Abstract
Affective neuroscience research suggests that maturational changes in reward circuitry during adolescence present opportunities for new learning, but likely also contribute to increases in vulnerability for psychiatric disorders such as depression and substance abuse. Basic research in animal models and human neuroimaging has made progress in understanding the normal development of reward circuitry in adolescence, yet, few functional neuroimaging studies have examined puberty-related influences on the functioning of this circuitry. The goal of this study was to address this gap by examining the extent to which striatal activation and cortico-striatal functional connectivity to cues predicting upcoming rewards would be positively associated with pubertal status and levels of pubertal hormones (dehydroepiandrosterone, testosterone, estradiol). Participants included 79 adolescents (10–13 year olds; 47 girls) varying in pubertal status who performed a novel reward cue processing task during fMRI. Pubertal maturation was assessed using sex-specific standardized composite measures based on Tanner staging (self-report and clinical assessment) and scores from the Pubertal Development Scale. These composite measures were computed to index overall pubertal maturation as well as maturation of the adrenal and gonadal axes separately for boys and girls. Basal levels of circulating pubertal hormones were measured using immunoassays from three samples collected weekly upon awakening across a three-week period. Results indicated greater striatal activation and functional connectivity between nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) to reward cue (vs. no reward cue) on this task. Also, girls with higher levels of estradiol showed reduced activation in left and right caudate and greater NAcc-putamen connectivity. Girls with higher levels of testosterone showed greater NAcc connectivity with the anterior cingulate cortex and the insula. There were no significant associations in boys. Findings suggest that patterns of activation and connectivity in cortico-striatal regions are associated with reward cue processing, particularly in girls. Longitudinal follow-up neuroimaging studies are needed to fully characterize puberty-specific effects on the development of these neural regions and how such changes may contribute to pathways of risk or resilience in adolescence.
Keywords: reward cue processing, fMRI, puberty, pubertal hormones, cortico-striatal regions, sex differences
1.0. Introduction
The onset of adolescence is characterized by a cascade of maturational changes in physical, hormonal, psychological, neural, and social domains (Crone and Dahl, 2012; Dorn et al., 2006). According to animal and human neuroimaging studies, adolescents show a peak in reward- and sensation-seeking behaviors and sensitivity to reward or stimuli that signal potential receipt of reward (Braams et al., 2015; Galvan, 2013). Such propensities toward reward and reward-signaling stimuli are thought to be associated with greater activation in striatal regions to reward stimuli (Galvan, 2013). Understanding neurodevelopment of reward circuitry is important in order to elucidate adolescent vulnerability to depression and substance abuse as well as pathways to resilience (Crone and Dahl, 2012; Dahl et al., 2018). Because most studies have focused on age-related changes, the influence of puberty on reward circuitry functioning remains poorly understood.
Puberty occurs over a period of approximately 4–5 years, with large individual differences in maturation rates. Adrenarche typically begins around five to seven years of age when levels of the androgens dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S), secreted from the adrenal glands, begin to increase. Physical changes during adrenarche include growth of pubic hair, changes in body odor, and appearance of acne. Gonadarche, which typically follows the onset of adrenarche, is associated with maturation of primary (i.e., ovaries and testes) and secondary sexual characteristics (e.g., breast and genital development) driven by production of gonadal steroid hormones like testosterone and estrogen. Pubertal hormones have been associated with physical and physiological changes (McAnarney et al., 1992), including influences on the developing adolescent brain (Schulz and Sisk, 2016). Measuring how pubertal maturation and hormones are associated with neural activation to rewards adds critical information regarding puberty-specific effects on reward circuitry functioning in ways that could deepen our understanding of vulnerability or resilience to psychopathology.
The ventral striatum (VS), which includes the nucleus accumbens (NAcc), is a target of dopaminergic midbrain neurons (i.e., ventral tegmental area, VTA) and plays an important role in reward anticipation and encoding of relative gains and losses (Haber and Knuston, 2010). Dopamine release from VTA neurons projecting to cortical and subcortical regions is increased during reward expectancy (Ikemoto and Panksepp, 1999), and thus drives activity in the VS and the medial prefrontal cortex (mPFC), including the orbitofrontal cortex (OFC) and rostral regions of the anterior cingulate cortex (rACC) (Diekhof et al., 2011). The dopamine system undergoes important maturational changes in early adolescence (Wahlstrom et al., 2010). Animal research has shown that the firing rate of dopamine neurons increases across early adolescence, peaks in late adolescence, and declines into adulthood (McCutcheon and Marinelli, 2009). With some exceptions (Bjork et al., 2010), neuroimaging studies indicate that compared to adults and children, adolescents show elevated VS responses when anticipating, viewing, or receiving rewarding stimuli (Galvan, 2013). Functional connectivity studies suggest that compared to adults, adolescents show reduced functional coupling between VS and prefrontal cortex during decision making in the context of reward (Teslovich et al., 2014), suggesting that modulation of this circuitry may be less efficient during this developmental period (van den Bos et al., 2012). The insula, which has dense reciprocal connections with the striatum (Haber and Knuston, 2010), also plays an important role in reward processing. In particular, neuroimaging studies have shown that this region is activated along with the striatum during gain anticipation in adults (Liu et al., 2011; Samanez-Larkin et al., 2007) and adolescents (Silverman et al., 2015).
The pubertal rise in hormones is thought to play an important role in the development of cortico-striatal regions (Crone and Dahl, 2012) in ways that could impact reward sensitivity and motivated behavior (van Duijvenvoorde et al., 2014). The few human neuroimaging studies focused on puberty-related effects suggest the process of puberty influences the structure and function of these neural regions in typically developing adolescents. Longitudinal studies suggest maturational changes in brain structure associated with pubertal stage and pubertal hormones (Goddings, 2015), but such findings have not been consistent across studies (Koolschijn et al., 2014). With regard to neural function, Braams et al. (2015) reported that longitudinal changes over a 2-year period in self-report measures of pubertal maturation and testosterone were associated with NAcc activation to reward (win>loss) on a gambling task in a large sample of 8–27 year-olds. Using a cross-sectional design, Forbes et al. (2010) reported reduced striatal and greater mPFC activation when receiving monetary reward on a card-guessing task in healthy 11–13 year-olds who were more advanced in pubertal maturation compared to their peers. They also reported a positive correlation between testosterone and striatal activation to reward anticipation in boys (Forbes et al., 2010). In contrast, higher levels of testosterone were positively correlated to striatal activation across both sexes in 10–16 year olds when risky decision was rewarded (Op de Macks et al., 2011). In the same study, greater task-related risk taking behavior in girls (11–13 years) was indirectly predicted by testosterone levels through heightened mPFC and by estradiol levels through reduced NAcc activation to risk-taking trials on the same task (Op de Macks et al., 2016). These findings suggest that there may be sex-specific influences of pubertal hormones on patterns of neural activation to reward in the context of guessing or gambling/risk-taking tasks.
Tasks used to examine puberty-related influences on reward circuitry involve processing certain levels of unpredictability, which have been shown to modulate striatal activation (Berns, 2001). Such modulatory effects tend to vary as a function of the individual’s tolerance of and response to uncertainty (Nelson et al., 2016; Tanovic et al., 2018). Thus, it remains unclear to what extent pubertal maturation may influence the functioning of cortico-striatal regions associated with processing a cue that predicts reward without the potential confounds related to unpredictability.
The current study aimed to address this gap by developing a task designed to examine the influence of puberty and pubertal hormones on patterns of activation and functional connectivity in cortico-striatal regions associated with reward cue processing, including bilateral striatum (i.e., NAcc, caudate, and putamen), mPFC (i.e., rACC and OFC) as well as the insula (Haber and Knuston, 2010; Silverman et al., 2015). To examine pubertal influences on the functional connectivity of these regions, we used seed-based functional connectivity analysis with the NAcc as the seed, using coordinates from a meta-analysis in adolescents (Silverman et al., 2015). The NAcc has been shown to be involved in processing cues that predict reward (Berridge et al., 2009). We hypothesized that pubertal maturation and levels of pubertal hormones would be positively associated with heightened striatal and insular activation and reduced NAcc-mPFC functional connectivity when processing reward cues. Because we wanted to examine specific influences of adrenarche and gonadarche, we proceeded in conducting analyses separately in boys and girls as changes along these axes of puberty are different in males and females.
2.0. Materials and Methods
2.1. Participants
The final sample included seventy-nine healthy adolescents (47 girls) aged between 10 and 13 years old and with no history of psychiatric or neurological disorders participated in the study (see Table 1). Participants were recruited from the community through advertisements and flyers. Girls were recruited between 10 and 12 years old and boys between 11 and 13 years old; these ages were based on findings that girls in the United States begin adrenarche (Byrne et al., 2017) and gonadarche (Dorn et al., 2006) approximately one year earlier than boys. The sample comprised of 65% Caucasian, 22% African American, 4% Hispanic, 1% Asian, and 10% mixed-ethnicity.
Table 1.
Participant characteristics
| Variables | Boys | Girls |
|---|---|---|
| n | 32 | 47 |
| Age | 12.54 (0.95) | 11.52 (0.83) |
| BMI | 20.90 (3.15) | 20.33 (3.80) |
| Tanner stage 1–2 (%) | 43 | 41 |
| Tanner stage 3–5 (%) | 57 | 59 |
| PDS – totala | 2.77 (1.05) | 3.07 (0.99) |
| PDS – adrenal axisa | 2.66 (1.21) | 2.99 (1.07) |
| PDS – gonadal axisa | 2.88 (1.24) | 3.17 (1.13) |
| Composite z-score adrenal axis | 2.73 (1.02) | 2.89 (0.95) |
| Composite z-score gonadal axis | 2.81 (0.81) | 3.09 (0.95) |
| Composite z-score total | 2.77 (0.82) | 2.99 (0.89) |
| DHEAraw | 119.32 (21.82) | 172.93 (15.18) |
| DHEAlogb | 5.49 (0.59) | 5.85 (0.64) |
| Testosteroneraw | 100.86 (15.47) | 55.63 (3.71) |
| Testosteronelogb | 5.12 (0.57) | 4.71 (0.44) |
| Estradiolraw | n/a | 1.56 (0.09) |
| Estradiollogb | n/a | 1.02 (0.16) |
Note: Table values represent means, with standard deviations in parentheses, unless otherwise reported. DHEA: Dehydroepiandrosterone. BMI: Body Mass Index (n=30 boys and n=47 girls); Tanner staging data was available for 74 participants. DHEA and testosterone: n=30 boys and n=46 girls; estradiol: n=45 girls.
Ranging from 1 (development has not yet begun) to 5 (development seems complete).
Empirical Bayesian estimation scores that have been log transformed
Exclusion criteria included presence of psychiatric disorder, visual disturbance, being pregnant, MRI contraindications (e.g., metal in the body, claustrophobia), or taking oral steroids. After screening based on exclusion criteria, a total of 117 adolescents were eligible and agreed to participate in the larger study. Data from 38 adolescents were not included in the analyses due to the following reasons: a) technical issues (n=10), b) structural abnormalities (n=2), c) less than 70% accuracy rate on the fMRI task (n=2), or d) excessive motion artifact (i.e., motion > 3mm/3° on any volume) (n=24).
Written parental informed consent was provided and written adolescent assent was obtained prior to scanning. The study was approved by the University of Pittsburgh Institutional Review Board. Participants received performance-based earnings in addition to compensation for their participation in the larger study focused on puberty-specific influences on fronto-limbic circuitry.
2.2. Screening Measures
The Columbia Diagnostic Interview for Children (CDIS-C) (Shaffer et al., 2000), a computerized structured interview used with adolescents and their parent to screen for presence of psychiatric diagnoses.
2.3. Pubertal Maturation Measures
2.3.1. Tanner Stage Assessment
Tanner stage represents 5 stages (ranging from 1=prepubertal to 5=adult-like) of genital and pubic hair development for boys, and breast and pubic hair development for girls (Marshall and Tanner, 1969, 1970). Tanner stage was assessed using both self-report and clinical observation by staff trained in the scientific study of puberty (e.g., medical student). The assessment was performed in a medical exam room located within the neuroimaging center. At the beginning of the assessment, research staff provided information about the normative pubertal development and a description of the Tanner stages to the adolescent using the picture-based interview about puberty (PBIP) (Dorn and Susman, 2004) (unpublished script, described in Shirtcliff et al., 2009). Research staff then left the room so adolescents could privately compare their physical development with photographs from the PBIP for their self-ratings. Upon returning to the room, a brief assessment was completed by research staff trained to complete Tanner stage using visual observation for research purposes (See Supplementary Material Figure 1 for histograms depicting distributions of Tanner staging scores for boys and girls).
2.3.2. Pubertal Development Scale (PDS)
Adolescents completed the PDS as it queries about several additional secondary sexual characteristics not captured by Tanner stage (e.g., skin changes, growth spurt, facial hair). The PDS is a self-report measure of pubertal maturation that contains five questions about physical development that are scored from 1 (development has not yet begun) to 4 (development seems complete). A scoring algorithm converts the PDS to the Tanner metric (1 to 5), and provides a sensitive way to differentiate between physical changes driven by adrenarche and gonadarche, respectively (Shirtcliff et al., 2009). Three scores were computed: an overall PDS score (PDSS), an adrenal axis score (PDSA), and gonadal axis score (PDSG) (See Supplementary Material Figure 1 for histograms depicting distributions of PDS adrenal, gonadal, and total scores for boys and girls).
2.3.3. Pubertal Composite Z-score
Following Shirtcliff and colleagues (Shirtcliff et al., 2009), we created composite puberty scores that captured pubertal maturation assessment from the observational and self-report Tanner stage measures (ranging from 1 to 5) and the PDS adrenal, gonadal, and total scores (ranging from 1 to 5). Each scale was weighed equally so that the composite equally reflected maturation on the adrenal and gonadal axes. The adjusted scores were computed separately for males and females. The reason for this approach was to capitalize on all of the data available to assess pubertal status and to ensure that each data point was equally weighted and on the Tanner score metric. The adrenal composite score was calculated by averaging the adrenal PDS scale with the observational and self-report Tanner stage for pubic hair, (M=2.72, SD=1.01, Cronbach’s α=.88). The gonadal composite score was calculated by averaging the gonadal PDS scale with the observational and self-report measures based on Tanner stage for breast or genital development in females and males, respectively (M=2.95, SD=.82, Cronbach’s α=.75). An overall pubertal maturation composite score was created by averaging the adrenal and gonadal scores across the PDS and Tanner stage measures (self and observational) (M=2.83, SD=.83, Cronbach’s α=.79). These composite scores representing adrenal, gonadal, and overall composite scores were then standardized (separately for males and females) so that the mid-point of each distribution represented the average level of pubertal maturation for each sex (see Table 1) (See Supplementary Material Table 1 for correlations between PDS measures of adrenal and gonadal axes and Tanner staging scores and Table 2 for correlations between composite scores and factor scores).
Table 2.
Mean accuracy and reaction times (with standard error in parentheses) for the Gopher task
| Variables | Boys | Girls |
|---|---|---|
| Accuracy (%) | ||
| Reward cue trials | 91.2 (1.3) | 91.4 (1.1) |
| No Reward cue trials | 90.6 (1.4) | 90.8 (1.2) |
| Reaction Times (ms) | ||
| Reward cue trials | 1437.9 (45) | 1499.2 (31) |
| No Reward cue trials | 1490.0 (48) | 1535.2 (32) |
2.3.4. Pubertal Hormones
Circulating levels of testosterone and DHEA (for both sexes) as well as estradiol (girls only) were assessed using salivary assays following published protocols (Shirtcliff et al., 2000). During their initial visit in the lab, adolescents and their parents were provided with instructions for saliva collection, storage, and shipping protocols as well as home saliva collection kits, which included a cardboard box that contained 3 pre-labeled vials, a saliva collection diary, an ice pack, and a plastic bag. Adolescents provided saliva samples at home immediately upon wakening (7am-9am) on 3 separate days over a three-week period (one sample per week) following the initial visit. The duration of 3 weeks was requested to minimize the influence of menstrual cyclicity because pubertal hormones cycle even in premenarcheal girls (Biro et al., 2014). The first saliva sample was collected within 1 week of the MRI scan. Adolescents expectorated a minimum of 1.8 ml of saliva via passive drool into a 2 ml vial using short straws. To prevent (blood) contamination of the saliva samples, participants were asked to avoid (1) brushing their teeth or eating a major meal for at least 1 h prior to collection, (2) eating anything acidic or high-sugar within 20 min. before collection, and (3) consuming something that stimulated the production of saliva (e.g., chewing gum). They were also asked to rinse their mouth with water about 10 min. prior to collection. Adolescents then completed a brief saliva diary. With supervision from parents, adolescents were instructed to store the samples in their home-freezer immediately upon collection and then to return the vials, on ice-pack to minimize freeze-thaw cycles, at the visit.
Saliva samples were stored in an ultra-cold −80°C freezer. Samples were batch shipped overnight on dry ice to the Stress Physiology Investigative Team laboratory, and remained frozen at −80°C until the day of assay. Commercially available and well-validated salivary enzyme-immunoassays kits (www.salimetrics.com) were used for each respective hormone. Samples were assayed in duplicate and values that disagreed by more than intra-assay coefficient of variation (CV)=7% were re-run. The averaged intra-assay CVs were: testosterone (M=2.54, SD=0.68), DHEA (M=2.37, SD=0.48), and estradiol (M=2.23, SD=0.64). All hormones for a participant were assayed on the same day to minimize freeze-thaw cycles, and all samples from a participant were assayed on the same plate to minimize variation in hormone concentrations across plates. The average inter-assay CVs across plates were 20.82 for testosterone, 25.24 for DHEA, and 18.80 for estradiol. As expected, the distributions of hormones were positively skewed, with skewness of 4.68 (SE=.13) in testosterone, of 2.60 (SE=.13) in DHEA, and of .88 (SE=.18) in estradiol. Therefore, hormones over 2.5 SD of the sample mean, separately for boys and girls (< 4%), were winsorized to 2.5 SD values, and then scores were log-transformed, as is common in prior literature (Dismukes et al., 2015). Few samples (5 for testosterone and 2 for DHEA) were below the level of detection for boys leading to the exclusion of 1 boy with low testosterone for all three samples; for girls, few samples (2 for DHEA and none for testosterone or estradiol) were below the level of detection of the assay and no girls were excluded for having low hormones for all three samples.
To derive a single basal score for each hormone, we extracted Empirical Bayesian (EB) estimation scores using Hierarchical Linear Modeling (HLM™ v.7, Scientific Software International, Inc.). Prior research (Shirtcliff and Ruttle, 2010) has demonstrated that use of EB estimates increases reliability of salivary hormones. The EB estimates are ideal for hormones because it “shrinks” the scores toward the individual (level 1) and population mean (level 2), thereby minimizing the influence of a particularly high or low sample without excluding the data. The intra-class correlation (ICC) was computed for boys and girls separately to describe fluctuation of hormone levels over the three weeks. For boys, the ICC for DHEA was 0.56 and for testosterone 0.71. For girls, the ICC for DHEA was 0.56, for testosterone 0.68, and for estradiol 0.46, suggesting moderate stability in each of the hormones across weeks.
2.4. Pre-Task Practice
Immediately prior to the scanning session, participants read instructions and completed a shortened version of the task in a mock scanner. The practice session also included training participants to map numbers 1–4 to corresponding buttons on the response glove as well as exposing participants to the sounds of the scanner and teaching them how to remain still during the scan.
2.5. fMRI Reward Cue Processing Task
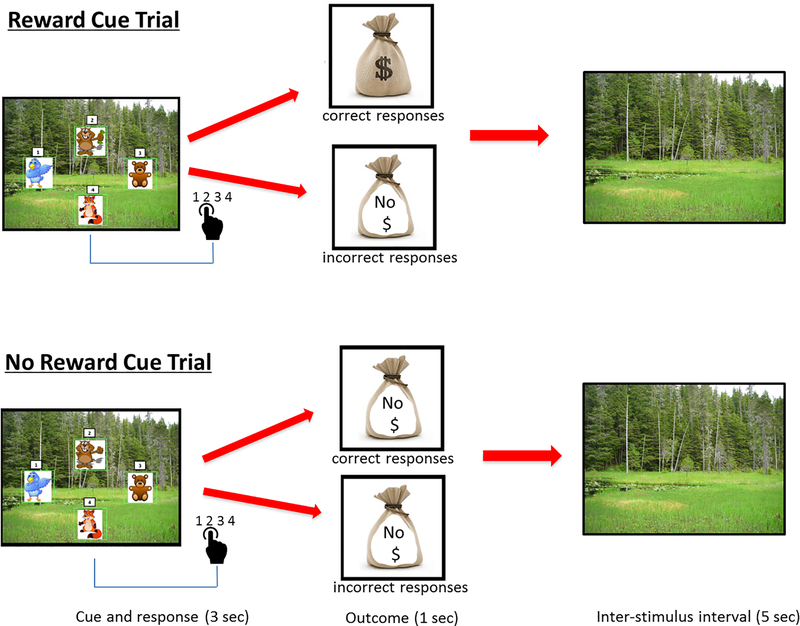
Participants underwent a 90-minute MRI scan at the Magnetic Resonance Research Center, University of Pittsburgh Medical Center Health System, USA. They completed a newly designed 9-minute reward cue processing task called the Gopher task (see Figure 1). The event-related task consisted of one run of 60 pseudo-randomized trials including 31 reward cue and 30 no reward cue trials. Each trial began with the presentation of a wooded scenery upon which was presented four white squares with a green frame. In each of the squares, which were positioned at the top, bottom, left and right of the scenery, one of four cartoon characters (a blue bird, a fox, a toy bear, or a gopher (i.e., burrowing rodent) were presented. The numbers 1 through 4, presented in smaller white squares above each of the larger white squares, were organized in clockwise sequence starting with the number 1 at the top of the white box on the left. The gopher served as the primary response target (i.e., cue for reward and no reward). The gopher appeared with his left paw raised in all trials. For 31 trials, the gopher was holding up green dollar bills (reward cue trials) and for 30 trials, the gopher was not holding dollar bills (no reward cue trials) (3 sec). Each reward cue slide was followed by a feedback slide (a brown money bag with “$” indicating earning $0.10 reward on correct reward cue trials or a money bag with “No $” on it indicating no reward) (1 sec). The feedback slide with a money ($) indicating a reward was presented on correct reward cue trials. Following the feedback slide was a slide with the wooded scenery that served as the interstimulus interval (5 sec). Because the duration of each trial was 9 s and our TR was 2 s, the task was not time-locked to acquisitions. Participants were told that that may receive money on reward cue trials but that they would never win money on the no reward cue trials. They received money to correct responses on reward cue trials. Participants were instructed to respond as quickly and as accurately as possible to the spatial location of the gopher by pressing the button on the response glove that corresponded to the number above the white box where the gopher appeared. The amount of money earned was presented at the end of the task and added to the participants’ “Wepay” cards at the end of the session. Participants were required to achieve a mean accuracy ≥70% across trial types in order to be included in the analyses. Analyses of the fMRI data focused primarily on correct trials in the reward cue vs. no reward cue conditions.
Figure 1.
Illustration of the Gopher Reward Processing task.
2.6. Behavioral Data Analysis
Mean percent accuracy scores and correct-trial reaction times were computed for each condition (Reward cue, No reward cue) for each participant. Data were analyzed using repeated measures ANOVA in SPSS 23.
2.7. Hormonal Analysis
Correlational analyses (Pearson r coefficient) were performed in boys and girls separately to assess the relationship between hormone levels and sex-specific composite z-scores pubertal maturation (adrenal, gonadal, total).
2.8. fMRI Data Acquisition and Analysis
Scanning was performed on a 3T Siemens Biograph mMR scanner at the Magnetic Resonance Research Center, University of Pittsburgh Medical Center, Pittsburgh, PA. Functional T2* weighted images were acquired using an echo-planar imaging sequence (TR:2000 ms; TE:26 ms; flip angle:90°; FOV:205 × 205 mm; acquisition matrix: 64 × 64; slice thickness:3.1 mm; number of slices:38 slices). Field map images were also acquired during the scanning session (TR:40 ms; TE:4.92 ms; TE2:7.38 ms; flip angle:35°; FOV:256 × 256mm; acquisition matrix:128 × 128; slice thickness:3.1 mm; number of slices:38). Both the functional T2* weighted and field mapping images were acquired as axial slices aligned with the Anterior Commissure-Posterior Commissure (AC-PC) line at midline. A high-resolution, T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) anatomical scan was acquired in the axial plane for each participant at the beginning of the functional scanning session (slice thickness-1mm, voxel size=1×1×1 mm3, 160 slices, TR=2300 ms, TE=2.47 ms, flip angle=9°, matrix=256×256, FOV=256 mm).
Image preprocessing was performed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, UK). These steps included correction for differences in acquisition time between slices, correction for motion (realignment using six-parameter rigid body) and unwarping using a field map, coregistration of the unwarped image to the participant’s structural image. Images were normalized using the ‘unified segmentation’ approach, including segmentation of the structural image and registration into MNI space, and then registration of functional images to MNI space was performed based on the parameters of the structural registration. Finally, normalized images were smoothed using a 6 mm FWHM Gaussian kernel.
First level analyses including model specification and estimation were performed using a general linear model (GLM) within SPM8. For first-level, a fixed-effects model was defined and trials with correct responses (incorrect trials were not modeled) on reward and no reward trials (Reward cue, No reward cue, Reward outcome, No reward outcome) and their corresponding onset times, were entered as multiple conditions in an event-related design matrix. Correct trials were modeled using the canonical hemodynamic response function. A high-pass filter of 128 s was used to remove low frequency drifts. To control for residual motion-related variance, motion regressors were included as covariates of no interest into the GLM.
For second-level analyses, a one-sample t-test was used to examine brain activation to the Reward cue>No reward cue. Given our a priori hypotheses and focus on the neural circuitry of reward cue processing in adolescence (Silverman et al., 2015), a regions-of-interest (ROIs) mask was created using the Automated Anatomic Labeling atlas of the WFU Pickatlas toolbox (Maldjian et al., 2003) that encompassed the striatum (including nucleus accumbens, caudate and putamen), medial PFC (including BA10, subgenual ACC, perigenual ACC and OFC), and the insula. Correction for multiple comparisons was implemented using a combined voxel-wise and cluster-size threshold. The statistical map was corrected for multiple comparison based on cluster extent (p<.001, family-wise α=.05). This was computed based on Monte Carlo simulation via the latest version of AFNI’s 3dClustSim tool (-acf methods; 10,000 iterations with first nearest neighbor clustering), which was developed to address concerns of inflated error rates (Cox et al., 2017; Eklund et al., 2016). These simulations resulted in a cluster size of 23 voxels in order to achieve a corrected alpha of .05. Finally, to extend these results, secondary ROI analyses were performed on Reward outcome>No reward outcome using the same correction method described above and a standard exploratory whole-brain analysis was performed with voxelwise threshold of p<.001 and k=50. Results from these additional analyses are included in Supplementary Material Table 3 and 4.
2.9. Functional Connectivity Analyses
To examine functional connectivity between the NAcc and target regions included in the ROI anatomical mask, we conducted a generalized psychophysiological interaction (gPPI) analysis (gPPI; http://www.nitrc.org/projects/gppi (McLaren et al., 2012). gPPI is a valuable method for examining how brain regions are functionally connected in a task-dependent manner (McLaren et al., 2012). Specifically, gPPI analyses reflect changes in a regression slope associated with the differential blood oxygenation level dependent (BOLD) response from one neural region (i.e. physiological response) under the influence of experimental contexts (i.e. psychological condition). For this analysis the NAcc was chosen as a seed region, given its role in reward cue processing in adolescence (Silverman et al., 2015). At the first-level, the psychophysiological (PPI) variable was computed for each participant by forming an interaction term between the vectors of each task condition (i.e., Reward cue, No reward cue) and the extracted, deconvolved BOLD signal from the NAcc. To be certain that the NAcc seed did not contain activation from surrounding regions, we drew a 6 mm sphere around the coordinates 12, 8, −6 (right hemisphere) −12, 8, −6 (left hemisphere) (Silverman et al., 2015). The mean time series across voxels within this seed region was extracted for each participant. Interaction terms were then entered into a new GLM with the original eigenvariate time-series, task onset vectors and six movement parameters which were regressors of no interest. The resultant gPPI interaction terms for each participant were positively weighted, given that our analysis focused on positive NAcc functional connectivity. The contrast images from the PPI analysis were then entered into second-level random-effects analyses in SPM8 to determine main effects of functional connectivity. As done in our analysis above, results of the single-subject analysis were entered into second-level one-sample t-test to examine changes in connectivity during reward cue vs. no reward cue conditions within our ROI mask. Results were thresholded at p<.001 with a FWE (p<.05) cluster-wise correction using AFNI’s –acf 3dClustSim. The estimated minimum cluster size for the gPPI analysis was 22 voxels.
2.10. Analyses with Pubertal Maturation
2.10.1. Pubertal Status
To determine if overall pubertal maturation was associated with brain activation and connectivity to reward cues in the entire sample, we used regression analysis with the standardized puberty composite score as an independent variable. Then, to examine if puberty associations were driven by the adrenal or gonadal axis within each sex, multiple linear regression models were performed in girls and boys separately with the standardized adrenal and gonadal composite scores, respectively, included as independent variables. For these analyses, we employed the same ROI mask as described above and results for each of the models were thresholded at p<.001 with a FWE (p<.05) cluster-wise correction using the same 3dClustSim procedures. For these analyses, the minimum cluster size was 22 for girls and 24 for boys.
2.10.2. Pubertal Hormones
To examine associations between brain activation and connectivity to reward cues with pubertal hormones, separate linear regression models for each of the pubertal hormones were computed in boys and girls separately. The model with estradiol was only computed in girls as salivary estradiol assays in boys are not reliable (Shirtcliff et al., 2000). Consistent with the analyses above, results for each of the models were thresholded at p<.001 with a FWE (p<.05) cluster-wise correction using the same 3dClustSim procedures. The minimum estimated cluster sizes were 22 for girls and 24 for boys.
3.0. RESULTS
3.1. Participant Characteristics
Table 1 shows characteristics of the participants with respect to age, Body Mass Index (BMI), pubertal status, and hormone levels. Age, but not BMI, was significantly correlated with measures of pubertal status as well as levels of testosterone and estradiol (see Supplementary Material Table 5).Thus, we controlled for age in the regression models with these pubertal measures.
3.2. Pubertal Data
Mean levels were similar to previously reported norms for adolescents (Shirtcliff et al., 2009). Correlational analyses indicated that, in boys, there were significant correlations between testosterone and gonadal and total composite measures. In girls, there were significant correlations between testosterone and adrenal, gonadal and total composite measures. There were also significant correlations between DHEA and all three composites measures (all ps <.05) (see Supplementary Material Table 6).
3.3. Behavioral Data
3.3.1. Accuracy
There was no significant condition (Reward cue, No reward cue) by sex interaction, F(1, 77)=.001, p=.99, or main effect of reward, F(1, 77)=.56, p=.46 (see Table 2). There were no significant correlations between accuracy and any measures of puberty or levels of pubertal hormones.
3.3.2. Reaction time
There was no significant condition by sex interaction, F(1, 77)=.38, p=.54. However, there was a significant main effect of condition, F(1, 77)=10.52, p=.002 such that participants responded faster on correct Reward cue trials compared to No reward cue trials (see Table 2). There were no significant correlations between reaction times and any measures of puberty or levels of pubertal hormones.
3.3. Neuroimaging Data: Neural Activation and NAcc Functional Connectivity to Reward Cues in Adolescents
3.3.1. Reward Cue Processing
Figure 3A highlights significantly greater activation to the Reward cue compared to the No reward cue in bilateral regions of the NAcc, putamen, and left insula (see Table 3). Figure 3B shows significant positive functional connectivity between the bilateral NAcc seed region and two clusters in the right and left regions of the rACC (see Table 3).
Figure 3.
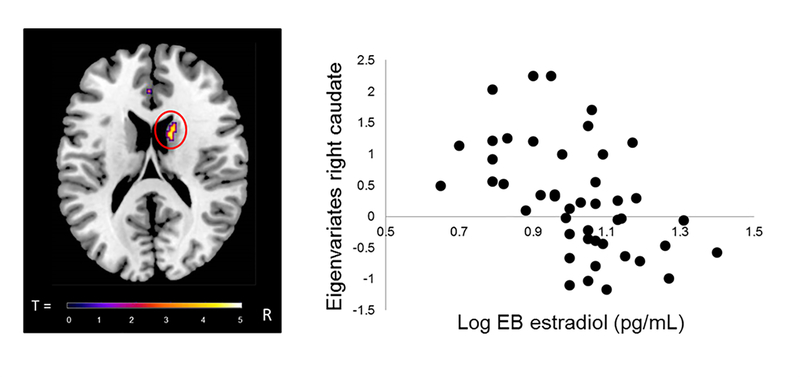
Results from regression analyses with estradiol in girls. A) Statistical map from regression analysis for Reward cue>No reward cue with levels of estradiol (log Empirical Bayes) in girls depicting a significant cluster in right caudate (peak coordinates x=16, y=4, z=20; T=4.14; k=34 voxels). Voxelwise threshold p<.001, with family-wise correction p<.05 and a cluster threshold of 23 voxels. B) Scatterplot illustrating negative association between girls’ level of estradiol (log Empirical Bayes) and neural activation in right caudate. EB = Empirical Bayes.
Table 3.
Regions-of-interest showing significant differences in neural activation and cortico-striatal functional connectivity to Reward cue>No reward cue.
| MNI | |||||||
|---|---|---|---|---|---|---|---|
| Coordinates |
|||||||
| Region-of-interest | K | X | y | z | T | Z | Voxel p |
| Neural Activation | |||||||
| Right nucleus accumbens | 130 | 10 | 14 | −2 | 4.80 | 4.48 | <.001 |
| Left insula | 172 | −32 | 0 | 12 | 4.39 | 4.13 | <.001 |
| Left putamen | 32 | −14 | 6 | −6 | 4.36 | 4.11 | <.001 |
| Right putamen | 32 | 26 | 10 | 4 | 4.11 | 3.90 | <.001 |
| 32 | 28 | −12 | 8 | 4.08 | 3.87 | <.001 | |
| Left nucleus accumbens | 53 | −6 | 6 | −2 | 3.94 | 3.75 | <.001 |
| Functional Connectivitya | |||||||
| Right rACC | 111 | 4 | 34 | −8 | 4.80 | 4.48 | <.001 |
| Left rACC | 71 | −4 | 30 | 0 | 4.11 | 3.90 | <.001 |
Note: k=number of voxels; rACC=rostral anterior cingulate cortex.
Nucleus accumbens served as the seed region: 6 mm sphere around the coordinates 12, 8, −6 (right hemisphere) −12, 8, −6 (left hemisphere) (Silverman et al., 2015).
3.3.2. Overall Effects of Pubertal Maturation
Regression analysis revealed no significant associations between overall pubertal maturation measures and neural activation or NAcc functional connectivity to Reward cue compared to No reward cue, in the entire sample, all p>.001, FWE corrected.
3.3.3. Sex-Specific Effects of Pubertal Maturation
3.3.3.1. Girls
Neural activation
Regression analyses, controlling for age, with pubertal hormones revealed a significant negative association between circulating levels of estradiol and brain activation to the Reward cue>No reward cue difference in the left (peak coordinates: x=−8, y=4, z=8; T=4.23; k=22 voxels) and right caudate (peak coordinates: x=16, y=4, z=20; T=4.14; k=34 voxels) (see Figure 3). Specifically, higher levels of estradiol were associated with reduced BOLD activation in these regions to reward cues. Linear regression analyses revealed no significant associations with any of the pubertal maturation composite z-scores, levels of DHEA or testosterone.
Functional connectivity
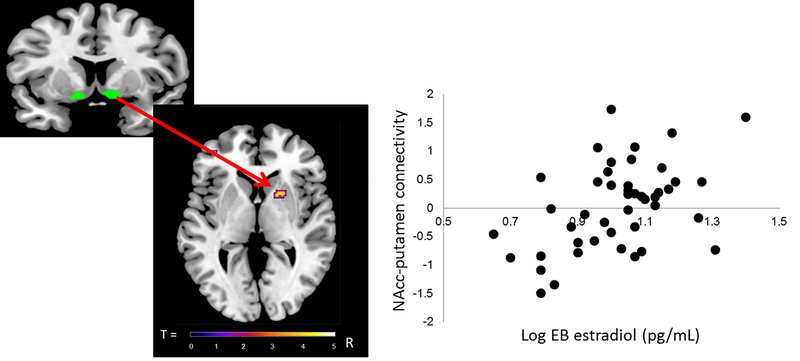
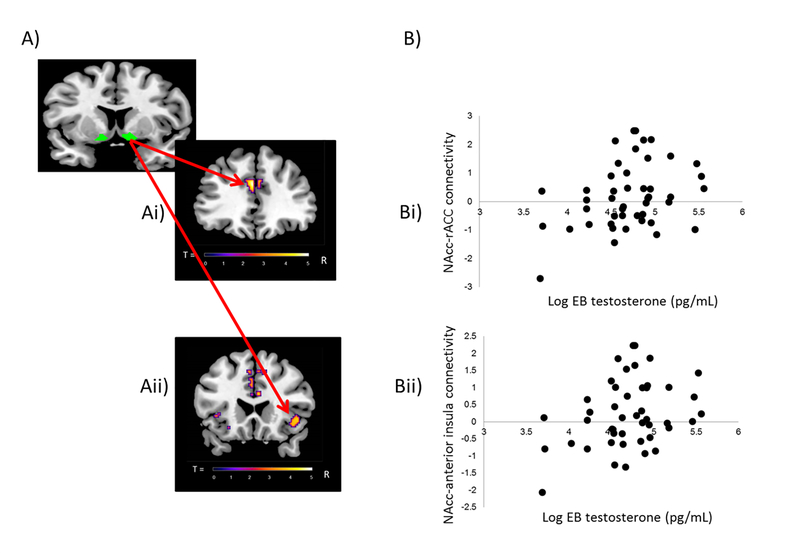
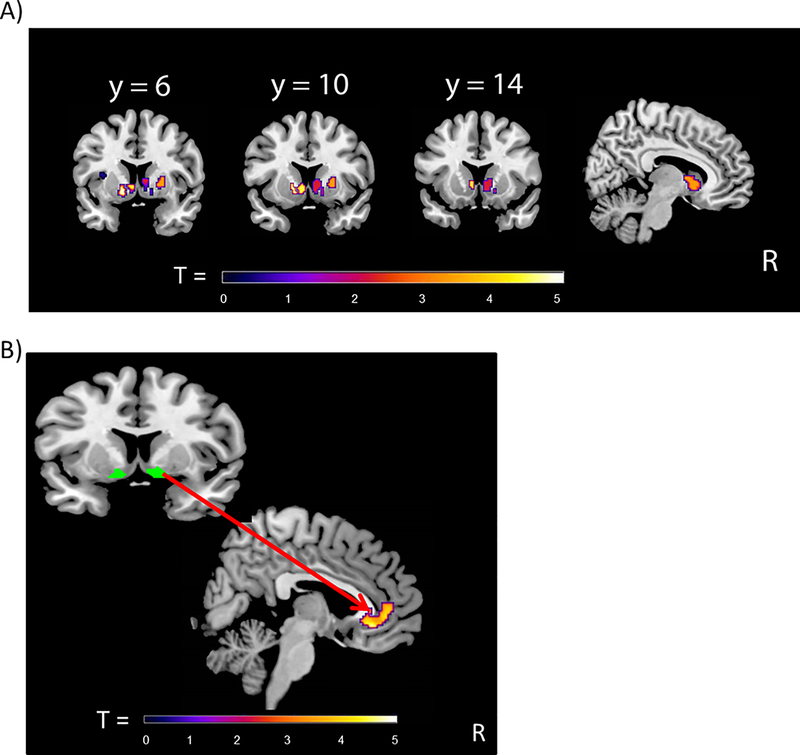
Regression analyses, controlling for age, with pubertal hormones revealed a significant positive association between circulating levels of estradiol and NAcc-putamen connectivity (peak coordinates: x=20, y=10, z=2; Z=3.66; k=36 voxels) in girls (see Figure 4). Also in girls, there was a significant positive association between circulating levels of testosterone and connectivity between the NAcc and clusters in the rACC (cluster 1: peak coordinates: x=−2, y=36, z=24; T=5.22; k=48 voxels; cluster 2: peak coordinates: x=6, y=40, z=20; T=4.29; k=35 voxels; cluster 3: x=−2, y=26, z=36; T=4.01; k=36 voxels) (see cluster 1 in Figure 5 Ai and Bi) and between the NAcc and the anterior insula (peak coodinates: x=44, y=22, z=−4; T=4.07; k=41 voxels) (Figure 5 Aii and Bii). Linear regression analyses revealed no significant associations with any of the pubertal maturation composite z-scores or levels of DHEA.
Figure 4.
Results from gPPI analyses with estradiol in girls. A) Depiction of functional connectivity to Reward cue>No reward cue between nucleus accumbens as a seed region [6 mm sphere around the coordinates x=12, y=8, z=−6 (right hemisphere); x= −12, y=8, z=−6 (left hemisphere) (Silverman et al., 2015)] and the right putamen (peak coordinates x=20, y=10, z=2; T=4.01; k=36 voxels. Voxelwise threshold p<.001, with family-wise correction p<.05 and a cluster threshold of 22 voxels. B) Scatterplot illustrating a positive association between girls’ level of estradiol (log Empirical Bayes) and gPPI eigenvariates extracted from the peak voxel of the cluster of the right putamen. gPPI = generalized psychophysiological interaction; EB = Empirical Bayes.
Figure 5.
Results from gPPI analyses with testosterone in girls. A) Depiction of functional connectivity to Reward cue>No reward cue between nucleus accumbens as a seed region [6 mm sphere around the coordinates x=12, y=8, z=−6 (right hemisphere); x= −12, y=8, z=−6 (left hemisphere) (Silverman et al., 2015)] and Ai) the left rACC (peak coordinates: x=−2, y=36, z=24; T=5.22; k=48 voxels) as well as Aii) the right anterior insula (peak coordinates: x=44, y=22, z=−4; T=4.07; k=41 voxels). Voxelwise threshold p<.001, with family-wise correction p<.05 and a cluster threshold of 22 voxels. B) Scatterplot illustrating a positive association between girls’ level of testosterone (log Empirical Bayes) and gPPI eigenvariates extracted from the peak voxel of the cluster of the Bi) left rostral anterior cingulate cortex and the Bii) right anterior insula. gPPI = generalized psychophysiological interaction; NAcc = nucleus accumbens; rACC = rostral anterior cingulate cortex; EB = Empirical Bayes.
3.3.3.2. Boys
Regression analyses revealed no significant associations between measures of pubertal maturation or levels of pubertal hormones and measures of neural activation or functional connectivity to Reward cue compared to No reward cue in boys, all p>.001, FWE corrected.
4.0. Discussion
Findings from this study indicate that task-related activation and functional connectivity successfully engaged the expected neural regions associated with processing reward cues (Haber and Knuston, 2010). Our hypotheses pertaining to pubertal maturation and pubertal hormones were only partially supported. Results indicate that girls with higher levels of estradiol show reduced striatal activation and greater connectivity within striatal regions when processing reward cues. In addition, girls with higher levels of testosterone show greater connectivity between the NAcc and the rACC as well as the anterior insula. Findings did not yield any significant associations in boys or in measures of pubertal maturation and DHEA across the sexes.
Of the studies that have examined puberty-related changes in reward circuitry in adolescence, few have examined hormonal influences on the functioning of neural regions supporting reward cue processing. Our findings of negative association between striatal activation to reward cues and levels of estradiol in girls are inconsistent with those from functional neuroimaging studies in adolescents performing guessing or risky decision making tasks (Forbes and Dahl, 2010; Op de Macks et al., 2016; Op de Macks et al., 2011). For instance, Op de Macks and colleagues (2011) reported a positive association between testosterone levels and ventral striatum response to reward vs. loss on a probabilistic decision making task (i.e., Jackpot Gambling task) in boys and girls but the reported positive relationship between striatal response and estradiol in girls did not reach statistical threshold. In another study using the same task, a positive association between estradiol levels and NAcc activation to Play>Pass trials was reported in girls (Op de Macks et al., 2016). Others studies reported no association between estradiol levels and reward processing (Alarcón et al., 2017; Forbes and Dahl, 2010). Certain factors need to be taken into consideration when interpreting the discrepancy in the relationship between estradiol levels and striatal activation in girls across studies. First, girls in the above studies were slightly older. For example, some samples were recruited between the ages of 12–17 (Alarcón et al., 2017), 10–16 (Op de Macks et al., 2011), and 11–13 (Forbes and Dahl, 2010; Op de Macks et al., 2016). Second, it is unclear to what extent the level of pubertal hormones assessed in those studies reflects maturational changes or individual differences in hormone levels, as few collected samples across the menstrual cycle. Third, the experimental paradigms differed considerably, with the current study focusing on reward cue processing and the other studies focusing on risky decision making that included different phases of reward processing (e.g., choice and outcome-related phases of reward processing) (Op de Macks et al., 2016; Op de Macks et al., 2011). Thus, it is possible that pubertal hormones may influence different phases of reward processing differently and that such influences may be sex-specific.
Negative associations between estradiol levels and reward sensitivity measures have been reported. For instance, higher levels of estradiol at mid-cycle in healthy women (18–40 years old) has been linked with reduced sensitivity to reward as assessed using a temporal discounting task (Smith et al., 2014). Although the mechanisms underlying the effects of estradiol on reward circuitry function remain unknown in humans, evidence from animal studies indicates that estrogens act directly in the mesolimbic reward circuitry and that higher levels of estradiol have a modulatory effect on dopaminergic systems (Sárvári et al., 2014). However, administration of microinjections of estradiol in the VTA in mice, which projects to the NAcc, was recently reported to be associated with reduced food reward behavior (Richard et al., 2017). Thus, it is possible that levels of estradiol could influence different phases of reward processing and/or interact with varying baseline levels of DA. For instance, opposite effects of estradiol levels on activation in prefrontal cortical regions supporting working memory were reported in healthy women as a function of variations in the gene for catechol-o-methyltransferase (COMT), the enzyme that metabolizes synaptic dopamine (Jacobs and D’Esposito, 2011). These findings suggest that the influence of estradiol on mPFC activation during working memory varies as a function of circulating dopamine levels. Taken together, our findings suggest that estradiol may not be behaving merely as an indicator of advanced maturation, but variations in levels of estradiol in early adolescence may be having a more direct modulatory role on the functioning of the reward circuitry.
With regard to functional connectivity, we focused analyses on the NAcc as a seed region given evidence demonstrating that the NAcc is implicated in processing of reward cues (Haber and Knuston, 2010) and was strongly activated by reward cues in our task. The NAcc receives glutamatergic inputs from the hippocampus, prefrontal cortex, and amygdala as well as GABAergic connections that are involved in action selection and coordination of motor outputs (Kelley, 2004). Findings from task-based functional connectivity studies indicate that regions of the prefrontal cortex become increasingly interconnected to striatal regions from childhood to adulthood (Somerville et al., 2011). Findings from the current study indicate that levels of estradiol and testosterone in girls, but not boys, modulated functional connectivity of the NAcc with some of the target regions implicated in reward cue processing. Specifically, higher levels of estradiol in girls were associated with positive NAcc-putamen connectivity. Given our findings of reduced striatal activation to reward cues as a function of higher levels of estradiol in girls, it is possible that NAcc-putamen positive connectivity reflects overall reduced response across these regions. In addition, higher levels of testosterone were associated with positive connectivity between the NAcc and regions of the ACC as well as the anterior insula. While few studies have examined the influence of testosterone on striatal activation to reward cues in girls, one study did not report any associations between striatal activation during reward anticipation and testosterone levels in girls but did report reduced striatal response to reward receipt with higher levels of testosterone (Forbes et al., 2010). Another study in 33 girls and 17 boys reported that greater striatal activation was associated with higher levels of testosterone when a risky decision was rewarded (Op de Macks et al., 2011). However, these studies did not include functional connectivity analyses. The anterior insula plays an important role in the detection of behaviorally relevant stimuli whereas the ACC plays an important role in modulating responses in the sensory, motor, and association cortices (Menon and Uddin, 2010) and both regions are considered to be part of the so-called salience network (Menon and Uddin, 2010; Seeley et al., 2007). Thus, it is possible that higher levels of testosterone in girls modulate connectivity within the saliency network when processing reward cues. More research is needed to deepen our understanding of the influence of pubertal maturation and pubertal hormones on reward circuitry neurodevelopment in adolescent girls.
Contrary to our hypotheses, results did not yield any association between levels of testosterone and neural activation during reward cue processing, especially in boys. Such results are inconsistent with evidence from previous neuroimaging studies demonstrating a link between circulating testosterone and VS response during reward processing (Braams et al., 2015; Op de Macks et al., 2011). In particular, longitudinal findings recently suggested a linear association between testosterone levels and NAcc activation to reward in 8 to 27-year olds assessed at two time points over a 2-year period (Braams et al., 2015). It is important to note, however, that the tasks used in the neuroimaging studies that reported such positive associations involved guessing or gambling (i.e., uncertain receipt of reward) (Braams et al., 2015; Forbes et al., 2010) and typically contrasted reward receipt versus loss (Braams et al., 2015; Op de Macks et al., 2011). Such neural activation to uncertain rewards could reflect reward prediction errors (Schultz, 2016), which signal the difference between expected and received reward and have shown in human neuroimaging studies to be associated with activity in cortico-striatal circuits, including the striatum, midbrain and prefrontal cortex (Garrison et al., 2013). Given that pubertal hormones may influence neural regions involved in action tendencies towards sensation seeking behavior (Crone and Dahl, 2012), it is possible that associations between measures of pubertal maturation or pubertal hormones might be more apparent when fMRI paradigms engage such motivationally salient stimuli and/or sensation-seeking behavioral tendencies that implicate a certain level of surprise or uncertainty. Research studies focusing on neural activation to reward cues assessed using versions of the Monetary Incentive Delay (MID), which focuses on reward cue processing, demonstrate that striatal activation is lower in adolescents compared to adults (Bjork et al., 2010) but that it tends to increase with age (Lamm et al., 2014). Thus, it is possible that striatal activation to reward cues may not be modulated as strongly by testosterone compared to tasks that engage risk taking action tendencies. Examining brain-behavior relationships using a longitudinal design with neuroimaging paradigms that assess different phases of reward processing are needed to better understand the developmental mechanisms underlying reward processing in adolescence.
The lack of association between neuroimaging measures and levels of DHEA is challenging to interpret given that few studies have examined the relationship between DHEA and reward processing. Although there is evidence suggesting that DHEA may play an important role in brain development, the few neuroimaging studies that examined the influence of DHEA on brain function focused on emotional information processing and varied in age range (Byrne et al., 2017; Whittle et al., 2015). Thus, further research is needed to elucidate how changes in DHEA levels may influence reward processing.
The findings of reduced striatal activation in girls as a function of levels of estradiol may have some implications regarding vulnerability pathways to depression. Numerous studies have documented blunted striatal activation to reward in adolescents with depression (Forbes, 2011). Blunted striatal activation to reward has also been reported in offspring at risk for depression (Olino et al., 2014). Given evidence that estradiol levels have been linked with reward circuitry functioning, it is possible that variation in estradiol levels in girls could shape the development of the reward circuitry in ways that may explain why girls may be more vulnerable to depression. Such an interpretation stems from evidence that girls more advanced in pubertal maturation than their peers are at heightened risk for depression (Angold et al., 1995). For instance, the Avon Longitudinal Study in the UK in typically developing adolescents demonstrated that 10-year-old girls with earlier breast development, which is related to a rise in levels of estrogens, report higher levels of depressive symptoms at age 14 (Joinson et al., 2012). It is possible that the observed findings in girls could represent a potential vulnerability marker for future onset of depression symptoms. However, the relationship between estradiol and depression may be mediated by other factors such as the role of estrogenic hormones in amplifying the stress response (Arnsten & Shanksky, 2004). Conversely, it is also possible that higher levels of estradiol in adolescent girls may represent vulnerability to risk taking that may be mediated by reduced striatal activation (Op de Macks et al., 2016). Future longitudinal research should evaluate whether these patterns of cortico-striatal functioning are associated with psychopathology in girls, particularly in those at high familial risk.
Findings from the current study must be considered in light of certain limitations. First, the moderate sample size may have limited our ability to detect variability across measures of pubertal maturation within each sex group. Second, the use of a cross-sectional design precludes drawing conclusions about the direction of the relationship between pubertal maturation, pubertal hormones, and reward circuitry functioning. Third, use of a categorial design precludes testing of possible interaction effects. Finally, the lack of measurement of estradiol levels in boys (due to previously observed poor correspondence between serum-saliva sample (Shirtcliff et al., 2000)) precludes concluding that the influence of estradiol on reward circuitry is specific to girls. Nevertheless, the current findings will serve to guide hypotheses for longitudinal studies examining how within-subject changes in pubertal maturation and levels of pubertal hormones in boys and girls are associated with changes in reward circuitry functioning and to what extent such changes may mediate increases in rates of psychopathology.
In conclusion, our findings indicate that compared to same-aged peers, young adolescent girls with higher levels of estradiol show patterns of reduced striatal activation and positive connectivity within striatal regions when processing reward cues. Such sex-specific patterns of neural response to reward cues could represent potential markers of vulnerability for future depression, given evidence of similar neural patterns in youth with or at-risk for depression. Girls with higher levels of testosterone also show patterns of greater connectivity between the NAcc and the ACC and the insula, which may be associated with modulation of the saliency network to reward cues. Examining brain-behavior relationships using a longitudinal design with neuroimaging paradigms that assess different phases of reward processing are needed to deepen our understanding of puberty-specific influences on reward circuitry functioning and identify potential developmental mechanisms underlying pathways of vulnerability or resilience in adolescence.
Supplementary Material
Figure 2.
Task-related neural activation and functional connectivity. Panel A highlights regional activation to Reward cue>No reward cue in bilateral regions of the nucleus accumbens (NAcc), putamen, and left insula. Voxelwise threshold p<.001, with family-wise correction p<.05 and a cluster threshold of 23 voxels. Panel B highlights functional connectivity to Reward cue>No Reward cue between the NAcc as a seed region [6 mm sphere around the coordinates 12, 8, −6 (right hemisphere) −12, 8, −6 (left hemisphere) (Silverman et al., 2015)] and two regions in rostral anterior cingulate cortex (rACC) [peak coordinates: Left rACC (x, y, z: −4, 30, 0) and Right rACC (x, y, z: 4, 34, −8)]. Voxelwise threshold p<.001, with family-wise correction p<.05 and a cluster threshold of 22 voxels. Statistical maps are overlaid on the skull stripped Colin brain (ch2better.nii). R=right.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health (R01 MH099007, PI: Ladouceur) and the National Institutes of Health (UL1TR001857). The authors would also like to thank Monique Ernst, PhD for her insightful comments as well as the adolescents and their families for participating in this research project.
References
- Alarcón G, Cservenka A, Nagel BJ, 2017. Adolescent neural response to reward is related to participant sex and task motivation. Brain and cognition 111, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM, 1995. Pubertal changes in hormone levels and depression in girls. Psychological Medicine 29, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G & Montague PR, 2001. Predictability modulates human brain response to reward. Journal of Neuroscience 21, 2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW, 2009. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current opinion in pharmacology 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, Dorn LD, 2014. Hormone Changes in Peripubertal Girls. The Journal of Clinical Endocrinology and Metabolism 99, 3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW, 2010. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE [Electronic Resource] 5, e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA, 2015. Longitudinal Changes in Adolescent Risk-Taking: A Comprehensive Study of Neural Responses to Rewards, Pubertal Development, and Risk-Taking Behavior. The Journal of Neuroscience 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB, 2017. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci 25, 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connectivity 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE, 2012. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience 13, 636–650. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, Suleiman AB, 2018. Importance of investing in adolescence from a developmental science perspective. Nature 554, 441–450. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O, 2011. The orbitofrontal cortex and its role in the assignment of behavioural significance. Neuropsychologia 49, 984–991. [DOI] [PubMed] [Google Scholar]
- Dismukes AR, Shirtcliff EA, Hanson JL, Pollak SD, 2015. Context influences the interplay of endocrine axes across the day. Developmental Psychobiology 57, 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F, 2006. Defining boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science 10, 30–56. [Google Scholar]
- Dorn LD, Susman EJ, 2004. Puberty Script: Assessment of Physical Development in Boys and Girls, Cincinatti, OH. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academic of Sciences USA 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, 2011. fMRI Studies of Reward Processing in Adolescent Depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 372–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, 2010. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and Cognition 72, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarriallo SR, Dahl RE, 2010. Healthy adolescents’ neural responses to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academic Child Adolescent Psychiatry 49, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, 2013. Sensitivity to reward in adolescence. Current directions in Psychological Science 22, 100–105. [Google Scholar]
- Garrison J, Erdeniz B, Done J, 2013. Prediction error in reinforcement learning: A meta-analysis of neuroimaging studies. Neuroscience & Biobehavioral Reviews 37, 1297–1310. [DOI] [PubMed] [Google Scholar]
- Goddings A-L, 2015. The Role of Puberty in Human Adolescent Brain Development, in: Bourguignon JP, Carel JC, Christen Y (Eds.), Brain Crosstalk in Puberty and Adolescence. Research and Perspectives in Endocrine Interactions. Springer, Switzerland. [Google Scholar]
- Haber SN, Knuston B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J, 1999. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews 31, 6–41. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M, 2011. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joinson C, Heron J, Araya R, Paus T, Croudace T, Rubin M, Marcus M, Lewis G, 2012. Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychological Medicine 12, 1–11. [DOI] [PubMed] [Google Scholar]
- Kelley AE, 2004. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & Biobehavioral Reviews 27, 765–776. [DOI] [PubMed] [Google Scholar]
- Koolschijn PCMP, Peper JS, Crone EA, 2014. The Influence of Sex Steroids on Structural Brain Maturation in Adolescence. PLoS ONE 9, e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Benson BE, Guyer AE, Perez-Edgar K, Fox NA, Pine DS, Ernst M, 2014. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain and cognition 89, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J, 2011. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews 35, 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Kraft R, Burdette J, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood 44, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1970. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnarney ER, Kreipe RE, Orr DP, G.D. C, 1992. Textbook of adolescent medicine. W.B. Saunders Company, Philadelphia. [Google Scholar]
- McCutcheon JE, Marinelli M, 2009. Age matters. The European journal of neuroscience 29, 997–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC, 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain structure & function 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Kessel EM, Jackson F, Hajcak G, 2016. The impact of an unpredictable context and intolerance of uncertainty on the electrocortical response to monetary gains and losses. Cognitive, Affective, & Behavioral Neuroscience 16, 153–163. [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Williamson DE, Dahl RE, Ryan ND, Forbes EE, 2014. Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental cognitive neuroscience 8, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, Dahl RE, 2016. Risky decision-making in adolescent girls: The role of pubertal hormones and reward circuitry. Psychoneuroendocrinology 74, 77–91. [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Moor BG, Overgaauw S, Guroglu B, Dahl RE, Crone EA, 2011. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience 1, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, López-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP, 2017. Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78, 193–202. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B, 2007. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience 10, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárvári M, Deli L, Kocsis P, Márk L, Maász G, Hrabovszky E, Kalló I, Gajári D, Vastagh C, Sümegi B, Tihanyi K, Liposits Z, 2014. Estradiol and isotype-selective estrogen receptor agonists modulate the mesocortical dopaminergic system in gonadectomized female rats. Brain Research 1583, 1–11. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2016. Dopamine reward prediction-error signalling: a two-component response. Nature reviews. Neuroscience 17, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL, 2016. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience and biobehavioral reviews 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of Neuroscience 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME, 2000. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: correspondence between hormonal and physical development. Child Abuse and Neglect 80, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran M, Booth A, Overman W, 2000. Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocol, reliability, and comparative validity. Hormones and Behavior 38, 137–147. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Ruttle PL, 2010. Immunological and neuroendocrine dysregulation following early deprivation and stress, in: Brisch H (Ed.), Attachment and Early Disorders of Development. Klett-Cotta & Stuttgart, Munich. [Google Scholar]
- Silverman MH, Jedd K, Luciana M, 2015. Neural Networks Involved in Adolescent Reward Processing: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies. NeuroImage 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA, 2014. Ovarian Cycle Effects on Immediate Reward Selection Bias in Humans: A Role for Estradiol. The Journal of Neuroscience 34, 5468–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ, 2011. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of cognitive neuroscience 23, 2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanovic E, Gee DG, Joormann J, 2018. Intolerance of uncertainty: Neural and psychophysiological correlates of the perception of uncertainty as threatening. Clinical Psychology Review 60, 87–99. [DOI] [PubMed] [Google Scholar]
- Teslovich T, Mulder M, Franklin NT, Ruberry EJ, Millner A, Somerville LH, Simen P, Durston S, Casey BJ, 2014. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Developmental Science 17, 59–70. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Cohen BM, Kahnt T, Crone EA, 2012. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex 22, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Op de Macks ZA, Overgaauw S, Gunther Moor B, Dahl RE, Crone EA, 2014. A cross-sectional and longitudinal analysis of reward-related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain and Cognition 89, 3–14. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M, 2010. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews 34, 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Byrne ML, Strikwerda-Brown C, Kerestes R, Seal ML, Olsson CA, Dudgeon P, Mundy LK, Patton GC, Allen NB, 2015. Associations between early adrenarche, affective brain function and mental health in children. Social Cognitive and Affective Neuroscience 10, 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.