Abstract
Background
Loss of muscle mass with age may be a key player in metabolic dysregulation.
Objective
To examine associations between abdominal muscle area and density with lipids and lipoproteins.
Methods
1868 adults completed health history and physical activity questionnaires, provided venous blood samples for lipids and inflammatory biomarkers, and underwent computed tomography to quantify body composition. Associations between muscle area and density with multiple lipid measures were assessed with multivariable linear and logistic regression.
Results
The mean age and body mass index of participants was 65 years and 28 kg·m2, respectively, and 50% were female. After adjustment for demographics, cardiovascular disease risk factors, lipid-lowering medications, physical activity, sedentary behavior, inflammatory biomarkers and central obesity, a 1-standard deviation (SD) increase in total abdominal, stability, and locomotor muscle areas were associated with a 13%, 11%, and 8% lower high-density lipoprotein cholesterol level, respectively (p<0.05). With similar adjustment, a 1-SD increase in total abdominal and stability muscle area was associated with a 13% and 12% lower total cholesterol level, respectively (p<0.01). Compared to the lowest quartiles of total, stability and locomotor muscle area, those in the higher quartiles of muscle area had over a 40% reduction in the odds of triglyceride levels greater than 150 mg/dl (p<0.05). Total abdominal muscle density was positively associated with total cholesterol (p<0.05) but was not associated with the other lipid outcomes.
Conclusion
Maintaining adequate skeletal muscle mass with age may decrease specific lipid levels related to hyperlipidemia and development of cardiometabolic disease.
Keywords: Muscle mass, aging, sarcopenia, dyslipidemia, body composition, myosteatosis
Introduction
Obesity, a significant and growing problem in the United States, is an important risk factor in the pathogenesis of cardiometabolic disease [1]. Much of the research investigating factors associated with an increased risk of cardiometabolic disease has focused on total body fat and central obesity, with the majority of the literature reporting a robust association between these markers and cardiometabolic disease [2,3]. Recently there has been a growing interest in understanding the role of skeletal muscle in metabolic health. In this regard, skeletal muscle is the largest insulin sensitive tissue compartment and plays an important role in glucose homeostasis [4]. Given this, the loss of muscle mass and muscle quality with age may be a key player in metabolic dysregulation and subsequent cardiometabolic disease [5,6].
Emerging research suggests age-related declines in skeletal muscle mass may be associated with significant metabolic consequences for older adults. Associations between skeletal muscle mass and metabolic syndrome [7–11], insulin resistance [7], and inflammation [12] have been reported but results are conflicting. Several investigations suggest that low levels of skeletal muscle mass increase risk of developing metabolic syndrome [7,8,11] and a metabolically-obese phenotype over time [13]. In contrast, others suggest that high levels of muscle mass are associated with elevated levels of triglycerides [9,14] and low-density lipoprotein cholesterol (LDL-c) [14], as well as development of metabolic syndrome [9,10].
Computed tomography (CT) provides a direct measure of the amount of tissue within a given volumetric slice of an anatomic location, which is denoted by tissue area (e.g., muscle area) from the 2-dimensional rendering on a computer screen [15]. Muscle area measured by CT is analogous to muscle mass/content. X-ray attenuation is a radiological characteristic that differentiates muscle tissue from fat tissue and provides a measure of muscle density [15].
Notably, muscle density, an indicator of muscle quality, may be more important than muscle area when examining metabolic alterations. Muscle quality refers to various aspects of the structure, composition, metabolic function and performance of skeletal muscle [16]. In this respect, the density of muscle derived from CT has been inversely associated with total body fat, fat infiltration of muscle, and both muscle strength and function [17,18].
Importantly, few studies have investigated the associations between muscle area with levels of several different lipoproteins and only two [10,14] have reported on muscle density and these outcomes. Given this, the purpose of the current study was to examine the associations of abdominal muscle area and density with different lipoproteins and triglyceride levels. CT scans were conducted on the abdomen for body composition assessment in this study. The abdomen was chosen to capture visceral adiposity and other measures to include muscle area and density. Abdominal muscle area and density derived from CT are strongly associated with total body muscle mass, density and voluntary strength [19–21]. Further, abdominal muscle density derived from CT attenuation values has been associated with voluntary muscle strength, independent of the muscle cross-sectional area, in older adults [17]. Thus, studying abdominal muscle area and density have implications for skeletal muscle health in older adults.
Materials and Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of adults from six regions across the US. The overall design of the MESA study has been published [22]. In brief, the cohort included a total of 6814 men and women who were aged 45–84 years and free from clinically apparent cardiovascular disease at the time of enrollment (July 2000 to August 2002). The racial/ethnic groups of participants included African American (28%), Chinese American (12%), Hispanic (22%) and non-Hispanic white (38%). Participants who were enrolled in the study returned for follow-up clinic visits approximately 2, 4, 6, and 10 years after the baseline clinic visit.
At clinic visits 2 and 3 (from 2002 to 2005), a random subset of 1970 participants were enrolled in an ancillary study where abdominal computed tomography scans were obtained and subsequently used to quantify the area and density of abdominal skeletal muscle, visceral fat, and subcutaneous fat. Approximately half of the 1970 participants had their scan at visit 2 and the other half at visit 3. To make the measurements contemporaneous, demographic, biomarker, and physical activity data obtained during visit 2 or 3 (corresponding to when the CT scan was conducted) were used in this study. The MESA studies were approved by the Institutional Review Board of each study site and all participants provided written informed consent.
Standard questionnaires were used to obtain information on participant demographics and health history. Cigarette smoking was defined as current, former, or never smoker. Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively. Body mass index, waist circumference and blood pressure were measured using standard procedures [22].
Participants self-reported their activity levels using the Typical Week Physical Activity Survey. This survey was adapted from the Cross-Cultural Activity Participation Study [23] and designed to identify the frequency of and time spent in leisure sedentary behavior and in various physical activities during a typical week in the previous month. The survey includes 28 items in categories of household chores, yard/lawn/garden/farm care, care of children/adults, transportation, walking, dancing and sport activities, conditioning activities (e.g., aerobics, cycling, jogging, rowing, and swimming), leisure activities, and occupational and volunteer activities. Where appropriate, questions differentiated between light-, moderate-, and heavy-intensity activities. Questions specific to sedentary behavior included time spent reading, sitting, watching television and recreational computer use. Survey responses were quantified into MET-minutes per week of sedentary behavior and moderate-to-vigorous physical activity defined as moderate and vigorous activities from all categories.
Laboratory
Venous blood was collected after a 12-hour fast. Samples were shipped to the MESA central laboratory (Laboratory for Clinical Biochemistry Research, University of Vermont, Burlington, VT) for measurement of total cholesterol, high-density lipoprotein cholesterol (HDL-c), LDL-c, triglycerides, glucose, and inflammatory biomarker concentrations. Total and HDL-c levels were measured using the cholesterol oxidase method (Roche Diagnostics, Indianapolis, Indiana) and triglycerides were measured using the triglyceride GB reagent (Roche Diagnostics, Indianapolis, Indiana). For triglyceride levels < 400 mg/dL, LDL-c was calculated using the Friedewald formula, while when triglycerides were > 400 mg/dl, nuclear magnetic resonance spectroscopy was used. Glucose was measured using a Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, New York). C-reactive protein (CRP), adiponectin, leptin, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and resistin concentrations were measured using Bio-Rad Luminex flow cytometry (Millipore, Billerica, MA). Average analytic coefficients of variation across several control samples ranged from 6.0% to 13.0%. We defined dyslipidemia as a total cholesterol/HDL-c ratio >5.0 or if the participant was taking medication to reduce cholesterol, while hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or taking antihypertensive medication [24], and diabetes was defined as fasting glucose ≥ 126 mg·dL−1 or use of diabetes medication [25].
Abdominal muscle measurements
Abdominal muscle, as well as visceral and subcutaneous fat, were measured from CT scans obtained at visit 2 or 3. Abdominal slices from these scans were processed using MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD) that measured fat, lean and total tissue using a semi-automated method. Fat tissue was identified as being between −190 and −30 Hounsfield units (HU), whereas lean tissue was identified as being between 0 and 100 HU. Densities between 0 and −30 HU were labeled as undefined tissue type. Six transverse cross sectional slices were analyzed at the following spine levels: 2 at L2/L3, 2 at L3/L4 and 2 at L4/L5.
Using the pixel intensities of a single slice obtained at L4/L5, and the HU criteria provided above, fat and muscle areas were calculated for the abdominal muscle groups and the subcutaneous and visceral compartments. Bilateral oblique, rectus abdominis, paraspinal and psoas muscles were defined within their unique fascial planes. These muscles were grouped into muscles of stabilization (oblique, rectus abdominis, paraspinal muscles), muscles of locomotion (psoas muscle), and total abdominal muscle (oblique, rectus abdominis, paraspinal muscles, and psoas). For each muscle, area was determined by summing the number of pixels of 0 to 100 HU within that muscle’s corresponding fascial plane. Muscle density was the average HU measurement of the pixels classified as muscle and within the muscle’s distinct fascial plane. Subcutaneous adipose tissue was defined as the fat outside of the visceral cavity, not including the fat located within the muscular fascia. Visceral fat area was computed as the sum of the pixels of the appropriate HU range within the visceral cavity.
CT imaging was interpreted by staff who were blinded to participants’ clinical information. Inter and intra-rater reliability for total abdominal, subcutaneous, and visceral cavity areas were 0.99 for all measurements. Inter and intra-rater reliability for all muscle groups ranged from 0.93 to 0.98.
Statistical Analysis
Characteristics of the population are summarized with mean and standard deviation (SD) for continuous variables and frequency and percentage of the study population for categorical variables. Skewed variables are presented as median with interquartile range. Muscle area and density are treated as continuous and categorical variables for all analyses (i.e., quartiles). Analysis of covariance was used to determine the differences in means of lipids by quartile of muscle area and density, after adjusting for age, sex, and race/ethnicity.
Mulivariable linear regression was used to determine the associations of muscle area and density (total, stability, and locomotor) with lipid levels (triglycerides; HDL-c, LDL-c, and total cholesterol). Logistic regression was used to determine the odds of having high LDL-c (>160 mg/dL), high triglyceride (>150 mg/dL) or low HDL-c (<40 mg/dL), while controlling for covariates. These cutpoints were based on the National Cholesterol Education Program ATP III guidelines [24]. The initial model (Model 1) adjusted for age, sex, race/ethnicity, diabetes, smoking, hypertension, lipid-lowering medication use, moderate-to-vigorous physical activity, sedentary behavior, height, subcutaneous abdominal fat area, visceral fat area and visceral fat density. Model 2 included Model 1 plus inflammatory markers (leptin, adiponectin, CRP, IL-6, resistin, TNFα). Model 3 included Model 2 plus muscle area or density for the specific muscle group (i.e., if the predictor variable of interest was total abdominal muscle area, total abdominal muscle density was added into the model). When HDL-c was the outcome variable, all models additionally included LDL-c and triglycerides as covariates. When LDL-c or VLDL-c was the outcome variable all models included HDL-c as a covariate and when triglycerides were the outcome variable all models included HDL-c and LDL-c as covariates.
Using multiplicative interaction terms, we tested for significant differences in the magnitude of the associations between muscle area and density with each lipid outcome by race/ethnicity, sex and BMI. There were no significant or robust differences across race/ethnicity, sex or BMI. As such, the data were unstratified for all analyses.
Among the 1970 participants, 1868 had complete data on muscle area, muscle density and all lipid outcomes. There were participants who were missing values for the covariates, resulting in an analytic sample for Model 3 of 1497 when total cholesterol was the outcome variable and 1475 when HDL-c, LDL-c, and triglycerides, respectively, were the outcome variables in regression analyses.
In a sensitivity analysis, we examined potential confounding of lipid-lowering medications by running the associations described above using only participants who were not taking lipid-lowering medications. Additionally, we conducted sensitivity analyses to determine if the associations differed for LDL-c and VLDL-c calculated using the method of Martin et al. [26]. All statistical analyses were conducted using Stata (Version 13; StataCorp, College Station, TX, USA) and a p-value of < 0.05 was used to determine statistical significance.
Results
The study cohort characteristics are presented in Table 1. Overall, the mean age of participants was 65 years and 50% were female. Forty percent of participants were non-Hispanic White, 21% were African American, 26% were Hispanic/Latino, and 13% were Chinese American. Mean BMI was 28 kg/m2. Thirty percent of participants had a BMI greater than 30 kg/m2, while approximately 38% of participants had dyslipidemia, 14% had diabetes mellitus, and almost half (48%) had hypertension.
Table 1.
Characteristics of the participants (n=1868)
| Characteristic | Mean (SD)/Percent (n) |
|---|---|
| Age (years, M [SD]) | 64.7 (9.8) |
| Female (% [n]) | 50.3 (940) |
| Ethnicity (% [n]) | |
| White | 40.0 (747) |
| Chinese American | 13.2 (247) |
| African American | 21.1 (394) |
| Hispanic | 25.7 (480) |
| Ever Smoker (% [n]) | 54.0 (1008) |
| Dyslipidemia (% [n]) | 37.6 (702) |
| Diabetes (% [n]) | 13.7 (256) |
| Hypertension (% [n]) | 46.7 (872) |
| Taking lipid lowering medications (% [n]) | 24.1 (451) |
| Body Composition | |
| BMI (M [SD]) | 28.0 (5.1) |
| Subcutaneous fat area (cm2, M [SD]) | 253.7 (117.4) |
| Visceral fat area (cm2, M [SD]) | 145.7 (67.9) |
| Abdominal muscle area (cm2, M [SD]) | 98.3 (27.6) |
| Stability muscle area (cm2, M [SD]) | 74.6 (21.8) |
| Locomotor muscle area (cm2, M [SD]) | 23.7 (7.4) |
| Abdominal muscle density (HU, M [SD]) | 42.2 (5.5) |
| Stability muscle density (HU, M [SD]) | 39.6 (6.1) |
| Locomotor muscle density (HU, M [SD]) | 50.3 (5.2) |
| Activity Levels | |
| Sedentary Behavior (min·wk−1, Mdn [IQR]) | 1470 (1455) |
| MVPA (MET-min·wk−1, Mdn [IQR]) | 3600 (4549) |
| Cardiovascular Disease Risk Factors | |
| Systolic blood pressure (mmHg, M [SD]) | 124 (21) |
| Diastolic blood pressure (mmHg, M [SD]) | 70 (10) |
| Triglycerides (mg·dL−1, M [SD]) | 126.8 (66.2) |
| Total cholesterol (mg·dL−1, M [SD]) | 189.3 (35.2) |
| High-density lipoprotein (mg·dL−1, M [SD]) | 51.8 (15.1) |
| Low-density lipoprotein (mg·dL−1, M [SD]) | 112.0 (31.3) |
| Very low-density lipoprotein (mg·dL−1, M [SD]) | 26.4 (16.4) |
| Non-high-density lipoprotein (mg·dL−1, M [SD]) | 138.0 (35.5) |
| Total to HDL cholesterol ratio | 3.9 (1.2) |
| Inflammatory Markers | |
| C-reactive protein (mg·L−1, Mdn [IQR] | 1.5 (2.6) |
| Adiponectin (μg·mL−1, Mdn [IQR]) | 17.5 (14.6) |
| Leptin (ng·mL−1, Mdn [IQR]) | 13.2 (22.5) |
| Resistin (ng·mL−1, Mdn [IQR]) | 15.0 (7.2) |
| Interleukin-6 (pg·mL−1, Mdn [IQR]) | 1.8 (1.7) |
| TNF-α (pg·mL−1, Mdn [IQR]) | 4.6 (2.9) |
M, mean; SD, standard deviation; %, percent; n, sample size; Mdn, median; IQR, interquartile range; BMI, body mass index; HU, Hounsfield units; MVPA, moderate-to-vigorous physical activity; MET, metabolic equivalents; TNF-α, tumor necrosis factor alpha. Note: SI conversion factors: To convert glucose, cholesterol, and triglycerides to mmol·L−1, multiply values by 0.0555, 0.0259, and 0.0113, respectively. To convert C-reactive protein to nmol·L−1, multiply values by 9.524.
Characteristics by quartiles of muscle area and density
Mean age was lower, and the percentage of men was higher, across increasing quartiles of total abdominal muscle area and density (p<0.001, Table 2). After adjusting for age, sex, and race/ethnicity, mean levels of HDL-c were lower across increasing quartiles of total abdominal muscle area, such that HDL-c was 22% lower in the highest compared to the lowest quartile (p<0.001). The prevalence of dyslipidemia was higher across increasing quartiles of total abdominal muscle area, such that dyslipidemia was 33% higher in the highest compared to the lowest quartile (p<0.01). Similar results were found across quartiles of stability muscle area (p<0.05) and trends were similar but not significant for locomotor muscle area (data not shown).
Table 2.
Adjusted mean characteristics by quartile of total abdominal muscle area and density.
| Total abdominal muscle area | Total abdominal muscle density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P | Q1 | Q2 | Q3 | Q4 | P | |
| Age (years) | 69.1 | 65.8 | 64.0 | 59.8 | <0.001 | 70.3 | 66.5 | 63.3 | 58.5 | <0.001 |
| Sex (% men) | 6.5 | 32.2 | 66.3 | 94.1 | <0.001 | 25.6 | 40.1 | 55.2 | 78.2 | <0.001 |
| HDL-c (mg·dL−1) a | 57.9 | 54.1 | 49.4 | 45.1 | <0.001 | 53.1 | 52.3 | 51.4 | 49.7 | <0.001 |
| LDL-c (mg·dL−1) a | 112.7 | 112.4 | 111.3 | 111.8 | 0.35 | 109.2 | 111.1 | 113.1 | 114.7 | 0.06 |
| VLDL-c (mg·dL−1) a | 24.9 | 25.6 | 26.3 | 28.8 | 0.05 | 28.6 | 26.2 | 27.1 | 23.8 | 0.001 |
| Non-HDL (mg·dL−1) a | 137.7 | 138.1 | 137.1 | 139.3 | 0.85 | 137.7 | 136.3 | 140.4 | 137.7 | 0.35 |
| TC (mg·dL−1) a | 196.2 | 192.3 | 186.8 | 183.5 | 0.05 | 189.5 | 189.9 | 190.3 | 189.2 | 0.03 |
| Triglycerides (mg·dL−1) a | 130.7 | 131.8 | 133.7 | 137.1 | 0.21 | 141.9 | 136.1 | 130.3 | 125.0 | <0.001 |
| Dyslipidemia (%) a | 33.8 | 37.0 | 41.2 | 44.8 | 0.003 | 41.6 | 39.8 | 38.5 | 37.0 | 0.11 |
Q, quartile; HDL-c, high-density lipoprotein cholesterol
adjusted for age, sex, race/ethnicity
LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol
Muscle area quartile cutpoints (cm2): Q1 < 77.02; Q2 77.02–94.66; Q3 94.67–116.84; Q4 ≥ 116.85.
Muscle density quartile cutpoints (HU): Q1 < 38.17; Q2 38.17–42.69; Q3 42.70–46.34; Q4 ≥ 46.35.
For muscle density, and after adjusting for age, sex, and race/ethnicity, mean levels of HDL-c, VLDL-c and triglycerides were lower across increasing quartiles of total abdominal muscle density (p≤0.001). More specifically, HDL-c, VLDL-c and triglycerides were 6%, 17%, and 12% lower in the highest compared to the lowest quartile of total abdominal muscle density, respectively. Similar results were found across stability and locomotor muscle density quartiles (data not shown).
Multivariable associations of muscle area and density with lipid outcomes
With adjustment for age, sex, race/ethnicity, height, diabetes, smoking, hypertension, lipid-lowering medication use, moderate-to-vigorous physical activity, sedentary behavior, subcutaneous abdominal fat area, visceral fat area and visceral fat density (Model 1), a 1-SD increase in total abdominal, stability, and locomotor muscle area was associated with an 11%, 10%, and 8% lower HDL-c, respectively (p<0.05 for all, Table 3). These associations were slightly attenuated, but remained significant, with the addition of inflammatory markers (Model 2). With the addition of muscle density as a covariate (model 3), a 1-SD increase in total abdominal, stability and locomotor muscle area was associated with a 13%, 11%, and 8% lower HDL-c, respectively (p<0.05 for all). Only locomotor muscle density was associated with HDL-c, with a 1-SD increase associated with a 5% lower HDL-c (Model 1, p=0.02). This association was attenuated with the addition of inflammatory markers (p=0.50) and locomotor muscle area (p=0.98). Total abdominal and stability muscle density were not associated with HDL-c (p>0.05).
Table 3.
Multivariable linear regression for the associations between continuous muscle area and density with lipid outcomes.
| HDL cholesterol | LDL cholesterol | VLDL cholesterol | Total cholesterol | Triglycerides | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 SD = 15.1 mg·dL−1 | 1 SD = 31.4 mg·dL−1 | 1 SD = 16.4 mg·dL−1 | 1 SD = 35.5 mg·dL−1 | 1 SD = 94.8 mg·dL−1 | ||||||
| Model 1 | B [95% CI] | St β | B [95% CI] | St β | B [95% CI] | St β | B [95% CI] | St β | B [95% CI] | St β |
| Muscle area | ||||||||||
| Total abdominal | −0.06 [−0.10, −0.03] | −0.11 | −0.04 [−0.13, 0.04] | −0.04 | −0.04 [−0.08,0.01] | −0.06 | −0.10 [−0.20, −0.005] | −0.08 | −0.05 [−0.22, 0.11] | −0.02 |
| Stability | −0.07 [−0.11, −0.03] | −0.10 | −0.07 [−0.16, 0.03] | −0.05 | −0.04 [−0.09, 0.10] | −0.05 | −0.13 [−0.24, −0.02] | −0.08 | −0.05 [−0.23, 0.14] | −0.02 |
| Locomotor | −0.16 [−0.30, −0.01] | −0.08 | 0.16 [−0.18, 0.50] | 0.04 | −0.13 [−0.29, 0.04] | −0.06 | 0.004 [−0.38, 0.39] | 0.001 | −0.26 [−0.91, 0.39] | −0.03 |
| Muscle density | ||||||||||
| Total abdominal | −0.12 [−0.28, 0.04] | −0.04 | 0.18 [−0.19, 0.55] | 0.03 | 0.07 [−0.12, 0.26] | 0.02 | 0.22 [−0.2, 0.64] | 0.03 | 0.59 [−0.12, 1.30] | 0.05 |
| Stability | −0.07 [−0.22, 0.07] | −0.03 | 0.08 [−0.26, 0.42] | 0.02 | 0.02 [−0.15, 0.19] | 0.01 | 0.12 [−0.26, 0.50] | 0.02 | 0.46 [−0.19, 1.10] | 0.04 |
| Locomotor | −0.16 [−0.31, −0.02] | −0.05 | 0.33 [−0.0003, 0.66] | 0.05 | 0.17 [0.002, 0.33] | 0.05 | 0.34 [−0.03, 0.71] | 0.05 | 0.54 [−0.09, 1.17] | 0.04 |
| Model 2 | ||||||||||
| Muscle area | ||||||||||
| Total abdominal | −0.06 [−0.09, −0.02] | −0.10 | −0.05 [−0.14, 0.03] | −0.05 | −0.04 [−0.09, 0.001] | −0.07 | −0.11 [−0.21, −0.01] | −0.09 | −0.07 [−0.24, 0.09] | −0.03 |
| Stability | −0.06 [−0.10, −0.02] | −0.08 | −0.08 [−0.18, 0.02] | −0.06 | −0.05 [−0.10, 0.005] | −0.06 | −0.15 [−0.26, −0.03] | −0.09 | −0.07 [−0.26, 0.12] | −0.02 |
| Locomotor | −0.16 [−0.30, −0.02] | −0.08 | 0.17 [−0.18, 0.52] | 0.04 | −0.13 [−0.31, 0.04] | −0.06 | 0.01 [−0.39, 0.40] | 0.001 | −0.27 [−0.94, 0.40] | −0.03 |
| Muscle density | ||||||||||
| Total abdominal | 0.02 [−0.14, 0.19] | 0.01 | 0.20 [−0.20, 0.60] | 0.04 | <0.01 [−0.20, 0.20] | <0.01 | 0.29 [−0.16, 0.74] | 0.04 | 0.31 [−0.45, 1.08] | 0.03 |
| Stability | 0.04 [−0.10, 0.19] | 0.02 | 0.08 [−0.28, 0.44] | 0.02 | −0.05 [−0.23, 0.14] | −0.02 | 0.15 [−0.25, 0.56] | 0.03 | 0.21 [−0.48, 0.90] | 0.02 |
| Locomotor | −0.05 [−0.19, 0.09] | −0.02 | 0.39 [0.04, 0.74] | 0.06 | 0.13 [−0.05, 0.31] | 0.04 | 0.45 [0.05, 0.84] | 0.06 | 0.35 [−0.32, 1.02] | 0.03 |
| Model 3 | ||||||||||
| Muscle area | ||||||||||
| Total abdominal | −0.07 [−0.11, −0.03] | −0.13 | −0.09 [−0.18, 0.01] | −0.08 | −0.05 [−0.10, −0.004] | −0.09 | −0.17 [−0.28, −0.06] | −0.13 | −0.12 [−0.31, 0.06] | −0.05 |
| Stability | −0.08 [−0.12, −0.03] | −0.11 | −0.11 [−0.21, −0.001] | −0.08 | −0.05 [−0.12, 0.008] | −0.06 | −0.20 [−0.32, −0.07] | −0.12 | −0.12 [−0.33, 0.09] | −0.04 |
| Locomotor | −0.16 [−0.31, −0.01] | −0.08 | 0.05 [−0.32, 0.42] | −0.01 | −0.20 [−0.38, −0.01] | −0.09 | −0.16 [−0.57, 0.26] | −0.03 | −0.43 [−1.14, 0.28] | −0.05 |
| Muscle density | ||||||||||
| Total abdominal | 0.16 [−0.02, 0.34] | 0.06 | 0.37 [−0.07, 0.81] | 0.07 | 0.10 [−0.12, 0.32] | 0.03 | 0.61 [0.12, 1.11] | 0.09 | 0.55 [−0.30, 1.39] | 0.05 |
| Stability | 0.16 [−0.003, 0.32] | 0.06 | 0.25 [−0.15, 0.64] | 0.05 | 0.02 [−0.17, 0.22] | −0.06 | 0.45 [−0.0001, 0.89] | 0.08 | 0.39 [−0.37, 1.14] | 0.04 |
| Locomotor | 0.001 [−0.15, 0.15] | 0.01 | 0.38 [0.006, 0.74] | 0.06 | 0.19 [0.009, 0.38] | 0.06 | 0.50 [0.08, 0.92] | 0.07 | 0.49 [−0.22, 1.20] | 0.04 |
SD, standard deviation; B, unstandardized beta; CI, confidence interval; St β, standardized beta; Bold indicates p<0.05; Data are adjusted for Model 1: age, sex, race/ethnicity, height, diabetes, smoking, hypertension, lipid lower medication use, moderate-to-vigorous physical activity, sedentary behavior, subcutaneous abdominal fat area, visceral fat area, visceral fat density. Model 2: Model 1 plus leptin, resistin, C-reactive protein, interleukin-6, adiponectin and TNF-α. Model 3: Model 2 plus muscle area or density for the specific muscle group. HDL-c additionally adjusted for LDL-c and triglycerides; LDL-c and VLDL-c additionally adjusted for HDL-c; Triglycerides additionally adjusted for HDL-c and LDL-c
With adjustment for variables in Model 1, a 1-SD increase in total abdominal and stability muscle area was associated with an 8% lower total cholesterol (p<0.05). With the addition of inflammatory markers and muscle density (Model 3), a 1-SD increase in total abdominal and stability muscle area was associated with a 13% and 12% lower total cholesterol, respectively (p<0.01 for both). Locomotor muscle area was not associated with total cholesterol. For muscle density and with adjustment for variables in Model 3, a 1-SD increase in total abdominal and locomotor muscle density was associated with a 9% and 7% higher total cholesterol, respectively (p=0.02). Stability muscle density was not associated with total cholesterol.
With adjustment for variables in Model 3, a 1-SD increase in total abdominal and locomotor muscle area was associated with a 9% lower VLDL-c (p=0.04 for both). For muscle density and with adjustment for variables in Model 3, a 1-SD increase in locomotor muscle density was associated with a 6% higher VLDL-c (p=0.04). There were no meaningful and consistent significant linear associations between muscle area or density with triglycerides and LDL-c.
Multivariable associations of quartiles of muscle area and density with lipid outcomes
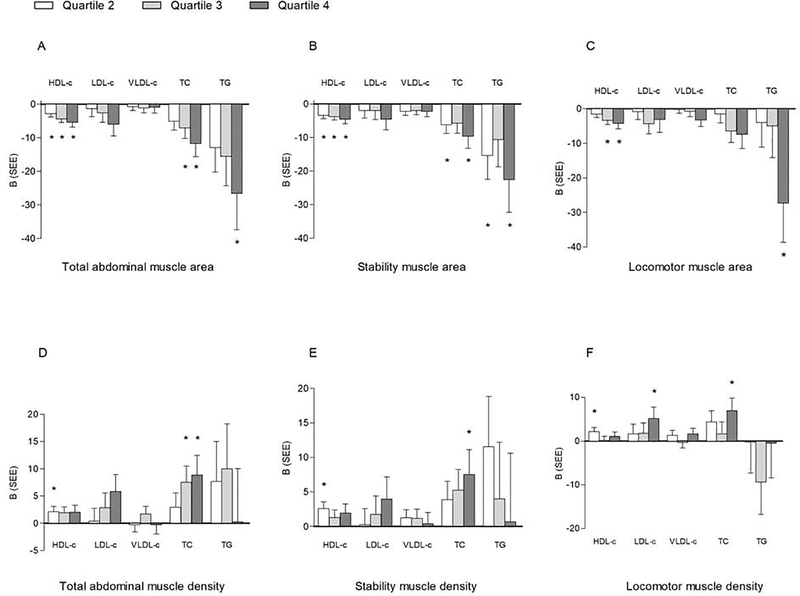
Compared to the lowest quartile, and after adjustment for variables in Model 3, there was a stepwise decrease in HDL-c with each higher quartile of total abdominal (−2.9, −4.4, and −5.4 mg/dl, respectively, p<0.01, Figure 1A) and stability (−3.4, −3.8, and −4.6 mg/dl, respectively, p<0.001, Figure 1B) muscle area, while for locomotor muscle area, only quartiles 3 (−3.3 mg/dl, p<0.001) and 4 (−4.3 mg/dl, p<0.001, Figure 1C) had a significantly lower HDL-c than quartile 1. The associations between HDL-c and quartiles of the different muscle density groups were essentially all non-significant (Figure 1D–F).
Figure 1.
Multivariable-adjusted linear associations between quartiles of muscle area and density with lipid outcomes; *P < .05; referent category: Quartile 1. Muscle area quartile cutpoints (cm2): total abdominal: Q1<77.02, Q2 = 77.02–94.66, Q3 = 94.67–116.84, Q4≥116.85; stability: Q1<58.20, Q2 = 58.20–72.12, Q3 = 72.13–89.13, Q4≥89.14; locomotor: Q1<17.88, Q2 = 17.88–22.75, Q3 = 22.76–28.83, Q4 ≥ 28.84; muscle density quartile cutpoints (HU): total abdominal: Q1 < 38.17, Q2 = 38.17–42.69, Q3 = 42.70–46.34, Q4 ≥ 46.35; stabilization: Q1 < 35.24, Q2 = 35.24–39.86, Q3 = 39.87–44.06, Q4 ≥ 44.07; locomotor: Q1 < 47.15, Q2 = 47.15–50.84, Q3 = 50.85–53.89; Q4 ≥ 53.90. Data are adjusted for model 3: age, sex, race/ethnicity, height, diabetes, smoking, hypertension, lipid-lowering medication use, moderate-to-vigorous physical activity, sedentary behavior, subcutaneous abdominal fat area, visceral fat area, visceral fat density, leptin, resistin, C-reactive protein, interleukin-6, adiponectin, TNF-α, and total abdominal muscle density. HDL-c additionally adjusted for LDL-c and triglycerides; LDL-c and VLDL-c additionally adjusted for HDL-c; triglycerides additionally adjusted for HDL-c and LDL-c. B, slope; SEE, standard error of the estimate; B, slope; SEE, standard error of the estimate; VLDL-c, very-low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-alpha; TC, total cholesterol; TG, triglyceride.
Compared to the lowest quartile, and after adjustment for variables in Model 3, the third and fourth quartiles of total abdominal muscle area (−7.1 and −11.7 mg/dl, respectively, p<0.05, Figure 1A) and the second and fourth quartiles of stability muscle area (−6.2, −9.7 mg/dl, respectively, p<0.05, Figure 1B) were associated with lower total cholesterol levels. However, compared to the lowest quartile, and with adjustment for variables in Model 3, the third and fourth quartiles of total abdominal muscle density (7.5 and 8.9 mg/dl, respectively, p=0.01, Figure 1D) and the fourth quartiles of stability (7.5 mg/dl, p=0.04, Figure 1E) and locomotor (7.0 mg/dl, p=0.02, Figure 1F) muscle density were associated with higher total cholesterol levels. Quartiles of muscle area were not associated with LDL-c or VLDL-c levels (p>0.05, Figure 1D–F).
Compared to the lowest quartile, and after full adjustment (Model 3), the fourth quartiles of total abdominal (−26.6 mg/dl, p=0.01, Figure 1A), stability (−22.6 mg/dl, p=0.02, Figure 1B) and locomotor (−27.3 mg/dl, p=0.02, Figure 1C) muscle area were associated with significantly lower triglyceride levels. Quartiles of muscle density were not associated with LDL-c, VLDL-c or triglyceride levels (p>0.05, Figure 1D–F).
Logistic regression models of quartiles of muscle area and density with lipid outcomes
Compared to the lowest quartile, and after adjustment for variables in Model 1, the odds of having HDL-c levels less than 40 mg/dl were higher for quartiles 2, 3 and 4 of stability muscle area, quartiles 3 and 4 of total abdominal muscle area and quartile 4 of locomotor muscle area (p<0.05, Table 4). With the addition of inflammatory markers and muscle density, these higher odds remained significant for all quartiles except quartile 2 of stability muscle area (Model 3, p<0.05).
Table 4.
Multivariable logistic regression models of the associations between quartiles of muscle area and lipid outcomes
| HDL-c < 40 mg·dL−1 | LDL-c > 160 mg·dL−1 | Triglycerides > 150 mg·dL−1 | ||||
|---|---|---|---|---|---|---|
| Model 1 | OR [95% CI] | P | OR [95% CI] | P | OR [95% CI] | P |
| Total Abdominal Muscle area | ||||||
| Q2 | 1.49 [0.82, 2.69] | 0.19 | 0.60 [0.31, 1.17] | 0.14 | 0.64 [0.44, 0.95] | 0.03 |
| Q3 | 2.47 [1.33, 4.57] | <0.01 | 0.92 [0.46, 1.86] | 0.83 | 0.61 [0.39, 0.96] | 0.03 |
| Q4 | 2.46 [1.23, 4.93] | 0.01 | 0.54 [0.21, 1.36] | 0.19 | 0.62 [0.36, 1.07] | 0.09 |
| Stability Muscle area | ||||||
| Q2 | 1.84 [1.04, 3.27] | 0.04 | 0.37 [0.18, 0.77] | 0.01 | 0.58 [0.39, 0.85] | 0.01 |
| Q3 | 2.82 [1.57, 5.06] | <0.01 | 0.92 [0.48, 1.75] | 0.79 | 0.63 [0.41, 0.95] | 0.03 |
| Q4 | 2.38 [1.26, 4.51] | 0.01 | 0.51 [0.22, 1.18] | 0.12 | 0.63 [0.38, 1.03] | 0.07 |
| Locomotor Muscle area | ||||||
| Q2 | 1.33 [0.75, 2.36] | 0.33 | 1.18 [0.62, 2.22] | 0.62 | 0.78 [0.54, 1.14] | 0.20 |
| Q3 | 1.66 [0.86, 3.23] | 0.14 | 1.19 [0.53, 2.68] | 0.67 | 0.54 [0.33, 0.89] | 0.02 |
| Q4 | 2.31 [1.10, 4.87] | 0.03 | 1.35 [0.48, 3.79] | 0.57 | 0.45 [0.24, 0.82] | 0.01 |
| Model 2 | ||||||
| Total Abdominal Muscle area | ||||||
| Q2 | 1.43 [0.76, 2.67] | 0.27 | 0.59 [0.29, 1.20] | 0.15 | 0.63 [0.42, 0.93] | 0.02 |
| Q3 | 2.61 [1.36, 5.03] | <0.01 | 0.98 [0.48, 2.02] | 0.96 | 0.60 [0.38, 0.95] | 0.03 |
| Q4 | 2.73 [1.31, 5.03] | 0.01 | 0.55 [0.21, 1.41] | 0.22 | 0.57 [0.33, 1.00] | 0.05 |
| Stability Muscle area | ||||||
| Q2 | 1.66 [0.90, 3.07] | 0.10 | 0.36 [0.17, 0.77] | 0.01 | 0.58 [0.39, 0.86] | 0.01 |
| Q3 | 2.75 [1.48, 5.12] | <0.01 | 0.92 [0.47, 1.79] | 0.81 | 0.59 [0.38, 0.92] | 0.02 |
| Q4 | 2.43 [1.24, 4.78] | 0.01 | 0.49 [0.21, 1.14] | 0.10 | 0.60 [0.36, 1.00] | 0.05 |
| Locomotor Muscle area | ||||||
| Q2 | 1.61 [0.87, 2.96] | 0.13 | 1.26 [0.65, 2.42] | 0.50 | 0.77 [0.52, 1.13] | 0.18 |
| Q3 | 1.97 [0.97, 4.01] | 0.06 | 1.32 [0.57, 3.01] | 0.52 | 0.54 [0.32, 0.89] | 0.02 |
| Q4 | 2.62 [1.19, 5.76] | 0.02 | 1.43 [0.50, 4.09] | 0.50 | 0.44 [0.24, 0.81] | 0.01 |
| Model 3 | ||||||
| Total Abdominal Muscle area | ||||||
| Q2 | 1.61 [0.83, 3.15] | 0.16 | 0.63 [0.30, 1.30] | 0.21 | 0.59 [0.39, 0.89] | 0.01 |
| Q3 | 3.08 [1.51, 6.28] | <0.01 | 1.02 [0.47, 2.20] | 0.96 | 0.57 [0.35, 0.91] | 0.02 |
| Q4 | 3.39 [1.50, 7.64] | <0.01 | 0.53 [0.19, 1.48] | 0.23 | 0.55 [0.30, 1.01] | 0.05 |
| Stability Muscle area | ||||||
| Q2 | 1.75 [0.93, 3.29] | 0.08 | 0.39 [0.18, 0.83] | 0.01 | 0.55 [0.37, 0.82] | <0.01 |
| Q3 | 2.91 [1.52, 5.60] | <0.01 | 0.97 [0.48, 1.97] | 0.93 | 0.57 [0.36, 0.89] | 0.01 |
| Q4 | 2.69 [1.30, 5.59] | <0.01 | 0.48 [0.19, 1.20] | 0.12 | 0.60 [0.35, 1.04] | 0.07 |
| Locomotor Muscle area | ||||||
| Q2 | 1.61 [0.87, 3.00] | 0.13 | 1.27 [0.65, 2.47] | 0.49 | 0.73 [0.49, 1.08] | 0.12 |
| Q3 | 2.01 [0.97, 4.15] | 0.06 | 1.33 [0.57, 3.11] | 0.51 | 0.49 [0.29, 0.82] | 0.01 |
| Q4 | 2.69 [1.19, 6.09] | 0.02 | 1.46 [0.49, 4.32] | 0.49 | 0.38 [0.20, 0.72] | <0.01 |
HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; OR, odds ratio; CI, confidence interval. Referent category: Quartile 1. Muscle area quartile cutpoints (cm2): total abdominal: Q1<77.02, Q2= 77.02–94.66, Q3=94.67–116.84, Q4≥116.85; stability: Q1<58.20, Q2=58.20–72.12, Q3=72.13–89.13, Q4≥89.14; locomotor: Q1<17.88, Q2=17.88–22.75, Q3=22.76–28.83, Q4≥28.84; Data are adjusted for Model 1: age, sex, race/ethnicity, height, diabetes, smoking, hypertension, lipid lower medication use, moderate-to-vigorous physical activity, sedentary behavior, subcutaneous abdominal fat area, visceral fat area, visceral fat density. Model 2: Model 1 plus leptin, resistin, C-reactive protein, interleukin-6, adiponectin and TNF-α. Model 3: Model 2 plus muscle density for the specific muscle group. HDL-c additionally adjusted for LDL-c and triglycerides; LDL-c additionally adjusted for HDL-c; Triglycerides additionally adjusted for HDL-c and LDL-c.
Compared to the lowest quartile, and after adjustment for variables in Model 1, the odds of having triglyceride levels greater than 150 mg/dl were lower for quartiles 2 and 3 of total abdominal muscle area and stability muscle area, as well as locomotor muscle area quartiles 3 and 4 (p<0.05). These lower odds remained significant in the fully adjusted model (p<0.05).
There were no meaningful and consistent associations between quartiles of muscle density with any of the categorical lipid outcomes (Supplemental Table 1).
In sensitivity analysis, we examined potential effect modification of lipid-lowering medications on these associations. Including only participants not taking lipid-lowering medications in analyses did not materially alter the associations between muscle area or density with any of the lipid outcome variables (data not shown). Additionally, we conducted analyses for the associations between muscle area or density and LDL-c and VLDL-c calculated with the Martin et al. [26] equation. The results were also not materially different from those obtained with LDL-c and VLDL-c calculated with the Friedewald equation (data not shown).
Discussion
In this cross-sectional analysis of a relatively large, multi-ethnic cohort from 6 centers located across the United States, we found that higher levels of abdominal muscle area were associated with lower levels of HDL-c, VLDL-c, total cholesterol and triglycerides. Notably, these associations were independent of relevant covariates including cardiovascular disease risk factors, physical activity, sedentary behavior, visceral adiposity, and markers of inflammation. Specifically, higher total abdominal muscle area was associated with significantly lower levels of HDL-c, VLDL-c, and total cholesterol. Additionally, the odds of having triglyceride levels above 150 mg/dl were significantly lower in those in the higher quartiles of total abdominal, stability and locomotor muscle areas. Overall, the associations were more robust for total abdominal muscle area when compared to stability and locomotor muscle areas. Furthermore, total abdominal muscle density was positively associated with total cholesterol. Our findings have clinical relevance and suggest that changes in skeletal muscle area may contribute to higher levels of specific lipids and the risk of cardiometabolic disease.
Little is known about the precise role of muscle area and density on lipid levels. Notably, we found a strong, independent association between abdominal muscle area and triglyceride levels. Our results indicate a greater than 40% reduction in the odds of having a triglyceride level above 150 mg/dL in those falling within higher quartiles of abdominal muscle area compared to those in the lowest quartile. This finding is consistent with the literature [8,11] and is clinically relevant given the growing epidemiological evidence that indicates fasting triglyceride levels are strong and independent predictors of atherosclerotic cardiovascular disease and all-cause mortality [27,28]. Similar to our results, Baek et al. [8] reported that older Korean men and women with sarcopenic obesity, a combination of both low muscle mass and obesity, had 2.5 and 1.5 greater odds of hypertriglyceridemia, respectively, than controls. Moreover, Scott et al. [11] reported increased odds of high triglycerides (OR 1.78) in a pooled sample of Korean and Australian adults with low muscle mass, defined as appendicular muscle mass divided by body mass index.
Our results also indicate greater abdominal muscle area is associated with lower HDL-c levels. HDL-c is thought to be protective for atherosclerotic cardiovascular disease but accumulating evidence suggests that this risk may be elevated in those with very high levels of HDL-c. Recent population-based studies [29–31] have shown a U-shaped association between HDL-c and cardiovascular disease mortality in both men and women, with significant increases in mortality rates with HDL-c above 97 mg/dL and 135 mg/dL, respectively. Our results are consistent with the few studies that have demonstrated an inverse association between muscle mass and HDL-c [8,10]. Although the mean value of HDL-c in our sample was within a healthy range (51.6 mg/dL), the statistical range of values was 144 mg/dL, with 5% of our sample at an HDL-c level between 80 and 161 mg/dL. Additionally, evidence suggests that variability in HDL particle size, composition and function may explain differences in risk of atherosclerotic cardiovascular disease with high or low levels of HDL-c and should be evaluated in future studies [31].
Our data suggest different types of associations between muscle area and density with total cholesterol levels. We found that a higher total abdominal muscle area, but lower density, was associated with lower total cholesterol levels. The inverse association between muscle area and total cholesterol is consistent with Baek and others [8] who reported a 1.88 greater odds of hypercholesterolemia in older adults with low levels of appendicular muscle mass, measured by dual-energy x-ray absorptiometry. The association between muscle density and cholesterol levels was unexpected but somewhat consistent with the literature. In one of the only studies to assess the associations between skeletal muscle area and density with lipid levels, Miljokvoic et al. [14] reported positive associations between calf muscle area and density with lipid and lipoprotein levels in a cohort of African American men. Specifically, in age-adjusted analyses, a higher skeletal muscle area was associated with higher levels of triglycerides and LDL-c. In fully adjusted models, the authors reported calf muscle density was positively and independently associated with LDL-c. Total cholesterol was not reported in this study. Others have reported no significant associations between calf and forearm muscle density and lipid and lipoprotein levels [10].
Our findings indicate the associations of muscle density and total cholesterol levels were independent of subcutaneous and visceral obesity, as well as other factors associated with high levels of cholesterol. These findings, along with others, suggest that increased fat infiltration (i.e., lower density) of certain muscles may not be detrimental for lipid levels and that muscle type, morphology and function may affect the associations with lipid levels. Further studies are needed to better understand the association between skeletal muscle density and lipid levels.
The potential protective effects of skeletal muscle on lipid and lipoprotein levels are unknown but may be related to the role of skeletal muscle in insulin sensitivity and glucose control. Loss of muscle mass with age may lead to reductions in resting metabolic rate and physical activity. This decreased total energy expenditure can result in a positive energy balance and increases in overall and ectopic fat deposition and inflammatory cytokines that contribute to further protein catabolism, insulin resistance and metabolic dysregulation [4,12,31–33]. Further, a recent study [34] reported that skeletal muscle mass was related to improved lipid metabolism through higher levels of circulating lipoprotein lipase and high-density lipoprotein binding protein 1. Our data support the importance of exercise, particularly resistance training, for promoting the maintenance of skeletal muscle area and density with age. Although studies suggest that aerobic exercise (e.g., brisk walking) combined with resistance training may prevent the loss of muscle mass and density with age [35,36], resistance training has been shown to be superior for increasing both muscle mass and density [37,38].
Strengths of this study include a well-characterized, multi-ethnic sample of men and women, the use of objective measures of abdominal muscle area and density via computed tomography scan, careful assessment of many potentially confounding factors, and levels of lipid and lipoprotein that were analyzed at a central laboratory, with a high level of reproducibility. The primary limitation is the cross-sectional study design that limits our ability to establish temporal associations between skeletal muscle area and density with lipid and lipoprotein levels. Although diet is an important determinant of lipid and lipoprotein levels it was not assessed in the study.
In summary, abdominal muscle area was inversely associated with triglycerides, HDL-c, VLDL-c and total cholesterol, whereas abdominal muscle density was positively associated with total cholesterol. Further, higher levels of muscle area significantly decreased the odds of hypertriglyceridemia. Our data suggest that maintenance of skeletal muscle mass with age may decrease risk of hyperlipidemia and development of cardiometabolic disease. Future prospective studies are needed to elucidate the effects of increasing skeletal muscle area and density on lipid and lipoprotein metabolism.
Supplementary Material
Highlights.
Abdominal muscle area was inversely associated with triglycerides and lipoproteins
Abdominal muscle density was positively associated with total cholesterol
Higher levels of muscle area decreased the odds of hypertriglyceridemia
Maintenance of skeletal muscle mass with age may decrease risk of hyperlipidemia
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This research was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and R01HL088451) and National Center for Advancing Translational Sciences (UL1-TR-000040 and UL1-TR-001079).
Abbreviations
- HDL-c
high-density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
- MESA
Multi-ethnic Study of Atherosclerosis
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res 2016;118:1752–70. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- [2].Melanson KJ, McInnis KJ, Rippe JM, Blackburn G, Wilson PF, Cheitlin MD. Obesity and cardiovascular disease risk: Research update. Cardiol Rev 2001;9:202–7. doi: 10.1097/00045415-200107000-00005. [DOI] [PubMed] [Google Scholar]
- [3].Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- [4].Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: Role of skeletal muscle metabolism. Ann Med 2006;38:389–402. doi: 10.1080/07853890600888413. [DOI] [PubMed] [Google Scholar]
- [5].Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care 2010;13:260–4. doi: 10.1097/MCO.0b013e328337d826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Integr Comp Physiol 2008;294:R673–80. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- [7].Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 2009;58:1013–22. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- [8].Baek SJ, Nam GE, Han KD, Choi SW, Jung SW, Bok AR, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: The 2008–2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest 2014;37:247–60. doi: 10.1007/s40618-013-0011-3. [DOI] [PubMed] [Google Scholar]
- [9].Park BS, Yoon JS. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J 2013;37:458–64. doi: 10.4093/dmj.2013.37.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mesinovic J, McMillan LB, Shore-Lorenti C, De Courten B, Ebeling PR, Scott D. Metabolic syndrome and its associations with components of sarcopenia in overweight and obese older adults. J Clin Med 2019;8. doi: 10.3390/jcm8020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scott D, Park MS, Kim TN, Ryu JY, Hong HC, Yoo HJ, et al. Associations of low muscle mass and the metabolic syndrome in Caucasian and Asian middle-aged and older adults. J Nutr Health Aging 2016;20:248–55. doi: 10.1007/s12603-015-0559-z. [DOI] [PubMed] [Google Scholar]
- [12].Vella CA, Cushman M, Van Hollebeke RB, Allison MA. Associations of abdominal muscle area and radiodensity with adiponectin and leptin: The Multiethnic Study of Atherosclerosis. Obesity 2018;26:1234–41. doi: 10.1002/oby.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee MJ, Kim EH, Bae SJ, Choe J, Jung CH, Lee WJ, et al. Protective role of skeletal muscle mass against progression from metabolically healthy to unhealthy phenotype. Clin Endocrinol (Oxf) 2019;90:102–13. doi: 10.1111/cen.13874. [DOI] [PubMed] [Google Scholar]
- [14].Miljkovic I, Kuipers AL, Kuller LH, Sheu Y, Bunker CH, Patrick AL, et al. Skeletal muscle adiposity is associated with serum lipid and lipoprotein levels in Afro-Caribbean men. Obesity 2013;21:1900–7. doi: 10.1002/oby.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol 2014;210:489–97. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Heal 2014;3:9. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- [18].Mikkelsen UR, Agergaard J, Couppé C, Grosset JF, Karlsen A, Magnusson SP, et al. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: The influence of ageing. Exp Gerontol 2017;93:54–67. doi: 10.1016/j.exger.2017.04.001. [DOI] [PubMed] [Google Scholar]
- [19].Gomez-Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: A step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr 2016;40:308–18. doi: 10.1177/0148607115604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- [21].Shen W, Punyanitya M, Wang Z, Gallagher D, St.-Onge M-P, Albu, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- [22].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux A V., Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [23].Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: The Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med 1999;8:805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- [24].National Cholesterol Education Program ATP III. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- [25].American Diabetes Association ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013;310:2061. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–63. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- [28].Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag 2016;12:171–83. doi: 10.2147/VHRM.S104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wilkins JT, Ning H, Stone NJ, Criqui MH, Zhao L, Greenland P, et al. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc 2014;3:1–7. doi: 10.1161/JAHA.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478–86. doi: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- [31].Singh K, Rohatgi A. Examining the paradox of high high-density lipoprotein and elevated cardiovascular risk. J Thorac Dis 2018;10:109–12. doi: 10.21037/jtd.2017.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hunter GR, Singh H, Carter SJ, Bryan DR, Fisher G. Sarcopenia and its implications for metabolic health. J Obes 2019;2019:1–10. doi: 10.1155/2019/8031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arner P Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 2002;18:S5–9. doi: 10.1002/dmrr.254. [DOI] [PubMed] [Google Scholar]
- [34].Matsumoto R, Tsunekawa K, Shoho Y, Yanagawa Y, Kotajima N, Matsumoto S, et al. Association between skeletal muscle mass and serum concentrations of lipoprotein lipase, GPIHBP1, and hepatic triglyceride lipase in young Japanese men. Lipids Health Dis 2019;18:1–9. doi: 10.1186/s12944-019-1014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 2008;105:1498–503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- [37].Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. Exercise and physical activity for older adults. Med Sci Sport Exerc 2009;41:1510–30. doi: 10.1249/mss.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- [38].Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol 1995;78:334–40. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.