Abstract
Glucagon is historically described as the counterregulatory hormone to insulin, induced by fasting/hypoglycemia to raise blood glucose through action mediated in the liver. However, it is becoming clear that the biology of glucagon is much more complex and extends beyond hepatic actions to exert control on glucose metabolism. We discuss the inconsistencies with the canonical view that glucagon is primarily a hyperglycemic agent driven by fasting/hypoglycemia and highlight the recent advances that have reshaped the metabolic role of glucagon. These concepts are placed within the context of both normal physiology and the pathophysiology of disease and then extended to discuss emerging strategies that incorporate glucagon agonism in the pharmacology of treating diabetes.
The Current View of Glucagon Biology in the Pathophysiology of Diabetes
Glucagon: The Opposing Force to Insulin
Glucagon, the predominant product of α-cells within islets, was originally identified in 1923 during efforts to purify insulin, where it was identified as a contaminant hyperglycemic factor (1). Further research determined that the hyperglycemic action of glucagon was mediated by increased hepatic glycogenolysis and gluconeogenesis, thereby increasing endogenous glucose production (2). This aspect of glucagon biology has been leveraged for pharmacological treatment of insulin-induced hypoglycemia in patients with diabetes (3). This historical progression has positioned insulin and glucagon as opposing hormones with respect to glycemic control (4), with imbalances in the insulin-to-glucagon ratio predicted to disrupt euglycemia. Diabetic hyperglycemia is often described to arise from both impaired insulin action and inappropriately elevated levels of glucagon (5–7). The perceived hyperglycemic effects of glucagon were reinforced by studies demonstrating that reduction of glucagon receptor (GCGR) activity blunts hyperglycemia in rodent models of insulinopenic diabetes (8,9). These observations have fostered the development of GCGR antagonists (GRAs) for the treatment of hyperglycemia (10). Consequently, the evolution of glucagon biology has fostered the general perspective that glucagon is a hypoglycemia/fasting-induced hormone that has the primary action of increasing glycemia. This perception has limited the interest in or investigation into the potential benefits of glucagon agonism for the treatment of type 2 diabetes (T2D). Here, we highlight a selection of findings that we believe contribute to a repositioning of glucagon away from its classical dogma of being a hypoglycemia-responsive opposing hormone to insulin. We also discuss how these somewhat paradoxical, underappreciated aspects of glucagon biology can be leveraged for the pharmacological treatment of T2D.
Does Glucagon Only Exist to Prevent Hypoglycemia?
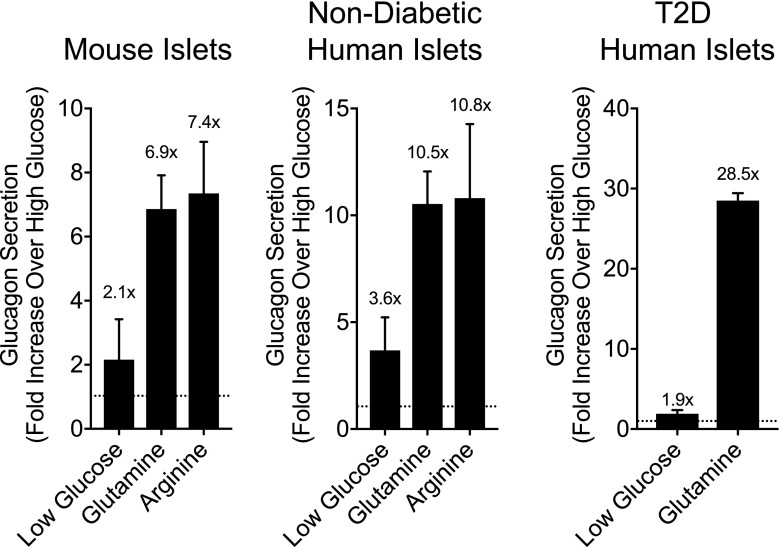
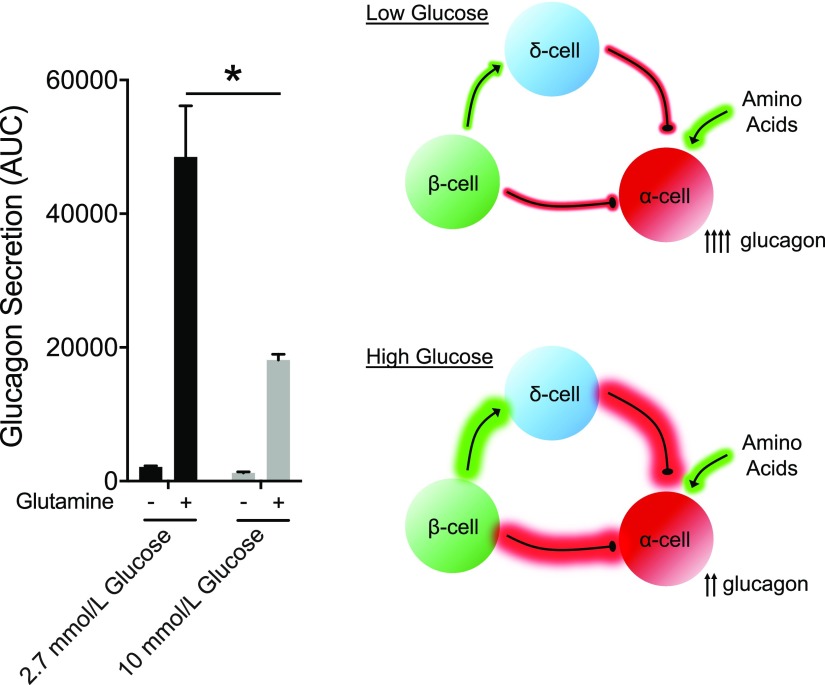
Reevaluation of historical data, along with a number of recent advances in our understanding of glucagon biology, has questioned whether the primary role of glucagon is to guard against hypoglycemia. First, it is important to consider the relationship between glycemia and glucagon levels. Although glucagon levels initially rise following the onset of a fast, concurrent with decreasing glycemia, with prolonged fasting (>3 days) circulating glucagon levels fall progressively to postprandial values despite persistent low blood glucose (11). Moreover, the administration of glucagon in hypoglycemic humans that have been fasting for >3 days does not produce any meaningful changes in glycemia (12), likely because of depleted glycogen stores. Hence, low plasma glucose does not always associate with elevated glucagon levels, raising the possibility that hypoglycemia is not the primary driver of α-cell secretory function. Furthermore, blocking glucagon action with genetic interruption (13,14) or pharmacological antagonism in mice (15), nonhuman primates (16), or humans (17,18) lowers glucose but does not necessary alter the susceptibility to hypoglycemia. On the other hand, a much tighter relationship is seen between glucagon levels and a subset of amino acids (19). Clinical studies often utilize amino acids, primarily arginine, as a α-cell secretagogue, which induces significant increases in circulating glucagon regardless of ambient glycemia. Alanine infusion also induces a robust increase in glucagon secretion (20), while branch-chain amino acids do not have a direct effect on glucagon secretion (21). Reducing glucagon action in hepatocytes drives hyperaminoacidemia, which is the precipitating factor for α-cell hyperplasia and hyperglucagonemia (14,16,22,23). We have found in isolated mouse and human islets that the amino acids glutamine, arginine, and alanine are potent inducers of glucagon secretion concentrations (21). Furthermore, physiological concentrations (0.5–1.0 mmol/L) of these amino acids can increase glucagon secretion up to 10-fold, while changes in glucose within physiological ranges only modify glucagon secretion twofold (Fig. 1). This observation that α-cells are more responsive to amino acids compared with changes in glucose concentrations questions the primacy of glycemia for glucagon secretion. In fact, it is unclear whether glucose serves as a direct signal for α-cells. It is difficult to dissociate any potential direct effects of glucose on α-cells from those mediated indirectly through either β- or δ-cells. Interestingly, isolated α-cells paradoxically increase glucagon secretion in response to glucose (24), which would indicate that the inhibitory actions of glucose on α-cells are indirect. Glucose increases β-cell activity and the secretion of products (insulin, zinc, γ-aminobutyric acid) that dampen α-cell function through direct paracrine inhibition mediated by β- to α-cell signaling. Similarly, δ-cell secretory activity also inhibits glucagon through somatostatin. Thus, the ability for glucose to modulate glucagon secretion is likely to be primarily driven by indirect paracrine interactions originated from β- and δ-cells. This model also suggests that the role of ambient glucose is to dictate the tone of α-cells, rather than serving as a direct, dose-related stimulus for glucagon secretion. In support of this, stimulation of glucagon secretion by amino acids is greater at low glucose, where inhibitory tone is low, compared with high glucose, where inhibitory tone is high (Fig. 2). Whether glycemic levels dictate the α-cell tone and response to other key regulators of α-cell function (25) such as fatty acids or CNS input is unknown.
Figure 1.
Effects of glucose versus amino acids on glucagon secretion in isolated perifused islets. Glucagon secretion was measured in perifused islets, calculated as the incremental area under the curve, and expressed as fold change relative to the values collected at high glucose (10 mmol/L). Low glucose conditions were at 2.7 mmol/L glucose, while the glucagon responses to both glutamine and arginine were collected under high-glucose conditions (10 mmol/L).
Figure 2.
Impact of glucose concentration on amino acid–stimulated glucagon secretion. Glucagon secretion was measured in perifused human islets from donors with T2D and calculated as the incremental area under the curve. The schematic illustrates the hypothesis that high-glucose conditions result in more inhibitory tone on the α-cell through paracrine interactions that originate from either β- or δ-cells, with the net effect of decreased α-cell tone and decrease glucagon secretion in response to the same amino acid stimulus.
A second factor that challenges the dogma that glucagon is primarily driven by hypoglycemia is the consistent observations that plasma glucagon levels increase postprandially, coinciding with an increase in glycemia. Postprandial rises in glucagon seen in T2D (26) have been described as pathogenic and a cause of hyperglycemia (27). Yet similar rises in postprandial glucagon are seen in individuals without diabetes (28,29). The amino acid component of a mixed meal is likely a major contributor to postprandial increases in glucagon secretion; however, both healthy individuals and individuals with T2D often display a modest increase immediately following oral glucose alone (30–32). The effect of oral glucose to stimulate glucagon is more pronounced in people with T2D, generally of short duration, and typically followed by a decrease in glucagon levels after 30 min. Furthermore, the mechanisms by which oral glucose stimulates α-cells are unknown but potentially involve enteroendocrine hormones such as GIP (33), a peptide that tends to have higher circulating concentrations among persons with T2D. The importance of postprandial rises in glucagon for metabolic homeostasis has not been extensively tested.
α-Cell Hyperplasia: Metabolic Adaptation Versus Pathogenic?
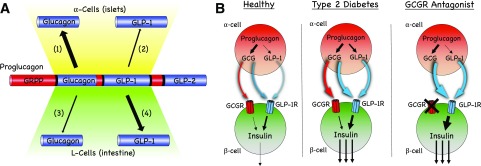
A key question in this revisionary model of α-cell function is as follows: why would metabolism evolve to increase α-cell function in response to metabolic stress and hyperglycemia if the primary actions of glucagon were to enhance endogenous glucose production? The argument has been made that this is a precipitating event that drives metabolic dysfunction (27) rather than an adaptation to help correct it. However, this argument does not align with the recent demonstrations that α-cells are essential for determining β-cell (21,34,35) and glycemic tone (36). Interruption of proglucagon input to β-cells drastically dampens the magnitude of nutrient-stimulated insulin secretion. The insulinotropic properties of glucagon are essential for β-cell function, which establishes a direct and critical relationship between α- and β-cells that is manifested in both mouse (21,34,35) and human (21,36) islets. These new studies raise the possibility that the hyperglucagonemia present in T2D is a compensatory mechanism to enhance β-cell function, but this has yet to be formally tested. However, this hypothesis provides an alternative perspective to position α-cell hyperplasia and increased glucagon secretion observed in T2D as a mechanism to correct, rather than induce, dysregulated homeostasis. This is similar to the well-accepted observation that insulin resistance drives hyperinsulinemia. The α-cell model whereby α-cell function in subjects with diabetes is a compensatory response to metabolic stress that stimulates β-cell function to maintain homeostasis is plausible based on available data. Mice chronically fed a high-fat diet demonstrate increased α-cell mass (37), supporting the hypothesis that α-cell hypertrophy compensates for metabolic stress; whether this occurs in humans is difficult to test. People with T2D consistently demonstrate an elevated α-cell–to–β-cell mass ratio (38), but this may result from decreased β-cell mass rather than an increase in α-cell mass (39). However, it is interesting to note that metabolic stress increases glucagon concentrations, while there is little evidence to support that undernutrition or chronic fasting impacts α-cell function. Finally, in addition to increased α-cell mass and glucagon secretion, metabolic stress also alters α-cell function to produce GLP-1 by increasing the expression of the prohormone convertase (PC)1 isoform (40–42). This enables the processing of proglucagon peptide to generate GLP-1, which is a much more potent insulin secretagogue compared with glucagon (21) (Fig. 3).
Figure 3.
Proglucagon processing and the impact on β-cell function. A: Proglucagon is posttranslationally modified by PC enzymes to produce glucagon and GLP-1 (1). α-Cells express high levels of PC2 to produce glucagon, a primary product of proglucagon (2). GLP-1 production by PC1/3 in α-cells is low in healthy states but can be induced by metabolic stress to increase the secretion of islet GLP-1 (3). Glucagon production and PC2 expression in enteroendocrine L-cells are low or absent in healthy states. Interventions such as bariatric surgery or pancreatectomy may induce PC2 expression and subsequent glucagon production in the gut (4). GLP-1 is the primary product of proglucagon in the gut under most conditions. B: α-Cells can use both glucagon and GLP-1 to stimulate insulin secretion in β-cells. In healthy islets, glucagon is the major product that mediates α- to β-cell communication but can do so through both the glucagon receptor (GCGR) and GLP-1R. Metabolic stress and T2D increase proglucagon production and the expression of PC1/3 in α-cells. Under these conditions, both glucagon and GLP-1 mediate α- to β-cell communication predominantly through the GLP-1R. Treatment with a GCGR antagonist substantially increases both glucagon and GLP-1. It is anticipated that α- to β-cell communication is enhanced through GLP-1R activity as long as the antagonist remains engaged with the GCGR.
How can the perspective that increased α-cell activity is a compensatory response to metabolic stress be reconciled with the data demonstrating that pharmacological or genetic reductions in glucagon action consistently lower glycemia? GRAs lower glycemia in humans with T2D (43) and in preclinical models of hyperglycemia (15). This effect of blocking glucagon activity would argue against the essential contribution of the α-cell toward postprandial glucose metabolism. However, while blocking glucagon activity in hepatocytes reduces endogenous glucose production, it is unclear whether this is the primary mechanism to facilitate glucose lowering in response to GRAs. A universal outcome of inhibiting glucagon action, either globally or specifically in hepatocytes, is α-cell hyperplasia and pharmacological levels of circulating glucagon and GLP-1 (14,22,44,45). Both glucagon and GLP-1 elevate β-cell tone and insulin secretion primarily through the GLP-1 receptor (GLP-1R) (21) (Fig. 3 [discussed in detail below]). Thus, GRAs would be expected to enhance β-cell tone and insulin secretion by substantially increasing activity at the GLP-1R (Fig. 3). Whether this is a mechanism that can account for the glucose-lowering response to GRA therapy has not been formally tested. However, a number of studies have demonstrated that the glucose lowering in response to a GRA or following genetic deletion of the GCGR is severely diminished in the absence of GLP-1R signaling (44,46–48). Moreover, pharmacological or genetic elimination of glucagon action requires some level of endogenous β-cell function in order to lower glycemia (49). Thus, there is evidence that β-cell GLP-1R activity and insulin secretion meaningfully contribute to the metabolic effects following inactivation of the GCGR, suggesting that the mechanism of glucagon lowering in response to GRAs potentially expands beyond reductions in hepatic glucose production.
Promiscuity or Versatility: Glucagon Signaling Through the GLP-1R
One Hormone, Two Receptors
The GCGR shares significant homology with the GLP-1R, particularly in the sequences that encode for the transmembrane and extracellular domains (50). The ligands for these receptors (glucagon and GLP-1) are also highly conserved in sequence. These similarities enable glucagon to engage with the GLP-1R, albeit at ∼10-fold decreased potency compared with GLP-1. GLP-1 also binds to the GCGR but with considerably less affinity than glucagon at GLP-1R, requiring concentrations that are unlikely to be achieved even with pharmacology (21,51,52). These structural similarities also serve as the basis for cross-reactive agonism of oxyntomodulin at the GCGR and the GLP-1R, albeit with substantially reduced affinity compared with the cognate ligands. Recent structural information regarding the binding mode of these α-helical peptides to their cognate class B GPCRs confirms the bipartite activation mechanism. This involves tethering the peptide C-terminal end to the N-terminal extracellular domain of the receptor and the subsequent orientation of the peptide N-terminal domain within the orthosteric activation site formed by the transmembrane helices of the receptor (53,54). These structural findings are congruent with the structure-activity relationship recently demonstrated in medicinal peptide chemistry studies (55). However, these structural studies stop short in addressing the differences in promiscuity observed for glucagon and GLP-1 to their related receptors. Although there is more sequence divergence in the C-terminal portion of the peptides between GLP-1 and glucagon, the amphipathic α-helix is conserved between the two hormones. The hydrophobic face of this helix interacts with the extracellular domain of the receptor, which notably also has a high degree of sequence homology between the two receptors. As the engagement of the peptide α-helix with the hydrophobic pocket of the extracellular domain is the primary step in the two-step activation process, the evolutionary conservation of these structural motifs provides ancillary support to the structural studies in explaining the promiscuous activity of the proglucagon peptides. Although this can explain the structural basis for cross-reactivity to paralogous receptors, these studies do not delineate why there is increased potency of glucagon at GLP-1R relative to GLP-1 at GCGR. The differential orientation of the peptide N-terminus within the activation site is the likely explanation. The orthosteric activation pocket of GLP-1R appears more accommodating of the inverted electrostatic charge at the third amino acid position of the peptides (Glu3 in GLP-1 to Gln3 in glucagon), which is the pivotal N-terminal residue that governs the divergent potency of cross-receptor activation. Interestingly, zebrafish glucagon has Glu3 and the zebrafish GLP-1R has near equal affinity to zebrafish-derived GLP-1 and glucagon, as well as to both human-derived peptides (56). Thus, better understanding of the evolution and function of the zebrafish glucagon–GLP-1 receptor signaling system seems like a fruitful way to approach the structural basis for differential cross-reactive binding of glucagon and GLP-1.
Glucagon Activity at the GLP-1R: Physiology or Pharmacology?
While it is clear that glucagon can engage the GLP-1R, it is important to understand whether this has any biological relevance. In vitro activity assays provide some estimation of the concentrations of peptides needed to produce activity at either receptor. For instance, activity assays using the human GCGR produced approximate half-maximal effective concentration (EC50) values of 2,500 nmol/L and 0.3 nmol/L for GLP-1 and glucagon, respectively (57). This indicates that even elevated concentrations of GLP-1 brought about by either GLP-1 pharmacotherapy (∼4–12 nmol/L) (58) or bariatric surgery (100 pmol/L) (59) are likely insufficient to engage the GCGR in vivo. Conversely, activity assays at the GLP-1R produced approximate EC50 values of 0.03 nmol/L and 3 nmol/L for GLP-1 and glucagon, respectively (57). While the value for GLP-1 aligns with plasma concentrations reported by multiple groups (∼5–30 pmol/L), the value for glucagon activity at the GLP-1R is substantially higher than normal plasma concentrations (5–30 pmol/L). However, circulating concentrations of glucagon achieved by pharmacotherapy or bariatric surgery may be sufficient to permit activity at the GLP-1R. Chronic rodent pharmacology experiments in loss-of-function models are required to determine the contribution of GLP-1R cross-reactivity to the therapeutic benefits of glucagon-based pharmacotherapies.
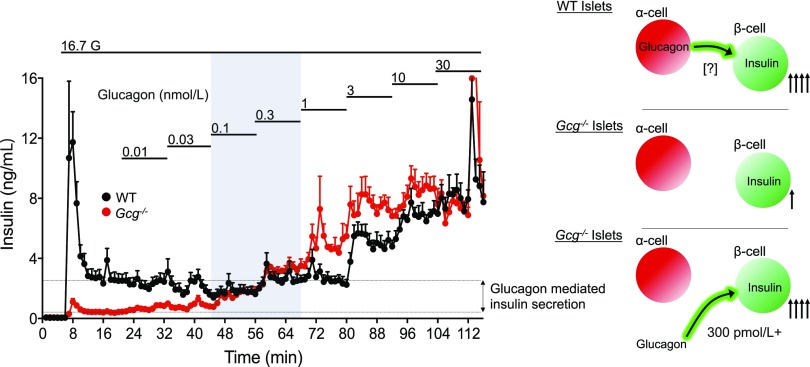
It is also likely that plasma levels of glucagon do not reflect the interstitial concentrations in islets, where α-cell production of glucagon occurs. We, and others, have recently reported that α-cell production of glucagon is essential to maintain β-cell function (21,34,35). Importantly, we demonstrated that the GLP-1R is the primary mediator of glucagon-stimulated insulin secretion and the essential component of α- to β-cell communication (21). One model used to demonstrate this relationship was Gcg−/− islets, which lack the production of all proglucagon-derived peptides (13). Gcg−/− islets produced impaired insulin secretion in response to glucose and amino acids, demonstrating the essential contribution of proglucagon peptides for β-cell function in response to nutrients. However, Gcg−/− islets produced insulin secretion similar to that of control islets when stimulated with glucagon, GLP-1, or GIP (21), showing normal β-cell function following restoration of GPCR ligands. This emphasizes that the impaired glucose-stimulated insulin secretion (GSIS) seen in Gcg−/− islets is due to impaired α-cell input, rather than defective β-cell per se, and that this defect is ameliorated upon restoration of glucagon. We used this model to titrate glucagon in perifusion experiments as a means of estimating interstitial glucagon concentrations, reasoning that glucagon concentrations that restored GSIS to wild-type (WT) levels would reflect interstitial concentrations of glucagon. The results of these experiments gave estimates of insulinotropic interstitial glucagon concentrations to be ∼0.3–1 nmol/L (Fig. 4)—up to 30-fold higher than circulating concentrations. However, Gcg−/− islets also appeared to have increased sensitivity to glucagon, making this an imperfect estimation, and likely an underestimation. Nonetheless, this value suggests that interstitial glucagon concentrations are likely sufficient to engage the GLP-1R in β-cells, concurrent with our functional data demonstrating that α-cell control of β-cell function is mediated by the GLP-1R (21). Furthermore, given the potential that glucagon is a physiological agonist of the GLP-1R, one must consider the effects of glucagon in other GLP-1R–expressing tissues such as the gut and CNS, particularly in the context of glucagon pharmacology. Specifically, it seems essential to consider glucagon activity at the GLP-1R when evaluating coagonists that incorporate glucagon and achieve pharmacological levels for sustained periods of time, or in the context of GRAs, which induce pharmacological levels of circulating native glucagon.
Figure 4.
Estimation of islet interstitial glucagon levels. Gcg−/− islets have impaired GSIS that has been attributed to lack of proglucagon input from paracrine interactions with α-cells (16). Titration of glucagon to rescue insulin secretion to WT levels provides an estimation of the interstitial glucagon levels in WT islets. Glucagon concentration at −300 pmol/L rescued insulin secretion, although Gcg−/− islets were more sensitive to glucagon, making this an imprecise and likely underestimation of interstitial glucagon concentrations. G, glucose.
What Is the Rationale for Chronic Use of Glucagon Analogs to Treat T2D?
Glucagon Pharmacotherapies: Antagonize Versus Agonize?
There is a long history of using the glucose-elevating effects of glucagon to treat hypoglycemia in patients with diabetes. Medicinal chemistry strategies to enhance the solubility and rapid onset of action, as well as alternative formulation strategies to support nasal administration (3), have improved the effectiveness of glucagon as an acute therapeutic rescue for patients with low blood glucose, usually as a result of overtreatment with glucose-lowering agents. Pharmacological agents that enhance endogenous glucagon secretion, such as GPR119 agonists (60) and isoform-selective somatostatin receptor antagonists (61), are other seemingly viable research strategies to harness GCGR agonism to treat hypoglycemia, the limiting side effect of diabetes treatment. There is a consensus that α-cell dysfunction in type 1 diabetes (T1D) manifests as impaired glucagon secretion in response to insulin-induced hypoglycemia (62). Consequently, utilization of exogenous glucagon to correct this impairment is generally efficacious in combating hypoglycemia in T1D. Emergency use of glucagon to treat hypoglycemia in T2D is less clear. It is important to consider the mechanism driving the hypoglycemia in T2D in order to predict the effect of glucagon. Among the antihyperglycemic medications available to treat T2D, exogenous insulin and sulfonylureas have the strongest association with hypoglycemia. The interaction between sulfonylureas and glucagon has the potential to be counterintuitive. Sulfonylureas stimulate insulin secretion through direct actions on KATP channels in β-cells to induce depolarization, which renders the β-cell sensitive to GPCR input even at low glucose. Consequently, while incretin activity in β-cells is commonly described as glucose dependent, activation of β-cell activity through direct manipulation of the KATP channel independent from elevated glucose eliminates the glucose dependency of incretins action in β-cells (63). Application of glucagon in this scenario can induce robust insulin secretion (64), since the cAMP generated by GCGR and/or GLP-1R can potentiate the β-cell activity induced by KATP closure, despite the ambient hypoglycemia. As such, it is not always appropriate to utilize glucagon as a counterregulatory hormone to combat hypoglycemia in T2D (65–67).
GCGR agonism continues to be developed and optimized for alleviating life-threatening hypoglycemia in T1D. However, with the rapid rise in the prevalence of T2D over the last few decades, a significant amount of effort has been placed in antagonizing glucagon to lower glycemia. GRAs are conceptually well accepted, despite adverse effects to promote liver fat accumulation (68). It has only been recently that strategies that enhance glucagon action for the treatment of T2D have been pursued. Given the dogmatic view that the primary role of glucagon is to raise blood glucose, enhancing glucagon action as a means of lowering glucose was initially met with resistance. However, coagonists incorporating GCGR activity are being developed as antidiabetes and antiobesity medications with promising results for glycemic control and weight loss (69), providing compelling evidence to support an increase in glucagon activity as a therapeutic strategy. Coagonists incorporating glucagon action were originally conceived of as being able to take advantage of glucagon’s ability to increase energy expenditure while buffering the glycemic effects with GLP-1R activity, and inspired by the promiscuity of oxyntomodulin at these two receptors. The tissue location(s) of glucagon action and mechanism(s) of action that potentially enable positive metabolic benefits are incompletely understood, as are the dose-limiting side effects of chronic glucagon action. This has limited the development of these agonists, as the biological and pharmacological properties of glucagon have not been fully appreciated.
Metabolic Benefits of Pharmacological Glucagon Agonism
It is becoming increasingly clear that glucagon can engage a number of physiological processes that support compounds that increase GCGR activity as a viable therapeutic approach for T2D. As discussed above, glucagon stimulates insulin secretion in β-cells through activity at both the GCGR and GLP-1R, with the balance leaning toward GLP-1R activity in mice and the balance potentially more even in humans (21). Interestingly, coinfusion of both glucagon and GLP-1 provides a synergistic effect on insulin secretion in humans (70). Whether this reflects synergistic activity at the level of a single β-cell, which could be governed by broadening intracellular signaling cascades (71), or a greater enhancement of β-cell activity through recruitment and activation of more β-cells is unknown. However, the ability of glucagon to potently stimulate insulin secretion through actions that compliment incretin peptide activity provides support for enhancing glucagon action as a diabetes therapy. A similar synergistic effect of glucagon and GLP-1 is seen for reductions in food intake in humans (70). While it is clear that various regions of the brain express the GLP-1R and engage anorectic signaling pathways (72), it is less clear whether any regions of the brain express GCGR. Central administration of glucagon to rodents inhibits food intake (73) and suppresses hepatic glucose production (74). Studies that demonstrate glucagon action in the hindbrain suggest that glucagon can bind receptors in the brain but do not rule out the possibility that these receptors are the GLP-1R. The anorectic effects of oxyntomodulin (a GCGR/GLP-1R agonist) remain intact in Gcgr−/− mice but are not present in Glp1r−/− mice (75), supporting the notion that GLP-1R mediates the ability for glucagon to reduce food intake. However, studies with long-acting glucagon monoagonists are required to clarify the permissive role of GLP-1R on the anorectic effects of glucagon therapy. Understanding how synergy can be achieved between two ligands on a single receptor would help unravel the mechanism by which glucagon inhibits food intake. Still, the ability of GLP-1/glucagon coagonists to stimulate insulin secretion and inhibit food intake has largely been attributed to the GLP-1 activity of these agents. Reconsideration of the role of glucagon in these events is necessary, especially given the exciting preliminary findings suggesting that synergistic actions can be seen between the two peptides.
Glucagon also stimulates an increase in energy expenditure in rodents and humans, providing rationale for the use of glucagon for weight loss. Where and how glucagon induces energy expenditure is unclear, particularly whether increased energy expenditure is due to direct cellular actions or indirect endocrine actions. Intracerebroventricular infusion increases energy expenditure (76), suggesting brain-mediated actions. Increased thermogenesis in brown adipose tissue has been proposed to occur through both direct actions via a GCGR in brown adipocytes and through indirect actions mediated by hepatocyte GCGR activity, farnesoid X receptor (77), and the induction of FGF21 (78). A direct action of glucagon on brown adipocytes was recently ruled out in rodents (79), which supports evidence that glucagon can increase energy expenditure independent of brown adipose activity in humans (80). The GCGR is also expressed in white adipocytes; however, their role in mediating the thermogenic effects of glucagon action is unknown, but lipolytic effects are likely involved. Glucagon action promotes futile macronutrient substrate cycling in target tissues (81), which in theory can drive nonthermogenic energy expenditure. Thus, understanding the mechanism driving these observations is essential to fully leverage this biology as a weight loss strategy. Finally, glucagon has well-documented actions for lipid metabolism (25,82), providing additional benefit for targeting hepatic steatosis. Interestingly, the ability for glucagon to promote lipid catabolism over storage may intersect with the actions of glucagon to drive ketogenesis (83).
In rodents, acute glucagon action induces immediate yet transient hyperglycemia, which is followed by improved insulin-mediated glucose disposal (45). Acute glucagon action enhances whole-body insulin sensitivity independent from its insulin secretory effect, as well as independent from prior hyperglycemia and hepatic glycogenolysis in these experimental settings. Further, the improved insulin sensitivity was independent of GLP-1R and FGF21, but action through other receptors or other endocrine signals cannot be dismissed. Although paradoxical at first glance, it seems rational that since glucagon levels are elevated in a fasted state, which is a state of heightened insulin sensitivity, that glucagon is well positioned according to its physiological regulation to contribute to discrete aspects of insulin action as opposed to being an all-encompassing counterregulatory hormone to insulin. Thus, glucagon action improves glucose tolerance by amplifying insulin action in addition to the intraislet paracrine effects to enhance insulin sensitivity.
How Much Glucagon Is Too Much?
Perhaps the most important design aspect for GLP-1 and glucagon coagonists has been the optimum amount of glucagon activity relative to GLP-1. This has mostly hinged on balancing the additional body weight–lowering efficacy driven by glucagon, which appears to have a steep dose response based on preclinical studies, versus the potential hyperglycemic liability of glucagon. Based on this seemingly narrow therapeutic window despite the ability of concurrent GLP-1 activity to partially mitigate the hyperglycemic effects of glucagon action, it appears that pharmaceutical companies have been particularly cautious in the amount of relative GCGR activity engineered into the clinical assets. Notably, the three GLP-1/glucagon coagonists that have advanced to later-stage clinical testing and on which clinical reports have been published all favored GLP-1R potency over GCGR potency. The general consensus on the potency ratio of those compounds (LY2944876, MEDI0382, and SAR425899) is that they are imbalanced in respective potencies where the potency at GLP-1R is universally greater than potency at GCGR (84,85). However, it is important to note that the determinants of potency ratio can vary depending on the assay type and calculation algorithms. Nonetheless, the clinical results from all of these coagonists showed body weight loss and lowering of glycosylated hemoglobin in patients with T2D. However, none of the studies had as an active comparator an appropriately matched GLP-1R monoagonist, and the effect sizes did not necessarily suggest superiority to what can be achieved by GLP-1 analogs (85,86). In order to achieve optimal outcome efficacy for both glucose control and weight loss, these compounds may require further adjustment of the GLP-1R-to-GCGR ratio. The molecular design of optimum coagonists could actually incorporate more aggressive GCGR agonism relative to GLP-1R in order to achieve additional body weight-lowering efficacy without compromising glycemic efficacy. Although the ancillary actions of glucagon discussed above suggest that glucagon has less deleterious effects on glycemia than initially contrived, the hyperglycemic liability is still a practical concern. Thus, to be more aggressive with the relative GCGR activity in these multifunctional agonists, and thus permit greater therapeutic efficacy, additional activities independent from GLP-1R action may be required to further buffer from the hyperglycemic propensity. We have shown that activity at the glucose-dependent insulinotropic polypeptide receptor (GIPR) can be recruited to provide a second mechanism that mitigates the hyperglycemic effects of fully potent GCGR agonism. This triple combination, particularly the high GCGR potency that is in balance with potencies at GLP-1R and GIPR, as well as the additional GIPR-mediated effects on systemic metabolism, results in unprecedented body weight loss not before reported in preclinical pharmacology studies, which rival the efficacy of bariatric surgeries (57). As hyperglycemia has been the predominant adverse effect of concern, secondary liabilities of chronic GCGR agonism have been understudied and will require special attention in additional clinical trials.
Summary and Future Directions
The discovery of glucagon was largely framed by the context that glucagon was a contaminant interfering with the purification of insulin from pancreatic tissue. The observation that this contaminant induced hyperglycemia promptly led to the idea that glucagon was the “anti-insulin,” a hormone that prevented hypoglycemia by buffering the actions of insulin. The evolution of this concept was extended to place glucagon in the center of the pathogenesis of diabetes, and it is been proposed that dysregulated α-cell function and hyperglucagonemia are essential contributors to hyperglycemia. Here, we propose a broader physiologic context for glucagon that may extend to the treatment of diabetes. This model incorporates new consideration of the secretion of glucagon, its insulinotropic actions, and activation of the GLP-1R and effectiveness in combination with GLP-1 as a therapy for T2D (69). This model extends the physiologic role of glucagon beyond the fasting and hypoglycemic states to a set of actions in prandial metabolism that may be useful for correcting hyperglycemia. While there are certainly many details to resolve, and an element of divergent or opposing metabolic effects of glucagon that are context specific, a thorough understanding of the physiological and pharmacological actions of glucagon has considerable potential in the development of therapeutic interventions. To accomplish this, the role of glucagon must expand beyond the current vantage of an “anti-insulin” hormone invoked by fasting and hypoglycemia.
Article Information
Funding. M.E.C. receives funding from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (F32-DK116542). J.E.C. receives funding from the American Diabetes Association (1-18-JDF-017) and is a Borden Scholar.
Duality of Interest. B.F. is an employee of Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.E.C. conducted the experiments. M.E.C. and J.E.C. analyzed data. All authors contributed to writing and editing of the manuscript. J.E.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Gosmanov NR, Gosmanov AR, Gerich JE. Glucagon physiology. In Endotext. Feingold KR, Anawalt B, Boyce A, et al., Eds. South Dartmouth, MA, 2000 [Google Scholar]
- 2.MacCuish AC, Munro JF, Duncan LJ. Treatment of hypoglycaemic coma with glucagon, intravenous dextrose, and mannitol infusion in a hundred diabetics. Lancet 1970;2:946–949 [DOI] [PubMed] [Google Scholar]
- 3.Seaquist ER, Dulude H, Zhang XM, et al. Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real-world setting. Diabetes Obes Metab 2018;20:1316–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherrington AD, Chiasson JL, Liljenquist JE, Jennings AS, Keller U, Lacy WW. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest 1976;58:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 6.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970;283:109–115 [DOI] [PubMed] [Google Scholar]
- 7.Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014;57:1919–1926 [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Berglund ED, Wang MY, et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci USA 2012;109:14972–14976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 2011;60:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JE, Drucker DJ. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat Rev Endocrinol 2015;11:329–338 [DOI] [PubMed] [Google Scholar]
- 11.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF Jr. Glucagon levels and metabolic effects in fasting man. J Clin Invest 1970;49:2256–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher M, Sherwin RS, Hendler R, Felig P. Kinetics of glucagon in man: effects of starvation. Proc Natl Acad Sci USA 1976;73:1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers AP, Sorrell JE, Haller A, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 2017;25:927–934.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longuet C, Robledo AM, Dean ED, et al. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 2013;62:1196–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto H, Cavino K, Na E, et al. Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc Natl Acad Sci U S A 2017;114:2753–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto H, Kim J, Aglione J, et al. Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 2015;156:2781–2794 [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner B, Esteves B, Dell V, et al. Single and multiple ascending-dose study of glucagon-receptor antagonist RN909 in type 2 diabetes: a phase 1, randomized, double-blind, placebo-controlled trial. Endocrine 2018;62:371–380 [DOI] [PubMed] [Google Scholar]
- 18.Kazierad DJ, Chidsey K, Somayaji VR, Bergman AJ, Calle RA. Efficacy and safety of the glucagon receptor antagonist PF-06291874: a 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes Metab 2018;20:2608–2616 [DOI] [PubMed] [Google Scholar]
- 19.Felig P, Owen OE, Wahren J, Cahill GF Jr. Amino acid metabolism during prolonged starvation. J Clin Invest 1969;48:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller WA, Faloona GR, Unger RH. The effect of alanine on glucagon secretion. J Clin Invest 1971;50:2215–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capozzi ME, Svendsen B, Encisco SE, et al. β cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:126742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean ED, Li M, Prasad N, et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab 2017;25:1362–1373.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Okamoto H, Huang Z, et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab 2017;25:1348–1361.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem 2010;285:14389–14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The new biology and pharmacology of glucagon. Physiol Rev 2017;97:721–766 [DOI] [PubMed] [Google Scholar]
- 26.Rauch T, Graefe-Mody U, Deacon CF, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther 2012;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YH, Wang MY, Yu XX, Unger RH. Glucagon is the key factor in the development of diabetes. Diabetologia 2016;59:1372–1375 [DOI] [PubMed] [Google Scholar]
- 28.Bock G, Dalla Man C, Micheletto F, et al. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010;95:872–878 [DOI] [PubMed] [Google Scholar]
- 30.Aulinger BA, Bedorf A, Kutscherauer G, et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes 2014;63:1079–1092 [DOI] [PubMed] [Google Scholar]
- 31.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA Trial. J Clin Endocrinol Metab 2015;100:3702–3709 [DOI] [PubMed] [Google Scholar]
- 32.Rhee NA, Østoft SH, Holst JJ, Deacon CF, Vilsbøll T, Knop FK. The impact of dipeptidyl peptidase 4 inhibition on incretin effect, glucose tolerance, and gastrointestinal-mediated glucose disposal in healthy subjects. Eur J Endocrinol 2014;171:353–362 [DOI] [PubMed] [Google Scholar]
- 33.Holst JJ, Christensen M, Lund A, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab 2011;13(Suppl. 1):89–94 [DOI] [PubMed] [Google Scholar]
- 34.Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;4:e127994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018;27:549–558.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingsgaard H, Ehses JA, Hammar EB, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A 2008;105:13163–13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita Y, Kozawa J, Iwahashi H, et al. Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. J Diabetes Investig 2018;9:1270–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marroqui L, Masini M, Merino B, et al. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. EBioMedicine 2015;2:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 2011;17:1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie Y, Nakashima M, Brubaker PL, et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J Clin Invest 2000;105:955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traub S, Meier DT, Schulze F, et al. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Reports 2017;18:3192–3203 [DOI] [PubMed] [Google Scholar]
- 43.Kazda CM, Ding Y, Kelly RP, et al. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care 2016;39:1241–1249 [DOI] [PubMed] [Google Scholar]
- 44.Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 2011;121:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T, Holleman CL, Nason S, et al. Hepatic glucagon receptor signaling enhances insulin-stimulated glucose disposal in rodents. Diabetes 2018;67:2157–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu W, Yan H, Winters KA, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 2009;331:871–881 [DOI] [PubMed] [Google Scholar]
- 47.Jun LS, Millican RL, Hawkins ED, et al. Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes 2015;64:819–827 [DOI] [PubMed] [Google Scholar]
- 48.Rivero-Gutierrez B, Haller A, Holland J, et al. Deletion of the glucagon receptor gene before and after experimental diabetes reveals differential protection from hyperglycemia. Mol Metab 2018;17:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damond N, Thorel F, Moyers JS, et al. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife 2016;5:e13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Gu S, Sun X, Li W, Tang Y, Liu G. Computational insight into conformational states of glucagon-like peptide-1 receptor (GLP-1R) and its binding mode with GLP-1. RSC Advances 2016;6:13490–13497 [Google Scholar]
- 51.Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic β-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 1998;47:66–72 [DOI] [PubMed] [Google Scholar]
- 52.Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol 2003;138:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Qiao A, Yang D, et al. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 2017;546:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Sun B, Feng D, et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017;546:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day JW, Gelfanov V, Smiley D, et al. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers 2012;98:443–450 [DOI] [PubMed] [Google Scholar]
- 56.Oren DA, Wei Y, Skrabanek L, Chow BK, Mommsen T, Mojsov S. Structural mapping and functional characterization of Zebrafish class B G-protein coupled receptor (GPCR) with dual ligand selectivity towards GLP-1 and glucagon. PLoS One 2016;11:e0167718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med 2015;21:27–36 [DOI] [PubMed] [Google Scholar]
- 58.Elbrønd B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care 2002;25:1398–1404 [DOI] [PubMed] [Google Scholar]
- 59.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li NX, Brown S, Kowalski T, et al. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 2018;67:1401–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karimian N, Qin T, Liang T, et al. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes 2013;62:2968–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 63.De León DD, Li C, Delson MI, Matschinsky FM, Stanley CA, Stoffers DA. Exendin-(9-39) corrects fasting hypoglycemia in SUR-1-/- mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion. J Biol Chem 2008;283:25786–25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thoma ME, Glauser J, Genuth S. Persistent hypoglycemia and hyperinsulinemia: caution in using glucagon. Am J Emerg Med 1996;14:99–101 [DOI] [PubMed] [Google Scholar]
- 65.Dougherty PP, Klein-Schwartz W. Octreotide’s role in the management of sulfonylurea-induced hypoglycemia. J Med Toxicol 2010;6:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langford NJ, Krentz AJ, Martin U, Elliott S, Ferner RE. Severe relapsing sulphonylurea-induced hypoglycaemia: a diagnostic and therapeutic challenge. Postgrad Med J 2003;79:120, 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lheureux PE, Zahir S, Penaloza A, Gris M. Bench-to-bedside review: antidotal treatment of sulfonylurea-induced hypoglycaemia with octreotide. Crit Care 2005;9:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzman CB, Zhang XM, Liu R, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:1521–1528 [DOI] [PubMed] [Google Scholar]
- 69.Capozzi ME, DiMarchi RD, Tschöp MH, Finan B, Campbell JE. Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocr Rev 2018;39:719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cegla J, Troke RC, Jones B, et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 2014;63:3711–3720 [DOI] [PubMed] [Google Scholar]
- 71.Khajavi N, Finan B, Kluth O, et al. An incretin-based tri-agonist promotes superior insulin secretion from murine pancreatic islets via PLC activation. Cell Signal 2018;51:13–22 [DOI] [PubMed] [Google Scholar]
- 72.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–837 [DOI] [PubMed] [Google Scholar]
- 73.Quiñones M, Al-Massadi O, Gallego R, et al. Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Mol Metab 2015;4:961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mighiu PI, Yue JT, Filippi BM, et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med 2013;19:766–772 [DOI] [PubMed] [Google Scholar]
- 75.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004;127:546–558 [DOI] [PubMed] [Google Scholar]
- 76.Atrens DM, Menéndez JA. Glucagon and the paraventricular hypothalamus: modulation of energy balance. Brain Res 1993;630:245–251 [DOI] [PubMed] [Google Scholar]
- 77.Kim T, Nason S, Holleman C, et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes 2018;67:1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Habegger KM, Stemmer K, Cheng C, et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 2013;62:1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beaudry JL, Kaur KD, Varin EM, et al. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol Metab 2019;22:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salem V, Izzi-Engbeaya C, Coello C, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab 2016;18:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyoshi H, Shulman GI, Peters EJ, Wolfe MH, Elahi D, Wolfe RR. Hormonal control of substrate cycling in humans. J Clin Invest 1988;81:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longuet C, Sinclair EM, Maida A, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 2008;8:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, Browning JD. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 2019;5:127737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henderson SJ, Konkar A, Hornigold DC, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab 2016;18:1176–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab 2019;21:120–128 [DOI] [PubMed] [Google Scholar]
- 86.Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018;391:2607–2618 [DOI] [PubMed] [Google Scholar]