Abstract
Adherence to antiretroviral therapy (ART) among youth remains low. We piloted an adapted active visualization device that demonstrates how ART works in the body. Youth living with HIV were randomized to: 1) standard care (n=14) or the 2) adapted active visualization intervention (n=14) and 71% of the sample (n=19) were re-assessed on viral load, adherence behaviors, and illness perceptions 2.5 months later. Intervention youth had lower viral loads, reported less difficulty in adhering to ART, and more motivation and control over their HIV than standard care at follow-up. Active visualization may be an acceptable tool to address ART adherence among youth.
Keywords: Youth living with HIV, antiretroviral therapy, medication adherence, multisensory learning, viral load
Introduction
Despite the many benefits of antiretroviral therapy (ART) for people living with HIV including extended life spans and better quality of life [i], adherence to the strict daily routine required by this medication is challenging, especially for youth. From 2010 to 2015, the rates of HIV infection decreased for all age groups, except for youth aged 25 to 34 [ii]. A recent meta-analysis reports that the rate of adherence for youth living with HIV in North America ranges from 28% to 75% [1]. This rate is far from the UNAIDS 90/90/90 goal [iii]. Youth living with HIV report several barriers to ART adherence including low perceived need for medication and poor motivation [iv]. Innovative approaches that consider cognitive processes involved in learning and motivation can be applied to address barriers to ART adherence and achieve viral suppression.
Applying multisensory learning theory [v] offers the opportunity to address several of these barriers. Activating multiple senses while presenting information supports knowledge acquisition and behavior change [11]. A recent RCT reports that non-adherent adults in South Africa who viewed an active visualization device explaining how ART works inside the body [vi] had improved viral loads eight months later [vii]. The current pilot examines whether this device could be adapted for use within a sample of youth living with HIV in the United States. In the current study, the device was adapted to be more interactive and stimulate multisensory learning, by allowing youth to visualize how ART works in the body while simultaneously practicing medication adherence and interacting with their healthcare provider. The current pilot examines the acceptability of this device among youth living with HIV and its potential influence on medication adherence.
Methods
The Institutional Review Board of University of California Los Angeles approved this study (IRB#16–001858-AM-00007).
2 Participants
All youth living with HIV aged between 12 and 29 years were invited to participate by clinic staff in Riverside (California), Panorama City (California), and New Orleans (Louisiana). When youth expressed interest in participating, research staff invited them to provide voluntary and informed consent. Youth were randomized to: 1) the intervention (standard care and the adapted active visualization device; n=14) or 2) standard care (n=14). A similar proportion in the intervention (n=11) and standard care (n=9) were reassessed about 2.5 months later (+/−14 days); the follow-up rate was 71%. Two more youth were recruited but one decided not to participate after consenting and the other chose not to finish the baseline assessment.
2Standard Care and Medication Adherence Trainer Conditions
Standard care Nurses have one-on-one conversations with patients about HIV, sexual behaviors, adherence, and viral load as part of standard care in the HIV clinics in Riverside, Panorama City, and New Orleans.
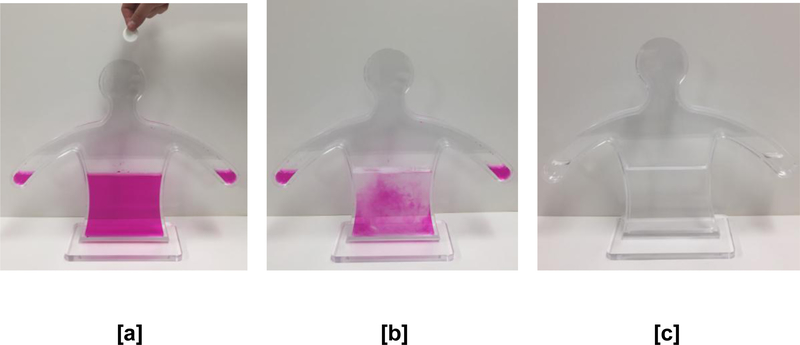
Intervention In addition to standard care, youth in the intervention received a ten-minute interactive one-on-one demonstration by a trained researcher (See Figure 1). The authors consulted with Jones et al. [6, 7] and built upon their original design of the active visualization device. The adapted demonstration used a similar human-body shaped container and procedure of changing the pH balance and color of the liquid inside the container by adding “medication’ (represented by an aspirin tablet) and actively changing the liquid from a pink color (indicating the presence of the virus), to colorless (indicating controlled viral load). The demonstration stepped through a number of days of adherence and non-adherence. In the current adaptation, the researcher asked the participant to add the ‘medicine’ to the device to represent days of adherence (rather than the researcher doing this themselves as in [7]). For days depicting nonadherence, the researcher added pink solution to the container to represent the increase in viral load that can follow consistent non-adherence. Another adaptation from the original device [6,7] in the current study was the use of an additional indicator solution (thymolphthalein) to turn the liquid into a different color (blue/purple), to represent the possible mutation of the HIV virus after inconsistent medication adherence. The resulting solution remained blue/purple even after subsequent tablets were added by the participant to demonstrate how currently prescribed medication could be ineffective after viral mutation.
Figure 1.
Adapated Active Visualization Device demonstrating the need for adherence to ART (after several medication ‘tablets’ [b], the virus is controlled and the solution turns colorless [c]).
2Measures
All participants self-reported the following assessments in-person at baseline or via text or email at follow-up (2.5 months later). Intervention youth also completed an assessment immediately after the demonstration that included questions about the acceptability of the adapted active visualization and their perceptions about HIV.
Demographics assessed included gender, age, ethnicity, and sexual orientation.
Viral load HIV diagnoses were verified and viral loads were obtained from clinical records at baseline and six months later. For all three clinics, viral loads under 20 copies/mL were considered undetectable.
Medication adherence Youth reported whether they took their medication as prescribed and how many times they missed their medication in the past three months.
Perceptions of HIV and ART Items from the Brief Illness Perception Questionnaire [viii] adapted for HIV infection were used to assess the participant’s perceptions about HIV (consequences, timeline, personal control, identity, concern, understanding, emotional response, and treatment control) [ix]. Beliefs about the necessity of ART and concerns about ART were assessed using items based on the necessity-concerns framework [x]. Additional Likert scale items on the same response format asked participants about other related perceptions, including their treatment motivation, treatment concerns, difficulties taking treatment, perceived risk of HIV severity, seriousness beliefs about HIV, and their relationship to their healthcare provider.
Acceptability of the adapted active visualization Immediately after the demonstration, youth reported how interesting and helpful the demonstration was in understanding ART and how motivated they felt to take their medication on a 10-point-Likert-type scale [7]. Youth also reported if the demonstration was a feasible approach to learn about ART in clinics (yes/no) [7].
2 Analyses
An a priori power analysis was conducted using G*Power 3.1.9.4 with an effect size estimate of 0.5 using a F-test, within-between interaction with 2 groups, 4 measurements, alpha error probability of 0.05, and power of 0.95. The current sample met the minimum total sample size of 12 participants based on the effects reported in a recent trial of a visual intervention aimed to improve ART adherence [xi]. However, there are many limitations to consider regarding sample size estimates for pilots [xii]. To test for group differences between youth living with HIV in the intervention and standard care across measures at baseline and follow-up, χ2 analyses or t-tests were used, where appropriate. To test for changes in viral loads, medication adherence, and HIV illness perceptions from baseline to follow-up, 2 (group: intervention vs. standard care) x 2 (time-point: baseline versus follow-up) mixed analyses of variance (ANOVAs) were used. Both viral-load measurements had positively skewed distributions with some youth having undetectable values (20 copies/mL). For the ANOVA, undetectable values were assigned the lower detection limit (20 copies/mL) and all viral loads were then log10 transformed.
Results
At baseline and follow-up, intervention and standard care youth were similar across all measures. Youth were on average 24 years old (SD=2.7) and the majority were male (n=26), Black or African American (n=17) or Latino (n=7), and self-identified as homosexual (n=16) or bi-sexual (n=4).
Intervention Youth reported that the demonstration was helpful in understanding their HIV infection (M=9.93, SD=0.27) and medication (M=9.64, SD=1.33). They also reported that the adapted device was interesting (M=9.79, SD=0.58) and motivating (M=10.0, SD=0.00). Youth reported feeling somewhat anxious about their infection (M=4.71, SD=4.14) and medication (M=4.79, SD=4.10). All youth (n=14) reported that the adapted device was an effective and feasible way to learn about ART adherence in clinics.
Baseline viral loads At baseline, half of the youth (n=14) had undetectable viral loads (<20 copies/mL), one participant had a viral load of 28 copies/mL, and the remaining had viral loads between 120–146,000 copies/mL. Viral loads of intervention (M=1.95 log10 copies/mL, SD=0.81) and standard care (M=1.90 log10 copies/mL, SD=1.08) youth were not significantly different at baseline, t(25)=−0.15, p=.884. Of the 28 youth, 19 had new viral load counts six months later (intervention: n=9; standard care: n=10). Of those that had detectable viral loads (> 20 copies/mL) at baseline, three intervention youth but none of the standard care youth reached undetectable viral loads (<20 copies/Ml) at follow-up. There were no significant main effects of group or time-point; however, there was a significant interaction between group and time-point, F(1,17)=6.95, p=.017, partial η2= 0.29. Viral loads of intervention youth were lower on average at follow-up (M=1.62 log10 copies/mL, SD=0.83) from baseline (M=2.13 log10 copies/mL, SD=0.84), t(9)=−2.24, p=.052. Viral loads of standard care were not significantly different at follow-up (M=2.08 log10 copies/mL, SD=1.01) from baseline (M=1.66 log10 copies/mL, SD=0.58), t(8)=1.54, p=.163.
Medication adherence Self-reported ART adherence behaviors and HIV perceptions were not significantly different between groups at baseline. Table 1 summarizes ART Adherence Behaviors and HIV Perceptions among YLH in the intervention and standard care groups over time. At baseline and follow-up, youth reported taking their ART as prescribed on average five days in the past week.However, a significantly lower proportion of intervention youth (2/10) reported missing a dose in the past three months when compared to standard care (7/9), χ2=6.34, p=.023.
Table 1.
Summary of ART Adherence Behaviors and HIV Perceptions among Youth Living with HIV in the Intervention and Standard Care Over Time
| Baseline (n = 28) | Follow-up (n = 20) | |||
|---|---|---|---|---|
| Standard Care (n=14) |
Intervent ion (n=14) |
Standard Care (n=9) |
Intervent ion (n=11) |
|
| Medication Adherence | ||||
| Days taken medication as prescribed, last 7 days | 5.71 (2.37) | 5.57 (1.74) | 5.27 (2.80) | 5.60 (1.90) |
| Missed medication at least once, last 3 months* | 50% (7) | 36% (5) | 78% (7/9) | 20% (2/10) |
| Medication Perceptions | ||||
| Perceived Need for HIV Medication | 9.71 (0.83) | 9.36 (1.45) | 9.22 (1.56) | 9.55 (1.21) |
| Motivation to adhere to ART* | 8.93 (1.82) | 9.79 (0.58) | 8.33 (2.18) | 9.91 (0.30) |
| Concern about ART | 7.29 (3.27) | 6.64 (3.48) | 6.56 (3.36) | 5.81 (4.45) |
| Seriousness of HIV | 8.43 (2.90) | 8.86 (2.25) | 8.78 (3.31) | 8.45 (3.17) |
| Difficulty in Medication Adherence, Overall* | 4.44 (3.94) | 2.64 (2.80) | 6.44 (4.30) | 0.45 (0.82) |
| Personal Risk for Illness Progression | 4.14 (3.57) | 5.00 (3.82) | 5.22 (4.02) | 2.64 (2.98) |
| HIV Illness Perceptions | ||||
| Consequences | 4.92 (3.50) | 5.35 (3.20) | 3.89 (3.76) | 4.82 (3.95) |
| Timeline | 7.43 (4.13) | 7.42 (3.55) | 8.89 (3.33) | 8.18 (3.37) |
| Personal control | 7.43 (2.59) | 7.79 (2.00) | 7.89 (1.96) | 8.45 (1.81) |
| Treatment control* | 9.64 (0.93) | 9.63 (0.63) | 8.78 (2.05) | 10.0 (0.00) |
| Identity | 1.64 (2.17) | 1.65 (2.41) | 2.33 (2.50) | 1.36 (1.29) |
| Concern | 6.79 (4.00) | 8.30 (3.00) | 5.89 (3.79) | 8.45 (3.14) |
| Understanding | 7.78 (2.64) | 8.27 (2.37) | 8.33 (2.12) | 9.46 (0.81) |
| Emotional Response | 6.00 (3.86) | 6.64 (3.75) | 4.44 (2.79) | 4.64 (3.78) |
| Relationship with Healthcare Provider (0–5) | 4.36 (0.93) | 4.64 (0.63) | 4.56 (0.73) | 4.80 (0.42) |
Note.
p<.05
There was a significant interaction between group and time-point in the degree of difficulty experienced in adhering to their medication, F(1,18)=4.48, p=.048, partial η2=.200. Specifically, intervention youth reported less difficulty in adhering to their HIV medication at follow-up (M=0.45, SD=0.82) when compared to baseline (M=2.64, SD=2.80), t(10)=−2.56, p=.028. There was no significant difference in the average degree of difficulty reported by the standard care youth from baseline to follow-up, t(8)=1.04, p=.330. Intervention youth (M=0.45, SD=0.82) reported significantly less difficulty in adhering to their medication at follow-up when compared to standard care (M=6.44, SD=4.30), t(18)=4.54, p<.001.
Intervention youth reported being more motivated to take their medication across time-points when compared to standard care, F(1,18)=7.10, p=.016, partial η2= 283. Specifically, intervention youth reported being more motivated to take their medication at follow-up (M−9.91, SD-0.30) than standard care (M-8.33, SD-2.18), t(18)=2.39, p=.028. Despite group, youth’s perceived need for their medication, the seriousness of HIV, their personal risk for illness progression, and their relationships with their healthcare providers were similar.
HIV illness perception Youth had similar illness perceptions about their HIV across measures and time-points. However, intervention youth felt that they had more control over their infection across time-points than standard care, F(1,18)=4.62, p=.046, partial η2= 204 (refer Table 1).
Discussion
This pilot provides empirical evidence that active visualization may be an acceptable and feasible tool to improve ART adherence among youth living with HIV. All youth who took part in the intervention report that the demonstration is helpful, interesting, and motivating. Viral loads assessed at follow-up support these findings. Intervention youth have lower viral loads and report less difficulty in adhering to their medication at follow-up versus baseline compared to standard care. This provides evidence that the adapted device may lead to improvements in adherence behaviors reflected through this biological marker. These findings also extend the results found in the previous RCT in South Africa using the original device [7].
Individuals tend to avoid risk in response to positive frames [xiii]. The adapted active visualization device may have encouraged medication adherence through employing positive framing by presenting adherence as a means to stay healthy and prevent transmission. These findings support such applications of positive framing through multisensory learning in ART medication adherence [11].
Several limitations of the current pilot limit the generalizability of the results, particularly the small sample size. Although the attrition rate is higher than expected (8/28), participant demographics are similar across groups. Several participants were unresponsive to the electronic follow-up; at least three participants provided contact information that was no longer valid and had not returned to the clinic for care. Future applications may consider running demonstrations in community settings where youth frequently visit (e.g. youth groups, homeless shelters) and provide cell phones and text reminders as part of the study to support retention and electronic follow-up assessments. The results of the pilot provide initial support for the feasibility of using the adapted active visualization device with youth living with HIV. The intervention is brief, portable and could be easily disseminated in clinics to educate youth about ART and HIV. Future research should expand upon the current study by using the device in a larger RCT, and examining whether long-term changes persist in adherence and viral load
Refrences
- [i].Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS (London, England). 2014. August 24;28(13):1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [ii]. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-23-1.pdf.
- [iii]. http://www.unaids.org/en/resources/909090.
- [iv].MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS and Behavior. 2013. January 1;17(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [v].Shams L, Seitz AR. Benefits of multisensory learning. Trends in Cognitive Sciences. 2008. November 1;12(11):411–7. [DOI] [PubMed] [Google Scholar]
- [vi].Jones AS, Petrie KJ. I Can See Clearly Now: Using Active Visualisation to Improve Adherence to ART and PrEP. AIDS and Behavior. 2017. February 1;21(2):335–40. [DOI] [PubMed] [Google Scholar]
- [vii].Jones AS, Coetzee B, Kagee A, Fernandez J, Cleveland E, Thomas M, Petrie KJ. The Use of a Brief, Active Visualisation Intervention to Improve Adherence to Antiretroviral Therapy in Non-adherent Patients in South Africa. AIDS and Behavior. 2018. September 26:1–9. [DOI] [PubMed] [Google Scholar]
- [viii].Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006. 60:631–637. [DOI] [PubMed] [Google Scholar]
- [ix].Broadbent E, Wilkes C, Koschwanez H, Weinman J, Norton S, Petrie KJ. A systematic review and meta-analysis of the Brief Illness Perception Questionnaire. Psychology & Health. 2015. November 2;30(11):1361–85. [DOI] [PubMed] [Google Scholar]
- [x].Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS ONE. 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xi].Perera AI, Thomas MG, Moore JO, Faasse K, Petrie KJ. Effect of a smartphone application incorporating personalized health-related imagery on adherence to antiretroviral therapy: a randomized clinical trial. AIDS patient care and STDs. 2014. November 1;28(11):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xii].Hertzog MA. Considerations in determining sample size for pilot studies. Research in nursing & health. 2008. April;31(2):180–91. [DOI] [PubMed] [Google Scholar]
- [xiii].Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981. January 30;211(4481):453–8. [DOI] [PubMed] [Google Scholar]