Abstract
Introduction:
Keratoconus is described as a degenerative bilateral, progressive, noninflammatory corneal disorder characterized by ectasia, thinning, and increased curvature. Keratoconus progression classification 1 year after performed crosslinking method in this study is based on the ABCD keratoconus grading system.
Aim:
To evaluate the possible keratoconus progression one year after performed a crosslinking (CXL) method based on the ABCD keratoconus grading system. Methods: Seventeen keratoconus patients (22 eyes) were included in this prospective study. CXL procedure was performed using the standard Dresden protocol at Eye Clinic Svjetlost Sarajevo with the inclusion period from January 2017 to January 2018. Twelve patients had monocular, and 5 patients had binocular treatments with follow up of 12 months. Preoperative and postoperative stages were compared using the ABCD keratoconus grading system measured on rotating Scheimpflug corneal tomography-based machine - Pentacam (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany).
Results:
Out of 22 eyes, one eye had keratoconus stage I-II, 6 eyes had stage II, 4 eyes had stage III, and 9 eyes had stage III-IV. There was no statistically significant gradient change of keratoconus in comparison to one month after the surgery, p>0.05. There was no progression of the gradient when comparing to the preoperative stage.
Conclusions:
Corneal cross-linking could effectively stabilize the progression of keratoconus, as assessed by key corneal topographic parameters. Analyzing the trend of stage change in 12 months follow up after the crosslinking procedure of keratoconus patients there was no progression of a gradient in comparison to the preoperative stage. According to our results, we can conclude that CXL is a safe and effective procedure in treating keratoconus.
Keywords: keratoconus, cross linking, ABCD gradation system, pentacam
1. INTRODUCTION
Keratoconus is described as a degenerative bilateral, progressive, noninflammatory corneal disorder characterized by ectasia, thinning, and increased curvature (1). It is associated with loss of visual acuity particularly concerning progressive corneal irregularity, (2) and usually is manifested asymmetrically between the two eyes of the same patient (3, 4). Keratoconus is the most common primary ectasia. Corneal thinning normally occurs in the inferotemporal as well as the central cornea, (5) although superior localizations have also been described (6). Corneal protrusion causes high myopia and irregular astigmatism, affecting visual quality. It usually becomes apparent during the second decade of life, normally during puberty, (7) although the disease has also been found to develop earlier (8) and later in life, and it typically progresses until the fourth decade of life, when it usually stabilizes. In the past, the diagnosis of this disease was based on clinical findings and typical slit-lamp signs (i.e. Fleischer ring, Vogt striae, Munson sign or Rizzuti sign) (1).
Corneal topography measurements represented a true revolution in the diagnosis and management of corneal disease (6, 7). Several classification systems for keratoconus have been proposed in the literature (4, 8). The Amsler-Krumeich (AK) system is amongst the oldest, and the severity of keratoconus is graded from stage 1–4 using spectacle refraction, central keratometry, presence or absence of scarring, and central corneal thickness (4). According to the Global Consensus on Keratoconus and Ectatic Diseases (2015), there is currently no clinically adequate classification system for keratoconus (9). The AK classification fails to recognize any changes other than on the anterior corneal surface. The Belin-Ambrosio Enhanced Ectasia Display (BAD) display (available on the Pentacam, OCULUS GmbH, Wetzlar, Germany) utilizes both anterior and posterior elevation data and pachymetric data to screen for an ectatic change. It displays the elevation data against the commonly used best-fit sphere (BFS) taken from the central 8.0 mm zone and also uses a reference surface called the “Enhanced Reference Surface” (10-11).
The goal of the BAD display was to develop a classification/staging system that had some similarities to the AK system for anterior data but addressed the deficiencies. Corneal UV crosslinking (CXL) has been established as an effective treatment of keratoconus. This technique increases the corneal rigidity and improves the biomechanical properties of the cornea by strengthening the crosslinks between collagen lamellae and stabilizes the corneal tissue (12). Nowadays, the cross-linking (CXL) procedure using the standard Dresden protocol is established as the gold standard for the treatment of progressive keratoconus (13).
2. AIM
To evaluate the possible keratoconus progression one year after performed crosslinking (CXL) method based on the ABCD keratoconus grading system.
3. PATIENTS AND METHODS
This was a prospective study that included 22 eyes of 17 patients. Of 17 patients included in the study, 12 of them had a procedure performed on one eye, and 5 patients had binocular treatments. The study was conducted at Eye Clinic Svjetlost Sarajevo from January 2017 to January 2018. Inclusion criteria were: patients diagnosed with keratoconus and progression of steepest meridian of 1 diopter (D) or more within a year, but not more than 60 D, subjective decrease of visual acuity (VA), CDVA of 0.8 or less, age frame of 15 to 40 years, and pachymetry of 400 microns or more. Exclusion criteria were: prior corneal surgeries, pachymetry less than 400 microns, scars or clouding of the cornea, chemical injuries, severe dry eye, delayed epithelial healing and any inflammatory eye surface process before CXL procedure.
Preoperative examination
Every patient had a complete preoperative ophthalmologic examination before deciding if the patient met the criteria for the study. The examination included uncorrected and corrected distant visual acuity (UDVA, CDVA), manifest and cycloplegic refraction, corneal topography measured on pentacam (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany), tonometry (Auto Non-Contact Tonometer, Reichert Inc., Buffalo, NY, USA), slit-lamp and dilated funduscopic examination. Visual acuity was measured using a standard Snellen acuity chart at 6 m and presented in decimal format. The patients were asked to discontinue the use of contact lenses for up to 4 weeks before this examination, depending on the type of lenses they were using.
Crosslinking method
Presently, corneal collagen cross-linking (CXL) with riboflavin A is the only pharmacological treatment available for keratoconus. This treatment is intended to reinforce the corneal architecture by cross-linking the stromal lamellae in a transversal way (14). Several studies have proven that the treatment is safe, effective, and provides the desired stability of the condition (15). Corneal collagen cross-linking (CXL) theoretically strengthens the cornea and subsequently halts or slows keratoconus progression through a photochemical reaction that alters the collagen matrix of the corneal stroma, creating riboflavin ultraviolet-A (UVA)–induced intrafibrillar and interfibrillar covalent bonds (14). Mild to moderate progressive keratoconus is still the most common indication for CXL treatment but the literature is scarce in confirming the safety and effectiveness of this method for patients with advanced keratoconus, particularly those with stage 3 and 4 of the Amsler-Krumeich classification (4).
Surgical procedure
Before the procedure started, the treated eye was cleaned with povidone iodide. Topical anesthesia of the cornea was obtained using tetracaine drops, alternating every 3 minutes for 9 minutes. During this time period, pilocarpine 2% eye drops were also instilled twice. After a lid speculum was inserted, a 9.0-mm diameter corneal abrasion was created. Riboflavin 0.1% (Riboflavin, Ricrolin, Peschke Meditrade) drops were instilled every 2 minutes for 30 minutes with balanced salt solution (BSS). Central corneal pachymetry was performed using ultrasound. In eyes with a CCT (without epithelium) less than 400 microns (μm), additional riboflavin 0.1% drops without dextran (0,1% in sterile solution, Medio Cross hypotonic) were applied until the thickness was 400 μm. The eye was irradiated for 30 minutes with an ultraviolet-A irradiance of 3 mW/cm2 (UV-X; Peschke Meditrade GmbH, Hunenberg, Switzerland). During irradiation, the cornea was moistened every 2 minutes with riboflavin 0.1% drops and BSS drops. After the surgery antibiotic and corticosteroids, both topically are used, and bandage contact lens is inserted. Contact lens is removed 3-5 days after the treatment, depending on epithelium healing. Drops are used during the first postoperative month. Patient follow-up was first 3 days in a row, then 1 week, 1 month, 3 months, 6 and 12 months after the procedure.
Keratoconus gradation system
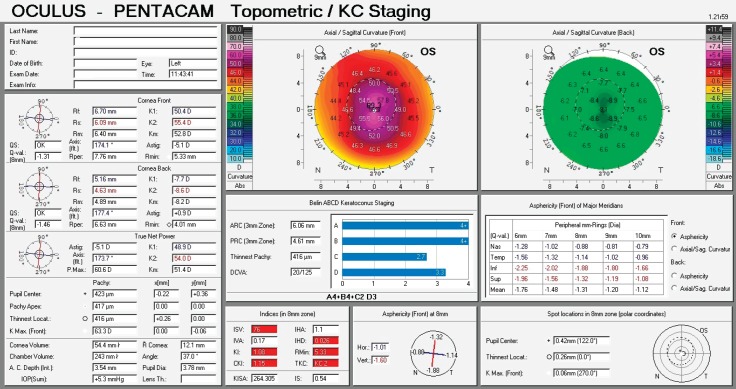
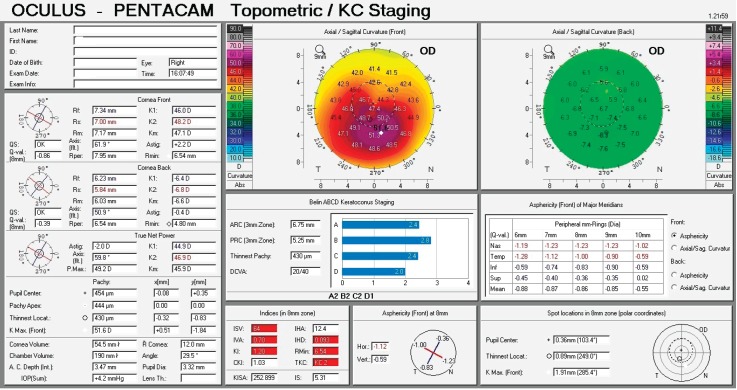
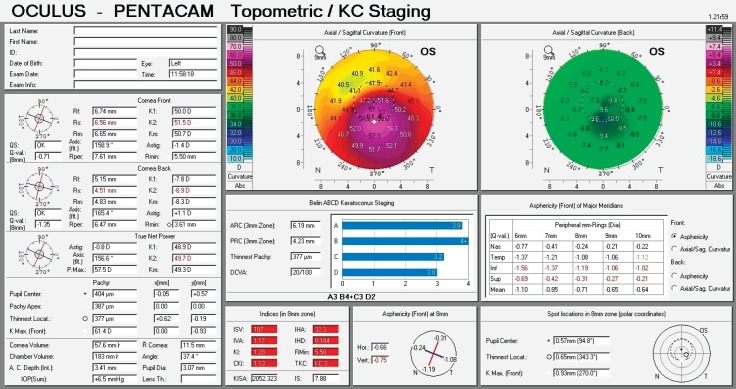
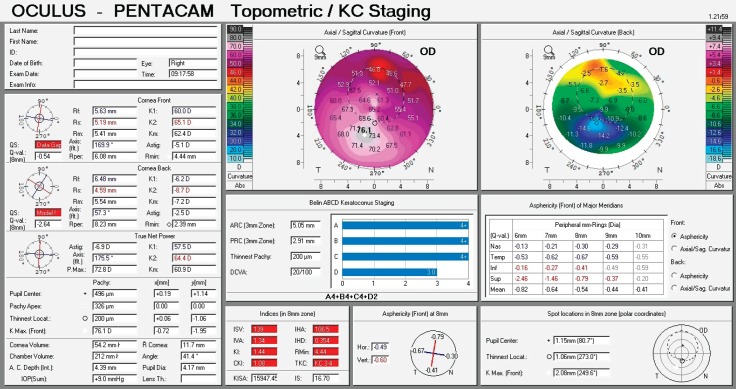
For keratoconus gradation system we used the rotating Scheimpflug corneal tomography system Pentacam (Oculus Optikgeräte GmbH, Heidelberg Germany). A rotating camera captures the diffuse volume scattering of a monochromatic slit light source projected onto the cornea and the anterior eye segment. Scheimpflug imaging creates a series of keratoconus specific indices. All files were analyzed with Pentacam software version 6.08r13. The newer software has stricter criteria. This software was developed to compute the radius of curvature for the anterior surface (ARC) and posterior surface (PRC) for a 3.0 mm zone centered on the thinnest point. The 3.0 mm zone was chosen as this is the exclusion zone size utilized in the BAD software for most keratoconus corneas. ARC and PRC from the3.0 mm zone centered on the thinnest point is not currently available on the Pentacam. The data generated from this database was used to develop a classification system that approximated stages 1–4 on the AK system for anterior data and corneal thickness, but added a fifth stage (stage 0), representing values more typically seen in normal eyes. Similar gates based on the standard deviations derived from the anterior surface were utilized for the posterior surface. The measurement results were checked under the quality specification (QS) window, and only the correct measurements (“QS” reads OK) were accepted (16).
Table 1. Proposed ABCD keratoconus grading system. Stages (0 to IV) are based on anterior and posterior radius of curvature (ARC, PRC), thinnest pachymetry, corrected distance visual acuity (CDVA) (mm- millimeter, μm- micrometer, D – diopter)).
| ABCD criteria | A | B | C | D |
|---|---|---|---|---|
| ARC (3 mm Zone) | PRC (3 mm Zone) | Thinnest pach μm | CDVA | |
| Stage 0 | > 7.25 mm (< 46.5 D) | > 5.90 mm | > 490 μm | ≥ 20/20 (≥ 1.0) |
| Stage I | > 7.05 mm (< 48.0 D) | > 5.70 mm | > 450 μm | < 20/20 (< 1.0) |
| Stage II | > 6.35 mm (< 53.0 D) | > 5.15 mm | > 400 μm | < 20/40 (< 0.5) |
| Stage III | > 6.15 mm (< 55.0 D) | > 4.95 mm | > 300 μm | < 20/100 (< 0.2) |
| Stage IV | < 6.15 mm (> 55.0 D) | < 4.95 mm | ≤ 300 μm | < 20/400(< 0.05) |
4. RESULTS
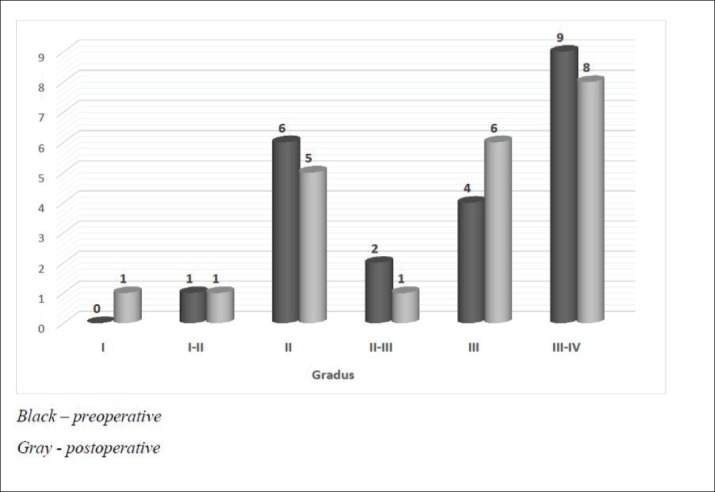
The 22 keratoconus eyes treated with CXL method using the Dresden protocol from January 2017 to January 2018, were subjected to keratoconus ABCD stages grading(16) by the Pentacam software at Eye Clinic Svjetlost Sarajevo. The resulting grading designated one eye as keratoconus Stage I-II, 6 eyes as Stage II, 4 eyes as Stage III and 9 eyes as stage III-IV. There is no statistically significant gradient change of keratoconus in comparison to one month after the surgery, p>0.05. There is no progression of the gradient when comparing to the preoperative stage.
Figure 1. Keratoconus stage (gradation) changes in 12 months of follow up after CXL treatment.
5. DISCUSSION
The first keratoconus classification was carried out by Marc Amsler in 1950 based on clinical signs. Central radius, visual acuity with glasses and contact lenses, corneal thickness and transparency were used to classify keratoconus into the stages: normal, suspect, mild, moderate or severe (stage 0 to 4). In 1984, Muckenhirn added the corneal eccentricity as an additional indicator for classification. Krumeich created a new classification scheme based on induced myopia, corneal radius, corneal thickness, and corneal slit-lamp findings classify into stages 0-4. A summary is shown in the paper of Kanellopoulos et al. (17) Topographic and tomographic measurements are essential to estimate the capacity of these parameters to assess the treatment effectiveness. This finding confirms the efficacy of corneal biomechanical changes and collagen lamellae organization. In this study, the change of keratoconus gradation corneal was measured by Oculus Pentacam before and after CXL treatment. The Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) is an anterior segment tomography device, based on a rotating Scheimpflug camera. Corneal tomography can measure not only the anterior corneal surface but also the posterior surface, allowing a three-dimensional image of the cornea. This technology provides significantly more information than anterior surface topography, as tomography utilizes data from anterior and posterior surfaces of the cornea, as well as pachymetric mapping (18, 19). The keratoconus grading system named ABCD uses the anterior and posterior radius of curvature taken from the 3 mm zone centered on the thinnest point (“A” for anterior, “B” for back surface) and the corneal thickness at the thinnest point (“C” for corneal thickness) as well as best-corrected distance visual acuity (“D” for distance visual acuity). This classification/grading system has advantages over the older Amsler-Krumeich classification in that it recognizes the importance of the posterior corneal surface and each component (anterior, posterior, thickness, visual acuity) are individually graded. This classification system conveys both anatomical and functional data that is missing from the Amsler-Krumeich classification. It conveys information on both anterior and posterior corneal surfaces, is centered on the thinnest point which is typically the region of the cone and adds a visual acuity measurement as well as an indication of corneal scarring (16).
Figure 1. Corneal topography display of keratoconus grade II - central location of the cone.
Figure 2. Corneal topography display of keratoconus grade II - inferotemporal location of the cone.
Table 2 shows the abnormal and pathological values. All data were measured preoperatively and 1, 3, 6, and 12 months postoperatively. Out of 22 eyes, one eye had keratoconus stage I-II, 6 eyes had stage II, 4 eyes had stage III, and 9 eyes had stage III-IV. There is no statistically significant gradient change of keratoconus in comparison to one month after the surgery, p>0.05. There is no progression of the gradient when comparing to the preoperative stage. Wollensak, Spoerl, and Seiler (15) first reported on the use of the CXL procedure to halt or reduce the progression of keratoconus in patients. Their work has been confirmed in multiple studies including in a recent randomized, prospective, double-masked, sham-controlled clinical trial. Our findings show that there are no statistically significant differences between the parameters and indices of keratoconus before and 1 year after treatment. Based on the fact that the maximal progression of keratoconus occurs during the second and third decades of life, (7) and also based on our participants’ age (16-33 years old), our results indicate that over the ensuing 12 months from the procedure, there was no significant progress in keratoconus and the progressive process of this disease had been stopped during 1 year after CXL. This study shows that most of the parameters and indices have not changed during 1 year after CXL, pointing toward the efficacy of this method in halting the disease progression at least for the first year after surgery, though in other studies, there had been improvement in some parameters and indices and the corneas had become more regular 1 year after CXL. Further research is needed to clarify the effects of this procedure during 2, 5, 10, and more years after surgery. Kanellopoulos reported that in his research after CXL keratoconus was stabilized in all cases (20). We found that corneal cross-linking could effectively stabilize the progression of KC, as assessed by key corneal topographic parameters. Analyzing trend of stage change in 12 months follow up after crosslinking procedure of keratoconus patients, our results showed that one eye (4,5%) had an increase of staging from II-III to III, while 3 eyes (13,6%) had an increase of one stage. Eighteen eyes (81,8%) remained in their preoperative stage. According to our results, we can say that CXL is a safe and effective procedure in treating keratoconus. This procedure should be preferably used for patients with progressive disease. Younger patients tend more aggressive course of the disease, that is why this treatment should be offered as much as possible. As a safe procedure without any serious complications, the CXL protocol could largely reduce the need for corneal transplantation.
Table 2. Trend in keratoconus stage changes in 12 months of follow up after CXL treatment.
| Gradient | Eyes | ||||||
|---|---|---|---|---|---|---|---|
| I | I-II | II | II-III | III | III-IV | ||
| Preoperative | 0 | 1 | 6 | 2 | 4 | 9 | 22 |
| 12 months | 1 | 1 | 5 | 1 | 6 | 8 | 22 |
Figure 3. Corneal topography display of keratoconus grade III - Inferotemporal location of the cone.
Figure 4. Corneal topography display of keratoconus grade III-IV inferior location of the cone.
6. CONCLUSION
Corneal cross-linking could effectively stabilize the progression of keratoconus, as assessed by key corneal topographic parameters. Analyzing the trend of stage change in 12 months follow up after the crosslinking procedure of keratoconus patients there was no progression of a gradient in comparison to the preoperative stage. According to our results, we can conclude that CXL is a safe and effective procedure in treating keratoconus.
Competing interest statement:
‘Declarations of interest: None.
Author’s contribution:
A.P, F.G. and S.G gave substantial contributions to the conception or design of the work in acquisition, analysis, or interpretation of data for the work. M.A.P., A.B and A.P had a part in article preparing for drafting or revising it critically for important intellectual content, and A.B and M.B gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
None.
REFERENCES
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio R, Jr, Caldas DL, da Silva RS, Pimentel LN, de Freitas VB. Impacto da análise do “wavefront” na refratometria de pacientes com ceratocone [Impact of the wavefront analysis in refraction of keratoconus patients] Rev Bras Oftalmol. 2010;69(5):294–300. [Google Scholar]
- 3.Zadnik K, Steger-May K, Fink BA, et al. CLEK Study Group. Between-eye asymmetry in keratoconus. Cornea. 2002;21(7):671–679. doi: 10.1097/00003226-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Amsler M. The ‘forme fruste’ of keratoconus. Wien Klin Wochenschr. 1961;73(1):842–843. [PubMed] [Google Scholar]
- 5.Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984;25(12):1426–1435. [PubMed] [Google Scholar]
- 6.Wilson SE, Lin DT, Klyce SD. Corneal topography of keratoconus. Cornea. 1991;10(1):2–8. [PubMed] [Google Scholar]
- 7.Villavicencio OF, Gilani F, Henriquez MA, et al. Independent population validation of the Belin/Ambrosio enhanced ectasia display: Implications for keratoconus studies and screening. Int J Kerat Ect Cor Dis. 2014;3(1):1–8. [Google Scholar]
- 8.Rabinowitz YS, Rasheed K. KISA % index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25(1):1327–1335. doi: 10.1016/s0886-3350(99)00195-9. [DOI] [PubMed] [Google Scholar]
- 9.Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic disease. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 10.Orucoglu F, Toker E. Comparative Analysis of Anterior Segment Parameters in Normal and Keratoconus Eyes Generated by Scheimpflug Tomography. J Ophthalmol. 2015. Article ID 925414, 8 pages. [DOI] [PMC free article] [PubMed]
- 11.Belin MW, Villavicencio OF, Ambrosio R., jr Tomographic parameters for the detection of keratoconus: Suggestions for screening and treatment parameters. Eye and Contact Lens. 2014;40(2):326–330. doi: 10.1097/ICL.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 13.Belin MW, Lim L, Rajpal RK, Hafezi F, Gomes JAP, Cochener B. Corneal Cross-Linking: Current USA Status: Report From the Cornea Society. Cornea. 2018 Oct;37(10):1218–1225. doi: 10.1097/ICO.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 14.Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28(5):510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 15.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol. 2010;149(2):585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Belin M, Duncan J. Keratoconus: The ABCD Grading System. Klinische Monatsblätter Für Augenheilkunde. 2016;233(6):701–707. doi: 10.1055/s-0042-100626. [DOI] [PubMed] [Google Scholar]
- 17.Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7(1):1539–1548. doi: 10.2147/OPTH.S44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luz A, Ursulio M, Castaneda D, Ambrosio R., Jr Corneal thickness progression from the thinnest point to the limbus: Study based on a normal and a keratoconus population to create reference values. Arquivos Brasileiros de Oftalmologia. 2006;69(4):579–583. doi: 10.1590/s0004-27492006000400023. [DOI] [PubMed] [Google Scholar]
- 19.Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(10):1475–1484. doi: 10.1016/j.ophtha.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Kanellopoulos AJ. Management of progressive keratoconus with partial topography-guided PRK combined with refractive, customized CXL – a novel technique: the enhanced Athens protocol. Clin Ophthal. 2019;13(2):581–588. doi: 10.2147/OPTH.S188517. [DOI] [PMC free article] [PubMed] [Google Scholar]