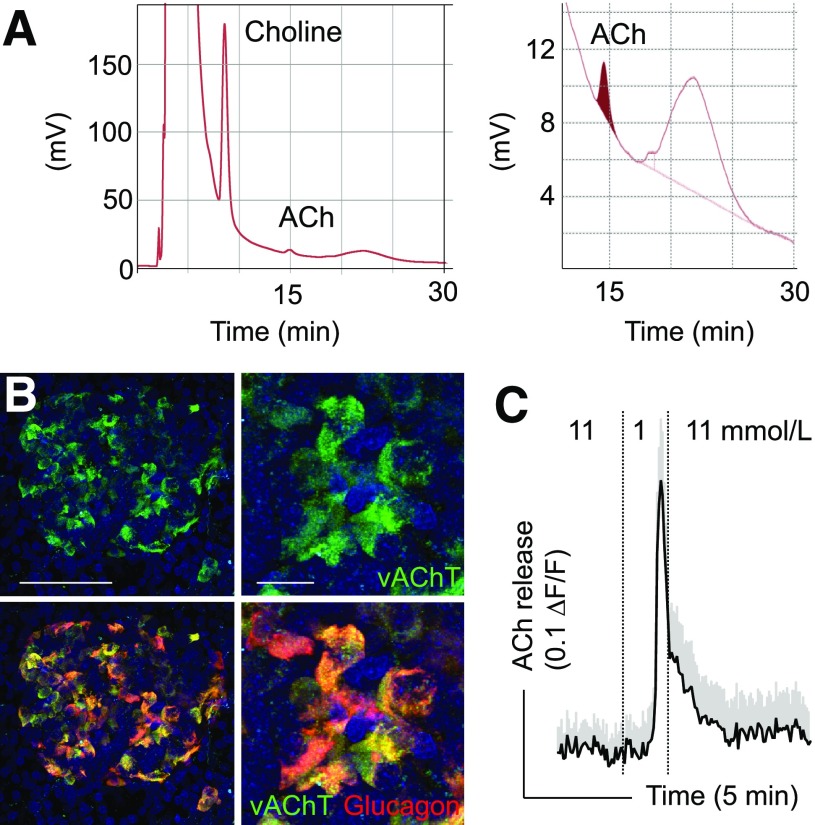
Figure 1.
Detection of acetylcholine (ACh) secretion from human islets using high-performance liquid chromatography and biosensor cells. A: Chromatogram showing acetylcholine detected from a 20-μL human islet sample. The acetylcholine peak area corresponds to ∼1–3 nmol/L and is accompanied by a larger choline peak. A blow up is shown on the right. Human islets were homogenized, protein was denatured, spun down, and placed in high-performance liquid chromatography column. Data contributed by Dr. Ed Phelps, University of Florida. B: Confocal images of vAChT (green) and glucagon (red) immunostaining in pancreas section from a monkey (Macaca fascicularis). As in the human, α-cells are labeled for this cholinergic marker. Panels on the right are higher magnifications of those on the left; bottom panels are merged images of vAChT and glucagon immunostaining. Scale bars = 100 μm (left), 20 μm (right). C: Trace of Ca2+ responses in biosensor cells expressing the muscarinic receptor M3 to monitor acetylcholine secretion from monkey islets (M. fascicularis). Reducing the glucose concentration from 11 mmol/L to 1 mmol/L elicited strong acetylcholine secretion. The dotted line denotes time of changes in glucose concentration. For these experiments, monkey islets were placed on a carpet of biosensor cells (trace shows mean ± SEM of 15 biosensor cells).
Because acetylcholine is efficiently and rapidly degraded by cholinesterases, the paracrine interactions of acetylcholine are extremely local and may occur preferentially via the interstitial space between endocrine cells and not through the vascular route. The effects of acetylcholine on insulin secretion from isolated human islets are greatly amplified in the presence of cholinesterase blockers (60,61,63), indicating 1) that acetylcholine is released endogenously and 2) that cells in the islet express cholinesterases. The interplay among acetylcholine, its receptors, and cholinesterase is the subject of active research in our laboratory because it may represent a target for intervention to promote insulin secretion. Indeed, there are many clinically approved cholinesterase blockers that could be repurposed to enhance insulin secretion in type 2 diabetes.
Is There a Neural Source of Acetylcholine in the Human Islet?
Several studies, including our own, show cholinergic innervation of the human islet, but this innervation is sparse compared with that of the mouse islet (64–66). This is consistent with studies showing that the contribution of neural input to insulin secretion during the cephalic phase is relatively small in humans (67,68). Along similar lines, vagotomized patients have normal postprandial serum insulin levels (69), and patients with type 1 diabetes who have undergone pancreas transplantation (and thus have denervated islets) remain euglycemic without therapy (70–72). The human β-cell may thus rely on local paracrine acetylcholine to get cholinergic input. Because it is not exclusively dependent on nervous input, cholinergic signaling in human islets is probably activated under circumstances other than those elicited by activation of the parasympathetic system.
Of course, it is not possible to rule out that parasympathetic nerves contribute cholinergic input to the human islet. It has been reported extensively that activation of parasympathetic nerves increases plasma levels of pancreatic polypeptide, which is released from pancreatic polypeptide–secreting islet γ-cells (73,74). Indeed, this physiological effect is widely used to demonstrate the impact of parasympathetic input on islet function during the cephalic phase. Interestingly, the highest levels of muscarinic receptor expression can be found in the γ-cell (31). We are revisiting the cholinergic innervation of the human islet with a particular emphasis on the γ-cell and found in preliminary experiments that cholinergic innervation of this cell is particularly strong (Fig. 2). If confirmed, these findings would explain why parasympathetic input predominantly affects the secretion of pancreatic polypeptide, a hormone with strong effects on pancreatic acinar cells and motility of the gastrointestinal tract (75). There are already indications that in humans the cephalic phase response may be strongest for pancreatic polypeptide and minor for insulin, glucagon, GLP-1, GIP, and ghrelin (76,77). Whether parasympathetic nerves can regulate other islet cells indirectly via γ-cells remains to be determined.
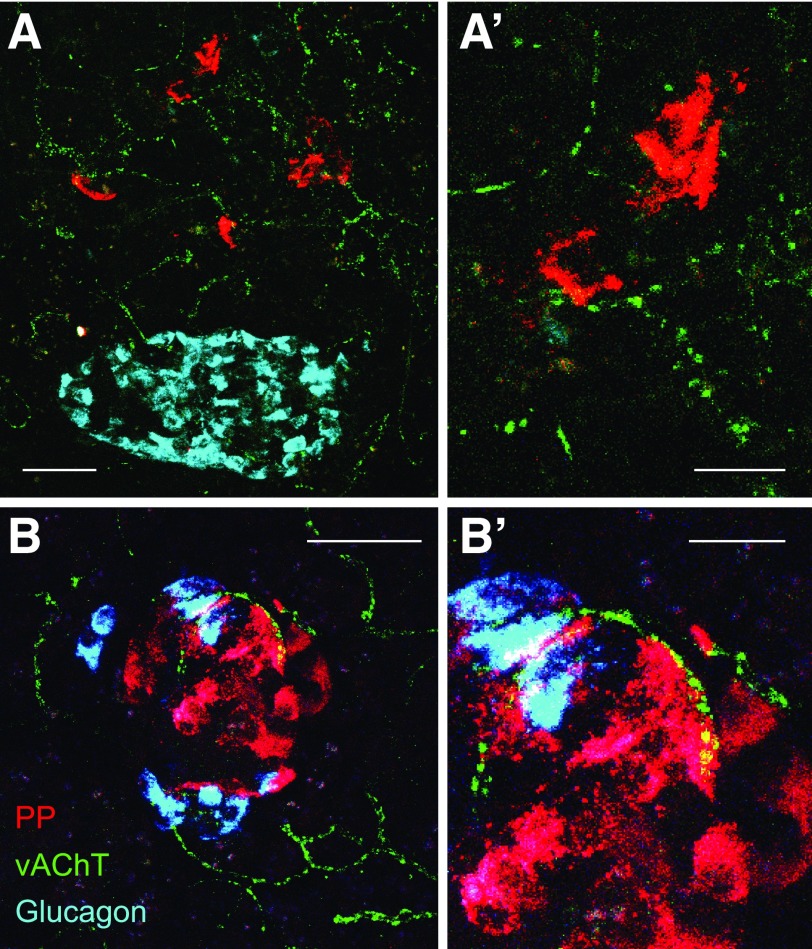
Figure 2.
Cholinergic innervation of pancreatic polypeptide (PP)-secreting islet γ-cells. A: Maximum projection of a z-stack of confocal images showing cholinergic nerves labeled for vAChT (green) in a human pancreas section. Note that the density of nerves is low in the islet region demarcated by α-cells (glucagon [cyan]). By contrast, many cholinergic axons can be seen in pancreatic polypeptide–rich regions (red [at low and high magnification in A and A’]). B: As in A, cholinergic axons can be seen closely apposed to pancreatic polypeptide–labeled cells. Shown is a rare islet mainly composed of pancreatic polypeptide cells from the posterior part of the human pancreatic head. Scale bars: 50 μm (A and B), 10 μm (A’ and B’).
Revealing a Cholinergic Phenotype Is Not Easy
It is important to mention here that confirming a cholinergic phenotype is challenging. The levels of transcripts for acetylcholine-synthesizing enzymes can be extremely low. Cells may contain only four molecules of ChAT transcripts and still exhibit ChAT enzymatic activity that produces acetylcholine (78). Visualizing the presence of ChAT with immunostaining is also not straightforward because the enzyme is found at low amounts (79,80). In our hands, visualizing ChAT required signal amplification with either the avidin-biotin complex method or tyramide signal amplification (Fig. 3). We successfully used antibodies to vAChT to reveal the cholinergic phenotype of α-cells, but the immunostaining results depended on the processing and quality of the human pancreas preparation (60,64). It is likely that these technical limitations have precluded critical demonstrations of the cholinergic phenotype in the islet and other tissues.
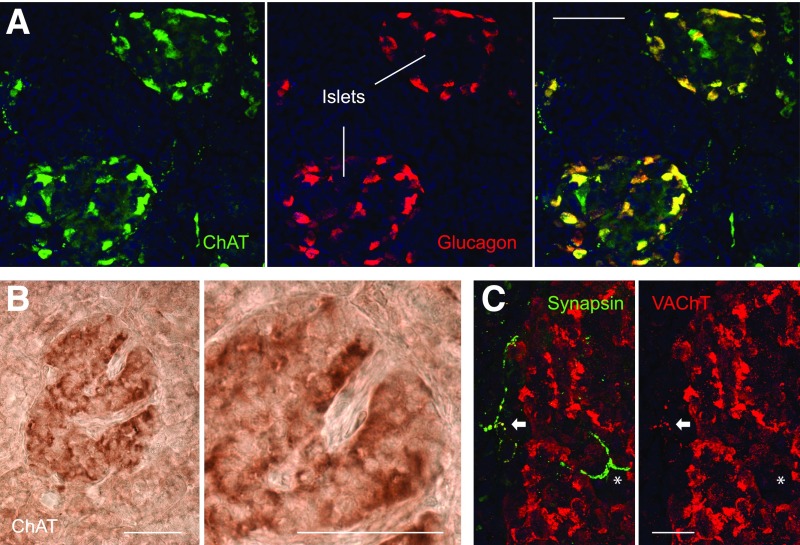
Figure 3.
Cholinergic markers in the human pancreatic islet. A: Confocal images showing immunostaining for the acetylcholine-synthesizing enzyme ChAT (green [shown alone in left panel]) in human pancreas sections using tyramide signal amplification. Glucagon staining is shown in middle panel (red). Most of the ChAT staining colocalizes with glucagon (merged image in right panel). Note that some ChAT-labeled axons can be seen in the exocrine regions. B: Brightfield images showing immunostaining for ChAT using the avidin-biotin complex method. Note that a subset of islet cells is stained in the islet. Right panel is a magnification of left panel. C: Confocal images showing staining for the axonal marker synapsin (green) and the cholinergic marker vAChT (red). An axon close to the islet is labeled for both markers (arrow [staining appears yellow in the merged image on the left]). By contrast, an axon penetrating the islet is only labeled for synapsin (*). Scale bars: 100 μm (A and B), 10 μm (C).
α-Cells Release Glutamate as a Positive Autocrine Signal
What Is the Major Brain Neurotransmitter Doing in the Islet?
Glutamate is the major excitatory neurotransmitter in the central nervous system. Even though it has been proposed as an islet paracrine signal for >20 years (81), the functional role of glutamate in the islet is still unresolved. One problem is that glutamate may originate from different sources such as the plasma, nerve terminals, or endocrine cells. Glutamate has been shown to present in glucagon secretory granules (81), and vesicular glutamate transporters (vGluT1-3) are expressed in α-cells of rodent, monkey, and human islets (82,83). Initial studies of rodent islets indicated that glutamate is secreted together with glucagon (81). Findings in human islets later confirmed that glutamate is released under conditions that also stimulate glucagon secretion (83). However, glutamate derived from metabolic pools, and not from granules, may be also released via membrane glutamate transporters by uptake reversal (84), suggesting multiple mechanisms for glutamate secretion.
Extracellular glutamate can act on ionotropic (AMPA/kainate and N-nitrosodimethylamine [NMDA] receptors) and metabotropic receptors. Results of the expression of glutamate receptors in islet cells are conflicting (85). Databases show that all endocrine cells, but not exocrine cells, of the human pancreas express one or more glutamate receptors (31). Glutamate has been reported to activate α-cells via AMPA/kainate receptors (83,86), inhibit α-cells via metabotropic receptors (87), activate AMPA/kainate and metabotropic receptors in β-cells to increase insulin secretion (88,89), or stimulate δ-cells via AMPA/kainate receptors (90). We compared glutamate signaling in human, monkey, and mouse islets and found that in all three species glutamate activated AMPA/kainate receptors on α-cells and stimulated glucagon secretion. Β-cells and insulin secretion, by contrast, could not be stimulated with glutamate agonists, either at basal or stimulatory glucose concentrations. These results have been confirmed for mouse islets (86). Activation of AMPA/kainate receptors leads to membrane depolarization, opening of voltage-gated Ca2+ channels, and an increase in glucagon secretion due to a rise in intracellular Ca2+ (83,86). We further found that blocking AMPA/kainate receptors in vivo reduces glucagon release and exacerbates insulin-induced hypoglycemia in mice (83). These results suggest that α-cells use glutamate to potentiate its secretory activity.
As always, the effects of extracellular glutamate in the islet may be more complex. The concentrations of glutamate in the islet may be strongly affected by reverse transport from a cell’s metabolic pool (84). Glutamate derived from such a mechanism could act as an extracellular signal as well as an intracellular metabolite that affects hormone secretion. Also, involvement of metabotropic receptors cannot be dismissed (87), and other endocrine cells within the islet (e.g., δ-cells) may also respond to glutamate (90). Clearly, more research is needed to further define the function of glutamate in the islet. Last but not least, glutamate may activate NMDA receptors to regulate insulin secretion and thus blood glucose control (91).
An Autocrine Loop That Boosts Glucagon Secretion
The findings described above indicate that glutamate participates in an autocrine positive feedback loop. This autocrine positive feedback ensures that even small decreases in plasma glucose concentration elicit glucagon secretion sufficient for avoidance of further hypoglycemia. Many of the paracrine and autocrine signals in the islet are involved in regulatory circuits that use feedback. Interestingly, most of the autocrine signals described for the islet provide positive feedback. That is, the autocrine signals reinforce the effects produced by the initial perturbation (i.e., a change in glucose concentration). As a result, a small perturbation at the input causes a much larger effect at the output. Small deviations in plasma glucose concentration (∼10%) are thus counteracted by sharp increases in insulin and glucagon secretion (threefold) (92). Positive feedback is often used in the rising phase of a physiological response to a perturbation. Not surprisingly, autocrine feedback loops in the islet are activated near the threshold for hormone responses to changes in glucose concentration. This helps make hormonal responses fast and robust.
In type 1 diabetes, α-cells lose their ability to sense decreases in glucose concentration. As a consequence of what is called glucose blindness, glucagon secretion is not stimulated when insulin treatment induces hypoglycemia. α-Cells, however, still respond to other stimuli and retain their potential to secrete glucagon. AMPA/kainate receptors expressed in the human α-cell thus represent a putative target for pharmacological intervention to prevent hypoglycemia. Safe drugs that positively modulate AMPA receptors (e.g., ampakines) are currently being developed for potential therapeutic applications to improve memory and cognition as well as to treat schizophrenia. Using these drugs to activate AMPA/kainate receptors in α-cells could be an adjuvant therapy in the management of drug-treated diabetes.
Concluding Remarks
Recent studies are revealing a new paracrine role for the α-cell in the islet (Fig. 4). For many years this role was overlooked, mostly because of technical limitations such as the inability to measure or manipulate glucagon signaling selectively in the islet. This led to the consensus that the contribution of α-cells to β-cell activity is negligible. Studies on human islets then showed that glucagon input from the α-cell to the β-cell affects insulin secretion and glucose homeostasis. These findings have now been confirmed and extended using novel genetic tools in mice. There is no doubt now that the α-cell impacts β-cell output, which is also reflected in mathematical models of glucose homeostasis. While we focused here on the direct actions on β-cells, the secretory products from α-cells also activate δ-cells. Indeed, glucagon, glutamate, and acetylcholine stimulate somatostatin secretion. Because δ-cells are activated by β-cell secretory products such as GABA, urocortin, and ATP, δ-cells may provide bidirectional communication between α- and β-cells. This can include electrical communication. Indeed, β-cells have been shown to regulate glucagon secretion through gap junction communication with δ-cells (93). Future models of how hormone secretion is orchestrated in the islet will have to include this ever-increasing complexity. It seems that the more we learn, the less we know.
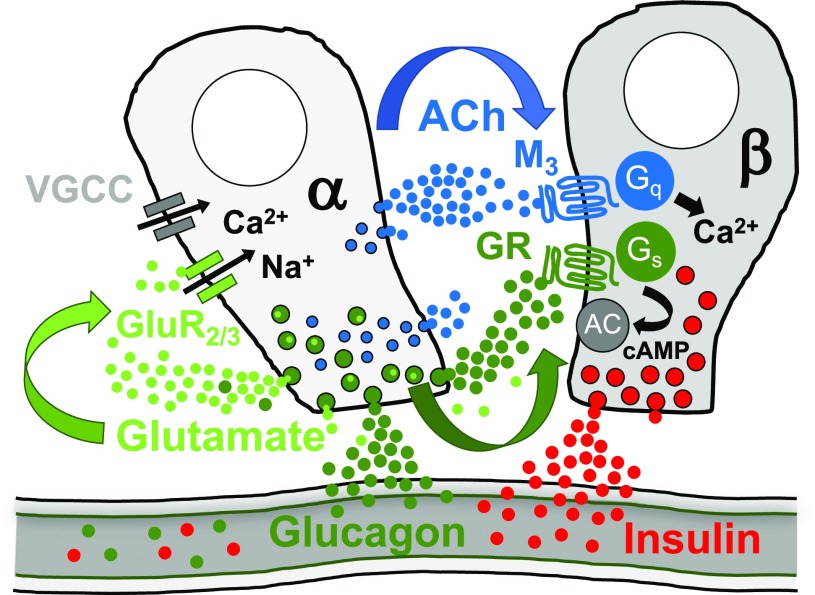
Figure 4.
Schematic for paracrine signaling originating from human α-cells. α-Cells secrete glucagon, acetylcholine, and glutamate, which all have been shown to have local paracrine or autocrine excitatory effects. Not depicted are additional receptors or non–β-cell targets for these signaling molecules within the islet (for more information, see acetylcholine: a shifting story). AC, adenylate cyclase; ACh, acetylcholine; GluR2/3, glutamate AMPA receptor 2/3; Gq, Gq alpha unit; GR, glucagon receptor; Gs, Gs alpha unit, G stimulatory; M3, acetylcholine muscarinic receptor 3; VGCC, voltage-gated Ca2+ channel.
Article Information
Acknowledgments. The authors thank all members of the Caicedo laboratory for their past and present contributions. The authors give special thanks to Dr. Ed Phelps for his generous sharing of the high-performance liquid chromatography data shown in Fig. 1 and to Drs. Dora Berman and Norma Kenyon for providing monkey islets.
Funding. This work was supported by the Diabetes Research Institute Foundation and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grants R56DK084321 (to A.C.), R01DK084321 (to A.C.), R01DK111538 (to A.C.), R01DK113093 (to A.C.), U01DK120456 (to A.C.), R33ES025673 (to A.C.), and R21ES025673 (to A.C.), and The Leona M. and Harry B. Helmsley Charitable Trust grants G-2018PG-T1D034 and G-1912-03552.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this work were presented at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988;37:413–420 [DOI] [PubMed] [Google Scholar]
- 2.Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M. Quantification of islet size and architecture. Islets 2012;4:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets 2009;1:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brissova M, Fowler MJ, Nicholson WE, et al. . Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 6.Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets 2015;7:e1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A 2010;107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasson A, Rachi E, Sakhneny L, et al. . Islet pericytes are required for β-cell maturity. Diabetes 2016;65:3008–3014 [DOI] [PubMed] [Google Scholar]
- 9.Epshtein A, Rachi E, Sakhneny L, Mizrachi S, Baer D, Landsman L. Neonatal pancreatic pericytes support β-cell proliferation. Mol Metab 2017;6:1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, et al. . Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol 2004;76:359–367 [DOI] [PubMed] [Google Scholar]
- 11.Purves D, Augustine GJ, Fitzpatrick D, et al. . Neuroscience. 3rd ed. Sunderland, MA, Sinauer Associates, Inc, 2008 [Google Scholar]
- 12.Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A 1996;93:3547–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer MK, Weihe E, Varoqui H, Eiden LE, Erickson JD. Distribution of the vesicular acetylcholine transporter (VAChT) in the central and peripheral nervous systems of the rat. J Mol Neurosci 1994;5:1–26 [DOI] [PubMed] [Google Scholar]
- 14.Chiu YH, Schappe MS, Desai BN, Bayliss DA. Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 2018;150:19–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 2018;98:117–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosco D, Armanet M, Morel P, et al. . Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes 2010;59:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konstantinova I, Nikolova G, Ohara-Imaizumi M, et al. . EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007;129:359–370 [DOI] [PubMed] [Google Scholar]
- 18.Meda P, Kohen E, Kohen C, Rabinovitch A, Orci L. Direct communication of homologous and heterologous endocrine islet cells in culture. J Cell Biol 1982;92:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meda P. Gap junction proteins are key drivers of endocrine function. Biochim Biophys Acta Biomembr 2018;1860:124–140 [DOI] [PubMed] [Google Scholar]
- 20.Arrojo E, Drigo R, Jacob S, García-Prieto CF, et al. . Structural basis for delta cell paracrine regulation in pancreatic islets. Nat Commun 2019;10:3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014;63:2714–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King AJ, Fernandes JR, Hollister-Lock J, Nienaber CE, Bonner-Weir S, Weir GC. Normal relationship of β- and non-β-cells not needed for successful islet transplantation. Diabetes 2007;56:2312–2318 [DOI] [PubMed] [Google Scholar]
- 23.Moens K, Berger V, Ahn JM, et al. . Assessment of the role of interstitial glucagon in the acute glucose secretory responsiveness of in situ pancreatic β-cells. Diabetes 2002;51:669–675 [DOI] [PubMed] [Google Scholar]
- 24.Shiota C, Prasadan K, Guo P, et al. . α-Cells are dispensable in postnatal morphogenesis and maturation of mouse pancreatic islets. Am J Physiol Endocrinol Metab 2013;305:E1030–E1040 [DOI] [PubMed] [Google Scholar]
- 25.Thorel F, Damond N, Chera S, et al. . Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 2011;60:2872–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 27.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 1965;2:415–416 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. . Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018;27:549–558.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000;43:1012–1019 [DOI] [PubMed] [Google Scholar]
- 30.Adriaenssens AE, Svendsen B, Lam BY, et al. . Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016;59:2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segerstolpe Å, Palasantza A, Eliasson P, et al. . Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelling RW, Vuguin PM, Du XQ, et al. . Pancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and mass. Am J Physiol Endocrinol Metab 2009;297:E695–E707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sørensen H, Winzell MS, Brand CL, et al. . Glucagon receptor knockout mice display increased insulin sensitivity and impaired β-cell function. Diabetes 2006;55:3463–3469 [DOI] [PubMed] [Google Scholar]
- 34.Hansen LH, Abrahamsen N, Hager J, et al. . The Gly40Ser mutation in the human glucagon receptor gene associated with NIDDM results in a receptor with reduced sensitivity to glucagon. Diabetes 1996;45:725–730 [DOI] [PubMed] [Google Scholar]
- 35.Hager J, Hansen L, Vaisse C, et al. . A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet 1995;9:299–304 [DOI] [PubMed] [Google Scholar]
- 36.Zhu L, Dattaroy D, Pham J, et al. . Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;5:e127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capozzi ME, Svendsen B, Encisco SE, et al. . β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svendsen B, Larsen O, Gabe MBN, et al. . Insulin secretion depends on intra-islet glucagon signaling. Cell Reports 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 39.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 [DOI] [PubMed] [Google Scholar]
- 40.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kharouta M, Miller K, Kim A, et al. . No mantle formation in rodent islets -- the prototype of islet revisited. Diabetes Res Clin Pract 2009;85:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YM, Guth PH, Kaneko K, Livingston EH, Brunicardi FC. Dynamic in vivo observation of rat islet microcirculation. Pancreas 1993;8:15–21 [DOI] [PubMed] [Google Scholar]
- 43.Nyman LR, Wells KS, Head WS, et al. . Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008;118:3790–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen MG, Cobelli C. Multiscale modelling of insulin secretion during an intravenous glucose tolerance test. Interface Focus 2013;3:20120085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo J, Choi MY, Koh DS. Beneficial effects of intercellular interactions between pancreatic islet cells in blood glucose regulation. J Theor Biol 2009;257:312–319 [DOI] [PubMed] [Google Scholar]
- 46.Koeslag JH, Saunders PT, Terblanche E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X complex. J Physiol 2003;549:333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 2013;24:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 2001;22:565–604 [DOI] [PubMed] [Google Scholar]
- 49.Gautam D, Han SJ, Hamdan FF, et al. . A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 2006;3:449–461 [DOI] [PubMed] [Google Scholar]
- 50.Gautam D, Gavrilova O, Jeon J, et al. . Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab 2006;4:363–375 [DOI] [PubMed] [Google Scholar]
- 51.Guenifi A, Simonsson E, Karlsson S, Ahrén B, Abdel-Halim SM. Carbachol restores insulin release in diabetic GK rat islets by mechanisms largely involving hydrolysis of diacylglycerol and direct interaction with the exocytotic machinery. Pancreas 2001;22:164–171 [DOI] [PubMed] [Google Scholar]
- 52.Doliba NM, Qin W, Vatamaniuk MZ, et al. . Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am J Physiol Endocrinol Metab 2004;286:E834–E843 [DOI] [PubMed] [Google Scholar]
- 53.Guo Y, Traurig M, Ma L, et al. . CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes 2006;55:3625–3629 [DOI] [PubMed] [Google Scholar]
- 54.Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia 2000;43:393–410 [DOI] [PubMed] [Google Scholar]
- 55.Woods SC, Porte D Jr. Neural control of the endocrine pancreas. Physiol Rev 1974;54:596–619 [DOI] [PubMed] [Google Scholar]
- 56.Coupland RE. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat 1958;92:143–149 [PMC free article] [PubMed] [Google Scholar]
- 57.Amenta F, Cavallotti C, de Rossi M, Tonelli F, Vatrella F. The cholinergic innervation of human pancreatic islets. Acta Histochem 1983;73:273–278 [DOI] [PubMed] [Google Scholar]
- 58.Ahrén B, Taborsky GJ Jr, Porte D Jr. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 1986;29:827–836 [DOI] [PubMed] [Google Scholar]
- 59.Brunicardi FC, Shavelle DM, Andersen DK. Neural regulation of the endocrine pancreas. Int J Pancreatol 1995;18:177–195 [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. . Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 2011;17:888–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Diaz R, Dando R, Huang YA, Berggren PO, Roper SD, Caicedo A. Real-time detection of acetylcholine release from the human endocrine pancreas. Nat Protoc 2012;7:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gautam D, Han SJ, Duttaroy A, et al. . Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab 2007;9(Suppl. 2):158–169 [DOI] [PubMed] [Google Scholar]
- 63.Del Rio G, Procopio M, Bondi M, et al. . Cholinergic enhancement by pyridostigmine increases the insulin response to glucose load in obese patients but not in normal subjects. Int J Obes Relat Metab Disord 1997;21:1111–1114 [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. . Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang SC, Baeyens L, Shen CN, et al. . Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia 2018;61:168–181 [DOI] [PubMed] [Google Scholar]
- 66.Butterworth E, Dickerson W, Vijay V, et al. . High resolution 3D imaging of the human pancreas neuro-insular network. J Vis Exp 2018;131:e56859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor IL, Feldman M. Effect of cephalic-vagal stimulation on insulin, gastric inhibitory polypeptide, and pancreatic polypeptide release in humans. J Clin Endocrinol Metab 1982;55:1114–1117 [DOI] [PubMed] [Google Scholar]
- 68.Teff KL, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism 1993;42:1600–1608 [DOI] [PubMed] [Google Scholar]
- 69.Becker HD, Börger HW, Schafmayer A. Effect of vagotomy on gastrointestinal hormones. World J Surg 1979;3:615–622 [DOI] [PubMed] [Google Scholar]
- 70.Pozza G, Bosi E, Secchi A, et al. . Metabolic control of type I (insulin dependent) diabetes after pancreas transplantation. Br Med J (Clin Res Ed) 1985;291:510–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diem P, Redmon JB, Abid M, et al. . Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest 1990;86:2008–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blackman JD, Polonsky KS, Jaspan JB, Sturis J, Van Cauter E, Thistlethwaite JR. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes 1992;41:1346–1354 [DOI] [PubMed] [Google Scholar]
- 73.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav 2011;103:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunicardi FC, Druck P, Sun YS, Elahi D, Gingerich RL, Andersen DK. Regulation of pancreatic polypeptide secretion in the isolated perfused human pancreas. Am J Surg 1988;155:63–69 [DOI] [PubMed] [Google Scholar]
- 75.Batterham RL, Le Roux CW, Cohen MA, et al. . Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 2003;88:3989–3992 [DOI] [PubMed] [Google Scholar]
- 76.Veedfald S, Plamboeck A, Deacon CF, et al. . Cephalic phase secretion of insulin and other enteropancreatic hormones in humans. Am J Physiol Gastrointest Liver Physiol 2016;310:G43–G51 [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Oestricker LZ, Wallendorf MJ, et al. . Cholinergic signaling mediates the effects of xenin-25 on secretion of pancreatic polypeptide but not insulin or glucagon in humans with impaired glucose tolerance. PLoS One 2018;13:e0192441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vandenbergh DJ, Mori N, Anderson DJ. Co-expression of multiple neurotransmitter enzyme genes in normal and immortalized sympathoadrenal progenitor cells. Dev Biol 1991;148:10–22 [DOI] [PubMed] [Google Scholar]
- 79.Schemann M, Sann H, Schaaf C, Mäder M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol 1993;265:G1005–G1009 [DOI] [PubMed] [Google Scholar]
- 80.Ratcliffe EM, deSa DJ, Dixon MF, Stead RH. Choline acetyltransferase (ChAT) immunoreactivity in paraffin sections of normal and diseased intestines. J Histochem Cytochem 1998;46:1223–1231 [DOI] [PubMed] [Google Scholar]
- 81.Hayashi M, Yamada H, Uehara S, et al. . Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 2003;278:1966–1974 [DOI] [PubMed] [Google Scholar]
- 82.Hayashi M, Otsuka M, Morimoto R, et al. . Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J Biol Chem 2001;276:43400–43406 [DOI] [PubMed] [Google Scholar]
- 83.Cabrera O, Jacques-Silva MC, Speier S, et al. . Glutamate is a positive autocrine signal for glucagon release. Cell Metab 2008;7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldmann N, del Rio RM, Gjinovci A, Tamarit-Rodriguez J, Wollheim CB, Wiederkehr A. Reduction of plasma membrane glutamate transport potentiates insulin but not glucagon secretion in pancreatic islet cells. Mol Cell Endocrinol 2011;338:46–57 [DOI] [PubMed] [Google Scholar]
- 85.Moriyama Y, Hayashi M. Glutamate-mediated signaling in the islets of Langerhans: a thread entangled. Trends Pharmacol Sci 2003;24:511–517 [DOI] [PubMed] [Google Scholar]
- 86.Cho JH, Chen L, Kim MH, Chow RH, Hille B, Koh DS. Characteristics and functions of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors expressed in mouse pancreatic alpha-cells. Endocrinology 2010;151:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uehara S, Muroyama A, Echigo N, et al. . Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by α-cells of islet of Langerhans. Diabetes 2004;53:998–1006 [DOI] [PubMed] [Google Scholar]
- 88.Bertrand G, Puech R, Loubatieres-Mariani MM, Bockaert J. Glutamate stimulates insulin secretion and improves glucose tolerance in rats. Am J Physiol 1995;269:E551–E556 [DOI] [PubMed] [Google Scholar]
- 89.Storto M, Capobianco L, Battaglia G, et al. . Insulin secretion is controlled by mGlu5 metabotropic glutamate receptors. Mol Pharmacol 2006;69:1234–1241 [DOI] [PubMed] [Google Scholar]
- 90.Muroyama A, Uehara S, Yatsushiro S, et al. . A novel variant of ionotropic glutamate receptor regulates somatostatin secretion from delta-cells of islets of Langerhans. Diabetes 2004;53:1743–1753 [DOI] [PubMed] [Google Scholar]
- 91.Marquard J, Otter S, Welters A, et al. . Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat Med 2015;21:363–372 [DOI] [PubMed] [Google Scholar]
- 92.Conn PM, Goodman HM, Kostyo JL. The Endocrine System. New York, Oxford University Press, 1998, p. 1–5 [Google Scholar]
- 93.Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. δ-Cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J Physiol 2018;596:197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]