Abstract
Aims.
Some patients with upper gastrointestinal symptoms have rapid gastric emptying (GE). We aimed to compare patients with normal and rapid GE and to identify phenotypes among patients with rapid GE.
Methods.
Among 2798 patients who underwent GE scintigraphy, we compared patients with normal and rapid GE and separately, patients with rapid GE at 1 hour (GE1), 2 hours (GE2), or both (GE12).
Results.
In 2798 patients, GE was normal (74%), delayed (18%), or rapid (8%). Among 211 patients with rapid GE, patterns were rapid GE1 (48%), 2 hours (17%), or 1 and 2 hours (35%); 42 (20%) had diseases that explain rapid GE. A combination of upper and lower gastrointestinal symptoms (54%) was more common that isolated upper (17%) or lower (28%) gastrointestinal symptoms (P<0.001). Constipation was more prevalent in patients with rapid GE 2 (72%) than rapid GE 1 (47%) or rapid GE12 hours (67%) (P<0.05). Among 179 diabetes mellitus (DM) patients, 15% had rapid GE, which not associated with the DM phenotype. By multivariable analysis, insulin therapy (odds ratio [OR], 0.36; 95% confidence interval [CI], 0.15-0.88) and weight loss (OR, 0.10; 95% CI, 0.01-0.78) were associated with a lower risk of rapid than normal GE in DM.
Conclusions.
Eight percent of patients undergoing scintigraphy had rapid GE, which is most frequently associated with upper and lower gastrointestinal symptoms; constipation is common. Insulin therapy and weight loss were associated with a lower risk of rapid than normal GE in DM patients.
Keywords: Dumping, rapid, constipation, dyspepsia, diabetes
Graphical Abstract
Introduction
Initially described in patients with dumping syndrome after gastric surgery, iatrogenic dumping syndrome (ie, after gastric bypass surgery and fundoplication) (1-3) is among the most common causes of rapid gastric emptying (GE). Other diseases (eg, diabetes mellitus (DM), autonomic neuropathy or postural orthostatic tachycardia syndrome [POTS]), and functional gastrointestinal disorders (eg, diarrhea, non-ulcer dyspepsia, and cyclic vomiting syndrome) are also associated with rapid gastric emptying (GE).(1, 3-11) Indeed, among patients with DM and upper gastrointestinal symptoms who undergo scintigraphy, approximately one in five have rapid GE.(6) Contrary to earlier studies, more recent studies did not find differences in the phenotype of DM among patients with normal, rapid, and delayed GE.(6, 11) However, treatment with insulin was associated with a lower risk of rapid versus normal GE in DM. This finding is interesting, needs to be confirmed, and may be explained by the finding that insulin reverses the reduced expression of neuronal nitric oxide synthase in diabetic mice.(12-15), Hence, insulin may improve gastric accommodation and prevent rapid GE.
Aside from post-surgical rapid GE, the pathogenesis of rapid GE is poorly understood. Increased gastric contractility is associated with, and may explain, idiopathic rapid GE.(16) Impaired postprandial gastric accommodation is associated with a higher gastric pressure, which may also predispose to accelerated GE.(17) However, impaired accommodation is not associated with rapid GE; among patients with non-ulcer dyspepsia, accommodation was reduced in 26% of patients with normal GE, 11% with rapid, and 6% with delayed GE.(18) Alternatively or in addition, it is conceivable but unknown if the mechanisms that normally retard GE of solids (ie, the pyloric sieve or duodenogastric neurohumoral feedback mechanisms) (19-21), possibly inherited,(22) may be impaired, thereby predisposing to rapid GE. In patients with dumping after gastric surgery, rapid GE of nutrients into the small intestine evokes the release, hence greater plasma concentrations, of hormones (eg, glucagon-like peptide [GLP-1], gastrin inhibitory peptide [GIP], catecholamines, and peptide YY [PYY]), which among other effects, activate neurohumoral feedback mechanisms (eg, the ileal brake) that delay gastrointestinal transit.(2) We postulated these differences in the underlying mechanisms and consequences of rapid GE among patients are manifest as phenotypes among patients with rapid GE, such as patients who have rapid GE only at 1 hour, only at 2 hours, or at 1 and 2 hours after a meal.(23) Hence, the aims of this study were to 1) compare the clinical features and disturbances of small intestinal and colonic transit between patients with normal GE and rapid GE, and 2) among patients who had rapid GE at 1 hour only, 2 hours only, and at 1 and 2 hours, and 3) to compare the factors associated with rapid and normal GE in DM patients with gastrointestinal symptoms.
Materials and Methods
Setting and identification of patients
This study was approved by the Institutional Review Board at Mayo Clinic. Between January 2013 and October 2017, gastric emptying was evaluated with scintigraphy in 4076 patients aged 18 years or older at Mayo Clinic, Rochester, MN. After excluding 6 patients who did not authorize review of their medical records for research, the diagnoses and surgical procedures were extracted from the electronic database using established methods in all 4070 patients.(24) Because our objectives were to identify phenotypes among patients who have rapid GE due to disordered gastrointestinal functions, patients with major gastrointestinal operations, anatomical abnormalities, intestinal obstruction or pseudoobstruction, or severe systemic diseases were excluded from this study. Hence, we excluded 480 patients who had a major gastrointestinal operation [ie, colonic resection (n = 98), gastrostomy (n = 85), ileostomy (n = 77), colostomy (n = 65), intestinal resection (n = 48), gastrectomy (n = 32), esophagectomy (n=1), fundoplication (n = 43), gastric band surgery (n=2) and Roux-En Y gastric bypass (n = 29)], 23 patients who had intestinal disease [ie, malrotation (n = 18), intestinal obstruction (n = 4) or intestinal pseudoobstruction (n = 1)], 372 who had a severe systemic condition [ie, ongoing malignancy (n = 281), history of liver, kidney or lung transplant (n = 46), chronic pulmonary disease with tracheostomy (n = 24), or carcinoid syndrome (n = 21)] and 397 patients in whom the results of GE at both 1 and 2 hours were not available. Of the remaining 2798 patients, 2075 (74%) had normal, 512 (18%) had delayed, and 211 (8%) had rapid GE. Since we sought to identify differences between patients with normal and rapid GE and among patients different patterns of rapid GE, 2286 patients with normal or rapid GE were studied further.
Scintigraphy
Antiemetic and opioid medications were discontinued for four half-lives before the study. After an overnight fast, GE was assessed with scintigraphy using a 300-kcal mixed meal containing 99mTc-sulphur colloid labelled eggs.(23, 25) Anterior and posterior images were acquired with a dual-head gamma camera, at 1, 2 and 4 hours. The upper limits of normal GE are 4-31% in women and 5-40% in men at one hour and 25-71% in women and 28-82% in men at two hours. To ensure a rigorous diagnosis, GE was considered rapid when it was more than 5% above the upper limit of normal; 89 patients with GE less than 5% above the upper limits of normal were considered to have normal GE. Patients with rapid GE were categorized into three groups: rapid GE at 1 hour only (rapid GE1), rapid GE at 2 hours only (rapid GE2) and rapid GE at 1 and 2 hours (rapid GE12) (Figure 1). In 2267 of 2286 patients (99%), small intestinal transit was also evaluated with a surrogate marker (ie, colonic filling at 6 hours [%]).
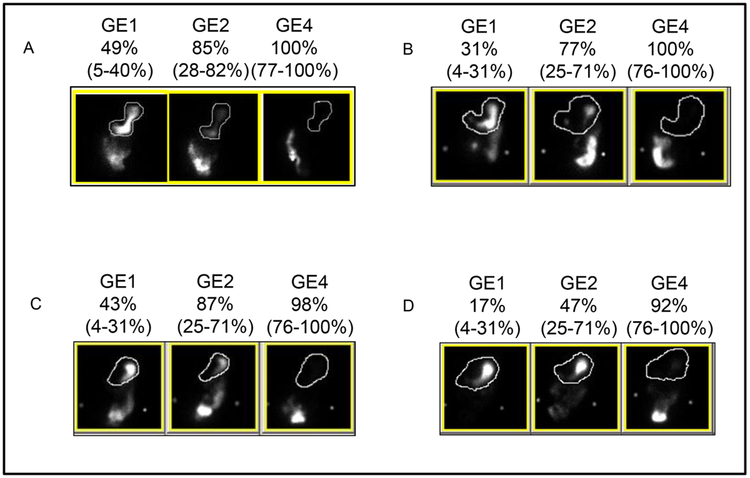
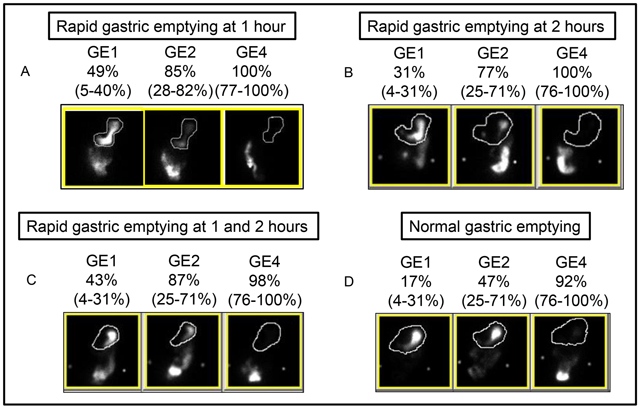
Figure 1. Scintigraphy results for different categories.
Panel A (top left) shows a patient with rapid gastric emptying at 1 hour only, panel B (top right) with rapid gastric emptying at 2 hours only, panel C (lower left) with rapid gastric emptying at 1 and 2 hours, and panel D (bottom right) with normal gastric emptying at 1 and 2 hours. GE1, 2, and 4 denote gastric emptying at 1, 2, and 4 hours. The numbers in parentheses represent the sex-matched normal values.
Colonic transit was also evaluated with 111In-labelled charcoal pellets contained in a capsule that had a pH-sensitive methacrylate coating which disintegrates in the alkaline terminal ileum.(26) After quantifying the 111In counts in the ascending, transverse, descending, and the rectosigmoid colon on the image at 24 hours, the weighted average of counts was summarized as the geometric center at 24 hours (GC24), which was evaluated in 1859 of 2286 patients (81%) with normal or rapid GE.
Assessment of gastrointestinal symptoms and associated conditions
Among the 2286 patients with normal or rapid GE, the conditions that are recognized to be associated with rapid GE (eg, diabetes mellitus, celiac disease, autonomic neuropathy), other gastrointestinal conditions (eg, inflammatory bowel disease), and medications were documented from the electronic database.(6, 7) Thereafter, the records of all 211 patients with rapid GE and 179 patients with DM were reviewed by SK to characterize the gastrointestinal symptoms and the characteristics of DM. Of these 211 patients, 207 (98%) had been evaluated by a gastroenterologist. In patients with multiple upper gastrointestinal symptoms, the principal indication was assigned using the following hierarchy ie, dyspepsia, nausea and/or vomiting, and other symptoms (ie, gastroesophageal reflux, atypical chest pain, and weight loss). For lower gastrointestinal symptoms, the hierarchy was constipation, diarrhea, chronic abdominal pain without bowel disturbances, and abdominal bloating. Peripheral neuropathy was identified by an abnormal physical examination (ie, diminished or absent knee or ankle reflexes or reduced sensation for touch, vibration or pinprick) or by an abnormal electromyography.(6) Nephropathy was defined by one or more abnormalities, ie, moderately increased albuminuria (ie, albumin excretion between 30 - 300 mg/day or 30 - 300 mg/g of creatinine in a random urine sample, markedly increased albuminuria (albumin excretion greater than 300 mg/day or greater than 300 mg/g of creatinine in a random urine sample), renal insufficiency with a GFR < 60 ml/min/1.73m2 (27) or an established prior diagnosis. A retinopathy was defined by a fundoscopic examination at our institution or an established prior diagnosis. Coronary artery disease was identified based on angiographic evidence of coronary artery blockage or history of angioplasty or a history of myocardial infarction.
Statistical analysis
The univariate associations between symptoms and categories defined by GE parameters (ie, rapid GE1, GE2, and rapid GE12) were analyzed with Fischer’s exact test for categorical variables and Kruskal-Wallis rank test for continuous variables. The correlations among continuous variables were compared with Spearman’s correlation coefficient. P value of less than 0.05 was considered significant. The data are expressed as numbers (percentages). A polychotomous multiple variable logistic regression model was used to identify factors independently associated with rapid versus normal GE in DM. Odds ratios are reported with 95% confidence intervals computed from the model. The predicted probabilities from this multiple predictor variable model were used to construct receiver operating characteristic (ROC) curves which illustrate the sensitivity and specificity of demographic, clinical features and therapy for discriminating normal from rapid GE in DM patients. All analyses used JMP software (JMP Pro, Version 14.1.0 SAS Institute Inc., version 9.4, Cary, NC).
Results
Baseline Demographics
In this cohort, 2075 patients had normal and 211 had rapid GE. Among patients with rapid GE, 102 (48%) had rapid GE1 only, 36 (17%) had rapid GE2 only, and 73 (35%) had rapid GE12 (Figure 1). In each group, approximately 75-85% of patients were women and approximately 40% were overweight or obese (Table 1). The distribution of age, sex and BMI was not significantly different among these groups. The BMI was not correlated with gastric emptying at 1 (r=0.10, P=.13), at 2 hours (r=0.03, P=.70) or 4 hours (r=−0.06, P=0.42).
Table 1. Demographics and clinical features.
| Normal GE (n=2075) |
Rapid GE (n=211) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Overall (n=211) |
Rapid GE1 only (n=102) |
Rapid GE2 only (n=36) |
Rapid GE1 and GE2 (n=73) |
||
| Age in years, Mean ± SD | 41 ± 15 | 44 ± 16* | 45 ± 16 | 42 ± 15 | 42 ± 15 | |
| Females, n (%) | 1588 (77%) | 167 (79%) | 78 (76%) | 28 (78%) | 61 (84%) | |
| BMI (kg/m2) a, Mean ± SD | 24.1 ± 6.7 | 24.3 ± 6.8 | 24.0 ± 7.2 | 24.5 ± 4.7 | 24.2 ± 7.1 | |
| <25, n (%) | 1239 (60%) | 129 (61%) | 60 (59%) | 23 (64%) | 46 (63%) | |
| 25-30, n (%) | 405 (20%) | 44 (21%) | 23 (23%) | 10 (28%) | 11 (15%) | |
| >30, n (%) | 364 (18%) | 38 (18%) | 19 (19%) | 3 (8%) | 16 (22%) | |
| Associated conditions | ||||||
| Autonomic function tests, n (%) | 265 (13%) | 22 (10%) | 12 (12%) | 4 (11%) | 6 (8%) | |
| POTS or autonomic neuropathy, n (%) | 136 (7%) | 14 (7%) | 8 (8%) | 2 (6%) | 4 (5%) | |
| Diabetes, n (%) | Type 1 | 55 (3%) | 8 (4%) | 8 (8%) | 0 | 0 |
| Type 2 | 97 (5%) | 19 (9%) | 9 (9%) | 1 (3%) | 9 (12%) | |
| Celiac disease, n (%) | 9 (0.4%) | 6 (3%)* | 2 (2%) | 1 (3%) | 3 (4%) | |
| Inflammatory bowel disease, n (%) | 43 (2%) | 3 (1%) | 1 (1%) | 0 | 2 (3%) | |
| Cholecystectomy, n (%) | 510 (25%) | 41 (19%) | 27 (26%) | 2 (6%) | 12 (16%) | |
| Scleroderma and/or Sjogren’s syndrome, n (%) | 54 (3%) | 3 (1%) | 1 (1%) | 0 | 2 (3%) | |
| Peripheral neuropathy or myasthenia gravis, n (%) | 109 (5%) | 13 (6%) | 7 (7%) | 4 (11%) | 2 (3%) | |
| Anxiety and/or depression, n (%) | 479 (23%) | 69 (33%)* | 28 (27%) | 15 (42%) | 26 (36%) | |
| Hypothyroidism, n (%) | 79 (4%) | 9 (4%) | 1 (1%) | 3 (8%) | 5 (7%) | |
P<0.05 for corresponding values of rapid vs normal GE
67 missing BMI values in normal GE and 2 in rapid GE
Small intestinal and colonic transit
Small bowel transit was not significantly different between patients with normal and rapid GE and among the GE categories (Table 2). However, a greater proportion of patients with normal GE had delayed colonic transit. The GE at 1, 2 and 4 hours was weakly correlated with colonic filling at 6 hours (Spearman correlation coefficients were respectively 0.10, 0.15, 0.13, P=.0001), and with GC24 (r=0.14, r=0.16, r=0.13, P=.001). Delayed colonic transit at 24 hours was observed in a greater proportion (P<.05) of patients with normal GE than rapid GE (Table 2). The small bowel transit was correlated with colonic transit at 24 hours (r=0.28, P=.0001). Compared to non-constipated patients, constipated patients were more likely (P<.05) to have delayed colon transit.
Table 2. Gastric, small intestinal, and colonic transit.
Unless stated otherwise, all values are Mean ± SD
| Rapid GE1
only (n=102) |
Rapid GE2
only (n=36) |
Rapid GE1
and GE2 (n=73) |
Normal GE1
and GE2 (n=2075) |
|
|---|---|---|---|---|
| Gastric emptying at 1 hour | 40 ± 8 | 29 ± 6 | 48 ± 14 | 19 ±7 |
| Gastric emptying at 2 hours | 67 ± 10 | 82 ± 6 | 85 ± 7 | 47±13 |
| Gastric emptying at 4 hours | 94 ± 7 | 98 ± 3 | 98 ± 2 | 86 ± 11 |
| Small bowel transit (CF6) | ||||
| n (%) | 99 (97%) | 35 (97%) | 71 (97%) | 2062 (99%) |
| Value | 45 ± 32 | 47 ± 29 | 51 ± 32 | 46 ± 29 |
| Geometric center at 24 hours (GC24) | ||||
| n (%) | 79 (78%) | 27 (75%) | 61 (84%) | 1692 (82%) |
| Value | 2.4 ± 1.1 | 2.1 ± 0.7 | 2.4 ± 1.1 | 2.1 ± 11 |
| Delayed GC24 (<1.6), n (%) | 13 (13%) | 3 (7%) | 5 (7%) | 406 (20%)* |
| Accelerated GC24 (>3.8), n (%) | 12 (12%) | 1 (1%) | 9 (12%) | 174 (8%) |
CF6 = colonic filling at hours; GC24 = geometric center for colonic transit at 24 hours
P<.05 versus rapid GE
Gastrointestinal symptoms
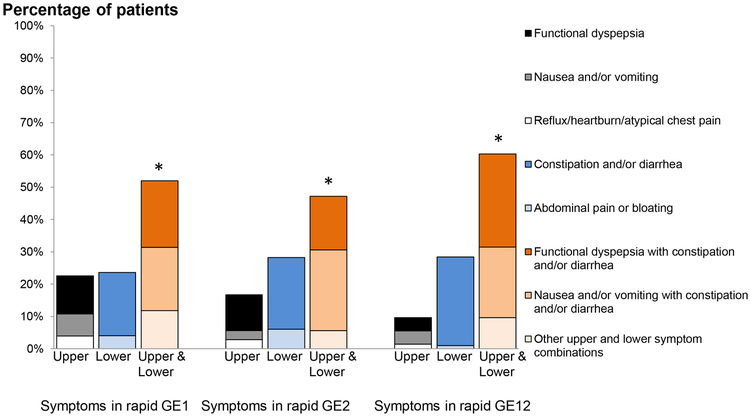
Among all 211 patients with rapid GE, more patients (ie, 113 [54%]) had upper and lower gastrointestinal symptoms than isolated upper (36 patients [17%]), or lower (59 patients, [28%]) gastrointestinal symptoms (P<0.001 Fisher’s exact test); 2 had abdominal wall pain and 1 had bulimia with constipation (Figure 2).
Figure 2. Distribution of gastrointestinal symptoms among patients with rapid gastric emptying at 1 hour, at 2 hours, and at 1 and 2 hours.
Vertical bars in each group represent isolated upper gastrointestinal, isolated lower gastrointestinal and both upper and lower gastrointestinal symptoms respectively. Colours in each bar represents the individual symptoms or symptom combinations. *P<0.05 within each group
Likewise, within each category of rapid GE (ie, GE1, GE2, and GE12), the proportion of patients with upper and lower GI symptoms was greater than the proportion of patients with only upper or only lower GI symptoms (P<0.05, Fisher’s exact test) (Figure 2).
Among patients with rapid GE, 58% had constipation . The proportion of patients with constipation, alone or in combination with upper GI symptoms, was associated (P<.05) with the pattern of rapid GE and greater in patients who had rapid GE 2 (26 patients [72%]) or rapid GE12 (49 patients [67%]) than rapid GE 1 (48 patients [47%]) . The proportion of patients with any lower GI symptom, with or without upper GI symptoms, was also associated (P<.05) with the category of rapid GE (ie, 65 patients [89%] with rapid GE12), 31 patients [86%] with GE2), and 76 patients [74%] with rapid GE1). By contrast, the proportion of patients who had isolated upper GI symptoms was not significantly different among rapid GE groups. Of 211 patients with rapid GE, 31 (15%) patients reported a greater than 10 pound weight loss in the last 12 months.
Associated conditions
Among 211 patients with rapid GE, only 42 (20%) had other conditions that are known to be or possibly associated with rapid GE ie, diabetes mellitus (27 patients [13%]), autonomic dysfunctions (14 patients [7%]) or celiac disease (6 patients [3%]). Another 17 patients (8%) had Crohn’s disease and/or ulcerative colitis (3 patients), neurological diseases (ie, myasthenia gravis (1 patient), peripheral neuropathy (12 patients), scleroderma and/or Sjogren’s syndrome (3 patients). (Table 1) Sixty nine (33%) had anxiety and/or depression, 41 (19%) had a cholecystectomy, and 9 (4%) had hypothyroidism.
Medications
Forty seven patients (22%) with rapid GE were taking proton pump inhibitors (39 patients [18%]) and/or H2 receptor antagonists (10 [5%] patients). Among these, 25 (12%) patients had rapid GE1, 6 (3%) patients had rapid GE2 and 16 (8%) patients had rapid GE12. Seventy five patients (36%) were taking other medications, including selective serotonin reuptake inhibitors (SSRI) (34 patients, 16%), other antidepressants (ie, mirtazapine, vilazodone, vortioxetine, bupropion, buspirone and trazodone) (17 patients, 8%), selective norepinephrine reuptake inhibitors (SNRI) (13 patients, 6%), tricyclic antidepressants (13 patients, 6%), and calcium channel blockers (7 patients, 3%). Opioids (79 patients, 37%) and antiemetic drugs (59 patients, 28%) were discontinued before the GE study. Only seven patients reported concurrent marijuana use.
Diabetes mellitus
Among DM patients, the distribution of patients with normal (152 patients, [85%]) or rapid (27 patients, [15%]) GE was not associated with age, sex, BMI, or the type or duration of DM (Table 3). Approximately one-third of patients each with normal and rapid GE had type 1 DM.
Table 3. Demographic features and characteristics of DM patients.
| Characteristic | Normal, N (%) (n=152) | Rapid, N (%) (n=27) |
|---|---|---|
| Demographics and lifestyle | ||
| Women | 107 (70%) | 20 (74%) |
| Age, mean ± SD, year | 50 ± 14 | 50 ± 15 |
| BMI, mean ± SD, kg/ma | 28 ± 9 | 30 ± 10 |
| Overweight | 31 (20%) | 6 (22%) |
| Obese | 59 (55%) | 14 (52%) |
| DM-related variables | ||
| Type 1 DM | 55 (36%) | 9 (33%) |
| Duration of Type 1 DMa | 21 ± 12 | 33 ± 17 |
| Duration of Type 2 DMa | 12.1 ± 9.1 | 11.1 ± 9.01 |
| HbA1c levels, mean ± SEMa | 7.6 ± 1.6 | 7.25 ± 1.8 |
| Treatment of DM | ||
| No medication | 8 (5%) | 2 (7%) |
| Only insulin therapy* | 89 (59%) | 9 (33%) |
| Only oral hypoglycemic agents (excluding GLP1 agonists, DPP IV inhibitors, pramlintide) | 38 (25%) | 11 (41%) |
| Only GLP1 agonists, DPP IV inhibitors, or pramlintide | 11 (7%) | 3 (11%) |
| More than one medication | 6 (4%) | 2 (8%) |
P<0.05 for univariate associations among groups
Values for BMI, duration of DM and HbA1c were missing in 2, 32 and 23 patients respectively.
All patients with DM had one or more gastrointestinal symptom. In the univariate analysis, diarrhea was more common (P<.05) in patients with rapid GE while weight loss was associated with normal GE (Table 4). Additionally, presence of both upper and lower GI symptoms was associated with rapid GE.
Table 4. Distribution of symptoms and extraintestinal complications among DM patients.
| Feature | Normal, n (%) (N=152) |
Rapid, n (%) (N=27) |
|---|---|---|
| Functional dyspepsia | 45 (30%) | 7 (26%) |
| Nausea and/or vomiting | 62 (41%) | 12 (44%) |
| Gastroesophageal reflux and/or atypical chest pain | 16 (11%) | 1 (4%) |
| Abdominal Pain | 7 (5%) | 4 (3%) |
| Constipation | 71 (47%) | 12 (44%) |
| Diarrhea* | 40 (26%) | 13 (48%) |
| Weight loss* | 43 (28%) | 1 (4%) |
| Extraintestinal complications | ||
| None | 48 (32%) | 10 (37%) |
| One | 54 (36%) | 9 (33%) |
| Two | 38 (25%) | 6 (22%) |
| Three | 12 (8%) | 2 (7%) |
| Peripheral neuropathya | 66 (43%) | 9 (33%) |
| Autonomic neuropathy | 21 (14%) | 4 (15%) |
| Retinopathya | 20 (13%) | 4 (15%) |
| Nephropathya | 79 (52%) | 14 (52%) |
| Proteinuriaa | 16 (11%) | 3 (19%) |
| Renal insufficiencya | 30 (11%) | 6 (11%) |
| Proteinuria and renal insufficiency | 7 (5%) | 2 (7%) |
| Hypertension | 53 (35%) | 6 (22%) |
| Coronary artery diseasea | 23 (15%) | 6 (22%) |
| Peripheral vascular disease | 10 (7%) | 3 (11%) |
P<0.05 for univariate associations among groups
Values for peripheral neuropathy, retinopathy, proteinuria, renal insufficiency and coronary artery disease were missing in 10, 34, 62, 19 and 10 patients respectively
Of 179 patients, 121 (68%) had one or more extra intestinal complications of DM (ie, retinopathy, nephropathy or neuropathy). The presence of any extra-intestinal complications and the number of extra-intestinal complications in each patient were not significantly associated with rapid GE (Table 4). Of the 58 patients who did not have extraintestinal complications, 10 had rapid and 48 had normal GE. The glycosylated hemoglobin was checked in 9 of the 10 patients with rapid GE and it was normal in 4 patients. Overall, glycosylated hemoglobin was abnormal in 91 of the 179 (ie, 51%) patients and was not significantly associated with the pattern of GE. Univariate analysis revealed a significant association between insulin therapy and the pattern of GE (P<.05). Specifically, 89 (59%) patients with normal versus 9 (33%) patients with rapid GE were treated with insulin alone.
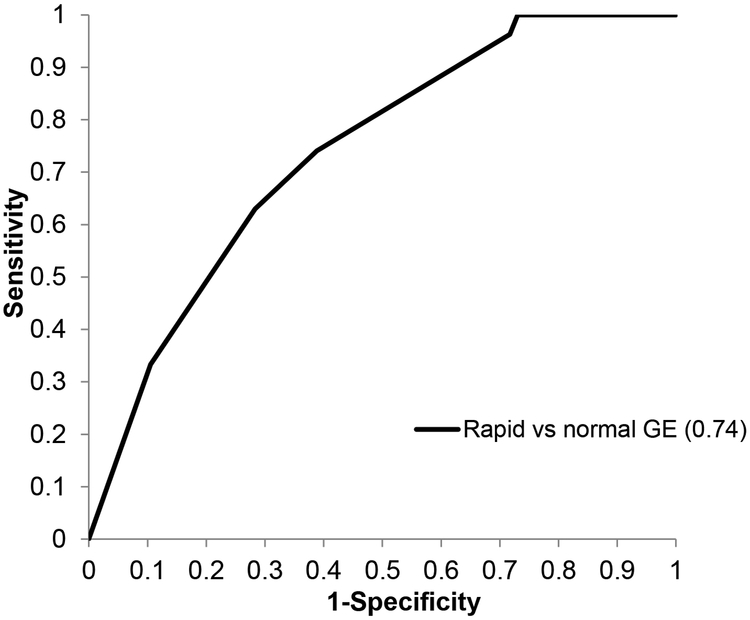
In the multivariable analysis of risk factors associated with GE disturbances, weight loss and insulin therapy alone were associated with a lower risk of rapid compared to normal GE (Table 5). The logistic regression model containing multiple variables had an area under the ROC curve of 0.74 for differentiating rapid from normal GE. (Figure 3) This suggests that at a specificity of 60%, clinical features and therapy were 74% sensitive for differentiating between rapid and normal GE.
Table 5. Multiple variable analysis of risk factors for rapid gastric emptying among patients with DM.
| Variable | Rapid vs. normal (Odds Ratio – 95% CI) |
|---|---|
| Insulin therapy alone | 0.36 (0.15, 0.88) |
| Weight loss | 0.10 (0.01, 0.78) |
| Diarrhea | 2.03 (0.84, 4.89) |
Figure 3. Receiver operating characteristic curve demonstrating the utility of clinical features and insulin therapy to differentiate DM patients with rapid and normal gastric emptying.
At a specificity of 60%, the clinical features and insulin therapy were 74% sensitive for differentiating between rapid and normal GE.
Discussion
In this large cohort of 2798 patients, 8% had rapid GE. Although we used more stringent criteria to define rapid GE and excluded patients who had prior gastric surgery, this proportion is comparable to the prevalence (ie, 10% of 750 patients, 9% of 545 patients and 9% of 2907 patients) in earlier reports that included patients who had gastric surgery.(3, 10, 28) In another study, 28% of 642 patients undergoing scintigraphy had rapid GE, perhaps because a less stringent criterion was used to define rapid GE (ie, gastric half-emptying time less than 70 minutes).(29)
Nearly one-third of patients with rapid GE1 had nausea and/or vomiting, perhaps because rapid delivery of nutrients distends and stimulates chemoreceptors in the duodenum. Bowel disturbances were also common; 26% of all patients with rapid GE had diarrhea. Among DM patients, diarrhea was associated with rapid GE by univariate but not multivariate analysis. In a previous study, 25 of 60 patients (40%) with non-organic diarrhea had rapid GE.(4) Patients with rapid small intestinal and/or colonic transit may benefit from treatment with muscarinic cholinergic or serotonin 5-HT3 receptor antagonists.(30-32)
While rapid gastrointestinal transit is associated with diarrhea,(33, 34), the correlations between GE1 and GE2 and CF6 and between CF6 and colonic transit in this study were significant but weak, perhaps partly because the exaggerated delivery of nutrients from the stomach to the small intestine activates the ileal brake, which is mediated by neurohumoral mechanisms.(21, 35) By contrast, two thirds of all patients reported constipation, which was associated with rapid GE2 and slow colon transit. One possible explanation is that rapid GE is associated with exaggerated release of humoral mediators of the ileal brake (ie, GLP-1, PYY, NPY, leptin, amylin), which may also delay colonic transit.(21, 36-40)
Only 20% of patients with rapid GE had conditions (ie, autonomic neuropathy or POTS, diabetes mellitus, celiac disease) that have been associated with rapid GE.(5-7) Among patients with an autonomic neuropathy or POTS, gastric emptying is more likely to be rapid than delayed.(5, 7) Approximately one in five patients with diabetes mellitus and gastrointestinal symptoms have rapid GE.(6, 41) Among patients who undergo assessment of GE and accommodation, this proportion was 37%.(11) Obesity was associated with rapid GE in asymptomatic individuals but not so in this study.(42, 43) By contrast to this study, those studies only included asymptomatic people; one study also excluded patients with systemic disorders, anxiety or depression. Conceivably, the weight loss associated with upper GI symptoms also attenuated the association between obesity and rapid GE in this study. While we did not evaluate temporal trends, this observation argues against the hypothesis that the obesity epidemic contributes to rapid GE. Thus, over 70% of patients had idiopathic rapid GE, assuming that their medications did not predispose to the condition. Conceivably, impaired duodeno-gastric feedback mechanisms, related perhaps to single nucleotide polymorphisms that are associated with impaired GLP-1 receptor functions may partly explain faster GE in some patients.(22)
Thirty six percent of all patients and 35 % of patients who did not have an underlying explanation for rapid GE or an organic gastrointestinal or neurological disease were taking medications that affect gastrointestinal motility. While opioids and antiemetic agents were discontinued before the study, other drugs (eg, SSRIs, tricyclic agents) were not. Administration of SSRIs at doses that are generally used in clinical practice (ie, buspirone [10 mg twice daily], venlafaxine [75 mg daily], and paroxetine [20 mg daily] did not affect GE in healthy people.(44) Neither amitriptyline (50 mg/day) nor escitalopram (10 mg/day) affected GE in patients with functional dyspepsia.(45) A higher dose of citalopram (ie, a single dose of 20 mg given intravenously) accelerated GE of solids in healthy people.(46) In this cohort, 13 patients (7%) with rapid GE were taking a SSRI at an oral dose of 40 mg or greater (ie, citalopram, escitalopram, sertraline and fluoxetine) and 3 (1%) patients were taking a SNRI (ie, venlafaxine and desvenlafaxine) at a dose of 75 mg or greater. The histamine-2 receptor antagonists increase gastric antral motor activity but delayed GE in humans.(47) Conceivably, these medications may predispose to rapid GE.
Fifteen percent of DM patients in this cohort had rapid GE. Allowing for the more stringent threshold for defining rapid GE, this prevalence, among 179 patients, is comparable to the corresponding prevalence of 22% in our previous study of all 129 patients who had scintigraphy between 2002 and 2006.(6) Similar to that study, two thirds of DM patients with rapid GE in this study had type 2 DM, for an average duration of 11 years. Nearly two thirds of patients with rapid GE had one or more extra-intestinal complication of DM. Reaffirming earlier observations, in this study (i) weight loss was associated with a lower risk of rapid versus normal GE,(6) (ii) the phenotype (ie, type of DM, prevalence of complications) did not discriminate between patients with normal and rapid GE,(10, 11, 48) and (iii) insulin therapy was associated with a lower risk of rapid (versus normal GE).(6) Insulin reverses the reduced expression of neuronal nitric oxide synthase (nNOS), which should improve gastric accommodation and gastric emptying, in diabetic mice.(12-15) Conceptually, reduced gastric accommodation predisposes to rapid GE. By increasing nitrergic neurotransmission, insulin therapy may preserve gastric accommodation, hence reduce the risk of rapid GE. Our understanding of the pathogenesis of rapid GE in DM is limited. To speculate, similar to a mouse model of type 2 DM (ie, obese, hyperglycemic, hyperinsulinemic female Lepr db/db mice), perhaps hyperglycemia increases the interstitial cells of Cajal and thereby accelerates GE.(49)
This was a large cohort of consecutively studied patients in whom GE was evaluated with standardized techniques. Nonetheless, there were limitations. The assessment of symptoms by physicians was not standardized. Non-gastrointestinal symptoms of rapid GE (eg, flushing, palpitations, and light-headedness) were not evaluated.
To conclude, 8% of patients in this large cohort had rapid GE. Patients with rapid GE were more likely to have upper and lower gastrointestinal symptoms than isolated upper or lower gastrointestinal symptoms. Among patients with rapid GE, 58% had constipation, which was more common in patients with rapid GE 2 (72%) than rapid GE 1 (47%) or rapid GE12 hours (67%). Rapid GE was observed in 15% of DM patients, of whom two thirds had type 2 DM, and nearly two thirds had extra-intestinal complications of DM. Compared to patients with normal GE, insulin therapy and weight loss were associated with a lower risk of rapid GE but the phenotype of DM was not. These studies should compare the pathogenic mechanisms (eg, gastric contractility and neurohumoral duodenogastric feedback mechanisms) among the GE phenotypes.
Acknowledgements and Disclosures
Funding. This study was supported in part by USPHS NIH Grant R01 DK068055 to Dr. Bharucha.
Abbreviations.
- GE
gastric emptying
- POTS
postural orthostatic tachycardia syndrome
- CCK
cholecystokinin
- GLP-1
glucagon like peptide-1
- GE1
gastric emptying at 1 hour
- GE2
gastric emptying at 2 hours
- GE12
gastric emptying at 1 and 2 hours
- GC24
colonic transit geometric center at 24 hours
- PYY
peptide YY
- CF6
colonic filling at 6 hours
Footnotes
Competing Interests. The authors have no competing interests.
References
- 1.Sawyers JL, Herrington JL Jr., Burney DP. Proximal gastric vagotomy compared with vagotomy and antrectomy and selective gastric vagotomy and pyloroplasty. Ann Surg 1977; 186: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nature Reviews Gastroenterology & Hepatology 2009; 6: 583–590. [DOI] [PubMed] [Google Scholar]
- 3.Hejazi RA, Patil H, McCallum RW. Dumping syndrome: establishing criteria for diagnosis and identifying new etiologies. Dig Dis Sci 2010; 55: 117–123. [DOI] [PubMed] [Google Scholar]
- 4.Charles F, Phillips SF, Camilleri M, Thomforde GM. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc 1997; 72: 323–328. [DOI] [PubMed] [Google Scholar]
- 5.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am J Gastroenterol 2007; 102: 618–623. [DOI] [PubMed] [Google Scholar]
- 6.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009; 70: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loavenbruck A, Iturrino J, Singer W, et al. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol Motil 2015; 27: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty S, Halland M, Burton D, et al. GI Dysfunctions in Diabetic Gastroenteropathy, Their Relationships With Symptoms, and Effects of a GLP-1 Antagonist. J Clin Endocrinol Metab 2019; 104: 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty S, Desai A, Halland M, et al. Relationship between Symptoms during a Gastric Emptying Study and Intestinal Chemosensitivity with Daily Symptoms Neurogastroenterol Motil 2019: e13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez Cifuentes J, Radetic M, Lopez R, Gabbard S. Clinical Predictors of Rapid Gastric Emptying in Patients Presenting with Dyspeptic Symptoms. Dig Dis Sci 2019; 13: 13. [DOI] [PubMed] [Google Scholar]
- 11.Chedid V, Brandler J, Vijayvargiya P, Park SY, Szarka LA, Camilleri M. Characterization of Upper Gastrointestinal Symptoms, Gastric Motor Functions, and Associations in Patients with Diabetes at a Referral Center. Am J Gastroenterol 2019; 114: 143–154. [DOI] [PubMed] [Google Scholar]
- 12.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy.[erratum appears in J Clin Invest 2000 Sep;106(6):803]. Journal of Clinical Investigation 2000; 106: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu WJ, Juang SW, Chin WT, Chi TC, Chang CJ, Cheng JT. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci 2000; 68: 625–634. [DOI] [PubMed] [Google Scholar]
- 14.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nature Clinical Practice Gastroenterology & Hepatology 2007; 4: 336–346. [DOI] [PubMed] [Google Scholar]
- 15.Hoybergs YM, Meert TF. The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett 2007; 417: 149–154. [DOI] [PubMed] [Google Scholar]
- 16.Bharucha AE, Manduca A, Lake DS, et al. Gastric Motor Disturbances In Patients With Idiopathic Rapid Gastric Emptying. Neurogastroenterol Motil 2010; 23: 617–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen P, Verschueren S, Ly HG, Vos R, Van Oudenhove L, Tack J. Intragastric pressure during food intake: a physiological and minimally invasive method to assess gastric accommodation. Neurogastroenterol Motil 2011; 23: 316–322, e153-314. [DOI] [PubMed] [Google Scholar]
- 18.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol 2003; 1: 264–272. [PubMed] [Google Scholar]
- 19.Imaging Schulze K. and modelling of digestion in the stomach and the duodenum. Neurogastroenterol Motil 2006; 18: 172–183. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M Integrated upper gastrointestinal response to food intake. Gastroenterology 2006; 131: 640–658. [DOI] [PubMed] [Google Scholar]
- 21.Goyal RK, Cristofaro V, Sullivan MP. Rapid gastric emptying in diabetes mellitus: Pathophysiology and clinical importance. J Diabetes Complications 2019: 107414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson B, Carlson P, Laurenti M, et al. Association between allelic variants in the glucagon-like peptide 1 and cholecystokinin receptor genes with gastric emptying and glucose tolerance. Neurogastroenterol Motil 2019: e13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai A, O’Connor M, Neja B, et al. Reproducibility of gastric emptying assessed with scintigraphy in patients with upper GI symptoms. Neurogastroenterol Motil 2018: e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am J Gastroenterol 2015; 110: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012; 24: 1076–e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil 2010; 22: 415–423, e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011; 171: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh A, Gull H, Singh RJ. Clinical significance of rapid (accelerated) gastric emptying. Clin Nucl Med 2003; 28: 658–662. [DOI] [PubMed] [Google Scholar]
- 29.Balan K, Sonoda LI, Seshadri N, Solanki C, Middleton S. Clinical significance of scintigraphic rapid gastric emptying. Nucl Med Commun 2011; 32: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE, Ravi K, Zinsmeister AR. Comparison of selective M3 and nonselective muscarinic receptor antagonists on gastrointestinal transit and bowel habits in humans. American Journal of Physiology - Gastrointestinal & Liver Physiology 2010; 299: G215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharucha AE, Isowa H, Hiro S, Guan Z. Differential effects of selective and non-selective muscarinic antagonists on gastrointestinal transit and bowel function in healthy women. Neurogastroenterol Motil 2013; 25: e35–43. [DOI] [PubMed] [Google Scholar]
- 32.Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014; 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy SN, Mena I, Snape WJ Jr., Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology 1991; 101: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 34.Manabe N, Nakamura K, Hara M, et al. Impaired gastric response to modified sham feeding in patients with postprandial distress syndrome. Neurogastroenterol Motil 2011; 23: 215–219, e112. [DOI] [PubMed] [Google Scholar]
- 35.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Current Gastroenterology Reports 2006; 8: 367–373. [DOI] [PubMed] [Google Scholar]
- 36.Sjolund K, Fasth S, Ekman R, et al. Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol Motil 1997; 9: 143–150. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Gourcerol G, Yuan PQ, et al. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am J Physiol Gastrointest Liver Physiol 2010; 298: G45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tough IR, Forbes S, Tolhurst R, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y(1) and Y(2) receptors. Br J Pharmacol 2011; 164: 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen A, Lund A, Knop FK, Vilsboll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 2018; 14: 390–403. [DOI] [PubMed] [Google Scholar]
- 40.Tough IR, Forbes S, Cox HM. Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol Motil 2018; 30: e13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bharucha AE, Kudva YC, Basu A, et al. Relationship Between Glycemic Control And Gastric Emptying In Poorly Controlled Type 2 Diabetes. Clin Gastroenterol Hepatol 2014; 13: 466–476.e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology 2006; 131: 1717–1724. [DOI] [PubMed] [Google Scholar]
- 43.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 2015; 148: 537–546.e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. American Journal of Physiology - Gastrointestinal & Liver Physiology 2003; 284: G130–137. [DOI] [PubMed] [Google Scholar]
- 45.Talley NJ, Locke GR, Saito YA, et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 2015; 149: 340–349.e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen P, Van Oudenhove L, Casteels C, Vos R, Verbeke K, Tack J. The effects of acute citalopram dosing on gastric motor function and nutrient tolerance in healthy volunteers. Aliment Pharmacol Ther 2011; 33: 395–402. [DOI] [PubMed] [Google Scholar]
- 47.Parkman HP, Urbain JL, Knight LC, et al. Effect of gastric acid suppressants on human gastric motility. Gut 1998; 42: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term Type 2 diabetes mellitus. Diabet Med 1998; 15: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi Y, Toyomasu Y, Saravanaperumal SA, et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017; 153: 521–535.e520. [DOI] [PMC free article] [PubMed] [Google Scholar]