Abstract
The objective of this study is to understand the effect of sustained release of vitamin C from β-tricalcium phosphate (β-TCP) scaffold on proliferation, viability and differentiation of human fetal osteoblast cells (hFOB). The influence of pH, drug concentration, and presence of polymer on the sustained release of vitamin C from polycaprolactone (PCL) coated β-TCP scaffolds are studied. Prolonged and sustained release of vitamin C, over 60 days is observed in PCL coated β-TCP scaffolds compared to uncoated scaffolds. Presence of PCL helps to minimize the burst release of vitamin C from β-TCP scaffolds in the initial 24 hours of release. To evaluate the osteogenic potential of vitamin C incorporated β-TCP scaffolds, osteoblast cells are cultured and cell morphology, proliferation, viability, and differentiation are assessed. Morphological characterization shows layer like osteoblast cell attachment in the presence of vitamin C compared to the control. MTT cell viability assay shows 2 folds increase in osteoblast cell density in the presence of vitamin C after 3,7 and 11 days of culture. Furthermore, increased ALP activity at 11 days of culture indicates the possible role of vitamin C on osteoblast differentiation. Additionally, a preliminary study shows vitamin C loaded scaffolds suppress osteosarcoma (MG-63) cell proliferation to 4 folds after 3 days compared to control. These results show a sustained release of vitamin C from PCL coated β-TCP scaffolds improve proliferation, viability, and differentiation of osteoblasts cell as well as mitigate osteosarcoma cell proliferation, suggesting its potential application as synthetic bone graft substitutes in tissue engineering application.
Keywords: In vitro Vitamin C release, Tricalcium phosphate (TCP), Bone tissue engineering scaffold, Osteoblast cell culture, Osteosarcoma cell culture
Graphical Abstract
1. Introduction
With increasing human lifespan, osteoporosis and bone fractures from related bone diseases have recently become a major clinical problem. In every three seconds, an osteoporotic fracture is reported resulting in more than 8.9 million fractures annually worldwide [1–2]. The term, osteoporosis is defined by reduced bone mass and microarchitectural degradation of the skeletal structure that leads to fragility fractures [3]. Multiple pathogenic factors such as post-menopausal estrogen deficiency, insufficient calcium content, and aging of the skeletal system are the most significant and relevant causes for the development of osteoporosis [4].
Calcium phosphate (CaP) biomaterials are the most extensively studied ceramics utilized in bone tissue engineering as synthetic grafts for bone repair, substitution or augmentation. Owing to their outstanding biocompatibility, good osteoconductive properties as well as the ability to form a strong bond between tissue-material interfaces, they are often used as drug carriers for bone tissue [5–6]. β-TCP, with its good resorption rate in physiological fluids, is widely used for a non-load bearing application, where the scaffold degrades over time and newly formed tissue replaces. The unique characteristics of β-TCP scaffolds make it suitable as a carrier to deliver biomolecules such as proteins / growth factors namely transforming growth factor beta (TGF-β), bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF), as well as drugs such as bisphosphonates and statins, which promote osteogenesis and bone tissue regeneration [7–8]. The main concern in the drug delivery system is to maintain a controlled and desired drug concentration at a minimum therapeutic level for a sustained period of time without reaching the toxic limit [8]. To control the release kinetics and suppress the burst release of biomolecules from the scaffolds, various polymers such as polycaprolactone (PCL), polylactide-co-glycolide (PLGA), chitosan, and collagen type I have been introduced [9]. PCL is an FDA approved, bioresorbable, biocompatible, and non-toxic polymer with a potential application in bone and cartilage repair [10–11]. The ester linkage present in PCL is readily hydrolysable, resulting in biodegradation of the whole moiety. However, the generated byproducts are either metabolized or eliminated by the body making it an excellent non-toxic and biocompatible polymer for numerous medical and drug delivery system. Various in vitro and in vivo studies have been carried out showing its potential and efficacy towards biomaterials and tissue engineering field [12–14].
Various micronutrients such as calcium, vitamin D, etc. are crucial for maintaining bone health as well as prevent osteoporosis [15–16]. Earlier studies have shown that vitamin D deficiency is the principal cause of bone loss among postmenopausal women in the northeastern United States [17]. Vitamin C or l-ascorbic acid is essential for collagen synthesis and normal bone development [18]. Studies have shown that vitamin C reduces the oxidative stress caused by reactive oxygen species, which leads to the process of bone resorption [19–20]. Additionally, vitamin C plays a major role during the formation of hydroxyproline residues, which stabilize the triple helix structure of collagen. It is worthwhile to mention that around 90–95% of bone is composed of collagen. Therefore, deficiency of vitamin C impedes collagen synthesis, which in turn leads to the inhibition of new bone formation [21]. Previous studies demonstrate that vitamin C also inhibited the differentiation of osteoclast precursor cells into mature osteoclasts by down-regulating RANKL expression [22]. Osteoclasts are multinucleated bone cells, which breaks down bone tissue at a molecular level, a process known as bone resorption. Receptor activator of nuclear factor-κB (RANK) binds to its ligand and thereby regulates the main pathway, which controls differentiation and proliferation of osteoclasts cells. Therefore, prevention of in vitro bone resorption by utilizing RANKL inhibitor has become an effective approach for the treatment of osteoporosis and other bone degenerative disorders. Additionally, various in vitro and in vivo reports demonstrated the inhibitory effect of vitamin C on various types of cancers, including lung cancer, pancreatic cancer, oral cancer, cervical cancer, and esophageal cancer [23–25]. The chemopreventive activity of vitamin C, although well-debated, is attributed to the antioxidant activity and ROS-mediated tumor cell damage.
In this study, β-TCP scaffolds are prepared by utilizing solid-state synthesis method. Vitamin C is loaded on scaffolds at two different concentrations, followed by PCL coating. In vitro release of vitamin C is investigated in both pH 7.4 phosphate buffer saline (PBS) and pH 5.0 acetic buffer solutions to mimic the acidic microenvironment after the surgery. On the contrary to the other reports, where vitamin C has been added directly to the cell culture medium, here, the gradual release of the biomolecule from TCP ceramics is studied. The interaction of vitamin C in cell media with MC3T3-E1 cells is previously studied [26]. Many reports also showed the effects of dietary or combined dietary and supplemental vitamin C intake on bone mineral density and prevalence of bone fractures [27–28]. However, a direct correlation of vitamin C with the bone-forming cells, i.e. osteoblasts has not been studied in detail so far. The objective of this study is to understand the effect of a biodegradable polymer like PCL on the release kinetics of vitamin C and its subsequent effect on morphology, viability, and differentiation of osteoblast and osteosarcoma cells. Cellular morphology, cell proliferation, and differentiation are studied as a function of vitamin C concentration, presence of PCL coating, and culture time. Our working hypothesis is that drug-polymer interaction and pH of the media may influence the release kinetics of vitamin C. A burst and uncontrolled release pattern may be exhibited for those scaffolds not coated with PCL, whereas a more controlled and sustained release pattern may be observed in the PCL coated scaffolds. Moreover, we postulate that a controlled release of vitamin C from TCP scaffolds may enhance osteoblast cell proliferation and prevents osteosarcoma cell proliferation.
2. Materials & Methods
2.1. TCP Sample Preparation
The solid-state synthesis method is utilized to prepare TCP scaffolds. In short, one mole of calcium carbonate and two moles of calcium phosphate dibasic are mixed by ball milling using a powder: milling media ratio of 1:4 for 2 hours. Calcination is carried out at 1050 °C. The powder is then crushed and ethanol is added to it. After 6 hours of milling, the powder is dried at 70 °C and pressed into discs with 12 mm diameter and 2.5 mm height by pressing uniaxially at 145 MPa.
2.2. In Vitro Vitamin C Loading and Release Study
Firstly, vitamin C (Sigma Aldrich, MO) is dissolved in DI water at two different concentrations of 4 and 8 mg/ml. Then, 50 μl of drug solution is loaded on both sides of TCP scaffolds, so that each sample contains either 400 or 800 μg of vitamin C. From now on, scaffolds loaded with 400 and 800 μg of vitamin C will be mentioned in this paper as 400C-TCP and 800C-TCP, respectively. After drug loading, the scaffolds are kept in the dark at room temperature for drying. 5% PCL (Mw=14000, Sigma Aldrich, MO) solution is prepared by dissolving in acetone. 50 μl of PCL solution is added dropwise to both sides of the scaffolds.
To study the in vitro release kinetics, a phosphate buffer and an acetate buffer with pH values of 7.4 and 5.0, respectively, are prepared. Three scaffolds from each group of composition (pure TCP, 400C-TCP and 800C-TCP) with and without PCL are kept in separate vials containing 4 ml of phosphate or acetate buffer. Vials are kept under continuous shaking at 150 rpm at 37 °C. Release media are collected after 1.5, 3, 6, 12, 24, 48, 96, and 144 h, and replaced with fresh buffer solution. Finally, 200 μl of release media from each sample are pipetted to a 96-well plate. To determine the concentration of the released drug, the absorbance of all the scaffolds are measured at 260 nm wavelength using the UV–Vis spectroscopy (BioTek).
2.3. In Vitro Cell-Material Interaction
2.3.1. Osteoblast Cell Culture
For cell culture study, all TCP scaffolds are sterilized in an autoclave at 121°C for an hour, prior to drug and polymer loading. Scaffolds are then moved to the biosafety cabinet. Vitamin C, suitable for cell culture (Sigma Aldrich, MO), is dissolved in filtered sterilized DI water and loaded in such a way that each sample contains either 2 or 5 μg of the drug. After drying, the PCL-coated scaffolds are prepared as explained before. The scaffolds for in vitro cell culture study are prepared in a sterilized condition to prevent any possible contamination during culture. The scaffolds are not exposed to any radiation such as UV, γ, or heat sterilization such as autoclaving after drug loading or polymer coating processes to prevent decomposition of biomolecules.
Human fetal osteoblast cells (hFOB 1.19, ATCC, Manassas, VA) are then seeded to scaffolds at a density of 35×103 cells/sample. The basal medium is prepared by dissolving 1:1 mixture of Ham’s F12 medium and Dulbecco’s Modified Eagle’s Medium (DMEM/F12, Sigma, St. Louis, MO), with 2.5 mM L-glutamine (without phenol red) in filtered sterilized DI water. The medium is then supplemented with 10% fetal bovine serum (FBS, ATCC, Manassas, VA) and 0.3 mg/mL G418 (Sigma, St. Louis, MO). Cultures are kept in an incubator at 34 °C temperature under an atmosphere of 5% CO2 and the cell medium is changed every 2–3 days during the entire experiment.
2.3.2. Osteosarcoma cell culture
All scaffolds are sterilized by autoclaving at 121 °C for 60 min. Human osteosarcoma cell line MG 63 (ATCC, USA) is used for this culture. Scaffolds are kept in 24 well plates and cells are seeded onto the scaffolds at a density of 4×105 cells/mL. Eagles minimum essential medium (EMEM) (ATCC, USA) is used to maintain the culture for the entire study. Cultures are kept in an incubator at 37°C under an atmosphere of 5% CO2 as recommended by ATCC for this particular cell line. The cell culture medium is changed every 2 days during the entire cell culture study.
2.3.3. Cellular Morphology
After 3, 7, and 11 days, scaffolds are collected from culture to evaluate the osteoblast cellular morphology, attachment, and proliferation. Scaffolds are fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer overnight at 4 °C. All scaffolds are then rinsed with 0.1 M phosphate buffer, followed by post-fixation with 2% osmium tetroxide (OsO4) for 2 h at room temperature. Scaffolds are rinsed again with 0.1 M phosphate buffer followed by dehydration in ethanol series (30, 50, 70, 95, and 100% three times). Hexamethyldisilane (HDMS) is used for the final drying step. A gold sputter coater is used to apply a layer of coating with a thickness of 10–15 nm. The morphology of scaffolds is then studied using FESEM.
2.3.4. Proliferation and Differentiation of Osteoblast Cells
The osteoblast cell viability is measured using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay after 3, 7, and 11 days of culture. Scaffolds are moved to a new 24-well plate and 100 μL of MTT (Sigma, St. Louis, MO) solution is added on top of each sample, followed by the addition of 900 μL of cell media. After 2 h of incubation at 34 °C, the solution is removed from each well and 600 μL of solubilizer solution (composed of 10 % Triton X-100, 0.1N HCl and isopropanol) is added to each sample to dissolve formazan crystals. Formazan is a purple-colored compound produced after reduction of MTT by cellular enzymes. Now, 100 μL of this solution is transferred to the 96-well plate and read by UV–Vis spectroscopy (BioTek) at 570 nm.
To evaluate the differentiation of osteoblast cells, ALP assay kit (Abcam, Cambridge, MA) is used according to the manufacturer’s protocol. Cells are detached from the surfaces of scaffolds using trypsin. After incubating for 3 min, the trypsin/cell media mixture is centrifuged for 5 min. Then, the supernatant is discarded and 400 μl of assay buffer is added to each sample, followed by centrifuging at 13,000 rpm for 3 min to remove any insoluble material. 80 μl of the solution is added to 96-well plate followed by addition of 50 μl of the 5 mM p-nitrophenyl phosphate (pNPP) to each well. Thereafter, it is incubated for 60 min at 25 °C. Finally, 20 μl of stop solution is added and the optical density is measured at 405 nm using a microplate reader.
2.4. Statistical Analysis
Vitamin C loading, PCL coating, in vitro release, MTT, and ALP assays are analyzed in triplicate of scaffolds. Measurements of each are also performed in three biological replicates. Data are presented as mean ± standard deviation. Student’s t-test is used to perform statistical analyses and P values < 0.05 are considered significant.
3. Results
3.1. Microstructure of TCP and PCL coating morphology
Figure 2 shows the morphological characterization of bare TCP scaffolds and TCP scaffolds with 5% PCL coating before vitamin C release study. The scaffolds have 32.1 and 24.8% of porosity and the pore size ranges from 5–10 μm and 3–7 μm for both uncoated and coated scaffolds respectively. Bare TCP sample shows characteristics of porous surface morphology. Addition of 5% PCL as a coating on bare TCP scaffolds blocked some pores. A flaky uneven surface can be observed due to the presence of PCL. The surface porosity in bare TCP sample results in initial burst release and an overall higher release of vitamin C.
Figure 2.
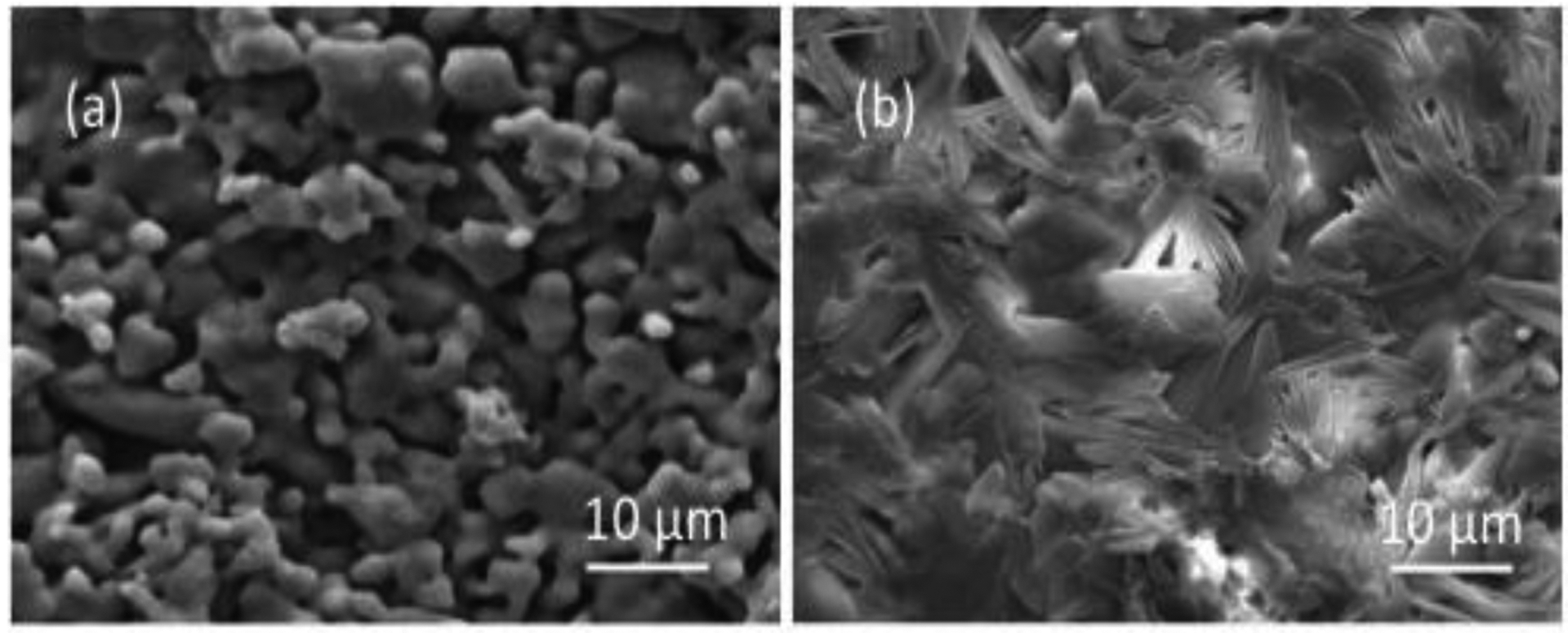
Microstructures of (a) bare TCP scaffolds and (b) TCP scaffolds with 5% PCL before vitamin C release.
3.2. In Vitro Vitamin C Release
Figure 3 depicts the cumulative release of vitamin C from TCP scaffolds with and without PCL coating in pH 7.4, phosphate buffer. A burst release of vitamin C for both coated and uncoated scaffolds are observed from TCP scaffolds within first 1.5 h, leading to a more slow and controlled release in later time points. For uncoated scaffolds, the cumulative release of vitamin C is 6.87 and 8.18 μg for 400C-TCP and 800C-TCP, whereas for PCL-coated scaffolds, the released vitamin C amount is 2.81 and 4.22 μg for 400C-TCP+PCL and 800C-TCP+PCL, respectively after 1.5 h. After 60 days, the total vitamin C released accumulates to 9.51 and 22.54 μg for 400C-TCP and 800C-TCP scaffolds and 6.18 and 14.01 μg for 400C-TCP+PCL and 800C-TCP+PCL, respectively.
Figure 3.
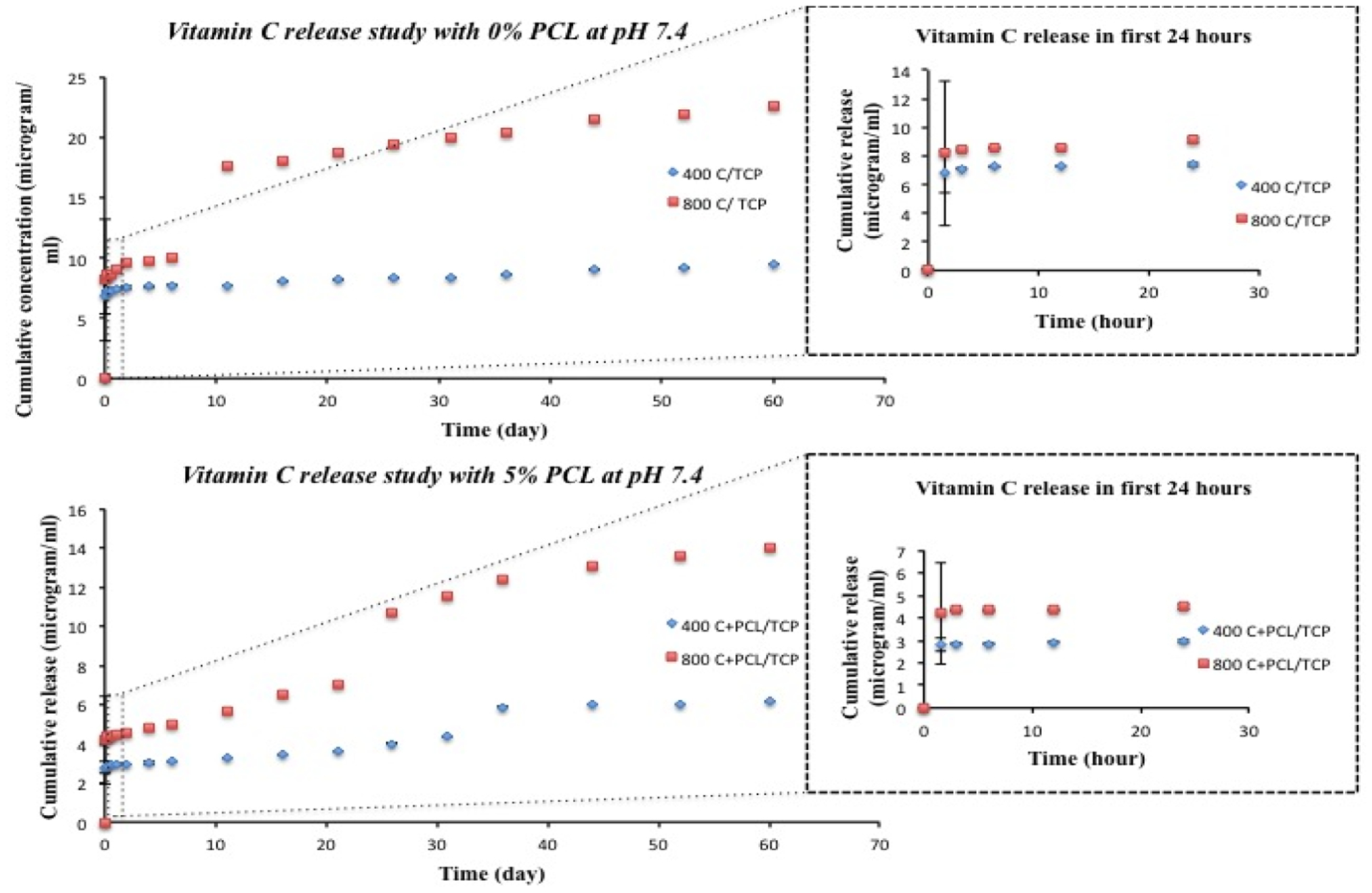
Cumulative release of vitamin C with respect to time (release study continued up to 60 days) from bare TCP scaffolds and 5% PCL coated TCP scaffolds at pH 7.4 (inset shows initial 24 h cumulative release of vitamin C with respect to time (hour)). Presence of PCL shows more control over vitamin C release kinetics and showed more sustained release compared to scaffolds without polymer.
Figure 4 represents the in vitro release kinetics of vitamin C from uncoated and coated scaffolds at pH 5.0, acetate buffer. Similar to pH 7.4, more sustained and controlled release of vitamin C can be seen in the case of PCL coated scaffolds than uncoated scaffolds. Both coated and uncoated scaffolds show a burst release of vitamin C in the first 1.5 h. PCL coated scaffolds show significantly less burst release than uncoated scaffolds. The initial release of 3.11 and 4.49 μg is found for 400C-TCP+PCL and 800C-TCP+PCL scaffolds, respectively after 1.5 h. For uncoated scaffolds, the initial release amount of vitamin C reaches to 4.42 and 14.40 μg, for 400C-TCP and 800C-TCP scaffolds, respectively. For scaffolds without PCL coating, the cumulative drug release after 60 days increases up to 33.43 μg and 50.12 μg for 400C-TCP and 800C-TCP, respectively. In the case of PCL coated scaffolds, total 26 and 31 μg of vitamin C has been released for 400C-TCP+PCL and 800C-TCP+PCL, respectively.
Figure 4.
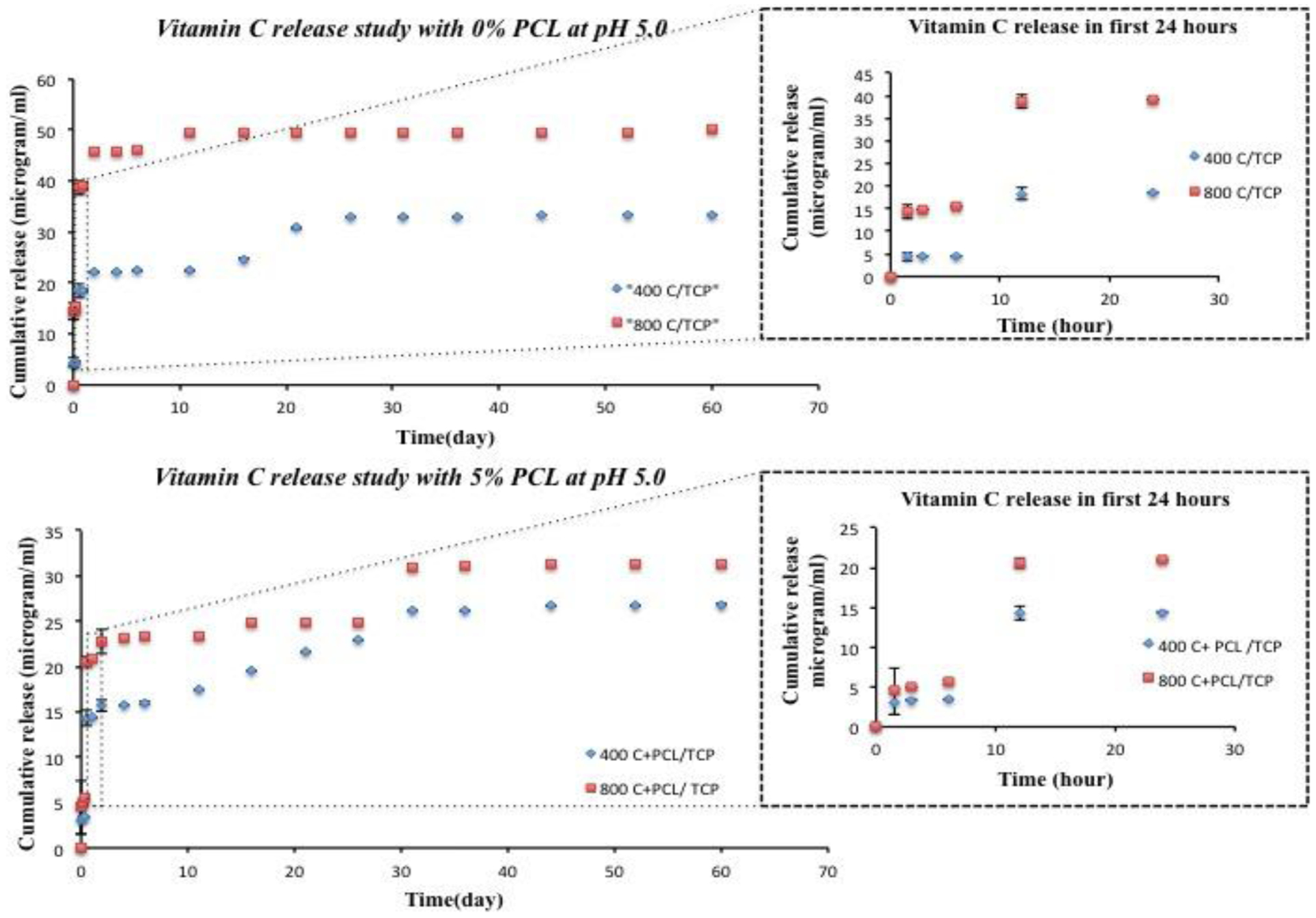
Cumulative release of vitamin C with respect to time (release study continued up to 60 days) from bare TCP scaffolds and 5% PCL coated TCP scaffolds at pH 5.0 (inset shows initial 24 h cumulative release of vitamin C with respect to time (hour)). The result shows the presence of PCL controlled the release of vitamin C and showed more sustained release compared to scaffolds without polymer.
Figure 5 shows the surface morphologies of TCP scaffolds after 60 days of vitamin C release at pH 7.4. No detectable morphological difference is observed in the scaffolds, which attributes to the low solubility of TCP and limited degradation of PCL at pH 7.4.
Figure 5.
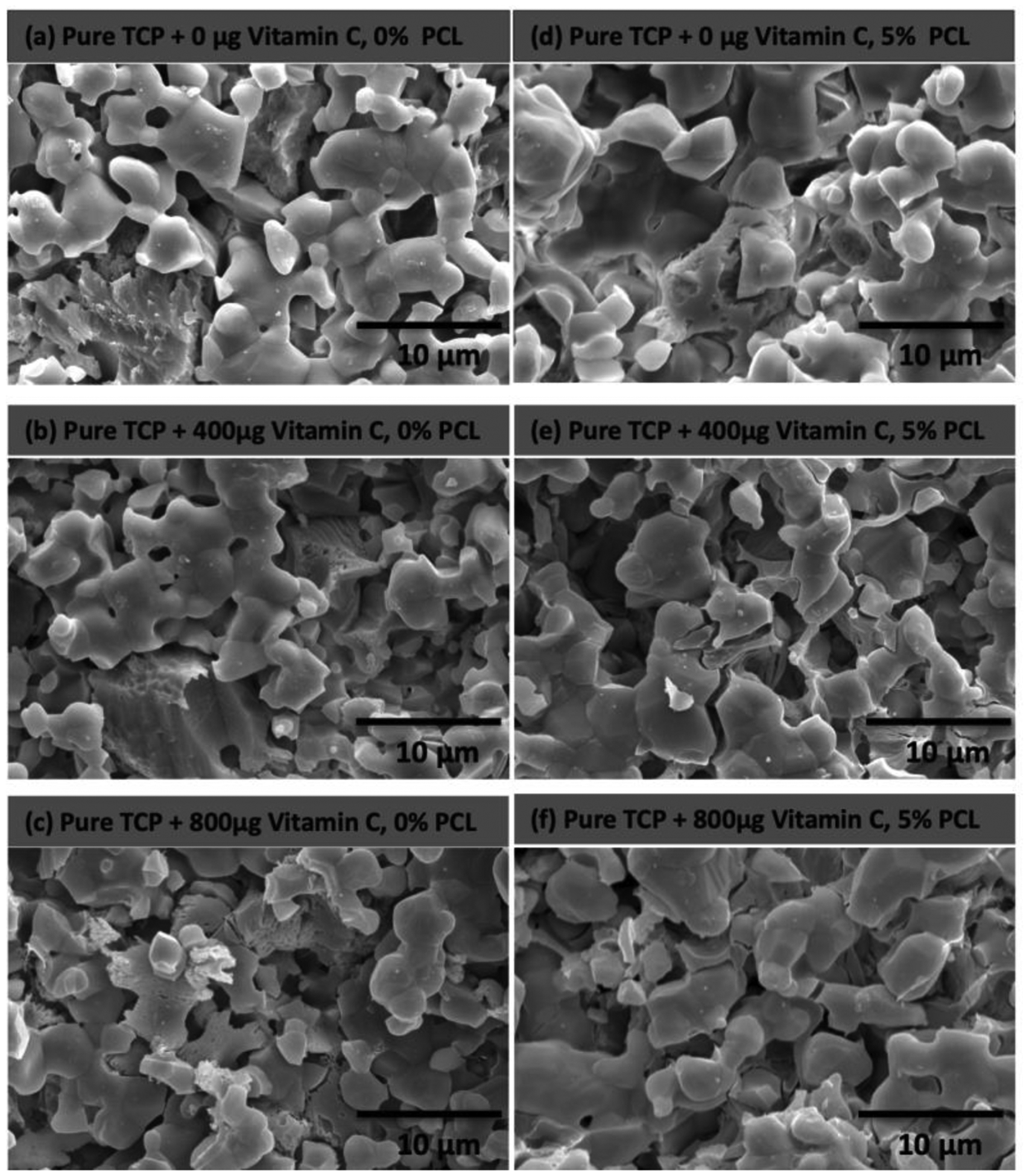
Microstructures of bare TCP scaffolds and 5% PCL coated TCP scaffolds after 60 days of vitamin C release at pH 7.4 showing very little surface degradation of the sample resulting in a slower release of vitamin C compared to scaffolds in pH 5.0
Figure 6 represents the morphological characterization of TCP scaffolds after 60 days of vitamin C release at pH 5.0. More rough, porous and non-homogeneous surface characteristics can be observed in uncoated and coated scaffolds at pH 5.0. The high solubility of TCP in acidic pH results in a higher amount of drug release, which is in accordance with the release kinetics in figure 4. Porous nature of PCL coating can be observed in figure 6 (d), (e) and (f), which can be attributed to the chemical degradation of PCL caused by acidic pH of the release media. Rough or uneven surface morphology is due to degradation of the PCL coating followed by rapid dissolution of TCP.
Figure 6.
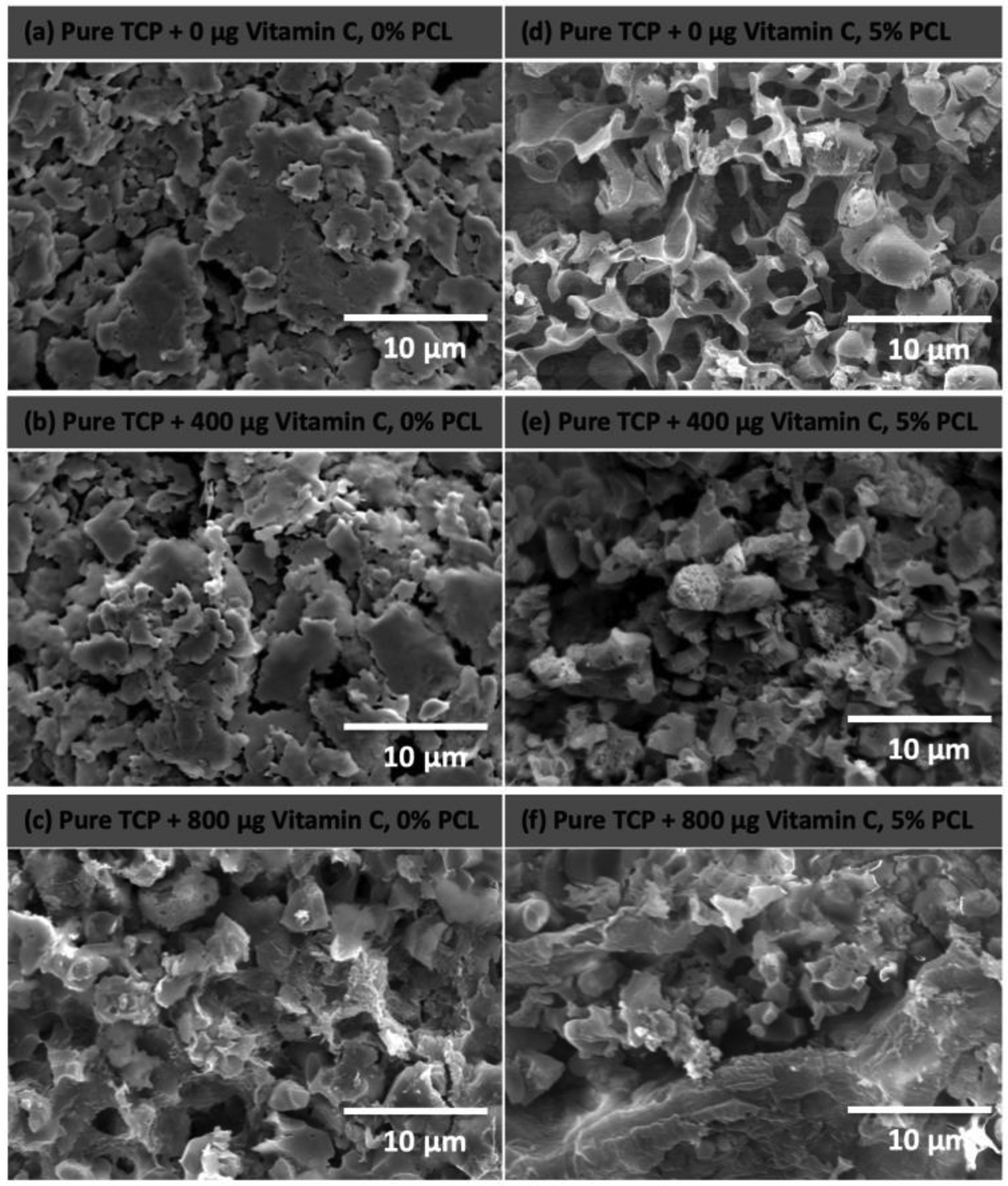
Microstructures of bare TCP scaffolds and 5% PCL coated TCP scaffolds after 60 days of vitamin C release at pH 5.0 shows higher surface degradation of the scaffolds resulting in a higher release of vitamin C compared to scaffolds at pH 7.4
3.2. Vitamin C Interaction with Osteoblast and Osteosarcoma Cells in vitro
To understand the effects of vitamin C on in vitro bone formation, the interaction between the osteoblast and vitamin C loaded TCP scaffolds in the absence and presence of PCL is studied. The morphology of the osteoblast cells after 3 days of culture is analyzed and osteoblast cell-material interaction is reported in figure 7. The FESEM images show firm attachment and higher proliferation of osteoblast cells on the surface of vitamin C-loaded scaffolds, compared to the control-TCP scaffolds. Similarly, an abundant number of cells can be seen on the surface of all PCL-coated vitamin C loaded scaffolds. At 7 days of culture, more cellular proliferation is found on all scaffolds compared to day 3, as shown in figure 8. Presence of PCL shows a multilayer-like osteoblast growth and attachment on the sample surface. Figure 9 represents similar morphology of cells on scaffolds after day 11. However, the crystal formation is found in all compositions, which is a key feature of calcium-deficient apatite formation. Comparing different compositions, the apatite formation is more abundant on control and PCL coated scaffolds.
Figure 7.
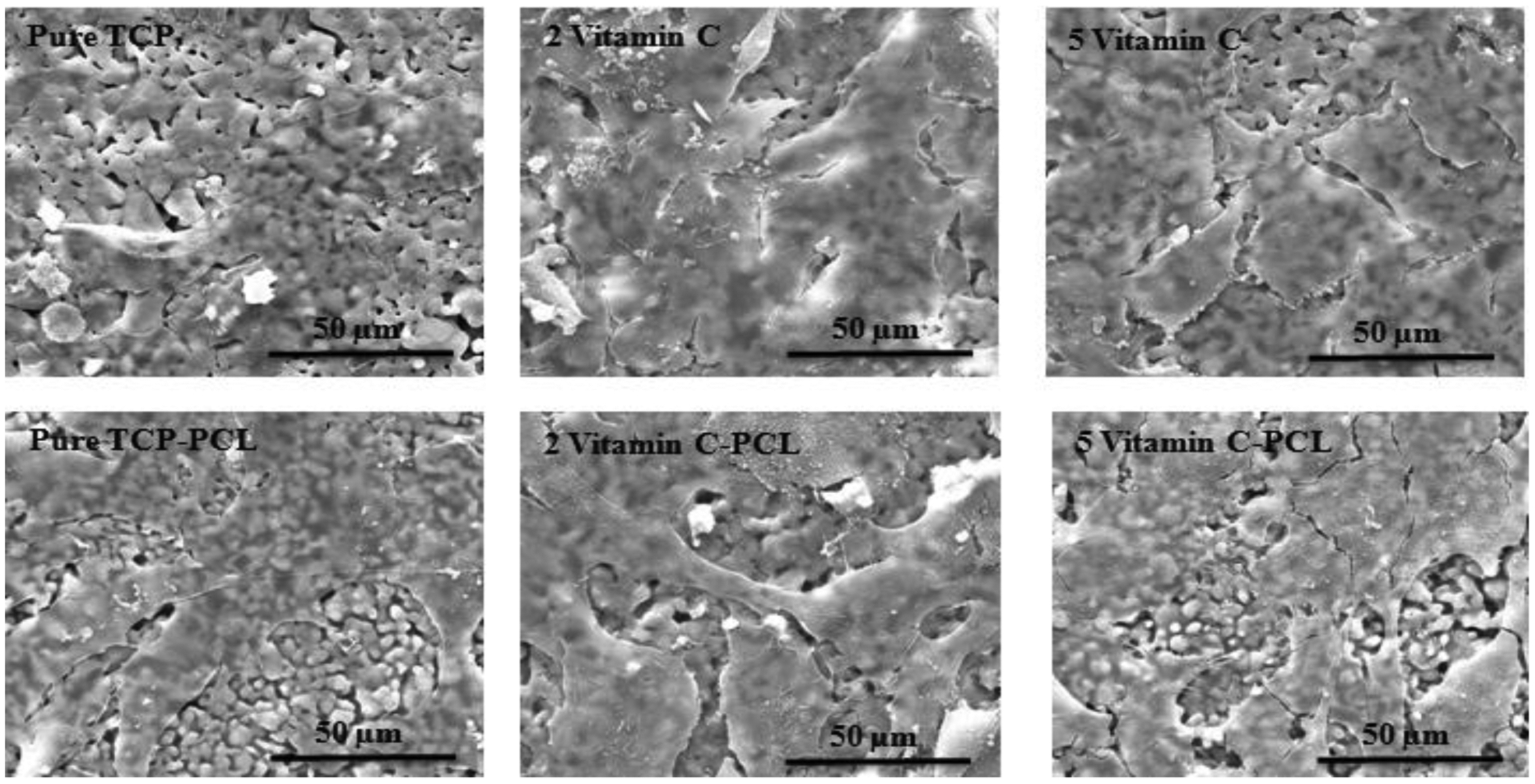
Osteoblast cellular morphology on scaffolds after 3 days of culture showing increased cell proliferation in scaffolds loaded with vitamin C compared to control.
Figure 8.

Osteoblast cellular morphology on scaffolds after 7 days of culture showing a clear distinction between vitamin C scaffolds with the control. Layers of osteoblast cells can be seen in scaffolds containing vitamin C compared to control.
Figure 9.
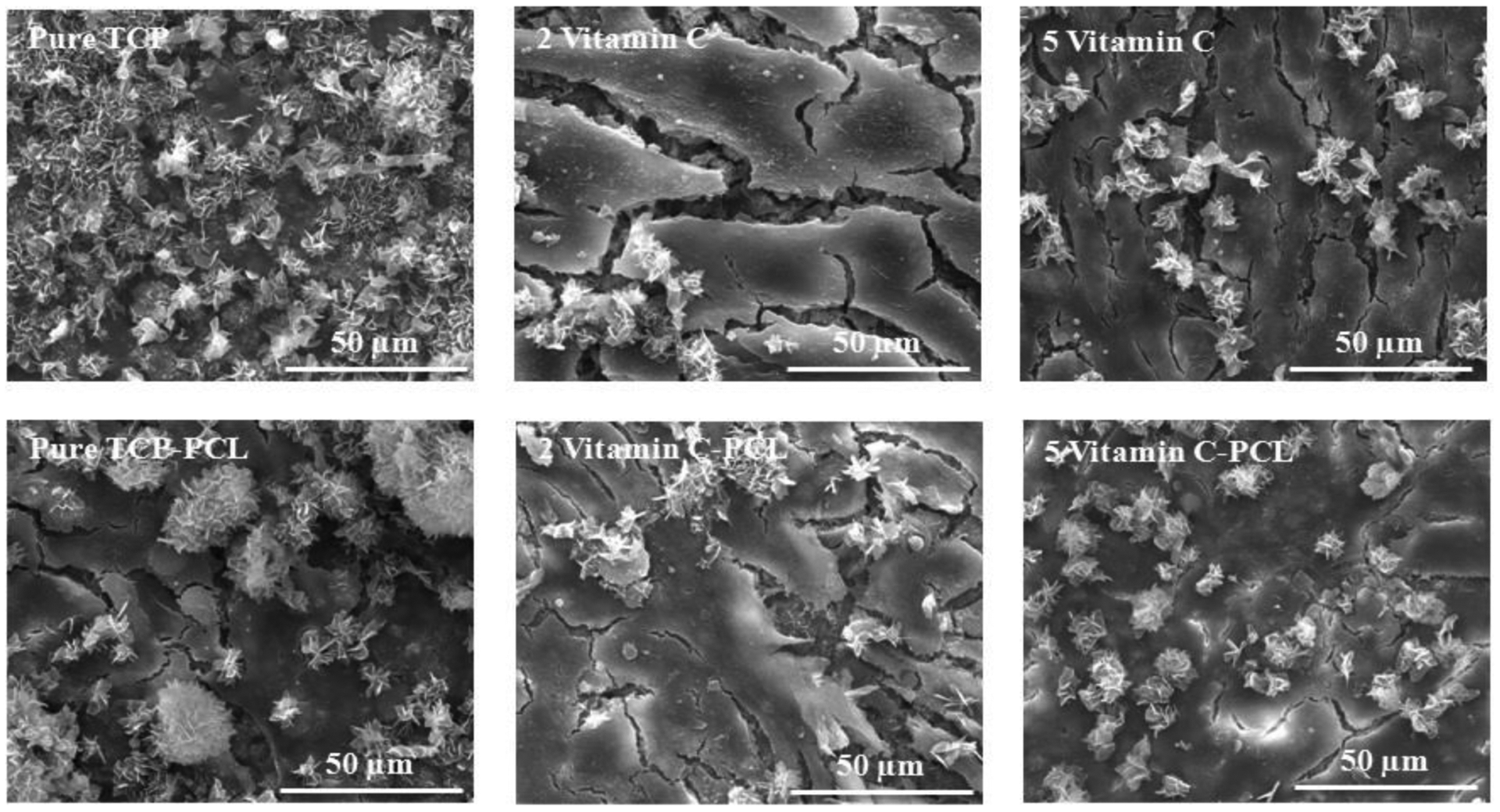
Osteoblast cellular morphology on scaffolds after 11 days of culture shows apatite formation.
MTT assay is performed to assess the cell viability at different time points, as shown in figure 10. After 3 days, the optical cell density is significantly higher on all scaffolds loaded with vitamin C compared to the control TCP and PCL coated TCP, which shows the efficacy of vitamin C on attachment and proliferation of osteoblast cells. After 7 days of culture, cell density increases for all scaffolds with the presence of vitamin C. These data are in agreement with the SEM images where the surfaces of scaffolds with vitamin C and PCL are covered with multiple layers of osteoblast cells. After 11 days of culture, cellular density is even more increased on vitamin C loaded scaffolds compared to day 7.
Figure 10.
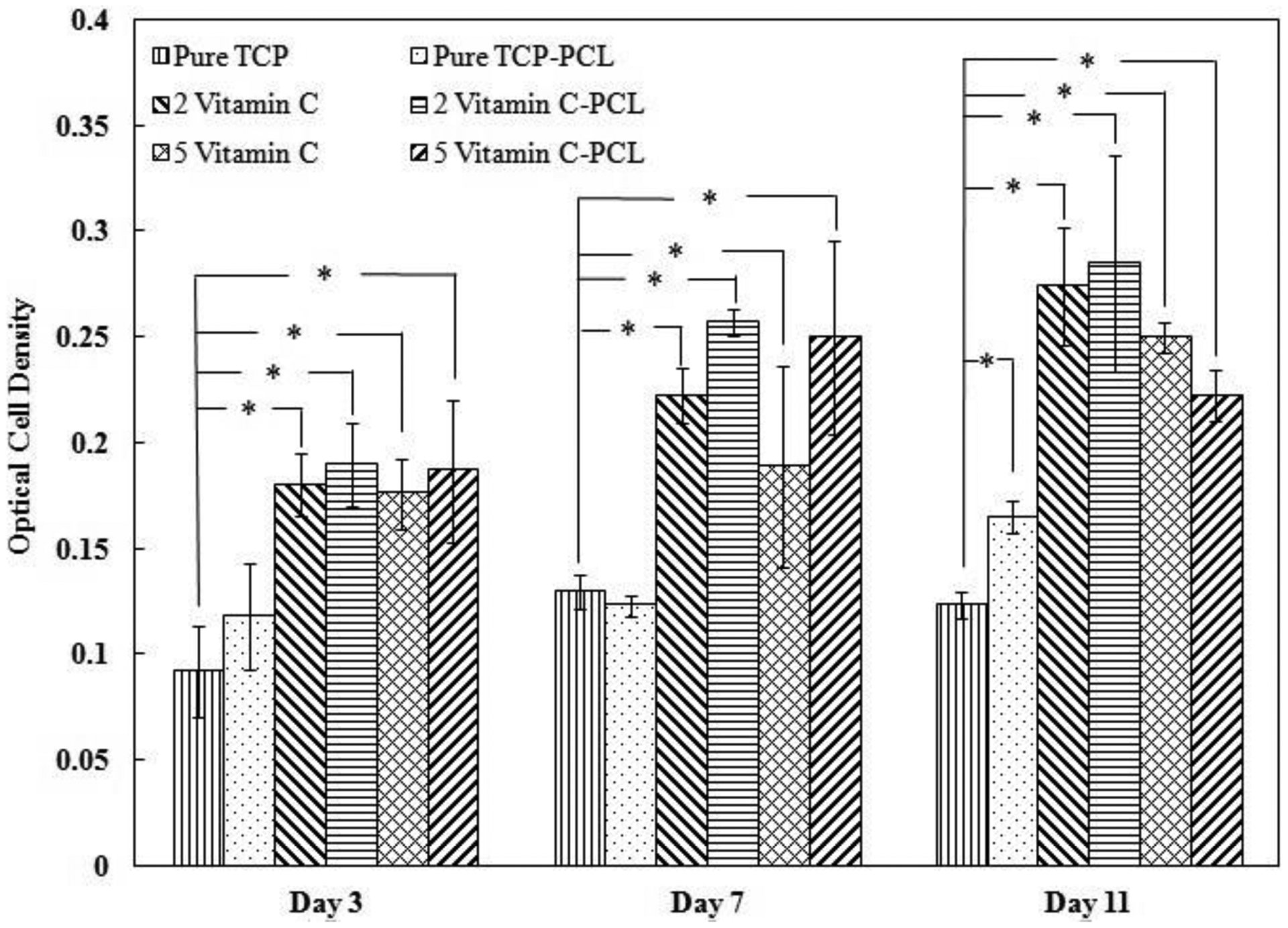
Osteoblast cell viability on control and vitamin C loaded scaffolds after 3, 7, and 11 days of culture showing a pronounced increase in osteoblast cell proliferation in the presence of vitamin C compared to control. Data are presented as mean ± standard deviation (* P < 0.05).
ALP assay is used to evaluate the differentiation of osteoblast cells and results are presented in Figure 11. No statistically significant difference in osteoblast differentiation is observed after 7 days of culture, indicating the time point being too early to initiate the differentiation process. After 11 days, cellular differentiation is significantly higher on TCP scaffolds loaded with 2 μg of vitamin C.
Figure 11.
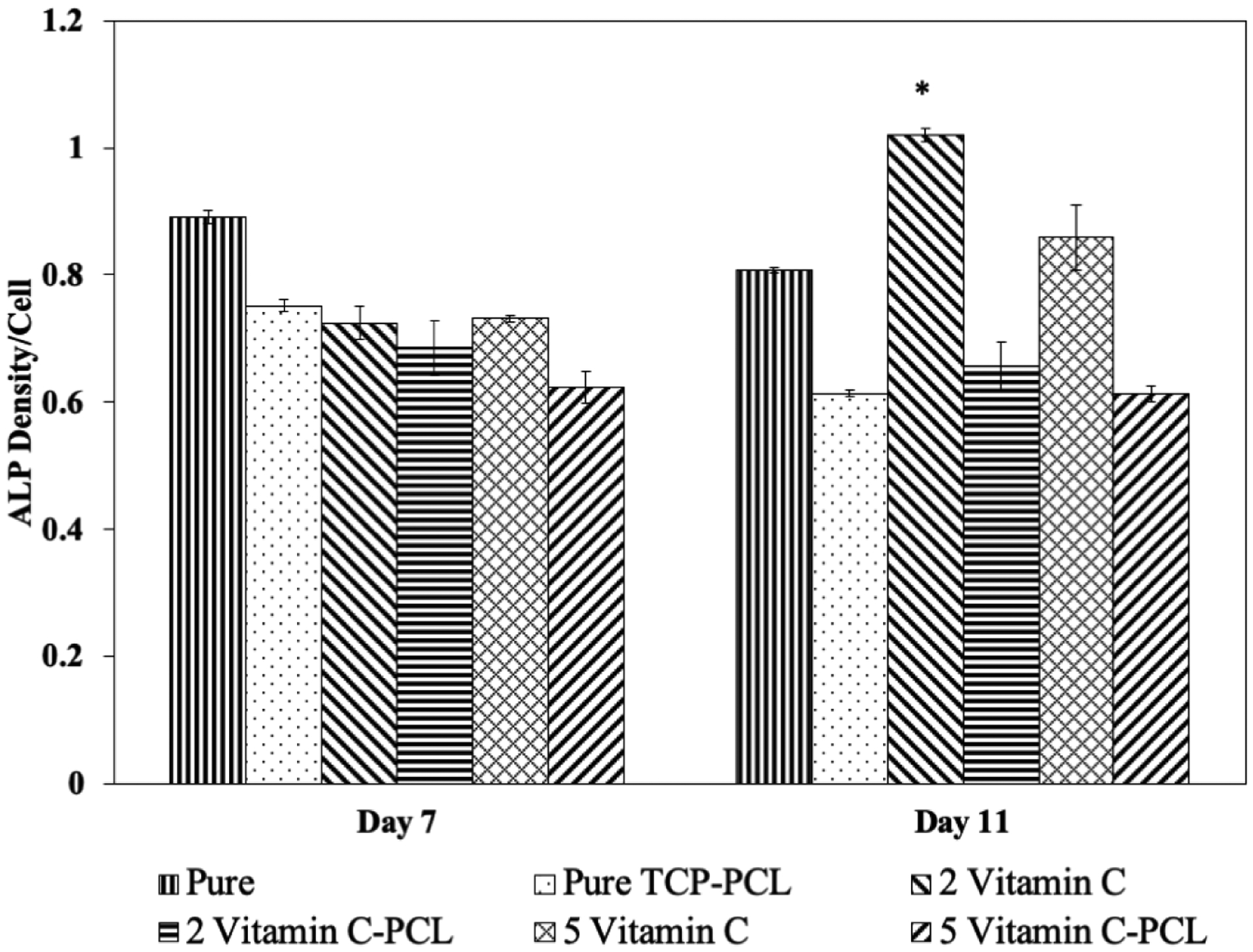
ALP activity on control and vitamin C loaded TCP scaffolds after 7, and 11 days of culture showing increased osteoblast cell differentiation in the presence of 2 μg of vitamin C at day 11 compared to the control scaffolds. Data are presented as mean ± standard deviation (* P < 0.05).
Fig 12 shows scanning electron microscopic images of day 3 osteosarcoma (MG 63) cell culture on control and 5 μg vitamin C loaded on TCP scaffolds. We can see layers of osteosarcoma cells in the sample without vitamin C. A significant change in osteosarcoma cell proliferation is shown in scaffolds loaded with vitamin C, supporting our hypothesis. ImageJ is utilized to analyze the SEM images quantitatively. It is found that the presence of vitamin C suppresses osteosarcoma cell proliferation up to 4 folds compared to the control scaffolds.
Figure 12.
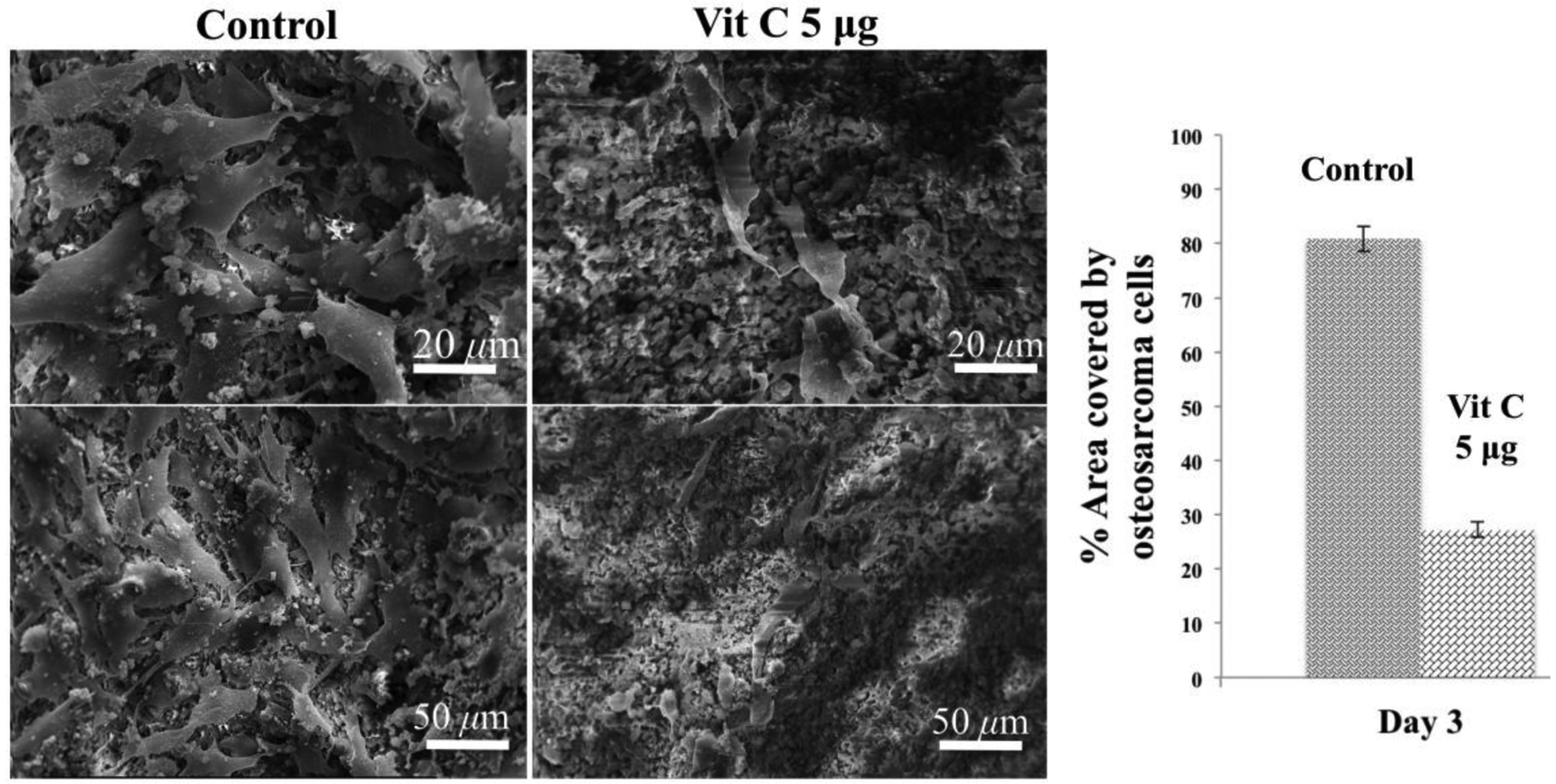
Osteosarcoma cell proliferation on control and vitamin C loaded scaffolds after 3 days of culture showing distinctively lower osteosarcoma cell proliferation in the presence of vitamin C compared to control. Quantitative analysis of the SEM images by imageJ clearly shows the presence of vitamin C has a pronounced effect on the inhibition of osteosarcoma cell proliferation.
4. Discussion
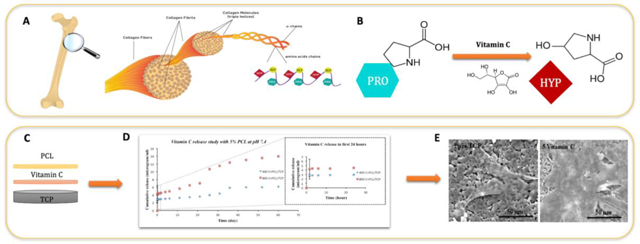
Localized delivery of osteogenic biomolecules has been extensively utilized for the treatment of osteoporotic fracture since it can stimulate rapid bone formation and significantly reduce the bone healing period [29]. Whereas systemic treatment of osteoporosis acts slowly and causes adverse effects, localized drug delivery exhibits enhanced fracture repair by providing high drug concentration at the surgery site for a longer period of time without systemic side effects [30]. Understanding the release behavior of biomolecules depends on the chemical structure of the biomolecule, presence of coating, the interaction between the biomolecule and substrate, and the pH of release media. In our study, a strong influence of pH can be observed on the vitamin C release kinetics. It has been reported that an increase in the release of vitamin C in the acidic condition is due to the fact that vitamin C exhibits maximum stability within pH 4–6 [31]. At neutral pH, vitamin C is present in the form of ascorbate monoanion, along with a low concentration of ascorbic acid and ascorbate dianion, whereas, in the acidic pH, the predominant form of vitamin C is ascorbic acid, which is a more stable form, as shown in figure 1 [32]. Additionally, accelerated dissolution of TCP in the acidic condition also results in increased release of vitamin C in acetate buffer media. Higher release of drug in acidic pH is especially useful to promote enhanced bone regeneration during the initial time points after implantation.
Figure 1.
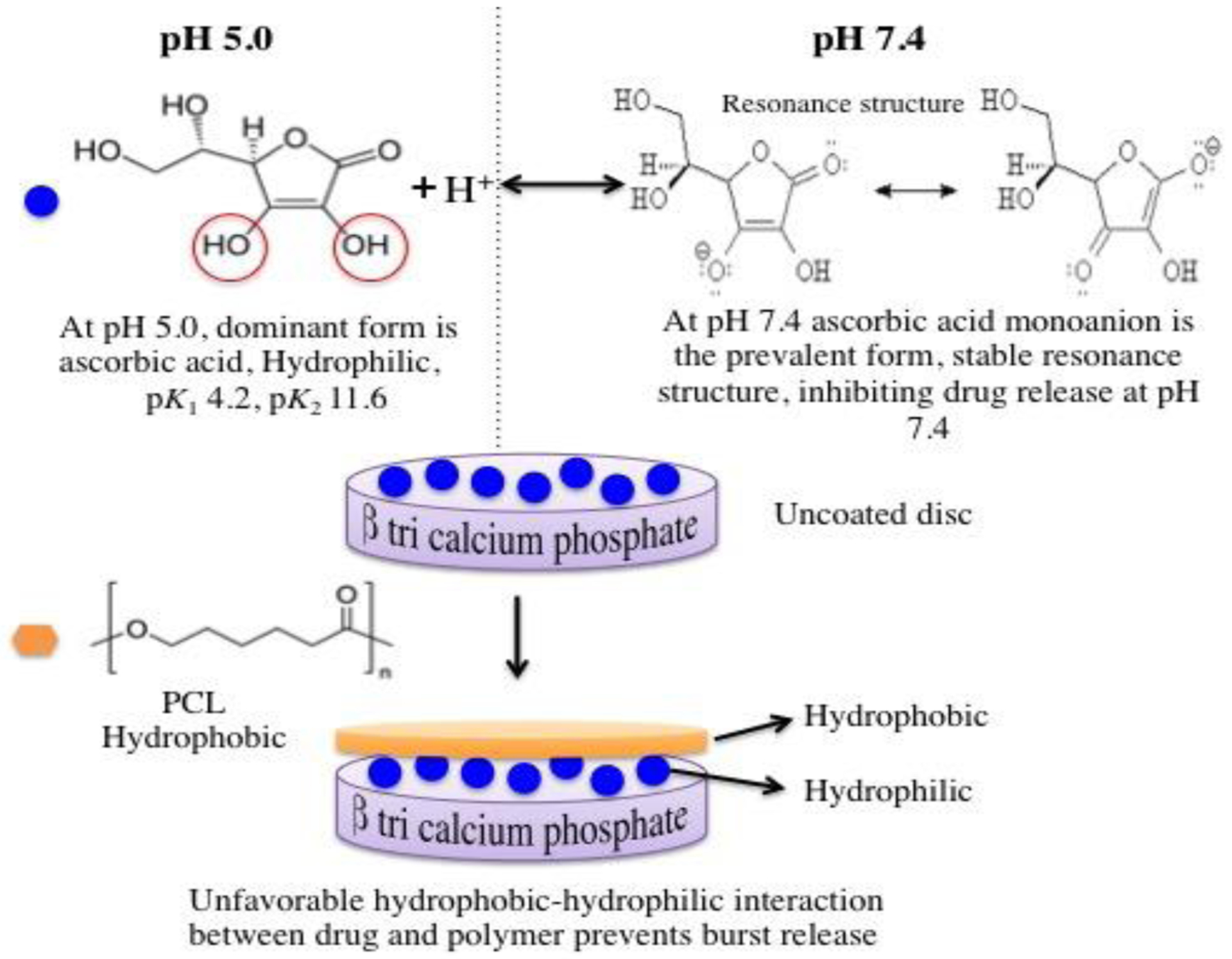
Schematic diagram showing mechanism of action involving the release kinetics of vitamin C from polymer-coated β-TCP scaffolds. Once the proton gets removed from the vinyl alcoholic group, the negative charge on the oxygen gets delocalized and moves to the carbonyl oxygen. This resonance structure stabilizes the conjugate base [34].
In recent years, PCL has received great attention for its use as a vehicle for slow and controlled-delivery of therapeutic biomolecules. Earlier studies from our group reported that PCL coatings not only provide controlled and sustained release of proteins from scaffold but also enhances its mechanical properties by decreasing the brittleness of TCP ceramics [33–34]. In this study, the presence of PCL has demonstrated lower burst release at the earlier time points and an overall slow but steady release of vitamin C from the TCP scaffold. Degradation of PCL is slow and takes several months to years depending on molecular weight, degree of crystallinity and degradation condition. The degradation occurs in two steps: the first step begins with the water diffusion into the amorphous region and subsequent degradation followed by an increase in crystallinity, while the second step involves hydrolytic degradation of the crystalline region [35]. Moreover, PCL coating acts as a physical barrier and delays the dissolution of TCP in acidic environment. Therefore, in the presence of PCL, TCP degradation occurs more slowly, resulting in a more sustained and controlled release of the drug [36].
After achieving a sustained release from vitamin C, its efficacy on in vitro proliferation, viability and differentiation of osteoblast cells has been demonstrated. The SEM results and MTT assay are in accordance to the literature, where significant evidence is presented indicating vitamin C not only promotes the formation of a collagenous extracellular matrix but also induce expression of particular genes related to the osteoblast phenotype. It is reported that presence of ascorbic acid induces up-regulation of osteoblast proliferation marked by an enhanced level of osteoblast cell-growth related genes (H4 histone, c-myc, c-fos), followed by a down-regulation phase, where increased expression of ALP, osteopontin, and osteocalcin is observed due to the onset of mineralization [37–38]. Though a pronounced effect of PCL has not been seen on cellular morphology on day 3, it is interesting to note that the presence of PCL, enhances osteoblastic activity after day 7. The effect of PCL on increased cellular viability is also observed in the MTT assay. This can be attributed to the osteoconductive affinity of PCL. Impressively, PCL has shown accelerated proliferation and differentiation in fibroblast-like MSCs by activating Wnt/β-catenin and Smad3 signaling pathways, related to osteogenesis [39].
Furthermore, the presence of 2 μg of vitamin C resulted in a high level of ALP expression at day 11, which denotes osteoblast differentiation and subsequent mineralization of the new bone. Studies have reported that vitamin C is the primary factor for the differentiation of some connective tissues such as muscle, cartilage, and bone, which are generally derived from the mesenchymal cell [40]. Additionally, the chemopreventive ability of vitamin C has been also evaluated from a short osteosarcoma cell culture study. Although a long-term in vitro and in vivo study is necessary to conclude its efficacy to kill cancer cells, these preliminary data appears to be promising for osteosarcoma management. These results indicate that a prolonged and sustained release of vitamin C drug delivery system is designed, which promotes in vitro osteoblast cell proliferation and differentiation, prevents osteosarcoma cell growth and therefore can be considered as an effective bone tissue-engineering scaffold for defect repair.
Summary
In this study, a controlled and sustained release of vitamin C from TCP scaffolds is observed with the presence of PCL coating. The effects of acidic and physiological pH, the concentration of the drug, and the presence of polymer coating, on in vitro release kinetics to vitamin C are evaluated. Increase in vitamin C release is found with lower pH and higher amount of vitamin C loading. PCL coating prevents the burst release of the drug from TCP scaffolds and results in a more controlled and sustained release of vitamin C over 60 days. In addition, the presence of vitamin C demonstrates enhanced attachment, proliferation, and viability of human osteoblast cells in TCP scaffolds. Microstructural characterization exhibit the formation of multilayered osteoblast cells on the vitamin C incorporated sample surface compared to control, which are also in accordance with the MTT assay. Osteoblast cell viability shows two folds increase in the presence of vitamin C, regardless of the time point. Moreover, an increase in ALP density has been shown in day 11 suggesting the possible influence of vitamin C on osteoblast differentiation. A preliminary in vitro osteosarcoma cell culture study shows 4 folds decrease in cell proliferation at day 3 in the presence of vitamin C compared to the control. The results show that sustained release of vitamin C from PCL coated TCP scaffolds can enhance the proliferation and differentiation of osteoblast cells and can be considered as an effective drug delivery system for bone tissue engineering applications with minimal to no side effects on the body.
Highlights.
Controlled release of vitamin C from TCP scaffolds is observed with PCL coating.
PCL coating prevents burst release of the drug from TCP scaffolds.
Presence of vitamin C demonstrates enhanced proliferation and viability of hFOB.
Vitamin C increases ALP density at day 11 suggesting osteoblast differentiation.
Vitamin C decreases osteosarcoma cell proliferation by 4 folds at day 3.
Acknowledgments
Researchers would like to acknowledge financial assistance from NIH [Grant # 1R01-AR-066361]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gronholz M.Jill, “Prevention, diagnosis, and management of osteoporosis-related fracture: a multifactorial osteopathic approach.” J. Am. Osteopath. Assoc 2008, 108, 575. [PubMed] [Google Scholar]
- 2.Johnell Olof, and Kanis JA, “An estimate of the worldwide prevalence and disability associated with osteoporotic fractures.” Osteoporosis Int. 2006, 17, 1726. [DOI] [PubMed] [Google Scholar]
- 3.Raisz Lawrence G, “Pathogenesis of osteoporosis: concepts, conflicts, and prospects.” J. Clin. Invest 2005, 115, 3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs B. Lawrence, “The mechanisms of estrogen regulation of bone resorption.” J. Clin. Invest 2000, 106, 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeGeros Racquel Zapanta, “Properties of osteoconductive biomaterials: calcium phosphates.” Clin. Orthop. Relat. Res 2002, 395, 81. [DOI] [PubMed] [Google Scholar]
- 6.Burg Karen JL, Porter Scott, and Kellam James F., “Biomaterial developments for bone tissue engineering.” Biomaterials 2000, 21, 2347. [DOI] [PubMed] [Google Scholar]
- 7.Urist Marshall R., Lietze Arthur, and Dawson Edgar, “[Beta]-tricalcium Phosphate Delivery System for Bone Morphogenetic Protein.” Clin. Orthop. Relat. Res 1984, 187, 277. [PubMed] [Google Scholar]
- 8.Ginebra Maria-Pau, Traykova Tania, and Planell JA, “Calcium phosphate cements as bone drug delivery systems: a review.” J. Controlled Release 2006, 113, 102. [DOI] [PubMed] [Google Scholar]
- 9.Tarafder Solaiman, Nansen Kelly, and Bose Susmita. “Lovastatin release from polycaprolactone coated β-tricalcium phosphate: Effects of pH, concentration and drug–polymer interactions.” Mater. Sci. Eng. C 2013. 33 3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff Maria Ann, and Hutmacher Dietmar Werner, “The return of a forgotten polymer— polycaprolactone in the 21st century.” Prog. Polym. Sci 2010, 35, 1217. [Google Scholar]
- 11.Williams Jessica M., et al. , “Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering.” Biomaterials 2005, 26, 4817. [DOI] [PubMed] [Google Scholar]
- 12.Kweon HaeYong, et al. , “A novel degradable polycaprolactone networks for tissue engineering.” Biomaterials 2003, 24, 801. [DOI] [PubMed] [Google Scholar]
- 13.Hutmacher Dietmar W., et al. , “Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling.” J. Biomed. Mater. Res, Part A 2001, 55, 203. [DOI] [PubMed] [Google Scholar]
- 14.Shor Lauren, et al. , “Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro.” Biomaterials 2007, 28, 5291. [DOI] [PubMed] [Google Scholar]
- 15.Gennari C, “Calcium and vitamin D nutrition and bone disease of the elderly.” Public Health Nutr. 2001, 4, 547. [DOI] [PubMed] [Google Scholar]
- 16.Gass Margery, and Dawson-Hughes Bess, “Preventing osteoporosis-related fractures: an overview.” Am. J. Med 2006, 119, S3. [DOI] [PubMed] [Google Scholar]
- 17.LeBoff Meryl S., et al. , “Occult vitamin D deficiency in postmenopausal US women with acute hip fracture.” J. Am. Med. Assoc 1999, 281, 1505. [DOI] [PubMed] [Google Scholar]
- 18.Sahni Shivani, et al. , “High vitamin C intake is associated with lower 4-year bone loss in elderly men.” J. Nutr 2008, 138, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett IR, et al. , “Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo.” J. Clin. Invest 1990, 85, 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu Samar, et al. , “Association between oxidative stress and bone mineral density.” Biochem. Biophys. Res. Commun 2001, 288, 275. [DOI] [PubMed] [Google Scholar]
- 21.Peterkofsky Beverly, “Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy.” Am. J. Clin. Nutr 1991, 54, 1135S. [DOI] [PubMed] [Google Scholar]
- 22.Xiao XH, et al. , “Ascorbic acid inhibits osteoclastogenesis of RAW264. 7 cells induced by receptor activated nuclear factor kappaB ligand (RANKL) in vitro.” J. Endocrinol. Invest 2005, 28, 253. [DOI] [PubMed] [Google Scholar]
- 23.Block Gladys. “Vitamin C and cancer prevention: the epidemiologic evidence.” Am. J. Clin. Nutr 1991, 53, 270S–282S. [DOI] [PubMed] [Google Scholar]
- 24.Cameron Ewan, and Linus Carl Pauling. Cancer and vitamin C: a discussion of the nature, causes, prevention, and treatment of cancer with special reference to the value of vitamin C. Linus Pauling Institute of Science and Medicine, 1979. [Google Scholar]
- 25.Lee Ki Won, et al. “Vitamin C and cancer chemoprevention: reappraisal.” Am. J. Clin. Nutr 2003, 78, 1074–1078. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi Renny T., Iyer Bhanumathi S., and Cui Yingqi, “Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells.” J. Bone Miner. Res 1994, 9, 843. [DOI] [PubMed] [Google Scholar]
- 27.Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM, Dietary vitamin C and bone mineral density in postmenopausal women in Washington State, USA. Br. J. Prev. Soc. Med 1997, 51, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton Deborah J., Barrett‐ Connor Elizabeth L., and Schneider Diane L., “Vitamin C supplement use and bone mineral density in postmenopausal women.” J. Bone Miner. Res 2001, 16, 135. [DOI] [PubMed] [Google Scholar]
- 29.Bose Susmita, and Tarafder Solaiman, “Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review.” Acta Biomater. 2012, 8, 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kylloenen Laura, et al. “Local drug delivery for enhancing fracture healing in osteoporotic bone.” Acta biomater. 2015. 11 412. [DOI] [PubMed] [Google Scholar]
- 31.Naidu K, “Vitamin C in human health and disease is still a mystery? An overview.” Nutr. J 2003, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Juan, Cullen Joseph J., and Buettner Garry R., “Ascorbic acid: chemistry, biology and the treatment of cancer.” Biochim. Biophys. Acta, Rev. Cancer 2012, 1826, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouriño Viviana, and Boccaccini Aldo R., “Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds.” J. R. Soc., Interface 2010, 7, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue Weichang, Bandyopadhyay Amit, and Bose Susmita, “Polycaprolactone coated porous tricalcium phosphate scaffolds for controlled release of protein for tissue engineering.” J. Biomed. Mater. Res, Part B 2009, 91, 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondal Debasish, Griffith May, and Venkatraman Subbu S.. “Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges.” Int. J. Polym. Mater 2016. 65 255. [Google Scholar]
- 36.2. Tarafder Solaiman, and Bose Susmita. “Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis.” ACS Appl. Mater. Interfaces 2014. 6 9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen Thomas A., et al. , “Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix.” J. Cell. Physiol 1990, 143, 420. [DOI] [PubMed] [Google Scholar]
- 38.Franceschi Renny T., and Iyer Bhanumathi S., “Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3‐ E1 cells.” J. Bone Miner. Res 1992,7, 235. [DOI] [PubMed] [Google Scholar]
- 39.Xue Ruyue, et al. “Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells.” Stem Cell Res. Ther 2017. 8 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaiswal Neelam, et al. , “Osteogenic differentiation of purified, culture- expanded human mesenchymal stem cells in vitro.” J. Cell. Biochem 1997, 64, 295. [PubMed] [Google Scholar]


