Abstract
Sodium–glucose cotransport 2 inhibitors (SGLT2i) lower plasma glucose but stimulate endogenous glucose production (EGP). The current study examined the effect of dapagliflozin on EGP while clamping plasma glucose, insulin, and glucagon concentrations at their fasting level. Thirty-eight patients with type 2 diabetes received an 8-h measurement of EGP ([3-3H]-glucose) on three occasions. After a 3-h tracer equilibration, subjects received 1) dapagliflozin 10 mg (n = 26) or placebo (n = 12); 2) repeat EGP measurement with the plasma glucose concentration clamped at the fasting level; and 3) repeat EGP measurement with inhibition of insulin and glucagon secretion with somatostatin infusion and replacement of basal plasma insulin and glucagon concentrations. In study 1, the change in EGP (baseline to last hour of EGP measurement) in subjects receiving dapagliflozin was 22% greater (+0.66 ± 0.11 mg/kg/min, P < 0.05) than in subjects receiving placebo, and it was associated with a significant increase in plasma glucagon and a decrease in the plasma insulin concentration compared with placebo. Under glucose clamp conditions (study 2), the change in plasma insulin and glucagon concentrations was comparable in subjects receiving dapagliflozin and placebo, yet the difference in EGP between dapagliflozin and placebo persisted (+0.71 ± 0.13 mg/kg/min, P < 0.01). Under pancreatic clamp conditions (study 3), dapagliflozin produced an initial large decrease in EGP (8% below placebo), followed by a progressive increase in EGP that was 10.6% greater than placebo during the last hour. Collectively, these results indicate that 1) the changes in plasma insulin and glucagon concentration after SGLT2i administration are secondary to the decrease in plasma glucose concentration, and 2) the dapagliflozin-induced increase in EGP cannot be explained by the increase in plasma glucagon or decrease in plasma insulin or glucose concentrations.
Introduction
Sodium–glucose cotransport 2 inhibitors (SGLT2i) are a novel class of antidiabetic agents that lower the plasma glucose concentration by inhibiting renal glucose reabsorption and producing glucosuria (1,2). We (3,4) and others (5,6) have shown that members of the SGLT2i class stimulate basal endogenous glucose production (EGP) and that the increase in EGP offsets by ∼50% the amount of glucose excreted in the urine (3), thereby blunting the decrease in the plasma glucose concentration and attenuating the clinical efficacy of SGLT2i. Although the increase in EGP can be viewed as a compensatory response to urinary glucose loss to prevent hypoglycemia, in patients with type 2 diabetes mellitus (T2DM), it occurs while the plasma glucose concentration still is well within the hyperglycemic range (3).
We and others have demonstrated (3–6) that the increase in EGP caused by SGLT2i is associated with a small but significant decrease in the plasma insulin concentration and a larger (∼25–35%) increase in the plasma glucagon concentration. Thus, the plasma glucagon-to-insulin ratio increases markedly by ∼50%. Because of the important role of plasma glucagon and insulin concentrations in the regulation of EGP (7), we hypothesized that the decrease in plasma insulin and increase in plasma glucagon concentrations could at least partly explain the increase in EGP caused by SGLT2i. The current study examined this hypothesis by testing the effect SGLT2i on EGP while 1) clamping the plasma glucose concentration at its basal fasting level and 2) clamping the plasma glucagon and insulin concentrations at their fasting level with somatostatin infusion and basal replacement of insulin and glucagon.
Research Design and Methods
Subjects
Of 52 patients with T2DM who were screened, 47 eligible subjects were enrolled, and 38 subjects completed the study. Supplementary Fig 1 depicts the study profile. Except for diabetes, all subjects were in good general health based on medical history, physical examination, blood chemistry analysis, complete blood count, thyroid function, urinalysis, and electrocardiogram. Patients had stable (±1.5 kg) body weight over the 3 months before the study, and no subject participated in any excessively heavy exercise program. Patients were drug naive (n = 5) or on a stable dose of metformin with (n = 10) or without (n = 23) a sulfonylurea. The distribution of background medication in the two treatment groups is presented in Table 1. The results were similar whether subjects were drug naive or taking metformin with or without a sulfonylurea. All background medications were continued without change throughout the study. Subjects with evidence of proliferative diabetic retinopathy or serum creatinine >1.4 mg/dL (women) or >1.5 mg/dL (men), or with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 were excluded.
Table 1.
Clinical and anthropometric characteristics of study participants
| Dapagliflozin | Placebo | P value | |
|---|---|---|---|
| (n = 26) | (n = 12) | ||
| Age (years) | 53 ± 2 | 53 ± 2 | NS |
| Female sex, n | 8 | 4 | NS |
| BMI (kg/m2) | 32.5 ± 0.7 | 32.2 ± 1.5 | NS |
| Diabetes duration (years) | 6.9 ± 1.8 | 5.9 ± 1.5 | NS |
| FPG (mg/dL) | 138 ± 7 | 126 ± 6 | NS |
| HbA1c (%) | 7.4 ± 0.2 | 7.5 ± 0.1 | NS |
| eGFR (mL/min) | 97 ± 3 | 97 ± 4 | NS |
| Background therapy, n | |||
| Drug naive | 3 | 2 | |
| Metformin | 15 | 8 | |
| Metformin + sulfonylurea | 8 | 2 |
Values are mean ± SEM or as indicated.
The University of Texas Health Science Center at San Antonio Institutional Review Board approved the study, and informed written consent was obtained from all subjects before their participation. The study is registered at ClinicalTrials.gov NCT02592421.
Experimental Design
All studies were performed at the Texas Diabetes Institute Clinical Research Center at 6:00 a.m. after a 10-h overnight fast. After confirming eligibility, subjects were consecutively randomized to receive dapagliflozin or placebo in 2:1 ratio. Subject stratification was done according to the following parameters: age, BMI, diabetes duration, fasting plasma glucose (FPG), eGFR, and HbA1c. Each subject received three, 8-h measurements of EGP that were performed in random order with a 7- to 10-day interval between studies. Background medications (metformin and/or sulfonylurea) were withheld in the morning of the study day. In study 1, EGP was measured with prime-continuous [3-3H]glucose infusion. In study 2, EGP was measured under conditions when the plasma glucose concentration was clamped, and in study 3, the EGP measurement was performed under pancreatic clamp conditions.
Measurement of EGP
Study 1
Subjects reported to the Clinical Research Center at 6:00 a.m., after an overnight fast. A catheter was placed into an antecubital vein, and an adjusted prime (40 μCi × FPG/100)-continuous (0.4 μCi/min) infusion of [3-3H]glucose was started and continued until 2:00 p.m. At 8:00 am, a second catheter was inserted retrogradely into a vein on the dorsum of the hand, which was placed in a heated box (70°C) for sampling of arterialized blood. After 2.5 h of tracer equilibration (8:30 a.m.), arterialized blood samples were drawn at −30, −20, −15, −10, −5, and 0 (time zero = drug administration) min. At time zero (9:00 a.m.), subjects received dapagliflozin 10 mg (n = 26) or placebo (n = 12) on separate days within a 1- to 2-week interval. After 9:00 a.m. (time zero), plasma samples were obtained every 10–20 min for 300 min for determination of plasma glucose, insulin, C-peptide, and glucagon concentrations and [3-3H]glucose-specific activity. At 6:00 a.m., subjects voided, and the urine was discarded. Urine was collected from 6:00 a.m. to 9:00 a.m. (baseline period) and from 9:00 a.m. to 2:00 p.m. (drug treatment period). Urinary volume and glucose concentration were measured to determine the urinary glucose excretion (UGE) rate. At 2:00 p.m., subjects received a meal and returned home.
Study 2
The measurement of EGP was similar to that in study 1, with one difference. After time zero (drug administration), the plasma glucose concentration was measured every 5 min, and a variable infusion of 20% dextrose solution was adjusted to maintain the plasma glucose concentration at the fasting level.
Study 3
The measurement of EGP was similar to that in study 1, with the following exceptions. Somatostatin infusion (750 µg/h) was started 5 min before the start of the [3-3H]glucose infusion, and the basal plasma insulin and glucagon concentrations were maintained with infusion of insulin (0.1 mU/kg ⋅ min) and glucagon (0.3 ng/kg ⋅ min).
Analytical Methods
Plasma glucose was measured using the glucose-oxidase method (Analox Reagent Instruments, International Point of Care, Toronto, Ontario, Canada). Plasma insulin (IBL America, Minneapolis, MN) and C-peptide (MP Biomedicals, Santa Ana, CA) were measured with immunoradiometric assays. Plasma glucagon (MilliporeSigma, Burlington, MA) was measured by radioimmunoassay.
Data Analysis
Under steady-state postabsorptive conditions, the basal rate of EGP equals the [3-3H]glucose infusion rate (GIR) (disintegrations per minute/min) divided by steady-state plasma titrated glucose-specific activity (disintegrations per minute/mg). Supplementary Fig. 2 depicts [3-3H]glucose-specific activity during the three studies in subjects receiving dapagliflozin and placebo. After drug administration, nonsteady conditions for [3-3H]glucose-specific activity prevail, and the total body Ra is calculated using the Steele equation. The change in EGP during the last hour of the study (i.e., 240–300 min) from baseline was considered the drug effect on EGP and was compared between dapagliflozin and placebo with ANOVA. All values are presented as mean ± SEM. A P value <0.05 was considered statistically significant.
Data and Resource Availability
The data sets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Table 1 presents the baseline characteristics of subjects receiving dapagliflozin and placebo. Patients were well matched in age, sex, BMI, duration of diabetes, FPG, HbA1c, and eGFR.
In study 1, dapagliflozin increased UGE during the EGP measurement to 2.54 ± 0.20 g/h. In study 2, the increase in UGE was significantly greater (3.74 ± 0.41 g/h) than in study 1 (P = 0.02). In study 3, UGE (1.86 ± 0.26 g/h) was significantly less (P < 0.005) than in study 1.
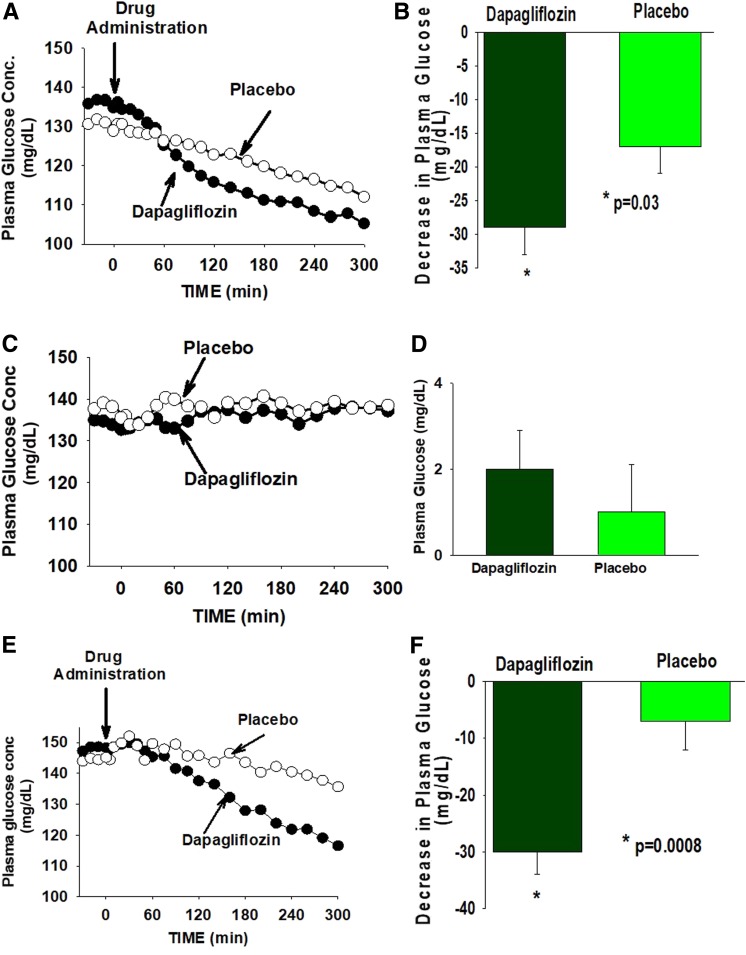
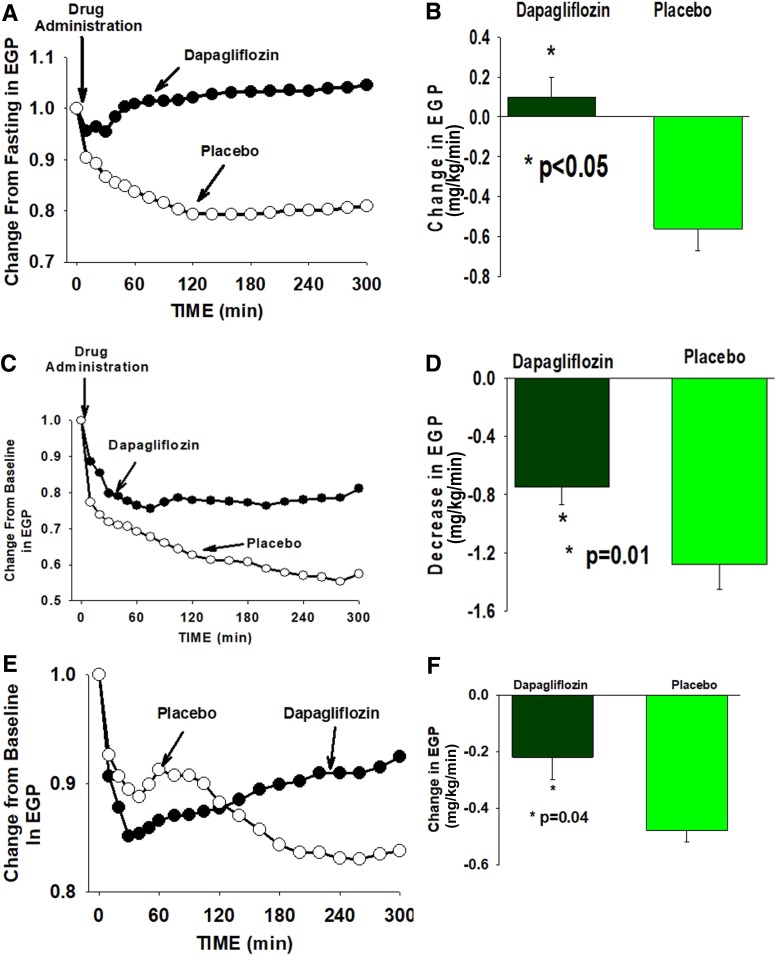
During EGP measurement in study 1, dapagliflozin produced a significant decrease in the plasma glucose concentration which, during the last hour (i.e., 240–300 min), was 29 ± 4 mg/dL below the FPG concentration, compared with a 17 ± 4 mg/dL decrease in subjects receiving placebo (Fig. 1A and Table 2). Thus, the placebo-subtracted decrease in the plasma glucose concentration brought about by dapagliflozin in study 1 was 12 mg/dL (−7.8%, P = 0.03). The basal rate of EGP at the last hour of the EGP measurement increased by +0.10 ± 0.10 mg/kg/min above the fasting level after dapagliflozin administration (+2.7%) compared with a −0.56 ± 0.11 mg/kg/min decrease (−19.6%) in subjects receiving placebo (Supplementary Fig. 3). Thus, compared with placebo, dapagliflozin caused a +0.66 mg/kg/min increase (+22.3%) (Table 2) in EGP (P < 0.03) (Fig. 2A). Consistent with previous studies (3), compared with placebo, dapagliflozin caused a small but significant increase in the plasma glucagon concentration (Table 3 and Supplementary Fig. 4) and a decrease in the plasma insulin concentration (Table 3 and Supplementary Fig. 5). Thus, the plasma glucagon-to-insulin ratio markedly increased (Table 3 and Supplementary Fig. 6).
Figure 1.
The time course of the change in the plasma glucose concentration (PGC) in subjects receiving dapagliflozin or placebo is shown in study 1 (A), study 2 (C), and study 3 (E). The change in PGC from baseline to the last hour of the study is shown in study 1 (B), study 2 (D), and study 3 (F).
Table 2.
Change from baseline to the last hour of the study (240–300 min) in plasma glucose concentration and EGP in study 1, study 2 (glucose clamp), and study 3 (pancreatic clamp)
| Dapagliflozin | Placebo | Difference (%) | P value | |
|---|---|---|---|---|
| Plasma glucose, mg/dL (%) | ||||
| Study 1: EGP | −29 ± 4 (−20.4) | −17 ± 3 (−12.6) | −12 (−7.8) | 0.03 |
| Study 2: EGP + glucose clamp | +3 ± 1 (+2.2) | 1 ± 1 (1) | +2 (+1.6) | NS |
| Study 3: EGP + pancreatic clamp | −30 ± 4 (−19.4) | −7 ± 5 (−3.1) | −23 (−16.3) | 0.0008 |
| EGP, mg/kg/min (%) | ||||
| Study 1: EGP | +0.10 ± 0.1 (+2.7) | −0.56 ± 0.11 (−19.6) | +0.66 (+22.3) | <0.0001 |
| Study 2: EGP + glucose clamp | −0.57 ± 0.12 (−26.1) | −1.28 ± 0.172 (−46.6) | +0.71 (+20.5) | 0.01 |
| Study 3: EGP + pancreatic clamp | −0.23 ± 0.09 (−7.9) | −0.48 ± 0.05 (−18.5) | +0.25 (+10.6) | 0.04 |
Values are mean ± SEM (%). The P value represents the difference between the change in plasma glucose concentration and the change in EGP between the dapagliflozin-treated and placebo-treated groups.
Figure 2.
Percentage change from baseline in total EGP measured with [3-3H]glucose infusion in subjects receiving dapagliflozin or placebo in study 1 (A), study 2 (glucose clamp) (C), and study 3 (pancreatic clamp) (E). The change in EGP from baseline to the last hour of the study is depicted in B (study 1), D (study 2), and F (study 3).
Table 3.
Plasma insulin and glucagon concentrations during measurement of EGP with dapagliflozin, with dapagliflozin plus glucose clamp, and with dapagliflozin plus pancreatic clamp versus placebo
| Insulin (µU/mL) | Glucagon (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Last hour | P value | ∆Insulin | Baseline | Last hour | P value | ∆Glucagon | |
| EGP | ||||||||
| Dapagliflozin | 15 ± 2 | 10 ± 1 | <0.0001 | 5 ± 1* | 57 ± 5 | 62 ± 6 | 0.03 | +5 ± 2* |
| Placebo | 12 ± 2 | 9 ± 1 | 0.02 | 3 ± 1 | 57 ± 6 | 56 ± 7 | NS | −1 ± 2 |
| Glucose clamp | ||||||||
| Dapagliflozin | 17 ± 2 | 17 ± 2 | NS | 0 ± 1 | 61 ± 4 | 55 ± 4 | 0.001 | −6 ± 2 |
| Placebo | 14 ± 2 | 14 ± 2 | NS | 0 ± 1 | 63 ± 10 | 56 ± 8 | 0.02 | −7 ± 3 |
| Pancreatic clamp | ||||||||
| Dapagliflozin | 12 ± 1 | 10 ± 1 | 0.002 | 2 ± 1 | 42 ± 3 | 41 ± 3 | NS | −1 ± 1 |
| Placebo | 9 ± 1 | 9 ± 1 | NS | 0 ± 1 | 37 ± 4 | 35 ± 4 | NS | −2 ± 2 |
∆Glucagon, plasma glucagon level at baseline minus last hour of the study; ∆Insulin, plasma insulin level at baseline minus last hour of the study.
P < 0.05 vs. placebo.
Under glucose clamp conditions (study 2), the plasma glucose concentration remained unchanged during the EGP measurement (Fig. 1C and Table 2) in both the placebo and dapagliflozin groups. Unlike study 1, the change in plasma insulin and glucagon concentrations under glucose clamp conditions was comparable in subjects receiving dapagliflozin and placebo (Table 3 and Supplementary Figs. 4 and 5), as was the plasma glucagon-to-insulin ratio (Table 3 and Supplementary Fig. 6). During the glucose clamp study, placebo-treated subjects experienced a precipitous decrease in EGP. The mean EGP during the last hour of study 2 (i.e., 240–300 min) was −1.28 ± 0.17 mg/kg/min (−46.6% decrease) below the fasting EGP. However, in dapagliflozin-treated subjects, the decrease in EGP was markedly attenuated −0.57 ± 0.12 mg/kg/min (−26.1% decrease). Thus, under glucose clamp conditions, dapagliflozin produced a +0.71 mg/kg/min (+20.4%, P = 0.01) increase in EGP compared with placebo (Fig. 2 and Table 2), even though the plasma insulin and glucagon concentrations were comparable in the dapagliflozin and placebo groups. The GIR during the glucose clamp (study 1) increased over time in both study groups. The mean GIR in subjects receiving dapagliflozin (1.45 ± 0.20 mg/kg/min) was greater compared with placebo (0.93 ± 0.10 mg/kg/min). However, the difference did not reach statistical significance (P = 0.10). The difference between the dapagliflozin and placebo groups was time-dependent (Supplementary Fig. 7A), with statistical significance being reached at 60 min. With time, the difference between the two groups diminished, most likely due to the increase in EGP. At 260 min, the difference was not statistically significant. The mean GIR in subjects receiving dapagliflozin strongly correlated with urinary glucose loss (r = 0.84, P < 0.001) (Supplementary Fig. 7B).
Under pancreatic clamp conditions (study 3), the plasma glucagon concentration remained unchanged (Supplementary Fig. 4) in subjects receiving placebo or dapagliflozin, while plasma insulin concentration remained unchanged in subjects receiving placebo (Supplementary Fig. 3). There was a small decrease in the plasma insulin concentration in subjects receiving dapagliflozin (12 ± 1 to 10 ± 1, P = 0.002) (Table 2 and Supplementary Fig. 5). However, the change from baseline to the last hour of the EGP measurement in plasma insulin concentration in subjects receiving dapagliflozin and placebo was not statistically different. Further, the glucagon-to-insulin ratio was comparable in the two groups (Table 3 and Supplementary Fig. 6B). Dapagliflozin produced a large decrease (−30 ± 4 mg/dL) in the plasma glucose concentration compared with placebo (−7 ± 5 mg/dL). Thus, the placebo-subtracted decrease in the plasma glucose concentration produced by dapagliflozin under pancreatic clamp conditions was −23 mg/dL (−16.3%, P = 0.0008), which was significantly greater than that in study 1 (P < 0.01).
Under pancreatic clamp conditions (study 3), EGP progressively decreased in subjects receiving placebo and, during the last hour, was reduced by −0.48 ± 0.05 mg/dL (−18.5%) below the fasting EGP. In subjects receiving dapagliflozin, EGP precipitously declined (−0.41 mg/kg/min) during the first hour after dapagliflozin administration and at 60 min was −8% below that in placebo. However, EGP progressively increased after 60 min and, from 60 to 300 min, the increase in EGP was 0.22 ± 0.08 mg/kg/min (P < 0.0001). During the last hour of EGP measurement (i.e., 240–300 min), EGP in subjects receiving dapagliflozin was 0.26 mg/kg/min (+10.6%, P = 0.04) (Fig. 2C) higher than in subjects receiving placebo.
Discussion
Consistent with previous studies (3–6), inhibition of renal SGLT2 transport with dapagliflozin was associated with a significant decrease in the plasma glucose concentration (−12 mg/dL) and a “paradoxical” increase in EGP with a concomitant increase in the plasma glucagon concentration and a decrease in the plasma insulin concentration. The present results demonstrate that prevention of the decrease in plasma glucose concentration blocked the decline in the plasma insulin concentration and inhibited the rise in the plasma glucagon concentration, indicating that the changes in the plasma insulin and glucagon concentrations after SGLT2i administration are secondary to the decrease in the plasma glucose concentration and are not due to direct action of the drug on the pancreatic islets (8). These results are consistent with recent studies (9–11) which demonstrated that prevention of the decrease in plasma glucose concentration blocked the increase in plasma glucagon concentration in patients with T2DM after dapagliflozin administration. Most importantly, despite the absence of any difference in the change in plasma insulin and glucagon concentrations between subjects receiving dapagliflozin and placebo under glucose clamp conditions, dapagliflozin administration still provoked an increase in EGP that was of similar magnitude to that caused by dapagliflozin without the glucose clamp in study 1 (Supplementary Fig. 6). The absolute difference between dapagliflozin-treated and placebo-treated subjects in the change in EGP from baseline to the last hour of EGP measurement (i.e., 240–300 min) was comparable in study 1 (glucose allowed to drop) and study 2 (glucose clamp condition), +0.66 vs. +0.71 mg/kg/min, respectively (Table 2 and Supplementary Fig. 8). This finding provides strong evidence against an important role for the increase in plasma glucagon and decrease in plasma insulin or change in plasma glucose concentration in mediating the acute increase in EGP caused by dapagliflozin. These results are consistent with a recent study in which we demonstrated that blocking the increase in plasma glucagon concentration and preventing the decrease in plasma insulin concentration with coadministration of a glucagon-like peptide 1 receptor agonist (liraglutide) plus SGLT2i (canagliflozin) failed to prevent the increase in EGP caused by canagliflozin (10). Collectively, these observations demonstrate that neither the change in plasma glucose concentration nor the change in pancreatic hormone (glucagon and insulin) concentrations can explain the acute stimulation of EGP by SGLT2i.
During the pancreatic clamp study with somatostatin and basal glucagon/insulin replacement (study 3), the plasma glucagon concentration remained unchanged in both study groups. While there was small decrease in plasma insulin concentration in subjects receiving dapagliflozin, the change from baseline to the last hour of the EGP measurement in plasma insulin concentration was comparable in both groups (Table 2 and Supplementary Fig. 5), as was the plasma glucagon-to-insulin ratio (Supplementary Fig. 6). However, the time pattern of change differed among the two groups. Dapagliflozin produced a large and rapid decline in EGP, such that at 60 min, EGP was significantly lower than in subjects receiving placebo. After 60 min, EGP rose progressively (Fig. 2E) to a level higher than in placebo. It should be emphasized that the increase in EGP from 60 to 300 min in subjects receiving dapagliflozin took place without a change in the plasma glucagon concentration. Further, it is unlikely that the small decrease in plasma insulin concentration is responsible for the progressive increase in EGP after dapagliflozin because the change in the plasma insulin concentration was comparable in dapagliflozin-treated and placebo-treated subjects.
Previous studies in experimental animals have reported a stimulatory action of somatostatin on renal gluconeogenesis (12). Although a similar stimulatory action of somatostatin in humans could have contributed to the increase in EGP in study 3, we believe that this is unlikely because we would have expected a similar increase in EGP in subjects receiving placebo, while in contrast, EGP dropped progressively throughout the study. More likely, the rise in EGP reflects the effect of dapagliflozin as was seen in studies 1 and 2.
It is noteworthy that the placebo-subtracted increase in EGP after dapagliflozin administration was attenuated in study 3 compared with studies 1 and 2 (Supplementary Fig. 8). It is well known that somatostatin markedly decreases splanchnic blood flow (13,14). Because of the important role of substrate supply in the regulation of hepatic gluconeogenesis (15), it is possible that decreased hepatic blood flow could have attenuated the increase in EGP caused by dapagliflozin under conditions of somatostatin infusion. Regardless of the mechanism(s) responsible for the attenuation of the increase in EGP by dapagliflozin, the placebo-subtracted decrease in the plasma glucose concentration in study 3 was significantly greater than in study 1 (23 vs. 12 mg/dL, P < 0.05). This emphasizes the clinical importance of the increase in EGP after SGLT2 administration in attenuating the clinical efficacy of SGLT2 inhibitors in lowering the plasma glucose concentration.
Urinary glucose loss caused by dapagliflozin varied significantly among the three studies. UGE under glucose clamp conditions (study 2) was significantly greater than in study 1. Conversely, under pancreatic clamp conditions (study 3), UGE was significantly less than in study 1. Because UGE is influenced by the amount of filtered glucose (product of the GFR and plasma glucose concentration) (16), the higher UGE under glucose clamp conditions (study 2) can be explained by the higher mean plasma glucose concentration compared with study 1 (136 vs. 116 mg/dL, P < 0.0001). However, the mean plasma glucose concentration from time 0–300 min under pancreatic clamp conditions in study 3 (134 ± 5 mg/dL) was comparable to that in study 2 (134 ± 5 mg/dL, P = ns) and was significantly higher than in study 1 (115 ± 3 mg/dL P > 0.0001). Nonetheless, UGE in study 3 was the smallest. This marked reduction in UGE in study 3 suggests a reduction in GFR by somatostatin secondary to decreased renal blood flow (17), neither of which were measured in the current study.
In summary, clamping the plasma glucose concentration at the fasting level prevented the change in plasma insulin and glucagon concentrations caused by SGLT2i but failed to prevent the dapagliflozin-induced increase in EGP. These results argue against an important role for changes in the plasma insulin and glucagon concentrations in mediating the increase in EGP caused by SGLT2i.
Supplementary Material
Article Information
Acknowledgments. The authors thank Khanh Horst for her excellent care of the patients throughout the study and Lorrie Albarado and Deena Murphy for their expert secretarial assistance in preparation of the manuscript.
Funding. This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-107680 to R.D.
Duality of Interest. R.D. receives grant support from AstraZeneca, Merck, and Janssen, is a member of the advisory boards of AstraZeneca, Janssen Pharmaceuticals, Intarcia Therapeutics, Boehringer Ingelheim, and Novo Nordisk, and is a member of the speakers bureaus of Novo Nordisk and AstraZeneca. E.C. receives grant support from AstraZeneca and Janssen Pharmaceuticals, is a member of the advisory boards of VeroScience, the Boehringer Ingelheim and Lilly Diabetes Alliance, and Sanofi, and is a member of the speakers bureaus of AstraZeneca, Janssen Pharmaceuticals, and the Boehringer Ingelheim and Lilly Diabetes Alliance. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A., N.L., R.M., C.A., A.M.A., H.A-J., O.L., and J.A. generated the data. C.T., R.D., and E.C. and reviewed and revised the manuscript. M.A.-G. analyzed the data and wrote the manuscript. M.A.-G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02592421, clinicaltrials.gov
This article contains Supplementary Data online at https://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0770/-/DC1.
M.A. and N.L. equally contributed to the study.
References
- 1.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11–26 [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol 2015;309:F889–F900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez R, Al-Jobori H, Ali AM, et al. Endogenous glucose production and hormonal changes in response to canagliflozin and liraglutide combination therapy. Diabetes 2018;67:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195 [DOI] [PubMed] [Google Scholar]
- 6.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999;48:1198–1214 [DOI] [PubMed] [Google Scholar]
- 8.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 9.Lundkvist P, Pereira MJ, Kamble PG, et al. Glucagon levels during short-term SGLT2 inhibition are largely regulated by glucose changes in patients with type 2 diabetes. J Clin Endocrinol Metab 2019;104:193–201 [DOI] [PubMed] [Google Scholar]
- 10.Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 2019;62:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali MA, Martinez R, Al-Jobori H, et al. Combination therapy with canagliflozin plus liraglutide exerts synergistic effect on weight loss, but not on HbA1c in T2DM patients. Diabetes Care. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dileepan KN, Khawaja AM, Wagle SR. Studies on the mechanism of action of somatostatin on renal gluconeogenesis: evidence for the involvement of alpha 1-adrenergic stimuli. Arch Biochem Biophys 1982;213:169–176 [DOI] [PubMed] [Google Scholar]
- 13.Price BA, Jaffe BM, Zinner MJ. Effect of exogenous somatostatin infusion on gastrointestinal blood flow and hormones in the conscious dog. Gastroenterology 1985;88:80–85 [DOI] [PubMed] [Google Scholar]
- 14.Cooper AM, Braatvedt GD, Qamar MI, et al. Fasting and post-prandial splanchnic blood flow is reduced by a somatostatin analogue (octreotide) in man. Clin Sci (Lond) 1991;81:169–175 [DOI] [PubMed] [Google Scholar]
- 15.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 2017;13:572–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Jobori H, Daniele G, Cersosimo E, et al. Empagliflozin and kinetics of renal glucose transport in healthy individuals and individuals with type 2 diabetes. Diabetes 2017;66:1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price BA, Jaffe BM, Zinner MJ. The effect of somatostatin on central hemodynamics, renal blood flow, and renal function in dogs. Surgery 1985;97:285–289 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.