Abstract
Cemtirestat, 3-mercapto-5H-[1,2,4]-triazino[5,6-b] indole-5-acetic acid was recently designed and patented as a highly selective and efficient aldose reductase inhibitor endowed with antioxidant activity. The aim of the present study was to assess the general toxicity of cemtirestat using in silico predictions, in vitro and in vivo assays. ProTox-II toxicity prediction software gave 17 “Inactive” outputs, a mild hepatotoxicity score (0.52 probability) along with a predicted LD50 of 1000 mg/kg. Five different cell lines were used including the immortalized mouse microglia BV-2, the primary human fibroblasts VH10, the insulinoma pancreatic β-cells INS-1E, the human colon cancer cells HCT116 and the human immortalized epithelial endometrial cell lines HIEEC. In contrast to the clinically used epalrestat, cemtirestat showed remarkably low cytotoxicity in several different cell culture viability tests such as MTT proliferation assay, neutral red uptake, BrdU incorporation, WST-1 proliferation assay and propidium iodide staining followed by flow cytometry. In a yeast spotting assay, the presence of cemtirestat in incubation of Saccaromyces cerevisiae at concentrations as high as 1000 μM did not affect cell growth rate significantly. In the 120-day repeated oral toxicity study in male Wistar rats with daily cemtirestat dose of 6.4 mg/kg, no significant behavioral alterations or toxicological manifestations were observed in clinical and pathological examinations or in hematological parameters. In summary, these results suggest that cemtirestat is a safe drug that can proceed beyond preclinical studies.
Keywords: cemtirestat, aldose reductase inhibitor, toxicity assessment
ABBREVIATIONS
- AKR1B1
Aldo-keto reductase family 1, member B1 (human aldose reductase)
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- BrdU
Bromodeoxyuridine
- DMEM
Dulbecco's modified eagle medium
- FBS
Fetal bovine serum
- LC−MS
Liquid chromatography–mass spectrometry
- MCF
Median corpuscular fragility
- MDA
Malondialdehyde
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NR
Neutral red
- P/S
Penicillin-streptomycin solution
- PBS
Phosphate-buffered saline
- RPMI-1640
Growth medium used in cell culture
- RBC
Red blood cells
- WST
Water soluble tetrazolium salt
- ZDF
Zucker diabetic fatty
- YPD
Yeast extract peptone dextrose
Introduction
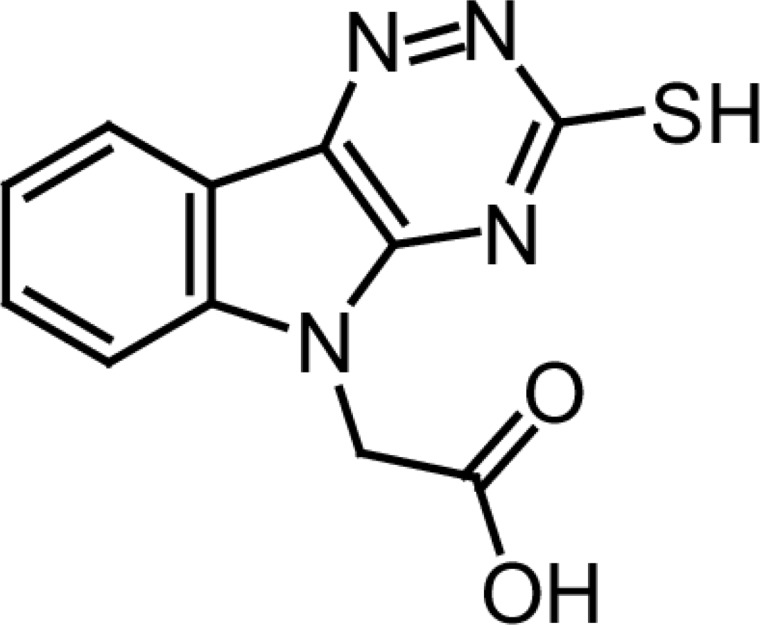
Cemtirestat, 3-mercapto-5H-1,2,4-triazino[5,6-b]indole-5-acetic acid (Figure 1), was recently designed and patented (Stefek et al., 2017) as a highly selective and efficient aldose reductase inhibitor. (Stefek et al., 2015; Stefek et al., 2016; Soltesova Prnova et al., 2015; Zhan et al., 2018). Aldose reductase (AKR1B1), the first enzyme of the polyol pathway, is a key mediator of glucose toxicity under hyperglycemic conditions (Yabe-Nishimura, 1998). Aldose reductase thus represents a promising therapeutic target and efficient aldose reductase inhibitors are sought as potential drugs to treat diabetic complications. In our recent study in ZDF rats, an animal model of type 2 diabetes, cemtirestat normalized symptoms of peripheral neuropathy with high significance (Soltesova Prnova et al. 2019). Inhibition of sorbitol accumulation in red blood cells and in the sciatic nerve along with markedly decreased plasma levels of thiobarbituric acid reactive substances pointed to the bifunctional effect of cemtirestat comprising both inhibition of flux of glucose through the polyol pathway and antioxidant action.
Figure 1.
3-Mercapto-5H-[1,2,4]-triazino[5,6-b]indole-5-acetic acid (cemtirestat).
Cemtirestat is presently a subject of preclinical studies as a promising agent with a therapeutic potential in relation to diabetic peripheral neuropathy. The aim of the present study was to assess a general toxicity of cemtirestat using in silico predictions, cell culture in vitro assays and in vivo animal investigations.
Material and methods
Substance
Cemtirestat (3-mercapto-5H-1,2,4-triazino[5,6-b]indole-5-acetic acid) was custom synthesized by Apollo Scientific Ltd, Bredbury, UK. A purity of >95% has been established for cemtirestat substance by the LC−MS technique as described in Stefek et al. (2015). The reference aldose reductase inhibitor epalrestat was obtained from Sigma-Aldrich (St. Louis, MO, USA).
In silico predictions
ProTox-II (http://tox.charite.de/protoxII), a webserver for the prediction of toxicity of chemicals was used.
Cell culture tests
Cell lines
The immortalized mouse microglial cell line BV-2 was kindly provided by Dr. Blasi at the University of Perugia (Blasiet al., 1990) and was cultured under standard conditions in Dulbecco’s modified eagle medium (DMEM, Sigma Aldrich), supplemented with 10% fetal bovine serum (FBS, PAA, Biotech, s. r. o., Bratislava, Slovakia), and 1% P/S (100 U/ml penicillin, 100 mg/ml streptomycin, K-Trade, s.r.o., Bratislava, Slovakia) and maintained in 5% CO2 at 37 °C. Cells were used for 10 passages at maximum (Mrvova et al., 2015).
Nonmalignant diploid human fibroblasts VH10 were obtained from Prof. A. Kolman (Laboratory of Radiology, University of Stockholm, Sweden) and were cultured under standard condition in DMEM, supplemented with 10% FBS, and 1% P/S (100 U/ml penicillin, 100 mg/ml streptomycin) and maintained in 5% CO2 at 37 °C as described elsewhere (Slamenova et al. 2009).
Rat INS-1E insulinoma pancreatic β-cells were kindly provided by Prof. Claes Wollheim, University of Geneva) and were cultured in RPMI 1640 (11 mM glucose, Sigma Aldrich) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 1 mM Na pyruvate, 55 μM 2-mercaptoethanol, 10 mM HEPES, 1% non-essential amino acids, and 10% fetal bovine serum, pH 7.0–7.4. The cells were grown in a humidified incubator containing 5% CO2 at 37 °C as described previously (Viskupicova et al., 2017).
Human colon cancer cells (HCT-116) were kindly provided by Prof. Sreeparna Banerjee, Department of Biological Sciences, Middle East Technical University, Ankara, Turkey and were cultured as described previously in RPMI-1640 (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS, Thermo Scientific, Waltham MA, USA), 2 mM L-glutamine and 1% penicillin/streptomycin (Enayat et al., 2016).
Human immortalized epithelial endometrial cell line (HIEEC) was kindly provided by Prof. Michael A. Fortier (Laval University, Qébec, Canada) and it was originally generated from a primary culture prepared from endometrial biopsy taken from a 37-year-old woman with confirmed absence of neoplasia and endometriosis, on day 12 of her menstrual cycle and were cultured in RPMI-1640 Medium (R5886; Sigma-Aldrich) as described elsewhere (Hevir-Kene and Rižner, 2015).
The Saccharomyces cerevisiae strain RDKY3615 (MAT a, ura3-52, his3Δ200, leu2Δ1, trp1Δ63, lys2ΔBgI, hom3-10, ade2Δ1, ade8, hxt13::URA3, Chen and Kolodner, 1999) was obtained from Dr. Hernan Flores Rozas from the College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Florida.
MTT viability test
To assess cell metabolic activity, the MTT assay was used which is based on the ability of cellular NAD(P) H-dependent oxidoreductases to reduce the tetrazolium dye MTT to insoluble formazan, which has a purple color (Stockert et al. 2018).The cells were grown in 96-well microplates in complete cell culture medium. At the end of the incubation with or without the compounds tested, the cells were incubated with MTT (0.5 mg/ml) for 120 min at 37 °C. Subsequently, 100 μl of 10% sodium dodecyl sulfate in HCl (0.01 M) was added and the cells were incubated at 37 °C overnight for complete dissolution of generated formazan. The absorbance was spectrophotometrically recorded at 570 nm using the reference 690 nm (Mrvova et al., 2015; Viskupicova et al., 2017).
Neutral red (NR) uptake assay
To determine the number of viable cells in culture, the NR uptake assay was used which is based on the ability of viable cells to incorporate neutral red dye within the lysosomes (Repetto et al., 2008). One hundred microliters of NR solution (0.003%) in complete cell culture medium was added to the cells and the dye accumulation was left to proceed for 4 hours. Next, the incubation medium was removed, the cells were washed twice with phosphate buffered saline (PBS) and the accumulated dye was solubilized with 2% acetic acid/ethanol mixture (1:1, v/v). The absorbance of the samples was read spectrophotometrically at 540 nm using a reference wavelength at 690 nm (Milackova et al. 2015).
BrdU incorporation assay
BrdU incorporation into the newly synthesized DNA (Vega-Avila and Pugsley, 2011) was used to detect proliferating HCT-116 cells according to the manufacturer’s instructions as described elsewhere (Enayat et al., 2016). Chemiluminescent BrdU cell proliferation kit was purchased from Roche (Mannheim, Germany).
WST-1 proliferation test
WST-1 proliferation test (Ishiyama, 1995) was used to determine the effect of cemtirestat on the proliferation of human immortalized epithelial endometrial cells (HIEEC). The reagent WST-1 (Roche Diagnostics, Germany) was employed following the manufacturer’s instructions as described elsewhere (Kljun et al. 2016).
Cell cycle analysis
VH10 fibroblasts cultured under standard condition (Slamenova et al., 2009) were fixed in 70% ethanol overnight at –20 °C; the cells were pelleted and resuspended gently in ice-cold phosphate-buffered saline (PBS) . After 2 washes, cells were suspended in propidium iodide staining solution (10 μg/ml propidium iodide, 100 μg/ml RNase, 0.1% (v/v) Triton-X in PBS for 15 min at 37 °C in the dark. The cell cycle was analyzed using a flow cytometer (Beckman Coulter FC500). Data were analyzed using MultiCycle AV software with a minimum of 5000 cells per sample.
Yeast spotting test
The yeast spotting test (Kwolek-Mirek and Zadrag-Tecza, 2014) was used to determine the effect of cemtirestat on yeast viability. An aliquot of the glycerol stock of the yeast strain was taken from –80 °C, streaked on a YPD plate and incubated at 30 °C for 16 h. A single colony was picked and inoculated into 3 ml of liquid yeast extract peptone dextrose (YPD) medium and grown overnight with shaking at 200 rpm at 30 °C. This overnight starter culture was then transferred to 10 ml of fresh liquid YPD and grown at 30 °C with shaking at 200 rpm until the OD 600 nm reached the value of 0.6. The culture was then divided into five separate tubes in equal numbers and either left untreated (Control) or treated with vehicle or 10, 100 and 1000μM of cemtirestat for 24h in YPD. During treatment the cells were incubated at 30 °C with shaking at 100 rpm. The cells in each tube were washed extensively followed by 10-fold serial dilution in 100 μl final volume (Enayat et al., 2016). 10 μl from each dilution was plated on an YPD-agar plate; the plate was air dried in a sterile manner and incubated at 30 °C for 48 h and photographed.
In vivo tests
Animals
Male Wistar rats were supplied by our own breeding facility at the Department of Toxicology and Laboratory Animal Breeding, Centre of Experimental Medicine, Slovak Academy of Sciences Dobra Voda. The animals were fed standard chow (protein, 19.2%; carbohydrate, 65.1%; fat, 4.0%; fiber, 4.0% and ash, 7.7% of weight). The animals were randomly assigned to two groups: (C) control rats (n=6); (T) rats (n=6) treated with cemtirestat (100 mg/l in drinking water). The drug treatment proceeded for 120 days. During the experiment the animals were housed in groups of two in cages of type T4 Velaz (Prague, Czech Republic) with bedding made of wood parings (changed every other day). Tap water and pelleted standard chow were supplied ad libitum. The animal room was air-conditioned with 10 air changes per hour; the environment was maintained, with continuous monitoring, at 23±1 °C temperature and 40–70% relative humidity. After the completion of behavioral testing for each group at the end of the experiment, the rats were anesthetized with chloral hydrate (20 mg/100 g i.p.), blood was collected into heparinized tubes by heart puncture and organ samples were collected for biochemical assays. All tissues were rapidly frozen in liquid nitrogen and stored at –80 °C.
The study was approved by the Ethics Committee of the Institute of Experimental Pharmacology and Toxicology, Centre of Experimental Medicine, Slovak Academy of Sciences and the State Veterinary and Food Administration of the Slovak Republic, and it was performed in accordance with the Principles of Laboratory Animal Care (NIH publication 83-25, revised 1985) and the Slovak law regulating animal experiments (Decree 289, Part 139, July 9th 2003).
Plasma assays
Frozen (–80 °C) samples of plasma were used for biochemical assays. Plasma glucose was analyzed by using an enzymatic colorimetric assay for glucose Glucose GOD 1500 (PLIVA-Lachema Diagnostika, Brno, CZ). Plasma cholesterol, triacylglycerides, urea, creatinine, Ca, P and Mg were assayed by Laboratoria s.r.o. Piešťany, Slovakia. Plasma levels of thiobarbituric acid reactive substances (TBARS), used as a marker of oxidative stress, were determined by modification of the method of Buege and Aust (Buege and Aust, 1978) as described previously (Soltesova Prnova et al., 2019).
Sorbitol assay
Erythrocyte sorbitol assay was performed by modified enzymatic assay as reported previously (Soltesova Prnova et al., 2019).
Behavioral tests
The rats were transferred to the experimental room and allowed to acclimatize for one hour. Hot water immersion tail-flick test, hot plate test and paw tactile responses test by using von Frey flexible filaments were performed as described previously (Soltesova Prnova et al., 2019).
Osmotic fragility
The osmotic fragility was determined by the degree of hemolysis induced by the changes of osmotic pressure using a step-down protocol with decreasing concentrations of NaCl, as reported previously (Prnova et al. 2015). The median corpuscular fragility (MCF), used as quantitative marker of osmotic fragility, was calculated as a concentration (mM) of NaCl at which 50% hemolysis occurred.
Statistical analysis
Statistical comparisons were carried out by one way ANOVA followed by Tukey’s post hoc test. p≤0.05 was considered statistically significant.
Results and discussion
Toxicity predictions in silico
The aim of computational approaches in toxicology is to complement in vitro and in vivo toxicity assays to minimize the need for animal testing and to reduce the cost and time of toxicity tests (Raies and Bajic, 2016). As shown in Table 1, ProTox-II toxicity prediction software (Banerjee et al., 2018) gave 16 “Inactive” outputs, a mild hepatotoxicity score (0.52 probability) along with a predicted LD50 of 1000 mg/kg. These outputs classify cemtirestat as a compound of acute toxicity class 4.
Table 1.
ProTox-II toxicity prediction of cemtirestat.
| No | Target | Prediction | Probability |
|---|---|---|---|
| 1 | Hepatotoxicity | Active | 0.52 |
| 2 | Carcinogenicity | Inactive | 0.52 |
| 3 | Immunotoxicity | Inactive | 0.99 |
| 4 | Mutagenicity | Inactive | 0.61 |
| 5 | Cytotoxicity | Inactive | 0.66 |
| 6 | Aryl hydrocarbon Receptor (AhR) | Inactive | 0.62 |
| 7 | Androgen Receptor (AR) | Inactive | 0.99 |
| 8 | Androgen Receptor Ligand Binding Domain (AR-LBD) | Inactive | 0.97 |
| 9 | Aromatase | Inactive | 0.82 |
| 10 | Estrogen Receptor Alpha (ER) | Inactive | 0.86 |
| 11 | Estrogen Receptor Ligand Binding Domain (ER-LBD) | Inactive | 0.98 |
| 12 | Peroxisome Proliferator Activated Receptor Gamma (PPAR-Gamma) | Inactive | 0.84 |
| 13 | Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | Inactive | 0.91 |
| 14 | Heat shock factor response element (HSE) | Inactive | 0.91 |
| 15 | Mitochondrial Membrane Potential (MMP) | Inactive | 0.81 |
| 16 | Phosphoprotein (Tumor Supressor) p53 | Inactive | 0.83 |
| 17 | ATPase family AAA domain-containing protein 5 (ATAD5) | Inactive | 0.94 |
Cell culture studies
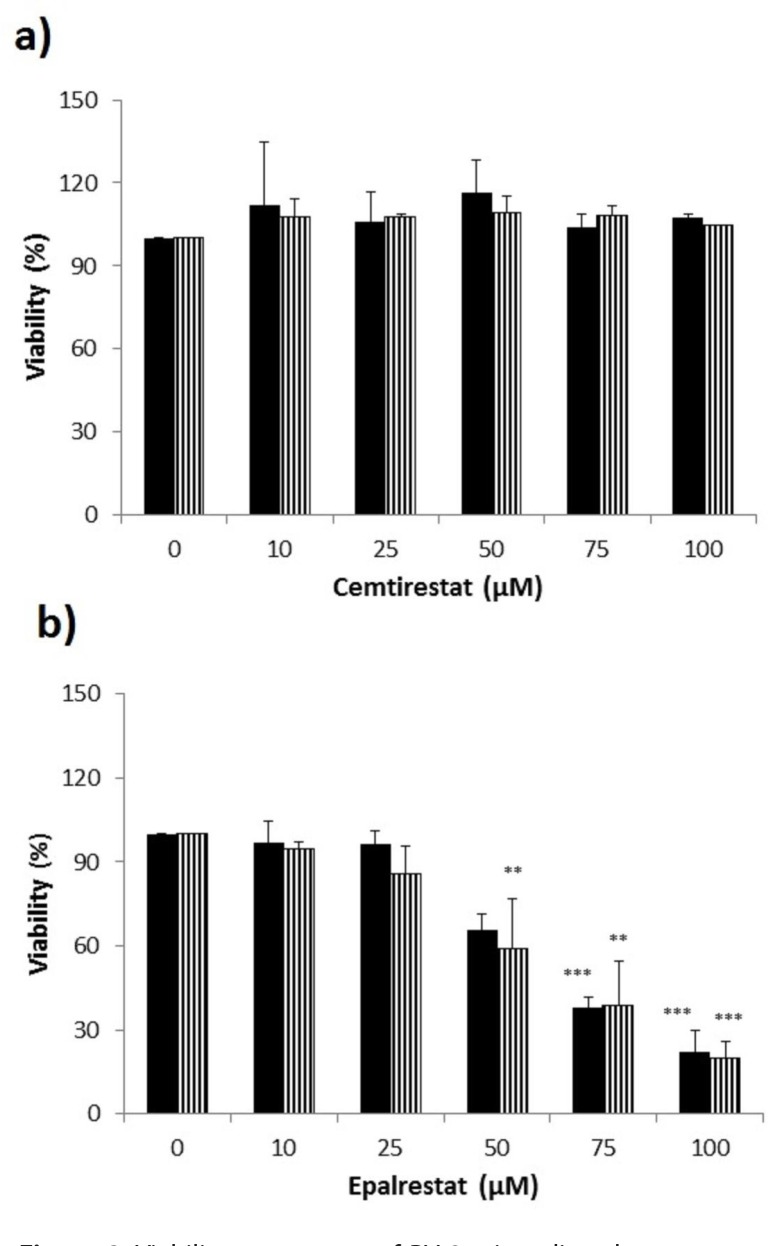
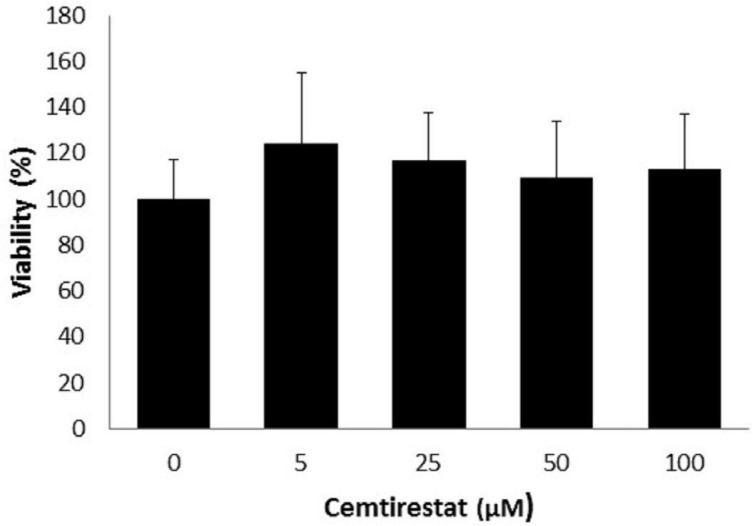
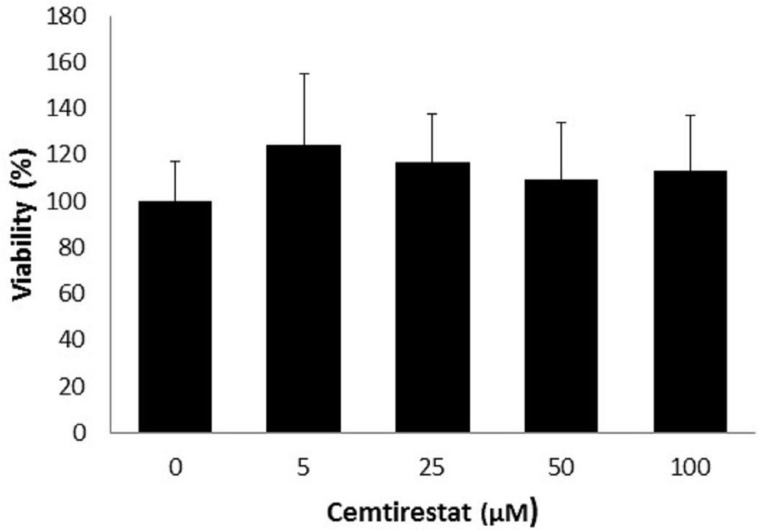
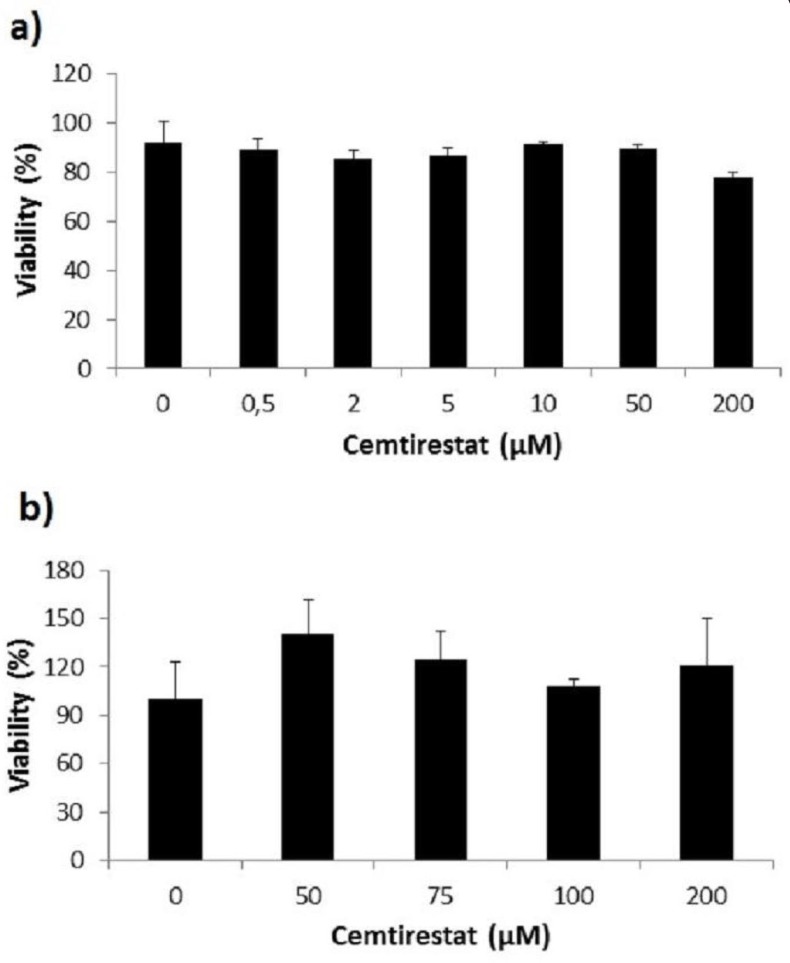
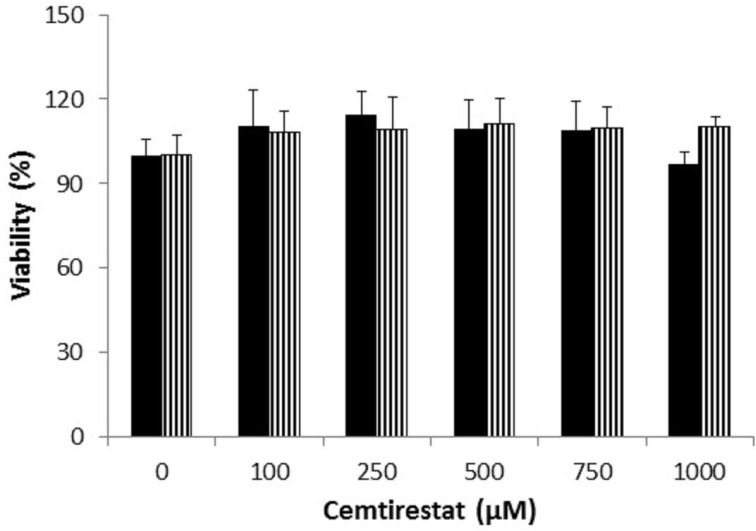
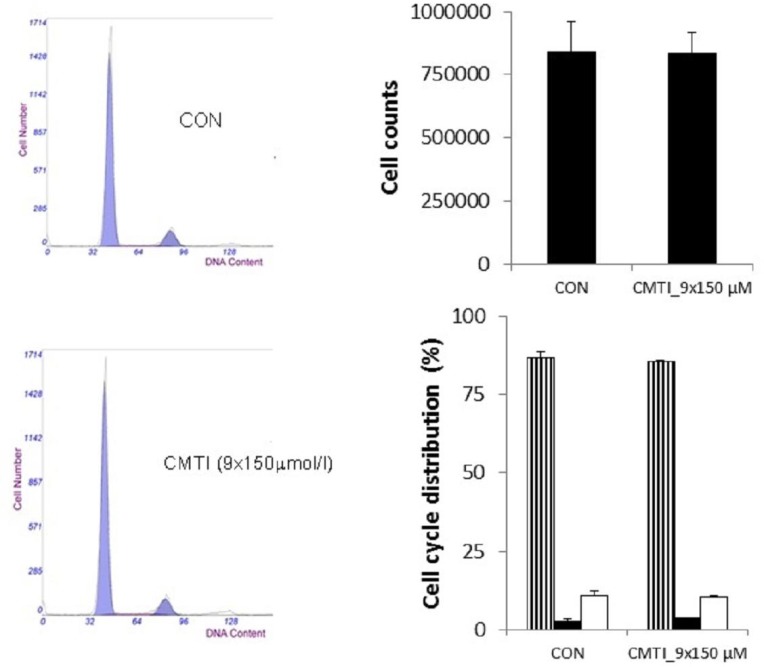
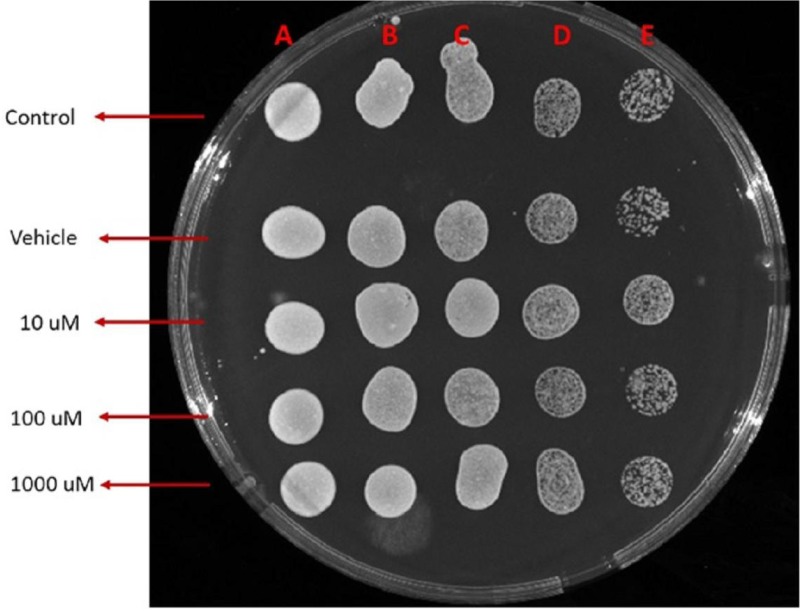
Remarkably low acute cytotoxicity of cemtirestat was observed in cell culture viability tests. To avoid method-related bias in viability tests (Pamies and Hartung, 2017) five principally different viability assays were used including MTT assay, NR uptake assay, BrdU incorporation assay, WST-1 proliferation test and propidium iodide DNA staining followed by flow cytometry. No significant effect of cemtirestat on cell viability was recorded with up to 100 μM of cemtirestat in the immortalized mouse microglia BV-2 (Figure 2a) and the human immortalized epithelial endometrial cell lines HIEEC (Figure 3). In contrast to cemtirestat, significant cell toxicity of the standard aldose reductase inhibitor epalrestat was recorded in the microglia at concentrations as low as 50 μM (Figure 2a). At this concentration the loss of viability estimated by MTT assay and NR uptake was 35% and 41%, respectively. A mild decrease in viability of Schwann cells in culture treated with 100 and 200 μM epalrestat was reported by Sato et al. (2014). Concentrations of cemtirestat up to 200 μM, were found to be without any effect on cell viability of the insulinoma pancreatic β-cells INS-1E (Figure 4) and on the proliferative capacity of the human colon cancer cells HCT-116 (Figure 5). Moreover, no significant cytotoxicity up to 1000 μM cemtirestat was recorded in the primary human fibroblasts VH10 (Figure 6). Chronic toxicity profile of cemtirestat applied every 12 hours over five consecutive days (9x 150 μM in total) in primary VH10 fibroblasts, shown in Figure 7, revealed no significant cell cycle-dependent cytotoxic effect. These experimental data are also in accordance with previously reported absence of any effect of cemtirestat on osmotic fragility of isolated erythrocytes up to 250 μM concentration (Prnova et al., 2015). As shown in Figure 8, cemtirestat up to 1000 μM concentration did not affect the viability of Saccharomyces cerevisiae in a yeast spotting test.
Figure 2.
Viability parameters of BV-2 microglia subsequent to 24 h exposure to cemtirestat (a) in comparison with standard epalrestat (b). MTT test (black columns), NR uptake test (striped columns). Results are mean ± SD of at least three independent experiments run in three replicates.***p≤0.001, **p≤0.01 vs. control. One way ANOVA followed by Tukey’s post hoc test.
Figure 3.
Viability parameters of HIEEC cells subsequent to 72 h exposure to cemtirestat. WST-1 proliferation test. Results are mean ± SD of at least three independent experiments run in three replicates. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between the groups.
Figure 4.
Viability parameters of pancreatic INS-1E cells subsequent to 24 h exposure to cemtirestat. MTT test. Results are mean ± SD of at least three independent experiments run in three replicates. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences the groups.
Figure 5.
Viability parameters of colon cancer HCT116 cells subsequent to a) 24 h (MTT test), b) 48 h (BrdU incorporation assay) exposure to cemtirestat. Results are mean ± SD of at least three independent experiments run in eight replicates. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between the groups.
Figure 6.
Viability parameters of VH-10 cells subsequent to 24 h exposure to cemtirestat. MTT test (black columns), NR uptake test (striped columns). Results are mean ± SD of at least three independent experiments run in three replicates. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between the groups.
Figure 7.
Effect of 5-day repetitive treatment with cemtirestat added into medium twice a day (150 μmol/l) on cell growth and cell cycle distribution in VH10 fibroblasts assessed by propidium iodide DNA staining and analysis of DNA content by flow cytometry. Data are mean ± SD of at least three independent experiments run in 3-4 replicates. One way ANOVA followed by Tukey’s post hoc test gave no significant differences between the groups. Representative cell cycle histograms; G1 phase (stripped columns), S phase (black columns), G2/M phase (empty columns).
Figure 8.
Yeast spotting test with cemtirestat (1 replicate). Concentration dependence. A: undiluted, B:1/10 diluted, C:1/100 diluted, D:1/1000 diluted, E:1/10000 diluted.
Animal studies
In our previous study in ZDF rats (Soltesova Prnova et al., 2019), there were two groups of animals treated with cemtirestat. The drug was administered as a water solution by oral gavage in daily doses of 2.5 and 7.5 mg/kg. The dose of 2.5 mg/kg corresponds to the recommended daily dose of clinically used aldose reductase inhibitor epalrestat (Sharma and Sharma, 2008). In the present study, cemtirestat was administered as a solution in drinking water and the required daily dose was 7.5 mg/kg. Based on preliminary measurements of daily water consumption, the required concentration of cemtirestat in drinking water was estimated to be 100 mg/l. Yet the final average dose calculated on the basis of actual water consumption (Table 4) during the 4-month treatment was slightly lower, approx. 6.4 mg/kg/day.
Table 4.
Average chow/water consumption, plasma biochemical markers and erythrocyte sorbitol and MCF values of male Wistar rats recorded at the end of the experiment. Effect of cemtirestat treatment*.
| C (6) | T(6) | |
|---|---|---|
| Average daily food consumption (g/100 g body weight) | 7.59±1.19 | 8.14±1.99 |
| Average daily water intake (g/100 g body weight) | 6.32±0.66 | 6.39±1.11 |
| Cholesterol (mM) | 1.63±0.25 | 1.74±0.29 |
| Triglycerides (mM) | 1.45±0.25 | 1.06±0.34 |
| Urea (mM) | 7.21±0.50 | 7.93±0.58 |
| Creatinine (μM) | 30.28±4.31 | 34.27±4.47 |
| TBARs in plasma (μM) | 5.60±0.54 | 5.76±0.31 |
| Ca | 2.30±0.16 | 2.36±0.12 |
| P | 1.48±0.27 | 1.49±0.41 |
| Mg | 0.72±0.05 | 0.82±0.07 |
| Sorbitol in red blood cells (nmol/ml packed RBC) | 1.91±0.98 | 2.28±0.74 |
| Erythrocyte MCF | 58.63±2.83 | 57.10±2.69 |
Measurements of chow and water consumption were made at 2-week intervals throughout the 4-month experiment. Group C, untreated control animals; Group T, rats treated by cemtirestat 6.4 mg/kg/day; MCF: The median corpuscular fragility was calculated as a concentration (mM) of NaCl at 50% hemolysis. Data are mean values±SD. Number of animals in each group is shown in parentheses. One-way ANOVA followed by the posthoc Tukey test gave no significant differences between C and T groups.
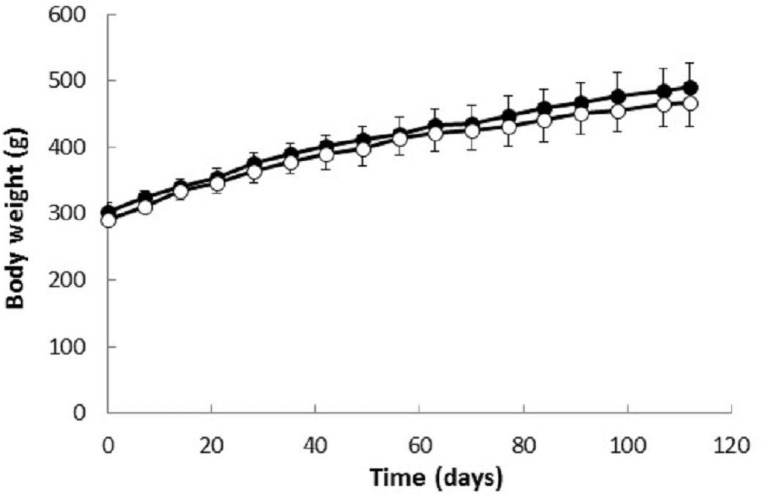
Experimental data summarized in Table 2 and Figure 9 show that cemtirestat treatment had no significant effect on body weight gains of the experimental animals during the 120-day experiment. In addition, as shown in Table 3, cemtirestat treatment had no significant effect on relative organ weights of the animals. In agreement with these findings, cemtirestat also did not affect daily food and water consumption (Table 4). No signs or symptoms of cemtirestat toxicity developed during the 120-day treatment period. These observations are in accordance with our previously reported findings that revealed no acute toxicological manifestation of cemtirestat administered intragastrically (50 mg/kg/day) to male Wistar rats for five consecutive days (Soltesova Prnova et al. 2015a).
Table 2.
Initial and final body weights and plasma glucose concentrations in male Wistar rats with or without cemtirestat treatment*.
| Body weight (g) | Blood glucose (mmol/l) | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| C (6) | 262.5±15.1 | 450.8±38.1 | 8.1±1.9 | 10.6±1.2 |
| T(6) | 275.8± 8.0 | 465.8±38.3 | 7.5±1.5 | 8.6±2.6 |
Group C, untreated control animals; Group T, rats treated by cemtirestat 6.4 mg/kg/day; Data are mean values±SD. Number of animals in each group is shown in parentheses. One way ANOVA followed by Tukey’s post hoc test gave no significant differences between C and T groups.
Figure 9.
Body weights of male Wistar rats treated with cemtirestat. Group C, untreated rats(●); group T, rats treated with cemtirestat (○, 6.4 mg/kg/day). Results are means ± SEM from 6 (C, T) animals. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between C and T groups.
Table 3.
Relative organ weights (g/100 g b.w.) male Wistar rats at the end of the experiment*.
| C (6) | T(6) | |
|---|---|---|
| Liver | 2.734±0.093 | 2.623±0.139 |
| Spleen | 0.189±0.055 | 0.180±0.010 |
| Adrenals | 0.0091±0.0012 | 0.0087±0.0013 |
| Kidney | 0.516±0.031 | 0.518±0.042 |
| Testes | 0.923±0.060 | 1.001±0.069 |
| Lens | 0.023±0.003 | 0.026±0.003 |
| Heart | 0.224±0.011 | 0.230±0.008 |
Group C, untreated control animals; Group T, rats treated by cemtirestat 6.4 mg/kg/day; Data are mean values±SD. Number of animals in each group is shown in parentheses. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between C and T groups.
Extensive plasma assays revealed no significant effect of cemtirestat on the levels of plasma glucose (Table 2), plasma cholesterol, triglycerides, urea, creatinine, TBARs, Ca, Mg and P levels (Table 4). Experimental value of the median corpuscular fragility (MCF, quantitative marker of osmotic fragility of the erythrocytes) was not affected by cemtirestat. This result is in agreement with previously published results showing absence of any effect of 250 μM cemtirestat on osmotic fragility of isolated red blood cells (Prnova et al., 2015).
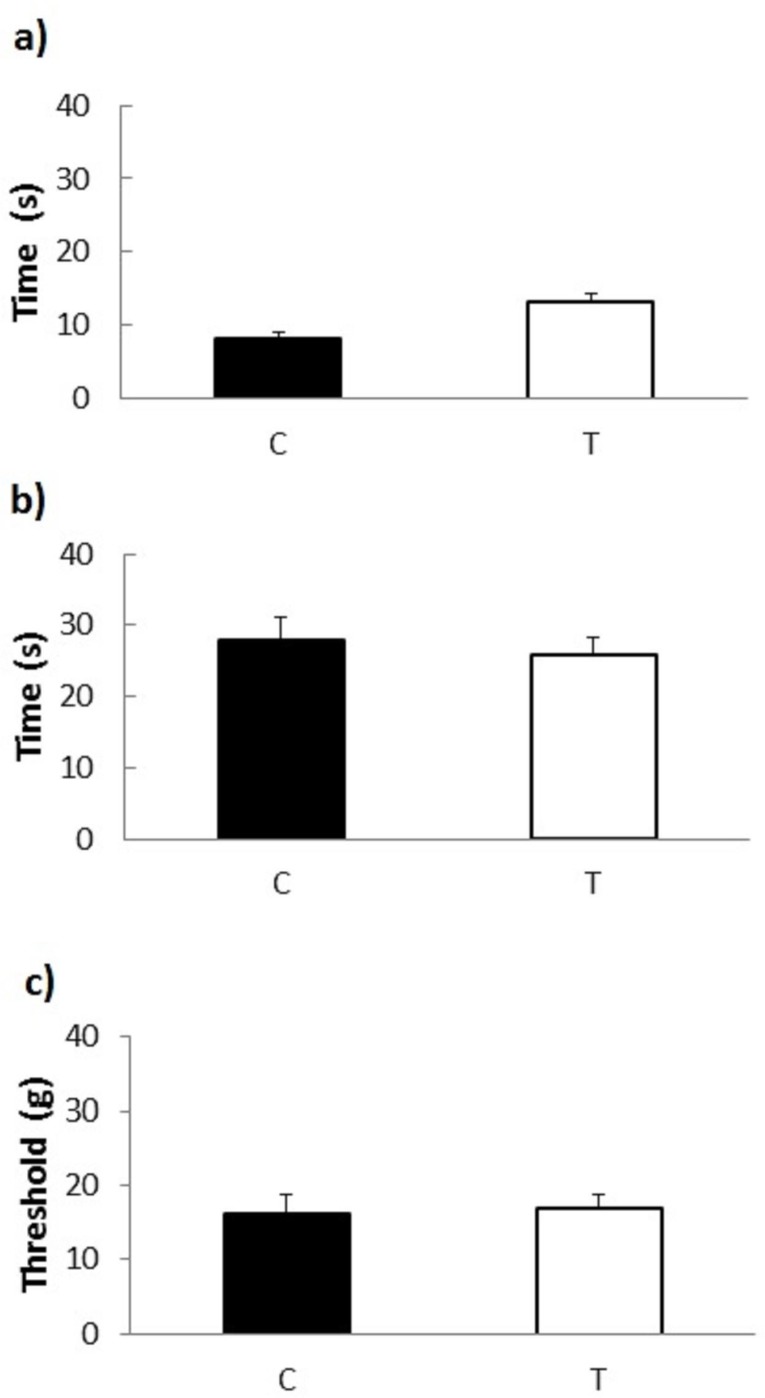
Sensitivity of the peripheral nerves to painful stimuli (tail flick and hot plate tests) and to non-painful stimulation with flexible von Frey filaments was not significantly affected by 120-day treatment of the experimental rats with cemtirestat (Figure 10).
Figure 10.
Peripheral nerve sensitivity tests in male Wistar rats treated with cemtirestat. Group C, untreated male Wistar rats; Group T, Wistar rats treated by cemtirestat (6.4 mg/kg/day); (a) Tail-flick test response latencies (50 °C); (b) Hot plate test response latencies (55 °C); (c) Tactile response thresholds as a result of stimulation with flexible von Frey filaments. Results are presented as means ± SEM from 6 (C, T) animals. One-way ANOVA followed by the post-hoc Tukey test gave no significant differences between C and T groups.
Conclusions
Based on ProTox-II toxicity prediction software, cemtirestat was classified as a compound of acute toxicity class 4 with mild hepatotoxicity. Cell culture viability tests performed on six different cell lines proved remarkably low cytotoxicity of cemtirestat. To avoid method-related bias in viability tests, five principally different viability assays were used. In the 120-day repeated oral toxicity study in male Wistar rats treated with the daily dose of 6.4 mg/kg of cemtirestat, no significant behavioral alterations or toxicological manifestations were observed in clinical and pathological examinations or in hematological parameters. In summary, these results suggest that cemtirestat is a safe drug that can proceed beyond preclinical studies.
Acknowledgements
Supported by VEGA 2/0005/2018, Slovak Research and Development Agency under the contract NO. APVV-15-0455, SAS-Tubitak JRP 2015/7 „GLUCOLIPOTOX“, TUBITAK Project No: 215S19, Science Academy of Turkey Young Investigators Award (BAGEP) and N1-0066 from the Slovenian Research Agency.
Declaration of interest
The authors report no conflict of interest.
REFERENCES
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46(W1):W257–W263. doi: 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Kolodner R.D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- Enayat S, Şeyma Ceyhan M, Taşkoparan B, Stefek M, Banerjee S. CHNQ, a novel 2-Chloro-1,4-naphthoquinone derivative of quercetin, induces oxidative stress and autophagy both in vitro and in vivo. Arch Biochem Biophys. 2016;596:84–98. doi: 10.1016/j.abb.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Hevir-Kene N, Rižner TL. The endometrial cancer cell lines Ishikawa and HEC-1A, and the control cell line HIEEC, differ in expression of estrogen biosynthetic and metabolic genes, and in androstenedione and estrone-sulfate metabolism. Chem Biol Interact. 2015;234:309–19. doi: 10.1016/j.cbi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Ishiyama M. Advantages of WST-1compared to MTT agents. In vitro Toxicology. 1995;8:187–189. [Google Scholar]
- Kljun J, Anko M, Traven K, Sinreih M, Pavlič R, Peršič Š, Ude Ž, Codina EE, Stojan J, Lanišnik Rižner T, Turel I. Pyrithione-based ruthenium complexes as inhibitors of aldo-keto reductase 1C enzymes and anticancer agents. Dalton Trans. 2016;45(29):11791–800. doi: 10.1039/c6dt00668j. [DOI] [PubMed] [Google Scholar]
- Kwolek-Mirek M, Zadrag-Tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014;14(7):1068–1079. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Mrvová N, Škandík M, Kuniaková M, Račková L. Modulation of BV-2 microglia functions by novel quercetin pivaloyl ester. Neurochem Int. 2015;90:246–54. doi: 10.1016/j.neuint.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Milackova I, Rackova L, Majekova M, Mrvova N, Stefek M. Protection or cytotoxicity mediated by a novel quinonoid-polyphenol compound? Gen Physiol Biophys. 2015;34(1):51–64. doi: 10.4149/gpb_2014028. [DOI] [PubMed] [Google Scholar]
- Pamies D, Hartung T. 1st Century Cell Culture for 21st Century Toxicology. Chem Res Toxicol. 2017;30(1):43–52. doi: 10.1021/acs.chemrestox.6b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prnova MS, Ballekova J, Majekova M, Stefek M. Antioxidant action of 3-mercapto-5H-1,2,4-triazino[5,6-b]indole-5-acetic acid, an efficient aldose reductase inhibitor, in a 1,1’-diphenyl-2-picrylhydrazyl assay and in the cellular system of isolated erythrocytes exposed to tert-butyl hydroperoxide. Redox Rep. 2015;20(6):282–288. doi: 10.1179/1351000215Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raies AB, Bajic VB. In silico toxicology: computational methods for the prediction of chemical toxicity. WIREs Comput Mol Sci. 2016;6:147–172. doi: 10.1002/wcms.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Sato K, Yama K, Murao Y, Tatsunami R, Tampo Y. Epalrestat increases intracellular glutathione levels in Schwann cells through transcription regulation. Redox Biol. 2013;2:15–21. doi: 10.1016/j.redox.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SR, Sharma N. Epalrestat, an aldose reductase inhibitor, in diabetic neuropathy: an Indian perspective. Ann Indian Acad Neurol. 2008;1(4):231–235. doi: 10.4103/0972-2327.44558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamenová D, Horváthová E, Wsólová L, Sramková M, Navarová J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat Res. 2009;677(1–2):46–52. doi: 10.1016/j.mrgentox.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Soltesova-Prnova M, Ballekova J, Gajdosikova A, Gajdosik A, Stefek M. A novel carboxymethylated mercaptotriazinoindole inhibitor of aldose reductase interferes with the polyol pathway in streptozotocin-induced diabetic rats. Physiol Res. 2015a;64(4):587–591. doi: 10.33549/physiolres.933034. [DOI] [PubMed] [Google Scholar]
- Soltesova-Prnova M, Majekova M, Milackova I, Díez-Dacal B, Pérez-Sala D, Ceyhan MS, Banerjee S, Stefek M. [5-(Benzyloxy)-1H-indol-1-yl]acetic acid, an aldose reductase inhibitor and PPARγ ligand. Acta Biochim. Pol. 2015a;62(3):523–528. doi: 10.18388/abp.2014_953. [DOI] [PubMed] [Google Scholar]
- Soltesova-Prnova M, Svik K, Bezek S, Kovacikova L, Karasu C, Stefek M. 3-Mercapto-5H-1,2,4-Triazino[5,6-b]Indole-5-Acetic Acid (Cemtirestat) Alleviates Symptoms of Peripheral Diabetic Neuropathy in Zucker Diabetic Fatty (ZDF) Rats: A Role of Aldose Reductase. Neurochem Res. 2019;44(5):1056–1064. doi: 10.1007/s11064-019-02736-1. [DOI] [PubMed] [Google Scholar]
- Stefek M, Milackova I, Díez-Dacal B, Pérez-Sala D, Soltesova-Prnova M. Use of 5-carboxymethyl-3-mercapto-1,2,4-triazino-[5,6-b]indoles and their pharmaceutical composition. Slovak Patent. 2017:No. 288508. [Google Scholar]
- Stefek M, Soltesova-Prnova M, Ballekova J, Majekova M. Cemtirestat, a novel aldose reductase inhibitor and antioxidant, in multitarget pharmacology of diabetic complications. International Journal of Advances in Science, Engineering and Technology. IRAJ. 2016;4(3):41–44. [Google Scholar]
- Stefek M, Soltesova-Prnova M, Majekova M, Rechlin C, Heine A, Klebe G. Identification of novel aldose reductase inhibitors based on carboxymethylated mercaptotriazinoindole scaffold. J Med Chem. 2015;58(6):2649–2657. doi: 10.1021/jm5015814. [DOI] [PubMed] [Google Scholar]
- Stockert JC, Horobin RW, Colombo LL, Blázquez-Castro A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochemica. 2018;120:159–167. doi: 10.1016/j.acthis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–14. [PubMed] [Google Scholar]
- Viskupicova J, Zizkova P, Rackova L, Horakova L. Pycnogenol Cytotoxicity in Pancreatic INS-1E β cells Induced by Calcium Dysregulation. Phytother Res. 2017;31(11):1702–1707. doi: 10.1002/ptr.5894. [DOI] [PubMed] [Google Scholar]
- Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21−33. [PubMed] [Google Scholar]