Abstract
INTRODUCTION
Transplant of fetal ventral mesencephalic tissue into the striatum of Parkinson’s disease (PD) patients has been performed to increase dopamine production and stimulate neuronal regeneration. Analysis of fetal graft tissue at autopsy has demonstrated 6 cases of α-synuclein pathology in PD patients, one case with both α-synuclein and tau pathology in a PD patient, and two cases of tau pathology within a Huntington’s Disease patient.
METHODS
A 49 year old man with PD underwent bilateral fetal ventral mesencephalic cell transplants into the striatum. Autopsy at age 70 included immunohistochemical staining of host and graft tissue with antibodies to phosphorylated α-synuclein and phosphorylated tau protein.
RESULTS
Autopsy confirmed the diagnosis of PD. Immunohistochemical staining of graft tissue demonstrated frequent neuronal perikaryal inclusions of phosphorylated α -synuclein and tau in the left graft only.
CONCLUSION
Speculations on the formation of pathology include: 1) α-synuclein and tau pathology spread from host to the graft in a neuron-neuron manner. 2) The nature of the fetal cells themselves, or transplantation process, may render fetal tissue more susceptible to the spontaneous generation of pathology. 3) Factors within host environment caused native tau and α-synuclein in fetal tissue graft to become phosphorylated.
Keywords: 1) Parkinson’s Disease, 2) Fetal Tissue Transplantation, 3) Alpha-synuclein, 4) Tau, 5) Lewy bodies
Introduction
The intracerebral transplant of fetal ventral mesencephalic tissue in patients with Parkinson’s disease (PD) was intended to increase neuronal growth and dopamine production. Clinical outcomes were mixed. While some showed symptomatic benefits, others progressed to uncontrolled dyskinesias [1–7]. Autopsy data from six transplanted PD patients revealed α-synuclein pathology within fetal graft tissue [1–6]. Additionally, one recent paper showed both α-synuclein and tau pathology within the fetal graft of one patient with PD and tau pathology alone in two patients with Huntington’s disease (HD) [7]. The purpose of this study is to report a second case of both α-synuclein and tau pathology in a fetal graft tissue at time of autopsy in a patient with PD.
Materials and Methods
A male with PD and REM sleep behavior disorder was followed in the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) until death and autopsy. He had signed written informed consent approved by the Banner Sun Health Institutional Review Board allowing both clinical assessments during life and several options for brain and/or bodily organ donation after death. PD symptoms began at age 45, predominantly left arm bradykinesia and eventually rest tremor, rigidity, and postural instability. Dopaminergic treatment was beneficial, but he quickly developed severe motor fluctuations and dyskinesias. At age 49 he underwent bilateral fetal ventral mesencephalic cell transplants into the striatum. He had no clinical benefit or side effects. He continued to progress, developed dementia at age 67, and passed away at age 70.
Fetal tissue was obtained using recommendations by the National Institute of Health and included informed consent by mother following elective abortion between 6 to 8 weeks gestation [8]. A 2 × 4 × 2 mm pieces of the rostral half of the ventral mesencephalon was removed, further dissected, and treated with antibiotics prior to transplantation. After transplant, all patients received immunosuppression with cyclosporin-A [8].
Complete pathological examination was performed using standard AZSAND [9, 10]. Lentiform nuclei collected at autopsy was fixed in neutral-buffered formalin and embedded in paraffin. All sections were stained with hematoxylin and eosin and primary antibodies against α-synuclein phosphorylated at serine 12 (p-α-synuclein; noncommercial antibody), Tyrosine hydroxylase (TH; Sigma catalog # T2928); phosphorylated tau protein (clone AT8; Thermo Scientist catalog # MN1020), TDP-43 phosphorylatedat residues 409 and 410 (pTDP-43; noncommercial antibody), MAP2 (EPR1969, abcam catalog #83830) and GFAP (Chemicon; catalog #AB5804). Primary antibody concentrations were 1:10,000 for p-synuclein and pTDP-43; 1: 3,000 for TH, GFAP and MAP3 and 1:1,000 for AT8. Immunohistochemical procedures were identical for all three methods, except for differing epitope exposure: 20 minutes proteinase K pretreatment for p-synuclein; 20 minutes in boiling citrate buffer for TH and TDP43, boiling in EDTA for MAP2, formic acid for AT8 and GFAP.
Results
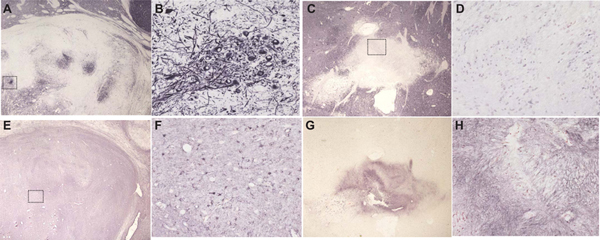
At autopsy, the diagnosis of PD was confirmed at Stage IV (neocortical stage) by the Unified Staging System for Lewy Body Disorders [11]. The brain weight was 1345 grams. Gross external examination showed mild cerebral gyral atrophy. Cerebral slices showed mild enlargement of the lateral ventricles. The lentiform nuclei were firm, whitish-tan nodules, 1.2 × 0.7 × 0.7 cm on the left and 0.3 × 0.3 × 0.3 cm on the right. The left nodule was centered on the globus pallidus external and extended slightly into the putamen. The right-sided nodule was centered within the ventral one-third of the putamen. The amygdala, the head and body of the hippocampus, and the parahippocampal gyrus showed no atrophy. The substantia nigra showed moderate depigmentation bilaterally. The brainstem and cerebellum were otherwise unremarkable.
Examination of the abnormal tissues with H & E stain showed the nodules as sharply demarcated from adjacent normal neuropil by circumferential zones of hypocellular fibrillary astrocytosis. The nodules contained disorganized neural tissue composed primarily of astrocytes and medium-sized multipolar neurons. The right nodule had fewer neurons and larger zones of fibrillary astrocytic tissue. Both nodules had scattered and focally frequent microcalcifications. There were no mitotic figures, endothelial proliferation, or areas of necrosis. The astrocytic nuclei were not hyperchromatic, enlarged or irregularly shaped.
Within the left nodule, multiple neurons contained eosinophilic perikaryal inclusions consistent with Lewy bodies, while others contained neurofibrillary tangles. Immunohistochemical staining of the abnormal striatal nodules for neurons with an antibody to MAP2 (EPR1969, abcam; catalog number ab 183830) showed abundant neuronal perikarya and fibers only within the left nodule (figure 1; A, B). These were absent on the right (figure 1; C, D). The left nodule had abundant GFAP-immunoreactive (Chemicon; catalog #AB5804) astrocytic cell bodies and fibers (figure 1; E, F). The right nodule showed positive fibers, consistent with a chronic astrocytic scar (figure 1; G, H).
Figure 1.
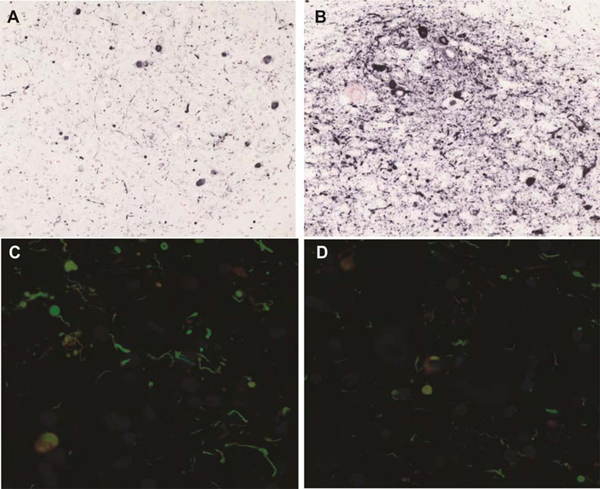
Stains for neurons (MAP2) and astrocytes (GFAP). Immunohistochemical staining of the abnormal striatal nodules for neurons with an antibody to MAP2 showed abundant neuronal perikarya and fibers only within the left nodule (A, B). These were absent on the right nodule(C, D). The left nodule had abundant GFAP-immunoreactive astrocytic cell bodies and fibers. The right nodule showed positive fibers, consistent with a chronic astrocytic scar (G, H).
(A) MAP2 (2x) of left nodule (B) MAP2 (20x) on left nodule (C) MAP2 (2x) of right nodule (D) MAP2 (20x) on right nodule (E) GFAP (2x) of left nodule (F) GFAP (20x) on left nodule (G) GFAP (2x) of right nodule (H) GFAP (20x) on right nodule
Staining for phosphorylated α-synuclein (antibody courtesy of Dr. H. Akiyama) and phosphorylated tau protein (AT8, Biolegend) showed, with both stains but only in the left-sided nodule, dense neuropil immunoreactivity in the form of puncta and fibers, and frequent neuronal perikaryal inclusions (figure 2). Some fibers were tortuous and focally enlarged. Tyrosine hydroxylase (TH) positive neuronal cell bodies and fibers were present in the left graft tissue, but not the right graft. TH positive fibers were also present in the host tissue surrounding the left graft nodule. Additionally in the left nodule there were rare pigmented neurons, approximately 1% or less of all neurons seen. A few pigmented neruons were positively stained for TH. Although colocalization of neuromelanin with phosphorylated tau and phosphorylated α-synuclein was not observed, due to very dark neuronal perikaryal staining for these, it is not possible to rule out colocalization.
Figure 2:
Stains for phosphorylated α-synuclein (α-syn) and phosphorylated tau (AT8).
(A) AT8 (20x) on left nodule (B) α-syn(20x) on left nodule (C) AT8 and α-syn double label on left (40x) (D) AT8 and α-syn double label on left
The Gallyas and thioflavin S stains showed sparse neurons with neurofibrillary tangles within the left nodule only. Staining for phosphorylated TDP-43 protein (antibody courtesy of Dr. H. Akiyama: Hasegawa M 2008) was negative in both nodules. Sparse fibers immunoreactive for phosphorylated α-synuclein and phosphorylated tau were seen in the host tissue surrounding the graft nodules, but at much lower densities than the left-sided graft. Around the right graft there were virtually no neurons (staining negative for MAP2 and GFAP). Host innervation of the right graft tissue was not apparent.
The remainder of the microscopic brain examination showed, in sections stained with Gallyas, Campbell-Switzer, and thioflavin S methods, focal concentrations of neocortical senile plaques, reaching moderate and frequent densities only in the inferomedial temporal lobe. The plaques were mostly of the diffuse type with only the temporal lobe containing focally sparse numbers of neuritic plaques. There were no plaques in the putamen, substantia nigra and cerebellar cortex.
Neurofibrillary tangles were rare or absent from all neocortical regions except the inferomesial temporal lobe, where they attained moderate densities in the fusiform and inferior temporal gyri. Tangle density ranged from moderate in the amygdala, frequent in the entorhinal area and hippocampal CA1 region, and rare in the putamen. The NIA-AA AD Neuropathological Change level was A1B2C1 “Low”[12]. Within the substantia nigra were focally moderate densities of neurofibrillary tangles and scattered oligodendroglial coiled bodies and frequent Lewy bodies and neurites. Phosphorylated alpha-synuclein staining was found in the olfactory bulb, brainstem, amygdala, and cingulate gyrus, with sparse to moderate densities in the cerebral neocortex, reaching frequent numbers only in the temporal lobe.
Discussion
Previously, six PD patients 12–24 years post-transplant were found to have phosphorylated α -synuclein within the fetal graft tissue at autopsy [1–6]. There is one case of a PD patient 16 years post-transplant with both phosphorylated tau and α-synuclein pathology and two HD patients 9–14 years post-transplant with phosphorylated tau pathology within the fetal graft tissues at autopsy [7]. We present a case of a fetal graft that has both phosphorylated α-synuclein and phosphorylated tau pathology at autopsy 21 years post-transplant. There are multiple theories on the development of the pathology.
In our case, both pathologies were much denser within the graft when compared to adjacent host tissue despite an equal density of neurons. One case report of a patient who received bilateral transplants four years apart revealed that the older graft had denser α-synuclein pathology with more “mature” Lewy bodies [2]. The transplanted fetal tissue, or the process of transplantation which is associated with trauma and inflammation, may make grafts more vulnerable to the spontaneous generation of pathology, or to more vigorous intracellular progression after initial seeding by host tissue [7].
The source of the pathology remains unclear. In prion disease, an abnormal protein causes normal proteins within a host to misfold, aggregate, and spread. One theory is that α-synuclein and tau pathology spread from host to graft in a neuron-neuron manner, similar to the spread of prions [7]. There are some limited models that may support this idea. In in vivo studies, human derived α-synuclein was injected into a distal location of rats with a dopamine depleted striatum that had received rat fetal ventral mesencephalic grafts [13]. Grafted cells demonstrated the human α-synuclein at autopsy [13]. In in vivo studies, when tissue from mouse brains that over produced human derived tau was injected into a wild type mouse, the human tau pathology propagated to the host mouse tissue [14]. In in vitro studies, exogenous fluorescently labeled extracellular tau aggregates were internalized and induced misfolding, and this pathologic tau was able to propagate between different cell lines [15].
Tau pathology is seen with a group of neurodegenerative diseases associated with cognitive decline. In our case, there was a nonspecific glial tauopathy in the host substantia nigra while the graft tau pathology appeared to be solely neuronal. It is important to emphasize that the fetal graft pathology in all of the cases was also in the host brain. One theory is that factors within host environment caused native tau and α-synuclein in the graft to become phosphorylated.
The independent finding of α-synuclein and tau pathology within the fetal graft tissue in our case does not support or refute any particular theory, but it does contribute to the growing body of knowledge surrounding the search for the pathogenesis of neurodegenerative disorders.
Highlights.
Both a-synuclein and tau pathology in fetal transplanted tissue in a Parkinson’s disease patient
One theory is that α-synuclein and tau pathology spread from host to graft in a neuron-neuron manner
Alternatively host environment could cause native graft proteins to become phosphorylated
Acknowledgments
Funding Sources: This study was funded by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05–901 and 1001 to the Arizona Parkinson’s Disease Consortium), the Michael J. Fox Foundation for Parkinson’s Research, and Mayo Clinic Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interset: none
References
- [1].Li W, Englund E, Widner H, Mattsson B, van Westen D, Lätt J, Rehncrona S, Brundin P, Björklund A, Lindvall O, Li J-Y, Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 6544–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li J-Y, Englund E, Widner H, Rehncrona S, Björklund A, Lindvall O, Brundin P, Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease, Mov. Disord. 25 (2010) 1091–1096. [DOI] [PubMed] [Google Scholar]
- [3].Li J-Y, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P, Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-tograft disease propagation, Nat. Med. 14 (2008) 501–503. [DOI] [PubMed] [Google Scholar]
- [4].Kordower JH, Chu Y, Hauser RA, Freeman TB, Warren Olanow C, Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease, Nat. Med. 14 (2008) 504–506. [DOI] [PubMed] [Google Scholar]
- [5].Kordower JH, Goetz CG, Chu Y, Halliday GM, Nicholson DA, Musial TF, Marmion DJ, Stoessl AJ, Sossi V, Freeman TB, Olanow CW, Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient, Ann. Neurol. 81 (2017) 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB, Transplanted dopaminergic neurons develop PD pathologic changes: a second case report, Mov. Disord. 23 (2008) 2303–2306. [DOI] [PubMed] [Google Scholar]
- [7].Cisbani G, Maxan A, Kordower JH, Planel E, Freeman TB, Cicchetti F, Presence of tau pathology within foetal neural allografts in patients with Huntington’s and Parkinson’s disease, Brain. 140 (2017) 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kopyov OV, Jaques D, Lieberman A, Duma CM, Rogers RL, Clinical Study of Fetal Mesencephalic Intracerebral Transplants for the Treatment of Parkinson’s Disease, Cell Transplantation. 5 (1996) 327–337. [DOI] [PubMed] [Google Scholar]
- [9].Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, and Rogers J The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007.2008a; 9:229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, and Sabbagh MN Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program.2015; 35:354–389.11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beach TG, the Arizona Parkinson’s Disease Consortium, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG, Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction, Acta Neuropathol. 117 (2009) 613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al. , National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012 (2011) 123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K, Collier TJ, Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat, Neurobiol. Dis. 43 (2011) 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M, Transmission and spreading of tauopathy in transgenic mouse brain, Nat. Cell Biol. 11 (2009) 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frost B, Jacks RL, Diamond MI, Propagation of tau misfolding from the outside to the inside of a cell, J. Biol. Chem. 284 (2009) 12845–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]


