Abstract
Introduction
Although diabetes and apolipoprotein E (apoE) are both significant risk factors for dementia, including Alzheimer's disease, it remains to be clarified how they are related to each other in contributing to the risk of dementia.
Methods
By reviewing the National Alzheimer's Coordinating Center (NACC) clinical records, we investigated whether diabetes affects cognitive decline depending on APOE genotype and their potential relationships with neuropathology.
Results
A significant interaction between diabetes and APOE genotype exists, where diabetes affected cognitive decline in APOE3 carriers and APOE2 carriers, but not APOE4 carriers. Moreover, the presence of vascular pathology was increased by diabetes in APOE3 carriers, while APOE4 carriers nearly reached plateau levels irrespective of diabetes.
Discussion
Diabetes accelerates cognitive decline, in part, through accelerating vascular impairment in non‐APOE ε4 carriers, but such effects are negligible in APOE4 carriers, who themselves are already vulnerable to vascular impairment.
Keywords: Alzheimer's disease, APOE, cognitive decline, dementia, diabetes
1. BACKGROUND
Diabetes is a significant risk factor for dementia.1, 2 Although the mechanism underlying this association remain elusive, several clinical and animal studies have indicated that diabetes may cause cognitive dysfunction by inducing changes in vascular integrity and function, brain structure, glucose metabolism, and insulin signaling, as well as by impairing amyloid β (Aβ)/tau metabolism.3, 4, 5, 6 Apolipoprotein E (APOE), the strongest genetic risk factor for Alzheimer's disease (AD), also affects these disease pathways, likely sharing common mechanisms with diabetes.7, 8, 9 On the other hand, the relationship between diabetes and APOE in contributing to AD or dementia has not yet been clarified fully despite some clinical observations.10, 11, 12 To understand the precise mechanism by which diabetes accelerates cognitive dysfunction, it is necessary to thoroughly address the effects of diabetes on clinical cognitive deterioration and the microscopic neuropathological changes in subjects depending on each APOE genotype.
The National Alzheimer's Coordinating Center (NACC) database contains a prospective standardized, longitudinal clinical evaluation of a large number of patients in the National Institute on Aging's Alzheimer's Disease Center (ADC) program across the United States with autopsy data for a subset of patients. Recently, we analyzed the NACC database and observed that APOE4 worsens cognitive decline during aging, while APOE2 protects against it, which can be independent of AD‐related neuropathology.13 In the present study, by using the same database, we investigated whether diabetes is associated with cognitive decline and neuropathological changes depending on the APOE genotype.
2. METHODS
2.1. Human clinical data
The clinical data in the NACC database, which were collected by the past and present ADCs from September 2005 to September 2018 as the longitudinal Uniform Data Set,14 were assessed in this study. NACC subjects are regarded as a referral‐based or volunteer case series, and the majority of the subjects are Caucasian and then Black or African Americans. This cumulative database contains clinical evaluations and neuropathological data when available for nondemented and demented individuals. To assess the effects of diabetes depending on the APOE genotype on the risk of cognitive decline during aging, we initially restricted our analysis to subjects who were genotyped for APOE and recruited at an age ≥60‐years‐old. Diabetes was defined as any history of diabetes from the subject's health history form. There were 36 subjects whose diabetic information was missing or unknown at all visits (≈0.14% of total subjects), and thus were excluded, resulting in 24,967 subjects (Figure 1). If a subject showed DIABETES = 1 (recent/active) or 2 (remote/inactive) at any visit, we classified such a person as “diabetes” (n = 3782). In these subjects, 459 subjects showed diabetes = 2 at any visit (12.1%), whereas 3323 subjects showed only diabetes = 1 (87.9%). If diabetes = 0 at all visits, we classified such a person as “non‐diabetes” or “normal” (n = 21,185). To maintain mutually exclusive groups, 672 subjects who had the APOE ε2/ε4 genotype were excluded from the analyses.15, 16 We also used neuropathological data from 4312 subjects to assess the relationship between diabetes and APOE genotype with the neuropathological assessments, which were collected by each ADC based on the NACC neuropathology data‐collection form.17
Figure 1.

Diagram of the selection of subjects in this study. Data collection was done with subjects who met the inclusion criteria (ie, having APOE genotype information and diabetic information and ≥60‐years‐old at recruitment). The remaining 24,967 subjects were used for clinical analysis and 4307 subjects for pathological data analysis after excluding subjects with APOE ε2/ε4 genotype
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources, such as PubMed. Although both diabetes and apolipoprotein E (APOE) are significant risk factors for dementia, including Alzheimer's disease (AD), the relationship between diabetes and APOE in contributing to AD or dementia has not yet been clarified fully in previous studies. Previous studies relevant to the current study are appropriately cited.
Interpretation: Our findings revealed novel aspects regarding the relationship between diabetes and APOE in contributing to AD or dementia, in terms of clinical symptoms and neuropathology.
Future directions: Our findings would stimulate additional clinical studies. Clinical biomarker studies would be warranted that can detect APOE genotype–specific effects of diabetes on accelerating cognitive decline through vascular impairments. In addition, in clinical trials targeting vascular aspects of diabetes‐associated cognitive decline, effects should be stratified by non–APOE4 carriers and APOE4 carriers.
2.2. Statistical analysis
To assess the risk of cognitive decline during aging in the NACC cohort, we used a Cox proportional hazard model with sex, race, years of education, diabetes, and APOE genotypes as covariates, the date of birth as the time of origin, and the age at onset of cognitive decline as the time of event. We used the NACC variable “DECAGE” to define the age at onset of cognitive decline, which was determined by clinicians after consulting with medical records, direct observation, and subject/informant report. When the NACC database held a different value at multiple visits within the same subjects, we used the record at their last visit (0.3% of total subjects). Subjects whose age at the onset of cognitive decline was unknown were eliminated from the analysis (0.8% of total subjects). Subjects who did not show any cognitive decline at their last visit were right‐censored (36.4% of total subjects). Hazard ratios (HRs), 95% confidence intervals (CIs) of the HRs, and associated P values are reported.
To define the presence/absence of cognitive impairment until their last visit, we used “NACCUDSD,” which described cognitive status (normal cognition, impaired but not mild cognitive impairment [MCI], MCI, or dementia). The effects of diabetes and APOE genotype on the Clinical Dementia Rating (CDR®) total and individual domain scores and Mini‐Mental State Exam (MMSE) neuropsychological battery scores were analyzed using a multivariable linear regression model adjusting for sex, race, years of education, and age at the cognitive test with/without considering the interaction between diabetes and APOE genotype. The effects of diabetes and APOE genotype on neuropathological scores were analyzed using a multivariable linear regression model adjusting for sex, race, and age at death with/without considering the interaction between diabetes and APOE genotype. Student t tests were conducted to assess the effects of diabetes depending on APOE genotypes, with applying a Bonferroni correction for multiple comparisons for the three APOE genotype statuses.
Statistical analyses were performed using JMP Pro software (version 12, SAS Institute Inc.), except for analyzing proportional hazard assumption of Cox proportional models, which were performed by weighted residuals18 function “cox.zph” of R (version 3.6.1, The R Foundation).
3. RESULTS
3.1. Diabetes is associated with earlier cognitive decline during aging in APOE2 and APOE3 carriers but not in APOE4 carriers
In the current study, to assess the relationship between diabetes and APOE genotype in contributing to the risk of cognitive decline during aging, we analyzed NACC database that follows a large number of middle‐ to older‐aged subjects with APOE genotype and diabetic information (n = 24,967, Figure 1 and Table 1). We analyzed the NACC variable “DECAGE” that indicates the age at which cognitive decline begins, and enabled us to assess APOE effects on cognitive decline during aging.13 In the model adjusting for sex, race, education, and APOE genotype, diabetes was indeed significantly associated with earlier cognitive decline across all NACC subjects (HR = 1.06, 95% CI = 1.02–1.11, P = 0.0068, Figure 2A), whereas we also observed a significant interaction between diabetes and the APOE genotype (P = 0.0012). Indeed, after separating each APOE genotype, diabetes was associated with earlier cognitive decline in APOE2 carriers (HR = 1.22, 95% CI = 1.06–1.42, P = 0.0080, median age at cognitive decline = 84‐years‐old [normal] vs 80‐years‐old [diabetes], Figure 2B) and APOE3 carriers (HR = 1.10, 95% CI = 1.03–1.17, P = 0.0045, median age at cognitive decline = 80‐years‐old [normal] vs 78‐years‐old [diabetes], Figure 2C) but not in APOE4 carriers (HR = 0.99, 95% CI = 0.93–1.06, P = 0.8322, median age at cognitive decline = 73‐years‐old [normal] vs 73‐years‐old [diabetes], Figure 2D). Proportional hazard assumption was not violated for the effects of diabetes in these analyses (in total subjects: P = 0.0658; in APOE2 carriers: P = 0.2027; in APOE3 carriers: P = 0.855; in APOE4 carriers: P = 0.1766). When we restricted the analysis to the 3323 subjects with diabetes who have recent/active, but no remote/inactive, record of diabetes, similar APOE genotype‐specific effects of diabetes on cognitive decline were observed (in APOE2 carriers: HR = 1.19, 95% CI = 1.02–1.39, P = 0.0306; in APOE3 carriers: HR = 1.11, 95% CI = 1.03–1.18, P = 0.0044; and in APOE4 carriers: HR = 1.00, 95% CI = 0.93–1.08, P = 0.9694). Although a significant interaction existed between APOE and sex (P < 0.0001), as previously reported,13 there was no interaction between diabetes and sex (P = 0.2252).
Table 1.
Demographic characteristics of clinically assessed subjects with/without diabetes categorized by APOE carrier status in the NACC database
| Clinically assessed cohorts | Total | Normal | Diabetes | P‐value |
|---|---|---|---|---|
| No. | 24,967 | 21,185 | 3782 | – |
| Sex (M:F) | 10,896:14,071 | 9110:12,075 | 1286:1996 | <0.0001 |
| Age at initial visit (years) | 74.3 ± 8.1 | 74.3 ± 8.2 | 74.1 ± 7.5 | 0.0734 |
| Age at last visit (years) | 77.9 ± 8.5 | 77.9 ± 8.6 | 77.7 ± 8.0 | 0.0872 |
| Education (years) | 15.1 ± 3.4 | 15.3 ± 3.3 | 14.2 ± 3.8 | <0.0001 |
| White:Black:other | 20,805:3185:977 | 18,242:2250:693 | 2563:935:284 | <0.0001 |
| Demented:non‐demented | 11,094:13,873 | 9525:11,660 | 1569:2213 | <0.0001 |
| By APOE carrier status | ||||
| ε2/ε2 or ε2/ε3 (APOE2) | ||||
| No. | 2390 | 1974 | 416 | – |
| Sex (M:F) | 1010:1380 | 824:1150 | 186:230 | 0.2661 |
| Age at initial visit (years) | 74.9 ± 8.6 | 74.9 ± 8.8 | 74.6 ± 7.9 | 0.4816 |
| Age at last visit (years) | 78.9 ± 9.2 | 79.0 ± 9.4 | 78.4 ± 8.3 | 0.1866 |
| Education (years) | 15.3 ± 3.3 | 15.4 ± 3.1 | 14.4 ± 3.6 | <0.0001 |
| White:Black:other | 1891:412:87 | 1635:276:63 | 256:136:24 | <0.0001 |
| Demented:non‐demented | 693:1697 | 572:1402 | 121:295 | 0.9642 |
| ε3/ε3 (APOE3) | ||||
| No. | 12,415 | 10,440 | 1975 | – |
| Sex (M:F) | 5406:7009 | 4473:5967 | 933:1042 | 0.0003 |
| Age at initial visit (years) | 74.8 ± 8.3 | 74.9 ± 8.4 | 74.3 ± 7.7 | 0.0031 |
| Age at last visit (years) | 78.5 ± 8.8 | 78.6 ± 8.9 | 78.0 ± 8.2 | 0.0031 |
| Education (years) | 15.1 ± 3.5 | 15.3 ± 3.4 | 14.1 ± 4.0 | <0.0001 |
| White:Black:other | 10,488:1337:590 | 9113:923:404 | 1375:414:186 | <0.0001 |
| Demented:non‐demented | 4574:7841 | 3871:6569 | 703:1272 | 0.2092 |
| ε3/ε4 or ε4/ε4 (APOE4) | ||||
| No. | 9490 | 8212 | 1278 | – |
| Sex (M:F) | 4209:5281 | 3594:4618 | 615:663 | 0.0036 |
| Age at initial visit (years) | 73.5 ± 7.5 | 73.5 ± 7.6 | 73.7 ± 7.0 | 0.525 |
| Age at last visit (years) | 76.9 ± 7.9 | 76.8 ± 8.0 | 77.0 ± 7.5 | 0.4768 |
| Education (years) | 15.1 ± 3.4 | 15.2 ± 3.3 | 14.3 ± 3.7 | <0.0001 |
| White:Black:other | 7916:1284:290 | 7038:956:218 | 878:328:72 | <0.0001 |
| Demented:non‐demented | 5530:3960 | 4826:3386 | 704:574 | 0.0133 |
For continuous data, values are the mean ± SD.
P‐values are from one‐way ANOVA (continuous data) or chi‐square test (categorical value). M = male, F = female.
Figure 2.

Diabetes is associated with earlier cognitive decline during aging depending on APOE genotype. Survival plot for the onset of cognitive decline in subjects with/without diabetes history across all subjects (A), APOE2 carriers (B), APOE3 carriers (C), and APOE4 carriers (D). Each figure shows the hazard ratio with 95% confidence interval and P‐value, calculated by Cox regression model adjusting for sex, race, years of education, and APOE genotypes (A) or sex, race, and years of education (B‐D)
3.2. Diabetes is associated with worsened cognitive status in non‐demented subjects with APOE2 or APOE3 status but not those with APOE4 status
To further confirm the effects of diabetes on cognitive decline depending on the APOE genotype, we next assessed the CDR score, including the individual domain and total scores. As the effects of diabetes could be different depending on cognitive status, we first analyzed the non‐demented subjects. As expected, diabetes was generally associated with exacerbated CDR total and individual scores, including memory and judgment, in both APOE2 and APOE3 carriers but not in APOE4 carriers, whereas there were some trends that APOE4 per se was associated with worse CDR scores and APOE2 per se was associated with better CDR scores (Figure 3 and Table S1). However, such harmful effects of diabetes on CDR scores were not observed in the demented subjects (Table S2). We also analyzed the potential interaction between diabetes and APOE genotype with the MMSE scores. Although we did not generally observe such interactions with the MMSE scores, some components of the MMSE, that is, those related to orientation, attention, and language, showed some interactions, where diabetes had more harmful effects in APOE3 carriers but less harmful effects in APOE4 carriers (Table S3).
Figure 3.

Diabetes is associated with worse cognitive status in non‐demented subjects depending on APOE genotype. Effects of diabetes on CDR memory score (A), CDR judgment score (B), CDR orientation score (C), CDR community affairs score (D), CDR home and hobbies score (E), CDR personal care score (F), CDR sum of boxes score (G), and global CDR score (H) depending on APOE carrier status (APOE2, APOE3, or APOE4) by adjusting for sex, race, years of education, and age at cognitive test. Data are presented as the adjusted mean ± standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001; by t test with Bonferroni correction for multiple comparisons for the three APOE genotype statuses. N.S. = not significant, E2 − DM = APOE2 carrier without diabetes history, E2 + DM = APOE2 carrier with diabetes history, E3 − DM = APOE3 carrier without diabetes history, E3 + DM = APOE3 carrier with diabetes history, E4 − DM = APOE4 carrier without diabetes history, and E4 + DM = APOE4 carrier with diabetes history
3.3. Effects of diabetes on neuropathological changes depending on APOE genotype
To understand the mechanism by which diabetes affects cognitive decline in an APOE genotype–dependent manner, we next analyzed the effects of diabetes on neuropathological changes in all available subjects depending on APOE genotypes (Table 2). The results were summarized in Table S4. As the number of APOE2 carriers with neuropathology data was much lower (n = 361) compared to APOE3 carriers (n = 2019) and APOE4 carriers (n = 1811), we focused on APOE3 and APOE4 carriers. Unexpectedly, diabetes was associated with lower scores of AD major neuropathology, including amyloid accumulation and neurofibrillary tangle (NFT) formation, and this was especially significant in APOE3 carriers (Figure 4A,B). On the other hand, diabetes was generally associated with increased vascular pathology, including infarcts or lacunes adjusting for APOE genotype (Figure 4C). Notably, the presence of any vascular pathology was increased by diabetes only in APOE3 carriers, whereas APOE4 carriers nearly reached plateau levels irrespective of diabetes (Figure 4D). Such effects were also observed in the model including only non‐demented subjects (Table S5). These results suggest that APOE genotype–specific effects of diabetes on cognitive decline might be mediated through vascular impairments for which APOE4 carriers per se are already vulnerable (Table S6).
Table 2.
Demographic characteristics of pathologically assessed subjects with/without diabetes categorized by APOE carrier status in the NACC database
| Pathologically assessed cohorts | Total | Normal | Diabetes | P‐value |
|---|---|---|---|---|
| No. | 4307 | 3755 | 552 | – |
| Sex (M:F) | 2298:2009 | 1960:1795 | 338:214 | <0.0001 |
| Age at death (years) | 82.7 ± 9.3 | 82.7 ± 9.5 | 82.3 ± 8.3 | 0.3273 |
| White:Black:other | 4099:158:50 | 3589:130:36 | 510:28:04 | 0.0029 |
| Demented:non‐demented | 3306:1001 | 2908:847 | 398:154 | 0.0064 |
| By APOE carrier status | ||||
| ε2/ε2 or ε2/ε3 (APOE2) | ||||
| No. | 361 | 307 | 54 | – |
| Sex (M:F) | 180:181 | 150:157 | 30:24 | 0.3638 |
| Age at death (years) | 83.9 ± 10.5 | 84.2 ± 10.7 | 82.6 ± 9.4 | 0.2715 |
| White:Black:other | 340:18:3 | 293:12:2 | 47:6:1 | 0.0929 |
| Demented:non‐demented | 214:147 | 183:124 | 31:23 | 0.7618 |
| ε3/ε3 (APOE3) | ||||
| No. | 2015 | 1735 | 280 | – |
| Sex (M:F) | 1067:948 | 892:843 | 175:105 | 0.0005 |
| Age at death (years) | 83.8 ± 9.6 | 84.0 ± 9.7 | 82.7 ± 9.0 | 0.0197 |
| White:Black:other | 1932:51:32 | 1677:36:22 | 255:15:10 | 0.0005 |
| Demented:non‐demented | 1402:613 | 1222:513 | 180:100 | 0.0405 |
| ε3/ε4 or ε4/ε4 (APOE4) | ||||
| No. | 1810 | 1605 | 205 | – |
| Sex (M:F) | 993:817 | 868:737 | 125:80 | 0.0606 |
| Age at death (years) | 80.9 ± 8.5 | 80.9 ± 8.7 | 81.7 ± 6.9 | 0.1287 |
| White:Black:other | 1711:85:14 | 1514:79:12 | 197:6:2 | 0.3818 |
| Demented:non‐demented | 1592:218 | 1415:190 | 1,77:28 | 0.458 |
For continuous data, values are the mean ± SD.
P‐values are from one‐way ANOVA (continuous data) or chi‐square test (categorical value). M = male, F = female.
Figure 4.
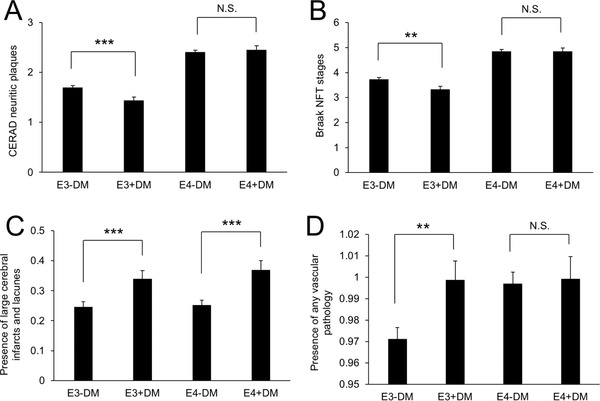
Diabetes is associated with neuropathological changes depending on APOE genotype. Effects of diabetes on CERAD neuritic plaques (A), Braak NFT stages (B), presence of large cerebral infarcts and lacunes (C), and presence of any vascular pathology (D), depending on APOE carrier status (APOE3 or APOE4) by adjusting for sex, race, and age at death. Data are presented as the adjusted mean ± standard error of the mean. **P < 0.01, ***P < 0.001; by t test with Bonferroni correction for multiple comparisons for the three APOE genotype statuses. N.S. = not significant, E3 − DM = APOE3 carrier without diabetes history, E3 + DM = APOE3 carrier with diabetes history, E4 − DM = APOE4 carrier without diabetes history, and E4 + DM = APOE4 carrier with diabetes history, CERAD = the Consortium to Establish a Registry for Alzheimer's Disease
4. DISCUSSION
In the present study, by analyzing the NACC subjects, we observed a significant interaction between diabetes and the APOE genotype: diabetes associated with earlier cognitive decline in APOE2 carriers and APOE3 carriers but not in APOE4 carriers. These results are consistent with previous population‐based studies, which have shown an association between diabetes and increased risk of dementia only among the non‐APOE4 carriers.10, 11 Marseglia et al. reported an association between diabetes and lower performance of a few specific cognitive functions in cognitively normal elderly people without APOE4, but not with APOE4.12 On the other hand, we observed significant effects of diabetes on more general cognitive functions as well as decline, likely due to the much larger cohort size of NACC database, which also allowed us to evaluate effects of diabetes in APOE2 carriers. Notably, we observed that diabetes hastened median age at cognitive decline by 4 years in APOE2 carriers and 2 years in APOE3 carriers, but not in APOE4 carriers (Figure 2). Such effects are fully meaningful in that medical cost is significantly reduced even when onset of AD is delayed by just 1 year.19
Of interest, diabetes is associated with less major AD neuropathology, including amyloid accumulation and NFT formation, and this is especially significant in the APOE3 carriers. Several neuropathological studies have reported no association between diabetes and amyloid accumulation or NFT formation,20, 21, 22, 23 whereas some studies have indicated that there was less amyloid accumulation in AD patients with diabetes than in those without diabetes.24, 25, 26 We observed that amyloid and tau accumulation scores are lower in APOE3 carriers, but not in APOE4 carriers, with diabetes than in those without diabetes. Such APOE genotype–specific effects may explain discrepancies between previous studies.
On the other hand, neuropathological studies have shown repeatedly the association of diabetes with increased cerebrovascular lesions such as infarcts.21, 22, 26, 27, 28 Consistent with this, we observed that diabetes is generally associated with increased vascular pathology, including infarcts or lacunes, irrespective of APOE genotype (Table S4). In contrast, we observed that the presence of any vascular pathology is increased by diabetes only in APOE3 carriers, whereas APOE4 carriers nearly reached a plateau irrespective of diabetes. The presence of any vascular pathology was defined by the NACC as “NACCVASC” if the subjects had shown at least one vascular pathology reported on the neuropathology form.17 Although further studies are required, this result appears to be consistent with the finding that the presence of APOE4 is associated with an increased risk of several vascular impairments, including cerebral amyloid angiopathy and atherosclerosis in humans.7, 29, 30 Although some conflicting results were reported, mice with APOE4 also show disrupted vascular function and integrity.31, 32, 33 Moreover, neurovascular regulation was impaired after brain ischemia in such mice.34 Thus, harmful effects of diabetes on cognitive decline as observed in our study may already be masked by the presence of APOE4, which itself increases the risk of vascular impairment.
There are several limitations in this study. First, information about whether the subjects had diabetes was collected by the main subject and participant report. Diabetes is clinically diagnosed based on blood glucose levels in the fasting state or after a challenge with oral glucose or blood hemoglobin A1c levels.35 Thus, blood glucose levels and hemoglobin A1c levels are required to validate our conclusions drawn from this study. Other information on severity of diabetes, including the type, duration, and timing, were also not considered in this study. Second, the subjects were the participants in the National Institute on Aging's ADC program across the United States; therefore, our analysis was not population based. Although such limitations would not affect our results regarding APOE genotype–specific effects of diabetes on cognitive decline, future studies resolving these limitations might address more precise effects on how diabetes interacts with APOE when contributing to the risk of dementia.
In summary, using the NACC database, we observed that (1) diabetes is associated with earlier deterioration of cognitive function in APOE3 and APOE2 carriers, but not in APOE4 carriers; (2) diabetes is associated with worsened cognitive function in non‐demented subjects with APOE3 or APOE2 carrier status, but not those with APOE4 carrier status; (3) amyloid and tau accumulation scores were lower, especially in APOE3 carriers with diabetes than those without diabetes; and (4) diabetes is associated with increased vascular pathology scores, especially in APOE3 carriers compared to APOE4 carriers. Our results suggest that diabetes accelerates cognitive decline, in part, through vascular impairment in non‐APOE4 carriers, but such effects are negligible in APOE4 carriers, who themselves are already vulnerable to vascular impairment. Understanding the precise mechanisms of these observations could provide a novel approach to the treatment of dementia.
AUTHOR CONTRIBUTIONS
MS and NS contributed to the concept and study design. MS, YT, KS, AF, GB, and NS contributed to data acquisition and analysis. MS and NS contributed to drafting the manuscript and figures. All authors edited and reviewed the final manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest with the content of this article.
Supporting information
Table S1‐S7
ACKNOWLEDGMENTS
We would like to thank the contributors to the NACC database for collecting the data. We would like to thank Dr. Wataru Ohashi for helping statistical analyses, and the lab members in the department of aging neurobiology and Dr. Takashi Sakurai for discussions. This work was supported in part by the Research Funding for Longevity Sciences from National Center for Geriatrics and Gerontology (28‐45 to Naoyuki Sato); Grants‐in‐Aid from Japan Promotion of Science (MEXT26293167, MEXT15K15272, and MEXT17H04154 to Naoyuki Sato and 17H07419 and 18H02725 to Mitsuru Shinohara); a Takeda Science Foundation Research Encouragement Grant (to Naoyuki Sato and Mitsuru Shinohara); a SENSHIN Medical Research Foundation Research Grant (to Naoyuki Sato); a Novartis Foundation for Gerontological Research Award (to Naoyuki Sato); an Annual Research Award Grant from the Japanese Society of Anti‐aging Medicine (to Naoyuki Sato); a NACC Junior Investigator Award (to Mitsuru Shinohara); a Takeda Medical Research Foundation Research Grant (to Naoyuki Sato); a research grant from the Japan Foundation for Aging and Health (to Mitsuru Shinohara); a research grant from the Uehara Memorial Foundation (to Mitsuru Shinohara); a research grant from the Hori Sciences and Arts Foundation (to Mitsuru Shinohara); and National Institutes of Health (NIH) grants R37AG027924, RF1AG057181, and R01AG035355 (to Guojun Bu). The NACC database is funded by NIH grant U01AG016976. NACC data are contributed by the NIA‐funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Shinohara M, Tashiro Y, Suzuki K, Fukumori A, Bu G, Sato N. Interaction between APOE genotype and diabetes in cognitive decline. Alzheimer's Dement. 2020;12:e12006 10.1002/dad2.12006
Contributor Information
Mitsuru Shinohara, Email: shinohara@ncgg.go.jp.
Naoyuki Sato, Email: nsato@ncgg.go.jp.
REFERENCES
- 1. Sato N, Morishita R. Brain alterations and clinical symptoms of dementia in diabetes: abeta/tau‐dependent and independent mechanisms. Front Endocrinol (Lausanne). 2014;5:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329‐340. [DOI] [PubMed] [Google Scholar]
- 3. Shinohara M, Sato N. Bidirectional interactions between diabetes and Alzheimer's disease. Neurochem Int. 2017;108:296‐302. [DOI] [PubMed] [Google Scholar]
- 4. Sutherland GT, Lim J, Srikanth V, Bruce DG. Epidemiological approaches to understanding the link between type 2 diabetes and dementia. J Alzheimers Dis. 2017;59:393‐403. [DOI] [PubMed] [Google Scholar]
- 5. Sato N, Morishita R. Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer disease: short‐ and long‐term modification by non‐genetic risk factors. Front Aging Neurosci. 2013;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold SE, Arvanitakis Z, Macauley‐Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shinohara M, Sato N. The roles of apolipoprotein E, lipids, and glucose in the pathogenesis of Alzheimer's disease In: Nakabeppu Y, Ninomiya T, eds. Diabetes Mellitus: A risk Factor for Alzheimer's Disease. Singapore: Springer Singapore; 2019:85‐101. [DOI] [PubMed] [Google Scholar]
- 9. Zhao N, Liu C‐C, Van Ingelgom AJ, et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96:115‐129.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer's disease. Diabetes. 2007;56:211‐216. [DOI] [PubMed] [Google Scholar]
- 11. Borenstein AR, Wu Y, Mortimer JA, et al. Developmental and vascular risk factors for Alzheimer's disease. Neurobiol Aging. 2005;26:325‐334. [DOI] [PubMed] [Google Scholar]
- 12. Marseglia A, Fratiglioni L, Laukka EJ, et al. Early cognitive deficits in type 2 diabetes: a population‐based study. J Alzheimers Dis. 2016;53:1069‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shinohara M, Kanekiyo T, Yang L, et al. APOE2 eases cognitive decline during aging: clinical and preclinical evaluations. Ann Neurol. 2016;79:758‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson RS, Bienias JL, Berry‐Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73:672‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Besser LM, Kukull WA, Teylan MA, et al. The Revised National Alzheimer's Coordinating Center's Neuropathology Form—available data and new analyses. J Neuropathol Exp Neurol. 2018;77:717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515‐526. [Google Scholar]
- 19. Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014;18:25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heitner J, Dickson D. Diabetics do not have increased Alzheimer‐type pathology compared with age‐matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306‐1311. [DOI] [PubMed] [Google Scholar]
- 21. Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960‐1965. [DOI] [PubMed] [Google Scholar]
- 22. Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer's disease neuropathology. Alzheimers Dement. 2016;12:882‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dos Santos Matioli MNP, Suemoto CK, Rodriguez RD, et al. Diabetes is not associated with Alzheimer's disease neuropathology. J Alzheimers Dis. 2017;60:1035‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalaria RN. Neurodegenerative disease: diabetes, microvascular pathology and Alzheimer disease. Nat Rev Neurol. 2009;5:305‐306. [DOI] [PubMed] [Google Scholar]
- 25. Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer's disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson PT, Smith CD, Abner EA, et al. Human cerebral neuropathology of type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population‐based neuropathologic study. Neurology. 2010;75:1195‐1202. [DOI] [PubMed] [Google Scholar]
- 28. Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shinohara M, Murray ME, Frank RD, et al. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer's disease. Acta Neuropathol (Berl). 2016;132:225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paternoster L, Martinez Gonzalez NA, Lewis S, Sudlow C. Association between apolipoprotein E genotype and carotid intima‐media thickness may suggest a specific effect on large artery atherothrombotic stroke. Stroke. 2008;39:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bien‐Ly N, Boswell CA, Jeet S, et al. Lack of widespread BBB disruption in Alzheimer's disease models: focus on therapeutic antibodies. Neuron. 2015;88:289‐297. [DOI] [PubMed] [Google Scholar]
- 32. Alata W, Ye Y, St‐Amour I, Vandal M, Calon F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood—brain barrier function in mice. J Cereb Blood Flow Metab. 2014;35:86‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koizumi K, Hattori Y, Ahn SJ, et al. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun. 2018;9:3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S7


