Abstract
Background
Morbidity and mortality from acquired immunodeficiency syndrome (AIDS) are often associated with the reactivation of a herpes virus infection. Human herpesvirus-6 (HHV-6) is usually common in childhood infections that remain latent and can act as opportunists during immunosuppression to reactivate and cause disease. In human immunodeficiency virus (HIV)-infected patients, the impact of HHV-6 infection can be an up-regulator of HIV replication and accelerate progress towards AIDS. However, studies on HHV-6 infection have never been done in Surabaya, Indonesia.
Purpose
To determine the presence of HHV-6 infection among HIV-infected individuals residing in Surabaya, Indonesia.
Patients and Methods
Plasma and peripheral blood mononuclear cells (PBMCs) were collected from 85 HIV-infected individuals in Universitas Airlangga Hospital, Surabaya, as well as 85 healthy controls. DNA extracted from PBMCs was subjected to PCR to determine HHV-6 infection, while plasma of HIV-infected individuals was used for viral RNA quantification using real-time PCR.
Results
HHV-6 infection was detected in 17.6% (15/85) of HIV-infected individuals, and in 3.53% (3/85) of healthy controls. Thus, HHV-6 infection was more likely to be found in HIV-infected individuals than in healthy controls (odds ratio 5.85; 95% confidence interval, 1.6–21). The HHV-6B was the most common subtype identified in both HIV-infected individuals (12/15) and healthy controls (3/3). The HHV-6A and co-infection between HHV-6A and HHV-6B were only found in HIV-infected individuals (2/15 and 1/15, respectively). Viral RNA load of HIV-infected individuals was not correlated to HHV-6 infection.
Conclusion
Our results indicate the emergence of HHV-6 infection among HIV-infected individuals residing in Surabaya, Indonesia, and the risk of HHV-6 infection was higher in HIV-infected individuals than in healthy controls.
Keywords: HHV-6, HIV-infected individuals, healthy controls, Surabaya, Indonesia
Introduction
Indonesia had the second-highest acquired immunodeficiency syndrome (AIDS)-related death among Asia and the Pacific countries in 2018.1 The human immunodeficiency virus (HIV) primarily attacks T helper (CD4+) lymphocytes. The human immunodeficiency virus (HIV) infection causes the immune system to weaken so the host becomes more susceptible to various infections and malignancies.2 The spectrum of diseases that can lead to morbidity and mortality in HIV infection is very broad. The causes of opportunistic infections in HIV/AIDS can be protozoan, bacterial, viral, or fungal infections.3 Mortality from AIDS is often associated with the reactivation of a herpes virus infection. Human herpesvirus-6 (HHV-6) is not usually associated with disease in the immunocompetent, but a major cause of opportunistic infection in HIV-infected individuals. HHV-6 infection can upregulate HIV replication and accelerate progress towards AIDS.
Human herpesvirus-6 (HHV-6) is a T lymphotropic herpesvirus belonging to the Betaherpesvirinae subfamily.4 HHV-6 is one of the most prevalent herpesviruses in humans. It is a causative agent for exanthema subitum in children.5 The two variants of HHV-6A and HHV-6B have an overall genetic identity of 90%.6 HHV-6 infections generally occur asymptomatically in healthy people and may also cause infection with mononucleosis-like syndrome and chronic fatigue syndrome in adults, and cause sixth disease (exanthema subitum) in children.7,8 In patients infected with HIV, the impact of HHV-6 infection can be an up-regulator of HIV replication and accelerate progress towards AIDS. There is clearly a relationship between Human Herpesvirus-6 (HHV-6) and HIV-1, where these two viruses have primary CD4+ T cell receptors.9
HIV plasma viral load has become the standard for monitoring antiretroviral (ARV) therapy. This is used as a marker of ARV responses and the development of HIV disease that has been used to manage and monitor HIV infection. Pre-ARV viral load levels and the decrease of viral load after ARV initiation can provide the prognostic information on the possibility of developing the disease.10 An HHV-6 infection that occurs in people with immunosuppression is the most serious clinical manifestation associated with HHV-6 infection or reactivation.11
We, therefore, decided to investigate the presence of this virus by using a highly sensitive and variant-specific PCR method and its correlation with HIV plasma viral load. Hence, the aim of our study was to evaluate the virus in the PBMC of HIV-infected individuals and healthy controls from Surabaya because no epidemiological data is available in Indonesia.
Materials and Methods
Study Area and Design
Samples were collected from HIV-positive adults at Universitas Airlangga Hospital between October and December 2016. The healthy control samples were collected from people living in Surabaya in June 2018. This study is an observational analytic study with a hospital-based, sex-matched case-control approach. All the samples were tested with HIV-1/2 antibody using Abbexa HIV-1/2 antibody Kit (Cat.no. 364861) (dx.doi.org/10.17504/protocols.io.7gyhjxw). There were 79 patients undergoing antiretroviral therapy and 6 naïve patients from HIV-infected group. Eighty-five plasma and PBMC samples were collected and used a control from healthy controls, which was the same number as the HIV-infected samples.
Informed Consent and Ethical Approval
Basic clinical details were collected from the patients to provide general information. Subsequently, informed consent was obtained from each patient before any samples were collected. The authors confirm that all participants were adequately informed of the aims of the study and voluntarily provided written informed consent for their details to be used in this study. We also ensured that all patient-identifying information/data were anonymized, and only code numbers were used for matter of confidentiality. The ethics approval for the study was obtained from the Ethics Committee of Universitas Airlangga Hospital.
Inclusion and Exclusion Criteria
Inclusion Criteria (Case Samples)
Patients confirmed positive of HIV by ELISA antibody’s, not in conditions of long-term use of immunosuppressive drugs such as corticosteroids or cytostatics and confirmed with written informed consent.
Inclusion Criteria (Control Samples)
The negative HIV samples were checked by ELISA antibody’s, not in conditions of long-term use of immunosuppressive drugs such as corticosteroids or cytostatics and confirmed with written informed consent.
Exclusion Criteria (Case and Control Samples)
Pregnant women and refused to include in this research.
PCR Detection
DNA was extracted from PBMC (https://dx.doi.org/10.17504/protocols.io.7j5hkq6) using the Promega Wizard Genomic DNA Purification Kit (Cat.no. A1120) (dx.doi.org/10.17504/protocols.io.7j6hkre), while RNA was extracted from plasma using the Qiagen QIAamp DSP virus Kit (Cat.no. 61704), both according to manufacturer instructions. Nested PCR was performed on DNA extracted from peripheral blood cells. Nested gB genes primer sets were designed to detect HHV-6 and IE genes to determine the subtype. The primer was set and provided by the Department of Clinical Virology, Kobe University. Outer primer of gB genes 5ʹ-TTCGGATGATTATATCAGAG-3ʹ and 5ʹ- ACTAAACACCGTACCATC-3ʹ and inner genes 5ʹ-CGGTTCGACAGAGATATTTCG-3ʹ and 5ʹ-ATCGCTACGGCTGAATAACACTG-3ʹa generated 260 bp. The PCR amplification of HHV-6 was done at 94 °C for 1 min, followed by 35 cycles of 1 min at 94 °C, 1 min at 55 °C and 1 min at 72 °C, with a final extension step (10 mins at 72 °C) to allow complete extension of the amplicons. To distinguish the virus, we used a set nested primer, outer primer 5ʹ- GAAGGAGTGACCTCTGGTGGTGAA-3ʹ and 5ʹ- GAATCTATCCATGAAGATGATGA-3ʹ. And inner primer 5ʹ- GGTGCTGAGTGATCAGTTTCA-3ʹ and 5ʹ- CAAACAAGCCCTAACTGTGTA-3ʹ generating a 206 bp product HHV-6A and 431 bp product HHV-B. PCR products were run in a 1.5% agarose gel and visualized by ethidium bromide staining. RNA HIV viral load was quantified using commercial kit real-time PCR (artus HI Virus-1 RG RT-PCR, Qiagen, Germany) and was performed according to the manufacturer’s instruction. The data obtained were analyzed using statistical software (SPSS version 21.0).
Results
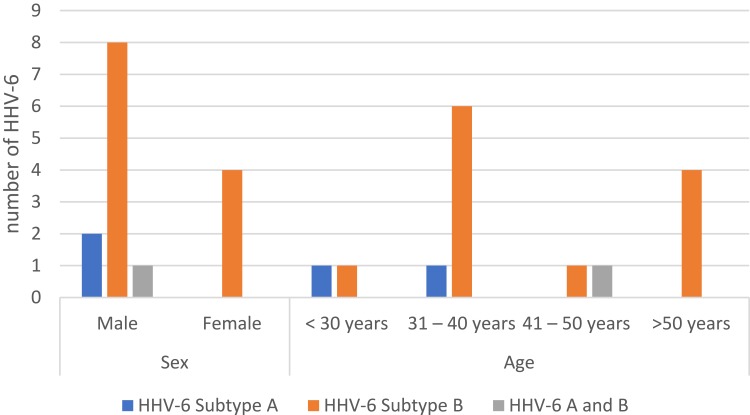
The HIV-infected individuals involved in this study comprised of 64 males and 39 females, with a mean age of 37 years (range 18–64 years). We found HHV-6 17.6% (15/85) of HIV-infected individuals and 3.5% (3/85) in healthy controls (Table 1). HHV-6B was found in 80% (12/15) of the patients, HHV-6A in 13.3% (2/15) and both were found in 6.6% (1/15) of HIV-infected individuals, while HHV-6B was detected in all samples from the healthy controls (Figure 1). None of the HHV-6 infected from HIV-positive were naïve patients. The results of the HHV-6 detection of HIV-infected individuals and healthy controls have differ amongst them on the SPSS Chi-Square test, and obtained a value of p= 0.003 (odds ratio 5.85; 95% confidence interval, 1.6–21), but here was no correlation between the DNA HHV-6 and HIV plasma viral load (Table 2), where p= 0.28.
Table 1.
Infection of HHV-6 Based on Sex and Age
| HHV-6 | |||||
|---|---|---|---|---|---|
| HIV-Infected | Healthy Controls | ||||
| n | % | n | % | ||
| Sex | Male (n=64) | 11 | 20.7 | 2 | 3.1 |
| Female (n=39) | 4 | 12.5 | 1 | 2.7 | |
| Age (years) | 18 – 30 (n=23) | 2 | 8.7 | 2 | 8.7 |
| 31 – 40 (n=36) | 6 | 16.6 | 0 | 0 | |
| 41 – 50 (n=20) | 3 | 15 | 0 | 0 | |
| >50 (n=6) | 4 | 66.6 | 1 | 25 | |
Figure 1.
Subtype of HHV-6 based on sex and age.
Table 2.
Correlation of HHV-6 to HIV Viral Load
| Viral Load HIV (Copies/mL) | Total | p value | ||||
|---|---|---|---|---|---|---|
| 0–>50 | 50–5000 | >5000 | ||||
| HHV-6 infection | Negative | 34 | 27 | 5 | 70 | 0.28* |
| Positive | 5 | 5 | 5 | 15 | ||
| Total | 39 | 32 | 10 | 85 | ||
Note: *correlation is not significant.
Discussion
Herpesvirus infections are common in HIV-infected individuals. Immunosuppression due to advancing HIV disease has been associated with the increasing frequency and prolonged duration of the reactivation of herpesvirus infections. In vivo studies about the interaction between HIV and HHV-6 proves an increase in the progress of HIV replication.9 In this study, 17.6% and 3.5% of HHV-6 DNA was detected among HIV-infected individuals and healthy controls PBMC samples, respectively. These results were lower than previous studies conducted in America, where it amounted to 75% of HIV-infected,14 while in healthy persons are 29%,15 and even 90%.16 In other countries, Latvia 8%17 and France 16.5%18 of healthy donors were positive for HHV-6.
The detection of HHV-6 using qualitative PCR in PBMC and serum does not indicate latent infection or active infection.19 Detection using real-time PCR is more sensitive to indicate active or latent infection based on the HHV-6 viral load number in PBMCs. Viral loads over 1000–100,000 copies/mL indicate an active infection.19 This study was unable to indicate latent or active infection. In another study by Flamand et al (2008), the detection of HHV-6 in body fluids including plasma, serum, and cerebrospinal fluid indicated active HHV-6 infection.12 Conversely, the use of plasma, although easier, creates controversy regarding the origin of viral DNA present in the blood compartment, which is associated with the production of viruses from the lymphoid tissue or incidental release of DNA from circulating cell lysis.12 The high sensitivity of real-time PCR and the presence of latent HHV-6 infections in adults leads to a high frequency of qualitative positive detection of viral DNA. Therefore, for proper interpretation, the amount of HHV-6 DNA in the blood must be calculated precisely. To date, no threshold has been formally defined as the boundary between latent and active infection. As a preliminary estimation, the 1000 copies genome equivalent per milliliter threshold of all blood represents a fluctuating gray zone that separates the two stages of HHV-6 infection.13
Previous studies explain that HHV-6 can be an important factor in the pathogenesis of immunological damage and accelerate the development of HIV-1 into AIDS.9 The cytopathic effect of two viruses synergistically used the damage of CD4+ T cells.9 HHV-6 infection or reactivation in AIDS patients increases the HHV-6 viral load in lymph nodes, blood (viremia), and the infections can spread to many organs, active CNS infections, pneumonitis, and retinitis and can cause death.11 In addition to co-infection, when observed in vivo and in vitro, HHV-6 promotes HIV replication through increased regulation of cytokines (for example, TNF and IL-1ß) and transactivation of long terminal repeat by IE-A and IE-B.20 In the 2015 research report of Agut et al, cases of HHV-6 active infections associated with primary infection, reactivation, and exogenous reinfection are asymptomatic and will cause serious infections in immunocompromised people.19
These results show that HHV-6B is dominant in the HIV-positive samples at 80% (12/15). The HHV-6A subtype was found in only 13.3% (2/15), while both HHV-6A and HHV-6B were found in only one sample 6.6% (1/15). All HHV-6 detected in donor samples were HHV-6B.
HHV-6 infection is usually acquired in infants, between 6 months and 2 years, following the loss of maternal antibodies.11 Primary infection can also occur later, in adults. In most countries, primary HHV-6B infection occurs first, in many cases related to clinical symptoms, whereas HHV-6A is obtained later, through asymptomatic infection. Finally, it can be detected that HHV-6A and HHV-6B in the blood or tissue of adults shows that both viruses infect many chronically.16
The analysis of the results of the highly active antiretroviral (HAART) treatment showed that patients on ARV treatment were the most infected with HHV-6. HIV-positive patients on a highly active antiretroviral treatment would not act on a herpes virus infection but could prevent the reactivation of these viruses.21 In an EBV infection, HAART treatment to improve CD4+ T cell restoration while contributing to lower HIV viral load does not affect.22 A herpesvirus infection is more likely to be found in HAART patients with high CD4 T cell counts. This finding can support that the coinfection of HIV and herpesviruses enhances CD4 T cell proliferation and thus broadens the types of target cells susceptible to HIV infection.22–26
In this study, HIV immunocompromised patients showed amounts of HIV plasma viral loads ranging from undetected to a high viral load. HHV-6 infection in PBMCs shows no correlation with the amount of HIV viral load. This is indicated by the quality of life of people living with HIV. Factors affecting the quality of life of people with HIV include regularity of the use of antiretroviral therapy, the influence of nutrition, patient environment, and support from surrounding people. The antiretroviral therapy used is beneficial for the quality of life of the patient. The quality of life of the patient is optimally enhanced and maintained for as long as possible.27
Conclusion
The presence of HHV-6 in HIV-infected people was higher than healthy persons. This study made it possible for the circulating HHV-6 among them. It indicates the emergence of HHV-6 infection among HIV-infected individuals residing in Surabaya, Indonesia.
Acknowledgments
Thanks to Japan Initiative for Global Research Network on Infectious Disease (J-GRID); Institute of Tropical Disease, Universitas Airlangga; Department of Clinical Virology, Kobe University, Japan. Financial support for this study was provided by Ministry of Research Technology and Higher Education Republic of Indonesia under Grant number 43/E/KPT/2017 and in part by a Grant-in-Aid from Dato’ Sri Prof. Tahir for supporting this research through the Tahir Professorship Program, Indonesia.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no potential conflict of interest, financial or other.
References
- 1.Joint united nations programme on HIV/AIDS (UNAIDS): UNAIDS Data 2018. Available from: www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed February5, 2019.
- 2.Grant I, Sacktor N, McArthur J. HIV neurocognitive disorders In: Gendelman HE, Grant I, Everall I, Lipton SA, Swindells S, editors. The Neurology of AIDS. 2nd London: Oxford University Press; 2005:357–370 [Google Scholar]
- 3.Isselbacher K. Harrison’s Principles of Internal Medicine. Singapore: McGraw Hill Book Co.; 2013:1022–1025. [Google Scholar]
- 4.Salahuddin SZ, Ablashi DV, Markham PD. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234(4776):596–601. doi: 10.1126/science.2876520 [DOI] [PubMed] [Google Scholar]
- 5.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet Oncol. 1988;1:1065–1067. doi: 10.1016/S0140-6736(88)91893-4 [DOI] [PubMed] [Google Scholar]
- 6.Dominguez G, Dambaugh TR, Stamey FR, et al. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol. 1999;73(10):8040–8052. doi: 10.1128/JVI.73.10.8040-8052.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy JA, Ferro F, Greenspan D, Lennette ET. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335(8697):1047–1050. doi: 10.1016/0140-6736(90)92628-U [DOI] [PubMed] [Google Scholar]
- 8.Okuno T, Takahashi K, Balachandra N, et al. Seroepidemiology of human herpesvirus- 6 infection in normal children and adults. J Clin Microbiol. 1989;27:651–655. doi: 10.1128/JCM.27.4.651-653.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lusso P, Crowley RW, Malnati MS, et al. Human herpesvirus 6A accelerates AIDS progression in macaques. Proc Natl Acad Sci. 2003;104(12):5067–5072. doi: 10.1073/pnas.0700929104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray JS, Elashoff MR, Iacono-connors LC, et al. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS. 1999;13(7):797–804. doi: 10.1097/00002030-199905070-00008 [DOI] [PubMed] [Google Scholar]
- 11.Tesini BL, Epstein LG, Caserta MT. Clinical impact of primary infection with roseoloviruses. Curr Opin Virol. 2014;9:91–96. doi: 10.1016/j.coviro.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flamand L, Stefanescu I, Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest. 1996;97(6):1373–1381. doi: 10.1172/JCI118557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutolleau D, Fernandex C, Andre E, et al. Human herpesvirus (HHV)-6 and HHV-7: two closely related virus with different infection profiles in stem cell transplantation recipients. J Infect Dis. 2003;187(2):179–186. doi: 10.1086/367677 [DOI] [PubMed] [Google Scholar]
- 14.Fairfax MR, Schacker T, Cone RW, et al. Human herpesvirus 6 DNA in blood cells of human immunodeficiency virus-infected men: correlation of high level with high CD4 cell counts. J Infect Dis. 1994;169(6):1342. doi: 10.1093/infdis/169.6.1342 [DOI] [PubMed] [Google Scholar]
- 15.Dolcetti R, Di Luca D, Carbone A, et al. Human herpesvirus 6 in human immunodeficiency virus-infected individuals: association with early histologic phases of lymphadenopathy syndrome but not with malignant lymphoproliferative disorders J Med Virol. 1996;48:344–353. doi: [DOI] [PubMed] [Google Scholar]
- 16.Cone RW, Huang ML, Hackman RC, et al. Coinfection with human herpesvirus 6 variants A and B in lung tissue. J Clin Microbiol. 1996;34(4):877–881. doi: 10.1128/JCM.34.4.877-881.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozireva S, Nemceva G, Danilane I, et al. Prevalence of blood-borne viral infections (cytomegalovirus, human herpesvirus-6, human herpesvirus-7, human herpesvirus-8, human retrovirus-5) among blood donors in Latvia. Ann Hematol. 2001;80(11):669–673. doi: 10.1007/s002770100359 [DOI] [PubMed] [Google Scholar]
- 18.Gèraudie B, Charrier M, Bonnafous P, et al. Quantitation of human herpesvirus-6A, −6B and −7 DNAs in whole blood, mononuclear and polymorphonuclear cell fractions from healthy blood donors. J Clin Virol. 2012;53:151–155. doi: 10.1016/j.jcv.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 19.Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28(2):313–335. doi: 10.1128/CMR.00122-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusso P. HHV-6 and HIV-1 Infection, In: Perspectives in Medical Virology. Elsevier; 2006:263–277. [Google Scholar]
- 21.Traore L, Nikiema O, Ouattara AK, et al. EBV and HHV-6 circulating subtypes in people living with HIV in Burkina Faso, impact on CD4 T cell count and HIV viral load. Mediterr J Hematol Infect Dis. 2017;9(1):e2017049. doi: 10.4084/mjhid.2017.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piriou ER, Dort KV, Nanlohy NM, et al. Novel method for detection of virus specific CD4+ T cells indicates a decrease EBV-specific CD4+ T cell response in untreated HIV-infected subjects. Eur J Immunol. 2005;35:3. doi: 10.1002/eji.200425792 [DOI] [PubMed] [Google Scholar]
- 23.Lusso P, Ensoli B, Markham PD, et al. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989;337(6205):370–373. doi: 10.1038/337370a0 [DOI] [PubMed] [Google Scholar]
- 24.Ensoli B, Lusso P, Schachter F, et al. Human herpes virus 6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989;8(10):3019–3027. doi: 10.1002/embj.1989.8.issue-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lusso P, De Maria A, Malnati M, et al. Induction of CD4 and susceptibility ti HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991;349(6309):533–535. doi: 10.1038/349533a0 [DOI] [PubMed] [Google Scholar]
- 26.Lusso P, Malnati MS, Garzino-demo A, et al. Infection of natural killer cells by human herpesvirus 6. Nature. 1993;362(6419):458–462. doi: 10.1038/362458a0 [DOI] [PubMed] [Google Scholar]
- 27.Nasronudin LA. HIV & AIDS Pendekatan Biologi Dan Molekuler, Klinis, Dan Sosial, Edisi Kedua. Surabaya: Airlangga University Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Joint united nations programme on HIV/AIDS (UNAIDS): UNAIDS Data 2018. Available from: www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed February5, 2019.