Abstract
The aim of the present study was to investigate the effects of Cichorium intybus on lipid peroxidation activities of both enzymatic and non-enzymatic antioxidants, inflammatory mediators, myocardial enzymes and histopathology of cardiac tissues in experimental diabetic cardiomyopathy (DCM). DCM was induced by single intraperitoneal injection of streptozotocin (STZ) (40 mg/kg) combined with high energy intake in rats. Seed extract of Cichorium intybus (CIE) (250 mg/kg & 500 mg/kg) was administered orally once a day for 3 weeks. Phytochemical investigations of seed extract revealed presence of some active ingredients such as alkaloids, tannins, saponin, phenols, glycosides, steroids, terpenoids and flavonoids. Seed extract of Cichorium intybus confirmed a significant potency towards restoring the blood glucose, an elevation of the levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), superoxide dismutase (SOD), thiobarbituric acid reactive substances (TBARS), blood glutathione (GSH), TNF-α and IL-6 and a reduction in the levels of catalase (CAT) was observed following the STZ treatment. Oxidative stress was accompanied by myocardial degeneration as evidenced by histopathological examination of cardiac tissues. Administration of CIE reduced the lipid peroxides level in heart. Serum levels of AST, GSH, LDH and SOD were brought down to physiological levels by CIE in STZ induced DCM rats. CIE also markedly down-regulated serum TNF-α and IL-6 levels. Catalase that was reduced in serum was brought back to near normal level. The extensive necrotic changes of cardiac tissue by STZ was minimized to normal morphology upon CIE administration. The study demonstrates the cardioprotective effect of CIE via inhibition of oxidative stress and pro-inflammatory cytokines.
Keywords: Cichorium intybus, diabetic cardiomyopathy, streptozotocin
Introduction
India is pertinently considered as the diabetic capital of the world with the 60 million of diabetics when contrasted with the quantity of diabetic patients around the globe which is increasing quickly and is expected to achieve 439 million by 2030 (Shaw et al., 2010). The long term complications of diabetes are of great concern. However, there is an increasing recognition that diabetes patients suffer from an additional complication termed diabetic cardiomyopathy (DCM). Diabetic cardiomyopathy, as an independent diabetic cardiovascular complication, is characterized by hypertension or valvular heart disease, the myocardial dysfunction in the absence of coronary artery disease. Past investigations reported that the pathophysiology of DCM results in increment of lipid accumulation, hyperglycemia, excessive generation of reactive oxygen species, cardiac inflammation and accumulation of cardiac fibrosis (Westermann et al., 2009; Falcão-Pires & Leite-Moreira, 2012). The existence of oxidative stress has been postulated in patients with diabetes. Lipid peroxidation (LPO) has been implicated in the pathogenesis of naturally occurring or induced diabetes (Baynes & Thorpe, 1996). Oxidative stress may be increased in diabetes owing to hyper-production of reactive oxygen species (ROS), such as O2•, OH• & H2O2 and/or a deficiency in the antioxidant defense system (Ahmed et al., 2015). The cytotoxic action of STZ is allied with the generation of reactive oxygen species causing oxidative damage (Szkudelski, 2001). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and reduced glutathione (GSH) provides support in scavenging the free radicals by breaking down the ROS (Kim JK et al., 2009). Past investigation have reported a flaw in the scavenging machinery in diabetic patients as compared to the healthy control (Anwer et al., 2012; Anwer et al., 2007).
It has been revealed after extensive investigations within the last two decades that inflammation itself mediated most chronic illnesses such as cancer, neurological, autoimmune, diabetes and cardiovascular diseases. Series of genes expressed gets stimulated by activated macrophages during inflammation in host defense, which conclude the release of different inflammatory mediators, cyclooxygenase-2, nitric oxide, pro inflammatory cytokines etc. (Yoon et al., 2009). Therefore, to prevent delay or treat the above ailment, inflammation should be suppressed. (Begum et al., 2015).
In India, plants, either medicinal or non-medicinal, have been a vital source for treatment of various ailment along with diabetes. Cichorium intybus, usually called as Kasni, is used in Unani and Ayurvedic traditional system of medicine to treat hyperglycemia as well as other diseases in India. The genus Cichorium (Asteraceae) is made up of six species and it is a wild plant with major geographical native in Europe and is common in Australia, North America and China. Chicory, a common name to Cichorium intybus, is well known as a coffee substitute and also widely used medicinally to treat various diseases ranging from diabetes to common wounds. In Eurasian countries and some parts of Africa, Cichorium intybus is most commonly used medicinal plant. In spite of its traditional use, chicory is not described in the European Pharmacopoeia or in any official Pharmacopoeia of a European Union member state (European Medicines Agency, 2013). However, due to its ubiquitous distribution several parts of the plant have been used globally in traditional medicines (Süntar et al., 2012).
With its wide therapeutic index different preparations of this plant are employed to treat various diseases. The juice extract is said to be a traditional remedy for tumors and for uterus cancer (Judžentienė & Būdienė, 2008). In South Africa, chicory syrup is used as a purifying medicine and tonic for infants (Van Wyk et al., 1997) and stems, leaves, weed, and roots are made into a tea to treat jaundice. In Turkey, an ointment is made for wound healing from the leaves of chicory (Sezik et al., 2001). The flowers of the chicory plant are used as an herbal treatment of everyday complaint such as appetite defects, sinus problems, cuts and bruises, and treatment of gallstones and gastroenteritis (Judžentienė & Būdienė, 2008). According to the European monograph, utilization of chicory roots includes the relief of symptoms related to mild digestive disorders like flatulence, slow digestion, abdominal fullness etc. and temporary loss of appetite (European Medicines Agency, 2010). The anti-malarial compounds, light-sensitive sesquiterpene lactones lactucin and lactucopicrin, were isolated and identified from chicory roots (Bischoff et al., 2004, Pieroni, 2000). In India, various categories of folk medicine from chicory plant are used. Folkloric use of c. intybus has been well documented acting as a hepato-protectant. In a commercial Unani product of India, chicory seeds are one of the main ingredients of Jigrine, used for the treatment of various diseases of the liver (Ahmed et al., 2003). Liv-52, a traditional Indian tonic used widely for hepato-protection (Huseiniet al., 2005) is also one of the herbal components of Ayurvedic system of Indian medicine.
Cichiroum intybus seed consist of Chicoric acid which is a major compound in methanolic extracts of chicory. Aliphatic compounds and their derivatives represent the major fractions while minor constituents of the plant comprised of terpenoids. The flowers of chicory comprise flavonoids, saccharides, essential oils, methoxy-coumarin cichorine etc. (Judžentienė & Būdienė, 2008). Numerous studies on Cichiroum intybus plant indicated that it possesses antimalarial, antiinflammatory, antimicrobial, antihelmintic, analgesic activity, antiallergic activity, antioxidant, tumor-inhibiting activity, gastro and hepatoprotective effects besides its positive influence in diabetes (Ahmed et al., 2003; Huseiniet al., 2005; Nandagopal & Kumari, 2007; Miller et al., 2011; Gürbüz et al., 2002; Cavin et al., 2005; Wesołowska et al., 2006; Heimler et al., 2009; Conforti et al., 2008; Kim et al., 1999; Pushparaj et al., 2007). The antidiabetic effect of the aqueous seed extract of Cichiroum intybus has also been investigated in early-stage diabetic rats, chicory treatment led to the increase in insulin levels pointing toward the insulin-sensitizing action of chicory (Ghamarian et al., 2012). However, there is a lack of experimental evidence on the favorable role of Cichorium intybus in STZ induced diabetic cardiomyopathy. In view of all the above reports we chose to evaluate chicory for possible beneficial actions in STZ induced diabetic cardiomyopathy.
Materials and methods
Experimental animals
Healthy Albino Wistar rats (8–10 weeks old), weighing about 150–180 g were procured from the Central Animal House Facility, Hamdard University, New Delhi, India. The animals had free access to standard laboratory food and water ad libitum, and they were housed in a natural light-dark cycle (12 h each). The experimental protocol was approved by the Institutional Animal Ethics Committee and the care of laboratory animal was taken as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Drugs and chemicals
The seeds of Cichorium intybus were procured from Hamdard Wakf Laboratories, India and was authenticated by chief scientist, Raw Material Herbarium and Museum, CSIR- NISCAIR ( Ref. no. NISCAIR/RHMD/Consult/2014/2538/117). streptozotocin (STZ) was purchased from Sigma Aldrich, St. Louis, USA. The AST, LDH, SOD and CAT diagnostic kits were purchased from Span diagnostics, Surat, India. Rat TNF-α and IL-1β cytometric assay kits were purchased from BD Biosciences, India. All the other biochemicals and chemicals used in the present study were of analytical grade.
Induction of experimental diabetes
Experimental diabetic rats were induced by feeding with high fat diet (HFD) during the whole experimental period. HFD was prepared by adding 20% sucrose (w/w) and 20% lard (w/w) into normal pellet diet. After 8 weeks of dietary manipulations, rats were intraperitoneally injected with STZ (40 mg/kg) dissolved in 100 mM citrate buffer pH 4.5 bolus of STZ. Control rats were administered an equivalent volume of citrate buffer (Wang et al., 2007). Blood glucose levels were measured 72 h after STZ injection and rats which had blood glucose values ≥11.6 mmol/l were used in the groups III–V.
Preparation of extract and preliminary phytochemical studies
Chicory seeds were cleaned and powdered using an electric mill. Every 200 g was soaked in 1 l of distilled water and refluxed for 20 minutes in a boiling water bath to make a 20% solution. The solution was allowed to cool at room temperature before being vacuum filtered through Whatman No. 1 filter paper. The filtrate was lyophilized and stored at −20 °C; every 100 g of powdered seed yielded about 8.2 g of lyophilized substance. The extract was suspended in distilled water and was administered orally to the animals using feeding tubes in doses of 250 mg/kg and 500 mg/kg. Different secondary metabolites are present in plant materials which exhibit various pharmacological activities. Crude extracts were subjected to phytochemical analysis for identification of alkaloids, cardiac glycosides, steroids, tannins, and saponins following standard protocol (Trease & Evans, 2002).
Experimental design
The rats were divided into five groups comprising of six animals in each group as follows:
Group I: Healthy control, received citrate buffer (1 ml/kg, i.p.)
Group II: per se, received only CIE (500 mg/kg, p.o.)
Group III: Diabetic cardiomyopathy (DCM), STZ single dose (40 mg/kg, i.p.) + HFD
Group IV: STZ + HFD + CIE (250 mg/kg, p.o.)
Group V: STZ + HFD + CIE (500 mg/kg, p.o.)
Diabetic animals were treated with chicory seed extract (250 mg/kg and 500 mg/kg, p.o.) for 3 weeks. On the last day of experiment, blood samples were collected by retro-orbital puncture for biochemical estimations and animals were sacrificed by cervical decapitation under light chloroform anesthesia and organs were collected for histopathological examination.
Measurements of glycemic level in serum
By using digital glucometer (Gluco One) blood glycemic level was determined using serum.
Measurements of myocardial enzymes and inflammatory cytokines in serum
Aspartate Aminotransferase (AST) and Lacate Dehydrogense (LDH) were determined by colorimetric analysis using a spectrophotometer with the associated detection kits (Reitman & Frankel, 1957; McLauchlan DW et al., 1988). TNF-α and IL-6 in serum were determined by cytometric method following the manufactures instructions (Brouckaert et al., 1993).
Estimation of Superoxide Dismutase (SOD) and Malondialdehyde (MDA) in heart tissue
SOD activity was measured according to the method of Marlund and Marklund (Marklund & Marklund, 1974). The enzyme activity was expressed in unit/mg of protein and 1 unit of enzyme is defined as the enzyme activity that inhibits autoxidation of pyrogallol by 50%. The concentration of MDA was measured in heart using the method of Ohkawa et al. 1979 (Ohkawab et al., 1979). Briefly, the heart tissues collected soon after sacrificing the rats, were suspended in 150 mM KCl and homogenized in Teflon homogenizer. The concentration of thiobarbituric acid reactive substances (TBARS) was expressed as nmol of MDA/mg of protein using 1,1,3,3,-tetraethoxypropane as standard. The protein were estimated by the method of Lowry et al. 1951 (Lowry et al., 1951).
Determination of reduced and total glutathione (GSH)
Freshly collected heart tissues were weighed and 10% (w/v) homogenates were made in 1.15 M KCl using a motor driven Teflon-pestle. GSH was estimated using the method of Sedlak and Lindsay 1968 (Sedlak & Lindsay, 1968) that uses 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB). The total glutathione in blood was estimated by the method of Beutler et al. 1963 (Beutler et al., 1963). The absorbance was read at 412 nm.
Determination of CAT
CAT activity was estimated following the method of Claiborne (1985) based on a decrease in absorbance at 240 nm due to consumption of hydrogen peroxide (H2O2).
Determination of SGOT
Recombinogen kit was used for SGOT estimation. 2 ml of blood samples was collected in centrifuge tube and left to stand for 1 hour at 37 °C and further samples was cooled in refrigerator for 3 hours. The clot formed was then removed and serum samples were decanted out. These serum samples were then centrifuged at 3000 rpm for 15 minutes. The supernatant after centrifugation was the serum sample used for analysis.
Histopathology
10% neutral buffered formalin fixed hearts were dehydrated in ascending grades of isopropyl alcohol embedded in paraffin wax and 5 μm sections were stained by haematoxylin and eosin stain.
Statistical analysis
Data were expressed as the mean ±SEM. For a statistical analysis of the data, group means were compared by one way ANOVA with post hoc analysis. The Tukey-Karmer post hoc test was applied to identify significance among groups; p<0.05 was considered to be statistically significant.
Results
Preliminary phytochemical screening
The results of the preliminary phyto-chemical screening of cichorium intybus seeds extract (CIE) showed the presence of alkaloids, phenolics, flavonoids, tannins, steroids, anthraquinones and saponin glycosides.
Chemical constituents
Various compounds from the extraction of Cichorium intybus have been isolated and identified and a summary of isolated and identified chemicals are listed below in (Table 1). (Street et al. 2013)
Table 1.
Isolated and identified compounds from the extraction of Cichorium intybus seed.
| S.No. | Compound |
|---|---|
| 1. | 11,13-Dihydrolactucopicrin |
| 2. | 11β,13-Dihydrolactucin |
| 3. | Caffeic acid |
| 4. | Chicoric acid |
| 5. | Chlorogenic acid |
| 6. | Cichorioside |
| 7. | Cichorioside B |
| 8. | Cyanidin |
| 9. | Kaempferol-3-O-glucoside |
| 10. | Kaempferol-3-O-glucosyl-7-O-(6”-O-malonyl)-glucoside |
| 11. | Kaempferol-7-O-glucoside |
| 12. | Lactucin |
| 13. | Lactucopicrin |
| 14. | Malic acid |
| 15. | Pelargonidin-3-O-monoglucuronide |
| 16. | Quercetin-3-O-glucuronide-7-O-(6”-O-malonyl)-glucoside |
| 17. | Quercetin-7-O-(6”-O-acetyl)-glucoside |
| 18. | Quercetin-7-O-galactoside |
| 19. | Tricin-3-O-glucoside |
| 20. | β-Sitosterol |
Effect of CIE on blood glucose in STZ diabetic rats
In group III (Diabetic group) the blood glucose was at highest level and after the administration of chicory decrease of level of blood glucose was observed. (Table 2).
Table 2.
Effect of Cichorium intybus seed extract on blood glucose of rats.
| S.No. | Treatment | Glucose (mg/dl) |
|---|---|---|
| 1. | Control (1 ml/kg. i.p.) | 100.67±6.19 |
| 2. | Per se (500 mg/kg, p.o.) | 116.33±2.4 |
| 3. | STZ Single dose (40 mg/kg, i.p.)+HFD | 306.33±10.18* |
| 4. | STZ+HFD+CIE (250 mg/kg, p.o.) | 121.33±4.01** |
| 5. | STZ+HFD+CIE (500 mg/kg, p.o.) | 106.67±3.26** |
Data shown as means±SEM from 6 animals. All comparisons of group IV and V with group III by student’s t test (*p<0.001). All comparisons of group I, II, IV and V with group III by ANOVA (**p<0.001).
Effect of CIE on myocardial enzymes in STZ diabetic rats
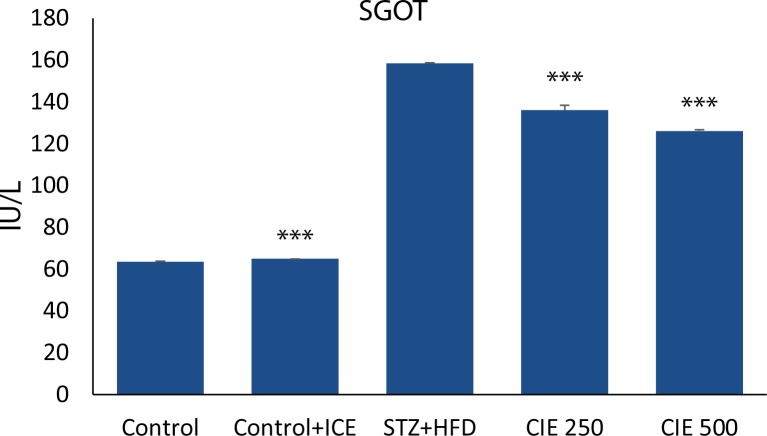
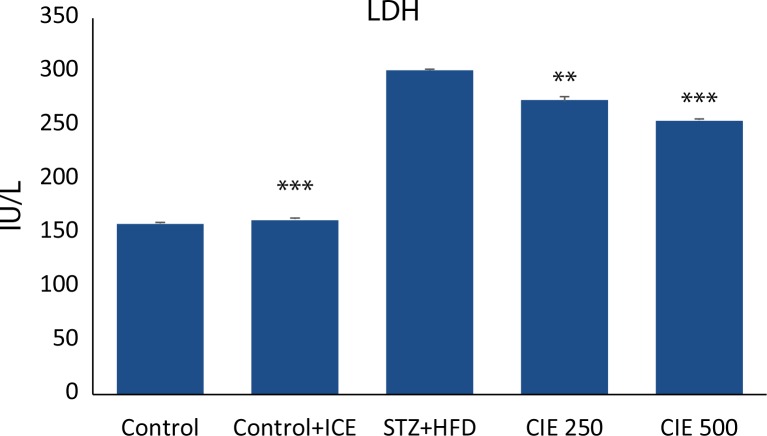
Myocardial enzymes SGOT and LDH are biochemical indicators of myocardial injury. A significant increase (p<0.01) ( Figures 1–2) in the levels of these enzymes was observed in animals treated with STZ. Administration of CIE at doses 250 mg/kg and 500 mg/kg significantly reduced (p<0.01) the level of these enzymes when compared to STZ treatment alone.
Figure 1.
Effect of Cichorium intybus treatment on SGOT level in rats. Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
Figure 2.
Effect of Cichorium intybus treatment on LDH level in rats. Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
Effect of CIE on STZ induced LPO
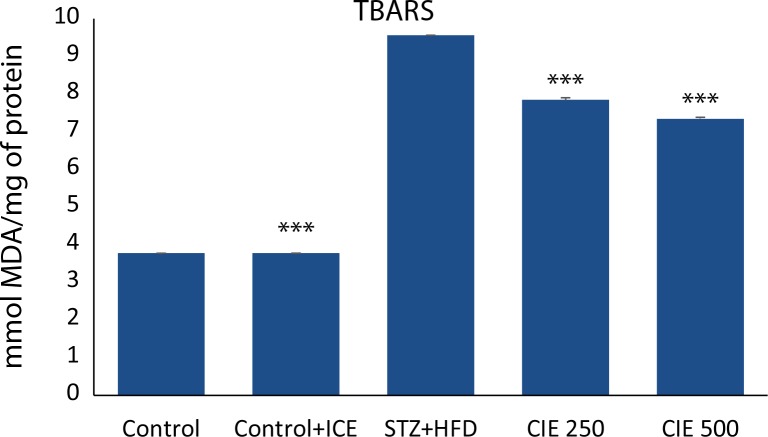
Freshly prepared heart homogenates were studied for the concentration of malondialdehyde (MDA). Rats treated with STZ alone had a significant increase (p<0.001) in the level of MDA. Simultaneous treatment of CIE at two concentrations reduced the level of MDA in heart (p<0.01) as shown in (Figure 3).
Figure 3.
Effect of Cichorium intybus treatment on TBARS in the heart of rats. Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
Effect of CIE on STZ induced changes in GSH contents
The glutathione levels in blood and reduced glutathione in heart homogenates are shown in (Table 3). The concentration of glutathione in animals treated with STZ was significantly increased both in blood and in homogenates of heart (p<0.001) as compared to control rats. Co-administration of CIE extract in two doses (250 mg/kg and 500 mg/kg) decreased the concentration of total and reduced glutathione in blood and heart (p<0.001). However, the values remained at higher than physiological levels at the time of estimation.
Table 3.
Effect of Cichorium intybus seed extract on glutathione in blood and heart muscle of rats.
| Groups | Treatment | Whole Blood glutathione (mg %) | Tissue GSH (μmole/g. wt of tissue) |
|---|---|---|---|
| I | Control (1 ml/kg. i.p.) | 1.09±0.053 | 1.89±0.082 |
| II | Per se (500 mg/kg, p.o.) | 1.14±0.107** | 1.97±0.077** |
| III | STZ Single dose (40 mg/kg, i.p.)+HFD | 2.10±0.071** | 3.77±0.047** |
| IV | STZ+HFD+CIE (250 mg/kg, p.o.) | 1.61±0.064**, * | 3.28±0.098**,* |
| V | STZ+HFD+CIE (500 mg/kg, p.o.) | 1.56±0.033*** | 2.87±0.08**, * |
Data shown as means ± SEM from 6 animals. All comparisons of group IV and V with group III by student’s t test (*p<0.001). All comparisons of group I, II, IV and V with group III by ANOVA (**p<0.001).
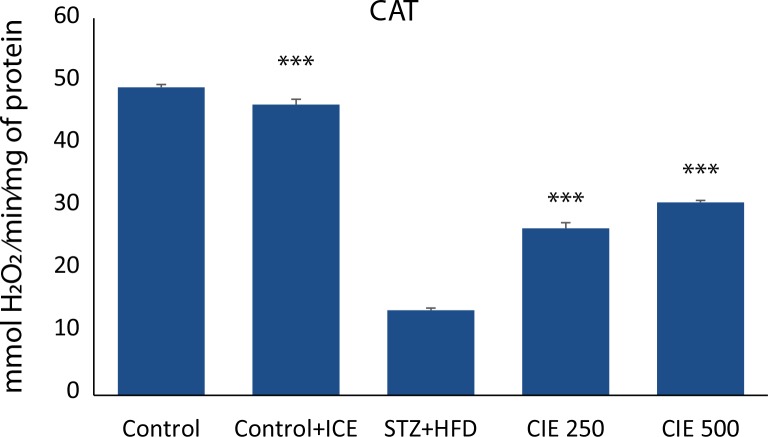
Effect of CIE on STZ-induced changes in activity of antioxidant enzymes
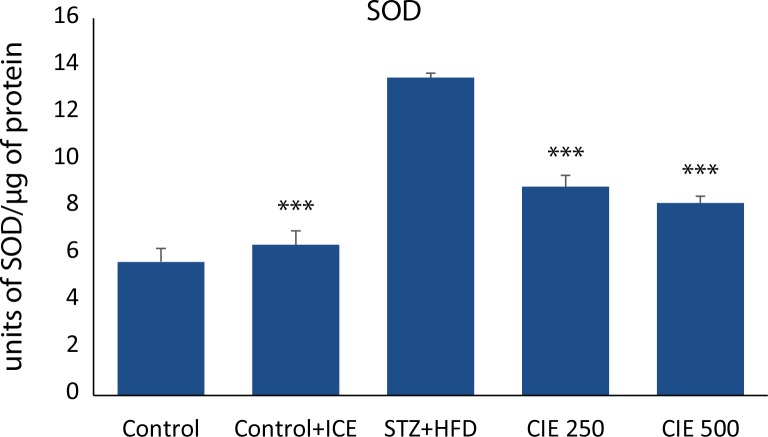
Figure 4 and 5 demonstrate the activities of SOD and CAT in cardiac tissue of healthy control, CIE only treated rats, diabetic control, and CIE treated diabetic rats. Only CIE treatment did not register any significant change in the activities of antioxidant enzymes when compared with healthy control rats. A significant (p<0.001) decrease in the activity of CAT and a significant increase in the activity of SOD activity was a notable manifestation of STZ toxicity. Administration of CIE significantly (p<0.001) improved the activities of these enzymes in diabetic rats when compared with diabetic control rats.
Figure 4.
Effect of Cichorium intybus treatment on SOD in the heart of rats. Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
Figure 5.
Effect of Cichorium intybus treatment on CAT in the heart of rats.Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
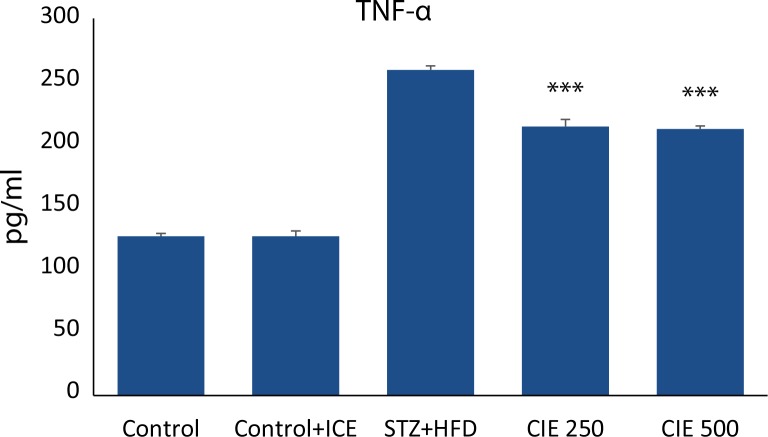
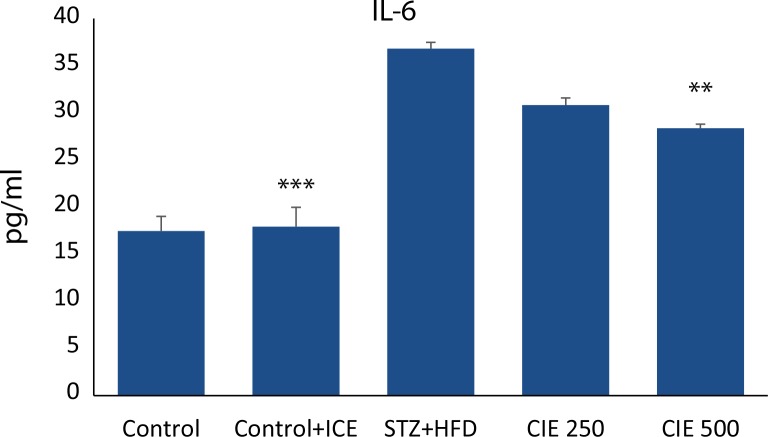
Effect of CIE on inflammatory biomarkers in STZ diabetic rats
To further elucidate the mechanism of action of CIE in myocardial inflammation, pro-inflammatory cytokines such as TNF-α and IL-6 were determined using Cytometric Bead Array technique. The serum level of TNF-α and IL-6 in STZ induced diabetic group was significantly (p<0.001) increased compared with the healthy control group as shown in ( Figure 6–7). However, the levels were significantly lowered in animals treated concomitantly with two doses of CIE (p<0.001). No significant difference in IL-6 level was found at the lower dose of CIE.
Figure 6.
Effect of Cichorium intybus treatment on TNF-α levels in the heart of rats. Values are expressed as mean ± SEM (n= 6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
Figure 7.
Effect of Cichorium intybus treatment on IL-6 level in the heart of rats. Values are expressed as mean ± SEM (n=6). Significant differences are indicated by ***p<0.001; **p<0.01; *p<0.05 vs. group III.
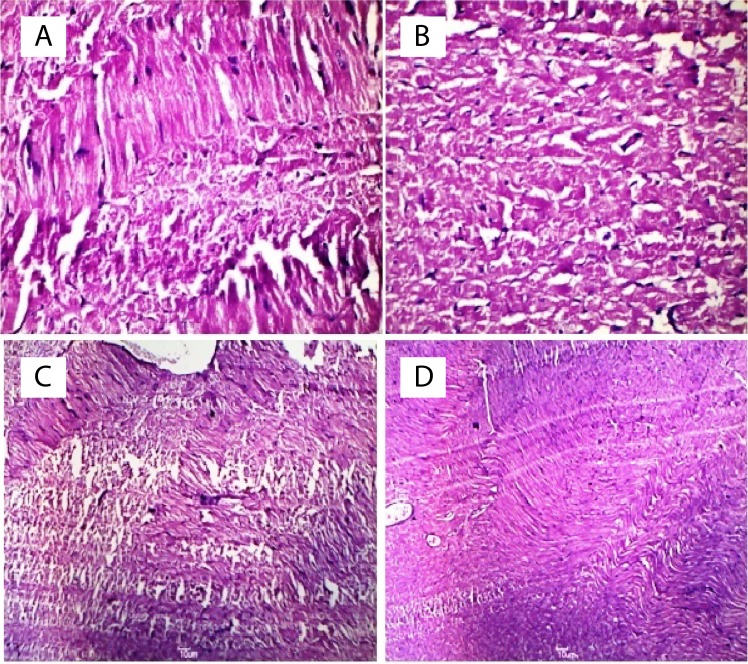
Effect of CIE on STZ induced histological changes on heart
Intraperitoneal injection of STZ along with HFD, induced myocardiopathy, characterized by extensive myocardial necrosis associated with massive leucocytic infiltrations predominantly lymphocytes with occasional polymorphs ( Figure 8B). The changes were predominantly observed in the ventricular myocardium and subendocardial myocardium. CIE at the dose of 500 mg/kg had remarkable cardioprotective effect as evidenced by minimal myocardial necrosis and infiltrative changes ( Figure 8D). CIE at dose 250 mg/kg was less effective in providing the protective effect on heart from STZ induced necrosis.
Figure 8.
Histopathological changes in heart of rat and in animal treated with Cichorium intybus. Figure A- Normal rat heart shows normal morphology of cardiomyocyte. Figure B- Degenerative changes in many myocardial fibres and massive leucocytic infilterations are seen in heart of STZ diabetic rats. Figure C- CIE-250mg/kg treated heart shows mild peripheral necrosis and edema with occasional polymorphs. Figure D- A few myocardial fibres show degenerative changes and necrosis in CIE-500mg/kg treated rats and a minimal infilterative changes. Myocardial tissue sections stained with hematoxylin and eosin (magnification=400X).
Discussion
The present study was undertaken to evaluate the protective effect of cichorium intybus on STZ induced diabetic cardiomyopathy in rats. Experimental diabetes produced by low dose of STZ combined with high energy intake is regarded as a general strategy to obtain type-2 diabetes animal model, since it stimulates the real course of human type-2 DM (Wang et al., 2007; Ti et al., 2011). The high energy intake induces insulin resistance at first and subsequently an injection of low dose STZ makes partial dysfunction of beta cells to suppress insulin secretion which works as a compensation to insulin resistance.
The cytotoxic action of STZ is associated with generation of ROS causing oxidative damage (Szkudelski, 2001). Disturbances of antioxidant defense systems in diabetes have been demonstrated, including alterations in the activities of antioxidant enzymes such as SOD, CAT and impaired glutathione metabolism (Maritim & Sanders, 2003). Chemicals with antioxidant properties and free radical scavengers may help in the regeneration of beta cells and protect pancreatic islets against cytotoxic effects of STZ (Alvarez et al., 2004). Decreased LPO and improved antioxidant status may be one mechanism by which dietary treatment contributes to the prevention of diabetic complications (Armstrong et al., 1996). Myocardial necrosis produced by STZ is also the consequence of increase LPO due to generation of free radicals. Cichorium seed extract protects the myocardium from peroxidative damage.
Chicory has reported antidiabetic activity (Pushparaj et al., 2007; Ghamarian et al., 2012) and as evidenced in the present study chicory seed extract possesses a strong antioxidant property. Decreased LPO and improved antioxidant status may be one mechanism by which dietary treatment contributes to the prevention of diabetic complications. The glucose lowering capacity of chicory has been attributed to its chemical composition including antioxidant compounds (Hassan & Yousef, 2010). Chicoric acid, a compound isolated from chicory, is also a new potential antidiabetic agent exhibiting both insulin-sensitizing and insulin-secreting properties (Tousch et al., 2008).
The LDH and AST levels, which were significantly increased following myocardial injury by STZ, were reduced to physiological level in serum upon administration of CIE in two doses. However, the 500 mg/kg dose of chicory had better protective effect than the 250 mg/kg dose group. An elevation of reduced GSH in heart muscle and total glutathione in whole blood was observed in STZ diabetic rats. The demonstration of increased myocardial levels of total and reduced glutathione is consistent with an activation of the glutathione-peroxidase redox pathway secondary to the generation of and subsequent detoxification of STZ induced free radicals (Jackson et al., 1984). Increase in cardiac muscle GSH suggest that the myocardial detoxification system is not overwhelmed as has been suggested. The presence of myocyte damage, as demonstrated histologically, suggests that injury has occurred despite the elevation of GSH level. If the rise in GSH is in response to free radicals, it would appear that the system is able to cope adequately with their production indicating contributory effect of other mechanisms in cardiac damage by STZ. The lowering of the level of GSH by chicory suggests scavenging effect of chicory on free radicals responsible for the elevation of GSH in heart muscle. The role of chicory in this context requires further study.
Furthermore, cardiac inflammation, characterized by increased levels of pro-inflammatory cytokines, was suppressed by chicory as well. Pro-inflammatory cytokines such as TNF-α and IL-6 are chief mediators of inflammatory reaction and critically participate in the manifestation of DCM. Chicory has been used as an anti-inflammation agent for generations (Cha et al., 2011). TNFα plays several important roles in inflammation based on its appearance at the inflammatory site and ability to induce certain mechanisms including activation and chemotaxis of leukocytes, expression of adhesion molecules on neutrophils and endothelial cells, and regulation of the secretion of other pro-inflammatory cytokines (Collins & Grounds, 2001). The TNF-α stimulates hyperlipidemia and hepaticlipogenesis simultaneously reducing the sensitivity to insulin in muscle tissues and finally the necrosis of target organs (Khanra et al., 2015).
Biochemical observations were in keeping with the morphological changes in the heart. Supplementation with CIE prevented the disorganization of cell plates and restored the cardiac cytoarchitecture nearly similar to that of normal rats. The extensive necrotic and abundant infiltrative changes in heart, which were consistently observed in STZ diabetic animals, were reduced to minimum with chicory co-treatment and further substantiated the protective effect of chicory in heart.
In summary, the present study shows that the administration of chicory extract shows remarkable hyperglycemic effect as well as reduces the tissue specific marker enzymes, lipid peroxides, GSH and pro-inflammatory cytokines which are elevated in response to acute administration of STZ and thereby demonstrates its cardioprotective effect. Analytical studies determining the active component of chicory responsible for cardioprotective effect are yet to be isolated and confirmed. Detailed mechanistic action of chicory, particularly the flavonoids present in CIE, on different free radicals requires further study.
Acknowledgements
Authors wish to acknowledge Jamia Hamdard for providing necessary facilities and other documentary works.
REFERENCES
- Ahmed B, Al-Howiriny TA, Siddiqui AB. Antihepatotoxic activity of seeds of Cichoriumintybus. J Ethnopharmacol. 2003;87:237–240. doi: 10.1016/s0378-8741(03)00145-4. [DOI] [PubMed] [Google Scholar]
- Ahmed D, Sharma M, Kumar V, Bajaj HK, Verma A. 2β-hydroxybetulinic acid 3β-caprylate: an active principle from Euryale FeroxSalisb. seeds with antidiabetic, antioxidant, pancreas & hepatoprotective potential in streptozotocin induced diabetic rats. J Food Sci Tech. 2015;52(9):5427–5441. doi: 10.1007/s13197-014-1676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JF, Barbera A, Nandal B, Barcello-Batllori S, Piquer S, Claret M, Guninovart JJ, Gomis R. Stable and functional regeneration of pancreatic beta cell population in n-STZ rats treated with tungstate. Diabetologia. 2004;47:470–477. doi: 10.1007/s00125-004-1332-8. [DOI] [PubMed] [Google Scholar]
- Anwer T, Sharma M, Pillai K, Haque S, Alam M, Zaman M. Protective effect of bezafibrate on streptozotocin-induced oxidative stress and toxicity in rats. Toxicology. 2007;229:165–172. doi: 10.1016/j.tox.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Anwer T, Sharma M, Pillai KK, Khan G. Protective effect of Withaniasomnifera against oxidative stress and pancreatic beta-cell damage in type 2 diabetic rats. Acta Pol Pharm. 2012;69:1095–1101. [PubMed] [Google Scholar]
- Armstrong AM, Chestnutt JE, Gormley MJ, Young IS. The effect of dietary treatment on lipid peroxidation and antioxidant status in newly diagnosed noninsulin dependent diabetes. Free Rad Biol Med. 1996;21:719–726. doi: 10.1016/0891-5849(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. The role of oxidative stress in diabetic complications. Current Opinion in Endocrinology. Diabetes and Obesity. 1996;3:277–284. [Google Scholar]
- Begum R, Sharma M, Pillai K, Aeri V, Sheliya MA. Inhibitory effect of Careyaarborea on inflammatory biomarkers in carrageenan-induced inflammation. Pharmaceutical Biol. 2015;53:437–445. doi: 10.3109/13880209.2014.923005. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clinic Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bischoff TA, Kelley CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Arefi AG. Antimalarial activity of Lactucin and Lactucopicrin. sesquiterpene lactones isolated from Cichoriumintybus L. J Ethnopharmacol. 2004;95:455–457. doi: 10.1016/j.jep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Brouckaert P, Libert C, Everaerdt B, Takahashi N, Cauwels A, Fiers W. Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiol. 1993;187:317–329. doi: 10.1016/S0171-2985(11)80347-5. [DOI] [PubMed] [Google Scholar]
- Cavin C, Delannoy M, Malnoe A, Debefve E, Touche A, Courtois D, Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem and Biophy Res Communic. 2005;327:742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Cha DS, Eun JS, Jeon H. Anti-inflammatory and antinociceptive properties of the leaves of Eriobotrya japonica. J Ethnopharmacol. 2011;134:305–12. doi: 10.1016/j.jep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook for methods for Oxygen Radical Research. Boca Raton F L: CRC Press; 1985. pp. 283–284. [Google Scholar]
- Collins RA, Grounds MD. The role of tumour necrosis factor –alpha (TNF-α) in skeletal muscle regeneration. (2001). Studies in TNF-α (–/–) and TNF-α (–/–)/LT-α (–/–) mice. J Histochem Cytochem. 49:989–1001. doi: 10.1177/002215540104900807. [DOI] [PubMed] [Google Scholar]
- Conforti F, Ioele G, Statti G, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food ChemToxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . “Assessment report on Cichoriumintybus L., radix,”. 2013. EMA/HMPC/113041/2010. [Google Scholar]
- European Medicines Agency . “Community herbal monograph on Cichoriumintybus L., radix,”. 2012. EMA/HMPC/121816/2010. [Google Scholar]
- Evans WC. Trease and Evans Pharmacognosy. 15 ed. Vol. 60. London: Saunders Publishers; 2002. pp. 357–359. [Google Scholar]
- Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Failure Reviews. 2012;17:325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU J Pharmaceutical Sci. 2012;20:1–9. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürbüz Il, Üstün O, Yeşilada E, Sezik E, Akyürek N. In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions. J Ethnopharmcol. 2002;83:241–244. doi: 10.1016/s0378-8741(02)00248-9. [DOI] [PubMed] [Google Scholar]
- Hassan HA, Yousef MI. Ameliorating effect of chicory (Cichoriumintybus L)- supplemented diet against nitrosamine precursors- induced liver injury and oxidative stress in rats. Food Chem Toxicol. 2010;48:2163–2169. doi: 10.1016/j.fct.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Heimler D, Isolani L, Vignolini P, Romani A. Polyphenol content and antiradical activity of Cichoriumintybus L. from biodynamic and conventional farming. Food Chemistry. 2009;114:765–770. [Google Scholar]
- Huseini HF, Alavian S, Heshmat R, Heydari M, Abolmaali K. The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomed. 2005;12:619–624. doi: 10.1016/j.phymed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Reeves JP, Muntz KH, Kruk D, Prough RA, Willerson JT, Buja L. M. Evaluation of free radical effects and catecholamine alterations in adriamycin cardiotoxicity. American J of Pathol. 1984;117:140–153. [PMC free article] [PubMed] [Google Scholar]
- Judžentienė A, Būdienė J. Volatile constituents from aerial parts and roots of Cichorium intybus L. (chicory) grown in Lithuania. Chemija. 2008;19:25–28. [Google Scholar]
- Khanra R, Dewanjee S, Dua TK, Sahu R, Gangopadhyay M, De Feo V, et al. Abromaaugusta L.(Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Translational Med. 2015;13:1–14. doi: 10.1186/s12967-014-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Kim HW, Lyu YS, Won JH, Kim DK, Lee YM, Morii E, Jippo T, Kitamura Y, An NH. Inhibitory effect of mast cell-mediated immediate-type allergic reactions by Cichorium intybus. Pharmacological Res. 1999;40:61–65. doi: 10.1006/phrs.1999.0474. [DOI] [PubMed] [Google Scholar]
- Kim KW, Suh SJ, Kim JD, Kim SS, Lee IS, Kim JK, Chang GT, Kim DS, Kim CH. Effects on lipid peroxidation and antioxidative enzymes of Euonymus alatus in cultured rat hepatocytes. Basic & Clinic Pharmacol & Toxicol. 2009;104:60–70. doi: 10.1111/j.1742-7843.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maritim AC, Sanders RA. Effects of alpha lipoic acid on biomarkers of oxidative stress in streptozotocin induced diabetic rats. J Nutr Biochem. 2003;14:288–294. doi: 10.1016/s0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochemn. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McLauchlan DW. Enzymes. In: Gowenlock AH, McMurry JR, McLauchlan DM, editors. Varley’s. Practical Clinical Biochemistry. 6th ed. London: Heinemann; 1988. pp. 477–549. [Google Scholar]
- Miller M, Duckett S, Andrae J. The effect of forage species on performance and gastrointestinal nematode infection in lambs. Small Ruminant Res. 2011;95:188–192. [Google Scholar]
- Nandagopal S, Kumari BR. Phytochemical and antibacterial studies of Chicory (Cichorium intybus L.)-A multipurpose medicinal plant. Adv Biol Res. 2007;1:17–21. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J Ethnopharmacol. 2000;70:235–273. doi: 10.1016/s0378-8741(99)00207-x. [DOI] [PubMed] [Google Scholar]
- Pushparaj P, Low H, Manikandan J, Tan B, Tan C. Anti-diabetic effects of Cichoriumintybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transminases. American J Clin Pathol. 1957;28:53–56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Bio chem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in central Anatolia. J Ethnopharmacol. 2001;75:95–115. doi: 10.1016/s0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Practical. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Street RA, Sidana J, Prinsloo G. Cichorium intybus: Traditional uses, Phytochemistry, Pharmacology and Toxicology. Evidence-Based Complimentary and Alternative Medicine. 2013;579319:1–13. doi: 10.1155/2013/579319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süntar I, Akkol EK, Keles H, Yesilada E, Sarker SD, Baykal T. Comparative evaluation of traditional prescriptions from Cichoriumintybus L. for wound healing: stepwise isolation of an active component by in vivo bioassay and its mode of activity. J Ethnopharmacol. 2012;143:299–309. doi: 10.1016/j.jep.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research. 2001;50:537–546. [PubMed] [Google Scholar]
- Ti Y, Xie GL, Wang ZH, Bi XL, Ding WY, Wang J, Jiang GH, Bu PL, Zhang Y, Zhong M, Zhang W. TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes. 2011;60:2963–2974. doi: 10.2337/db11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousch D, Lajoix AD, Hosy E, Azay-Milhau J, Ferrare K, Jahannault C, Cros G, Petit P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Bio chemic and Bio phy Res Communic. 2008;377:131–135. doi: 10.1016/j.bbrc.2008.09.088. [DOI] [PubMed] [Google Scholar]
- Van Wyk B-E, Oudtshoorn Bv, Gericke N. Medicinal Plants of South Africa: Briza. Pretoria, South Africa: Briza Publications; 1997. [Google Scholar]
- Wang HJ, Jin YX, Shen W, Neng J, Wu T, Li YJ, Fu ZW. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac J Clin Nutr. 2007;16:412–417. [PubMed] [Google Scholar]
- Wesołowska A, Nikiforuk A, Michalska K, Kisiel W, Chojnacka-Wójcik E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J Ethnopharmacol. 2006;107:254–258. doi: 10.1016/j.jep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Westermann D, Walther T, Savvatis K, Escher F, Sobirey M, Riad A, Bader M, Schultheiss HP, Tschöpe C. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–1381. doi: 10.2337/db08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon W-J, Ham YM, Kim S-S, Yoo B-S, Moon J-Y, Baik JS, Lee NH, Hyun C-G. Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW 264.7 macrophages. Eur Asia J Bio Sci. 2009;3:130–143. [Google Scholar]