Abstract
Background
Pelvic adhesions can form secondary to inflammation, endometriosis, or surgical trauma. Strategies to reduce pelvic adhesion formation include placing barrier agents such as oxidised regenerated cellulose, polytetrafluoroethylene, and fibrin or collagen sheets between pelvic structures.
Objectives
To evaluate the effects of barrier agents used during pelvic surgery on rates of pain, live birth, and postoperative adhesions in women of reproductive age.
Search methods
We searched the following databases in August 2019: the Cochrane Gynaecology and Fertility (CGF) Specialised Register of Controlled Trials, MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, the Cochrane Central Register of Controlled Trials (CENTRAL), Epistemonikos, and trial registries. We searched reference lists of relevant papers, conference proceedings, and grey literature sources. We contacted pharmaceutical companies for information and handsearched relevant journals and conference abstracts.
Selection criteria
Randomised controlled trials (RCTs) on the use of barrier agents compared with other barrier agents, placebo, or no treatment for prevention of adhesions in women undergoing gynaecological surgery.
Data collection and analysis
Three review authors independently assessed trials for eligibility and risk of bias and extracted data. We calculated odds ratios (ORs) or mean differences (MDs) with 95% confidence intervals (CIs) using a fixed‐effect model. We assessed the overall quality of the evidence using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) methods.
Main results
We included 19 RCTs (1316 women). Seven RCTs randomised women; the remainder randomised pelvic organs. Laparoscopy (eight RCTs) and laparotomy (11 RCTs) were the primary surgical techniques. Indications for surgery included myomectomy (seven RCTs), ovarian surgery (five RCTs), pelvic adhesions (five RCTs), endometriosis (one RCT), and mixed gynaecological surgery (one RCT). The sole indication for surgery in three of the RCTs was infertility. Thirteen RCTs reported commercial funding; the rest did not state their source of funding.
No studies reported our primary outcomes of pelvic pain and live birth rate.
Oxidised regenerated cellulose versus no treatment at laparoscopy or laparotomy (13 RCTs)
At second‐look laparoscopy, we are uncertain whether oxidised regenerated cellulose at laparoscopy reduced the incidence of de novo adhesions (OR 0.50, 95% CI 0.30 to 0.83, 3 RCTs, 360 participants; I² = 75%; very low‐quality evidence) or of re‐formed adhesions (OR 0.17, 95% CI 0.07 to 0.41, 3 RCTs, 100 participants; I² = 36%; very low‐quality evidence).
At second‐look laparoscopy, we are uncertain whether oxidised regenerated cellulose affected the incidence of de novo adhesions after laparotomy (OR 0.72, 95% CI 0.42 to 1.25, 1 RCT, 271 participants; very low‐quality evidence). However, the incidence of re‐formed adhesions may have been reduced in the intervention group (OR 0.38, 95% CI 0.27 to 0.55, 6 RCTs, 554 participants; I² = 41%; low‐quality evidence).
No studies reported results on pelvic pain, live birth rate, adhesion score, or clinical pregnancy rate.
Expanded polytetrafluoroethylene versus oxidised regenerated cellulose at gynaecological surgery (two RCTs)
We are uncertain whether expanded polytetrafluoroethylene reduced the incidence of de novo adhesions at second‐look laparoscopy (OR 0.93, 95% CI 0.26 to 3.41, 38 participants; very low‐quality evidence). We are also uncertain whether expanded polytetrafluoroethylene resulted in a lower adhesion score (out of 11) (MD ‐3.79, 95% CI ‐5.12 to ‐2.46, 62 participants; very low‐quality evidence) or a lower risk of re‐formed adhesions (OR 0.13, 95% CI 0.02 to 0.80, 23 participants; very low‐quality evidence) when compared with oxidised regenerated cellulose.
No studies reported results regarding pelvic pain, live birth rate, or clinical pregnancy rate.
Collagen membrane with polyethylene glycol and glycerol versus no treatment at gynaecological surgery (one RCT)
Evidence suggests that collagen membrane with polyethylene glycol and glycerol may reduce the incidence of adhesions at second‐look laparoscopy (OR 0.04, 95% CI 0.00 to 0.77, 47 participants; low‐quality evidence). We are uncertain whether collagen membrane with polyethylene glycol and glycerol improved clinical pregnancy rate (OR 5.69, 95% CI 1.38 to 23.48, 39 participants; very low‐quality evidence).
One study reported adhesion scores but reported them as median scores rather than mean scores (median score 0.8 in the treatment group vs median score 1.2 in the control group) and therefore could not be included in the meta‐analysis. The reported P value was 0.230, and no evidence suggests a difference between treatment and control groups.
No studies reported results regarding pelvic pain or live birth rate.
In total, 15 of the 19 RCTs included in this review reported adverse events. No events directly attributed to adhesion agents were reported.
Authors' conclusions
We found no evidence on the effects of barrier agents used during pelvic surgery on pelvic pain or live birth rate in women of reproductive age because no trial reported these outcomes.
It is difficult to draw credible conclusions due to lack of evidence and the low quality of included studies. Given this caveat, low‐quality evidence suggests that collagen membrane with polyethylene glycol plus glycerol may be more effective than no treatment in reducing the incidence of adhesion formation following pelvic surgery. Low‐quality evidence also shows that oxidised regenerated cellulose may reduce the incidence of re‐formation of adhesions when compared with no treatment at laparotomy. It is not possible to draw conclusions on the relative effectiveness of these interventions due to lack of evidence.
No adverse events directly attributed to the adhesion agents were reported. The quality of the evidence ranged from very low to moderate. Common limitations were imprecision and poor reporting of study methods. Most studies were commercially funded, and publication bias could not be ruled out.
Plain language summary
Barrier agents for adhesion prevention after gynaecological surgery
Review question
This review of trials assessed the effects of barrier agents on pelvic pain, live birth rate, clinical pregnancy rate, adhesion formation, and adhesion score (a measure of adhesion severity) after pelvic surgery.
Background
A common problem following pelvic surgery is the occurrence of adhesions, where the surfaces of two separate pelvic structures (e.g. inner lining of pelvic wall or pelvic organs such as uterus, ovaries, bladder, or bowel) stick together. During pelvic surgery, strategies to reduce pelvic adhesion formation include placing a synthetic physical barrier between pelvic structures.
Study characteristics
We included 19 randomised controlled trials (RCTs) that included a total of 1316 women undergoing gynaecological surgery. These trials assessed different types of barrier agents for preventing adhesions and compared them with each other or with no treatment. The data are current to August 2019. Thirteen RCTs reported commercial funding; the other studies did not state their source of funding.
Key results
No studies reported the effects of barrier agents used during pelvic surgery on pelvic pain or live birth rate among women of reproductive age.
Low‐quality evidence suggests that oxidised regenerated cellulose and collagen membrane with polyethylene glycol plus glycerol may be more effective than no treatment in reducing the risk of adhesion formation following pelvic surgery.
One study reported the effect of collagen membrane with polyethylene glycol plus glycerol on postoperative adhesion score; however due to the way these data were reported, we are unable to interpret whether the intervention had any effect. No studies reported the effect of oxidised regenerated cellulose on adhesion score.
One study reported the effect of collagen membrane with polyethylene glycol plus glycerol on clinical pregnancy rate; however this evidence was found to be of very low quality. We are uncertain whether this intervention led to a higher clinical pregnancy rate than no treatment. No studies reported the effect of any other intervention on clinical pregnancy rate.
Two studies compared the effects of expanded polytetrafluoroethylene and oxidised regenerated cellulose on adhesion score and adhesion formation. However, this evidence was found to be of very low quality, and we are uncertain whether either intervention was more effective than the other. No studies compared the relative effects of these interventions on pelvic pain, live birth rate, or clinical pregnancy rate.
We found no conclusive evidence on the relative effectiveness of any reported interventions. No adverse events directly attributed to the adhesion agents were reported.
Quality of the evidence
The quality of the evidence ranged from very low to moderate. The most common limitations were imprecision (few participants and wide confidence intervals) and poor reporting of study methods. Most studies were commercially funded, and publication bias could not be ruled out.
Summary of findings
Background
Description of the condition
Pelvic adhesions can form as the result of pelvic inflammation, endometriosis, or surgical trauma. The incidence of pelvic adhesions at second‐look laparoscopy in the first few weeks after surgery has been reported to be between 25% and 92% (Okabayashi 2014). Consequences of adhesion formation include subfertility, development of chronic abdominal pain, and dyspareunia (difficult or painful sexual intercourse) (SRS 2007). A recent study demonstrated that in women with a known reason for small‐bowel obstruction, adhesions were the single most common cause (Ten Broek 2013).
Cutting, surgical denudation, ischaemia, desiccation, or abrasion can cause peritoneal trauma during surgery. Subsequent healing in the peritoneal cavity occurs through a combination of mesothelial regeneration and fibrosis, resulting in adhesion formation between damaged serosal surfaces (diZerega 1990).
Minimally invasive techniques such as laparoscopy reduce the risk of de novo (new) adhesion formation but do not eliminate it entirely. Studies included within this review therefore assessed both laparoscopy and laparotomy.
Description of the intervention
Several barrier agents with different characteristics are commercially available. Oxidised regenerated cellulose was the first tested synthetic mechanical barrier agent to cover traumatised peritoneum in the pelvis. It is applied over raw tissue surfaces at the last stage of surgery after haemostasis has been achieved, and it is designed to form a gelatinous protective coat within eight hours of application. Following this, it is broken down into its monosaccharide constituents and is designed to be absorbed within two weeks. Concerns with use of oxidised regenerated cellulose include migration and the need for meticulous haemostasis. Use of oxidised regenerated cellulose in the presence of bleeding may promote fibrin deposition at sites of incomplete haemostasis, resulting in adhesion formation rather than prevention (Wiseman 1999).
Another commercially available barrier agent is expanded polytetrafluoroethylene surgical membrane. This inert and permanent barrier acts by preventing cellular growth. However, it must be sutured to remain in place, and this may increase the incidence of adhesions while prolonging operating time. Much debate is ongoing regarding the need to remove expanded polytetrafluoroethylene after peritoneal healing is complete. This debate stems from studies investigating expanded polytetrafluoroethylene in vascular and pericardial grafts, which found no significant long‐term adverse effects when the barrier was not removed (Jacobs 1996). In addition, a prospective multi‐centre observational study investigating the long‐term use of expanded polytetrafluoroethylene without removal reported only one case of postoperative infection, which did not require removal of the membrane (Hurst 1998).
Other products include sodium hyaluronate with carboxymethylcellulose, an adhesion barrier agent composed of chemically derived sodium hyaluronate and carboxymethylcellulose. It is designed to be absorbed from the peritoneal cavity within seven days and completely excreted from the body within 28 days (Diamond 1996). Sodium hyaluronate with carboxymethylcellulose consists of chemically modified hyaluronic acid and carboxymethylcellulose. It separates denuded planes of tissue for up to seven days before it is absorbed. As with oxidised regenerated cellulose, migration is the cause of some concern; however no evidence suggests that its effectiveness is altered by the presence of blood. Although evidence for its use in gynaecological surgery is limited, sodium hyaluronate with carboxymethylcellulose has been widely studied in the context of colorectal surgery. Formation of adhesions and incidence of small‐bowel obstruction were reduced in five randomised controlled trials (RCTs) investigating its use in various colorectal and general surgical procedures (Ten Broek 2013).
Another product is a dual‐sided hydrophilic film. One side is a smooth surface consisting of porcine collagen, polyethylene glycol, and glycerol, and the opposite side is a porous surface made of lyophilised porcine collagen. The film is designed to degrade in the body within three weeks of application, and it has demonstrated significant reduction in adhesion formation in rat models (Gruber‐Blum 2011).
Fibrin sheet is a sheet‐type fibrin sealant with a solid layer of human fibrinogen, thrombin, and aprotinin coating the active surface of equine collagen stained with riboflavin (Mais 1995a; Pellicno 2003).
Fluid and pharmacological methods used to prevent adhesion formation are investigated in another Cochrane Review (Ahmad 2014).
How the intervention might work
Theoretically, inert physical materials that are able to prevent mechanical contact between serosal surfaces for longer than three days have the potential to be helpful in preventing adhesion formation. This would allow independent healing of each traumatised peritoneal surface. Some barrier agents (e.g. expanded polytetrafluoroethylene) need to be sutured into place, requiring extra operating time, especially during laparoscopic procedures.
Why it is important to do this review
This review is concerned with the effectiveness of barrier agents placed in the peritoneal cavity for preventing adhesions and in some cases for improving fertility. Pharmacological adjuncts are reviewed elsewhere. Women present with the secondary effects of adhesions, including dyspareunia, subfertility, bowel obstruction, and chronic pelvic pain. These problems can greatly impact quality of life and may necessitate further surgery. No clinical consensus or guidance is available regarding the most effective anti‐adhesion agent. Assessment of the evidence on effectiveness of barrier agents is therefore important.
Objectives
To evaluate the effects of barrier agents used during pelvic surgery on rates of pain, live birth, and postoperative adhesions in women of reproductive age.
Methods
Criteria for considering studies for this review
Types of studies
We included published or unpublished randomised controlled trials (RCTs) in which either women or pelvic structures were the unit of randomisation. Non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days or participant numbers) were excluded, as they are associated with high risk of bias. In the case of cross‐over trials, only data from before the cross‐over would have been included, as any additional pelvic surgery increases the risk of adhesion formation. However, no cross‐over trials were identified.
Types of participants
Women undergoing pelvic surgery for infertility or for other indications. Studies investigating adhesion prevention in non‐gynaecological specialties were excluded. Types of surgery performed could include open or laparoscopic procedures.
Types of interventions
Trials comparing physical barrier agents (oxidised regenerated cellulose, expanded polytetrafluoroethylene, sodium hyaluronate with carboxymethylcellulose, fibrin sheet, collagen membrane with polyethylene glycol and glycerol) used during pelvic surgery versus any other physical barrier agent or placebo or no treatment were included.
Studies of fibrin glue and Sepracoat (Genzyme Corporation) were excluded, as these are not physical barrier agents.
Types of outcome measures
Primary outcomes
Pelvic pain (improvement/worsening/no change in pain at second‐look laparoscopy (SLL)), as measured by validated pain scales, for example, visual analogue pain scale (VAS) scores, the McGill Pain Questionnaire (MPQ), a pain improvement rating scale, general pain experience, or a gynaecological pain questionnaire
Live birth rate
Secondary outcomes
Adhesion score, recorded on whichever scale the study authors used but with preference given to the modified American Fertility Society (mAFS) score
Number of participants with adhesions at SLL
Clinical pregnancy rate (pregnancy confirmed on ultrasound scan)
Miscarriage rate, defined as loss of pregnancy before 24 weeks of gestation
Ectopic pregnancy rate
Number of participants with improvement in quality of life (QoL) at SLL, recorded on whichever scale was chosen by study authors
Adverse events
Live birth rate and clinical pregnancy rate are relevant when studies have specifically investigated use of the barrier agent in procedures performed to improve fertility. This does not apply to some studies.
Articles that met the inclusion criteria but did not report any of the outcomes considered within this review were also included within the qualitative analysis.
Search methods for identification of studies
We searched for all published and unpublished RCTs comparing the use of barrier agents versus any other active intervention or placebo/no treatment without language restriction. Searches were designed and conducted by the Information Specialist for Cochrane Gynaecology and Fertility.
Electronic searches
We searched:
Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials; Procite platform, searched 21 August 2019 (Appendix 1);
Central Register of Controlled Trials (CENTRAL); OVID platform, searched 21 August 2019 (Appendix 2);
MEDLINE; OVID platform, searched from 1946 to 21 August 2019 (Appendix 3);
Embase; OVID platform, searched from 1980 to 21 August 2019 (Appendix 4); and
PsycINFO; OVID platform, searched from 1806 to 21 August 2019 (Appendix 5).
These searches were conducted by the Information Specialist for the Cochrane Gynaecology and Fertility Group.
Other electronic searches included trial registers for the US National Institutes of Health, the World Health Organization (WHO) international trial registry platform, the Database of Abstracts of Reviews of Effects (DARE), OpenGrey, PubMed, Epistemonikos, and Google Scholar.
We conducted the last search in August 2019.
Searching other resources
We also searched the reference lists of relevant publications, review articles, and included studies and contacted experts in the field to request additional data. We handsearched relevant journals and conference abstracts that were not covered in the CGF register, in liaison with the Information Specialist. Two review authors screened retrievals.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts identified by the search, we retrieved the full text of all potentially eligible studies. Three review authors (PA, KK, MT) independently examined these full‐text articles for compliance with inclusion criteria. Disagreements on study eligibility were resolved by consultation with a fourth review author (GA). Review authors corresponded with study investigators to clarify study eligibility (e.g. with respect to participant eligibility criteria and allocation method). Figure 1 shows a flow diagram of study selection.
1.
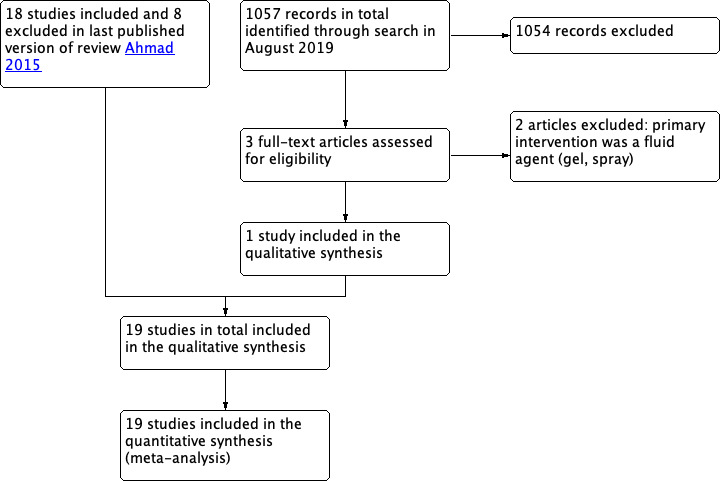
Study flow diagram.
Data extraction and management
Three review authors (PA, KK, MT) independently extracted data from eligible studies using a data extraction form that was designed and pilot‐tested by the review authors. Disagreements were resolved by consultation with a fourth review author (GA). Data extracted included study characteristics and outcome data. When studies were followed by multiple publications, the main trial report was used as the reference, and additional details were derived from secondary papers. We corresponded with study investigators to ask for further data on methods and/or results, as required.
Assessment of risk of bias in included studies
We assessed each included trial for the following criteria using the Cochrane risk of bias assessment tool: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias (Higgins 2011). We presented conclusions in the 'Risk of bias' table and incorporated them into the interpretation of review findings by performing sensitivity analyses. Two review authors (KK, MT) independently performed all assessments of the quality of clinical trials. All discrepancies were resolved by GA and PA.
Care was taken to search for within‐study reporting bias, as seen in trials failing to report obvious outcomes (e.g. pregnancy rate, major complications) or reporting them in insufficient detail.
Measures of treatment effect
Results of dichotomous variables are presented as Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CIs), and results of continuous variables are presented as mean differences (MDs).
We reversed the direction of effects of individual studies, when required, to ensure consistency across trials. We treated ordinal data as continuous outcomes. We presented 95% CIs for all outcomes. When data used to calculate ORs or MDs were not available, we planned to utilise the most detailed numerical data available, which might facilitate similar analyses of included studies. We compared the magnitude and direction of effects reported by studies versus how they are presented in the review, taking account of legitimate differences.
Unit of analysis issues
For within‐participant designs, 'effective sample sizes' were calculated to allow for statistical synthesis, that is, for a trial randomly assigning ovaries within each participant rather than randomly assigning participants, numbers were calculated to simulate as nearly as possible the odds ratio and the confidence interval as if the study design had randomly assigned participants, not ovaries. This was achieved as follows.
We calculated the odds ratio and the 95% CI for the matched design.
We constrained the control rate in the hypothetical parallel design to make it equal to that observed in the matched design.
We constrained group sizes for the parallel design to make them equal to each other.
Based on the two constraints above, we calculated the numbers of 'successes' and 'failures' in each group to reproduce as nearly as possible the OR (95% CI) of the matched design.
As a result of this type of re‐analysis of data, studies with within‐participant design that may have previously demonstrated significance may no longer do so. Several studies of within‐participant design comparing oxidised regenerated cellulose versus no treatment were presented and analysed wrongly as having a parallel design. We recognised that failure to account for pairing and failure to take account of doubling the sample size by using ovaries instead of participants as the unit of randomisation may have yielded spurious results. For these studies, the least favourable outcome for oxidised regenerated cellulose that was compatible with the reported results was assumed. We attempted to obtain correct results tables from study authors but were unsuccessful.
Dealing with missing data
Data were analysed on an intention‐to‐treat (ITT) basis as far as possible, and attempts were made to obtain missing data from the original investigators. When data for primary outcomes could not be obtained, we planned to undertake imputation of individual values. For secondary outcomes, only available data were analysed. If studies reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), we assumed that the outcome had a standard deviation equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
Clinical and methodological characteristics of individual studies were considered to ensure that any pooling was clinically meaningful. The I² statistic was calculated to assess statistical heterogeneity. An I² measurement over 50% was taken to indicate substantial heterogeneity.
Assessment of reporting biases
In view of the difficulties involved in detecting and correcting publication bias and other reporting biases, study authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies, and by remaining alert for duplication of data. We aimed to minimise the impact by ensuring that a robust and comprehensive search was performed. We planned to create a funnel plot of 10 or more studies that were included in a meta‐analysis to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to suggest that it is more beneficial in smaller studies).
Data synthesis
Statistical analysis was performed in accordance with the guidelines developed by Cochrane. Data from the primary studies were combined in RevMan using the fixed‐effect model. We planned to report standardised mean differences (SMDs) if similar outcomes were reported on different scales. An increase in OR, SMD, or MD was indicated to the right of the central line of the forest plot, and a decrease was indicated to the left of the central line. Whether this favoured treatment or no treatment depended on the outcome analysed, but the axes were labelled accordingly. Analyses were stratified by type of barrier agent, type of surgery, and type of control.
Subgroup analysis and investigation of heterogeneity
If we had detected substantial heterogeneity, we planned to explore possible explanations via sensitivity analyses. We planned to take any statistical heterogeneity into account when interpreting the results, especially if any variation in the direction of effect was evident.
Sensitivity analysis
We conducted sensitivity analyses for the important review outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility of studies and analysis. These analyses included consideration of whether review conclusions differed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted; or
relative risk had been used as the summary effects measure.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEpro software and Cochrane methods (GRADEpro GDT 2015; Higgins 2011). These tables evaluate the overall quality of the body of evidence for the main review outcomes (pelvic pain, live birth rate, clinical pregnancy rate, improvement or worsening of adhesion scores, and incidence of adhesions) using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). Judgements about evidence quality (high, moderate, or low) have been justified, documented, and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
At the 2020 update three studies were retrieved in full text. One new study was identified and was included within the meta‐analysis in this update (Canis 2014), and two were excluded; there are now 19 included studies and 10 excluded. See Figure 1 for an overview of search results.
At the 2015 update eighteen studies met inclusion criteria, and eight studies were excluded. See study tables under Characteristics of included studies. Reasons why studies were excluded are detailed in the Characteristics of excluded studies tables.
Included studies
Trial design and setting
Number of studies
We identified 19 RCTs that met the inclusion criteria (1309 participants).
Multi‐centre trials
Ten were multi‐centre studies (Azziz 1993; Canis 2014; Diamond 1996; Franklin 1995; Haney 1995; Myomectomy ASG 1995; Nordic APSG 1995; Sekiba 1992; Takeuchi 2005; Tinelli 2011).
Design
In seven parallel‐group trials, the unit of randomisation was the participant (Canis 2014; Diamond 1996; Mais 1995a; Mais 1995b; Takeuchi 2005; Tinelli 2011Wallweiner 1998). The remainder were within‐participant trials in which the unit of randomisation was the ovary, uterine sites, or the pelvic sidewall. One study was a multi‐arm trial comparing fibrin gel and a fibrin sheet versus control (Takeuchi 2005). Comparison of the fibrin sheet arm versus the control arm is included in this review.
Support/sponsorship
Thirteen trials stated sponsorship. Seven trials were sponsored by Johnson & Johnson (Azziz 1993; Franklin 1995; Li 1994; Nordic APSG 1995; Saravelos 1996; Sekiba 1992; van Geldorp 1994), one was sponsored by Genzyme (Diamond 1996), one by Covidien (Canis 2014), and a further two trials by manufacturers of expanded polytetrafluoroethylene (Haney 1995; Myomectomy ASG 1995). One trial received additional sponsorship from the Medical Research Council (MRC) in Canada (Greenblatt 1993). Another trial received support from Johnson & Johnson after the study was completed to help with the analysis, but the final content of the paper was under the sole control of the principal investigator (Keckstein 1996).
Participants
Primary indications for surgery
Myomectomy was the indication in six trials (Canis 2014; Diamond 1996; Mais 1995b; Myomectomy ASG 1995; Takeuchi 2005; Tinelli 2011), and ovarian surgery was the indication in four trials (Greenblatt 1993; Keckstein 1996; Saravelos 1996; van Geldorp 1994). Three trials were restricted to women undergoing adhesiolysis for infertility (Azziz 1993; Nordic APSG 1995; Sekiba 1992). Participants also underwent adhesiolysis in Li 1994, although the indications were subgrouped into infertility or pelvic pain. The indication for the Haney 1995 trial was also pelvic adhesions, although the primary reason for surgery was not exclusively infertility, and this trial was not subgrouped. The indications were endometriosis in Mais 1995a and Wallweiner 1998, and bilateral ovarian disease in Franklin 1995.
Type of surgery
Laparotomy was performed in nine trials (Azziz 1993; Canis 2014; Diamond 1996; Franklin 1995; Li 1994; Myomectomy ASG 1995; Nordic APSG 1995; Sekiba 1992; van Geldorp 1994), and laparoscopy was performed in the remainder (Greenblatt 1993; Haney 1995; Keckstein 1996; Korell 1994; Mais 1995a; Mais 1995b; Saravelos 1996; Takeuchi 2005; Wallweiner 1998). Tinelli 2011 performed both laparoscopy and laparotomy.
Suturing
Oxidised regenerated cellulose was occasionally sutured into place (Keckstein 1996; Li 1994; Sekiba 1992), whereas expanded polytetrafluoroethylene was always sutured into place (Haney 1995; Korell 1994; Myomectomy ASG 1995).
Adjuvants to surgery
One trial compared oxidised regenerated cellulose versus no treatment; hydrocortisone was also instilled in both treatment and control groups (Li 1994).
Microsurgical techniques
In three trials, it was noted that microsurgery was performed (Azziz 1993; Li 1994; Nordic APSG 1995).
Interventions
Thirteen RCTs compared oxidised regenerated cellulose with no treatment ‐ six at laparoscopy (Keckstein 1996; Mais 1995a; Mais 1995b; Saravelos 1996; Tinelli 2011; Wallweiner 1998), and seven at laparotomy (Azziz 1993; Franklin 1995; Li 1994; Nordic APSG 1995; Sekiba 1992; Tinelli 2011; van Geldorp 1994)
Two RCTs compared expanded polytetrafluoroethylene versus oxidised regenerated cellulose (Haney 1995; Korell 1994)
One RCT compared expanded polytetrafluoroethylene versus no treatment (Myomectomy ASG 1995)
One RCT compared sodium hyaluronate with carboxymethylcellulose versus no treatment (Diamond 1996)
One RCT compared fibrin sheet versus no treatment (Takeuchi 2005)
One RCT compared collagen film with polyethylene glycol and glycerol versus Ringer's lactate (Canis 2014)
Timing of second‐look laparoscopy (SLL)
Only two studies stated a mean time after laparoscopy (Diamond 1996 ‐ 23 days; Haney 1995 ‐ 30 days). Large variation was noted in timing of the SLL both within and between studies. Only three studies performed all SLL procedures within six weeks (Greenblatt 1993; Haney 1995; Myomectomy ASG 1995). Only four further studies performed SLL after eight weeks (Keckstein 1996; Mais 1995a; Mais 1995b; Wallweiner 1998). Remaining studies performed SLL after 10 days to 20 weeks.
Tinelli 2011 did not routinely perform SLL but instructed participants to have any further surgery completed within a six‐year period. Adhesions were assessed at the second surgery. Timing of the second surgery varied between 2.3 and 2.5 years.
Studied outcomes
Primary outcomes
Pelvic pain
No trials reported this outcome.
Live birth rate
No trials reported this outcome.
Secondary outcomes
Adhesion score
Only one study reported mean adhesion score as an outcome (Haney 1995). Another study reported median adhesion score as an outcome (Canis 2014).
Number of participants with adhesions at SLL
Nine trials reported the incidence of de novo adhesions as an outcome (Canis 2014; Diamond 1996; Greenblatt 1993; Korell 1994; Mais 1995b; Myomectomy ASG 1995; Saravelos 1996; Takeuchi 2005; Tinelli 2011). Ten trials reported the incidence of re‐formation adhesions as an outcome (Azziz 1993; Franklin 1995; Haney 1995; Keckstein 1996; Li 1994; Mais 1995a; Nordic APSG 1995; Sekiba 1992; van Geldorp 1994; Wallweiner 1998).
Clinical pregnancy rate
One study reported this outcome (Canis 2014).
Miscarriage rate
No trials reported this outcome.
Ectopic pregnancy rate
One study reported this outcome (Canis 2014).
Quality of life
No trials reported this outcome.
Adverse effects
Fourteen studies reported adverse effects as an outcome (Azziz 1993; Canis 2014; Diamond 1996; Franklin 1995; Haney 1995; Keckstein 1996; Mais 1995a; Mais 1995b; Myomectomy ASG 1995; Nordic APSG 1995; Saravelos 1996; Takeuchi 2005; Tinelli 2011; van Geldorp 1994). In the remaining studies, the absence of adverse effects was not stated.
Excluded studies
Ten studies were excluded. In nine studies, the agent used as the intervention was not a barrier agent, and one study did not investigate use of a barrier agent during a gynaecological procedure (To 1992).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary diagram.
2.
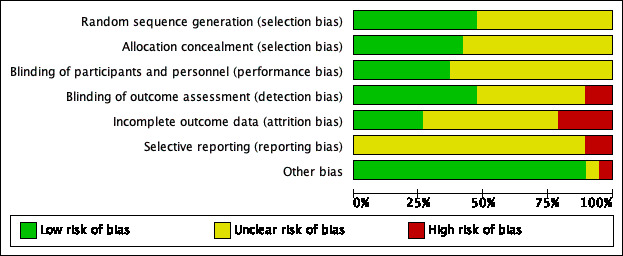
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
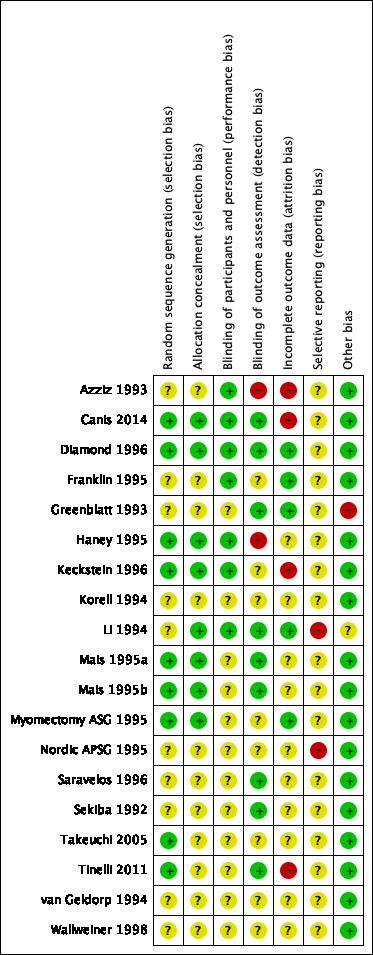
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Ten studies were rated as having unclear risk of bias. Study authors did not detail the randomly assigned component despite stating that these were randomised studies (Azziz 1993; Franklin 1995; Greenblatt 1993; Korell 1994; Li 1994; Nordic APSG 1995; Saravelos 1996; Sekiba 1992; van Geldorp 1994; Wallweiner 1998). Nine studies were deemed to have low risk of bias for sequence generation (Canis 2014; Diamond 1996; Haney 1995; Keckstein 1996; Mais 1995a; Mais 1995b; Myomectomy ASG 1995; Takeuchi 2005; Tinelli 2011).
Concealment of allocation
Eight studies were deemed to have low risk of selection bias (Canis 2014; Diamond 1996; Haney 1995; Keckstein 1996; Li 1994; Mais 1995a; Mais 1995b; Myomectomy ASG 1995). The remaining 11 studies were deemed to have unclear risk of selection bias. Although five of these studies used sealed envelopes, investigators did not detail the methods used to develop and monitor the allocation process with sealed envelopes (Azziz 1993; Franklin 1995; Nordic APSG 1995; Saravelos 1996; Sekiba 1992).
Blinding
Blinding of participants and personnel (performance bias)
Due to the nature of the intervention and the lack of comparable placebo agents, no study achieved blinding of the primary surgeon. However, by revealing the treatment allocation only after completing the initial surgical intervention, seven studies were deemed to have minimised performance bias (Azziz 1993; Canis 2014; Diamond 1996; Franklin 1995; Haney 1995; Keckstein 1996; Li 1994).
Twelve studies were rated to have unclear risk of performance bias (Greenblatt 1993; Korell 1994; Mais 1995a; Mais 1995b; Myomectomy ASG 1995; Nordic APSG 1995; Saravelos 1996; Sekiba 1992; Takeuchi 2005; Tinelli 2011; van Geldorp 1994; Wallweiner 1998), as study authors did not clearly state whether participants were blinded and/or whether treatment allocation was revealed before or during the initial surgery.
Blinding of outcome assessment (detection bias)
Nine studies clearly stated that assessors at SLL were blinded (Canis 2014; Diamond 1996; Greenblatt 1993; Li 1994; Mais 1995a; Mais 1995b; Saravelos 1996; Sekiba 1992; Tinelli 2011). Two of these studies recorded the SLL in video, and blinded independent assessors watched those videos to grade the severity of adhesions (Canis 2014; Diamond 1996). However, in Canis 2014, video recording was not performed in seven of the SLL patients, meaning that these patients were not assessed by a blinded independent surgeon, potentially increasing the risk of detection bias. Therefore, we included only the data from independently performed SLL from Canis 2014 in our final data analysis.
Eight studies did not clearly state that the surgeon performing the SLL was blinded to the initial intervention (Franklin 1995; Keckstein 1996; Korell 1994; Myomectomy ASG 1995; Nordic APSG 1995; Takeuchi 2005; van Geldorp 1994; Wallweiner 1998).
Two studies were deemed to have high risk of detection bias, as the surgeons at SLL were not blinded to the intervention group (Azziz 1993; Haney 1995).
Fourteen studies blinded surgeons to randomisation at second‐look laparoscopy, three studies did not discuss blinding (Haney 1995; Myomectomy ASG 1995; van Geldorp 1994), and two studies did not use blinding (Azziz 1993; Takeuchi 2005).
Incomplete outcome data
Four studies were rated as high risk, 10 as unclear risk, and five as low risk.
Dropouts
Azziz 1993 reported a large number of dropouts. A total of 198 participants were randomly assigned, but 64 were excluded from analysis because of inadequate documentation in 23, surgical technique and evaluation inconsistent with the protocol in 36, concurrent therapy in conflict with the protocol in three, and participant refusal to undergo second‐look laparoscopy in two (Wiseman 1999), demonstrating high risk of bias. Keckstein 1996 also reported a large number of dropouts, with 8 of 25 participants not returning for SLL. No reason was given for this. Canis 2014 reported a large number of dropouts as well, with 7 of 61 patients not undergoing second‐look laparoscopy and 21 of 61 not returning for three‐year follow‐up.
Tinelli 2011 reported participants undergoing a second surgical procedure within six years of initial surgery: 546 of 694 participants underwent a second surgery. In contrast to other trials, a second‐look laparoscopy at a specified time interval was not performed on all participants, leading to the exclusion of 148 participants from the results; this increases the risk of attrition bias.
Withdrawals and intention‐to‐treat analysis
Withdrawals were stated in eleven studies (Azziz 1993; Canis 2014; Diamond 1996; Greenblatt 1993; Haney 1995; Keckstein 1996; Li 1994; Myomectomy ASG 1995; Saravelos 1996; Takeuchi 2005; Tinelli 2011), and three studies did not state withdrawals (Franklin 1995; Nordic APSG 1995; Sekiba 1992); in five studies, no dropouts were reported (Mais 1995a; Mais 1995b; Takeuchi 2005; van Geldorp 1994; Wallweiner 1998). No studies with dropouts performed an ITT analysis. A total of six participants from all studies were reported to have been unable to undergo second‐look laparoscopy because of pregnancy.
Selective reporting
Two studies were rated as high risk (Li 1994; Nordic APSG 1995), and the other 17 studies were rated as unclear risk. The Nordic study reported larger numbers of participants randomly assigned in meeting abstracts before the study was published in a journal (Nordic APSG 1995). The explanation for this discrepancy was that some participants who did not meet inclusion criteria were recruited and were not included in the final analysis (personal communication, Wiseman 1999). In the United Kingdom, centres other than Sheffield were randomly assigning participants in a study of oxidised regenerated cellulose versus no treatment in the early 1990s (personal communication, A Watson), but the data have never been published (Li 1994). Attempts to identify these data by contacting investigators and the sponsoring company (Ethicon, Bridgewater, New Jersey, USA) have proved fruitless thus far. In van Geldorp 1994, another centre was recruiting participants (investigator: Trimbos‐Kemper, The Netherlands), but these results have been reported only briefly in a review (Wiseman 1999). Data regarding the use of oxidised regenerated cellulose must be interpreted with caution because of the high risk of bias.
Additional information was sought from some study authors; at the time of this writing, six replies had been received (Azziz 1993; Franklin 1995; Keckstein 1996; Myomectomy ASG 1995; Saravelos 1996; van Geldorp 1994).
Haney 1995 reported statistically significant reductions in the adhesion score, but it is not clear whether these analyses took account of the within‐participant design. It should be noted that the incidence of adhesions in the oxidised regenerated cellulose arm of the trial is greater than would be expected from the RCTs of oxidised regenerated cellulose versus no barrier agent. As it was not clear from the publication whether the surgeon was unblinded at the time of the second‐look laparoscopy, this result should be treated with some caution.
Other potential sources of bias
Statistical analysis
Of the 10 studies using a within‐participant design and reporting adhesion formation, only three presented and analysed matched data appropriately (Azziz 1993; Saravelos 1996; Sekiba 1992). Appropriate data were extracted from the reports of two others (Franklin 1995; Haney 1995), which were incorrectly analysed in the original. See Methods section for a full description. One further study failed to present comparative data and was excluded from all analyses (Greenblatt 1993).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Analysis 1 ‐ Oxidised regenerated cellulose vs no treatment at laparoscopy for adhesion prevention after gynaecological surgery.
| Oxidised regenerated cellulose vs no treatment at laparoscopy for adhesion prevention after gynaecological surgery | ||||||
|
Population: women having gynaecological surgery
Settings: surgical
Intervention: oxidised regenerated cellulose (Interceed) Comparison: no treatment at laparoscopy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Oxidised regenerated cellulose | |||||
| Pelvic pain | Not reported in any study in this comparison | |||||
| Live birth rate | Not reported in any study in this comparison | |||||
| Adhesion score | Not reported in any study in this comparison | |||||
| Incidence of adhesions ‐ de novo Incidence of adhesions at second‐look laparoscopy | 479 per 1000 | 315 per 1000 (216 to 433) | OR 0.50 (0.3 to 0.83) | 360 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d | |
| Incidence of adhesions ‐ re‐formation (or mixture) Incidence of adhesions at second‐look laparoscopy | 746 per 1000 | 333 per 1000 (171 to 546) | OR 0.17 (0.07 to 0.41) | 100 (3 studies) | ⊕⊝⊝⊝ Very Lowa,c,d | |
| Clinical pregnancy rate | Not reported in any study in this comparison | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to risk of bias; high level of attrition in one study. bDowngraded one level due to inconsistency; I² = 75%. cDowngraded one level due to unclear risk of publication bias. dDowngraded one level due to imprecision: small number of events and relatively wide confidence intervals.
Summary of findings 2. Analysis 2 ‐ Oxidised regenerated cellulose vs no treatment at laparotomy for adhesion prevention after gynaecological surgery.
| Oxidised regenerated cellulose vs no treatment at laparotomy for adhesion prevention after gynaecological surgery | ||||||
|
Population: women having gynaecological surgery
Settings: surgical
Intervention: oxidised regenerated cellulose Comparison: no treatment at laparotomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Oxidised regenerated cellulose | |||||
| Pelvic pain | Not reported in any study in this comparison | |||||
| Live birth rate | Not reported in any study in this comparison | |||||
| Adhesion score | Not reported in any study in this comparison | |||||
| Incidence of adhesions ‐ de novo Incidence at second‐look laparoscopy | 479 per 1000 | 399 per 1000 (279 to 535) | OR 0.72 (0.42 to 1.25) | 271 (1 study) | ⊕⊝⊝⊝ Very Lowa,b,c | |
| Incidence of adhesions ‐ re‐formation (or mixture) Incidence at second‐look laparoscopy | 746 per 1000 | 528 per 1000 (443 to 618) | OR 0.38 (0.27 to 0.55) | 554 (6 studies) | ⊕⊕⊝⊝ Lowc,d | |
| Clinical pregnancy rate | Not reported in any study in this comparison | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to risk of bias; high level of attrition in one study. bDowngraded one level due to imprecision: small number of events and wide confidence intervals. cDowngraded one level due to unclear risk of publication bias. dDowngraded one level due to inconsistency: moderate heterogeneity (I² = 41%).
Summary of findings 3. Analysis 4 ‐ Expanded polytetrafluoroethylene vs oxidised regenerated cellulose for adhesion prevention after gynaecological surgery.
| Expanded polytetrafluoroethylene vs oxidised regenerated cellulose (Interceed) for adhesion prevention after gynaecological surgery | ||||||
|
Population: women having gynaecological surgery
Settings: surgical
Intervention: expanded polytetrafluoroethylene Comparison: oxidised regenerated cellulose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oxidised regenerated cellulose | Expanded polytetrafluoroethylene | |||||
| Pelvic pain | Not reported in any study in this comparison | |||||
| Live birth rate | Not reported in any study in this comparison | |||||
| Adhesion score Non‐validated score out of 11 at SLL | Mean adhesion score was ‐3.79 lower (5.12 to 2.46 lower) in the expanded polytetrafluoroethylene group | 58 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | |||
| Incidence of adhesions ‐ de novo Incidence at second‐look laparoscopy | 149 per 1000 | 141 per 1000 (44 to 374) | OR 0.93 (0.26 to 3.41) | 38 (1 study) | ⊕⊝⊝⊝ Very lowc,d | |
| Incidence of adhesions ‐ re‐formation (or mixture) Incidence at second‐look laparoscopy | 567 per 1000 | 146 per 1000 (26 to 512) | OR 0.13 (0.02 to 0.8) | 23 (1 study) | ⊕⊝⊝⊝ Very lowc,d | Confidence interval crossed the line of no effect when a risk ratio rather than an odds ratio was calculated (RR 0.36, 95% CI 0.13 to 1.01) |
| Clinical pregnancy rate | Not reported in any study in this comparison | |||||
| *The basis for the assumed risk is the median oxidised regenerated cellulose group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to imprecision: small number of events and wide confidence intervals. bDowngraded one level due to risk of bias: non‐validated adhesion scoring system used. cDowngraded one level due to unclear risk of publication bias. dDowngraded two levels due to imprecision: small number of events and wide confidence intervals which cross the line of no effect.
Summary of findings 4. Analysis 7 ‐ Collagen membrane with polyethylene glycol and glycerol vs Ringer's lactate.
| Collagen membrane with polyethylene glycol and glycerol vs Ringer's lactate | ||||||
|
Patient or population: patients with adhesion prevention after gynaecological surgery Settings: surgical Intervention: collagen membrane with polyethylene glycol and glycerol Comparison: Ringer's lactate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ringer's lactate | Collagen membrane with polyethylene glycol and glycerol | |||||
| Pelvic pain | Not reported in any study in this comparison | |||||
| Live birth rate | Not reported in any study in this comparison | |||||
| Adhesion score | Median adhesion score in the control group was 0.8 compared with 1.2 in the intervention group | 47 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
|||
|
Incidence of adhesions ‐ de novo Incidence at second‐look laparoscopy |
479 per 1000 | 36 per 1000 (0 to 415) | OR 0.04 (0.00 to 0.77) | 47 (1 study) | ⊕⊕⊝⊝ Lowa,c | |
|
Incidence of adhesions ‐ re‐formation or mixture Incidence at second‐look laparoscopy |
Not reported in any study in this comparison | |||||
| Clinical pregnancy rate | 235 per 1000 | 637 per 1000 (298 to 879) | OR 5.69 (1.38 to 23.48) | 39 (1 study) | ⊕⊝⊝⊝ Very lowa,c,d | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to imprecision: small sample size and wide confidence intervals. bDowngraded one level due to risk of bias: adhesion scores reported as median (min‐max) rather than mean and standard deviation. cDowngraded one level due to unclear risk of publication bias. dDowngraded one level due to risk of bias: high level of attrition.
No studies reported either of our primary outcomes (pelvic pain and live birth).
1. Oxidised regenerated cellulose versus no treatment at laparoscopy
1.1. Analysis.
Comparison 1 Oxidised regenerated cellulose vs no treatment at laparoscopy, Outcome 1 Incidence of adhesions.
Primary outcomes
1.1 Pelvic pain
This was not assessed by any study.
1.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
1.3 Adhesion score
This was not assessed by any study.
1.4 Number of participants with adhesions at SLL
Six trials reported this outcome. Three reported de novo adhesion formation (Mais 1995b; Saravelos 1996; Tinelli 2011), and three reported re‐formation of adhesions following adhesiolysis (Keckstein 1996; Mais 1995a; Wallweiner 1998); Keckstein 1996 reported both de novo and re‐formation adhesions and was therefore included with the other studies reporting re‐formed adhesions.
We are uncertain whether the use of oxidised regenerated cellulose reduced the incidence of de novo adhesions when compared with no treatment at second‐look laparoscopy (OR 0.50, 95% CI 0.30 to 0.83, 3 trials, 360 participants; I² = 75%; very low‐quality evidence). This evidence would suggest that among women with a 48% chance of developing de novo adhesions with no treatment, the incidence of de novo adhesions using oxidised regenerated cellulose will be between 22% and 44%.
No clear explanation was provided for the high heterogeneity in this analysis (I² = 75%). However, it is possible that the small number of participants within the included studies may partially account for it. Differences in operative procedure were observed between studies, as were differences in wrapping and use of oxidised regenerated cellulose (Saravelos 1996). It is unclear, however, whether these differences can account for the very high level of heterogeneity observed in these studies.
We are also uncertain whether the use of oxidised regenerated cellulose reduced the incidence of re‐formed (or mixed) of adhesions (OR 0.17, 95% CI 0.07 to 0.41, 3 trials, 100 participants; I² = 36%; very low‐quality evidence). This evidence would suggest that among women with a 75% chance of developing re‐formed (or mixed) adhesions with no treatment, the incidence of adhesion formation using oxidised regenerated cellulose will be between 18% and 55%.
See Figure 4.
4.
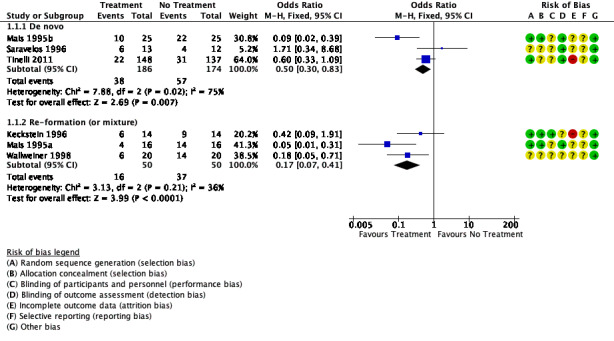
Forest plot of comparison: 1 Oxidised regenerated cellulose (Interceed) vs no treatment at laparoscopy, outcome: 1.1 Incidence of adhesions.
1.5 Clinical pregnancy rate
This was not assessed by any study.
1.6 Miscarriage rate
This was not assessed by any study.
1.7 Ectopic pregnancy rate
This was not assessed by any study.
1.8 Quality of life (QoL)
This was not assessed by any study.
1.9 Adverse outcomes
Tinelli 2011 reported the incidence of fever within 48 hours as 6.5% to 12.5% of cases; this was likely to be secondary to surgery rather than to use of the barrier agent. Otherwise, no adverse outcomes were identified among the 236 participants receiving the intervention across studies (Keckstein 1996; Mais 1995a; Mais 1995b; Saravelos 1996; Tinelli 2011; Wallweiner 1998).
2. Oxidised regenerated cellulose versus no treatment at laparotomy
2.1. Analysis.
Comparison 2 Oxidised regenerated cellulose vs no treatment at laparotomy, Outcome 1 Incidence of adhesions.
Primary outcomes
2.1 Pelvic pain
This was not assessed by any study.
2.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
2.3 Adhesion score
This was not assessed by any study.
2.4 Number of participants with adhesions at SLL
Six trials reported on re‐formation of adhesions following laparotomy for adhesiolysis ‐ Azziz 1993,Li 1994,Nordic APSG 1995,Sekiba 1992 ‐ and ovarian surgery ‐ Franklin 1995, van Geldorp 1994. Use of oxidised regenerated cellulose may have resulted in a reduction in the incidence of re‐formation adhesions compared with no treatment (OR 0.38, 95% CI 0.27 to 0.55, 6 trials, 554 participants; I² = 41%; low‐quality evidence). This suggests that among women with a 75% chance of adhesion re‐formation with no treatment, the incidence of adhesion re‐formation using oxidised regenerated cellulose will be between 45% and 62%.
We are uncertain whether oxidised regenerated cellulose affected the formation of de novo adhesions following laparotomy (OR 0.72, 95% CI 0.42 to 1.25, 1 trial, 271 participants; very low‐quality evidence). This suggests that among women with a 48% chance of developing de novo adhesions with no treatment, the incidence of de novo adhesions using oxidised regenerated cellulose will be between 28% and 54%.
See Figure 5.
5.
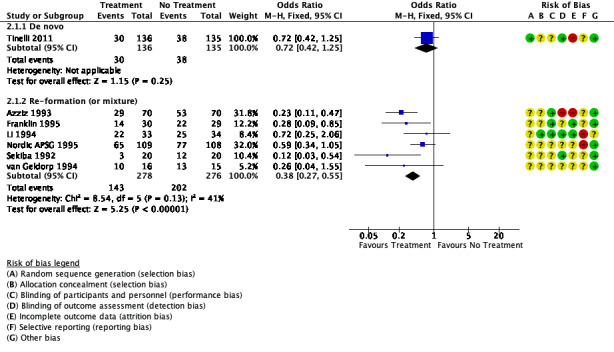
Forest plot of comparison: 2 Oxidised regenerated cellulose (Interceed) vs no treatment at laparotomy, outcome: 2.1 Incidence of adhesions.
2.5 Clinical pregnancy rate
This was not assessed by any study.
2.6 Miscarriage rate
This was not assessed by any study.
2.7 Ectopic pregnancy rate
This was not assessed by any study.
2.8 Quality of life (QoL)
This was not assessed by any study.
2.9 Adverse outcomes
In Azziz 1993, three adverse events occurred (atelectasis, postoperative ileus, fever and abdominal pain), and in van Geldorp 1994, a decrease in haemoglobin was reported. These adverse effects are related to the surgery rather than to the barrier agent. Otherwise, no adverse outcomes related to the agent were identified among 225 participants receiving the intervention across five studies (Azziz 1993; Franklin 1995; Nordic APSG 1995; Tinelli 2011; van Geldorp 1994). Two studies did not report on adverse outcomes (Li 1994; Sekiba 1992).
3. Expanded polytetrafluoroethylene versus no treatment
3.1. Analysis.
Comparison 3 Expanded polytetrafluoroethylene vs no treatment, Outcome 1 Incidence of adhesions.
Primary outcomes
3.1 Pelvic pain
This was not assessed by any study.
3.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
3.3 Adhesion score
This was not assessed by any study.
3.4 Number of participants with adhesions at SLL
Only one trial reported this comparison and described a reduction in new adhesion formation among women undergoing myomectomy at laparotomy (Myomectomy ASG 1995). Use of expanded polytetrafluoroethylene may have resulted in a reduction in new adhesion formation (OR 0.17, 95% CI 0.03 to 0.94, 1 trial, 42 participants; low‐quality evidence). This result was obtained by assuming the outcome least favourable to expanded polytetrafluoroethylene that was compatible with reported results.
3.5 Clinical pregnancy rate
This was not assessed by any study.
3.6 Miscarriage rate
This was not assessed by any study.
3.7 Ectopic pregnancy rate
This was not assessed by any study.
3.8 Quality of life (QoL)
This was not assessed by any study.
3.9 Adverse outcomes
No adverse outcomes were reported for 21 participants receiving the intervention in one trial (Myomectomy ASG 1995).
4. Expanded polytetrafluoroethylene versus oxidised regenerated cellulose
(Analysis 4.1 and Analysis 4.2; Table 3)
4.1. Analysis.
Comparison 4 Expanded polytetrafluoroethylene vs oxidised regenerated cellulose, Outcome 1 Mean adhesion score (non‐validated score).
4.2. Analysis.
Comparison 4 Expanded polytetrafluoroethylene vs oxidised regenerated cellulose, Outcome 2 Incidence of adhesions.
Primary outcomes
4.1 Pelvic pain
This was not assessed by any study.
4.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
4.3 Adhesion score
We are uncertain whether the use of expanded polytetrafluoroethylene affected the adhesion score at SLL when compared with oxidised regenerated cellulose (MD ‐3.79, 95% CI ‐5.12 to ‐2.46, 1 study, 62 participants; very low‐quality evidence).
4.4 Number of participants with adhesions at SLL
One trial reported no evidence of a difference in the formation of de novo adhesions following myomectomy at laparoscopy (OR 0.93, 95% CI 0.26 to 3.41, 1 trial, 38 participants; very low‐quality evidence) (Korell 1994). This suggests that among women with a 15% chance of developing de novo adhesions with oxidised regenerated cellulose, the incidence of de novo adhesions using expanded polytetrafluoroethylene will be between 5% and 38%.
One trial reported this comparison for a reduction in re‐formation of adhesions among women undergoing adhesiolysis at laparotomy (Haney 1995). We are uncertain whether the use of expanded polytetrafluoroethylene was associated with a reduction in adhesion re‐formation (OR 0.13, 95% CI 0.02 to 0.80, 1 trial, 23 participants; very low‐quality evidence). This suggests that among women with a 57% chance of adhesion re‐formation with oxidised regenerated cellulose, the incidence of adhesion re‐formation using expanded polytetrafluoroethylene will be between 3% and 52%. This finding was sensitive to choice of effect measure, just ceasing to show a reduction in adhesions when the risk of re‐formed adhesions was analysed using risk ratio (RR 0.36, 95% CI 0.13 to 1.01).
4.5 Clinical pregnancy rate
This was not assessed by any study.
4.6 Miscarriage rate
This was not assessed by any study.
4.7 Ectopic pregnancy rate
This was not assessed by any study.
4.8 Quality of life (QoL)
This was not assessed by any study.
4.9 Adverse outcomes
Participants in Haney 1995 experienced no adverse effects (three were treated with expanded polytetrafluoroethylene during surgery; nine were treated with oxidised regenerated cellulose). One study did not report whether adverse outcomes occurred (Korell 1994).
5. Sodium hyaluronate and carboxymethylcellulose versus no treatment
5.1. Analysis.
Comparison 5 Sodium hyaluronate and carboxymethylcellulose vs no treatment, Outcome 1 Mean adhesion score (non‐validated score).
Primary outcomes
5.1 Pelvic pain
This was not assessed by any study.
5.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
5.3 Adhesion score
Diamond 1996 reported adhesion severity score with use of sodium hyaluronate with carboxymethylcellulose in comparison with no treatment, using a non‐validated scoring method out of four. This showed that the use of sodium hyaluronate with carboxymethylcellulose probably resulted in a reduced adhesion severity score when compared with no treatment (MD 0.49, 95% CI 0.53 to 0.45, 1 trial, 127 participants; moderate‐quality evidence; Analysis 5.1).
5.4 Number of participants with adhesions at SLL
This was not assessed by any study.
5.5 Clinical pregnancy rate
This was not assessed by any study.
5.6 Miscarriage rate
This was not assessed by any study.
5.7 Ectopic pregnancy rate
This was not assessed by any study.
5.8 Quality of life (QoL)
This was not assessed by any study.
5.9 Adverse outcomes
No adverse outcomes were reported in the 59 participants undergoing the intervention in one trial (Diamond 1996).
6. Fibrin sheet versus no treatment
(Analysis 6.1 and Analysis 6.2)
6.1. Analysis.
Comparison 6 Fibrin sheet vs no treatment at laparoscopic myomectomy, Outcome 1 Mean adhesion score (non‐validated score).
6.2. Analysis.
Comparison 6 Fibrin sheet vs no treatment at laparoscopic myomectomy, Outcome 2 Incidence of de novo adhesions per participant.
Primary outcomes
6.1 Pelvic pain
This was not assessed by any study.
6.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
6.3 Adhesion score
One study assessed fibrin sheet versus no treatment at laparoscopic myomectomy. Study authors reported no difference in adhesion grading, using a non‐validated scoring method out of four (MD 0.14, 95% CI ‐0.67 to 0.39, 1 trial, 48 participants; very low‐quality evidence).
6.4 Number of participants with adhesions at SLL
Only one study assessed fibrin sheet versus no treatment at laparoscopic myomectomy (Takeuchi 2005). Study authors reported the frequency and severity of postoperative adhesions. No evidence was found of differences between groups in the incidence of postoperative adhesions per participant (OR 1.20, 95% CI 0.42 to 3.41, 1 study, 62 participants; very low‐quality evidence).
6.5 Clinical pregnancy rate
This was not assessed by any study.
6.6 Miscarriage rate
This was not assessed by any study.
6.7 Ectopic pregnancy rate
This was not assessed by any study.
6.8 Quality of life (QoL)
This was not assessed by any study.
6.9 Adverse outcomes
Participants in Takeuchi 2005 experienced no adverse effects (30 were treated with fibrin sheet during surgery).
7. Collagen membrane with polyethylene glycol plus glycerol versus Ringer's lactate
(Analysis 7.1, Analysis 7.2, Analysis 7.3, and Analysis 7.4; Table 4)
7.1. Analysis.
Comparison 7 Collagen membrane with polyethylene glycol and glycerol vs control at laparotomic myomectomy, Outcome 1 Incidence of adhesions.
7.2. Analysis.
Comparison 7 Collagen membrane with polyethylene glycol and glycerol vs control at laparotomic myomectomy, Outcome 2 Median adhesion score.
| Median adhesion score | ||
|---|---|---|
| Study | Treatment | Control |
| Canis 2014 | 0.8 (n=24) | 1.2 (n=23) |
7.3. Analysis.
Comparison 7 Collagen membrane with polyethylene glycol and glycerol vs control at laparotomic myomectomy, Outcome 3 Clinical pregnancy rate.
7.4. Analysis.
Comparison 7 Collagen membrane with polyethylene glycol and glycerol vs control at laparotomic myomectomy, Outcome 4 Ectopic pregnancy rate.
Primary outcomes
7.1 Pelvic pain
This was not assessed by any study.
7.2 Live birth rate
This was not assessed by any study.
Secondary outcomes
7.3 Adhesion score
One study assessed collagen membrane with polyethylene glycol plus glycerol versus Ringer's lactate in myomectomy at laparotomy (Canis 2014). The study reports median rather than mean mAFS scores at SLL; however results appear to demonstrate no difference between groups (0.8 vs 1.2, 1 trial, 47 participants; very low‐quality evidence).
7.4 Number of participants with adhesions at SLL
One study investigated the incidence of de novo adhesions with collagen membrane with polyethylene glycol plus glycerol versus Ringer's lactate in myomectomy at laparotomy (Canis 2014). This suggests that there may have been a lower incidence of adhesions at SLL in the collagen membrane with polyethylene glycol plus glycerol group when compared with the control group (OR 0.04, 95% CI 0.00 to 0.77, 1 trial, 47 participants; low‐quality evidence) (Analysis 7.1). This suggests that among women with a 48% chance of developing de novo adhesions with no barrier, the incidence of de novo adhesions using collagen membrane with polyethylene glycerol plus glycol will be between 0% and 42%.
See Figure 6.
6.
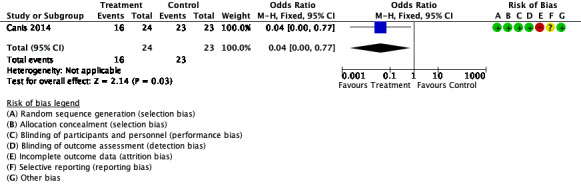
Forest plot of comparison: 7 Collagen membrane with polyethylene glycol and glycerol vs Ringer's lactate, outcome: 7.1 Incidence of adhesions.
7.5 Clinical pregnancy rate
One study assessed collagen membrane with polyethylene glycol and glycerol versus Ringer's lactate in myomectomy at laparotomy (Canis 2014). This suggests that collagen membrane with polyethylene glycol and glycerol may have improved clinical pregnancy rate at three‐year follow‐up when compared with the control (OR 5.69, 95% CI 1.38 to 23.48, 1 trial, 39 participants; low‐quality evidence; Analysis 7.3). This suggests that among women with a 23% chance of becoming clinically pregnant with no barrier, the incidence of clinical pregnancy using collagen membrane with polyethylene glycol plus glycerol will be between 29% and 87%.
7.6 Miscarriage rate
This was not assessed by any study.
7.7 Ectopic pregnancy rate
One study assessed collagen membrane with polyethylene glycol plus glycerol versus Ringer's lactate in myomectomy at laparotomy (Canis 2014). At three‐year follow‐up of 39 participants, no ectopic pregnancies were reported in either study group (Analysis 7.4).
7.8 Quality of life (QoL)
This was not assessed by any study.
7.9 Adverse outcomes
Canis 2014 reported four participants in the collagen membrane with polyethylene glycol plus glycerol group who were hospitalised due to adverse events: one due to intra‐abdominal bleeding, one with anaemia due to preoperative and postoperative bleeding, one with a parietal haematoma, and one with abdominal pain and digestive disorders.
Other analyses
Sensitivity analyses
Three studies reported an unclear randomisation procedure (Greenblatt 1993; Haney 1995; Wallweiner 1998) (although additional information has been requested). Excluding these studies from the analyses did not substantially affect findings.
Azziz 1993 reported a large number of dropouts (64/198), but excluding this study from the analyses did not affect findings.
In other sensitivity analyses, use of a random‐effects model, or of risk ratio rather than odds ratio, did not substantially change findings for any outcomes, except that in the comparison of expanded polytetrafluoroethylene versus oxidised regenerated cellulose (Analysis 4.2), the confidence interval crossed the line of no effect when a risk ratio rather than an odds ratio was calculated (RR 0.36, 95% CI 0.13 to 1.01).
Publication bias
Insufficient studies were included in any analysis to permit construction of a funnel plot, so we were unable to formally assess publication bias.
'Summary of findings' tables
'Summary of findings' tables for Analyses 3, 5, and 6 have not been included in this update of the review but can be found in the last published version (Ahmad 2015).
Discussion
Summary of main results
Review authors identified that no studies reported our primary outcomes of pelvic pain and live birth rate; therefore any conclusions discussed below relate solely to our secondary outcomes.
We found evidence that use of oxidised regenerated cellulose may reduce the incidence of re‐formation of adhesions at laparotomy (low‐quality evidence). We are uncertain whether use of oxidised regenerated cellulose reduces de novo adhesion formation or adhesion re‐formation at laparoscopy. We are also uncertain whether the use of oxidised regenerated cellulose reduces de novo adhesion formation at laparotomy.
We are uncertain whether polytetrafluoroethylene reduces adhesion formation when compared with oxidised regenerated cellulose.
One study suggested that use of sodium hyaluronate with carboxymethylcellulose probably reduces adhesion formation after laparoscopy (moderate‐quality evidence).
We are uncertain whether use of fibrin sheets reduces the formation of adhesions.
One study suggested that use of a collagen membrane with polyethylene glycol plus glycerol may reduce the formation of adhesions at laparotomy (low‐quality evidence). Based on evidence from the same study, we are uncertain whether collagen membrane with polyethylene glycol and glycerol improved clinical pregnancy rate (low‐quality evidence).
Overall completeness and applicability of evidence
Most gynaecological surgery is associated with risk of pelvic adhesions. Clinical consequences of adhesions include pain, subfertility, and small‐bowel obstruction, as well as complications of subsequent surgery. Minimally invasive techniques such as laparoscopy reduce the risk of de novo adhesion formation but do not eliminate it entirely. Studies included within this review therefore assessed both laparoscopy and laparotomy.
This review assessed the effectiveness of barrier agents in reducing adhesions following conservative gynaecological surgery. As noted above, pharmacological and fluid agents are assessed in a separate Cochrane Review.
Unfortunately, no studies in this review reported live birth rate or pelvic pain as a primary outcome but instead focused on extent of adhesion formation by incidence or by score. However, only six studies investigated the use of barrier agents in women seeking treatment to improve fertility. None of these studies reported adverse outcomes attributable to use of a barrier agent.
Quality of the evidence
The included studies were at unclear risk of bias in most domains. The most common limitation was failure to report sufficient details of study methods. Studies were at high risk of bias related to attrition rates (four studies), selective reporting (two studies), and lack of blinding (one study). Reporting of adverse effects was inadequate. We were unable to formally assess the risk of publication bias. However, we note that 13 studies reported sponsorship, 12 of which were sponsored by companies that manufactured adhesion agents. Furthermore, evidence suggests that unpublished data may exist regarding oxidised regenerated cellulose. Specifically, centres in the United Kingdom were known to be randomly assigning participants for a study in 1990 (personal communication, AW), although only results from Sheffield were identified by our search (Li 1994). Evidence of duplicate publication of data may contribute to increased risk of publication bias (Haney 1995).
Clinical heterogeneity between studies was observed, with differences in surgical technique and variable timing of second‐look laparoscopy ranging from 23 days to 2.5 years. Considerable statistical heterogeneity was observed in the comparison of oxidised regenerated cellulose versus no treatment at laparoscopy (I² = 75%), for which no clear explanation was provided.
We graded the overall quality of the evidence using GRADE methods. The quality ranged from very low to moderate but was low or very low for most comparisons. The main limitations were imprecision and poor reporting of study methods. See Table 1, Table 2, Table 3, and Table 4.
Summary of findings tables for Analyses 3, 5, and 6 have not been included in this update and can be found in the last published version (Ahmad 2015).
Potential biases in the review process
The review authors made every effort to identify all studies that should be considered for inclusion. However, it remains possible that some errors were made in the review process and some relevant publications may have been missed.
Since the time of the previous review, necessary details required for inclusion in Cochrane Reviews have increased substantially. Although all previously included studies were reassessed for bias, some information was required from the study authors themselves, and attempts were made to contact them.
Duplicate publication of data and existence of unreported data create a risk of bias. This is reflected in the GRADE rating of very low for oxidised regenerated cellulose versus no comparison at laparoscopy. This was often difficult to establish because the data were presented as abstracts at meetings (often by different study authors). For example, Haney 1995 had four abstract publications, and Keckstein 1996 had two abstract publications.
Agreements and disagreements with other studies or reviews
A review published in 2010 concludes that the "evidence is not adequate for definite conclusions to be drawn, either in terms of efficacy or in terms of safety" with regard to anti‐adhesion agents in gynaecological surgery (Pados 2010). This is consistent with the findings of this review, which has found very little evidence on clinically relevant endpoints. Furthermore, the generally low GRADE evidence quality ratings given to most of the outcomes in this review show how, on our assessment, considerable uncertainty remains regarding use of these agents for improving patient outcomes.
The guidelines of the Society of Obstetricians and Gynaecologists of Canada on use of adhesion agents in gynaecological surgery are consistent with the notion that evidence on the effects of barrier agents on long‐term clinical outcomes such as chronic pelvic pain and infertility is insufficient (Robertson 2010). These guidelines also state, "Oxidized regenerated cellulose adhesion barrier is associated with reduced incidence of pelvic adhesion formation at both laparoscopy and laparotomy when complete haemostasis is achieved." This information is somewhat consistent with that reported in this review, which found that oxidised regenerated cellulose may reduce the re‐formation of adhesions at laparotomy. However, this review determined that the quality of evidence obtained ranged from very low to low; and due to poor quality of evidence we were uncertain of the benefits of oxidised regenerated cellulose at laparoscopy.
The guidelines of the Society of Obstetricians and Gynaecologists of Canada also state, "Polytetrafluoroethylene barrier is more effective than no barrier or oxidized regenerated cellulose in preventing adhesion formation" (Robertson 2010). This again is partially consistent with our conclusions, as we identified one study suggesting that use of expanded polytetrafluoroethylene may reduce formation of de novo adhesions at laparoscopy. However, we rated the quality of evidence as low. We also found one trial that suggested superiority of expanded polytetrafluoroethylene over oxidised regenerated cellulose for incidence of re‐formation adhesions. We deemed this evidence to be of very low quality, as this was a very small, unblinded, privately funded study, and we are therefore uncertain whether polytetrafluoroethylene reduces adhesion formation when compared with oxidised regenerated cellulose.
Finally, Robertson 2010 states, "Chemically modified sodium hyaluronate/carboxymethylcellulose is effective in preventing adhesion formation, especially following myomectomies," although researchers warned that these results should be interpreted with caution on the basis of comments provided in the previous version of this review. Our updated review also agrees with this conclusion.
Authors' conclusions
Implications for practice.
We found no evidence on the effects of barrier agents used during pelvic surgery on either pain or fertility outcomes in women of reproductive age, as no studies were identified that reported these outcomes.
Low‐quality evidence suggests that oxidised regenerated cellulose, expanded polytetrafluoroethylene, sodium hyaluronate with carboxymethylcellulose, and collagen membrane with polyethylene glycol plus glycerol may all be more effective than no treatment in reducing the incidence of adhesion formation following pelvic surgery. We found no conclusive evidence on the relative effectiveness of these interventions. We are uncertain whether use of a fibrin sheet is more effective than no treatment. No adverse events directly attributed to the adhesion agents were reported. The quality of the evidence ranged from very low to moderate. The most common limitations were imprecision and poor reporting of study methods. Most studies were commercially funded, and publication bias could not be ruled out.
Implications for research.
Further high‐quality studies with improved blinding of surgeons/outcome assessors, reduced selective reporting, and use of intention‐to‐treat analyses are required. Research should focus on the effects of barrier agents on clinical outcomes, with emphasis on fertility outcomes among women trying to conceive. Future studies should include women as the unit of randomisation, live birth and pain as primary outcomes, and earlier second‐look laparoscopy (within four to six weeks).
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2019 | New search has been performed | Review has been updated; 1 new study has been included (Canis 2014) |
| 13 September 2019 | New citation required but conclusions have not changed | Evidence regarding new interventions has been included; however due to low‐quality evidence, we made no changes to our overall conclusions at this update |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 19 March 2015 | New search has been performed | Review has been updated; 1 new study has been included (Tinelli 2011) |
| 19 March 2015 | New citation required but conclusions have not changed | We made no change to our conclusions at this update |
| 2 June 2008 | Amended | Review has been converted to new review format |
| 20 December 2007 | New citation required and conclusions have changed | Substantive amendments have been made |
| 20 December 2007 | New search has been performed | Review has been updated |
Acknowledgements
The review authors would like to thank the members of Cochrane Gynaecology and Fertility, Auckland, New Zealand, who assisted with both the 1999 review and the 2007, 2015, and 2020 updates of the review.
We acknowledge the contributions of Dr Selma Mourad, Dr Sarah Lensen and Ms Katie Stocking who provided peer review comments.
We thank Cindy Farquhar, Patrick Vanderkerchove, Andy Vail, and David Wiseman for their contributions to the original 1999 version of the review.
Finally, we thank James Duffy, Cindy Farquhar, Andy Vail, Patrick Vanderkerchove, and David Wiseman for their contributions to the 2007 update to the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
Procite platform
Searched 21 August 2019
Keywords CONTAINS "adhesion"or "adhesions" or "adhesions outcome" or "gynecologic surgical procedure" or "surgery‐gynaecological" or "surgical complications" or "*Surgical‐Procedures,‐Laparoscopic" or "post‐operative adhesions" or "post operative complications" or "cell adhesion molecules" or"laparoscopic" or"laparoscopy" or"laparotomy"or "myomectomy"or"mini‐laparoscopy"or"mini‐laparotomy"or"minilaparotomic myomectomy"or"minilaparotomy"or"cystectomy"or"hysterectomy"or"uterine artery embolization"or"UAE"or"pelvic adhesions"or"ovarian adhesions"or"ovarian cystectomy"or"endometrial ablation"or"endometrial adhesions"or"endometrioma" or"electrosurgery"or"electroresection"or"laparotomy"or"*Laser Surgery"or"salpingectomy"or"*Salpingostomy‐"or"salpingotomy" or Title CONTAINS "adhesion"or "adhesions" or "adhesions outcome"or"surgical complications"or"*Surgical‐Procedures,‐Laparoscopic"or"post‐operative adhesions"or"endometrial adhesions"or "pelvic adhesions"
AND
Keywords CONTAINS "Barrier Membrane" or "Gore‐Tex" or "interceed" or "Polytetrafluoroethylene" or "Seprafilm" or "surgical membrane" or "dextran" or "adhesion barrier" or "adhesion barriers" or "adhesion prevention" or "adhesions outcome" or "surgical membrane" or"Oxiplex"or"SprayGel"or"adept"or "intercoat gel"or"sepracoat"or "adhesiolysis"or"polyethylene glycol"or"hydrogel"or "intergel" or "adhesions" or"adhesion"or"adhesiolysis"or Title CONTAINS "Barrier Membrane" or "Gore‐Tex" or "interceed" or "Polytetrafluoroethylene" or "Seprafilm" or "surgical membrane" or "dextran" or "adhesion barrier" or "adhesion barriers" or "adhesion prevention" or "adhesions outcome" or "surgical membrane" or"Oxiplex"or"SprayGel"or"adept"or "intercoat gel"or"sepracoat"or "adhesiolysis"or"polyethylene glycol"or"hydrogel"or "intergel"or"adhesions" or"adhesion"or"adhesiolysis" (291 records)
Appendix 2. CENTRAL search strategy
OVID platform
Searched 21 August 2019
1 exp gynecologic surgical procedures/ or exp endometrial ablation techniques/ or exp hysterectomy/ or exp hysteroscopy/ or exp ovariectomy/ or exp salpingostomy/ or exp uterine artery embolization/ (4091) 2 (gyn$ adj3 surg$).tw. (2937) 3 exp laparotomy/ (711) 4 exp laparoscopy/ (5248) 5 laparoscop$.tw. (17783) 6 laparotom$.tw. (2716) 7 (hysterectom$ or cystoscop$ or hysteroscop$).tw. (8399) 8 endometrial ablation$.tw. (285) 9 (ovariectom$ or salpingostom$).tw. (335) 10 (ovar$ adj2 surg$).tw. (459) 11 (uterine artery emboli?ation or UAE).tw. (527) 12 (pelv$ adj5 surg$).tw. (1618) 13 (ovar$ adj5 cystect$).tw. (144) 14 endometrioma$.tw. (282) 15 exp endometriosis/ (736) 16 endometriosis.tw. (2011) 17 fallopian$.tw. (1099) 18 exp Tissue Adhesions/ (431) 19 Adhesion$.tw. (5362) 20 myomectom$.tw. (682) 21 (ovar$ adj2 defect$).tw. (8) 22 (ovar$ adj2 cauter$).tw. (12) 23 microsurg$.tw. (761) 24 adhesiolysis.tw. (200) 25 electrosurg$.tw. (377) 26 or/1‐25 (39827) 27 Interceed.tw. (39) 28 tc7.tw. (24) 29 exp polytetrafluoroethylene/ or exp proplast/ (460) 30 (polytetrafluoroethylene$ or proplast$).tw. (507) 31 (gore‐tex or goretex).tw. (102) 32 (fibrin adj2 sheet$).tw. (29) 33 (surg$ adj2 membrane$).tw. (174) 34 exp Cellulose, Oxidized/ (54) 35 (Cellulose adj2 Oxidiz$).tw. (87) 36 (Cellulose adj2 Oxidis$).tw. (11) 37 70% dextran$.tw. (5) 38 (fibrin adj2 sealant$).tw. (422) 39 barrier$.tw. (15131) 40 seprafilm$.tw. (42) 41 exp Membranes, Artificial/ (1181) 42 (Artificial adj2 Membrane$).tw. (99) 43 (surg$ adj2 membrane$).tw. (174) 44 (anti adhesi$ or antiadhesi$).tw. (177) 45 (prevent$ adj7 adhesi$).tw. (541) 46 (manag$ adj5 adhesi$).tw. (102) 47 or/27‐46 (18089) 48 26 and 47 (1136)
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 21 August 2019
1 exp gynecologic surgical procedures/ or exp endometrial ablation techniques/ or exp hysterectomy/ or exp hysteroscopy/ or exp ovariectomy/ or exp salpingostomy/ or exp uterine artery embolization/ (80533) 2 (gyn$ adj3 surg$).tw. (10180) 3 exp laparotomy/ (18495) 4 exp laparoscopy/ (93301) 5 laparoscop$.tw. (119250) 6 laparotom$.tw. (47268) 7 (hysterectom$ or cystoscop$ or hysteroscop$).tw. (49659) 8 endometrial ablation$.tw. (1203) 9 (ovariectom$ or salpingostom$).tw. (27775) 10 (ovar$ adj2 surg$).tw. (2213) 11 (uterine artery emboli?ation or UAE).tw. (4443) 12 (pelv$ adj5 surg$).tw. (9814) 13 (ovar$ adj5 cystect$).tw. (667) 14 endometrioma$.tw. (2297) 15 exp endometriosis/ (20998) 16 endometriosis.tw. (21757) 17 fallopian$.tw. (10049) 18 exp Tissue Adhesions/ (12241) 19 Adhesion$.tw. (201767) 20 myomectom$.tw. (3417) 21 (ovar$ adj2 defect$).tw. (212) 22 (ovar$ adj2 cauter$).tw. (36) 23 microsurg$.tw. (24576) 24 adhesiolysis.tw. (1464) 25 electrosurg$.tw. (3462) 26 or/1‐25 (545400) 27 Interceed.tw. (131) 28 tc7.tw. (322) 29 exp polytetrafluoroethylene/ or exp proplast/ (11032) 30 (polytetrafluoroethylene$ or proplast$).tw. (8027) 31 (gore‐tex or goretex).tw. (2052) 32 (fibrin adj2 sheet$).tw. (121) 33 (surg$ adj2 membrane$).tw. (737) 34 exp Cellulose, Oxidized/ (846) 35 (Cellulose adj2 Oxidiz$).tw. (858) 36 (Cellulose adj2 Oxidis$).tw. (67) 37 70% dextran$.tw. (16) 38 (fibrin adj2 sealant$).tw. (1712) 39 barrier$.tw. (268850) 40 seprafilm$.tw. (223) 41 exp Membranes, Artificial/ (97866) 42 (Artificial adj2 Membrane$).tw. (2939) 43 (surg$ adj2 membrane$).tw. (737) 44 (anti adhesi$ or antiadhesi$).tw. (3049) 45 (prevent$ adj7 adhesi$).tw. (6025) 46 (manag$ adj5 adhesi$).tw. (470) 47 or/27‐46 (390027) 48 26 and 47 (17618) 49 randomized controlled trial.pt. (487576) 50 controlled clinical trial.pt. (93226) 51 randomized.ab. (452140) 52 placebo.tw. (205577) 53 clinical trials as topic.sh. (188022) 54 randomly.ab. (316594) 55 trial.ti. (203411) 56 (crossover or cross‐over or cross over).tw. (81335) 57 or/49‐56 (1262664) 58 (animals not (humans and animals)).sh. (4577069) 59 57 not 58 (1160093) 60 48 and 59 (759)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 21 August 2019
1 exp gynecologic surgery/ or exp pelvis surgery/ or exp uterine tube surgery/ or exp uterus surgery/ (148278) 2 exp endometrium ablation/ (2624) 3 exp hysterectomy/ or exp abdominal hysterectomy/ or exp vaginal hysterectomy/ or exp radical hysterectomy/ (66175) 4 exp hysteroscopy/ (11528) 5 exp ovariectomy/ (32790) 6 exp salpingoplasty/ or exp salpingostomy/ (1030) 7 exp uterine artery embolization/ (3510) 8 (gyn$ adj3 surg$).tw. (15000) 9 exp laparotomy/ (72923) 10 exp laparoscopy/ (149397) 11 laparoscop$.tw. (189510) 12 laparotom$.tw. (61388) 13 (hysterectom$ or hysteroscop$).tw. (61338) 14 endometrial ablation technique$.tw. (90) 15 (ovariectom$ or salpingostom$).tw. (30291) 16 (ovar$ adj2 surg$).tw. (3353) 17 (uterine artery emboli?ation or UAE).tw. (6932) 18 (pelv$ adj5 surg$).tw. (16269) 19 (ovar$ adj5 cystect$).tw. (1342) 20 endometrioma$.tw. (3724) 21 exp endometriosis/ (34438) 22 endometriosis.tw. (30644) 23 fallopian$.tw. (12290) 24 Adhesion$.tw. (249323) 25 myomectom$.tw. (6030) 26 (ovar$ adj2 defect$).tw. (282) 27 (ovar$ adj2 cauter$).tw. (54) 28 microsurg$.tw. (28653) 29 adhesiolysis.tw. (2733) 30 electrosurg$.tw. (4356) 31 or/1‐30 (743178) 32 exp oxidized regenerated cellulose/ (1418) 33 oxidized regenerated cellulose.tw. (369) 34 Interceed.tw. (213) 35 tc7.tw. (357) 36 exp politef/ (18380) 37 (polytetrafluoroethylene$ or proplast$).tw. (8747) 38 (gore‐tex or goretex).tw. (2848) 39 (fibrin adj2 sheet$).tw. (172) 40 (surg$ adj2 membrane$).tw. (859) 41 (Cellulose adj2 Oxidiz$).tw. (888) 42 (Cellulose adj2 Oxidis$).tw. (85) 43 70% dextran$.tw. (15) 44 (fibrin adj2 sealant$).tw. (2235) 45 barrier$.tw. (326178) 46 seprafilm$.tw. (322) 47 exp artificial membrane/ (44422) 48 (Artificial adj2 Membrane$).tw. (3128) 49 (surg$ adj2 membrane$).tw. (859) 50 exp tissue adhesive/ (18874) 51 tissue adhesive$.tw. (1869) 52 (prevent$ adj7 adhesi$).tw. (7131) 53 (anti adhesi$ or antiadhesi$).tw. (3717) 54 (manag$ adj5 adhesi$).tw. (612) 55 or/32‐54 (421885) 56 31 and 55 (24309) 57 Clinical Trial/ (951683) 58 Randomized Controlled Trial/ (561335) 59 exp randomization/ (83766) 60 Single Blind Procedure/ (36208) 61 Double Blind Procedure/ (161349) 62 Crossover Procedure/ (60191) 63 Placebo/ (326256) 64 Randomi?ed controlled trial$.tw. (209043) 65 Rct.tw. (33498) 66 random allocation.tw. (1900) 67 randomly allocated.tw. (33012) 68 allocated randomly.tw. (2466) 69 (allocated adj2 random).tw. (808) 70 Single blind$.tw. (23173) 71 Double blind$.tw. (194240) 72 ((treble or triple) adj blind$).tw. (988) 73 placebo$.tw. (288978) 74 prospective study/ (542433) 75 or/57‐74 (2066245) 76 case study/ (63393) 77 case report.tw. (378738) 78 abstract report/ or letter/ (1068360) 79 or/76‐78 (1500676) 80 75 not 79 (2014902) 81 56 and 80 (1731)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 21 August 2019
1 exp Postsurgical Complications/ (873) 2 Adhesion$.tw. (3445) 3 adhesiolysis.tw. (17) 4 exp Cell Adhesion Molecules/ (813) 5 or/1‐4 (4390) 6 Interceed.tw. (0) 7 (polytetrafluoroethylene$ or proplast$).tw. (11) 8 (gore‐tex or goretex).tw. (5) 9 (fibrin adj2 sheet$).tw. (0) 10 (surg$ adj2 membrane$).tw. (1) 11 (Cellulose adj2 Oxidiz$).tw. (1) 12 dextran$.tw. (590) 13 barrier$.tw. (66795) 14 (Artificial adj2 Membrane$).tw. (25) 15 antiadhesi$.tw. (5) 16 (prevent$ adj3 adhesion$).tw. (17) 17 anti adhesi$.tw. (12) 18 or/6‐17 (67377) 19 5 and 18 (238) 20 random.tw. (56000) 21 control.tw. (429452) 22 double‐blind.tw. (22320) 23 clinical trials/ (11415) 24 placebo/ (5327) 25 exp Treatment/ (1011240) 26 or/20‐25 (1395394) 27 19 and 26 (75)
Data and analyses
Comparison 1. Oxidised regenerated cellulose vs no treatment at laparoscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adhesions | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 De novo | 3 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.83] |
| 1.2 Re‐formation (or mixture) | 3 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.07, 0.41] |
Comparison 2. Oxidised regenerated cellulose vs no treatment at laparotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adhesions | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 De novo | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.42, 1.25] |
| 1.2 Re‐formation (or mixture) | 6 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.27, 0.55] |
Comparison 3. Expanded polytetrafluoroethylene vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adhesions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 De novo | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.94] |
Comparison 4. Expanded polytetrafluoroethylene vs oxidised regenerated cellulose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean adhesion score (non‐validated score) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐3.79 [‐5.12, ‐2.46] |
| 2 Incidence of adhesions | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 De novo | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.26, 3.41] |
| 2.2 Re‐formation (or mixture) | 1 | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.80] |
Comparison 5. Sodium hyaluronate and carboxymethylcellulose vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean adhesion score (non‐validated score) | 1 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.53, ‐0.45] |
Comparison 6. Fibrin sheet vs no treatment at laparoscopic myomectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean adhesion score (non‐validated score) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.67, 0.39] |
| 2 Incidence of de novo adhesions per participant | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.42, 3.41] |
Comparison 7. Collagen membrane with polyethylene glycol and glycerol vs control at laparotomic myomectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of adhesions | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.77] |
| 2 Median adhesion score | Other data | No numeric data | ||
| 3 Clinical pregnancy rate | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.69 [1.38, 23.48] |
| 4 Ectopic pregnancy rate | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azziz 1993.
| Methods | Unit of randomisation: pelvic sidewall Method of randomisation: sealed envelopes, computer‐generated Time of randomisation: at completion of surgery Blinding: second‐look laparoscopy performed by the same surgeon | |
| Participants | Patients undergoing pelvic adhesiolysis by laparotomy
N = 134 (268 pelvic sidewalls) Dropouts: 64 participants were excluded for surgical procedures and/or evaluations inconsistent with the protocol (39), for inadequate documentation (23), and for not returning for second‐look laparoscopy (2) Some participants underwent additional surgical procedures (salpingostomy, myomectomy, tubal anastomosis, endometriosis treatment) Indication for surgery: infertility Pre‐existing adhesions: yes Cause of adhesions: not stated (endometriosis included) Microsurgery: yes Age, years: > 18 (not further specified) Location: multi‐centre trial at 13 centres in North America Timing and duration: 1986 to 1989 |
|
| Interventions | Oxidised regenerated cellulose on pelvic sidewall vs uncovered opposite sidewall Other adjuvants used: none |
|
| Outcomes | Adhesions at second‐look laparoscopy: at 10 days to 14 weeks
Adverse effects No pregnancy outcomes |
|
| Notes | Trial supported by manufacturers of Interceed (Johnson & Johnson, Inc.) Additional information provided by first study author Documented with photographs and drawings Reports of an earlier trial with a smaller sample size were published in 1989 and 1990 (see references) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assignment performed by computerised algorithm; however study authors state that this was performed by the study's sponsor, who manufactured the intervention of interest |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used for allocation; however no further details regarding the process of concealment are provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unit of randomisation was by ovary, so each participant received both intervention and control Surgeon was not blinded, but treatment assignment was revealed only intraoperatively: "after completion of all operative procedures... a sealed envelope disclosed assignment of treated and untreated sidewall" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgeon who performed the initial laparotomy was also the surgeon who performed the second‐look laparoscopy. As no independent observers were used, this could be a source of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 64 participants were excluded for surgical procedures and/or evaluations inconsistent with the protocol (39), for inadequate documentation (23), and for not returning for second‐look laparoscopy (2) |
| Selective reporting (reporting bias) | Unclear risk | Data presented as numbers of cases and percentages; no data conversion. P values stated for outcomes. No omission of outcomes and no subsets of data. However, no pre‐published protocol for reference purposes |
| Other bias | Low risk | No other bias identified |
Canis 2014.
| Methods | Unit of randomisation: women Method of randomisation: random balanced table in numbered, sealed envelopes Time of randomisation: following operation, before closure of abdominal cavity Blinding: patients blinded, videotapes of surgery reviewed by independent blinded surgeons Multi‐centre trial |
|
| Participants | Patients undergoing myomectomy at laparotomy for excision of at least 1 myoma > 60 mm in diameter N = 61 33 randomised to intervention (collagen membrane with polyethylene glycol and glycerol (PREVADH)) and 28 randomised to control (Ringer's lactate) Dropouts: 2 patients from the intervention group withdrew due to subsequent operations; 5 patients (3 from the intervention group and 2 from the control group) did not undergo second‐look laparoscopy. Videos of surgery in 7 patients (4 from the intervention group and 3 from the control group) were not assessed by independent blinded surgeons Age, years: 20 to 42; median 34.0 in intervention group and 34.5 in control group Exclusion criteria: endometriosis (stage > 1), preoperative embolisation, submucous myoma, chronic corticotherapy, immunosuppressive or immunomodulatory treatment, treatment with a gonadotrophin‐releasing hormone (GnRH) analogue within 3 months of the study, adnexal adhesions discovered during surgery Baseline characteristics: no significant differences between groups stated Indication for surgery: preservation of fertility Pre‐existing adhesions: none Microsurgery: no Additional surgical procedures: none stated Location: 11 centres in France Timing: May 2006 to June 2008 |
|
| Interventions | Collagen membrane with polyethylene glycol and glycerol (PREVADH) vs instillation of 500 mL Ringer's lactate Other adjuvants used: none stated Second‐look laparoscopy:
|
|
| Outcomes | Adhesions at second‐look laparoscopy:
Pregnancy outcomes:
Adverse events |
|
| Notes | Study sponsored and funded by Covidien Intention to treat Surgery documented by video; however videos not analysed in 7 cases Power calculation: stated 34 patients needed in each arm Prospectively registered at US clinical trials database: NCT01388907 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed using a balanced random numbers table |
| Allocation concealment (selection bias) | Low risk | Blinded, sealed, numbered envelopes were opened in the operating theatre to decide treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study does not clearly state that patients were blinded, but most patients had the initial surgery and an SLL Surgeons were not blinded, but the randomisation process was applied intraoperatively to reduce the risk of performance bias: "after the uterus was sutured, but before closing the abdominal wall, patients were randomly allocated to one of two treatment groups, application of the anti‐adhesive film to the surgical site (P‐Group), or instillation of 500 mL Ringer's lactate solution into the pelvic cavity (R‐group, control)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent blinded surgeons were asked to review videos recorded during the second‐look laparoscopy to assess for adhesion development |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3‐year assessments completed in only 61% of patients |
| Selective reporting (reporting bias) | Unclear risk | No clear omissions of outcomes; data presented clearly, no subsets of data omitted. No protocol identified for comparison. |
| Other bias | Low risk | Coelioscopy second‐look adhesions performed later in control group than in intervention group (4.1 vs 2.9 months) Recruitment stopped early despite initial sample size calculations requiring 80 patients Interim analysis performed without type 1 error adjustment Effectiveness of anti‐adhesion film in preserving fertility interpreted with caution as confounding infertility factors unavailable and intention to preserve fertility may have changed during 3‐year follow‐up |
Diamond 1996.
| Methods | Unit of randomisation: women Method of randomisation: computer‐generated Timing of randomisation: end of surgery Blinding: surgeon unaware of randomisation at second‐look laparoscopy Multi‐centre trial | |
| Participants | Women undergoing uterine myomectomy at laparotomy with at least 1 posterior uterine incision ≥ 1 cm. Similar baseline characteristics, including age, size and number of myomas, and number of uterine incisions N = 127 Microsurgery: no Dropouts: 6 (withdrew or excluded because of new medications) Age, years: 34 Location: USA (19 centres) Timing: 1993 to 1995 Pre‐existing adhesions Cause of adhesions: not stated |
|
| Interventions | Sodium hyaluronate and carboxymethylcellulose vs no treatment | |
| Outcomes | Adhesion formation at the time of second‐look laparoscopy (mean days 23, range 7 to 70) Outcomes included number of adhesion sites, severity score, and surface area of uterine adhesions No pregnancy outcomes reported | |
| Notes | Power calculations: not stated Documented by video Multi‐centre trial Sponsored by Genzyme No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clear method of randomisation derived from computer‐generated randomisation list No clear source of bias Quote: "at each centre each enrolled patient was assigned consecutively a study number that corresponded to an identically numbered sealed study envelope, which determined the patient's treatment assignment via a computer derived randomisation list. After completion of myomectomy, patients were randomised at each centre into two groups" |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes created before patient enrolment used to provide allocation, decreasing risk of bias Quote: "at each centre each enrolled patient was assigned consecutively a study number that corresponded to an identically numbered sealed study envelope, which determined the patient's treatment assignment via a computer derived randomisation list. After completion of myomectomy, patients were randomised at each centre into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study authors do not explicitly state that patients were blinded, but all patients had the initial myomectomy and the SLL: "after completion of the myomectomy and associated procedures, but before closure of the abdominal cavity, patients were randomized at each center into two groups: no treatment controls and patients to receive the Seprafilm study device" Surgeons were not blinded, but randomisation took place intraoperatively to minimise performance bias: "after myomectomy, just before closure of the abdominal cavity, women randomized for no treatment (n = 68) received neither Seprafilm nor a placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Videotapes of the SLL were reviewed by an independent blinded observer: "videotapes recorded during laparoscopy subsequently were reviewed at a central site by a single independent observer blinded to the patient's treatment assignment, to serve as the basis for the efficacy data on Seprafilm" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 (out of 127) participants were withdrawn |
| Selective reporting (reporting bias) | Unclear risk | Data presented graphically and as percentages. P values stated for outcomes. No omission of outcomes and no subsets of data. Although this was an infertility study, no pregnancy outcomes stated |
| Other bias | Low risk | No other bias identified |
Franklin 1995.
| Methods | Unit of randomisation: ovary
Method of randomisation: blocks of 2 with sealed envelopes
Timing of randomisation: during surgery
Blinding: yes 57 participants randomly assigned 2 exclusions 55 participants (110 ovaries) analysed Trial supported by manufacturers of Interceed (Johnson & Johnson Medical, Inc.) |
|
| Participants | Patients undergoing bilateral ovarian surgery by laparotomy (N = 55)
Dropouts: not stated
Indication for surgery: bilateral ovarian disease (adhesions 37; tubal occlusion 7; PID history 4; endometriosis 5; other 2); not exclusively participants with infertility
Pre‐existing adhesions: some (39/55)
Cause of adhesions: not stated (endometriosis included)
Microsurgery at surgeon's discretion Age, mean, years: 29.9 (range 22 to 41) Location: 6 centres (Houston, Texas; Detroit, Michigan; Richmond, Virginia; Grand Rapids, Michigan ‐ USA; Sydney ‐ Australia; Danderyd ‐ Sweden) Timing and duration: 1990 to 1993 |
|
| Interventions | Oxidised regenerated cellulose on ovary vs uncovered opposite ovary Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: second or third week
Pregnancy outcomes: nil Adverse effects |
|
| Notes | Power calculations: nil Preliminary report of this trial published as abstract in 1993 (see references) Documentation: video Sponsored by Johnson & Johnson | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear description of method of randomisation provided Quote: "before beginning the study, a treatment randomisation scheme was generated. Patients were randomised in blocks of two to provide balance with respect to the left and right ovaries" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used to conceal allocations; unclear but appear to have been created after randomisation already decided Quote: "randomised treatment assignments were placed in sealed envelope for each patient, thereby blinding the surgeon to the treatment assignment during surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unit of randomisation was by ovary, so each participant received both intervention and control Surgeon was not blinded, but treatment assignment was revealed only intraoperatively: "randomized treatment assignments were placed in sealed envelopes for each patient, thereby blinding the surgeon to the treatment assignment during surgery. At that time, a sealed envelope was opened, disclosing the assignment of either the left or right ovary for wrapping with Interceed" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the surgeon performing SLL was blinded to which ovary received which intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 exclusions (out of 57 participants): 1 due to complete lysis of adhesions, and the second due to severe adhesions of pelvic wall at SLL |
| Selective reporting (reporting bias) | Unclear risk | Data presented as numbers of cases; no data conversion. P values stated for outcomes. No omission of outcomes and no subsets of data. No pre‐published protocol identified |
| Other bias | Low risk | No other bias identified |
Greenblatt 1993.
| Methods | Unit of randomisation: ovary Method of randomisation: not stated Time of randomisation: at completion of surgery Blinding: surgeon at second‐look laparoscopy unaware of side that was treated | |
| Participants | Patients undergoing bilateral ovarian cautery by laparoscopy
N = 8; 1 exclusion, 7 participants, 14 ovaries
Indication for surgery: infertility due to clomiphene‐resistant PCOS
Pre‐existing adhesions: no
Microsurgery: no Age, mean, years: 26.6 Duration of infertility, mean, years: 3.2 (range 1.5 to 5) Tubal status: normal HSG and laparoscopy Ovulatory status: BBT and serum progesterone Semen analysis: done but results not stated Location: Canada Timing and duration: not stated |
|
| Interventions | Oxidised regenerated cellulose on ovary vs uncovered opposite ovary Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: at 3 to 4 weeks
Adverse effects Pregnancy outcomes stated but not by intervention, as unit of randomisation was ovaries |
|
| Notes | Power calculation: nil Documentation: video Sponsored by MRC Canada (Interceed provided by Johnson & Johnson) No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement of method of random sequence generation. Quote: "Interceed was applied to one ovary, selected randomly" |
| Allocation concealment (selection bias) | Unclear risk | No clear statement of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unit of randomisation is ovary, so all women served as their own controls Surgeon was not blinded to intervention. Study authors also do not state whether treatment allocation took place intraoperatively |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was performed by an independent blinded surgeon: "three to 4 weeks later, subject returned to the surgical short‐stay unit. They underwent a second laparoscopy performed by a different surgeon, unaware of which side had been wrapped with Interceed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 exclusion due to technical difficulty in applying oxidised regenerated cellulose |
| Selective reporting (reporting bias) | Unclear risk | Data presented as numbers of cases and percentages. P values stated for significant outcomes. No omission of outcomes and no subsets of data. No pre‐published protocol identified |
| Other bias | High risk | Study used within‐participant design for reporting adhesion formation. However, study was unclear on presentation and analysis of matched data, and no comparative data were presented. As such, we were unable to extract appropriate data from the study |
Haney 1995.
| Methods | Unit of randomisation: pelvic sidewall Method of randomisation: computerised randomisation table ‐ sealed envelopes Timing of randomisation: at completion of surgery Blinding: not clear whether surgeon was aware of randomisation at second‐look laparoscopy | |
| Participants | Patients undergoing pelvic adhesiolysis and reconstructive pelvic surgery by laparotomy
N = 29; 32 participants randomly assigned but 3 excluded (failed second‐look laparoscopy)
29 participants (58 pelvic sidewalls) analysed
Surgical procedures, in addition to adhesiolysis: salpingostomy, fimbrioplasty, ovarian cystectomy, endometriosis surgery Indication for surgery: not stated whether exclusively for infertility Pre‐existing adhesions: yes Cause of adhesions: previous surgery 27; PID history 5 (endometriosis included) Microsurgery performed at surgeon's discretion Age, years, range: 18 to 40 (mean not stated) Location: USA (Durham, North Carolina; Atlanta, Georgia; Denver, Colorado; San Diego, California; Houston, Texas) Timing and duration: 1991 to 1993 |
|
| Interventions | Expanded polytetrafluoroethylene on pelvic sidewall vs oxidised regenerated cellulose on opposite pelvic sidewall Other adjuvants used: antibiotics Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy
Scoring (0 to 11) according to (1) extent: 0 = no adhesions; 1 = < 25%; 2 = 25% to 50%; 3 = 50% to 75%; 4 = > 75%; (2) type: 0 = none; 1 = filmy, transparent, avascular; 2 = opaque, transparent, avascular; 3 = opaque, capillaries present; 4 = opaque, large vessels; (3) tenacity: 0 = none; 1 = adhesions essentially fell apart; 2 = adhesions were lysed with traction; 3 = adhesions required sharp dissection Adverse effects Pregnancy outcomes: nil |
|
| Notes | Power calculations: nil
Sponsorship: trial supported by manufacturers of Gore‐Tex (Gore and Associates Inc., Flagstaff, Arizona, USA)
Documentation of adhesions: no video or photographic records stated Preliminary report of this trial published as abstract in 1992 and in 1994 (see references) Potential duplicate publication of preliminary data in March 1993: further information requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clear use of computerised randomisation: "randomization accomplished by RANDOM software; Digital Equipment Corporation, Manard, MA)" |
| Allocation concealment (selection bias) | Low risk | Sealed randomised envelopes concealed randomisation and were not opened until after patients had been intraoperatively deemed to be included in the study: "after adhesiolysis, the size of the resulting peritoneal defect was measured in square centimeters. At this point in the procedure, if the patient met the criteria for inclusion as a candidate for the study, a randomization envelope [...] was opened to reveal which sidewall was to be covered with PTFE and which was to be covered with oxidized cellulose" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unit of randomisation was either left or right pelvic sidewall, so every woman also served as her own control Surgeon was not blinded, but treatment allocation took place intraoperatively: "after adhesiolysis, the size of the resulting peritoneal defect was measured in square centimeters. At this point in the procedure, if the patient met the criteria for inclusion as a candidate for the study, a randomization envelope [...] was opened to reveal which sidewall was to be covered with PTFE and which was to be covered with oxidized cellulose" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgeon performing the SLL was not blinded to which pelvic sidewall received which intervention. The PTFE barrier had to be sutured on the pelvic sidewall and needed to be removed in most cases during SLL, but the oxidised regenerated cellulose barrier was not sutured and had mostly dissolved at the time of SLL |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: 32 participants randomly assigned but 3 excluded (failed second‐look laparoscopy) 29 participants (58 pelvic sidewalls) analysed |
| Selective reporting (reporting bias) | Unclear risk | Data presented as scores and graphically presented. P values stated for outcomes. No omission of outcomes and no subsets of data. However, no pre‐published protocol identified |
| Other bias | Low risk | No other bias identified |
Keckstein 1996.
| Methods | Unit of randomisation: ovary
Method of randomisation: toss of a coin
Time of randomisation: at completion of surgery Blinding: surgeon at second‐look laparoscopy not aware of allocation |
|
| Participants | Patients undergoing bilateral ovarian cystectomy by laparoscopy N = 25 14 participants with bilateral endometriomas, 6 with ovarian cysts and endometriosis, and 5 with ovarian cysts only Dropouts: 8, not stated why Some participants also underwent adhesiolysis and removal of endometriosis Indication for surgery: ovarian cysts; not specifically participants with infertility Pre‐existing adhesions: some (but participants with dense adhesions excluded) Cause of adhesions: not stated (endometriosis included) Microsurgery: no Timing not stated Country: Germany (1 centre) |
|
| Interventions | Oxidised regenerated cellulose on ovary vs uncovered opposite ovary Other adjuvants used: none Suturing: 9 of 17 in the oxidised regenerated cellulose group; 3 of 17 in the non‐oxidised regenerated cellulose group Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: 8 to 30 weeks
Adverse effects Pregnancy data nil |
|
| Notes | Power calculations: nil Analysis sponsored by Johnson & Johnson only after study was completed First presented as abstract in 1994 No photographic or video documentation No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin tosses were used as a simple method of randomisation Quote: "the random assignment of one ovary for wrapping with Interceed was revealed to the surgeon after a coin toss" |
| Allocation concealment (selection bias) | Low risk | Coin toss took place intraoperatively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unit of randomisation was each ovary, so all women acted as their own controls Surgeon was not blinded, but treatment allocation took place intraoperatively: "at the end of cystectomy the random assignment of one ovary for wrapping with Interceed [...] was revealed to the surgeon after a coin toss" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study does not state whether the assessor during SLL was blinded to initial treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 dropouts (n = 25); no reasons stated as to why participants did not return for SLL |
| Selective reporting (reporting bias) | Unclear risk | Data presented as numbers of cases and percentages, as well as graphically. P values stated for significant outcomes. No omission of outcomes and no subsets of data. However, no pre‐published protocol identified |
| Other bias | Low risk | No other bias identified |
Korell 1994.
| Methods | Unit of randomisation: participant Method of randomisation: unknown Time of randomisation: unknown Blinding: unknown | |
| Participants | Patients undergoing myomectomy by laparoscopy
N = 38
Dropouts: not known
Myomectomy details unknown Indication for surgery: fibroids Pre‐existing adhesions: no Microsurgery: no Age, mean, SD: unknown Location: Germany Timing and duration: unknown |
|
| Interventions | Oxidised regenerated cellulose applied to myomectomy incision(s) vs Gortex applied to myomectomy site(s) Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy
Adverse effects: unknown Pregnancy: unknown |
|
| Notes | Power calculations not stated Documentation not stated No source of funding stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated within study |
| Allocation concealment (selection bias) | Unclear risk | Not stated within study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated within study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated within study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data presented as numbers of cases and percentages. P values stated for significant outcomes. No omission of outcomes and no subsets of data. However, no pre‐published protocol identified |
| Other bias | Low risk | No other bias identified |
Li 1994.
| Methods | Unit of randomisation: pelvic sidewall
Method of randomisation: computer‐generated random numbers
Time of randomisation: at completion of surgery
Blinding: assessor unaware of which sidewall was treated 28 participants randomly assigned 1 exclusion (declined second‐look laparoscopy) 27 participants (54 pelvic sidewalls) analysed Trial supported by manufacturers of Interceed (Johnson & Johnson Medical, Ltd., UK) |
|
| Participants | Patients undergoing adhesiolysis by laparotomy (N = 28; 54 pelvic sidewalls, 1 withdrawal) Indication for surgery: infertility 21; chronic pelvic pain 6 Pre‐existing adhesions: yes Cause of adhesions: endometriosis 9; PID 10; previous surgery 5; uncertain 3 Microsurgery: yes Age, years, mean: 30.4 (SD 4.5) No details on participants with infertility Location: UK Timing and duration: 1989 to 1992 |
|
| Interventions | Oxidised regenerated cellulose on pelvic sidewall vs uncovered opposite sidewall (oxidised regenerated cellulose sutured in 3 cases) Other adjuvants used: intraperitoneal hydrocortisone in Intralipid Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: 3 to 14 weeks after initial surgery
Adverse effects Pregnancy outcomes: not stated |
|
| Notes | Documented with photographs and drawings at initial surgery and with video recording at second‐look laparoscopy Sponsored by Johnson & Johnson Video at second‐look laparoscopy No power calculations No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated but no details of method provided Quote: "the assignment of the test side for each woman was carried out before the study and was randomised blocks of two to provide balance" |
| Allocation concealment (selection bias) | Low risk | Allocation concealment using envelopes opened intraoperatively, reducing risk of bias Quote: "a sealed envelope was then opened revealing which of the side walls was to receive Interceed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unit of randomisation was either right or left side of pelvic wall, so every woman also acted as her own control Surgeon was not blinded, but treatment allocation was revealed only intraoperatively |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent blinded surgeon performed assessments at second‐look laparoscopy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 exclusion (declined second‐look laparoscopy) |
| Selective reporting (reporting bias) | High risk | Data presented as numbers of cases as well as graphically. P values stated for significant outcomes. No omission of outcomes and no subsets of data. Unclear whether power calculation performed Furthermore, despite 21 participants undergoing adhesiolysis for infertility, no pregnancy outcomes stated It should also be noted that centres other than Sheffield ‐ Li 1994 ‐ were randomly assigning participants around the UK for a study of oxidised regenerated cellulose and no treatment in the early 1990s (personal communication, A Watson). However, data have never been published |
| Other bias | Unclear risk | During surgery, "constant irrigation of the tissue with a physiological solution that contained heparin" was performed. Also, all patients received instillation of hydrocortisone in Intralipid |
Mais 1995a.
| Methods | Unit of randomisation: participant
Method of randomisation: random numbers table ‐ numbered sealed envelopes
Time of randomisation: at completion of surgery
Blinding: yes 32 participants No exclusions stated No source of funding stated |
|
| Participants | Patients undergoing pelvic adhesiolysis and endometriosis treatment by laparoscopy (N = 32)
Dropouts: nil Endometriosis treatment consisted of coagulation or resection of implants and endometriomas, followed by a 3‐month course of GnRH agonist Indication for surgery: not stated; not specifically participants with infertility Pre‐existing adhesions: yes Cause of adhesions: endometriosis (stage IV) Microsurgery: no Age, years, mean (SD): treatment group 30.1 (6.5); controls 29.3 (6.2) Location: Italy Timing and duration: January 1993 to June 1994 |
|
| Interventions | Oxidised regenerated cellulose on ovaries and de‐peritonealised areas vs surgery only Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy 12 to 14 weeks later
Adverse effects Pregnancy outcomes: nil |
|
| Notes | Power calculation done Documentation: video Sponsorship: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (using numbered sealed envelopes) to undergo surgery alone or surgery with an oxidised regenerated cellulose barrier. Treatment or control assignment was obtained by using a table of random digits. The envelope seal was broken in the operation room after completion of all operative procedures and before removal of laparoscopic ports |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to conceal allocation. The envelope seal was broken in the operation room after completion of all operative procedures and before removal of laparoscopic ports |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Does not state whether initial surgeon was blinded; however as control was no treatment, appears unlikely that they were. Not clearly stated whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent blinded surgeons performed second‐look laparoscopy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data reported in incidences/percentages. No range or CI reported. P values reported only for significant results. No conversion of data or subsets |
| Other bias | Low risk | No other bias identified |
Mais 1995b.
| Methods | Unit of randomisation: participant Method of randomisation: random numbers table ‐ numbered sealed envelopes Time of randomisation: at completion of surgery Blinding: double | |
| Participants | Patients undergoing myomectomy by laparoscopy
N = 50
Dropouts: nil
Myomectomy consisted of removal of 1 to 4 myomas; largest was 3 to 6 cm Indication for surgery: fibroids; not specifically participants with infertility Pre‐existing adhesions: no Microsurgery: no Age, years, mean (SD): treatment group 34.1 (5.7); control group 33.2 (5.5) Location: Italy Timing and duration: January 1993 to June 1994 |
|
| Interventions | Oxidised regenerated cellulose on myomectomy incision(s) vs uncovered myomectomy site(s) Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy 12 to 14 weeks later
Adverse effects Pregnancy: not stated |
|
| Notes | Power calculations: nil Documentation: not stated No source of funding stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment or control assignment was obtained by using a table of random digits |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes were used to conceal allocation. The envelope seal was broken in the operation room after completion of all operative procedures and before removal of laparoscopic ports |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Does not state whether initial surgeon was blinded; however as control was no treatment, appears unlikely that they were. Not clearly stated whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent blinded surgeons performed second‐look laparoscopy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data reported in incidences/percentages. No range or CI reported. P values reported only for significant results. No conversion of data or subsets |
| Other bias | Low risk | No other bias identified |
Myomectomy ASG 1995.
| Methods | Unit of randomisation: myomectomy site Method of randomisation: computerised randomisation table ‐ sealed envelopes Timing of randomisation: at completion of surgery Blinding: not stated whether surgeon was aware of randomisation at second‐look laparoscopy | |
| Participants | Patients undergoing myomectomy (2 or more) by laparotomy
N = 28
1 exclusion (declined second‐look laparoscopy)
27 participants (54 myomectomy sites) analysed
All had 2 myomectomy incisions of similar length and > 2 cm apart located on the fundus and on the posterior uterine wall Indication for surgery: fibroids; not exclusively participants with infertility Microsurgery: no Pre‐existing adhesions: no Age, years, range: 18 to 43 Location: USA (Durham, North Carolina; Houston, Texas; Atlanta, Georgia; San Diego, California) and Canada (Montreal) Timing and duration: 1991 to 1993 |
|
| Interventions | Expanded polytetrafluoroethylene over myomectomy site vs uncovered myomectomy site Other adjuvants used: antibiotics Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: 2 to 6 weeks after initial surgery
Scoring (0 to 11) according to (1) extent: 0 = no adhesions; 1 = < 25%; 2 = 25% to 50%; 3 = 50% to 75%; 4 = > 75%; (2) type: 0 = none; 1 = filmy, transparent, avascular; 2 = opaque, transparent, avascular; 3 = opaque, capillaries present; 4 = opaque, large vessels; (3) tenacity: 0 = none; 1 = adhesions essentially fell apart; 2 = adhesions were lysed with traction; 3 = adhesions required sharp dissection Adverse effects Pregnancy outcomes: nil |
|
| Notes | Documented with diagrams, photographs, and videotapes Sponsored by Gore‐Tex Surgical Membrane Multi‐centre trial Power calculations: nil Preliminary reports of this trial published as abstracts 1993 and 1994 No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a computerised randomisation table |
| Allocation concealment (selection bias) | Low risk | Assignments were concealed in sealed envelopes opened intraoperatively |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No clear statement whether initial surgeon or participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement whether surgeon performing second‐look laparoscopy or assessment at SLL was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrew from study before SLL |
| Selective reporting (reporting bias) | Unclear risk | Data presented as incidence of adhesions with CI and adhesion scores as means ± SEM. P values reported. No omissions or subsets of data. However, no pre‐published protocol identified |
| Other bias | Low risk | No other bias identified |
Nordic APSG 1995.
| Methods | Unit of randomisation: adnexae
Method of randomisation: sealed envelopes
Timing of randomisation: at completion of surgery
Blinding: yes 66 participants (132 ovaries) No exclusions stated Trial supported by manufacturers of Interceed (Johnson & Johnson, Stockholm, Sweden) |
|
| Participants | Patients undergoing adhesiolysis by laparotomy
N = 66 (14 more were erroneously entered, as they did not meet entry criterion of pre‐existing adhesions)
Indication for surgery: infertility
Pre‐existing adhesions: yes
Cause of adhesions: not stated (endometriosis excluded)
Microsurgery: yes Age: not stated No details on infertility status (duration of infertility, tubal status, ovulatory status, semen analysis) Location: Scandinavia, Sweden (Stockholm, Linkoping, Goteborg, Gavle, Umea, and Skovde); 1 in Finland (Oulo), 1 in Denmark (Aalborg) Timing and duration: 1991 to 1993 |
|
| Interventions | Oxidised regenerated cellulose on ovary and fallopian tube vs uncovered opposite side Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Outcomes analysed in review Adhesions at second‐look laparoscopy
Adverse effects Pregnancy outcomes: nil |
|
| Notes | Power calculations: nil
Sponsored by Johnson & Johnson
Multi‐centre trial
Duplicate publication with Larsson 93 (but different numbers) Preliminary reports of this trial published as abstracts in 1993 and 1994 (see references) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement regarding method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used for allocation concealment but unclear when sealed or opened. Quote: "a sealed envelope disclosed assignment of which side was to be treated with the adjuvant therapy" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unit of randomisation was right or left adnexa, so every woman also acted as her own control Not clearly stated whether surgeon was blinded, or when treatment allocation was revealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement whether surgeon performing second‐look laparoscopy or assessment at SLL was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | High risk | Data presented in graphical form, as well as in tables as means ± SEMs. P values reported in table. Data reported for adhesions on ovaries, fallopian tubes, fimbriae. Data reported elsewhere with different sample size. Despite being an infertility study, pregnancy outcomes not stated |
| Other bias | Low risk | No other bias identified |
Saravelos 1996.
| Methods | Unit of randomisation: ovaries Method of randomisation: computer‐generated in opaque envelopes Timing of randomisation: unclear Blinding: double | |
| Participants | Women (N = 21) undergoing treatment for polycystic ovarian syndrome by ovarian electrocautery
7 dropouts: 4 were pregnant, 2 withdrew, 1 had such dense adhesions at laparoscopy of both ovaries that no assessment could be made Pre‐existing adhesions: nil Mean age, years: 28 No other cause for infertility found Timing: 1994 to 1995 Country: UK (1 centre) |
|
| Interventions | Oxidised regenerated cellulose vs no treatment | |
| Outcomes | Adhesion formation
Further adhesiolysis Timing of second‐look laparoscopy: 2 to 11 weeks Pregnancy: outcomes given but ovaries randomly assigned, not participants |
|
| Notes | Supported by Johnson & Johnson Video taken Power calculations: nil No intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement provided regarding method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used for allocation concealment but unclear when sealed or opened. Quote: "a sealed envelope disclosed the assignment of which side was to be treated with Interceed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unit of randomisation was right or left ovary, so every woman also acted as her own control Not clearly stated whether surgeon was blinded, or when treatment allocation was revealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Second‐look laparoscopy was video‐recorded and assessed by independent blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant excluded, as underwent ovarian drilling; 4 withdrew because of pregnancy; 2 withdrew because of personal choice (n = 21) |
| Selective reporting (reporting bias) | Unclear risk | Data reported as incidences and percentages. No data conversion. No P values reported |
| Other bias | Low risk | No other bias identified |
Sekiba 1992.
| Methods | Unit of randomisation: pelvic sidewall
Method of randomisation: sealed envelopes
Time of randomisation: at completion of surgery
Blinding: double 63 participants (126 pelvic sidewalls) No exclusions stated Trial supported by manufacturers of Interceed (Johnson & Johnson Medical, KK, Tokyo, Japan) |
|
| Participants | Participants undergoing adhesiolysis by laparotomy
N = 63 Dropouts: not stated Indication for surgery: infertility, known presence of bilateral sidewall adhesions Pre‐existing adhesions: yes Cause of adhesions: severe endometriosis 28; previous surgery 15; PID 9; no obvious cause 19 Microsurgery: no Country: Japan Timing and duration: not stated |
|
| Interventions | Oxidised regenerated cellulose on pelvic sidewall vs uncovered opposite sidewall Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy at 10 days to 14 weeks
Pregnancy outcomes: nil |
|
| Notes | Documented with photographs and drawings
No power calculations
Duplicate publication
Supported by Johnson & Johnson
Multi‐centre trial Trial also published elsewhere in 1993 (see references) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement provided regarding method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used for allocation concealment but unclear when sealed or opened. Quote: "the pelvic sidewall to be covered was determined randomly using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unit of randomisation was right or left pelvic sidewall, so every woman also acted as her own control Not clearly stated whether surgeon was blinded, or when treatment allocation was revealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor performing second‐look laparoscopy was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data presented in graphical form for some outcomes. P values stated for significant outcomes. No data subsets reported. No pregnancy outcomes reported despite being an infertility study |
| Other bias | Low risk | No other bias identified |
Takeuchi 2005.
| Methods | Unit of randomisation: participant Method of randomisation: computer‐generated Time of randomisation: before surgery Blinding: absent | |
| Participants | Women (N = 146) undergoing laparoscopic myomectomy; 69 in control group, 68 in fibrin gel group, and 68 in fibrin sheet group 2 became pregnant, 23 had other surgical procedures, 29 women with fibroid < 5 cm were excluded Pre‐existing adhesions: nil Mean age: no statistically significant difference Timing: 2001 to 2002 Country: multi‐centric | |
| Interventions | Fibrin sheet placed on site of myomectomy wound vs uncovered wound Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy at 13 to 16 weeks
|
|
| Notes | Documentation of adhesions unclear No power calculations Support funding: unclear Multi‐centre trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using computer‐generated lists |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clearly stated whether participants or initial surgeons were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated whether surgeon performing or assessing second‐look laparoscopy was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition rate, with only 91 of 146 patients undergoing SLL Quote: "SLL was planned for 146 patients," "23 patients who underwent other surgical procedures (prior to SLL) were excluded," "29 patients in whom the maximum myoma diameter was < 5 cm were excluded," "[for] 2 women SLL was cancelled because they became pregnant," "consequently, 91 patients who underwent (surgery) alone were evaluated" |
| Selective reporting (reporting bias) | Unclear risk | Data reported as incidences and percentages. P values reported only for significant outcomes. No subsets of data. No pre‐published protocol identified. mAFS score done, but no standard deviations reported (review authors unable to extract standard deviations from data published in study paper because of the way they had been presented) |
| Other bias | Low risk | No other bias identified |
Tinelli 2011.
| Methods | Unit of randomisation: 546 participants
Time of randomisation: at completion of surgery
Blinding: single Method of randomisation: block randomisation was used to ensure balance in the numbers of participants in the 4 treatment arms. A statistician generated the entire randomisation sequence list in advance, and allocations were sequentially numbered from beginning to end Exclusion criteria for the investigation: previous uterine or pelvic surgery; previous abdominal general surgery; presurgical treatment with GnRH analogues; gynaecological malignancy; pregnancy; use of any instillation such as 32% dextran‐70, corticosteroids, anticoagulants, or non‐steroidal anti‐inflammatory drugs; haematological or coagulation disorders; presence of ongoing pelvic infection |
|
| Participants | 546 participants underwent surgery Type of surgery: laparoscopic or abdominal single or multiple intracapsular myomectomy Reasons for surgery: pelvic pain, menorrhagia and growth of fibroids as verified by ultrasound; some women requested myomectomy because of infertility Mean age between groups, years: 28.9 to 30.2 Timing of second‐look surgery between groups, years: 2.3 to 2.6 Dates: January 2003 to June 2009 |
|
| Interventions | Participants were subdivided into groups for laparoscopic or abdominal surgery. Participants underwent single or multiple intracapsular myomectomy. Participants were randomly assigned to placement of oxidised regenerated cellulose absorbable adhesion barrier to the uterine incision or to control without barriers | |
| Outcomes | Primary and secondary outcomes of analysis: presence and severity of adhesions for 4 groups: laparotomy with barrier, laparotomy without barrier, laparoscopy with barrier, and laparoscopy without barrier | |
| Notes | Funding: no external source stated Power calculation: stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list used to reduce bias Quote: "after completion of reconstructive uterine surgery, subjects were assigned to the treatment in a 1:1 ratio using a randomization list with random permutated blocks, length of 4" |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not stated in the text |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated whether initial surgeon was blinded; however as control was no treatment, this appears unlikely. Not clearly stated whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Surgeons who performed second‐look laparoscopy and assessment were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate, intentionally excluding patients not requiring repeat surgery, even though these patients may have had asymptomatic adhesions Quote: "of the 694 enrolled patients, 546 (78%) were available for assessment of adhesions during a subsequent operation" Adhesions were assessed at second surgery only if the participant had undergone subsequent surgery over the 6 years. Arguably, study data may be skewed by not including the 21% of participants who did not require subsequent surgery. Reasons for subsequent surgery include "laparotomy for cesarean section, laparotomy or laparoscopy for ovarian cysts, recurrent fibroids, appendectomy, cholecystectomy, extrauterine pregnancy and infertility" |
| Selective reporting (reporting bias) | Unclear risk | Data reported as incidences and percentages. P values reported only for significant outcomes. No differences identified between methods and reported results. However, no pre‐publication protocol identified for reference purposes |
| Other bias | Low risk | No other bias identified |
van Geldorp 1994.
| Methods | Unit of randomisation: ovary Method of randomisation: computer‐generated by telephone Time of randomisation: at completion of surgery Blinding: not stated | |
| Participants | Patients undergoing bilateral ovarian surgery by laparotomy
N = 20 participants (40 ovaries)
No dropouts Surgery consisted of adhesiolysis, ovarian cystectomy, and removal of endometriosis Indication for surgery: not stated; not specifically participants with infertility Pre‐existing adhesions: yes Cause of adhesions: endometriosis 14; others not stated Microsurgery: no Age, years, mean: 31 (range 22 to 38) Location: University Hospital, Rotterdam, The Netherlands Timing and duration: not stated |
|
| Interventions | Oxidised regenerated cellulose on ovary vs uncovered opposite ovary Other adjuvants used: not stated Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy
|
|
| Notes | Trial supported by Johnson & Johnson Documentation: not stated Power calculations: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At the end of the procedure and after haemostasis was achieved, 1 ovary was chosen on a random basis |
| Allocation concealment (selection bias) | Unclear risk | At the end of the procedure and after haemostasis was achieved, 1 ovary was chosen on a random basis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unit of randomisation was right or left ovary, so every woman also acted as her own control Not clearly stated whether surgeon was blinded, or when treatment allocation was revealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated whether surgeon performing or assessing second‐look laparoscopy was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data reported as mean area ± SEM. P values reported for significant outcomes. No subsets of data. No pre‐publication protocol for reference purposes |
| Other bias | Low risk | No other bias identified |
Wallweiner 1998.
| Methods | Unit of randomisation: participant Method of randomisation: not stated Time of randomisation: at completion of surgery Blinding: yes | |
| Participants | Women (N = 40) undergoing initial treatment for endometriosis by laparoscopy‐vaporisation of peritoneal deposits, excision of endometrioma, reconstructive surgery, and adhesiolysis
No dropouts Mean age, years: 27 Pre‐existing adhesions: yes Cause of adhesions: endometriosis Microsurgery: no Location: Germany Timing: not stated 7 dropouts: 4 were pregnant, 2 withdrew, and 1 had such dense adhesions at laparoscopy of both ovaries that no assessment could be made Pre‐existing adhesions: nil Mean age, years: 28 No other cause of infertility found Timing: 1994 to 1995 Country: UK (1 centre) |
|
| Interventions | Oxidised regenerated cellulose applied to all injured visceral peritoneal surfaces of ovary,tubes and uterus vs no treatment Other adjuvants used: none Second‐look laparoscopy
|
|
| Outcomes | Adhesions at second‐look laparoscopy: 3 to 6 months later
Adverse outcomes: unknown Pregnancy: not stated |
|
| Notes | Power calculations: nil Sponsorship: not stated Documentation: video | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not clearly stated |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated in text |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated whether initial surgeon was blinded; however as control was no treatment, this appears unlikely. Not clearly stated whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States that evaluator performing second‐look laparoscopy was not familiar with the initial operative site, but does not clearly state that evaluator was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of whether withdrawals or dropouts occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Data reported as numbers in a dichotomous score. P values stated. No conversion or subsets of data. No pre‐publication protocol for reference purposes |
| Other bias | Low risk | No other bias identified |
AFS: American Fertility Society.
BBT: basal body temperature.
CI: confidence interval.
GnRH: gonadotrophin‐releasing hormone.
HSG: hysterosalpingogram.
mAFS: modified American Fertility Society.
PID: pelvic inflammatory disease.
PTFE: polytetrafluoroethylene.
SD: standard deviation.
SEM: standard error of the mean.
SLL: second‐look laparoscopy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Diamond 1998 | Excluded, as trial intervention was a solution ‐ not a barrier |
| Dunn 2002 | Excluded, as trial intervention was a solution ‐ not a barrier |
| Korrell 1998 | Excluded, as trial intervention was a solution ‐ not a barrier |
| Korrell 2000a | Excluded, as trial intervention was a solution ‐ not a barrier |
| Korrell 2000b | Excluded, as trial intervention was a solution ‐ not a barrier |
| Liu 2015 | Excluded as trial intervention was a solution ‐ not a barrier |
| Reid 1993 | Not an intervention that was sought by the objectives of the review |
| Reid 1997 | Not an intervention that was sought by the objectives of the review |
| To 1992 | Excluded, as no outcomes of interest to this review (fever and need for antibiotics) |
| Trew 2016 | Excluded, as trial intervention was a solution ‐ not a barrier |
Differences between protocol and review
At the 2015 update:
the analysis method was changed from Peto ORs to Mantel‐Haenszel ORs; and
the outcomes of "improvement of adhesion score at SLL" and "worsening of adhesion score at SLL/mean adhesion score at SLL" were combined in the text for ease of presentation. This did not affect the analysis nor the conclusions of this review.
Contributions of authors
In the 2020 update, contributions of the review authors included the following.
-
Gaity Ahmad (GA).
Main review author of the 2020 update; undertook search, screened search results, organised retrieval of papers, screened papers against inclusion criteria, extracted data from papers, managed and interpreted data, and supervised KK, PA, and MT throughout the process.
-
Kyungmin Kim (KK).
Co‐review author of the 2020 update; organised retrieval of papers, screened papers against inclusion criteria, extracted data from papers, and managed and interpreted data.
-
Matthew Thompson (MT).
Co‐review author of the 2020 update; organised retrieval of papers, screened papers against inclusion criteria, extracted data from papers, and managed and interpreted data.
-
Priya Agarwal (PA).
Co‐review author of the 2020 update; organised retrieval of papers, screened papers against inclusion criteria, extracted data from papers, and managed and interpreted data.
-
Helena O'Flynn (HO'F).
Co‐review author of the 2015 update; assisted with writing of the 2020 update.
-
Akshay Hindocha (AH).
Co‐review author of the 2015 update; assisted with writing of the 2020 update.
-
Andrew Watson (AW).
Co‐review author of the 2015 update; assisted with writing of the 2020 update.
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
Yorkshire Regional Health Authority ‐ Research & Development Unit, Other.
Declarations of interest
Previous review author David Wiseman is a past employee of Ethicon Limited (manufacturer of oxidised regenerated cellulose) and is now a consultant to several companies, including Ethicon.
AW received a consultancy fee and lecture fees from the distributors of Adept in 2003.
GA, KK, MT, PA, HO'F and AH have no interests to declare and received no external funding.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Azziz 1993 {published data only}
- Azziz R, Cohen S, Curole D, Diamond M, Franklin R, Haney A, et al. Microsurgery alone or with Interceed absorbable adhesion barrier for pelvic side wall adhesion re‐formation. INTERCEED (TC7) Adhesion Barrier Study Group II. Surgery Gynecology & Obstetrics 1993;177(2):135‐9. [PubMed] [Google Scholar]
- Azziz R, Cohen S, Franklin R, Haney F, Russell L, Patton G, et al. Prevention of postsurgical adhesions by INTERCEED (TC7), an absorbable adhesion barrier: a prospective, randomized multicenter clinical study. INTERCEED (TC7) Adhesion Barrier Study Group. Fertility & Sterility 1989;51:933‐8. [PubMed] [Google Scholar]
- Malinak L. Interceed (TC7) as an adjuvant for adhesion reduction: clinical studies. Treatment of Surgical Adhesions. New York: Wiley‐Liss Inc, 1990:193‐206. [PubMed] [Google Scholar]
Canis 2014 {published data only}
- Canis MJ, Triopon G, Daraï E, Madelenat P, LeVêque J, Panel P, et al. Adhesion prevention after myomectomy by laparotomy: a prospective multicenter comparative randomized single‐blind study with second‐look laparoscopy to assess the effectiveness of PREVADH™. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;178:42‐7. [PUBMED: 24841647] [DOI] [PubMed] [Google Scholar]
Diamond 1996 {published data only}
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm* membrane (HAL‐F): a blinded, prospective, randomised, multicenter clinical study. The Seprafilm Adhesion Study Group. Fertility & Sterility 1996;66(6):904‐10. [PubMed] [Google Scholar]
Franklin 1995 {published data only}
- Franklin R, Jansen R, Larsson B, Malinak L, Rosenberg S, Webster B. A multicentre clinical evaluation of Interceed (TC7) absorbable adhesion barrier in the treatment of ovarian defects. Fertility & Sterility 1993;31:S31 (0‐063). [Google Scholar]
- Franklin RR, Diamond M, Malinak R, Larsson B, Jansen R, Rosenberg S, et al. Reduction of ovarian adhesions by the use of Interceed. Ovarian Adhesion Study Group. Obstetrics & Gynecology 1995;86(3):335‐40. [DOI] [PubMed] [Google Scholar]
Greenblatt 1993 {published data only}
- Greenblatt EM, Casper RF. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: lack of correlation with pregnancy rate. Fertility & Sterility 1993;60(5):766‐70. [PubMed] [Google Scholar]
Haney 1995 {published data only}
- Haney A, Hesla J, Hurst B, Kettel L, Murphy A, Rock J, et al. Expanded polytetrafluororethylene (Gore‐Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertility & Sterility 1995;63(5):1021‐6. [PubMed] [Google Scholar]
- Haney A, Hesla J, Hurst B, Kettel L, Murphy A, Rock J, et al. Prevention of pelvic side wall adhesion reformation using surgical barriers: expanded polytetrafluororethylene (Gore‐Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7). The Adhesion Barrier Study Group. Fertility & Sterility 1994;S210 (abstract):265. [Google Scholar]
- Haney A, Hesla J, Hurst B, Kettel M, Murphy A, Rock J, et al. Prevention of pelvic side wall adhesion reformation using surgical barriers: expanded polytetrafluororethylene (Gore‐Tex Surgical Membrane) is superior to oxidised regenerated cellulose (Interceed TC7). The Adhesion Barrier Study Group. Fertility & Sterility 1994;S210 (abstract):265. [Google Scholar]
- March C, Boyers S, Franklin R, Haney A, Hurst D, Lotze E, et al. Prevention of adhesion formation/reformation with Gore‐Tex Surgical Membrane. In: Diamond M, diZerega G, Linsky C, Reid RL editor(s). Gynecologic Surgery and Adhesion Prevention. New York: Wiley‐Liss Inc, 1993:253‐9. [Google Scholar]
- Schlaff W, Hurst B, Kettel M, Murphy A, Franklin R, Lotze E, et al. Prevention of pelvic side wall adhesions using barrier methods: e‐PTFE (Gore‐Tex Surgical Membrane) and oxidised regenerated cellulose (Interceed, TC7): preliminary report. The Adhesion Barriers Study Group. Fertility & Sterility 1992;S27 (abstract):58. [Google Scholar]
Keckstein 1996 {published data only}
- Keckstein J, Karageorgieva E, Sasse V, Roth A, Tuttlies F, Ulrich U. Reduction of postoperative adhesion formation after laparoscopic ovarian cystectomy. International Journal of Gynecology & Obstetrics 1994;46(Suppl 1):9. [DOI] [PubMed] [Google Scholar]
- Keckstein J, Ulrich U, Sasse V, Roth A, Tuttlies F, Karageorgieva E. Reduction of postoperative adhesion formation after laparoscopic ovarian cystectomy. Human Reproduction 1996;11(3):579‐82. [DOI] [PubMed] [Google Scholar]
Korell 1994 {published data only}
- Korell M. Reduction of adhesion by INTERCEED Barrier and Gortex Surgical Membrane after laparoscopic myomectomy. Presented at: "Moglichkeiten der Adhasionprophylaxe," April 1994, Munich, Germany. Munich.
Li 1994 {published data only}
- Li TC, Cooke ID. The value of an absorbable adhesion barrier, Interceed, in the prevention of adhesion reformation following microsurgical adhesiolysis. British Journal of Obstetrics & Gynaecology 1994;101(4):335‐9. [DOI] [PubMed] [Google Scholar]
Mais 1995a {published data only}
- Mais V, Ajossa S, Marongiu D, Peiretti R, Guerriero S, Melis G. Reduction of adhesion reformation after laparoscopic endometriosis surgery: a randomised trial with an oxidized regenerated cellulose absorbable barrier. Obstetrics & Gynecology 1995;86(4 Pt 1):512‐5. [DOI] [PubMed] [Google Scholar]
Mais 1995b {published data only}
- Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis G. Prevention of de‐novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Human Reproduction 1995;10(12):3133‐5. Also presented as an abstract at: 3rd International Congress on Pelvic Surgery; February 29‐March 2, 1996; San Diego, California (USA). [DOI] [PubMed] [Google Scholar]
Myomectomy ASG 1995 {published data only}
- Franklin R, Haney A, Kettel L, Eberhardt L, Murphy A, Rock J, et al. An expanded polytetrafluoroethylene barrier (Gore‐Tex Surgical Membrane) reduces post‐myomectomy adhesion formation. The Myomectomy Adhesion Multicenter Study Group. Fertility & Sterility 1995;63(3):491‐3. [PubMed] [Google Scholar]
- Tulandi T, Murphy A, Rock J, Franklin R, Rowe G, Lotze E. Effects of Gore‐Tex Surgical Membrane on post‐myomectomy adhesions. Human Reproduction 1993;8(Suppl 1):9. [Google Scholar]
- Tulandi T, Rowe G, Rock J, Murphy A, Lotze E, Kettel L, et al. Effects of expanded polytetrafluoroethylene Gore‐Tex Surgical Membrane on postmyomectomy adhesion. The Myomectomy Adhesion Study Group. Fertility & Sterility 1994;S210 (abstract):226. [Google Scholar]
Nordic APSG 1995 {published data only}
- Larsson B, Berg A, Bryman I, Thorburn J, Hogset K, Laatikainen T, et al. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Nordic Adhesion Prevention Study Group. Fertility & Sterility 1995;63(4):709‐14. [PubMed] [Google Scholar]
- Larsson B, Bryman I, Thorburn J, Hogset K, Laatikainen T, Martikainen H, et al. Beneficial effect of Interceed TC7 as adjuvant therapy in prevention of postoperative adhesions on ovaries and oviducts in microsurgical operations for fertility. Fertility & Sterility 1993;Suppl:172. [Google Scholar]
Saravelos 1996 {published data only}
- Saravelos H, Li T‐C. Post‐operative adhesions after laparoscopic electrosurgical treatment for polycystic ovarian syndrome with the application of Interceed to one ovary: a prospective randomised controlled study. Human Reproduction 1996;11(5):992‐7. [DOI] [PubMed] [Google Scholar]
Sekiba 1992 {published data only}
- Sekiba K. Use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion formation in infertility and endometriosis surgery. In: Diamond M, diZerega G, Linsky C, Reid R editor(s). Gynaecologic Surgery and Adhesion Prevention. New York: Wiley‐Liss Inc, 1993:221‐33. [PubMed] [Google Scholar]
- Sekiba K, Yoshida N, Fukaya T, Ono T, Mizunuma H, Osada H, et al. The use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. The Obstetrics and Gynecology Adhesion Prevention Committee. Obstetrics & Gynecology 1992;79(4):518‐22. [PubMed] [Google Scholar]
Takeuchi 2005 {published data only}
- Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakri J, Kmoshita K. Adhesion prevention effects of fibrin ceilings after laparoscopic myomectomy as determined by second look laparoscopy: a prospective randomised, controlled study. Journal of Reproductive Medicine 2005;50(8):571‐7. [PubMed] [Google Scholar]
Tinelli 2011 {published data only}
- Tinelli A, Malvas A, Guido M, Tsin DA, Hudelist G, Hurst B, et al. Adhesion formation after intracapsular myomectomy with or without adhesion barrier. Fertility & Sterility 2011;95(5):1780‐5. [DOI] [PubMed] [Google Scholar]
van Geldorp 1994 {published data only}
- Wiseman D, Geldorp H, Marks M, Diamond M. The effects of Interceed (TC7) absorbable adhesion barrier on the development of adhesions in patients with endometriosis. In: Nehzat C editor(s). Endometriosis: Advanced Management and Surgical Techniques. New York: Springer Verlag, 1995:200a‐e. [Google Scholar]
- Geldorp H. Interceed absorbable adhesion barrier reduces the formation of postsurgical adhesions after ovarian surgery. Fertility & Sterility 1994;P273:213‐4. [Google Scholar]
Wallweiner 1998 {published data only}
- Wallweiner D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: an explanation for the controversy on the use of autologous and alloplastic barriers?. Fertility & Sterility 1998;69(1):132‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Diamond 1998 {published data only}
- Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL‐C) solution: a prospective, randomized, blinded, placebo‐controlled multicenter study. The Sepracoat Adhesion Study Group. Fertility & Sterility 1998;69(6):1067‐74. [DOI] [PubMed] [Google Scholar]
Dunn 2002 {published data only}
- Dunn R, Johns D, Ferland R. A pilot, blinded evaluation of the adhesion barrier system. Human Reproduction Abstracts 2002;17(Abstract book 1):577. [Google Scholar]
Korrell 1998 {published data only}
- Korrell M, Obernitz N, Hepp H. Incidence of adhesions after endoscopic myomectomy ‐ Interceed TC7 versus Intergel. 7th Congress of the European Society for Gynaecological Endoscopy; 1998 Oct 7‐10; Vienna, Austria. 1998:25.
Korrell 2000a {published data only}
- Korrell M, Obernitz NV, Hepp H. Incidence of adhesions after endoscopic myomectomy ‐ Interceed TC7 versus Intergel. 9th Congress of the European Society for Gynaecological Endoscopy; 2000 October; Paris, France. 2000.
Korrell 2000b {published data only}
- Korrell M, Obernitz NV, Hepp H. Incidence of adhesions after endoscopic myomectomy ‐ Interceed TC7 versus Intergel. XV1 FIGO World Congress of O&G; September 3‐8, 2000; Washington, DC. 2000:25.
Liu 2015 {published data only}
- Liu C, Lu Q, Zhang Z, Xue M, Zhang Y, Zhang Y, et al. A randomized controlled trial on the efficacy and safety of a new crosslinked hyaluronan gel in reducing adhesions after gynecologic laparoscopic surgeries. Journal of Minimally Invasive Gynaecology 2015;22(5):853‐63. [DOI: ] [DOI] [PubMed] [Google Scholar]
Reid 1993 {published data only}
- Reid R, Spence J, Tulandi T, Yuzpe A. Clinical experience of Interceed (TC7) absorbable adhesion barrier and heparin. Fertility & Sterility 1993;S153:160. [DOI] [PubMed] [Google Scholar]
Reid 1997 {published data only}
- Reid RL, Hahn PM, Spence JEH, Tulandi T, Yuzpe AA, Wiseman DM. A randomised clinical trial of oxidised regenerated cellulose adhesion barrier (Interceed, TC7) alone or in combination with heparin. Fertility & Sterility 1997;67(1):23‐9. [DOI] [PubMed] [Google Scholar]
To 1992 {unpublished data only}
- To WK, Ho FC, Tang G. INTERCEED study: a preliminary report on immediate post‐operative response in cesarean section patients. Presented at: 2nd International Symposium on Adhesion Prevention; January 1992; Palm Beach, Florida (USA).
Trew 2016 {published data only}
- Trew GH, Pistofidis GA, Brucker SY, Krämer B, Ziegler NM, Korell M, et al. A first‐in‐human, randomized, controlled, subject‐ and reviewer‐blinded multicentre study of Actamax adhesion barrier. Archives of Gynaecology and Obstetrics 2016;295:383‐95. [DOI: 10.1007/s00404-016-4211-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmad 2014
- Ahmad G, Mackie FL, Iles DA, O'Flynn H, Dias S, Metwally M, et al. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD001298.pub4] [DOI] [PubMed] [Google Scholar]
diZerega 1990
- diZerega GS. The peritoneum and its response to surgical injury. Progress in Clinical & Biological Research 1990;358:1‐11. [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton, Ontario, Canada: McMaster University (developed by Evidence Prime, Inc.), 2015.
Gruber‐Blum 2011
- Gruber‐Blum S, Petter‐Puchner AH, Brand J, Fortelny RH, Walder N, Oehlinger W, et al. Comparison of three separate antiadhesive barriers for intraperitoneal onlay mesh hernia repair in an experimental model. British Journal of Surgery 2011;98:442‐9. [PUBMED: 21254024] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hurst 1998
- Hurst BS. Permanent implantation of expanded polytetrafluoroethylene is safe for pelvic surgery. Human Reproduction 1999;14(4):925‐7. [DOI] [PubMed] [Google Scholar]
Jacobs 1996
- Jacobs J, Iyer R, Weston J, Amato J, Elliott M, Leval M, et al. Expanded PTFE membrane to prevent cardiac injury during resternotomy for congenital heart disease. Annals of Thoracic Surgery 1996;62(6):1778‐82. [DOI] [PubMed] [Google Scholar]
Okabayashi 2014
- Okabayashi K, Ashrafian H, Zacharakis E, Hasegawa H, Kitagawa Y, Athanasiou T, et al. Adhesions after abdominal surgery: a systematic review of the incidence, distribution and severity. Surgery Today 2014;44:405‐20. [PUBMED: 23657643] [DOI] [PubMed] [Google Scholar]
Pados 2010
- Pados G, Venetis CA, Almaloglou K, Tarlatzis BC. Prevention of intra‐peritoneal adhesions in gynaecological surgery: theory and evidence. Reproductive BioMedicine Online September 2010;21(3):290‐303. [DOI] [PubMed] [Google Scholar]
Pellicno 2003
- Pellicno M, Bramamte S, Cirillo D. Effectiveness of auto‐cross linked hyaluronic acid gel after laparoscopic myomectomy in infertile patients: a prospective randomised controlled study. Fertility & Sterility 2003;80:441‐4. [DOI] [PubMed] [Google Scholar]
Robertson 2010
- Robertson D, Lefebvre G, Leyland N, Wolfman W, Allaire C, Awadalla A, et al. Adhesion prevention in gynaecological surgery. Journal of Obstetrics and Gynaecology Canada 2010;32(6):598‐608. [DOI] [PubMed] [Google Scholar]
SRS 2007
- Practice Committee of the American Society for Reproductive Medicine: Society of Reproductive Surgeons. Pathogenesis, consequences and control of peritoneal adhesions in gynecologic surgery. Fertility & Sterility 2007;88:21‐6. [DOI] [PubMed] [Google Scholar]
Ten Broek 2013
- Broek RP, Stommel MW, Strik C, Laarhoven CJ, Keus F, Goor H. Burden of adhesions in abdominal and pelvic surgery: systematic review and meta‐analysis. BMJ 2013;3:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiseman 1999
- Wiseman DM, Trout JR, Franklin RR, Diamond MP. Metaanalysis of safety and efficacy of an absorbable adhesion barrier (INTERCEED TC7) in laparotomy. Journal of Reproductive Medicine 1999;44(4):325‐31. [PubMed] [Google Scholar]
References to other published versions of this review
Ahmad 2015
- Ahmad G, O'Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD000475.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farquhar 1999
- Farquhar C, Vandekerchove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for preventing subfertility. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000475] [DOI] [PubMed] [Google Scholar]


