Abstract
The auditory system allows us to monitor background environmental sound patterns and recognizes deviations that may indicate opportunities or threats. The mismatch negativity (MMN) and P3a potentials have generators in auditory and inferior frontal cortex, and index expected sound patterns (standards) and any aberrations (deviants). The MMN and P3a waveforms show increased positivity for consecutive standards and deviants preceded by more standards. We hypothesized attenuated repetition effects in older participants, potentially due to differences in prefrontal functions. Young (23 ± 5 yrs.) and older (75 ± 5 yrs.) adults were tested in two oddball paradigms with pitch or location deviants. Significant repetition effects were observed in the young standard and deviant waveforms at multiple time windows. Except the earliest time window (30–100ms), repetition effects were absent in the older group. Repetition effects were significant at frontal but not temporal lobe sites, and did not differ among pitch and location deviants. However, P3a repetition was evident in both ages. Findings suggest age differences in the dynamic updating of sensory memory for background sound patterns.
Keywords: mismatch negativity, sensory memory, repetition effects, aging
Introduction
Certain facets of attention control are thought to decline during normal cognitive aging (Kramer & Madden, 2007). Attention can be guided by either voluntary control, such as consciously deciding to eavesdrop on a nearby conversation, or automatically, as when a loud noise captures attention regardless of one’s volition. Most attention models only distinguish between the controlled and automatic aspects of attention control. A recent model proposes a third category, called “selection history”, where attention control is automatically guided by information in short and long-term memory (Awh et al., 2012; Theeuwes, 2019, see Addleman & Jiang, 2019). The idea that attention can be guided by memory has a long heritage (Pillsbury, 1908; James, 1890). An example of a short-term influence on attention control is the lingering effects of recently attended information on current trial performance. Longer-term influences on attention control include the learned statistical properties, reward value, or personal significance of stimuli (Theeuwes, 2019; Brian Anderson, 2013; Moray, 1959). Behavioral studies show that aging is associated with decline in both controlled and automatic attention control (Hamm & Hasher, 1992; Escera et al., 2000). According to the framework of Awh and colleagues, automatic attention control can be driven by salience (e.g. loudness of an unexpected sound), or selection history (e.g. stimulus patterns, probability, and priming).
Selection history, includes aspects of implicit processing such as single stimulus or paired-stimulus repeats, which are thought to be well preserved in aging (Curran et al., 1997), while other implicit processes such as learning complex sequences show age decline (Bennett et al., 2006). Most of the repetition priming effects research includes investigation of retrieval strategies or response-speed/accuracy (Howard & Howard, 2013; Ikier et al., 2008), and therefore does not examine the initial sensory encoding and memory. Recent studies in younger participants have suggested that auditory repetition effects observed during the initial auditory encoding might tap into the neural correlates of memory traces underlying priming (Auksztulewicz & Friston, 2016; Cooper et al., 2013; Friston et al., 2005). However, no prior studies have addressed whether early auditory encoding processes might underlie priming processes in cognitive aging.
Automatic neural responses can be studied in a passive listening “oddball” paradigm. In oddball paradigms a repetitive “standard” sound is intermittently punctuated by a “deviant” sound that differs from the standard in terms of a stimulus feature, such as pitch or location (Näätänen et al., 1978). Any attention capture by deviants, in this case, would be guided by saliency.
Neural responses in the oddball task are commonly measured with EEG, by averaging responses shortly before and after each stimulus (“event-related potentials”, ERPs). Typically, the standard ERP is subtracted from the deviant ERP waveform. The resulting difference waveform is characterized by two prominent peaks: a negative peak, the “mismatch negativity” (MMN, ~100–250 ms latency), and a positive peak called the “P3a” (~250–280 ms latency). The MMN indexes change detection in the environment, such as a deviant sound (Halgren et al., 1995; Alain et al., 1998). The P3a reflects the orienting response, and is larger for deviant stimuli that are more distinct from the standards in both passive and active tasks (Friedman et al., 2001, Wronka et al., 2008).
Selection history has a straightforward connection to ERP dynamics as a function of how many standards have been presented in a row between two deviant stimuli (termed “repetition effects”). Repetition effects are a well-known property of ERPs and indicate nonstationary brain responses (Golob et al. 2001; Golob & Starr, 2000). Repetition Effects have been recorded at multiple spatial and temporal scales. Spatially, repetition effects capture auditory encoding as individual intra-cortical neuronal spiking in primates (Li et al., 1993; Miller et al., 1994), and the summated neural-activity of millions of neurons measured as BOLD fMRI signal changes in humans (Grill-Spector et al. 2006).
In passive oddball paradigms, repetition effects in standard sounds are evident as increasing positivity at the time of the MMN, which is “reset” after presentation of a deviant (termed “repetition positivity” or “repetition suppression”) (Cooper et al., 2013; Haenschel et al, 2005). For deviant stimuli, the negative potential becomes more positive after longer sequences of standard stimuli (Haenschel et al. 2005; Costa-Faidella et al., 2011). Repetition effects are thought to index the development of a sensory memory trace, where the strength of these cortical representations in the auditory cortex increases with repeat number, and is captured as a larger positivity in the standard waveform at the latencies of ~50–250 ms (Haenschel et al., 2005, Baldeweg et al., 2004; Cooper et al., 2013). Note that making grand average standard and deviant ERPs from all trials will obscure repetition effects.
We now turn from using the oddball task to index saliency and selection history to what is known about the cortical generators of standard and deviant ERP waves. Various studies suggest enhanced auditory cortex activity during MMN reflects prefrontal and temporo-parietal cortical sources (lesion studies: Alain et al., 1998; Alho et al., 1994; animal studies: Javit et al., 1994), where the prefrontal contributions to the MMN can be dissociated from auditory cortical activity (Deouell, 2007). A dissociation between frontal and temporal generators was initially provided by EEG current source density estimates of separate generators in frontal and temporal cortex (Rinne et al., 2000), and intracranial recordings in humans (Halgren et al., 1995). A functional dissociation between these two generators was shown by selectively reducing the frontal vs. the temporal generator under various conditions (Sleep: Sallinen & Lyytinen, 1997; Schizophrenia: Alain et al., 1998; and Alcohol: Jaaskelainen et al., 1996). Finally, interrelations between auditory and frontal MMN generators were supported by animal studies documenting reciprocal anatomical connections and short latency single-unit responses to sounds in prefrontal regions (Romanski et al., 1999; Azuma & Suzuki, 1984). Similarly, the P3a has multiple generator sites in association cortex, including prefrontal, anterior cingulate, and parietal areas (Polich, 2007; Halgren et al., 1998).
Separate temporal and frontal lobe generators of the MMN are relevant to aging because convergent evidence suggests that the prefrontal cortex undergoes substantial age-related structural changes at the cellular and regional levels (Sowell et al., 2003; Raz, 1997). Behavior studies of implicit sequence learning are germane to repetition effects, and white matter integrity of tracts to and from dorsolateral prefrontal cortex is positively associated with sequence learning and negativity associated with age (Bennett et al., 2011). In contrast, sensory and motor cortices, including primary and secondary auditory cortex, show little change with age (Flood & Coleman, 1988). Some prior studies found smaller MMN amplitudes and longer latencies in older (> ~65 years) relative to younger (~20–30 years) participants (Cheng et al., 2013; Ruzzoli et al., 2012; Gaeta et al., 1998). However, other reports did not find significant age differences in MMN measures, particularly when sounds were delivered at a rapid rate and had obvious differences between standards and deviants (Cooray, 2014; Pekkonen et al., 2007; Kisley, 2005). Studies investigating the P3a in passive oddball tasks generally report either longer latencies, or smaller amplitudes in older participants (Nowak et al, 2016; Friedman et al., 1998).
The mixed results in aging may be due, in part, to methodological issues. Typically, MMN is expressed as a difference wave, which provides a convenient metric, but also introduces three important ambiguities. Firstly, a difference ERP waveform prevents analysis of the absolute voltage values and shapes of the constituent standard and deviant waveforms. Consequently, important age differences may go unrecognized if differences in one waveform are counteracted by the other waveform. It is noteworthy however, that this did not happen in this dataset. Secondly, difference measures generally have a lower signal to noise ratio, relative to single measures, due to contributions from two sources of variability (Cronbach & Furby, 1970). A smaller signal-to-noise ratio results in lower statistical power and test-retest reliability. Thirdly, repetition effects are averaged out, which opens the possibility that age group differences may exist for the time course of repetition effects but not for grand averages.
We aim to test the prediction that MMN and P3a repetition effects for standards and deviants are evident in young adults, but will be attenuated or absent in older participants. According to this hypothesis, the frontal cortex involvement may be important for stimulus selection history to influence auditory stimulus processing, and its influence would be expressed as repetition effects. Since age-related declines are more prominent in frontal than auditory cortex, we predicted an absence or reduction of repetition effects in the older adults. Moreover, if repetition effects are less apparent in older participants, this may help explain some of the overall age differences observed in MMN and P3a measures when averaged across repetitions, as well as heterogeneity of findings across studies. Deviant stimuli were tested using two different stimulus dimensions (frequency, location), in separate blocks to check for generality and replicability of any age differences in repetition effects.
Methods
Participants:
Twenty-seven young undergraduates (mean = 23 ± 5 yrs., M/F = 8/19), and thirty older community residents (mean = 75 ± 5 yrs., M/F = 11/19) were recruited. Older participants have no history of major neurological and psychiatric conditions and received a battery of standardized cognitive tests to screen for cognitive decline (see Golob et al., 2009). One participant with a low mini-mental status test score (MMSE = 20) was excluded. Hearing thresholds were tested with an audiometer (Maico, Eden Prairie, MN), and all participants had thresholds < 25 dB (0.5– 4 kHz). Written informed consent was obtained from all participants for a protocol through the Tulane University IRB, and the experiments were consistent with the Declaration of Helsinki.
Study design:
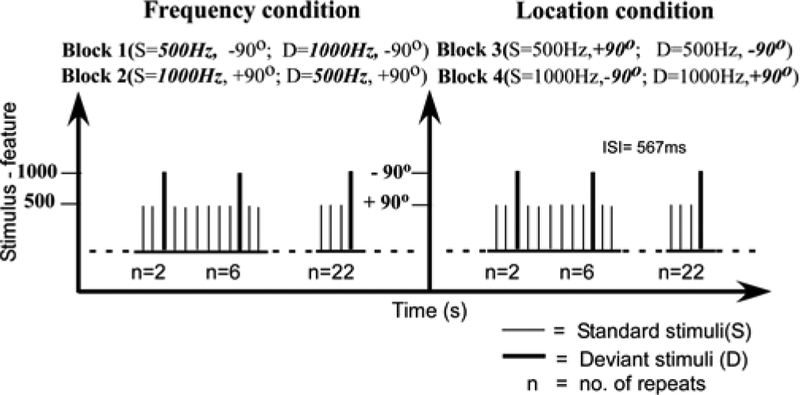
Two monoaural pure tones (500 Hz and 1,000 Hz; 100 ms duration; 10 ms rise/fall times, 80 dB SPL) were presented to either the left or right ear with an interstimulus interval (onset to onset) of 567 ms. Participants were instructed to watch a silent movie (“The sounds in the background. Spatial (monaural ear of presentation) and non-spatial (frequency) variables were chosen to tap into two major aspects of auditory processing. There were four stimulus blocks (1,000 trials/block). Two blocks examined spatial deviants, where in each block the same frequency tone was presented to one ear as the standard and the other as the deviant (ears counterbalanced across blocks) (Figure 1). The other two blocks examined monaural pitch deviants using high and low frequency tones, and the standard/deviant frequencies were counterbalanced across blocks. The left and right ears for frequency deviants and frequencies for spatial deviants were approximately counterbalanced across participants. Stimuli were pseudo-randomly presented to ensure adequate sampling of sequences and to avoid repetition of deviants. Stimulus probabilities were 0.945 for standards, and 0.055 for deviants. Repetition effects examined runs of standards after a deviant (standard repetition effects) and numbers of standards before a deviant (for deviant repetition effects). To analyze standard ERP repetition effects, four positions within the run of standards after deviants were tested: 2, 6, 12, and 22 & 23 standards in a row. The numbers were chosen to approximately double, with 2 being the first opportunity for a repetition in the sequence. For deviant ERP repetition effects, trials were collapsed over a range of positions because there were far fewer trials vs. the standards. The number of standards since the last deviant were examined for 9–12, 16–19, and 22 & 23. The grandaverage standard waveforms were obtained by averaging younger (71–82), older (62–73) trials out of 108 total trials for repeat conditions 2, 6 & 12; while younger (43–45), older (40) out of 60 total trials were averaged for repeat condition 22 & 23. The grandaverage deviant waveforms were obtained by averaging (22–28) trials out of 30 deviant trials in both younger and older participants. EEG was recorded from 64-channel electrode caps using standard methods (Compumedics-Neuroscan, Charlotte, NC, USA) (500 Hz sample rate, DC-100 Hz bandpass, was visually inspected for artifacts). The reference during recording was placed between Cz and CPz, and, re-referenced offline to the average reference (see Mock et al., 2015). Offline band pass filtering (.1, 30 Hz) of the waveforms was performed. Baseline from −100 to 0 ms. EEG was corrected for DC drift and eye blink artifacts in Neuroscan using an algorithm (Gratton et al., 1983). Event-related potentials to stimuli were averaged from EEG sweeps between −100 ms to 400 ms relative to stimulus onset.
Figure 1.
Schematic illustration of stimulus sequence structure.
Statistical analysis:
Prior research informed the selection of frontal (10/20 system, Fz) and temporal (M1, M2) electrode sites to measure the repetition effects in different temporal windows and MMN and P3a. Mean voltage (ERP amplitude) was quantified for each of the time windows as a function of repetition effect (Friedman et al., 2001; Näätänen et al., 2007). Analysis of standards used three-time windows (30–100 ms, 90–130 ms, 132–200 ms), which were common to both age groups. This common window approach was necessary to account for the latency differences between the ERP peaks for each age group. Additional analysis was performed for time windows that best captured these peaks in each age group, where age-specific non-overlapping time windows were used to capture the age appropriate window latencies, regardless of length, or trying to make the windows comparable for analysis. The two new standard windows were: 50–98 ms and 100–130 ms in the younger participants and 30–78 and 80–130 ms in the older participants.
Deviant waveforms are shown in Figure 2b, with two time window measures (90–130 ms MMN, 200–260 ms P3a). Separate analysis of variance tests (ANOVAs, significance = p < 0.05) were performed for each time window, using the factors of age (younger vs. older), stimulus type (frequency vs. location) and repetition ((standards: 2, 6, 12, 22 & 23); deviants: (9–12), (16–19) and (22–24)). Planned contrasts were used for post-hoc analyses involving the factor of repetition.
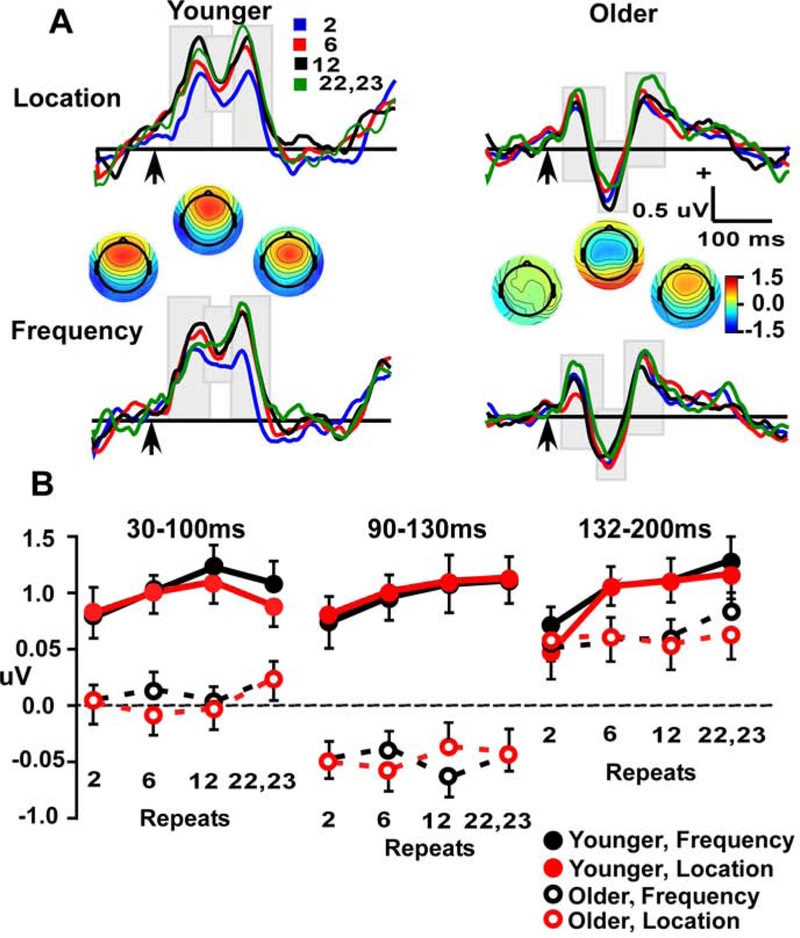
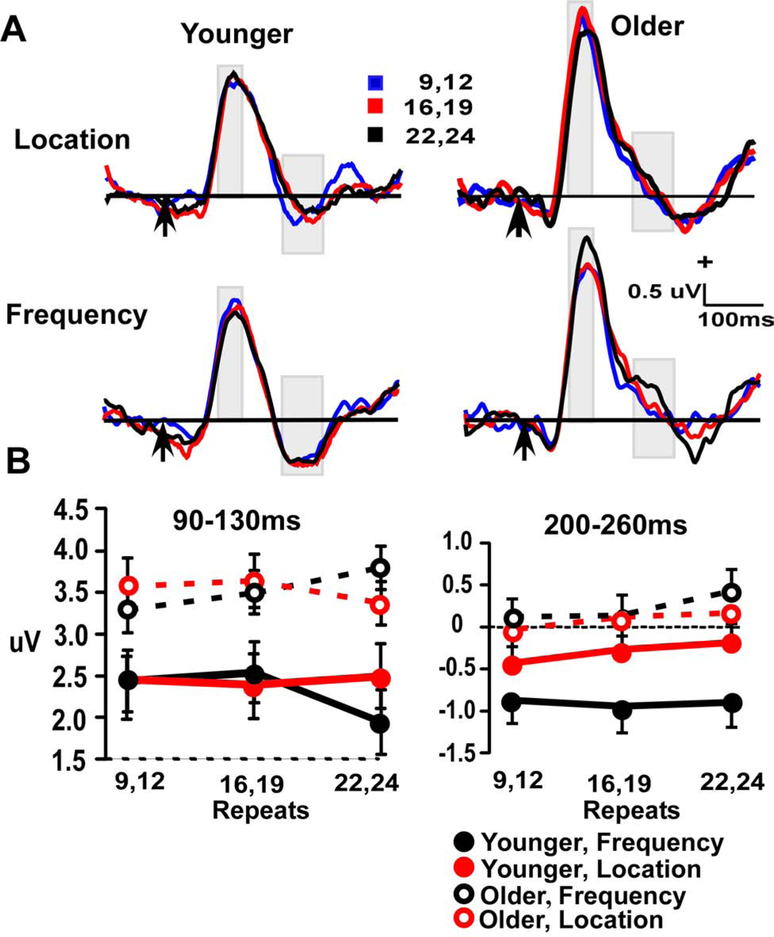
Figure 2.
A. Standard frontal repetition effects (at Fz electrode) in Location and Frequency waveforms across three windows (30–100 ms, 90–130 ms and 132–200 ms), for Younger (left) and Older (right) participants. Arrow indicates stimulus-onset. B. Line plots demonstrate repetition effect in aging across four repeat conditions (2, 6,12 & 22–23) in three time-windows (30–100 ms, 90–130 ms &132–200 ms).
Repetition effects in this study were compared with previous MMN studies through the classical MMN measure, obtained by subtracting standards from deviants (90–130 ms window). The 12 and 22 repetition points were common to both standards and deviants. Note that in deviants, 9–12 repetitions and 22–24 repetitions were summated in an ERP average due to fewer number of trials. These trial summations were done to ensure that each condition had at least 60 trials or more per condition.
Results
Standard tones at frontal site (Fz):
Event-related potentials and plots of amplitudes in each time window for standard tones are presented in Figure 2. Analysis of frontal activity used 2 (age) x 2 (stimulus type) x repetition position (4) ANOVAs, with separate analyses at three time-windows that corresponded to peaks of the waveforms (Figure 2A). The 30–100 ms window showed a main effect of age (F (1, = 24.6, p < 0.001) and a significant age x repetition interaction (F (3, 165) = 24.6, p < 0.001). The main effect was due to more positive amplitudes in the young group. Follow-up quadratic contrasts examined the age x repetition interaction and found significant effects in both age groups (both p’s < 0.05) (Figure 2, lower left panel). Repetition effects in young participants were manifest as progressive amplitude increases across repetition lengths of 2, 6, 12 standards in a row, and then plateaued or had a slight decrease between 12 and (22 & 23) repetitions. In contrast, amplitudes in older participants across 2, 6, and 12 repetitions were comparable, and only showed an amplitude increase for the longest repetition that was tested (22 & 23 standards in a row). Thus, the onset of repetition effects in the older adults was greatly delayed relative to the younger adults. There was no main effect or interaction involving the stimulus type factor.
The 132–200 ms window showed a significant main effect of repetition (F (3, 165) = 7.4, p < 0.001) that was qualified by age x repetition interaction (F (3, 165) = 3.6, p < 0.02). Planned contrasts showed a significant linear fit in the young (p < 0.001) but not in older participants (p > 0.25). As with the 30–100 ms window analysis, there were no significant differences among stimulus types, which indicates some generality to the findings. The same analysis was conducted with window measures more specific to each age group, and the same results were found (see supplementary methods). The 90–130 ms window showed a significant age effect (F (1, 55) = 39.3, p < 0.001), with more positive amplitudes in the young group.
Standard tones at mastoid sites (M1, M2):
Analysis of the mastoid sites used 2 (age) x 2 (stimulus type) x repetition position (4) x 3 (time window) x 2 (site: M1, M2) ANOVAs. As seen in Figure 3, the 30–100 ms window showed significant effects for age (F (1, 55) = 17.5, p < 0.001). The effect for site was significant in this window (F (1, 55) = 8.1, p < 0.01) (not shown in Fig 3), with more negative potentials in the young vs. older and left vs. right mastoids. The only significant effect for the 90–130 ms window was that of age (F (1, 55) = 41.4, p < 0.001, with young more positive than older). In the 132–200 ms window, there was a small effect of electrode site (F (1, 55) = 4.1, p < 0.05), with more negative potentials at the right site (M2) (Not shown in the Fig 3). Overall, the absence of repetition effects to standard tones at mastoid sites contrasts with the age differences in repetition effects seen at the frontal site.
Figure 3.
A. Standard temporal repetition effects (combined left and right Mastoid electrode sites) in Location and Frequency waveforms across three windows (30–100 ms, 90–130 ms and 132–200 ms), for Younger (left) and Older (right) participants. Arrow indicates stimulus-onset. B. Line plots demonstrate repetition effect in aging across four repeat conditions (2, 6, 12 & 22–23) in three time-windows (30–100 ms, 90–130 ms &132–200 ms).
Deviant tones at frontal site (Fz):
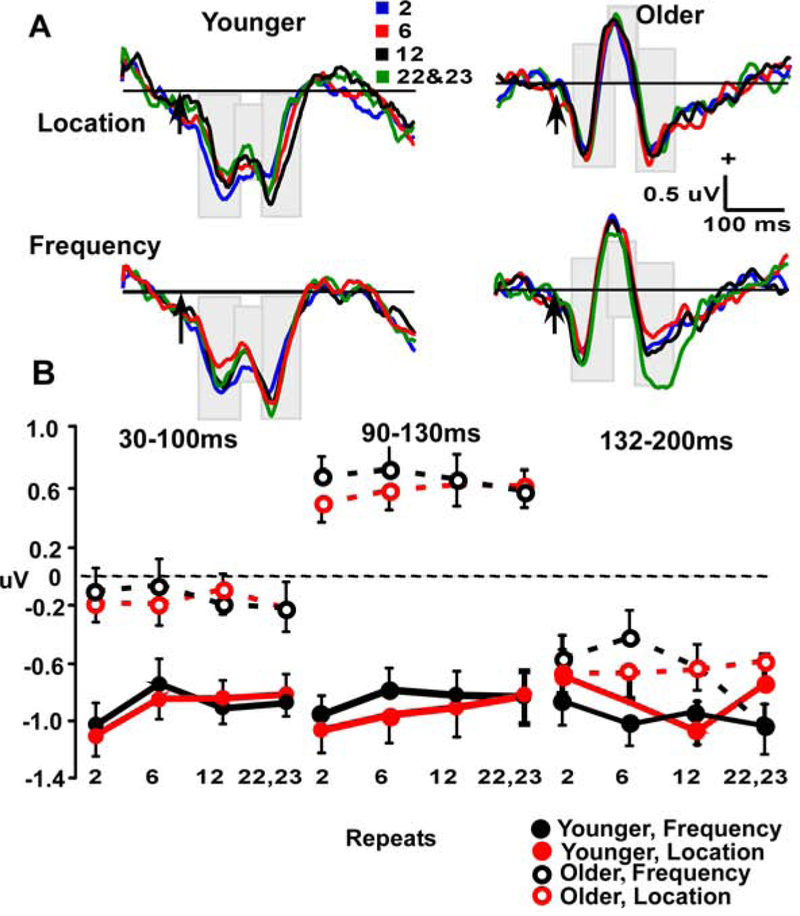
Plots of the results for deviant stimuli are shown in Figure 4. Recall that the 90–130 ms window indexes the MMN, while the 200–260 window captures the P3a peak. Analysis of frontal activity between 90–130 ms using a 2 (age) x 2 (stimulus type) x repetition position (3) x 2 (time window) ANOVA had significant effects of age (F (1, 55) = 16.2, p < 0.001) and an age x repetition interaction (F (2, 110) = 8.5, p < 0.05). The age effect was due to larger, more negative, amplitudes in older vs. younger participants. Contrasts to better understand the age x repetition interaction showed a linear fit over repetitions in the young (p < 0.001), which was not evident in older participants (p = 0.22). For the 200–260 ms window, there was an effect of age (F (1, 55) = 28.6, p < 0.001), with larger P3a amplitudes in the young.
Figure 4.
A. Deviant frontal repetition effects (at Fz electrode site) in Location and Frequency waveforms across 90–130 ms and 200–260 ms, P3a windows, in Younger (left) and Older (right) participants. Arrow indicates stimulus-onset. B. Line plots demonstrate repetition effect in aging across three repeat conditions (9–12, 16–19 & 22–24) in two time-windows (90–130 ms & 200–260 ms).
Deviant tones at mastoid sites (M1, M2):
As seen in Figure 5, In the MMN (90–130 ms) time window, there was only a significant effect of age (F(1, 55) = 12.9, p < 0.001), due to more positive potentials in the young participants. For the P3a (200–260 ms) window, there were main effects of age (F (1, 55) = 8.7, p < 0.01) and site (F (1, 55) = 14.7, p < 0.001), with more positive potentials in the young vs. older and for the right vs. left mastoid site. There was also a significant age x stimulus type interaction (F (1, 55) = 6.5, p < 0.02), because age differences were larger for the location vs. frequency deviants.
Figure 5.
A. Deviant temporal repetition effects (combined left and right Mastoid electrode sites) in Location and Frequency waveforms across 90–130 ms and 200–260 ms, P3a windows, in Younger (left) and Older (right) participants. Arrow indicates stimulus-onset. B. Line plots demonstrate repetition effect in aging across three repeat conditions (9–12, 16–19 & 22–24) in two time-windows (90–130 ms & 200–260 ms).
Subtraction waveform (deviant minus standard) at frontal site (Fz):
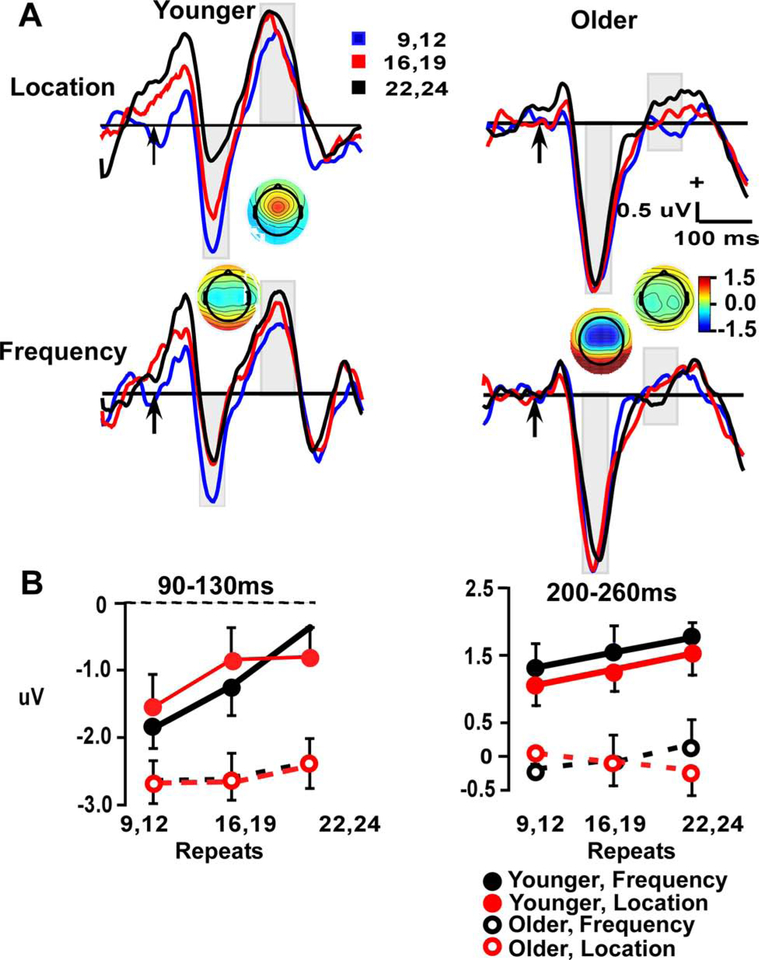
Since repetition effects were only observed at the frontal site for standards and deviants, they are presented in this paper in subtraction waveforms at just the frontal site to isolate the MMN (90–130 ms) and P3a (200–260 ms) (Figure 6). Limited number of trials were available for the infrequent deviants to create ERP averages. Therefore, repetition effects were measured after 12 and 22 standards in a row, the two repetition points common to both standards and deviants. For the MMN measure, a 2 (age) x 2 (stimulus type) x repetition position (2) ANOVA had a main effect of repetition (F (1, 55) = 10.8, p < 0.01) and an age x repetition interaction (F (1, 55) = 5.3, p < 0.03). As seen in Figure 6, the interaction was driven by a significant repetition effect in the young (p < 0.01), which was absent in the older participants (p < 0.42). There was no significant overall effect of age (p < 0.51).
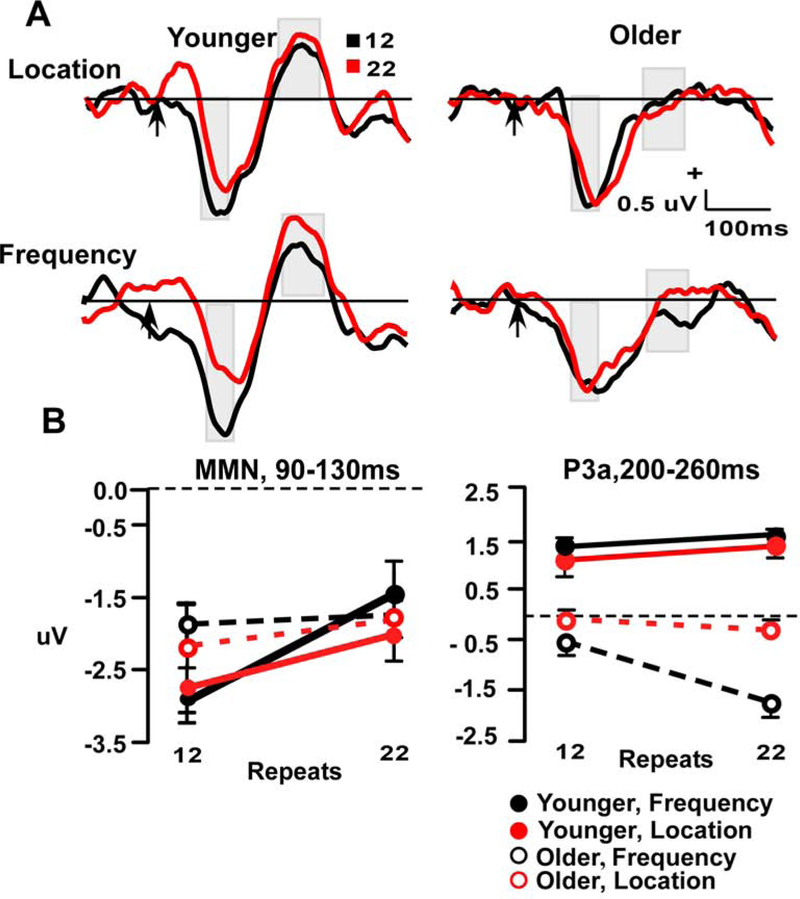
Figure 6.
A. Difference waveform frontal repetition effects (Deviant minus Standard) waveforms at electrode site Fz. Time windows index the MMN (90–130 ms) and P3a (200–260 ms). Arrow indicates stimulus-onset. B. Line plots demonstrate repetition effect in aging across two repeat conditions, 12 & 22 in two time-windows (90–130 ms & 200–260 ms).
For the P3a measure, there was a main effect of age (F (1, 55) = 63.82, p < 0.001: young > older), a trend for a repetition effect (F (1, 55) = 3.21, p = 0.075), and a significant repetition x stimulus type interaction (F (1, 55) = 4.11, p < 0.05). The age x repetition x feature was not significant (p = 0.87). The interaction between repetition and stimulus type was significant due to repetition effects in the frequency but not location condition, and is similar to earlier findings that show repetition seems to be significant at the same location (Leung et al., 2013).
See supplementary Figure 1 for analysis of repetition effects in MMN at the temporal sites, which generally did not yield significant findings.
Discussion
The main results demonstrated that the standard and deviant waveforms in the young had significant repetition effects in multiple time windows at frontal, but not temporal sites. In contrast, with one exception (standards: 30–100 ms), older participants did not have repetition effects. These findings indicate age differences in the degree to which the selection history influences the processing of incoming stimuli, with the young adults having a much greater influence of selection history. Age differences were significant for repetition effects regardless of the sensory feature used to define the deviant sound (frequency vs. location), which indicates some generality of the age differences. Similarly, the subtraction waveform (deviants minus standards) also only had significant MMN repetition effects in younger participants, while P3a repetition effects were seen in both age groups. Taken together, these findings provide new evidence of dynamic adjustments in processing of standard and deviant sounds in young but not older adults. The absence of such dynamics in older participants, in tandem with the large literature on generators of the MMN, suggests a fruitful new approach to understanding specific neural mechanisms for age differences in sensory memory and attention control.
Age differences in repetition effects for standards and deviants
Prior studies using passive oddball or roving paradigms, where standards and deviants vary over time, have consistently observed repetition effects to standards at frontal electrode sites in the young (Nowak et al., 2016; Horvath et al., 2008; Baldeweg et al., 2004; Haenschel et al., 2005). Repetition effects were observed at temporal sites in a study where participants actively discriminated among stimuli (Haenschel et al., 2005). According to the “repetition suppression” account, repetition effects seen in the standard waveform reflect auditory sensory memory trace formation (Haenschel et al, 2005; Cooper et al., 2013). However, since most repetition effect studies, including the present one, employ passive listening, the often-hypothesized relationship between echoic memory, as defined by behavioral measures, and ERP repetition effects is currently uncertain.
We propose that sequence effects reflect attention guidance by selection history, because there was no behavioral goal (participants passively listened to the sounds), and the same physical stimuli were presented across sequences, so there were no sequence differences in stimulus saliency. While most measures of standard and deviant ERPs showed repetition effects in young participants, there were generally no significant repetition effects in the older participants. The one exception was in the earliest time window for standards (30–100 ms), where repetition effects were observed in the older participants, but only after many (22 or 23) standards in a row. At the point repetition effects were first evident in the older participants, repetition effects in the young group had stopped.
The present findings suggest a distinction between sensory memory driving the presence of an MMN/P3a potential, and dynamic updating of these and other potentials over trials. When the MMN was calculated using standard methods by averaging across all trials and subtracting deviants from standards, the overall MMN amplitude was about the same in both age groups. Thus, the general lack of repetition effects in the older group is not due to an inability to generate robust potentials. Rather, age differences in repetition effects seem to be specific to the dynamic updating and maintenance of MMN related representations across trials (review, Irvine, 2018). Prior work showing age differences in mismatch negativity amplitude elicited by patterns across trials may also be relevant to selection history (Alain et al., 1999).
Auditory ERP repetition effects have been corroborated by several MEG studies (Todorvic et al., 2011; Aine et al., 2005; fMRI: Grady et al., 2011), and some report age differences in neural adaptation (Leung et al., 2013; Grady et al., 2011). Repetition effects and normal aging have been explored in task based priming experiments using 3 or 4 repetitions (Aine et al., 2005; Grady et al., 2011), or in passive listening tasks with two (Golob et al., 2001) or four (Leung et al., 2013) stimuli in a row. The current study focused on delineating the effects of longer repeats on automatic attention control mechanisms, with a wide range of repetition lengths.
In order to have a sufficient number of trials for deviant averages, sequence effects were not measured for each possible sequence position. Standards were measured after two standards in a row after a deviant, then 6, which then doubled to 12 and then nearly doubled again to 22 or 23 standards in a row. Future work would be needed to map in between the measured sequences here, and to determine if repetition effects are present in older participants for sequences longer than 22 or 23 standards. Note that earlier studies have reported age-related attenuation in amplitudes for N100 and P200 sensory potentials (Anderer et al., 1996), which have been thought to indicate age-related inhibitory decline (Boutros et al., 2000). Prior studies have shown attenuated N1 and P2 amplitudes after stimulus repetition, which are thought to indicate impaired inhibitory control (Näätänen & Picton, 1987; Boutros, 2000; Fuerst et al., 2007).
Repetition effects at the midline frontal site to deviants have been observed in a passive oddball condition (Heinemann et al., 2011), but there have been mixed findings under passive roving conditions (Recasens et al., 2015 vs. Haenschel et al., 2005; Cooper et al., 2013; Baldeweg et al., 2004). Cooper et al (2013) did not find repetition effects in deviants using sequences up to 16 repeats. In contrast, a study with a similar design except that repeat lengths ranged from 16 to 36, did find significant repetition effect in the deviants (Baldeweg et al., 2004), suggesting that perhaps a minimum number of repeats were needed. Despite the fact that there is no satisfactory explanation for Cooper’s findings, one could summarize that the absence of repetition in deviants during a passive roving task could be attributed to: 1) the fast turnover of the deviant features, and the cumulative effect of deviation in several features during a roving condition, or 2) the need for a minimum length of stimulus repeats preceding the deviant to extract repetition effects.
Age differences in repetition effects for subtraction waveforms (MMN, P3a)
The MMN repetition effects were significant in the younger participants in this study, and corroborate earlier study findings (Nowak et al., 2016; Costa-Faidella, 2011; Horvath et al., 2008; Haenschel et al., 2005). As with the separate measures of standard and deviant ERPs, MMN repetition effects were absent in older participants. In contrast, P3a repetition effects were observed in both age groups for the frequency domain. Note that repetition effects were not present in either group when analyzing the deviants, which are classically thought to elicit the P3a (Friedman et al., 2001). Nonetheless, in the subtraction waveform there were significant repetition effects reflecting the contrast between standard and deviant stimulus processing, at least from 100–130 ms.
Although it is counterintuitive, we speculate that the strength of sensory memory, as indicated by the MMN, might initially be stronger but static over trials in older participants. In younger adults, sensory memory may strengthen, and then exceed the level of older participants after enough repetitions. This would explain why there were no overall differences in MMN amplitude. It is possible that strengthening of memory traces during repetition suppression in the standards (young & old), and repetition enhancement in the deviants (young only), might also contribute to lowering the threshold for attention capture (P3a repetition effects) in young vs. older participants.
The lack of age differences in overall MMN amplitude is consistent with other reports (review by Cooray, 2014; Kisley, 2005), yet other studies have observed smaller MMN amplitudes and longer latencies in older vs. younger adults (Cheng et al., 2013; Ruzzoli et al., 2012; Gaeta et al., 1998). The mixed age-related findings in MMN are likely due to a wide variety of experimental designs, analysis techniques (Peter et al., 2010; Jacobsen & Schröger, 2003), and methods to control for age-related hearing deficits (Cheng et al., 2013). Finding comparable overall MMN amplitudes in young and older adults seems to be most likely when the sounds are delivered rapidly, and there are obvious differences in stimulus features, such as pitches differing by an octave or far left/right locations, among standards and deviants.
It is worth noting that even though the MMN measured from the subtraction waveform had no overall age differences here, there were large amplitude differences among age groups when the standard and deviant ERPs were examined separately. Hence, examining standard and deviant waveforms separately may help to better understand any mixed results regarding age effects in subtraction waveforms.
The P3a follows the MMN in passive oddball paradigms. In the present study the pattern of results was opposite to the MMN. While there was an overall age difference for the P3a amplitude and a modest trend for a repetition effect, these factors did not significantly interact. These age differences in overall P3a amplitude have been interpreted to reflect age-related reductions in the automatic orientation of attention (Nowak et al., 2016; Walhovd & Fjell, 2001). On the one hand, these results favor potential differences in automatic orienting, as the standard and deviant tones were readily distinguishable by pitch (one octave) and location (left or right ears). On the other hand, the age-related preservation of repetition suppression and enhancement effects in the older participants suggest that repetition effects might be influencing attention capture and need to be tested further.
The repetition effects in the MMN, are termed, “MMN memory trace effects” and are related to auditory sensory memory trace formation (Baldeweg et al., 2004). The MMN has classically been interpreted as indexing a comparison between the current stimulus and a memory template of recent stimuli (Näätänen et al., 2005; Alain et al., 1998). More recently, within the predictive coding framework, the MMN has been proposed to not only index recent sensory experience but also engender a prediction about upcoming stimuli (Friston, 2005). The absence of MMN repetition effects in older participants, suggests a crucial role of frontal cortex in mediating the repetition effects through selection history, during information processing after encoding, which further suggest building a context for the incoming background sounds. This assertion could be tested in future work by using the absent MMN repetition effects in the older group to distinguish template vs. predictive coding models for MMN and P3a functions (Schröger et al. 2015; Carbajal & Malmierca, 2018).
Recent studies of macaque cortical activity show that ascending and descending neural tracts might have different spectral information. For example, synchronization between cortical areas within the gamma band was related to ascending connections from hierarchically lower (sensory) to higher (frontal) regions (Bosman et al., 2012), while descending connections were related to inter-areal synchronization in the beta band (Bastos et al., 2015). Further exploration could elucidate mid-latency components (~10–50 ms), that were not directly observed in the ERP measures (see review Grimm et al., 2016), and other oscillatory components such as theta (Hsiao et al., 2009) or gamma band network dynamics (Nicol et al., 2011), particularly to examine interactions between auditory and prefrontal cortical generators. This would help clarify the exact timing of involvement of various regions in auditory information processing and selection history, which remains a bit vague as of now.
Conclusions
In summary, the findings support the idea that automatic auditory processing that is guided by selection history, is largely absent in the older participants. We speculate that this might be due to age differences in the interplay of auditory and inferior prefrontal circuits thought to generate the mismatch negativity and related responses. Our results from separately examining repetition effects in standards and deviants, along with the classical MMN/P3a difference wave, show that subtraction can obscure cortical dynamics underlying age differences. We suggest that repetition effects and their temporal dynamics not only explain some of the mixed results in aging and MMN/P3a, but also provide a noninvasive way to examine neural correlates of auditory sensory memory in the aging brain. The observed dynamics can be used in the future as a non-invasive way to probe interactions between sensory and association cortices, and in examining the functional implications of networks between regions with large age-related decline and their interactions with areas with little morphological differences during normal aging.
Supplementary Material
Acknowledgments
This research was supported by NIH grants P20GM103629 and R01DC014736
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Both authors report no conflict of interest.
References
- 1.Addleman DA and Jiang YV, 2019. The influence of selection history on auditory spatial attention. Journal of experimental psychology: human perception and performance, 45,474–488. 10.1037/xhp0000620 [DOI] [PubMed] [Google Scholar]
- 2.Alain C, Woods DL, Knight RT, 1998. A distributed cortical network for auditory sensory memory in humans. Brain Res. 812, 23–37. 10.1016/S0006-8993(98)00851-8 [DOI] [PubMed] [Google Scholar]
- 3.Aine CJ, Adair JC, Knoefel JE, Hudson D, Qualls C, Kovacevic S, Woodruff CC, Cobb W, Padilla D, Lee RR, Stephen JM 2005. Temporal dynamics of age-related differences in auditory incidental verbal learning, Cognitive Brain Research. 24,1–18. 10.1016/j.cogbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Alain C, Hargrave R, & Woods DL, 1998. Processing of auditory stimuli during visual attention in patients with schizophrenia. Biological Psychiatry, 44, 1151–1159. [DOI] [PubMed] [Google Scholar]
- 5.Alain C, Cortese F and Picton TW, 1999. Event-related brain activity associated with auditory pattern processing. Neuroreport, 10, 2429–2434. [DOI] [PubMed] [Google Scholar]
- 6.Alho K, Woods DL, Algazi A, Knight RT, & Näätänen R 1994. Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalography and clinical neurophysiology, 91, 353–362. [DOI] [PubMed] [Google Scholar]
- 7.Anderer P, Semlitsch HV, Saletu B 1996. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalogr. Clin. Neurophysiol, 99, 458–472. [DOI] [PubMed] [Google Scholar]
- 8.Azuma M, Suzuki H, 1984. Properties and distribution of auditory neurons in the dorsolateral prefrontal cortex of the alert monkey. Brain Res. 298, 343–346. 10.1016/0006-8993(84)91434-3 [DOI] [PubMed] [Google Scholar]
- 9.Awh E, Belopolsky AV, Theeuwes J, 2012. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn. Sci 16, 437–443. 10.1016/j.tics.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auksztulewicz R, & Friston K, 2016. Repetition suppression and its contextual determinants in predictive coding. Cortex, 80, 125–140. 10.1016/j.cortex.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldeweg T, Klugman A, Gruzelier J, Hirsch SR, 2004. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr. Res 69, 203–217. 10.1016/j.schres.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 12.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, DeWeerd P, Kennedy H, Fries P, 2015. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85, 390–401. 10.1016/j.neuron.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 13.Bennett IJ, Golob EJ, Parker ES, Starr A, 2006. Memory Evaluation in Mild Cognitive Impairment using Recall and Recognition Tests. J. Clin. Exp. Neuropsychol 28, 1408–1422. 10.1080/13803390500409583 [DOI] [PubMed] [Google Scholar]
- 14.Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Howard DV, 2011. White matter integrity correlates of implicit sequence learning in healthy aging. NBA 32, 2317.e1–2317.e12. 10.1016/j.neurobiolaging.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P, 2012. Attentional Stimulus Selection through Selective Synchronization between Monkey Visual Areas. Neuron 75, 875–888. 10.1016/j.neuron.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutros NN, Carrington RM, Petrakis I, Campbell D, Torello M, Krystal J 2000Similarities in the disturbances in cortical information processing in alcoholism and aging: A pilot evoked potential study. Internat Psychogeriat.12, 513–525. [DOI] [PubMed] [Google Scholar]
- 17.Carbajal GV, Malmierca MS, 2018. The Neuronal Basis of Predictive Coding Along the Auditory Pathway: From the Subcortical Roots to Cortical Deviance Detection. Trends Hear. 22, 1–33. 10.1177/2331216518784822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CH, Hsu WY, Lin YY, 2013. Effects of physiological aging on mismatch negativity: A meta-analysis. Int. J. Psychophysiol 90, 165–171. 10.1016/j.ijpsycho.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 19.Cooper RJ, Atkinson RJ, Clark RA, Michie PT, 2013. Event-related potentials reveal modelling of auditory repetition in the brain. Int. J. Psychophysiol 88, 74–81. 10.1016/j.ijpsycho.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Cooray G, Garrido MI, Hyllienmark L, Brismar T, 2014. A mechanistic model of mismatch negativity in the ageing brain. Clin. Neurophysiol 125, 1774–1782. 10.1016/j.clinph.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 21.Costa-Faidella J, Baldeweg T, Grimm S, Escera C, 2011. Interactions between “What” and “When” in the Auditory System: Temporal Predictability Enhances Repetition Suppression. J. Neurosci 31, 18590–18597. 10.1523/JNEUROSCI.2599-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronbach LJ, Furby L, 1970. How we should measure “change”- or should we? Psychol. Bull 74, 68–80. [Google Scholar]; Curran T, 1997. Effects of aging on implicit sequence learning: Accounting for sequence structure and explicit knowledge. Psychol. Res 60, 24–41. [DOI] [PubMed] [Google Scholar]
- 23.Deouell LY, 2007. The frontal generator of the mismatch negativity revisited. J. Psychophysiol 21, 188–203. 10.1027/0269-8803.21.34.188 [DOI] [Google Scholar]
- 24.Escera C, Alho K, Schröger E, Winkler IW, 2000. Involuntary Attention and Distractibility as Evaluated with Event-Related Brain Potentials. Audiol. Neurotol 5, 151–166. 10.1159/000013877 [DOI] [PubMed] [Google Scholar]
- 25.Flood DG, Coleman PD, 1988. Neuron numbers and sizes in aging brain: Comparisons of human, monkey, and rodent data. Neurobiol. Aging 9, 453–463. 10.1016/S0197-4580(88)80098-8 [DOI] [PubMed] [Google Scholar]
- 26.Friedman D, Cycowicz YM, Gaeta H, 2001. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev 25, 355–373. 10.1016/S0149-7634(01)00019-7 [DOI] [PubMed] [Google Scholar]
- 27.Friedman D, Kazmerski VA, Cycowicz YM, 1998. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology 35, 508–520. 10.1017/S00485772989706 [DOI] [PubMed] [Google Scholar]
- 28.Friston K, 2005. A theory of cortical responses. Philos. Trans. R. Soc. B Biol. Sci 360, 815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuerst DR, Gallinat J and Boutros NN 2007. Range of sensory gating values and test-retest reliability in normal subjects. Psychophysiology. 44, 620–626. doi: 10.1111/j.1469-8986.2007.00524.x [DOI] [PubMed] [Google Scholar]
- 30.Gaeta H, Friedman D, Ritter W, Cheng J, 1998. An event-related potential study of age-related changes in sensitivity to stimulus deviance. Neurobiol. Aging 19, 447–459. 10.1016/S0197-4580(98)00087-6 [DOI] [PubMed] [Google Scholar]
- 31.Golob E, Starr a, 2000. Effects of stimulus sequence on event-related potentials and reaction time during target detection in Alzheimer’s disease. Clin. Neurophysiol 111, 1438–1449. 10.1016/S1388-2457(00)00332-1 [DOI] [PubMed] [Google Scholar]
- 32.Golob EJ, Miranda GG, Johnson JK, Starr A, 2001. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging 22, 755–763. 10.1016/S0197-4580(01)00244-5 [DOI] [PubMed] [Google Scholar]
- 33.Golob EJ, Medina LD, Bright S, Ringman JM, Starr A, Schaffer B, Irimajiri R, 2009. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology 73, 1649–1655. 10.1212/wnl.0b013e3181c1de77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratton G, Coles MG, and Donchin E 1983. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol 55, 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- 35.Grady CL, Charlton R, He Y, Alain C 2011. Age differences in fMRI adaptation for sound identity and location. Front. Hum. Neurosci 2011;5:24 10.3389/fnhum.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm S, Escera C, Nelken I, 2016. Early indices of deviance detection in humans and animal models. Biol. Psychol 116, 23–27. 10.1016/j.biopsycho.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 37.Grill-Spector K, Henson R, & Martin A, 2006. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences, 10(1), 14–23. 10.1016/j.tics.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 38.Haenschel C, Vernon DJ, Dwivedi P, Gruzelier JH, Baldeweg T, 2005. Event-related brain potential correlates of human auditory sensory memory-trace formation. J. Neurosci 25, 10494–501. 10.1523/JNEUROSCI.1227-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halgren E, Baudena P, Clarke JM, Heit G, Liégeois C, Chauvel P, Musolino A, 1995. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr. Clin. Neurophysiol 94, 191–220. 10.1016/0013-4694(94)00259-N [DOI] [PubMed] [Google Scholar]
- 40.Halgren E, Marinkovic K, Chauvel P, 1998. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol 106, 156–164. 10.1016/S0013-4694(97)00119-3 [DOI] [PubMed] [Google Scholar]
- 41.Heinemann LV, Kaiser J, Altmann CF, 2011. Auditory repetition enhancement at short interstimulus intervals for frequency-modulated tones. Brain Res. 1411, 65–75. 10.1016/j.brainres.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 42.Hamm VP, Lynn H, 1992. Age and the availability of Inferences. Psychol. Aging 7, 56–64. [DOI] [PubMed] [Google Scholar]
- 43.Horváth J, Winkler I, Bendixen A, 2008. Do N1/MMN, P3a, and RON form a strongly coupled chain reflecting the three stages of auditory distraction? Biol. Psychol 79, 139–147. 10.1016/j.biopsycho.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 44.Howard JH, Howard DV, 2013. Aging mind and brain: is implicit learning spared in healthy aging? Front. Psychol 4, 1–6. 10.3389/fpsyg.2013.00817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiao F-J, Wu ZA, Ho L-T, Lin Y-Y, 2009. Theta oscillation during auditory change detection: A MEG study. Biol. Psychol 81, 58–66. 10.1016/j.biopsycho.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 46.Ikier S, Yang L, and Hasher L, 2008. Implicit proactive interference, age, and automatic versus controlled retrieval strategies. Psychol. Sci 19, 456–461. 10.1111/j.1467-9280.2008.02109.xIrvine, [DOI] [PubMed] [Google Scholar]; D.R.F., 2018. Plasticity in the auditory system. Hear. Res 362, 61–73. 10.1016/j.heares.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 47.Jääskeläinen IP, Pekkonen E, Hirvonen J, Sillanaukee P, Näätänen R, 1996. Mismatch negativity subcomponents and ethyl alcohol. Biol. Psychol 43, 13–25. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen T, Schröger E, 2003. Measuring duration mismatch negativity. Clin. Neurophysiol 114, 1133–1143. 10.1016/S1388-2457(03)00043-9 [DOI] [PubMed] [Google Scholar]
- 49.Javit DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, & Arezzo JC 1994. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain research, 667, 192–200. [DOI] [PubMed] [Google Scholar]
- 50.Kisley MA, Davalos DB, Engleman LL, Guinther PM, Davis HP, 2005. Age-related change in neural processing of time-dependent stimulus features. Cogn. Brain Res 25, 913–925. 10.1016/j.cogbrainres.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 51.Kramer, Arthur F; Madden DJ, 2007. Chapter 5: Attention, in: Craik, Fergus IM; Salthouse TA (Ed), The Handbook of Aging and Cognition: Third Edition. Taylor & Francis, pp. 189–249. [Google Scholar]
- 52.Leung AWS, He Y, Grady CL, Alain C 2013. Age Differences in the Neuroelectric Adaptation to Meaningful Sounds. PLoS ONE 8, e68892 10.1371/journal.pone.0068892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Miller EK, & Desimone R,1993. The representation of stimulus familiarity in anterior inferior temporal cortex. J. Neurophysio 69(6), 1918–29. 10.1152/jn.1993.69.6.1918 [DOI] [PubMed] [Google Scholar]
- 54.Mauritis NM, Elting JW, Jager DKRB, van der Hoeven JH, Brouwer WH, 2005. P300 component identification in auditory oddball and novel paradigms using source analysis techniques: reduced latency variability in the elderly. J. Clin. Neurophysiol 22, 166–75. 10.1097/00004691-200302000-00003 [DOI] [PubMed] [Google Scholar]
- 55.Mock JR, Foundas AL, Golob EJ, 2015. Speech preparation in adults with persistent developmental stuttering. Brain Lang. 149, 97–105. 10.1016/j.bandl.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller EK, Desimone R, 2019. Parallel Neuronal Mechanisms for Short-Term Memory. Science. 263, 520–522. 10.1126/science.8290960 [DOI] [PubMed] [Google Scholar]
- 57.Moray N, 1959. Attention in dichotic listening: Affective cues and the influence of instructions. Q. J. Exp. Psychol 56–60. 10.1080/1747021590841628 [DOI] [Google Scholar]
- 58.Näätänen R, Paavilainen P, Rinne T, Alho K, 2007. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol 118, 2544–90. 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 59.Näätänen R, Jacobsen T, Winkler I, 2005. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology 42, 25–32. 10.1111/j.1469-8986.2005.00256.x [DOI] [PubMed] [Google Scholar]
- 60.Näätänen R Gaillard AWK, Mantysalo S, 1978. Early Selective-Attention Effect on Evoked Potential Reinterpreted. Acta Psychol. 42, 313–329. [DOI] [PubMed] [Google Scholar]
- 61.Nicol RM, Chapman SC, Vértes PE, Nathan PJ, Smith ML, Shtyrov Y, Bullmore ET, 2011. Fast reconfiguration of high-frequency brain networks in response to surprising changes in auditory input. J. Neurophysiol 107, 1421–1430. 10.1152/jn.00817.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak K, Oron A, Szymaszek A, Leminen M, Näätänen R, Szelag E, 2016. Electrophysiological Indicators of the Age-Related Deterioration in the Sensitivity to Auditory Duration Deviance. Front. Aging Neurosci 8, 1–10. 10.3389/fnagi.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pekkonen E, Rinne T, Reinikainen K, Kujala T, Alho K & Naatanen R 1996. Aging Effects on Auditory Processing: An Event-Related Potential Study, Experimental Aging Research, 22:2, 171–184, 10.1080/03610739608254005 [DOI] [PubMed] [Google Scholar]
- 64.Peter V, McArthur G, Thompson WF, 2010. Effect of deviance direction and calculation method on duration and frequency mismatch negativity (MMN). Neurosci. Lett 482, 71–75. 10.1016/j.neulet.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 65.Polich J, 2007. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol 118, 2128–48. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raz N; Gunning Faith M., Head Denise, Dupuis James H, McQuain John, Briggs Susan D., Loken Wendy J., T. AE and A. JD, 1997. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb. Cortex 7, 268–282. 10.1093/cercor/7.3.268 [DOI] [PubMed] [Google Scholar]
- 67.Recasens M, Leung S, Grimm S, Nowak R, Escera C, 2015. Repetition suppression and repetition enhancement underlie auditory memory-trace formation in the human brain: A MEG study. Neuroimage 108, 75–86. 10.1016/j.neuroimage.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 68.Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R, 2000. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage 12, 14–19. 10.1006/nimg.2000.0591 [DOI] [PubMed] [Google Scholar]
- 69.Romanski LM; Tian B; Fritz J; Mishkin M; Goldman-Rakic PS, and, Rauschecker JP, 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci 2, 1131–1136. 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruzzoli M, Pirulli C, Brignani D, Maioli C, Miniussi C, 2012. Sensory memory during physiological aging indexed by mismatch negativity (MMN). Neurobiol. Aging 33, 625.e21–625.e30. 10.1016/j.neurobiolaging.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 71.Sallinen M, Lyytinen H, 1997. Mismatch Negativity during objective and subjective sleepiness. Psychophysiology 34, 694–702. [DOI] [PubMed] [Google Scholar]
- 72.Schröger E, Marzecová A, Sanmiguel I, 2015. Attention and prediction in human audition: A lesson from cognitive psychophysiology. Eur. J. Neurosci 41, 641–664. 10.1111/ejn.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, 2003. Mapping cortical change across the human life span. Nat. Neurosci 6, 309–315. 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- 74.Theeuwes J, 2019. Goal-driven, stimulus-driven, and history driven selection. ScienceDirect. Curr. Opin. Psychol 29, 97–101. 10.1016/j.copsyc.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 75.Tavakoli P, Campbell K, 2016. Can an auditory multi-feature optimal paradigm be used for the study of processes associated with attention capture in passive listeners? Brain Res. 1648, 394–408. 10.1016/j.brainres.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 76.Todorovic A, van Ede F, Maris E, de Lange FP, 2011. Prior Expectation Mediates Neural Adaptation to Repeated Sounds in the Auditory Cortex: A MEG Study. Journal of Neuroscience 31 (25), 9118–23. 10.1523/JNEUROSCI.1425-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walhovd KB, Fjell AM, 2001. Two- and three-stimuli auditory oddball ERP tasks and neuropsychological measures in aging. Neuroreport 12, 3149–3153. 10.1097/00001756-200110080-00033 [DOI] [PubMed] [Google Scholar]
- 78.Wronka E, Kaiser J, Coenen AML, 2008. The auditory P3 from passive and active three-stimulus oddball paradigm. Acta Neurobiol. Exp. (Wars). 68, 362–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.