Abstract
Background
Chronic pain and mood disorders share common neuroanatomical substrates involving disruption of the reward system. Although increase in negative affect (NA) and decrease in positive affect (PA) are well-known factors complicating the clinical presentation of chronic pain patients, our understanding of the mechanisms underlying the interaction between pain and PA/NA remains limited. Here, we used a validated task probing behavioral and neural responses to monetary rewards and losses in conjunction with functional magnetic resonance imaging (fMRI) to test the hypothesis that dysfunction of the striatum, a key mesolimbic structure involved in the encoding of motivational salience, relates to mood alterations comorbid with chronic pain.
Methods
Twenty-eight chronic musculoskeletal pain patients (chronic low back pain, n=15; fibromyalgia, n=13) and 18 healthy controls underwent fMRI while performing the Monetary Incentive Delay (MID) task. Behavioral and neural responses were compared across groups and correlated against measures of depression (Beck Depression Inventory) and hedonic capacity (Snaith-Hamilton Pleasure Scale).
Results
Compared to controls, patients demonstrated higher anhedonia and depression scores, and a dampening of striatal activation and incentive-related behavioral facilitation (reduction in reaction times) during reward and loss trials of the MID task (ps<0.05). In all participants, lower activation of the right striatum during reward trials was significantly correlated with lower incentive-related behavioral facilitation and higher anhedonia scores (ps<0.05). Finally, among patients, lower bilateral striatal activation during loss trials was correlated with higher depression scores (ps<0.05).
Conclusions
In chronic pain, PA reduction and NA increase are accompanied by striatal hypofunction as measured by the MID task.
Keywords: Chronic pain, mood alteration, monetary incentive delay task, reward circuitry, striatal hypofunction, functional magnetic resonance imaging (fMRI)
1. INTRODUCTION
Chronic pain is highly comorbid with mood disorders and is often accompanied by increased negative affect (NA) and decreased positive affect (PA), yielding poorer health-related quality of life and greater clinical burden than either condition alone (Albrecht et al., 2019b; Arnow et al., 2006; Bair et al., 2003; McWilliams et al., 2003).
Several lines of evidence support the presence of dysfunctional reward pathways in co-occurring pain and mood alteration. Anhedonia, defined as the loss of pleasure from ordinarily rewarding activities, is a cardinal symptom of major depressive disorder and is commonly associated with chronic pain (Manchikanti et al., 2002). A wealth of animal and human research indicates that pain and anhedonia share common neuroanatomical substrates involving the reward systems (de Heer et al., 2014; Garland et al., 2019; Leknes and Tracey, 2008), such as reduced activity in the striatum and anomalies in reward processing. While analgesia and pain relief are inherently rewarding and hedonic events, the experience of relief may differ in patients with chronic pain. For example, patients with fibromyalgia, a disorder with documented alterations in endogenous opioid analgesic activity (Harris et al., 2007), show dampened neural responses to anticipation of pain relief, which might reflect anhedonic response to rewarding stimuli (Loggia et al., 2014). Among the brain regions involved in the processing of rewards, the striatum is among those most consistently shown to be altered in chronic pain disorders, including chronic low back pain (Baliki et al., 2010; Baliki et al., 2012; Berger et al., 2014; Martikainen et al., 2015; Martikainen et al., 2013), burning mouth syndrome (Hagelberg et al., 2003a; Hagelberg et al., 2003b), and fibromyalgia (Wood et al., 2007b). The striatum is a key structure implicated in the learning of associations between stimuli, actions, and rewards, and motivational modulations of motor behavior (Liljeholm and O'Doherty, 2012), and as such its disruption in chronic pain may reflect different aspects of reward processing, including motivational salience and motor planning (Puglisi-Allegra and Ventura, 2012).
Notably, blunted responsiveness in regions of the reward system, including the striatum, predicts attenuated opioid-induced analgesia even in healthy participants (Wanigasekera et al., 2012). Thus, the investigation of striatal dysfunctions in chronic pain patients may enhance our understanding of the reduced efficacy of opioid treatments of chronic pain, and guide research identifying novel treatment targets that could help ameliorate the global opioid epidemic.
While a growing number of studies implicate striatal neurocircuitry in the pathophysiology of mood disorders (Epstein et al., 2006; Keedwell et al., 2005; Pizzagalli et al., 2009; Pizzagalli et al., 2008), including when comorbid with pain (Borsook et al., 2007), our knowledge remains limited. In this study, we used the monetary incentive delay (MID) task (Knutson et al., 2000), a validated functional task that probes behavioral and neural responses to monetary rewards and losses, to test the hypothesis that striatal dysfunction co-occurs with mood alterations in chronic pain. The MID task has been used in various clinical conditions linked with anhedonia or NA and alterations in the reward circuitry, such as major depression and substance use disorders (Beck et al., 2009; Knutson et al., 2008). It has been established as a reliable tool to assess incentive-specific behavioral and neurophysiological responses (Knutson et al., 2001b; Knutson and Greer, 2008) and a useful paradigm to investigate psychiatric phenotypes (Knutson and Heinz, 2015). Notably, while the MID task contains separate anticipation and outcome/consumption phases, in this study we focused only on the former, due to our specific interest in the striatum. In fact the anticipation phase is a more sensitive probe of striatal function (in healthy subjects, anticipation of reward or loss is ordinarily accompanied by strong activation of striatal regions, which is dampened in several psychiatric conditions, e.g., major depression), whereas the feedback phase of the task tends to recruit more the orbitofrontal and ventromedial prefrontal regions, with lesser striatal engagement (Oldham et al., 2018; Wilson et al., 2018).
2. METHODS AND MATERIALS
2.1. Participants
Twenty-eight patients diagnosed with chronic (>6 months) musculoskeletal pain and 18 healthy, pain-free controls (HC) completed all study procedures. Patients had either chronic low back pain (CLBP; n=15), with and without radicular pain complaints, or fibromyalgia (FM; n=13), a disorder characterized by widespread pain, muscle tenderness, and other symptoms (Wolfe et al., 1990). CLBP was defined as ongoing low back pain for more than 6 months, with a self-reported average pain intensity of at least 3 (on a 0-10 scale) during a typical week for at least half the week. All FM patients met the criteria of the American College of Rheumatology (Wolfe et al., 2011).
Exclusion criteria included any magnetic resonance imaging (MRI) contraindications (e.g., pregnancy, claustrophobia), history of notable medical disorders, illicit drug use confirmed by subjective report and urine drug screening, and routine moderate-to-high use of opioids (>60mg morphine equivalents). Because participants were simultaneously scanned with the PET radioligand [11C]PBR28, which binds to the 18kDa translocator protein (TSPO) (Albrecht et al., 2019a; Albrecht et al., 2019b; Loggia et al., 2015), we also excluded for the use of benzodiazepines (other than alprazolam, lorazepam, and diazepam, due to their documented low affinity for TSPO (Kalk et al., 2013). However, the PET results are beyond the scope of this investigation, which focuses solely on functional MRI (fMRI) responses to the MID task, and will not discussed further here.
The study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital. The participants in this dataset were included in research evaluating the role of neuroinflammation using PET in FM (Albrecht et al., 2019a) and CLBP (Albrecht et al., 2019b; Loggia et al., 2015), which did not report on any task-based fMRI results. All participants were enrolled between 10/30/2015 and 11/29/2017 and provided written informed consents to a protocol approved by the Partners Human Research Committee.
2.2. Procedure
After an initial phone screening, eligible participants completed a 2-hour visit for clinical assessment and training, including a medical history intake and a physical examination.
On a separate day, participants underwent a simultaneous PET/MRI scan in conjunction with the MID task. Periodically throughout the scan, participants rated their pain on a visual analog scale, anchored by 0 (“No pain at all”) and 100 (“Most intense pain tolerable”). One during-scan pain rating for one CLBP patient was missing. Participants were compensated for their participation in the study and received instructions that they could earn an additional $17-$22 depending on their task performance (see section 2.3 for detail). During the study, all participants (minus one CLBP patient) completed the Beck Depression Inventory (BDI-1A; Beck et al., 1961), which has shown good psychometric properties in individuals with chronic pain (Geisser et al., 1997). In addition, with the exception of 2 FM patients, all participants completed the Snaith-Hamilton Pleasure scale (Snaith et al., 1995), which specifically assesses anhedonia. Using a dimensional approach (Franken et al., 2007), each SHAPS item was scored on a 1-4 scale (1=“Strongly agree”; 2=“Agree”; 3=“Disagree”; 4=“Strongly disagree”). The scoring proposed in the original publication of the scale (Snaith et al., 1995) was also used, recoding the four response categories in dichotomous categories (i.e., 0 for either “Agree” or “Strongly agree”, 1 for either “Disagree” or “Strongly disagree”), but only to classify participants as anhedonic per the original cutoff (score > 2, original scoring).
2.3. MID Task
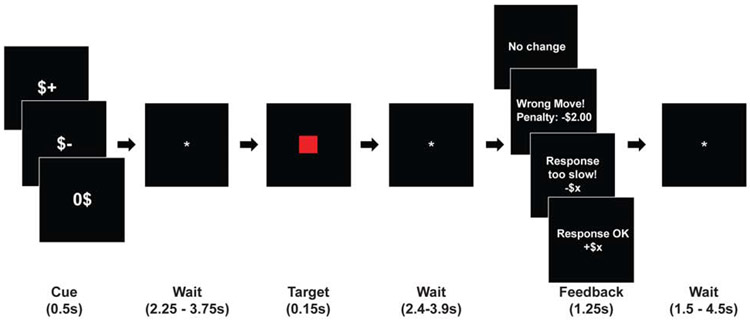
The specific trial structure of the MID task used in this study followed that of previous works (Admon et al., 2017) (see Figure 1). The task consisted of three runs, each lasting approximately five minutes and containing 24 trials (8 reward trials, 8 loss trials, and 8 no-incentive trials) in a pseudorandomized order, following an initial practice run. At the onset of each trial, the anticipatory visual cue appeared for 0.5s and indicated the potential outcome (“$+,” “$−” or “0$” for reward, loss or no-incentive trials, respectively). Following a variable jittered anticipatory period (2.25—3.75s), the participants saw a red target square appear for 0.15s and pressed a key as soon as possible upon seeing the target. A successful trial was defined as button press within the 70th percentile of the participant’s RT from the immediately preceding run. After a second variable interval (2.4—3.9s), visual feedback (1.25s) indicated the trial outcome. If successful, participants gained money ($1.98—2.32; pseudorandomized) on reward trials and avoided losing money (“no change”) on loss trials. Conversely, participants did not gain any money (“no change”) on reward trials and lost money ($1.82—2.19; pseudorandomized) on loss trials if their RTs fell outside of the 70th percentile. No-incentive trials always yielded “no change” feedback. “Wrong moves” (penalty: $2), occurred when participants either pressed the button before the target square appeared or gave no response. There was no feedback on cumulative earning, and a variable interval (1.5—4.5s) separated the trials. An initial calibration run, identical to the task runs but without any feedback, was completed immediately before the first experimental MID run to generate baseline RT calculations. In healthy subjects, anticipation of reward and loss is ordinarily accompanied by strong activation of striatal regions (including the ventral striatum, head of the caudate, and putamen), and faster incentive-related response time (RT) speed compared to that in the non-incentivized trials (Oldham et al., 2018; Wilson et al., 2018). In patients with major depression, however, the reward and loss anticipation has been associated with dampened striatal activations, and the cues lose their facilitatory effect on RTs (Pizzagalli et al., 2009).
Figure 1: MID trial structure.
In the MID task, for each trial, participants were asked to press a button as quickly as possible after a visual target appears on a screen. If the button press occurred within a pre-specified temporal window after target presentation, the trial was deemed successful and the participant could either gain a monetary reward (“reward” trials), avoid a monetary loss (“loss” trials), or induce no change to the cumulative earning (“no-incentive” trials), depending on trial type. At the beginning of each trial, a cue indicated the upcoming trial type ($+, $− and 0$, for “reward”, “loss” or “no-incentive” trials, respectively).
2.4. Neuroimaging Data Acquisition and Processing
Scans were performed on an integrated PET/MRI scanner consisting of a dedicated brain avalanche photodiode-based PET scanner in the bore of a Siemens 3T Tim Trio MRI (Siemens Corp, Erlangen, Germany) (Kolb et al., 2012). A multi-echo T1-weighted magnetization-prepared rapid acquisition with gradient echo (MEMPRAGE) volume was acquired prior to tracer injection (TR/TE1/TE2/TE3/TE4=2530/1.64/3.5/5.36/7.22ms, flip angle=7°, voxel size=1mm isotropic) for anatomical localization and spatial normalization of the imaging data (and generation of attenuation correction maps (Izquierdo-Garcia et al., 2014)).
The participants underwent four ~5-minute BOLD fMRI scans, each corresponding to one initial calibration and three experimental MID task runs (TR/TE=2s/30ms, flip angle=90°, voxel size=3.1x3.1x3mm, 37 slices, 142 volumes). The calibration imaging data were collected to generate the same acoustic and physical environment the participants would experience during the experimental MID runs, thus providing accurate calibration of the RTs to be used in the first run. This run also allowed us to probe the participants’ ability to comfortably complete the task. Although patients reported some pain during scanning, no participant reported discomfort significant enough to interfere with task completion. fMRI data were pre-processed and analyzed using FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl/), AFNI (Automated Functional NeuroImaging, http://afni.nimh.nih.gov/afni), and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) software packages. Data were corrected for slice-timing, motion, and B0 field inhomogeneities. Subsequently performed were brain extraction, co-registration to the MEMPRAGE, spatial smoothing with a 6mm Gaussian kernel, and nonlinear registration to Montreal Neurological Institute (MNI) standard space.
2.5. Statistical Analysis
All group effects in demographic, behavioral, and imaging outcomes were first tested by combining all patients into a singular “Pain” group, to maximize our statistical power (primary analysis). While CLBP and FM may have different etiology and pathophysiology, both are characterized by musculoskeletal pain. Combining data is justified in this context because 1) this project focuses on the impact of chronic pain on reward processing, and not on the somatic aspects of pain, and 2) disruptions in reward processing have been similarly hypothesized in multiple chronic pain disorders, including FM and CLBP (Berger et al., 2014; Loggia et al., 2014). To evaluate whether any effects observed in the primary analyses might be driven by one subgroup, follow-up secondary group analyses were performed, entering CLBP and FM separately in the statistical models.
2.5.1. Demographic and Behavioral Outcomes
Group differences in age and questionnaire scores were tested using an analysis of variance (ANOVA) or unpaired t-tests as applicable, while group differences in sex distribution were tested using a chi-square test.
RTs were averaged separately per trial type (reward, loss, no-incentive) after removal of “wrong moves,” as previously described (Pizzagalli et al., 2009). One FM participant was excluded from the behavioral analysis due to a calibration error where the 50th percentile was used instead of the 70th. While this calibration may have slightly affected the difficulty of the task, the same participant was however included in the imaging analyses, as it was presumed that the subjective experience of reward/loss anticipation (as opposed to the performance of the button press itself) would not have been meaningfully affected by a minor change in the difficulty of the task. Moreover, repeating the fMRI analyses after excluding the same patient still yielded significant group effects (see Results).
We first evaluated the RTs using analyses of covariance (ANCOVA) with Group and Trial as factors and age and sex as covariates of no interest. The aim of these analyses was to test for a significant Trial type effect on RTs, as a marker for a successful experimental manipulation (shorter RT in reward/loss trials indicating behavioral facilitation and thus a correct implementation of the MID task (Pizzagalli et al., 2009)). Although these analyses enabled observation of Group effects or Group×Trial interactions, the primary evaluation of these effects utilized a different ANCOVA model, in which the no-incentive RT was added as a covariate (rather than a level in the factor Trial). This model produced maximal sensitivity in assessing the expected incentive-related RT reduction, by correcting for the general RT differences across groups. While age and sex were not statistically different in the primary analyses (comparing all pain patients with controls), there were marginal or significant group differences when the pain groups were evaluated separately (see Supplementary Materials). Therefore, these variables were used as covariates in all group analyses.
Significant effects and interactions were analyzed using Tukey’s HSD. Pearson’s correlations were employed to investigate the relationship between behavioral measures (RT difference scores, BDI scores, SHAPS scores) in the patients. Demographic and behavioral data were analyzed using Statistica 13 (StatSoft).
2.5.2. Neuroimaging Data
In the first-level fMRI analysis, the anticipatory time period (including visual cue and anticipatory period prior to target presentation), button press, feedback/outcome (gain, loss, no change, wrong move) and six head motion parameters (3 translations, 3 rotations) were modeled as regressors per trial type. Parameter estimates for each contrast of interest (“reward > no-incentive” and “loss > no-incentive”) were computed for each run, then averaged for each subject using a fixed-effect analysis (3 runs for all subjects, except for one CLBP and two FM participants, for whom technical and calibration issues prevented the execution of the third run). Mixed-effect analyses (FSL’s FLAME1) were used to create group maps for each group, an omnibus group map generated by averaging all three groups (in order to functionally define region-of-interests (ROI); see below) and to compare HC against pain patients, both separately and combined, with sex and age as regressors of no interest. Results were corrected for multiple comparisons using a cluster-forming threshold of z≥3.1 and a corrected cluster significance threshold of p<0.05. Images were visualized with Freesurfer’s Freeview tool (https://surfer.nmr.mgh.harvard.edu/fswiki/FreeviewGuide).
In addition to the whole-brain voxelwise analyses, we pursued ROI analyses using functionally-defined striatal ROIs. These ROIs were obtained from a conjunction analysis of the entire sample using the omnibus activation maps for “reward > no-incentive” and “loss > no-incentive” anticipation contrasts applying easythresh_conj (https://warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/fsl/easythresh_conj.sh). The resulting regions were intersected with the binarized Oxford-Imanova Striatal structural atlas, then lateralized to define functionally-localized striatal ROIs. Note that this strategy was pursued instead of running masked voxelwise analyses using anatomically defined striatal masks because the striatal activations typically observed during the MID anticipatory phase do not completely overlap with the available anatomical striatal masks (e.g., they usually tend to load more on ventral striatum and ventromedial aspects of caudate and putamen, and typically extend slightly outside the anatomical boundary of striatal labels (Oldham et al., 2018)).
From these functionally defined striatal ROIs, we extracted the mean contrast of parameter estimates (COPEs), or beta weights. Applying statistical models analogous to those in the RT analyses, we first evaluated the striatal COPEs with ANCOVAs, setting Group and Trial as factors and age and sex as covariates of no interest. In contrast to the behavioral analyses, in these ROI analyses we do not report the statistical significance related to the Trial type effect, and data are only displayed to appreciate effect sizes. This omission is to avoid circularity, since the ROIs originated from the significant Trial type effects from the voxelwise analyses. Paralleling the RT analyses, the main evaluation of Group and Group×Trial type effects was performed including the no-incentive COPEs as a covariate (rather than a level in the factor Trial). This latter analysis enabled maximal sensitivity to the striatal activation expected in the reward and loss trials (Wilson et al., 2018), correcting for any general differences in striatal function.
Pearson’s correlations were calculated to investigate the relationship between change in brain activation within the ROI and change in RT (both compared to the no-incentive trials), and clinical symptoms (BDI and SHAPS scores) in patients. In all analyses and graphs, the RT difference is denoted as “no-incentive minus reward (or loss)” and the striatal activation difference as “reward (or loss) minus no-incentive”. Correlation analyses were performed across participants whenever the variable presented a continuous distribution and comparable range across groups (e.g., SHAPS, RT, brain activations). Correlations were evaluated in patients only, if variables had a discontinuous distribution across groups and/or presented limited range in the controls (e.g., BDI, pain ratings). ROI analyses were performed on Statistica 13.
3. RESULTS
3.1. Demographics
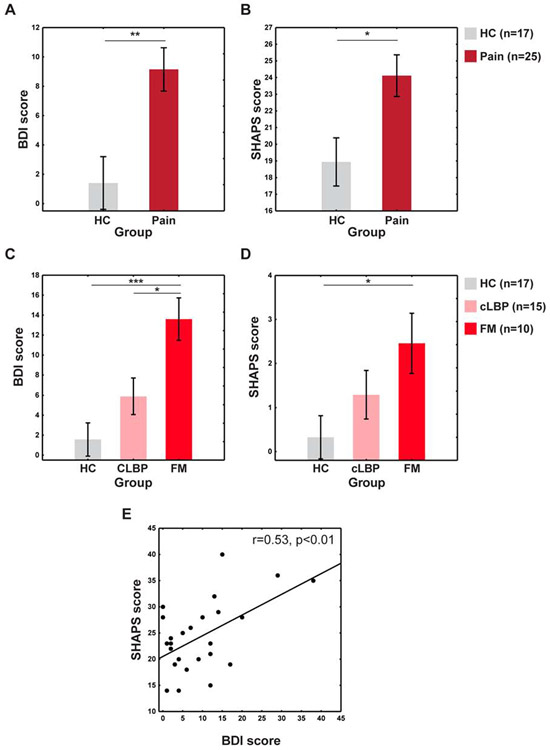
Demographic characteristics of the groups are presented in Table S1. No differences in age (F(1,44)=1.72, p=0.2) or sex (p=0.14) were observed between the pain group and healthy controls. When controlling for sex and age, patients had significantly higher BDI scores than controls (F(1,41)=10.76, p<0.01) (Figure 2A). The average BDI score for patients was 9.15, although 12 patients (4 CLBP and 8 FM patients) had a score of at least 10 (the threshold for mild-to-moderate depression for the version used in the current study (Beck et al., 1988)). Pain patients also had significantly higher SHAPS scores (i.e., higher anhedonia) (F(1,40)=7.18, p=0.01) (Figure 2B). Figures 2C-D display data separating the pain group into CLBP and FM subgroups (for more information, see Supplementary Materials). Using the original SHAPS scoring, 8 patients (2 CLBP and 6 FM) scored above a 2, indicating anhedonia (Snaith et al., 1995). BDI and SHAPS scores were significantly correlated in the pain group (r=0.53, p<0.01) (Figure 2E). Positive associations between pain ratings and BDI (r=0.31) and SHAPS (r=0.28) were observed but did not reach statistical significance (ps≥0.12).
Figure 2: BDI and SHAPS results.
A. BDI score comparison between pain and HC groups. B. SHAPS score comparison between pain and HC groups. C-D. BDI data (C) and SHAPS data (D) splitting the pain group into CLBP and FM subgroups. E. SHAPS and BDI score correlation within the pain group. *p<0.05, **p<0.01, ***p<0.001.
3.2. Behavioral Responses to the MID Task
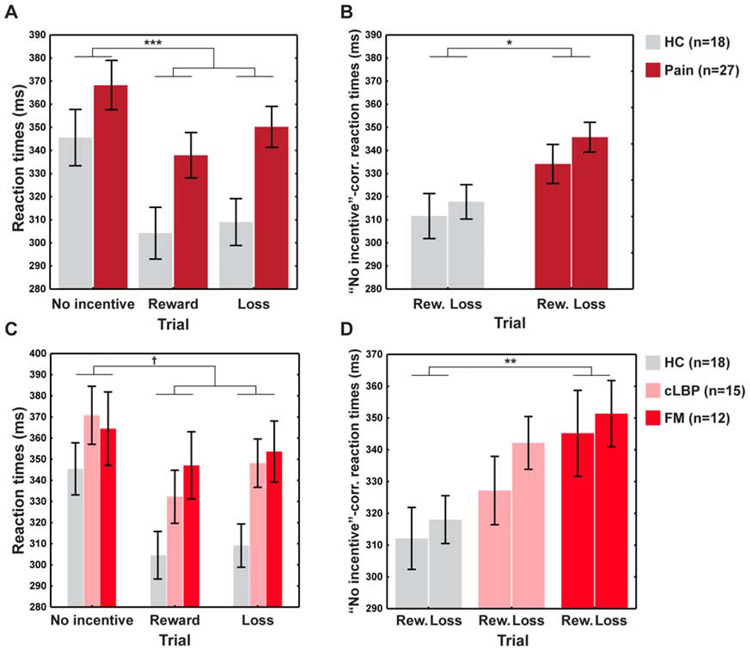
A significant effect of Trial type emerged using absolute RTs (F(2,82)=4.27, p=0.02). Post-hoc pairwise comparisons clarified that all participants were faster during reward (p=0.0001) and loss trials (p=0.0002) than during no-incentive trials, indicating that the task elicited the intended effects. RTs for reward and loss trials did not differ (p=0.19). The pain group was significantly slower than controls (F(1,41)=6.0, p=0.02), irrespective of trial type (Group×Type interaction: F(2,82)=1.13, p=0.33) (Figure 3A).
Figure 3: Behavioral MID results.
A. Analyses of the absolute RTs. B. Analyses of the “No-incentive”-corrected RT (i.e., RTs of the reward/loss trials, correcting for those of the no-incentive trials). C-D. Absolute RTs (C) and “No-incentive”-corrected RTs (D) splitting the pain group into CLBP and FM subgroups. †=0.0502, *p<0.05, **p<0.01, ***p<0.001.
When controlling for differences in RTs during the no-incentive trials, the RTs from reward or loss trials were significantly longer in patients (F(1,40)=5.48, p=0.02), indicating that the relative facilitatory effect of reward or loss cues on RTs was attenuated in patients. No significant Group×Trial interaction emerged from the relative RTs (F(1,40)=0.34, p=0.56), suggesting similar effects from loss and reward trials (Figure 3B). Neither BDI nor SHAPS correlated with RT difference scores (ps>0.33). Stratifying the pain patients into CLBP and FM subgroups (Figures 3C-D) revealed a gradient where CLBP patients performed intermediately to the HC and FM groups, paralleling the pattern observed in the depression and anhedonia scores (Figure 2C-D) (for more information, see Supplementary Materials).
Overall, our results are consistent with the hypothesis that chronic pain patients display dampened behavioral response to reward and loss cues, even after correcting for the general slowness of pain patients.
3.3. Neural Responses to the MID Task
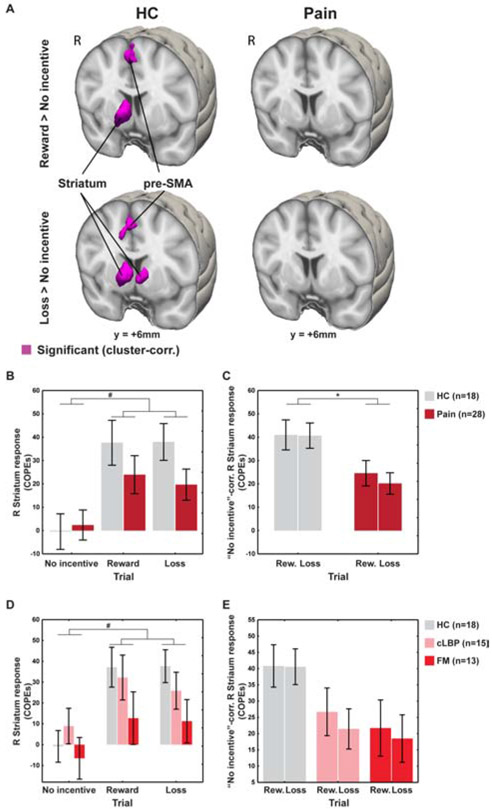
In a whole-brain voxelwise analysis of healthy controls, both reward and loss trials were associated, as expected, with significant activation of the striatum (in the caudate nucleus –right-sided for the former, bilateral for the latter–, right nucleus accumbens, and right putamen) and the supplementary and pre-supplementary motor areas (SMA/pre-SMA), compared to the no-incentive trials. Chronic pain patients, in contrast, did not demonstrate any significant activations (see Figure 4A; Table 1). Figure 4B displays the brain responses extracted from the striatum of both pain and HC groups.
Figure 4: Imaging MID results.
A. Whole-brain voxelwise analyses. B. Brain responses extracted from functionally-defined striatum in both pain and HC groups. C. ROI analyses of the functionally-defined right striatum, using “No-incentive”-corrected COPEs (i.e., COPEs of the reward/loss trials, correcting for those of the no-incentive trials). D-E. ROI analyses using COPEs (D) and “No-incentive”-corrected COPEs (E) splitting the pain group into CLBP and FM subgroups. *p<0.05. #Trial type effect significant in the voxelwise analyses.
TABLE 1:
MNI coordinates and cluster size from the voxelwise analyses.
| Local Maxima | ||||||
|---|---|---|---|---|---|---|
| Cluster Size (# voxels) |
Cluster size (p value) |
Z | MNI x (mm) |
MNI y (mm) |
MNI z (mm) |
Label |
| Reward minus no-incentive | ||||||
| Controls | ||||||
| 5.02 | 6 | 12 | 4 | R head of the caudate nucleus | ||
| 529 | 0.000819 | 4.42 | 8 | 16 | −2 | R nucleus accumbens |
| 3.79 | 14 | 12 | −6 | R putamen | ||
| 254 | 0.029 | 4.03 | 0 | −2 | 66 | SMA/pre-SMA |
| Pain | ||||||
| n.s. | ||||||
| Loss minus no-incentive | ||||||
| Controls | ||||||
| 5.02 | 8 | 8 | 4 | R head of the caudate nucleus | ||
| 768 | 1.26E-05 | 4.15 | −8 | 8 | 2 | L head of the caudate nucleus |
| 3.69 | 10 | 12 | −4 | R nucleus accumbens | ||
| 3.38 | 14 | 10 | −6 | R putamen | ||
| 270 | 0.0118 | 3.92 | 0 | −4 | 66 | SMA/pre-SMA |
| Pain | ||||||
| n.s. | ||||||
While the direct group comparison of these contrast maps was not significant in the whole-brain voxelwise analyses, ROI analyses (Figure 4C) showed that, when controlling for anticipatory brain responses in the no-incentive trials, the right striatal responses in the reward and loss trials were significantly smaller in patients than in controls (F(1,41)=7.21, p=0.01). Again, no significant Group×Trial interaction was observed for these contrasts, (F(1,41)=0.29, p>0.05), signifying that anticipatory striatal hypofunction was similarly observed in reward and loss trials. When the analyses were repeated after the exclusion of the FM patient with the RT calibration error, the Group effect remained significant (F(1,40)=8.34; p=0.006) and the Group×Trial interaction remained not significant (F(1,40)=0.23, p>0.05). Similar to the behavioral results, a gradient also appeared in the striatal responses, with the CLBP patients displaying intermediate activation between the HC and FM groups (Figures 4D-E; for more information, see Supplementary Materials).
Overall, the imaging results demonstrate a dampening in the brain responses to anticipation of reward and loss, paralleling the patterns observed in the behavioral results.
3.4. Association between imaging and behavioral/clinical measures
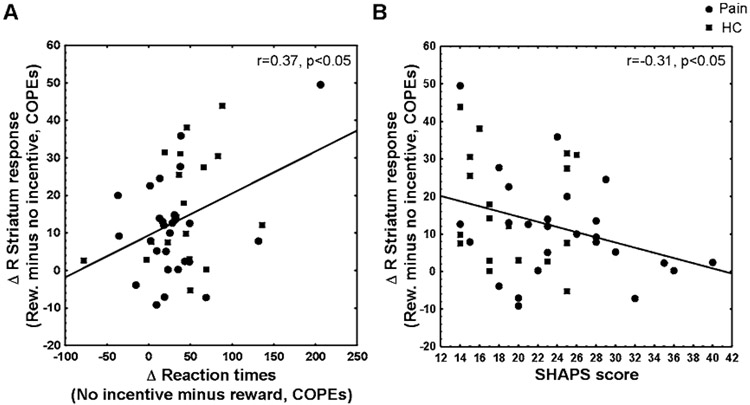
Across all participants, lower activation of the right striatum during anticipation of reward was significantly correlated with slower incentive-related behavioral facilitation (r=0.37, p=0.013; Figure 5A), and higher SHAPS scores (r= −0.31, p=0.037; Figure 5B). The same correlations did not reach statistical significance for the right striatum during loss trials (ps≥0.11), or for the left striatum in either reward or loss trials (ps≥0.09).
Figure 5: Correlation between imaging and behavioral measures in reward trials.
A. Change in reward COPEs (vs. no-incentive COPE) of the functionally-defined right striatum correlated with change in reward-trial RTs. B. Change in reward COPEs (vs. no-incentive COPE) of the functionally-defined right striatum correlated with BDI scores.
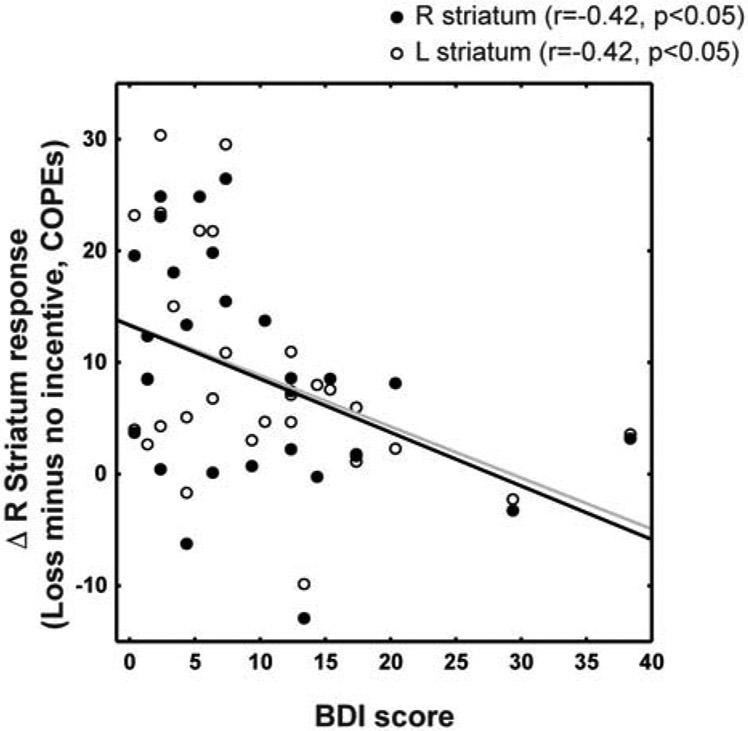
In patients, diminished striatal activation was correlated with higher BDI scores during loss trials (right striatum: r=−0.42, p=0.026; left striatum: r=−0.42, p=0.027) (Figure 6). The correlation between striatal activation and BDI scores was not significant in the reward trials, for both right (r=−0.37, p=0.056) and left striatum (r=−0.29, p=0.14). Pain ratings also were not significantly correlated with either reaction times ( −0.101<rs<−0.018, ps>0.64) or striatal responses (−0.185<rs<−0.085, ps>0.40) in partial correlation analyses correcting for sex and age.
Figure 6: Correlation between imaging and behavioral measure in loss trials.
Change in loss COPEs (vs. no-incentive COPE) of the functionally-defined right striatum correlated with BDI scores.
For results from the analyses separating patients into CLBP and FM subgroups, see Supplementary Materials.
4. DISCUSSION
In the current study, we report that chronic pain patients, when compared with healthy controls, show 1) increased negative affect and anhedonia, 2) smaller incentive-related behavioral facilitation to reward and loss cues and 3) lower anticipatory activation in the striatum. Striatal hypofunction across groups was associated with slower behavioral responses and greater levels of anhedonia, while in patients, higher depression scores were correlated with low bilateral striatal activation during loss trials.
The absence of robust striatal activations in chronic pain patients supports the general view that dysfunctions within the mesolimbic dopamine pathways play a role in the pathogenesis of chronic pain (Baliki et al., 2012), and its comorbidity with mood alterations (Schwartz et al., 2014). Notably, the most pronounced activation difference between pain patients and healthy controls was observed in the caudate nucleus, a region of the striatum known to control motivation (Delgado et al., 2004) and is highly innervated by dopaminergic neurons whose projections are sent from the substantia nigra pars compacta. We also observed activity in the nucleus accumbens, another striatal region with high density of dopaminergic neurons and whose activity is consistently associated with anticipation of reward as well as motivational salience and reward-oriented motor planning as probed by the MID task (Knutson et al., 2001a). While our understanding of the neurophysiological mechanisms underlying negative affect and anhedonia in chronic pain remains incomplete, preclinical pain models provide some clues. For instance, the excitatory synaptic transmission of D2 dopamine receptor expressing medium spiny neurons in the nucleus accumbens show a galanin receptor 1-mediated depression, implicating alterations in the indirect pathway of the basal ganglia (Schwartz et al., 2014). Human imaging studies in FM patients have shown hypoactivation in the mesolimbic dopamine systems (Loggia et al., 2014), low D2 dopamine receptor binding potential in the striatum (Wood et al., 2007b), and low presynaptic dopamine activity (Wood et al., 2007a), while CLBP patients have also demonstrated low striatal dopamine receptor binding potential (Martikainen et al., 2015). Even though the research is mixed in relating particular striatal substrates and subnuclei with specific dopaminergic functions and behavioral manifestations, converging lines of evidence support the role of dopaminergic alterations as mediators of anhedonia and NA comorbid with chronic pain (Finan and Smith, 2013; Jarcho et al., 2012; Scott et al., 2006; Taylor et al., 2016; Tiemann et al., 2014). Moreover, in healthy participants, ventral striatum activation positively covary with the activation of the periaqueductal gray and the pain reduction induced by positive mood change (Villemure et al., 2012), further providing a link between striatum, pain, and affect.
Although a previous study probed reward processing in FM patients using the MID task (Martucci et al., 2018), the current study differs from the existing study in important ways. In particular, the former study did not observe any significant group differences in RTs or striatal activation. A possible explanation for this discrepancy is the fact that while our study employed an anticipatory period of variable duration, the previous study used a fixed duration. The latter experimental choice could have rendered the task performance more dependent on the ability to correctly estimate the duration of the anticipatory period, than on the actual identification of the target itself. In addition, our use of shorter runs (three 5-minute 24-trial blocks, vs. 90 trials in 2 blocks) may have helped minimize attention fatigue, and the use of a functionally-defined striatal mask (as opposed to anatomically defined ROIs) may have afforded greater sensitivity to detect group differences. Our study also included both FM and CLBP patients, which enabled us to observe a HC>CLBP>FM gradient in all principal outcomes evaluated. The dampening of the behavioral and striatal responses to reward and loss cues, and the levels of negative affect and anhedonia were all greatest in FM patients, followed by CLBP patients. This gradient provides further support for the link between striatal hyporesponsiveness to incentives and mood alterations in chronic pain.
Among the limitations of this study, we note a relatively small sample size, particularly within each pain subgroup (CLBP and FM). Because in this study it was assumed that striatal hypofunction was a general feature of chronic pain, irrespective of specific etiology and clinical characteristics of the patients evaluated, we elected to combine data of patients with different pain disorders. While we feel that the focus on reward/motivational (and not somatosensory) processing justified this approach, it is certainly possible that some nuanced differences may exist in how striatal physiology is affected in different pain disorders. Future studies with larger sample sizes will be necessary to fully evaluate how certain features of chronic pain (e.g., etiology, degree of “centralization”) may relate to striatal dysfunction. Similarly, future studies will need to compare patients with or without pain, but comparable levels of anhedonia, in order to evaluate the specific contributions of pain state to the striatal alterations reported here. In addition, the increase in reaction times during the MID task was not selective for the reward and loss trials but was also observed during the neutral trials. This overall trend in the patients is suggestive of a generalized reduction in motor processing speed, which could be due to a number of factors including an effect of pain on cognition (Seminowicz et al., 2004), or the use of pain medications (Hienz et al., 2001). However, it should be noted that the group differences in the incentive-related trials remained significant even after correcting for differences in reaction times in the neutral trials, indicating that these effects go beyond a simple overall reduction in motor reactivity, and are genuinely reflective of a dampened incentive-related behavioral facilitation. Furthermore, the causal relationship between striatal hypofunction and pain-comorbid mood alterations cannot be resolved by this investigation and will likely require studies using interventions such as dopamine precursor depletion. Future research is also warranted to investigate the trial-type-dependent lateral activation of the striatum and the reproducibility of the findings in non-musculoskeletal pain conditions (e.g., neuropathic pain).
With the ever-growing demand for effective treatments of chronic pain, the need to advance our understanding of chronic pain is more critical than ever. Unraveling the interactions between chronic pain and the nervous systems could lend a new insight into identifying better predictors and novel treatment targets for pain and its comorbidities.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the participants for their kind contribution to this research. We would also like to thank Dr. Susanne Becker for helpful comments.
This research was supported by grants from the National Institute of Health (NIH) R01 NS094306-01A1 (MLL), R01 NS095937-01A1 (MLL), R21 NS087472-01A1 (MLL), and Department of Defense W81XWH-14-1-0543 (MLL) and an Early Career Award from IASP (MLL). VN was supported by grants from the Office of the Director (OT2-OD023867), P01 AT009965, R61 AT009306, R33 AT009306, R01 AT007550, and R01 AR064367. DAP was partially supported by R37 MH095809 and R01 MH101521.
Footnotes
DISCLOSURES
MK, IM, DSA, RA, AT-C, CB, EP, PK, RRE, AS, and VN have no biomedical financial interests or potential conflict of interest to report. Over the past three years, M.L.L. received consulting fees from Shionogi Inc for activities unrelated to the current study. D.A.P received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, CompassPathway, Posit Science and Takeda Pharmaceuticals USA and an honorarium from Alkermes for activities unrelated to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, Vitaliano G, Pizzagalli DA, 2017. Dopaminergic Enhancement of Striatal Response to Reward in Major Depression. Am J Psychiatry 174, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Forsberg A, Sandstrom A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Hoglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML, 2019a. Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation. Brain Behav Immun 75, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Kim M, Akeju O, Torrado-Carvajal A, Edwards RR, Zhang Y, Bergan C, Protsenko E, Kucyi A, Wasan AD, Hooker JM, Napadow V, Loggia ML, 2019b. The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry. [DOI] [PMC free article] [PubMed]
- Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C, 2006. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med 68, 262–268. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K, 2003. Depression and pain comorbidity: a literature review. Arch Intern Med 163, 2433–2445. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV, 2010. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV, 2012. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J, 2009. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry 66, 734–742. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG, 1988. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review 8, 77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Arch Gen Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Berger SE, Baria AT, Baliki MN, Mansour A, Herrmann KM, Torbey S, Huang L, Parks EL, Schnizter TJ, Apkarian AV, 2014. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res Notes 7, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA Jr., Shaw M, Renshaw P, Elman I, Levine J, 2007. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11, 7–20. [DOI] [PubMed] [Google Scholar]
- de Heer EW, Gerrits MM, Beekman AT, Dekker J, van Marwijk HW, de Waal MW, Spinhoven P, Penninx BW, van der Feltz-Cornelis CM, 2014. The association of depression and anxiety with pain: a study from NESDA. PLoS One 9, e106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA, 2004. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex 14, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA, 2006. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Finan PH, Smith MT, 2013. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev 17, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P, 2007. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord 99, 83–89. [DOI] [PubMed] [Google Scholar]
- Garland EL, Trøstheim M, Eikemo M, Leknes S, 2019. Anhedonia in Chronic Pain and Prescription Opioid Misuse. PsyArXiv Preprints. [DOI] [PubMed]
- Geisser ME, Roth RS, Robinson ME, 1997. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain 13, 163–170. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, Nagren K, Eskola O, Jaaskelainen SK, 2003a. Altered dopamine D2 receptor binding in atypical facial pain. Pain 106, 43–48. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, Luutonen S, Nagren K, Jaaskelainen S, 2003b. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 101, 149–154. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK, 2007. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 27, 10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienz RD, Zarcone TJ, Brady JV, 2001. Perceptual and motor effects of morphine and buprenorphine in baboons. Pharmacol Biochem Behav 69, 305–313. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Garcia D, Hansen AE, Forster S, Benoit D, Schachoff S, Furst S, Chen KT, Chonde DB, Catana C, 2014. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med 55, 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED, 2012. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 153, 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Owen DR, Tyacke RJ, Reynolds R, Rabiner EA, Lingford-Hughes AR, Parker CA, 2013. Are prescribed benzodiazepines likely to affect the availability of the 18 kDa translocator protein (TSPO) in PET studies? Synapse 67, 909–912. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML, 2005. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58, 843–853. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D, 2001a. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21, RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH, 2008. Neural responses to monetary incentives in major depression. Biol Psychiatry 63, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001b. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM, 2008. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci 363, 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Heinz A, 2015. Probing psychiatric symptoms with the monetary incentive delay task. Biol Psychiatry 77, 418–420. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D, 2000. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12, 20–27. [DOI] [PubMed] [Google Scholar]
- Kolb A, Wehrl HF, Hofmann M, Judenhofer MS, Eriksson L, Ladebeck R, Lichy MP, Byars L, Michel C, Schlemmer HP, Schmand M, Claussen CD, Sossi V, Pichler BJ, 2012. Technical performance evaluation of a human brain PET/MRI system. Eur Radiol 22, 1776–1788. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I, 2008. A common neurobiology for pain and pleasure. Nat Rev Neurosci 9, 314–320. [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O'Doherty JP, 2012. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci 16, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V, 2014. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol 66, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM, 2015. Evidence for brain glial activation in chronic pain patients. Brain 138, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Pampati V, Beyer C, Damron K, Barnhill RC, 2002. Comparison of psychological status of chronic pain patients and the general population. Pain Physician 5, 40–48. [PubMed] [Google Scholar]
- Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, Stohler CS, Zubieta JK, 2015. Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J Neurosci 35, 9957–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen IK, Pecina M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, Harris RE, Stohler CS, Zubieta JK, 2013. Alterations in endogenous opioid functional measures in chronic back pain. J Neurosci 33, 14729–14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci KT, Borg N, MacNiven KH, Knutson B, Mackey SC, 2018. Altered prefrontal correlates of monetary anticipation and outcome in chronic pain. Pain 159, 1494–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW, 2003. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 106, 127–133. [DOI] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yucel M, Lorenzetti V, 2018. The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping 39, 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty D, Iosifescu DV, Rauch SL, Fava M, 2009. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M, 2008. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 43, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Ventura R, 2012. Prefrontal/accumbal catecholamine system processes high motivational salience. Front Behav Neurosci 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC, 2014. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK, 2006. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci 26, 10789–10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD, 2004. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain 112, 48–58. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Becker S, Schweinhardt P, Cahill C, 2016. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157, 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann L, Heitmann H, Schulz E, Baumkotter J, Ploner M, 2014. Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS One 9, e96167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C, Laferriere AC, Bushnell MC, 2012. The ventral striatum is implicated in the analgesic effect of mood changes. Pain Res Manag 17, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, Tracey I, 2012. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A 109, 17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP, Colizzi M, Bossong MG, Allen P, Kempton M, Mtac, Bhattacharyya S, 2018. The Neural Substrate of Reward Anticipation in Health: A Meta-Analysis of fMRI Findings in the Monetary Incentive Delay Task. Neuropsychol Rev 28, 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, 2011. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. , 1990. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33, 160–172. [DOI] [PubMed] [Google Scholar]
- Wood PB, Patterson JC 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL, 2007a. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain 8, 51–58. [DOI] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA, 2007b. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 25, 3576–3582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.