Abstract
The prevalence of obesity is increasing globally, leading to significantly increased morbidity, mortality, and health care costs. However, there is a lack of effective treatment options that can treat patients with obesity less invasively than with bariatric surgery. Bariatric arterial embolization (BAE) is an image-guided, minimally invasive, percutaneous procedure that is currently being investigated in preclinical animal models and early clinical trials. If successful, BAE may represent a viable interventional approach for obesity treatment. The purpose of this article is to introduce the physiological and anatomical rationale for BAE, review techniques involved in performing BAE for weight modulation, and provide up-to-date preclinical evidence that supports the translation of BAE into patients.
Introduction
Over the past few decades, obesity has become a major global health epidemic affecting both adults and children.1 In the United States alone, nearly 93.3 million (39.8%) adults and 13.7 million (18.5%) children and adolescents are obese.2 Despite medical advances and conservative lifestyle changes, the obesity rate continues to rise rapidly, leading to a catastrophic impact on morbidity, mortality, and health care costs. Obesity has been increasingly recognized as one of the major contributors to myriad diseases, including cardiovascular disease, liver disease, type 2 diabetes, degenerative joint disease, and certain types of cancers.1, 2
Traditional lifestyle changes alone have shown a moderate benefit for obesity management, with an average weight loss of 5–10% in overweight (body mass index (BMI)=25 - 29.9 kg/m2) and obese (BMI=30 - 34.9 kg/m2) patients.3, 4 However, such benefits are often unsustained, with patients regaining any weight lost in as little as three years after initiating lifestyle changes.5 Pharmacotherapy with drugs, such as Phentermine, Orlistat, and Liraglutide, has also only led to modest weight loss of 5-10.5%.6 Given the side effects of these drugs and issues with patient compliance, pharmacotherapy has been used as an adjuvant therapy to lifestyle changes. To date, the only proven effective treatment with demonstrated long term, sustained weight loss is bariatric surgery, such as Roux-en-Y gastric bypass and sleeve gastrectomy.7 However, these surgical procedures are usually reserved for patients with BMI > 40 kg/m2, or BMI > 35 kg/m2 with obesity-associated comorbid conditions for which conventional therapies have failed.8 Due to the high invasiveness, cost, or concerns with procedure-related complications, only ~1% of morbidly obese patients are treated surgically. Therefore, less invasive therapies with potentially less complications that can target patients with obesity are warranted.
Bariatric Arterial Embolization: Rationale
The gastric fundus has long been recognized as the epicenter of the neuroregulatory pathway of the stomach by regulating hunger stimulation and satiety.9, 10 In particular, ghrelin, a 28-amino acid peptide produced by the X/A cells in the fundus11, is the only hormone discovered to date that directly stimulates appetite and induces positive energy balance, leading to body weight gain10, 12 In obese patients, ghrelin-producing cells have higher density and increased expression levels in the gastric musoca13. Correspondingly, ghrelin levels are markedly reduced after bariatric surgery,14, 15 suggesting that the modulation of ghrelin via less invasive means may provide an alternative, viable means for obesity treatment. However, ghrelin-inhibitory drugs have not been successful.
Bariatric arterial embolization (BAE) was originally proposed as an image-guided, minimally invasive, percutaneous technique performed by interventional radiologists to modulate ghrelin production in the gastric fundus. BAE involves transcatheter occlusion of the arteries supplying the gastric fundus, which contains the majority of ghrelin-producing cells.16 Because of the rapid turnover of gastric mucosal cells, selective occlusion of the fundal arteries was expected to cause mild ischemic damage, leading to reduced ghrelin production and thereby weight loss. The concept has been tested in large animal models (i.e., swine and canine) with demonstrated technical feasibility, safety, and short-term therapeutic benefits.17-24 Because of the similar vascular anatomy and vessel size to humans, swine were considered to be an appropriate model to develop and optimize the embolization protocols, test devices that could be directly translated to the clinic, and demonstrate safety and efficacy of the procedure. Recently, several prospective clinical trials have been initiated/performed with promising early outcomes.2, 25-28 The following sections will focus on preclinical studies that have laid the foundation for clinical translation.
Embolic Material Choices
Several types of embolic materials have been tested for transcatheter BAE to induce ischemic insult to ghrelin-producing mucosa of the stomach, including liquid sclerosants (e.g., sodium morrhuate, bleomycin A5 hydrochloride, and lipiodol emulsion),17-19 particle embolics, 19, 20, 23, 24, 29, 30 metallic coils, 20 and clips.31 In an initial proof-of-concept study, Arepally et al. used a liquid sclerosant, sodium morrhuate, to achieve distal occlusion of all fundal arteries in growing swine, termed “gastric artery chemical embolization” (GACE).17 This initial study demonstrated the ability to decrease weight gain in growing swine.18 However, liquid embolics were difficult to precisely target, which might explain the presence of stomach necrosis and ulcer development away from the targeted area.17 Subsequent studies have primarily employed particle embolics to enable more precise targeting to overcome these issues associated with liquid embolics.
The size of embolic particles is an important factor to consider during embolic selection in that it determines the embolic penetration depth, which in turn affects the therapeutic benefits and potential side effects, such as gastric ulcerations. To mimic the embolization characteristics of liquid embolics, Paxton et al. utilized smaller, 40 μm-calibrated, spherical microspheres (Embozene) for BAE in growing swine.23 Mixed with an equal amount of a non-ionic, iodinated contrast agent, these embolic particles could be delivered to distal arteries with reduced systemic toxicity as compared to a liquid sclerosant. With small-size embolic microspheres, mild gastric ulceration occurred in two of five pigs. Therefore, larger-size, non-spherical polyvinyl alcohol (PVA) particles 21 and tris-acryl gelatin (Embospheres) 24, 32 microspheres have been evaluated for weight and hormone production modulations. PVA is a biocompatible and highly compressible embolic agent that has been widely used for embolotherapy. However, the high coefficient of friction of non-spherical PVA particles suggests that they can aggregate. Therefore, the effective size of the particle may be significantly larger, leading to proximal rather than distal vessel embolization. In addition, the particles may “clump” within a microcatheter, impeding their precise delivery into the recipients.
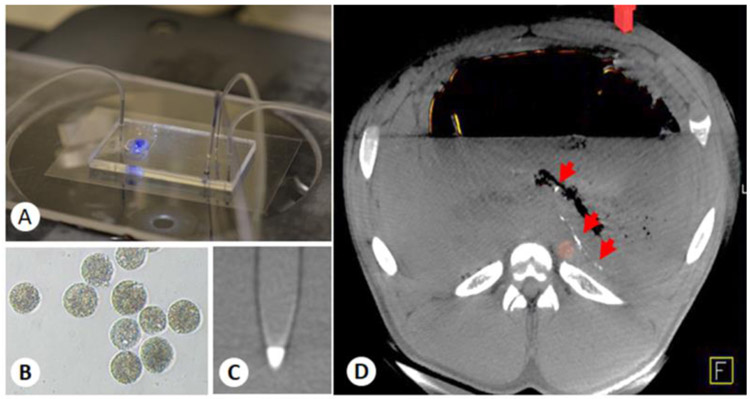
Recently, Embospheres have been evaluated for BAE in healthy growing swine.24, 32 Embospheres are made of a cross-linked tris-acryl hydrophilic copolymer impregnated with gelatin to facilitate endothelial adherence. Therefore, Embospheres may provide more complete vascular occlusion than PVA particles. Commercially available embolic microspheres have relatively large size variations that follow a Gaussian distribution within a given size range (e.g., 100–300 μm, 300-500 μm). Filtering of the synthesized particles through a series of sieves can allow one to collect microspheres within a specific diameter range. Recently, our group has developed a hydrogel microsphere with a narrow size distribution using a custom-made microfluidic device (Fig. 1A). Such microspheres are made with a biocompatible alginate hydrogel impregnated with an x-ray contrast agent (e.g., barium sulfate) to impart direct x-ray visibility (Figs. 1B, 1C). The ability to directly visualize the embolic microspheres can facilitate the assessment of embolic microspheres distribution (Fig. 1D) and non-target embolization.
Figure 1.
(A) Custom-made microfluidic device for x-ray-visible, small-size, monodisperse, embolic microsphere synthesis. (B) Microscopic image of microfluidic-prepared, barium sulfate-impregnated alginate microspheres. (C) Maximum-intensity projection of a barium alginate microsphere phantom acquired with a clinical x-ray flat panel angiographic system (Axiom Artis Zee), demonstrating the radiopacity of the microspheres. (D) Axial view of the CBCT image of a pig stomach embolized with 50 μm barium alginate microspheres, showing the distribution of the microspheres one week after embolization (arrows).
Other types of embolic materials used for BAE include clips and metallic coils made with platinum. For BAE, coils are used in combination with microspheres for embolization of the left gastroepiploic artery (LGEA) and right gastroepiploic artery (RGEA),20 while clips are used alone for laparoscopic clipping of LGEA to modulate blood flow and increase vascular supply to the gastroesophageal junction (GEJ).31
Equipment
Since BAE is an image-guided, minimally invasive, percutaneous technique for treating otherwise healthy patients, precise targeting of the gastric arteries is critically important. The major equipment involved the procedure include the following: 1) Ultrasound imaging for guiding percutaneous femoral artery access. Because an ultrasound machine is portable and suitable for an angiography suite, it is often placed on the left-hand side of the operator. When used for percutaneous femoral artery access, the probe is wrapped with a clear, disposable, sterile cover equipped with ultrasound transmission gel. The right groin is the preferred side for puncture. 2) Cone beam computed tomography (CBCT) for providing 3D high-resolution angiographic maps of the vascular architecture of the gastric fundus (Fig. 2). 3) Standard adult gastroscopy for assessing the integrity of the gastric mucosa after embolization. 4) Inhalational anesthesia machine for maintaining general anesthesia throughout the embolization procedure, as well as for controlling the breath-hold during imaging acquisition to reduce motion artifacts.
Figure 2.
Digital photograph of the C-arm CBCT used for BAE (Axiom Artis Zee).
Embolization Procedural Steps
Animal Preparation
All animal studies should be approved by the Institutional Animal Care and Use Committee (IACUC). All swine receive daily oral proton pump inhibitors (e.g., 40 mg omeprazole) starting three days prior to the procedure until the end of the study. On the day of the procedure, 40 mg intravenous pantoprazole is given to animals instead of omeprazole. All animals are switched to a liquid diet (Ensure, Abbott Laboratories, Chicago, IL) two days prior to embolization, and are fasted overnight prior to the procedure to ensure the stomach is empty of ingesta. Fasted pigs are sedated with a combination of ketamine (10 mg/mL), xylazine (100 mg/mL), and telazol (100 mg/mL) at a dose of 1 mL per 25 kg intramuscularly, and anesthesia is induced with intravenous propofol (~4 mg/kg). Animals are weighed, and ~10 mL of blood is drawn from the jugular vein for blood chemistry, complete blood count, and hormone assessment. Animals are intubated and mechanically ventilated for the duration of the procedure. General anesthesia is maintained with 1-3% isoflurane (Baxter Healthcare, Deerfield, IL). Prophylactic antibiotics may be administered immediately prior to percutaneous access.
Prior to embolization, animals are securely tied to a V-board in a supine position. The groin area is shaved and sterilely prepared for percutaneous access of the femoral artery.
BAE Procedures
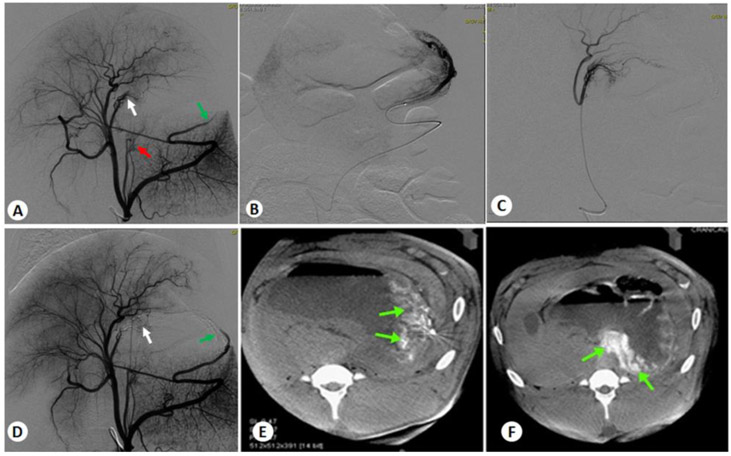
After the animals are draped, BAE or sham procedures are performed using sterile technique by an interventional radiologist and a veterinarian. First, femoral arterial access is obtained percutaneously under ultrasound guidance (Zonare Medical Systems, Inc., Mountain View, CA) using the Seldinger technique. For this purpose, a micropuncture set (needle and wire) is used to access the right femoral artery, and then a 5 F introducer sheath is placed. Under x-ray fluoroscopic guidance (Axiom Artis Zee, Forchheim, Germany), a 5 F angiographic guide catheter (SOS Angiographic Catheter, Angiodynamics, or Flexion Axis, Surefire Medical, Westminster, CO) is advanced over a 0.035-inch Bentson guidewire (Cook Medical, Bloomington, IN) under x-ray fluoroscopic guidance, and formed in the thoracic or abdominal aorta to select the celiac axis. A pre-embolization celiac digital subtraction angiogram (DSA) with iohexol injection (Omnipaque, GE Healthcare Inc., Princeton, NJ) at 4 mL/sec for 5 seconds is obtained to map the vessels feeding the gastric fundus (Fig. 3A). A dual-phase CBCT scan with a 30% (v/v) iohexol injection at 4 mL/sec for 10 seconds is also acquired to assess 3D vascular anatomy. An anti-reflux (Infusion System mT, Surefire Medical) or a high-flow (Renegade, Boston Scientific Corp, Marlborough, MA) microcatheter is then advanced over an appropriate guidewire (0.016-inch Fathom, Boston Scientific Corp.) into a fundal branch of the main gastric artery. If the catheter does not enter the targeted artery branches, twisting the angulations of the tip of the microcatheter or shaping the guide wire may be helpful. A DSA of the selected vessel is acquired with manual injection of 50% iohexol to confirm the subselected target artery (Figs. 3B, 3C). One hundred μg of nitroprusside is then delivered intra-arterially to prevent vasospasm during embolic deployment. The artery is then embolized with the test embolic beads diluted with 50-90% iohexol until five beats of stasis is achieved. Embolization is performed slowly with a 1 mL syringe. Intermediate single-shot images are also acquired to document the distribution of embolics beads during delivery. The volume of the embolic beads delivered is recorded. A final DSA is then acquired to confirm the embolization of the target arteries. If residual flow is observed, further embolic is administered. Post-embolization DSA and CBCT are acquired to confirm the success of embolization (Figs. 3D-F). The microcatheter is flushed with saline and repositioned before embolization of the next arterial branch.
Figure 3.
Representative angiograms of BAE in two fundal arteries. (A) Pre-embolization celiac DSA of the stomach showing RGA (white arrow), LGA (red arrow), and LGEA (green arrow) that supply the gastric fundus for embolization. (B) Sub-selection DSA of LGEA. (C) Sub-selection DSA of RGA. (D) Post-embolization celiac DSA after super-selective embolization of LGEA and RGA, showing the stasis of the blood flow (arrows). (E) Axial view of CBCT images of the stomach after LGEA embolization. (F) Axial view of CBCT images of the stomach after RGA embolization.
The BAE procedure time is recorded starting from femoral artery access to the removal of the catheter after BAE. Fluoroscopy time and radiation exposure are also recorded. After the procedure, all devices are removed and hemostasis at the femoral artery entry site is obtained via manual compression for 15-20 min, and the animals are transferred for recovery. Closure devices are not utilized due to the lack of abundant fat around the vessels in the groin in most swine and dogs.
In humans, the major blood supply to the fundus is through left gastric artery (LGA), while in pigs, LGEA and right gastric artery (RGA) contribute significantly to fundal perfusion. Therefore, in preclinical studies, BAE is usually performed in either LGEA alone, or in combination with other fundal arteries. Control swine receive saline in the corresponding gastric arteries.
Tips and Tricks for BAE
The following tips and tricks may facilitate the embolization procedure:
Keep all the embolization devices and materials in order on a separate table, and only place the items on the embolization table when needed.
Always keep separate bowls for saline, contrast, and nitroprusside. Label the containers clearly. We typically use sterile foil cups with different shapes for easy and clear identification.
Separate syringes with different sizes should be used for embolization and flush purpose.
As multiple vessels often supply the fundus in the pig and dog, a CBCT is highly recommended to avoid vessels that feed the diaphragm, liver, and pancreas, and also determine the degree of sub-selection that may be required to avoid the spleen and other non-target organs.
After vessel selection, contrast injection should be performed to assess catheter position and flow within the vessel for reflux potential.
Embolic materials should be delivered in small aliquots and dripped in slowly to enable maximal distal embolization.
After embolic delivery, the catheter should be flushed with saline to purge the device of agent. This will prevent aggregation or blood clotting at the tip of the microcatheter as well as accidental embolic delivery in non-target vessels.
Anti-reflux catheters may lead to more distal delivery of the embolic as well as distribution to LGA from RGA or vice versa.
A post-embolization DSA should be performed after initial contrast washout to re-evaluate the success of embolization, as the embolic materials may be redistributed, leading to incomplete embolization of that vessel.
Post-Procedural Assessment
The animals are monitored twice daily after BAE by a veterinarian for signs of post-operative pain or inappetence. Post-operative analgesia, such as buprenorphine, may be administered for any animals showing discomfort. The vascular access site is assessed daily for approximately one week following the embolization procedure for signs of infection, inflammation, and general integrity. Post-operative antibiotics for five days may be administered to prevent iatrogenic infection. At approximately one to three weeks post-embolization, upper gastrointestinal endoscopy is performed using a standard adult gastroscope (Pentax, Denver, CO) to examine the effect of BAE on the integrity of the gastric mucosa. Signs of gastric ulcerations are evaluated and recorded.
Preclinical Outcomes
Although percutaneous transarterial embolization has been routinely performed by interventional radiologists for treating upper gastrointestinal artery bleeding,33, 34 the concept of BAE as a minimally invasive technique to treat obesity is relatively new.17 To date, there are approximately a dozen preclinical studies for BAE reported. Excitingly, all the preclinical data have demonstrated that BAE is technically feasible and safe in large animal models, regardless of the embolic materials employed, the number of gastric arteries embolized, or the duration of the study period. However, the results regarding the effect of BAE on weight change and ghrelin modulation are mixed.
The first preclinical BAE study was reported by Arepally et al.17 This pilot study tested a liquid sclerosing agent, morrhuate sodium, in a dose-escalating manner (37.5-2000 μg) for BAE in eight healthy swine (BAE: N=6, control: N=2). All the fundal arteries, including LGA and all other accessory gastric arteries and vessels to the fundus, were embolized. Four weeks after BAE, animals that received the lowest doses of sodium morrhuate (37.5-62.5 μg) showed significantly elevated serum ghrelin levels as compared to baseline levels or to that of control animals. Animals that received an intermediate dose of sodium morrhuate (125 μg) experienced a decrease in serum ghrelin levels, while animals with the highest dose (2000 μg) died acutely after the procedure with perforated gastric ulcers noted. Fundal ghrelin expression levels in BAE animals were markedly decreased as compared to controls. Nevertheless, no significant difference in weight change between BAE and controls was observed. This early study demonstrated that BAE could be used to modulate serum ghrelin levels, and provided a reference dose for using sodium morrhuate in BAE.17
Subsequently, Arepally et al. evaluated the effect of BAE with 125 μg sodium morrhuate on weight change and ghrelin production in 10 heathy swine (BAE: N=5, control: N=5).18 The study showed that overall plasma ghrelin levels were significantly reduced in BAE animals as compared to either their baselines levels or to those of controls. Plasma ghrelin levels at week 4 slightly increased as compared to weeks 2 and 3 after embolization, suggesting that the embolized vessels might be recanalized, leading to the reestablishment of the blood flow. Follow-up angiography performed at week 4 confirmed this notion. Moreover, BAE animals gained significantly less weight than controls. The results from this study further supported that selectively embolizing the gastric fundus could modulate ghrelin production and weight change, and thus, BAE could potentially be used to treat obesity.
Considering the toxicity issues of sodium morrhuate, other embolic materials have been evaluated, including liquid and particle embolics, coils, and clips. In a canine model, bleomycin A5 hydrochloride emulsified with lipiodol was investigated as a liquid embolic in parallel with 500-700 μm PVA particles.19 During the eight-week study, animals that received either bleomycin or PVA particles experienced significantly reduced serum ghrelin levels (−15.8%, - 30.2%, respectively), while control animals had 13.6% increase in serum ghrelin levels. Both BAE arms demonstrated marked weight loss, which was directly correlated to the suppression of serum ghrelin levels. Moreover, no clinically significant adverse events or any evidence of gastric ulcerations were noted. While liquid embolics tend to penetrate deeper into the vascular bed, leading to better suppression of ghrelin production, they can also result in tissue damage, in particular when non-target embolization occurs. Thus, subsequent studies to advance BAE toward clinical translation have primarily focused on particle embolics. Paxton et al. investigated performing BAE with commercially available, calibrated, 40 μm microspheres (CeloNova Biosciences) mixed with equal amounts of a non-ionic contrast agent in growing swine.23, 35 Animals that underwent BAE experienced significantly less weight gain and reduced serum ghrelin levels. Yet, 50% of the experimental animals developed gastric ulcerations away from the embolized fundus, which could be attributed to non-target embolization or peri-procedural stress. These ulcers were mild and healed without any therapy or interventions, suggesting that particle-induced ulcers might be transient and have minimum long-term sequelae. Histological analysis of the gastric mucosa revealed significantly muted fundal ghrelin expression levels and increased fibrosis with fibrotic strands extending through the mucosa, which might alter gastric motility and absorption, further contributing to lack of weight gain in growing animals.35 Similar results were also observed in another study where yttrium-90 (90Y) resin microspheres were used for BAE.30 Bariatric radioembolization with 90Y resulted in a significant reduction in weight gain, decreased fundal ghrelin expression, and significant fibrosis of the fundus. However, gastric ulcers were noted in five of six embolized animals.30
Subsequently, two studies were performed to address the issue of gastric ulcerations. One study performed by Paxton et al. investigated whether the use of gastroprotective agents (sucralfate and omeprazole) and limiting the number of arteries embolized could mitigate the development of ulcers,22 while the other study performed by Kim et al. employed larger-size, non-spherical PVA particles (50-150 μm and 150-250 μm), which aggregated more proximally than the smaller 40 μm microspheres.21 Unexpectedly, both studies failed to prevent gastric ulcerations. BAE with 40 μm microspheres and gastroprotective agents resulted in 40-50% of gastric ulcerations, regardless the number of arteries embolized,22 while BAE with PVA particles exhibited 60% of gastric ulcerations.21 Although mild gastric ulcers are an unwanted side effect, the administration of gastroprotective agents might still provide some benefit and would be recommended in human clinical trials.
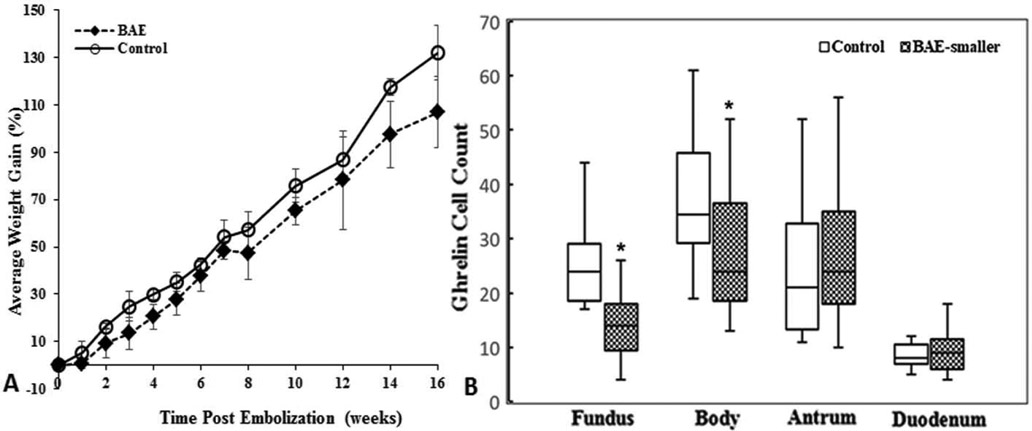
Of note, these BAE studies are limited to a duration of four to nine weeks. Recently, Fu et al. examined the effect of embolic size on weight change, ghrelin production, and gastric ulcerations in nineteen swine for a longer time period of 16 weeks.24 Animals were recruited in two phases due to the longer study period. Each phase had its own control group to minimize the confounding factors. Phase I BAE animals received 300-500 μm microspheres (Embospheres, Merit Medical Systems, BAE: N=5, control: N=5), and phase II BAE animals received 100-300 μm microspheres (Embospheres, BAE: N=5, control: N=4). In both phases, BAE and control animals exhibited steady increase in weight gain during the 16-week study period. However, only phase II BAE animals experienced significantly less weight gain than control animals. This weight gain suppression was correlated with markedly reduced fundal ghrelin expression (Fig. 4). In addition, 60% of phase I BAE animals and 100% of phase II BAE animals developed superficial gastric ulcers, suggesting that smaller-size embolics are more effective for weight suppression, but they are also associated with a potentially higher risk of gastric ulcerations. This study highlighted the need to balance safety and efficacy when choosing embolic particle size before clinical translation.
Figure 4.
(A) Graph shows mean percentage weight gains ± standard deviations after BAE with 100-300 μm microspheres. Mean weight gain was statistically significantly lower in BAE-treated pigs than in control pigs. (B) Box plot of ghrelin cell counts shows statistically significant reduction of ghrelin-expressing cells in the gastric fundus (P < .001) and body (P=.004) without compensatory upregulation in the duodenum (P=.66) and antrum (P=.84) after BAE with 100-300 μm microspheres as compared to controls. Reprinted with permission from Fu et al.24
Although a few prospective clinical investigations of BAE in patients have started with promising preliminary results reported, there are concerns from the bariatric surgery field that embolization of LGA would deprive patients from further bariatric surgery due to the damage of LGA or potential devascularization of GEJ, which depends mainly on LGA.36 However, recanalization of LGA along with collateral artery formation in the LGA territory is frequently seen in patients who undergo LGA embolization for treating upper gastrointestinal bleeding, and thus, BAE should not prevent patients from undergoing bariatric surgery in the future. In addition, BAE with particle embolics only embolizes the artery distally, leaving the proximal aspect of LGA intact. Preclinical studies reported by Diana et al. demonstrated that GEJ blood flow could be enhanced after laparoscopic clipping of LGEA31, or embolization with the combination of platinum coils and PVA microspheres.20 Therefore, BAE should not prevent future bariatric surgery procedures, if needed, and instead may provide a bridge for patients who are not initially candidates for bariatric surgery.
Conclusions
With refined imaging techniques that provide detailed gastric artery anatomy and better understanding of the performance of embolic agents, BAE can be safely performed to modulate the global function of the stomach. Preclinical studies in large animal models have shown that BAE can not only suppress ghrelin production in the embolized fundus, but also indirectly influence acid production, gastric motility, and possibly nutrient absorption. Therefore, like bariatric surgery (e.g., sleeve gastrectomy), BAE appears to regulate body weight through synergetic hormonal changes and alteration of the physiologic function of the stomach. The most common complication is the development of gastric ulcers, which are normally superficial and manageable.
Despite promising preclinical results, several fundamental questions remain to be addressed. These include optional embolic materials (size and composition), the ideal targeting arteries (one or more), and embolization techniques. Nevertheless, future studies and ongoing clinical trials may shed the light on the potential impact of this procedure on patients with obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Obesity and Overweight. World Health Organization; https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. 2019. [Google Scholar]
- 2.Weiss CR, Abiola GO, Fischman AM, et al. Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial: Results at 1 Year. Radiology 2019;291:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalle Grave R, Calugi S, El Ghoch M. Lifestyle modification in the management of obesity: achievements and challenges. Eat Weight Disord 2013;18:339–49. [DOI] [PubMed] [Google Scholar]
- 4.Jones LR, Wilson CI, Wadden TA. Lifestyle modification in the treatment of obesity: an educational challenge and opportunity. Clin Pharmacol Ther 2007;81:776–9. [DOI] [PubMed] [Google Scholar]
- 5.Montesi L, El Ghoch M, Brodosi L, et al. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes 2016;9:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol 2018;14:12–24. [DOI] [PubMed] [Google Scholar]
- 7.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014:Cd003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956–61. [PubMed] [Google Scholar]
- 9.Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg 2003;138:389–96. [DOI] [PubMed] [Google Scholar]
- 10.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 2004;18:439–56. [DOI] [PubMed] [Google Scholar]
- 11.Stengel A, Tache Y. Regulation of food intake: the gastric X/A-like endocrine cell in the spotlight. Curr Gastroenterol Rep 2009;11:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takiguchi S, Adachi S, Yamamoto K, et al. Mapping analysis of ghrelin producing cells in the human stomach associated with chronic gastritis and early cancers. Dig Dis Sci 2012;57:1238–46. [DOI] [PubMed] [Google Scholar]
- 13.Maksud FA, Alves JS, Diniz MT, et al. Density of ghrelin-producing cells is higher in the gastric mucosa of morbidly obese patients. Eur J Endocrinol 2011;165:57–62. [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. The New England Journal of Medicine 2002;346:1623–30. [DOI] [PubMed] [Google Scholar]
- 15.Geloneze B, Tambascia MA, Pilla VF, et al. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg 2003;13:17–22. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- 17.Arepally A, Barnett BP, Montgomery E, et al. Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: initial experience. Radiology 2007;244:138–43. [DOI] [PubMed] [Google Scholar]
- 18.Arepally A, Barnett BP, Patel TH, et al. Catheter-directed gastric artery chemical embolization suppresses systemic ghrelin levels in porcine model. Radiology 2008;249:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bawudun D, Xing Y, Liu WY, et al. Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovasc Intervent Radiol 2012;35:1460–6. [DOI] [PubMed] [Google Scholar]
- 20.Diana M, Pop R, Beaujeux R, et al. Embolization of arterial gastric supply in obesity (EMBARGO): an endovascular approach in the management of morbid obesity. proof of the concept in the porcine model. Obes Surg 2015;25:550–8. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Kim MD, Han K, et al. Bariatric Arterial Embolization with Non-spherical Polyvinyl Alcohol Particles for Ghrelin Suppression in a Swine Model. Cardiovasc Intervent Radiol 2017. [DOI] [PubMed] [Google Scholar]
- 22.Paxton BE, Arepally A, Alley CL, et al. Bariatric Embolization: Pilot Study on the Impact of Gastroprotective Agents and Arterial Distribution on Ulceration Risk and Efficacy in a Porcine Model. J Vasc Interv Radiol 2016;27:1923–1928. [DOI] [PubMed] [Google Scholar]
- 23.Paxton BE, Kim CY, Alley CL, et al. Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology 2013;266:471–9. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Weiss CR, Paudel K, et al. Bariatric Arterial Embolization: Effect of Microsphere Size on the Suppression of Fundal Ghrelin Expression and Weight Change in a Swine Model. Radiology 2018;289:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai ZB, Qin YL, Deng G, et al. Bariatric Embolization of the Left Gastric Arteries for the Treatment of Obesity: 9-Month Data in 5 Patients. Obes Surg 2018;28:907–915. [DOI] [PubMed] [Google Scholar]
- 26.Kipshidze N, Archvadze A, Bertog S, et al. Endovascular Bariatrics: First in Humans Study of Gastric Artery Embolization for Weight Loss. JACC Cardiovasc Interv 2015;8:1641–4. [DOI] [PubMed] [Google Scholar]
- 27.Syed MI, Morar K, Shaikh A, et al. Gastric Artery Embolization Trial for the Lessening of Appetite Nonsurgically (GET LEAN): Six-Month Preliminary Data. J Vasc Interv Radiol 2016;27:1502–8. [DOI] [PubMed] [Google Scholar]
- 28.Weiss CR, Akinwande O, Paudel K, et al. Clinical Safety of Bariatric Arterial Embolization: Preliminary Results of the BEAT Obesity Trial. Radiology 2017;283:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JM, Kim MD, Han K, et al. Bariatric Arterial Embolization with Non-spherical Polyvinyl Alcohol Particles for Ghrelin Suppression in a Swine Model. Cardiovasc Intervent Radiol 2017;40:744–749. [DOI] [PubMed] [Google Scholar]
- 30.Pasciak AS, Bourgeois AC, Paxton BE, et al. Bariatric Radioembolization: A Pilot Study on Technical Feasibility and Safety in a Porcine Model. J Vasc Interv Radiol 2016;27:1509–17. [DOI] [PubMed] [Google Scholar]
- 31.Diana M, Halvax P, Pop R, et al. Gastric supply manipulation to modulate ghrelin production and enhance vascularization to the cardia: proof of the concept in a porcine model. Surg Innov 2015;22:5–14. [DOI] [PubMed] [Google Scholar]
- 32.Fu Y, Paudel K, Kedziorek DA, et al. Histological Analysis of Gastric Ghrelin Expression in Selective Bariatric Embolized Swine. J Vasc Interv Radiol 2016;The society of Interventional Radiology annual scientific meeting supplement. [Google Scholar]
- 33.Beggs AD, Dilworth MP, Powell SL, et al. A systematic review of transarterial embolization versus emergency surgery in treatment of major nonvariceal upper gastrointestinal bleeding. Clin Exp Gastroenterol 2014;7:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CW, Liu KL, Wang HP, et al. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol 2007;18:209–16. [DOI] [PubMed] [Google Scholar]
- 35.Paxton BE, Alley CL, Crow JH, et al. Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model. J Vasc Interv Radiol 2014;25:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink JM, Martini V, Seifert G, et al. Left Gastric Artery Embolization for Weight Loss-a Dead-End Procedure. Obes Surg 2018;28:3623–3624. [DOI] [PubMed] [Google Scholar]