Abstract
Chronic occupational exposure to organophosphorus pesticides (OPs) is consistently associated with deficits on behavioral tests when compared to unexposed comparison groups. However, a dose-response relationship has yet to be established, leading some to doubt an association between occupational OP exposure and behavioral deficits. Pesticide application teams in Egypt who are primarily exposed to one OP, chlorpyrifos (CPF), were recruited into a field assessment. Trail Making A and the more challenging Trail Making B tests were administered to 54 engineers (who supervise the pesticide application process, usually from the side of the field), 59 technicians (who guide the pesticide applicators in the field), 31 applicators (who mix and apply pesticides using knapsack sprayers), and 150 controls (who did not work in the fields) at two different times during the OP application season as well as immediately after applications had ended and 1.5 months later. All participants were males since only males work on pesticide application teams in Egypt. Urinary levels of 3,5,6-trichloro-2pyridinol (TCPy), a specific metabolite of CPF, confirmed the pattern of lower to higher CPF exposures from engineers to technicians to applicators, and these were all greater than urinary metabolite levels in controls. A consistent relationship between job title and performance speed on the behavioral task was observed: Controls had the best (fastest) performance on Trail Making A and B tests throughout the application season, and applicators had significantly slower performance than engineers on Trail Making A (p= 0.015) and B (p =0.003). However, individual urinary TCPy, blood acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) levels did not predict individual performance. This study identifies a dose-related effect based on job title, which serves as a surrogate for chronic exposure in that differing job titles exhibit varying group exposure levels. The results establish that chronic occupational exposure to chlorpyrifos is neurotoxic and suggest that the classic biomarkers of recent CPF exposure are not predictive of chronic exposure effects.
Keywords: Chlorpyrifos, Organophosphorus pesticide, OP, Neurotoxicology, Dose response, Occupational exposure
1. Introduction
Human occupational exposures to organophosphorus pesticides (OPs), including chlorpyrifos (CPF), have been studied extensively in cross-sectional studies, and several meta-analyses or systematic analyses of these data have concluded that adverse behavioral effects are associated with occupational exposure to OP pesticides in adults (Ismail et al., 2012; Meyer-Baron et al., 2015; Munoz-Quezada et al., 2016; Rohlman et al., 2011; Ross et al., 2013) and developmental OP exposures in children (Gonzalez-Alzaga et al., 2014; Jurewicz and Hanke, 2008). However, only a few studies in adults have reported an association between biomarkers of exposure and behavioral outcomes in occupationally-exposed cohorts (e.g., Abdel Rasoul et al., 2008; Ismail et al., 2017b; Rohlman et al., 2016; Rothlein et al., 2006). One study in children (Rauh et al., 2011) identified declines in full scale IQ on the Wechsler Intelligence Scale for Children (WISC) in 7-year-olds that were correlated with individual CPF levels in umbilical blood plasma that had been collected prenatally. The association between OP exposures and behavioral deficits in humans, and specifically adults, is still questioned by some, in part because of the paucity of evidence of a correlation between the behavioral changes and biomarker(s) of exposure (as noted by Meyer-Baron et al., 2015), and because a dose-response relationship has yet to be demonstrated. Other reviews have emphasized inconsistent findings and limited exposure assessments as reasons for the continued uncertainty (Burns et al., 2013; Li et al., 2012) as summarized by Rohlman et al. (2016). A significant limitation of these assessments is that each of the epidemiological studies compared a single OP-exposed population to an unexposed or control population.
Thus, there is a need to identify workers with a range of OP exposures that allows an examination of the relationship between different occupational exposure levels and behavioral performance and to test their relationship with biomarkers of exposure collected at the time of testing: urinary 3,5,6-Trichloro-2-pyridinol (TCPy), a specific metabolite of CPF, and blood acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). All these are established biomarkers of recent OP exposure (Alexander et al., 2006; Wang et al., 2016).
Pesticide application teams working for the Ministry of Agriculture (MOA) in Egypt apply CPF and pyrethroids to the cotton crop (see Fig. 1). The MOA regulates pesticide applications to the cotton crop by supplying and maintaining the equipment; dictating the pesticides and concentration levels to be applied, as well as the application schedule; and monitoring the effects on pest infestation of the cotton plants. The pesticide application teams consist of applicators who mix, load and apply the pesticides, technicians who support the applicators by walking next to the applicators and directing their walking speed in areas of heavier vs. lighter pest infestation, and engineers who oversee the pesticide application process, including pesticide mixing and applications. CPF is the primary pesticide that is used in cotton production in Egypt, and it is applied in a routine of daily applications for up to 4 weeks, followed by an application of a pyrethroid pesticide, then an additional application cycle of CPF depending on the degree of infestation (T. M. Farahat et al., 2003; Rohlman et al., 2011). This cycle has been followed for at least 15 years prior to the time of this study. Adult engineers and technicians from the Egyptian MOA had been studied previously, revealing a broad range of adverse behavioral test deficits relative to control performance (T. M. Farahat et al., 2003). However, in that earlier study, comparisons were between exposed workers and non-exposed controls, so it was not possible to delineate a dose-response relationship.
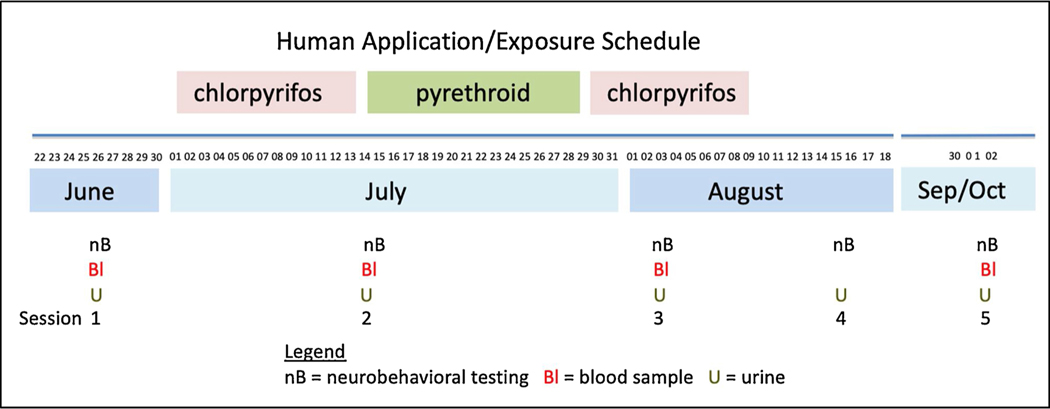
Fig. 1.
Pesticide applicators wearing backpack sprayers and technicians guiding the walking speed to increase or decrease pesticide concentration and to focus the spray direction of the applicators in Egyptian cotton fields.
The purpose of this study was to assess the impact of CPF exposures on the Trail Making Test, a prototypical behavioral test of search, scanning, processing speed, mental flexibility and executive function (Tombaugh, 2004) used in many prior studies examining OP effects and considered a valuable screening test for cognitive effects of pesticides (Lucero et al., 2019). The overall study was designed to assess cognitive and motor speed performance of worker job categories with different exposure histories and to test our primary hypothesis that the mechanisms mediating the behavioral effects of long-term or chronic exposures are unrelated to the classic biomarkers of recent CPF exposures. To address our primary hypothesis, we describe the results as a series of tests of eight hypotheses: 1) Higher urinary TCPy concentrations will be detected in applicators who walk directly into the pesticide spray than in technicians who walk beside them or engineers who are not often in the field during applications; 2) The average activity of blood cholinesterase, a biomarker of recent OP exposure, will be lower in applicators than in technicians or engineers during periods of CPF application; 3) Pesticide workers will demonstrate deficits (slower performance) on the Trail Making Test compared to controls, and job titles with higher CPF exposures will have greater deficits in performance than those with lower CPF exposures; 4) A greater number of years working for the MOA, and thus more years of exposure to CPF, will be associated with greater deficits on the Trail Making Test; 5) Peak exposures defined by urinary TCPy measures will be negatively associated with post-exposure Trail Making performance; 6) Higher CPF exposure concentrations (based on actual 2009 urinary TCPy concentrations) multiplied by years applying pesticides (i.e., cumulative exposure) will be associated with greater deficits in post-exposure Trail Making Test performance; 7) Trail Making performance will correlate in a dose-response fashion with urinary TCPy levels and both BChE and AChE cholinesterase inhibition; and 8) Trail Making Test deficits will persist after CPF exposure ends.
2. Materials and methods
2.1. Overview
A field study was conducted in 2009 and 2010 to examine the impact of CPF exposure on behavioral performance in pesticide application teams employed in Egyptian cotton fields for the Ministry of Agriculture (MOA) and controls working for the MOA but not in the fields.
2.2. Recruitment
The MOA in the Menoufia Governorate in Egypt’s Nile Delta provided access to the population that included members of pesticide application teams (applicators, technicians and engineers) and controls who had the same job titles as technicians and engineers but did not work in the agricultural fields. A team typically consisted of 1 engineer, 1 technician and 3 applicators, although additional technicians or applicators were assigned to a team depending on availability and the size of the field to be sprayed on any given day. Control participants worked in office jobs recording crop and pesticide application data and preparing informational materials for the local farmers or performing other job activities not related to pesticide application. Members of pesticide application teams were recruited at the MOA offices in the Menoufia governorate or at field stations operated by the MOA and tested in 2009 (the “2009 cohort”), with a subset retested in 2010 (the “2010 cohort”). The controls were recruited and tested in 2010. The applicators, engineers, technicians and controls were employed by the MOA year-round. However, some applicators who worked at these stations were adolescents who worked for the MOA only during the summer pesticide application season. Some participants also reported applying pesticides on family and neighbors’ farms (Lein et al., 2012; Rohlman et al., 2016). Only males work in these job categories in Egypt. In addition, a sample of 101 Menoufia University employees were recruited in 2009 to provide urine samples from Egyptians who did not work near the cotton fields.
2.3. Study setting
Study activities were conducted by staff in an open room with tables arranged along the walls in MOA offices in Menoufia Governorate. Participants received compensation equivalent to a day’s pay for the lower-paid workers. The project was approved by the Ethics Committee at Menoufia University and the IRB of Oregon Health & Science University. All participants signed a consent form prior to data collection.
2.4. Application/exposure schedule/study design
Five test sessions were conducted during the two years of the study that corresponded with specific time points within the application cycle (Fig. 2). Annually, CPF was applied from July 1 until July 15, pyrethroids were applied from mid-July to the end of July, and CPF was again applied from approximately August 1 until August 10. The exact dates on which applications occurred depended on the number of cotton fields assigned to a particular field station and the level of insect infestation in the cotton crop in those fields. Thus, the initial round of pesticide applications each summer began at some field stations on July 1 while at others it started as late as July 5, and the applications continued until all the assigned fields were treated, by July 15. The pyrethroid and second round of CPF applications followed the same pattern. Each field station director had control over the exact schedule of application on their assigned farms in collaboration with the MOA directorate. However, the pesticides and the concentrations to be applied were set by the MOA and these were constant from year to year.
Fig. 2.
Schedule (days of month listed above the month) of pesticide application, test administration and sample collection in 2009.
Test session 1 was conducted prior to the beginning of pesticide applications and represented baseline (although some applicators may have applied pesticides on private farms during this time). Sessions 2 and 3 were conducted in mid-July and early August during the CPF application. Session 4 was conducted shortly after CPF applications had ended (2nd to 3rd week of August, started one week earlier in 2010 than in 2009 because of the start of Ramadan), and Session 5 was in late September or early October, shortly after Ramadan had ended (several weeks after applications had concluded). Application team participants applied pesticides daily during the afternoons. All testing sessions were conducted in the morning when there were no pesticide applications.
At the start of each workday, technicians collected approximately 100 cotton bolls from different locations in each field scheduled to be sprayed that day to determine the number of weevils per boll in each field. This determination in turn led to adjustments in the pace walked by the applicators. The pesticide was applied at a standard walking pace using mist-blowers to apply 80 Liters of Pestban (CPF) to a feddan (0.42 ha, or 1.04 acres) in 20 min. This was designed to produce an application rate of 0.02 liters of diluted CPF per square meter (F. M. Farahat et al., 2011; Singleton et al., 2015, 2014). In areas of heavier infestation, technicians would direct applicators to walk more slowly in order to increase the concentration in those areas (the technicians are seen walking ahead and to the side of the applicators in Fig. 1). Engineers who oversaw the process typically observed from the side of the field where mixing/loading of the backpack sprayers occurred, although some would stand with the technicians and applicators during spraying in the field. Depending on the individual and the field station directives or traditions, the engineers may have participated in mixing/loading and/or walked in the fields near the applicators (which we observed in some instances).
2.5. Behavioral test
The Trail Making Test, parts A and B (US Army Individual Test Battery developed in 1944 (M. Lezak et al., 2004) was administered in Years 1 and 2 using the same protocol described in the prior study of this same population (T. M. Farahat et al., 2003) but to different people at different field stations. Non-standard instructions were used in Session 1 (2009) (viz., the instruction “try not to lift the pen as you move from one number or letter to the next” was not clear and participants were observed to lift the pen during testing), but this was corrected in sessions 2–5; the Year 1 data from Session 1 were discarded. The Trail Making Test measures complex visual scanning with a motor component. It assesses rapid visual search, visuospatial sequencing and cognitive set shifting; these are factors related to attention. Motor speed and agility contribute strongly to test performance. Performance on the test is particularly affected by brain injury, such as head trauma (M. D. Lezak, 1995).
Other behavioral tests were also administered, but they are not included here. Time to complete the Trail Making Test is the primary outcome measure. As this is a timed test, faster times reflect better performance. Errors were relatively infrequent, but not described here.
2.6. Urinary 3,5,6-trichloro-2-pyridinol (TCPy)
Urine samples were collected at the same time as behavioral testing (see Fig. 2) to analyze for 3,5,6-trichloro-2-pyridinol (TCPy), a CPF-specific metabolite excreted in the urine (Fenske et al., 2012). Once collected, urine samples were placed on wet ice in a cooler and transported to Menoufia University (Shebin El-Kom, Egypt), approximately 20 min from the study field stations, where they were stored at −20 °C. Urine samples were shipped on dry ice and analyzed for TCPy at the University at Buffalo (Buffalo, NY, USA) by negative-ion chemical ionization gas chromatography–mass spectrometry, using 13C-15N3,5,6TCPy as an internal standard (described previously by F. M. Farahat et al., 2011; T. M. Farahat et al., 2003). Creatinine concentrations were measured using the Jaffe reaction (Fabiny and Ertingshausen 1971). Urine TCPy concentrations are expressed as micrograms TCPy per gram creatinine.
2.7. Quantification of cholinesterase activity in the blood
Blood samples were collected at the same time as behavioral testing was conducted to analyze for acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), which are biomarkers of both exposure and effect (Rohlman et al., 2011). To establish the baseline ChE activity for each worker, a single pre-exposure blood sample was collected in June 2009 prior to the start of the MOA-regulated CPF application season (Session 1 in Fig. 2). Two additional blood samples were collected during the period of CPF applications (during behavioral testing sessions), and two post-CPF exposure blood samples were collected after CPF spraying had ended.
Blood samples were collected by venipuncture into 10 mL lavender top (EDTA) vacutainer tubes and immediately placed on wet ice in a cooler and transported to Menoufia University, where they were analyzed in triplicate for AChE and BuChE activity using an EQM Test-Mate kit (EQM Research Inc., Cincinnati, OH, USA). The EQM Test-Mate kit is a portable spectrophotometric analyzer developed for the determination of ChE activity in whole blood, based on the original Ellman method (McConnell et al., 1992). The intraclass correlation coefficient for BuChE with this method is 0.987 and for AChE is 0.898, indicating that there is very little variation among replicates relative to total variation.
2.8. Statistical analysis methods
Estimating equations (GEE) were used to model and test for differences in key responses of interest over multiple testing sessions while controlling for potential demographic and occupational characteristics. Specific analyses are described in each Results section.
3. Results
In Year 1 (2009) 144 members of pesticide application teams—31 applicators, 59 technicians, and 54 engineers—were recruited and tested in five sessions (Fig. 2). A subset of 4 applicators, 19 technicians, and 21 engineers was retested in 2010 (Year 2). Also in 2010, 150 subjects were recruited to serve as controls. Demographic characteristics of these groups are summarized in Table 1.
Table 1.
Mean years of age, education, and experience within MOA for study participants from 2009 and 2010, by job title; standard deviation (SD) given in parentheses.
| Cohort | Age | Education | MOA |
|---|---|---|---|
| 2009 | |||
| Applicator (n=31) | 39.2 (13.3) | 11.2 (2.5) | 12.5 (9.3) |
| Engineer (n=54) | 54.1 (4.1) | 14.4 (3.0) | 18.2 (8.6) |
| Technician (n=59) | 48.0 (4.9) | 12.3 (1.9) a | 14.8 (9.2) |
| 2010 (subset of above for applicators, engineers, and technicians) | |||
| Control (n=150) | 49.6 (6.0) | 13.0 (2.4) b | 20.1 (9.4) c |
| Applicator (n=4) | 40.7 (7.5) | 12.0 (0.0) | 16.5 (5.1) |
| Engineer (n=21) | 54.0 (4.0) | 13.5 (2.1) | 17.3 (9.2) |
| Technician (n=19) | 49.2 (3.8) | 12.4 (1.2) | 16.0 (9.7) |
1 missing, n =58.
10 missing, n= 140.
3 missing, n =147.
3.1. Association of urinary TCPy concentration with job title
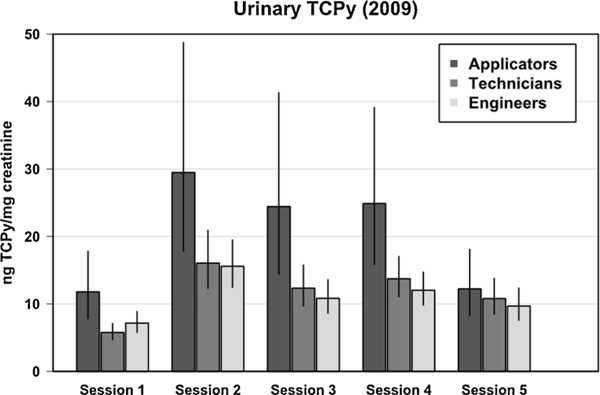
Among the 144 participants from the 2009 cohort, 142 (30 applicators, 58 technicians, 54 engineers) had measurable concentrations of urinary TCPy (ng/mg creatinine) in at least one of the five sessions during which urine samples were collected. Urinary TCPy concentration was log-transformed prior to the GEE analysis to improve symmetry, stabilize variance, and lessen the influence of outliers in the test of hypothesis 1.
The geometric mean (GM) concentration of urinary TCPy for engineers did not differ significantly (p = 0.43) from the GM concentration in technicians for any session, while the GM concentration for applicators was significantly higher than both engineers (p = 0.018) and technicians (p = 0.013) during Sessions 1–4. For Session 5, during which urine samples were collected in September (approximately 6 weeks after applications ended), there were no differences between the job type groups (p > 0.30 for each comparison) (Fig. 3). Urinary TCPy measures were not available for the 2010 cohort. The sample of 101 Menoufia University employees who did not work near the cotton fields, recruited in 2009, provided urine samples on June 28 and August 12–13 (corresponding respectively to the testing time of Session 1 and 4 in the 2009 field sample collection). The GM concentration of urinary TCPy from these two time points were 5.5 (95 % CI = 4.8–6.3) and 6.3 (95 % CI = 6.4–8.7) ng/mg creatinine; the difference between the time points was significant (p = 0.004). Even the high end of the CI for the control values is lower than the GM of urinary TCPy for applicators on all sessions except Session 1, whereas the GM of the University group is close to the levels seen in the technicians and engineers.
Fig. 3.
Geometric mean TCPy concentration (vertical axis; 95 % confidence interval as error bars) by job title and session during Year 1 (2009). Pesticide applications began a few days before Session 2 and ended a few days before Session 4.
These data support Hypothesis 1: Higher urinary TCPy concentrations will be detected in applicators who walk directly into the pesticide spray than in technicians who walk beside them or engineers who are not often in the field during applications. Based on the urinary TCPy measures in 2009, there are essentially two exposure groups: applicators and technicians + engineers.
3.2. Association of blood cholinesterase activity with job title
AChE activity (U/gram of hemoglobin [gHgb]) and BuChE (U/mL) was quantified for 139 (31 applicators, 54 engineers, 54 technicians) of the 144 workers from Year 1 (2009) at Session 1 (pre-application), Session 3 (during [the second period of] application), and Session 5 (post application); Session 3 was judged to be more representative of a chlorpyrifos application period than Session 2. Blood cholinesterase activity was measured in Year 2 (2010) for the 150 controls, but for only a few applicators and none of the engineers or technicians. Hypothesis 2, which focused on cholinesterase activity (AChE, BuChE) was also analyzed using GEE following log-transformation of these data. GEE models applied to these transformed outcomes estimate behavior of the geometric mean (GM) after transformation back to the original scale with additive changes estimated on the log scale becoming multiplicative effects (i.e., fold changes) concerning the GM when back transformed.
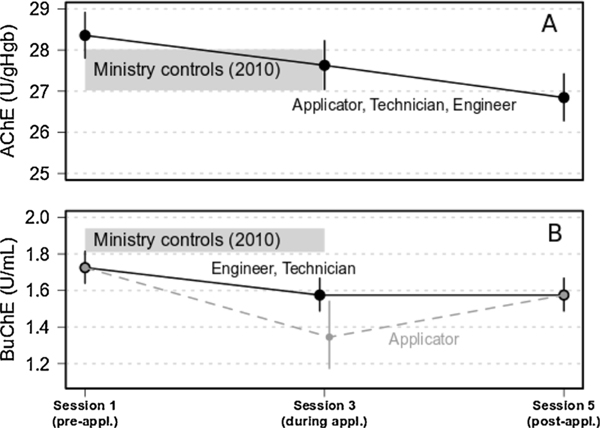
Occupation/job title did not significantly affect the GM of AChE activity over time (p = 0.43; test of occupation × session interaction), implying all three occupations shared a similar AChE profile over time. The GM of AChE activity for all three job titles decreased steadily over the pesticide application period compared to pre-application levels. Pre-application GM activity was 28.4 (95 % CI: 27.8–28.9) U/gHgb, and decreased 2.7 % (95 % CI: 1.9–3.4 %; p < 0.001) at each successive session (e.g., 2.7 % decrease at Session 3, followed by a further 2.7 % decrease from Session 3–5). The final concentration post-application (Session 5) was 94.7 % (95 % CI:93.2–96.2 %) of the initial pre-application level (Fig. 4, A). The sample of controls (2010) had an estimated GM AChE activity of 27.5 (95 % CI:27—28) U/gHgb over a period spanning Sessions 1–3.
Fig. 4.
GM of AChE (A) and BuChE (B) activity for applicators, engineers and technicians. The shaded bar represents 95 % confidence band for AChE and BuChE levels in controls from 2010.
BuChE activity for the same cohort exhibited a somewhat different pattern. Pre-application GM activity of BuChE was similar (p = 0.47) for all occupations, estimated to be 1.73 (95 % CI: 1.64–1.81) U/ml overall. Post-application activity levels also did not significantly differ (p = 0.41) among groups, though the GM activity post-application for all groups was 8.7 % (95 % CI: 6.0–11.3 %; p < 0.001) less than baseline. During Session 3 (application period), applicators had a GM activity decrease of 22 % (95 % CI: 11.4–31.4 %; p < 0.001) from baseline, which was 14.6 % (95 % CI: 2.7–25.0 %; p = 0.017) less than final post-application levels (in Session 5). For comparison, the sample of 150 controls (2010) had BuChE activity that remained relatively constant [X2(2df) = 3.77, p = 0.15; test for change over time] for Sessions 1–3, with a GM of 1.88 (95 % CI: 1.81–1.94) U/ml. All three job title groups had consistently lower levels of BuChE activity during 2009 than even the lower confidence limit for these controls (Fig. 4B).
These data partially support Hypothesis 2: The average activity of blood cholinesterase, a biomarker of recent OP exposure, will be lower in applicators than in technicians or engineers during periods of CPF application, was supported for BuChE, but not for AChE.
3.3. Association of job title with trail making performance
Of 144 pesticide team members recruited in Year 1 (2009) and described in Table 1, 139 (30 applicators, 58 technicians, 51 engineers) completed both Trail Making tests A and B at Sessions 2–5. Trail Making performance (A and B; Hypotheses 3, 4, 6, 7) over repeat testing sessions did not require any transformation prior to analysis by GEE. All GEE models presumed the (transformed) response followed an approximately normal distribution with exchangeable correlation structure of repeated measures taken over multiple testing sessions. Robust standard errors were used to guard against potential misspecification of the actual correlation structure.
For both Trail Making A and B, increasing age was significantly (p < 0.005) associated with slower performance; each additional decade of age was associated with an approximate 13 s increase in time to complete the test. After controlling for age, there was no indication that performance over time, compared to performance at Session 2, was modified by occupation for either Trail Making A [X 2 (6 df) = 4.62, p = 0.59] or B [X 2 (6df) = 4.67, p = 0.57; test of time × occupation interaction], thereby implying all three occupations shared parallel performance profiles over time. That is, across all job titles, and in controls, performance got faster with each successive session and slower with increased age of the participants.
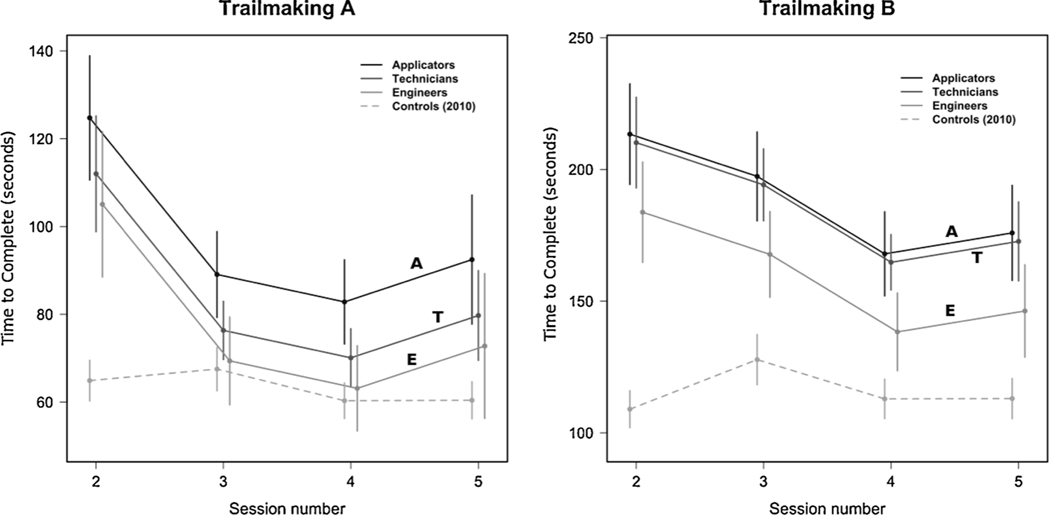
Among those of similar age, and regardless of session, applicators completed the Trail Making A test an average of 19.7 (95 % CI: 3.8–35.6; p = 0.015) s slower than engineers and 12.7 (95 % CI: 1.1–24.4; p = 0.032) s slower than technicians. Technicians averaged 7 s slower than engineers, which was an insignificant (p = 0.28) difference. Performance at Sessions 3, 4, and 5 was, respectively, 35.7 (95 % CI: 22.6–48.8), 41.9 (95 % CI: 29.0–54.8), and 32.3 (95 % CI: 14.8–49.8) s faster than at Session 2 (p < 0.001 for each). Compared to controls from 2010, applicators were slower at all sessions (p < 0.001 for each comparison) and technicians were also slower at each of the same four sessions (p < 0.04 for each); engineers only showed slower performance (p < 0.001) than controls at Session 2 but not at any later sessions (p > 0.15 for Session 3–5) (Fig. 5, left).
Fig. 5.
Performance (time to complete) of pesticide applicators and control subjects on Trail Making A (left) and B (right).
For Trail Making B, controls had faster performance (p < 0.001) at each session when compared to all other occupations, with only performance at Session 3 being significantly different from the other sessions (approximately 18 s slower, p < 0.001). Adjusted for age, engineers were 30 (95 % CI: 6.5–53; p = 0.012) s faster than applicators and 26 (95 % CI: 9–44; p = 0.003) s faster than technicians at each of the testing sessions. Performance for applicators and technicians did not significantly differ (p = 0.75). As was the case for Trail Making A, performance at Session 4 was fastest, being 45 s better than at Session 2 (95 % CI: 31–60 s; p < 0.001). Sessions 3 and 5 also had faster times than Session 2, by 16 and 37 s, respectively (p ≤ 0.05 for each) (Fig. 5, right).
These data support Hypothesis 3: Pesticide workers will demonstrate deficits (slower performance) on the Trail Making Test compared to controls, and job titles with higher exposures will have greater deficits in performance than those with lower exposures. These data also support Hypothesis 8: Trail Making Test deficits will persist after exposure ends. Hypothesis 8 is the subject of a more extensive analysis later in the Results (3.8).
3.4. Association of years working for the MOA with trail making performance
The hypothesis that an increased number of years of occupational CPF exposures is associated with greater deficits in Trail Making performance was tested by examining data from 2009 for the same subjects described in section 3.3 using GEE analyses that presumed the (transformed) response followed an approximately normal distribution with exchangeable correlation structure of repeated measures taken over multiple testing sessions. Robust standard errors were used to guard against potential misspecification of the actual correlation structure. Both years of work at the MOA (and thus exposure) and education are addressed. For both Trail Making A and B, after adjusting for age and occupation, neither years of work for the MOA nor years of education were significantly associated with performance (Trail Making A: p = 0.60 for years of education, p = 0.91 for years of work for MOA; Trail Making B: p = 0.70 for years of education, p = 0.25 for years of work for MOA). Each 5 years of experience working for the MOA was associated with a 0.2-s increase in Trail Making A performance, emphasizing the lack of impact of years working for the MOA on test performance.
These data do not support Hypothesis 4: A greater number of years working for the MOA, and thus more years of exposure to CPF, will be associated with greater deficits on the Trail Making Test.
3.5. Association of peak urinary TCPy levels with post-exposure trail making performance
We examined whether performance was associated with an individual’s peak urinary TCPy concentration observed over Sessions 1–5, to test whether this peak concentration was associated with average performance from Sessions 4 and 5, after exposure had ended. We thought peak exposure might be a better predictor of persistent effects than average exposure. Trail Making performance was tested using ordinary multivariable regression with robust standard errors.
Of the 144 subjects from 2009, 142 (30 applicators, 58 technicians, 54 engineers) had at least one urine sample collected for TCPy quantification during the five sessions from which a maximal or peak value could be obtained. Session 2 was most frequently associated with the highest urinary TCPy level, regardless of job title. The sample size decreases to 124 when peak urinary TCPy concentration is correlated with average performance from those participants who completed behavioral testing during Sessions 4 and 5, and these are the same subjects described in Section 3.6 and Table 2. Over the entire sample, the median and inter-quartile range (IQR) for peak TCPy concentration was 22.3 [13.0–46.2] ng/mg creatinine with a marginally significant (p = 0.05) difference among the three occupations. The median [IQR] peak was 38.8 ([15.6–102.4] for applicators, 21.7 [12.8–34.0] for technicians and 21.2 [11.8–40.2] for engineers.
Table 2.
Descriptive characteristics for 124 participants used to determine associations between post-exposure performance (Sessions 4 and 5) and demographic characteristics or measures of exposure from earlier Sessions (2 and 3) when CPF application was ongoing.
| Characteristic | Applicator (n = 30) | Technician (n = 47) | Engineer (n = 47) | p-value |
|---|---|---|---|---|
| Trails A, s mean (SD) | 83.2 (31.8) | 76.7 (16.9) | 78.7 (50.3) | 0.60 |
| Trails B, s mean (SD) | 168.1 (52.2) | 171.4 (36.8) | 152.9 (44.0) | 0.09 |
| Age, yrs mean (SD) | 38.9 (13.5) | 48.7 (4.6) | 54.1 (4.3) | <0.001 |
| Education, yrs mean (SD) | 11.0 (2.3) | 12.3 (1.2) | 14.5 (3.0) | <0.001 |
| Years MOA mean (SD) | 12.9 (9.2) | 14.7 (9.4) | 18.5 (8.3) | 0.02 |
| GMa TCPy, ng/mg creat. median [IQR] | 20.8 [11.8–50.3] | 12.5 [7.8–20.2] | 12.1 [8.9–20.6] | 0.03 |
| Relativeb AChE median [IQR] | 0.98 [0.93–1.01] | 0.98 [0.95–1.01] | 0.97 [0.94–1.00] | 0.63 |
| Relativeb BuChE median [IQR] | 0.86 [0.68–0.94] | 0.94 [0.86–1.00] | 0.94 [0.90–0.99] | 0.01 |
Geometric mean from Session 2 & 3.
Geometric mean activity from Session 2 & 3, divided by activity at Session 1.
There was some evidence to suggest that peak urinary TCPy concentration had a differential effect on Trail Making A performance according to job title (p = 0.09; test of TCPy × occupation interaction). After adjusting for age, the approximate 3.55-fold increase in TCPy from 13 to 46.2 ng/mg creatinine is associated with a 7.7 s decrease in completion time for applicators (p = 0.08), a 25.6 s increase in completion time for engineers (p = 0.19), and a 1.8 s increase in completion time for technicians (p = 0.59). This analysis also relied on GEE models and was expanded to contain all collected measures of CPF exposure (TCPy, AChE, BuChE) to explore their influence after controlling for other known demographic/occupational factors.
For Trail Making B, there was no indication that the effect of peak TCPy concentration was modified by occupation (p = 0.57; test of TCPy × occupation interaction), implying a similar trend for all occupations, after adjusting for age. This trend was associated with an increased completion time (i.e., worse performance) of approximately 10.6 (95 % CI: 1.1–20.2; p = 0.03) s over the central half-sample from 13 to 46.2 ng/mg creatinine. By comparison, this effect is only slightly smaller than the estimated effect associated with a 10 year increase in age, which was estimated to be a 12.3 (95 % CI: 2.4–22.1) s increase in time needed to complete Trail Making B. Even after adjusting for peak TCPy and age, there still remain persistent effects due to job, with applicators and technicians respectively taking an average of 26.6 (p = 0.06) and 24.4 (p = 0.005) s longer to finish than engineers.
Thus, Hypothesis 5: Peak exposures defined by TCPy measures will be negatively associated with post-exposure Trail Making performance, was not supported by the data.
3.6. Association of estimated cumulative exposures with post-exposure trail making performance
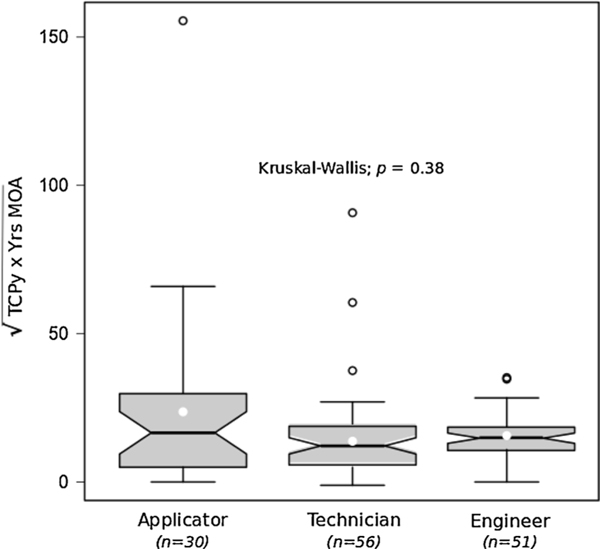
As employees have the same job and job title from year to year, years of work in the MOA served as the basis for defining cumulative exposure. The concept for this analysis was predicated on the assumption that TCPy exposure during the application periods (Sessions 2 and 3) in 2009 was reflective of a person’s typical exposure experience in all years they worked for the MOA (i.e., a person who carefully avoided skin exposure to pesticides during 2009 would have behaved similarly over their working lifetime, and those who, for example, stirred the pesticide mixture with their hands in 2009 would have done so over the years; these hypothetical application team members would have vastly different skin exposures). Thus, years working in the MOA was multiplied by their GM urinary TCPy concentration from the two application periods (Sessions 2 and 3 in 2009) to estimate each person’s cumulative exposure over their lifetime of working. This measure has units of ng TCPy*year/mg creatinine and was square-root transformed prior to analysis to improve symmetry. This transformation preserves order, so the ranking of individuals on the transformed scale is identical to that on the original scale. For this analysis, we created an additional explanatory variable defined as the product between the application specific (Sessions 2 & 3) GM TCPy concentration and years working for the MOA. GEE models of the sort previously described were then fit to Trail Making A / B performance over time while including this new composite predictor in addition to other demographic and occupational explanatory variables. Again, the models presumed the (transformed) response followed an approximately normal distribution with exchangeable correlation structure of repeated measures taken over multiple testing sessions. Robust standard errors were used to guard against potential misspecification of the actual correlation structure.
Fig. 6 shows the distribution of this cumulative exposure measure among the three job titles for a sample of 137 subjects. These are the same subjects examined in Section 3.3, but missing two applicators for whom there was no TCPy measures during the two sessions in question. The same models described in Section 3.3 (examining age and occupation) were used here as well, but with inclusion of the (transformed) cumulative exposure measure as well as an interaction between this cumulative exposure measure and session. Thus, the cumulative measure of exposure for each person derived from Sessions 2–3, was used as a predictor for performance at all sessions. This interaction was used to assess whether any trend associated with cumulative exposure fluctuates according to session. However, no difference was noted among job titles.
Fig. 6.
Distribution of cumulative exposure of TCPy (GM from Sessions 2 and 3) multiplied by years spent working for MOA (vertical axis; square-root scale). For each job title, the white circle is the mean and the open circles represent outliers. The black horizontal line in the center of each shaded area represents the median. The v-shaped notches formed by the shaded areas denote the 95 % CI for the median. The lower and upper edges of the shaded area represent the 25th and 75th percentiles. The whiskers extend to the largest and smallest nonextreme values (within 1.5 box links).
Transformed cumulative exposure was not associated with job title (p = 0.38), and overall had a median of 15 units (IQR from 9 to 20 units). Adjusted for job title and age, we found a significant (p = 0.05) interaction between cumulative exposure and session in relation to performance on Trail Making A, but not for Trail Making B (p = 0.67). The interpretation of the interaction was made by considering the observed IQR for the exposure and reporting the change in the average performance on Trail Making A over this range from 9 to 20 units (i.e., change in performance over the central half-sample of transformed cumulative exposure) at Sessions 2—5.
The effect associated with cumulative exposure alternates between (insignificant) increases and decreases in performance, with the trend for an 11 unit gain in cumulative exposure varying from just under a 3 s worsening in performance to a little more than a 2 s improvement. These measured effects related to cumulative exposure, however, are considerably smaller than the 13.2 (95 % CI: 8.3–18; p < 0.001) s increase in completion time per 10-year increase in age, and they are thus far less important than age.
These data do not support hypothesis 6: Higher CPF exposure concentrations (based on actual 2009 TCPy exposures) multiplied by years applying pesticides (i.e., cumulative exposure) will be associated with greater deficits in post-exposure Trail Making Test performance.
3.7. Association of TCPy, BuChE, and AChE with trail making performance
We tested whether Trail Making performance correlates with established biomarkers of CPF exposure (i.e, blood AChE, blood BuChE, and urinary TCPy) in a dose-response manner. The analysis used the same GEE models described in the methods, with Trail Making performance as the response and percent cholinesterase inhibition (or log-TCPy concentration), occupation, session, and age as explanatory characteristics. The analyses for hypothesis 7 also relied on GEE models and were expanded to contain all collected measures of CPF exposure (TCPy, AChE, BuChE) to explore their influence after controlling for other known demographic/occupational factors. Cholinesterase inhibition was determined by dividing each participant’s BuChE and AChE activity at Session 2, 3, and 5 by his activity at Session 1. Session 4 was excluded because no participant had cholinesterase activity measured during that session.
Of 144 participants described in Table 1, 132 (30 applicators, 51 technicians, 51 engineers) had blood BuChE and AChE activity recorded for Sessions 2, 3 and 5, and 138 (30 applicators, 57 technicians, 51 engineers) also had urinary TCPy recorded for Sessions 2, 3 and 5 [not every applicator provided a viable sample at each time point]. Mean years of age, education, and work experience for this slightly smaller cohort was not different from values given in Table 1 (differences were always less than 0.4 years).
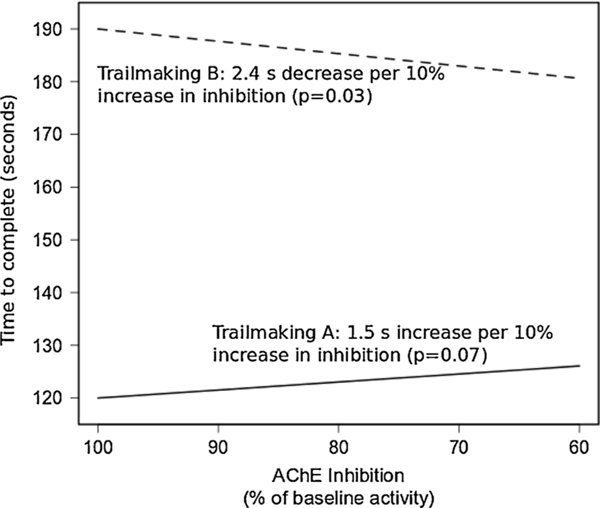
Controlling for age, occupation, and session, we found no association between percent BuChE inhibition and performance on Trail Making A (p = 0.24) or B (p = 0.65). For AChE, each 10 percentage point increase in inhibition was associated with a marginally significant (p = 0.073) 1.5 s increase in Trail Making A completion time and a 2.4 s decrease (95 % CI: 0.2–4.4; p = 0.029) in Trail Making B completion time (Fig. 7). To be suggestive of an effect, the direction of change would be expected to be in the same direction for Trail Making A and B, but it was not.
Fig. 7.
Association of AChE inhibition compared to baseline (Session 1) with time to complete Trail Making A or B.
Individual urinary TCPy concentration was not associated with contemporaneous participant performance on either Trail Making A (p = 0.95) or B (p = 0.83).
Thus, the data do not support Hypothesis 7: Trail Making Test performance will correlate in a dose-response fashion with the urinary TCPy biomarker and both BuChE and AChE biomarkers.
3.8. Persistence in trail making performance effects after exposures had ended
Data from Session 4 (7–10 days after CPF applications and occupational exposures ended) and Session 5 (~1.5 months post exposure in the cotton fields) were examined to test whether Trail Making A or B performance at these specific post-exposure periods was associated with job title or biomarkers of exposure/effect (urinary TCPy, blood AChE, blood BuChE) measured during Sessions 2 and 3 (mid-July, early August) when pesticide application teams were applying CPF. Prior analyses (Section 3.3) revealed no significant differences between Session 4 and 5 for any occupation with respect to Trail Making performance, after accounting for age (p = 0.11, Trails A; p = 0.22 Trails B). We subsequently averaged each participant’s performance from these two sessions and tested this average post-exposure performance measure for association with job title and three averaged measures of exposure from Session 2 and 3. The GM TCPy concentration from those two periods during CPF application and GM AChE and BuChE activity from those same sessions relative to activity at Session 1 were calculated to generate a percent inhibition of cholinesterase activity. Although the prior analyses found a strong effect due to age, but no effects due to education or years working at the MOA, we included these variables in this new analysis since it relies on a specific subset of testing sessions and involves a more limited cohort of individuals. Since this analysis involves summary measures of performance and exposure computed at the level of the individual subject (i.e., no longer any longitudinal component), ordinary multivariable regression was used to estimate and test the associations of interest, with models using robust standard errors to accommodate heteroscedasticity. Multivariable regression was used to model average post-exposure Trail Making performance (A / B; Sessions 4 and 5) as a function of average CPF exposure measures (TCPy, AChE, BuChE) from active application periods (Session 2 and 3) while controlling for other demographic/occupational factors.
Table 2 gives descriptive characteristics for the 124 subjects (30 Applicators, 47 Technicians, 47 Engineers) who met the criterion of having all the data used to address the hypothesis that post-exposure performance varies by job title and exposures. We considered several models in the process: Model 1 includes occupation and age (already known to be non-negligible) without adjustment for other factors; Model 2 expands Model 1 by also including years of education and years of work for MOA; Model 3 expands Model 2 by also including all exposure characteristics. These nested models allow for testing of differences among job titles with varying degrees of adjustment, and also determine whether inclusion of demographic or exposure characteristics significantly affects performance. The Bayesian Information Criterion [BIC], which quantifies the balance between model accuracy and parsimony/complexity, was computed (lower values preferred) to help determine when additional terms were irrelevant (Schwarz, 1978).
Without controlling for any other factors, post-exposure performance on Trail Making A does not differ significantly by job title (p = 0.60), while post-exposure performance on Trail Making B has some suggestive but inconclusive evidence of an effect due to job title (p = 0.09). All demographic characteristics show robust differences among job titles (p ≤ 0.02 for each), while exposure differences appear, at least in an unadjusted sense, limited to urinary TCPy (p = 0.03) and blood BuChE inhibition (p = 0.01). These last conclusions regarding exposure are similar to outcomes reported in Section 3.3 for the larger cohort of 139 or 144 participants.
By contrast, regression models for Trail Making A that accounted for age found applicators to be almost 20 s slower than engineers (p = 0.06) and just over 15 s slower than technicians (p = 0.05), with no real difference between engineers and technicians (less than 4 s of separation; p = 0.64). Each decade of age was associated with an average increase of 10 additional s to complete the task. Further attempts to include education, years working at the MOA, and the three measures of exposure found those five variables didn’t have a significant impact [F(5,115) = 1.12, p = 0.35] beyond the initial model, and more specifically, the exposures had no additional influence [F (3,115) = 0.60, p = 0.62] beyond a model that fully-adjusted for all demographic characteristics. The comparative size and direction of differences between job titles differs by almost 1.5 s between the crude (Model 1) and fully-adjusted model, suggesting the other demographic and exposure variables are not strong confounders with performance on Trail Making A. These results are summarized in Table 3.
Table 3.
Estimated change in mean performance (in s) for Trail Making A according to job title, adjusted for various demographic and exposure variables. All models use robust standard errors to account for non-constant variance; comparisons between models show significance of those additional terms used to adjust performance beyond job title. and age.
| Model 1 (M1) | Model 2 (M2) | Model 3 (M3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Occupation | |||||||||
| Eng. – Appl. | −19.7 | 10.2 | 0.06 | −18.9 | 8.5 | 0.03 | −18.2 | 8.6 | 0.04 |
| Tech. – Appl. | −16.3 | 8.2 | 0.05 | −15.3 | 8.8 | 0.09 | −15.3 | 8.5 | 0.08 |
| Eng. – Tech. | −3.5 | 7.2 | 0.64 | −3.6 | 4.5 | 0.43 | −2.9 | 5 | 0.56 |
| Age per 10 yrs | 10.1 | 3.6 | 0.01 | 8.6 | 4.0 | 0.04 | 8.6 | 4.1 | 0.04 |
| Education per 1 yr | −0.2 | 2.4 | 0.92 | −0.5 | 2.4 | 0.84 | |||
| Work for MOA per 1 yr | 0.4 | 0.2 | 0.05 | 0.4 | 0.2 | 0.05 | |||
| GMa TCPy per 15 ng/mg creat. | −0.6 | 0.5 | 0.21 | ||||||
| Relativeb AChEper 10 percentage point decrease | 0.6 | 4.4 | 0.88 | ||||||
| Relativeb BuChE per 10 percentage point decrease | 1.6 | 1.6 | 0.34 | ||||||
| [Bayesian Information | 1252.7 | 1261.2 | 1274.3 | ||||||
| Criterion (lower is better) | |||||||||
| Model Comparisons | |||||||||
| M2 vs M1 (simultaneous test of education, work) | F(2, 118) = 2.03, p = 0.14 | ||||||||
| M3 vs M2 (simultaneous test of all 3 exposures) | F(3, 115) = 0.60, p = 0.62 | ||||||||
| M3 vs M1 (simultaneous test of all effects other than age, occupation) | F(5, 115) = 1.12, p = 0.35 | ||||||||
Geometric mean from Session 2 & 3.
Geometric mean activity from Session 2 & 3, divided by activity at Session 1.
Similar models for Trail Making B found age-adjusted performance was best for engineers, who were on average 33 (p = 0.02) and 25 (p = 0.01) s faster than applicators and technicians, respectively. Each additional decade of age was associated with an approximate 12-s increase in completion time. Although additional demographic and exposure-related variables were not significant [F(5,115) = 0.62, p = 0.68], they are confounded with occupation and performance such that controlling for these five variables closes the performance gap between applicators and engineers by nearly 13 s while the performance gap between Engineers and Technicians is narrowed by 7 s. Table 4 summarizes these findings for Trail Making B.
Table 4.
Estimated change in mean performance (in s) for Trail Making B according to job title, adjusted for various demographic and exposure variables. All models use robust standard errors to account for non-constant variance; comparisons between models show significance of those additional terms used to adjust performance beyond occupation and age.
| Model 1 (M1) | Model 2 (M2) | Model 3 (M3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Occupation | |||||||||
| Eng. – Appl. | −32.6 | 14.2 | 0.02 | −23.3 | 14.8 | 0.12 | −19.8 | 17.2 | 0.25 |
| Tech. – Appl. | −7.7 | 12.4 | 0.54 | −4.3 | 12.3 | 0.72 | −2.0 | 14.3 | 0.89 |
| Eng. – Tech. | −24.9 | 8.8 | 0.01 | −19.0 | 9.7 | 0.05 | −17.8 | 10.0 | 0.08 |
| Age per 10 yrs | 12.2 | 5.8 | 0.04 | 11.9 | 6.3 | 0.06 | 11.6 | 6.6 | 0.08 |
| Education per 1 yr | −2.2 | 1.7 | 0.22 | −2.5 | 1.8 | 0.17 | |||
| Work for MOA per 1 yr | −0.3 | 0.5 | 0.54 | −0.3 | 0.5 | 0.52 | |||
| GMa TCPy per 15 ng/mg creat. | −0.3 | 2.2 | 0.90 | ||||||
| Relativeb AChE per 10 percentage point decrease | −0.6 | 4.3 | 0.90 | ||||||
| Relativeb BuChE per 10 percentage point decrease | 1.8 | 5.1 | 0.73 | ||||||
| BIC (lower is better) | 1288.4 | 1295.5 | 1309.3 | ||||||
| Model Comparisons | |||||||||
| M2 vs M1 (simultaneous test of education, work) | F(2, 118) = 1.06, p = 0.35 | ||||||||
| M3 vs M2 (simultaneous test all 3 exposures) | F(3, 115) = 0.05, p = 0.98 | ||||||||
| M3 vs M1 (simultaneous test of all effects other than age, occup.) | F(5, 115) = 0.62, p = 0.68 | ||||||||
Geometric mean from Session 2 & 3.
Geometric mean activity from Session 2 & 3, divided by activity at Session 1.
In sum, the differences in job title, which are associated with different exposure levels to CPF (section 3.3), differ at Sessions 4 and 5 (when exposures had ended) once age differences are taken into account in the analysis. Fig. 5 also depicts the differences between the job title groups, with a slightly larger N (because that analysis did not include all the variables and thus all the measures used in these more comprehensive models). Taking all other relevant variables (viz., education, years at the MOA, TCPy, AChE, BuChE) into account in the analysis, which was not done in the prior sections, does not add anything to the outcome of the analysis. Comparisons of model 2 vs. model 1 (p = 0.35), model 3 vs. model 2 (p = 0.96), or model 3 with model 1 (p = 0.68) are not significant and therefore do not improve on the explanatory power of model 1. As the analysis controls for more and more variables, the adjustments had little effect. In the M3 vs. M1 comparison, there is no real difference in the model performance (i.e., the lowest BIC is M1 and there is little difference in that added complexity without any added benefit). That is, the more comprehensive models don’t improve the explanatory power of the model – the added variables just don’t matter.
Hypothesis 8. Trail Making Test deficits will persist after exposure ends, is confirmed.
3.9. Trail making performance over time
While too few of the pesticide applicator group in 2009 returned for testing in 2010 to provide the same level of analysis that could shed light on the possibility of a year-to-year decline in performance, it is possible to illustrate applicator team performance relative to the Controls who were only tested in 2010 by combining the applicator team job titles (viz., applicators, technicians, engineers) into a single group. Trail Making Test performance is described for the 44 subjects (4 applicators, 19 technicians, 21 engineers) who completed both tests A and B during Years 1 and 2, together with 150 controls who took both A and B for the first time in Year 2 (2010). The mean time to complete the Trail Making Test was computed according to control (n = 150) and pesticide application teams (n = 44) (Fig. 8). Session 1 is included only for controls because they, unlike the application team members, received the correct testing instructions in Session 1. Though controls were tested only in 2010, the impact of learning the skill of Trail Making Test performance necessitates that their performance in 2010 be compared to agriculture workers from 2009 as both groups were experiencing testing for the first time. Fig. 8 shows the improvement over time with succeeding administrations of the test, reflecting learning and increasing familiarity with test performance. This is consistent with the neuropsychological literature (Duff et al., 2012; Fernandez-Ballesteros et al., 2005; Nguyen et al., 2015) and with test results from adolescent Egyptian pesticide applicators who worked in the cotton fields, and were tested repeatedly (Ismail et al., 2017b). The Egyptian Trail Making Test scores are considerably higher (slower) than those collected on community members in North America of roughly the same age and educational background (as reported by Tombaugh, 2004), though cultural differences may make comparisons to results from other geographical/cultural areas moot (Stanczak et al., 2001). Fig. 8 also shows that the applicator team members could achieve or exceed the same level of performance as controls, it just took longer to reach that level than was the case for the controls. Of course, if the controls were given an equal amount of practice their performance may have been far better.
Fig. 8.
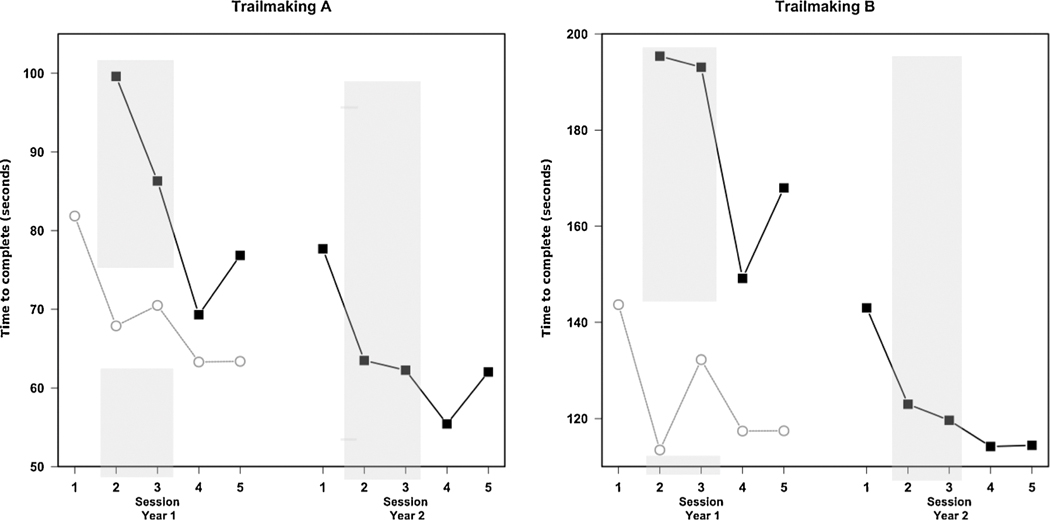
Average performance on Trail Making A (left) and B (right) over repeat sessions in years 2009 and 2010 for agricultural workers (black line, filled squares). MOA controls (gray line, open circle) are shown for comparison. The gray shaded columns identifiy the period during which pesticides were being applied.
4. Discussion
Previous research has demonstrated that Egyptian pesticide applicator team members exposed to CPF had impaired performance on Trail Making Tests A and B compared to controls (T. M. Farahat et al., 2003). Several other studies of occupational OP pesticide exposure have reported the same outcome on the Trail Making Test as well as on other behavioral tests (Blanc-Lapierre et al., 2013; Cole et al., 1997; Korsak and Sato, 1977; Mackenzie Ross et al., 2010; Richter et al., 1992; Rosenstock et al., 1991; Wesseling et al., 2002), whereas a smaller number of studies have not observed a similar impact of OP exposures (Fiedler et al., 1997; Roldan-Tapia et al., 2006). The results of the present study are consistent with those studies that report an association between occupational OP exposures and deficits in behavioral test performance, and significantly extend them by demonstrating a relationship between behavioral test deficits and two job titles with differing exposure levels plus control, which suggests a dose-response relationship. Pesticide applicators had significantly higher exposure levels than technicians or engineers based on urinary TCPy measures, and controls had significantly lower exposure levels than any of the occupational groups. Performance on the Trail Making Test A and B was poorest for the applicators, better for the technicians and engineers, and best for the controls in four testing sessions. The group differences in urinary TCPy measures did not persist after applications stopped, but the group differences on Trail Making Test performance observed during the time of applications were still evident up to a month after the applications had ended. These continued deficits after exposures ended are consistent with prior research (Rohlman et al., 2016). Urinary TCPy and blood cholinesterase measures taken at the time of testing did not correlate with the deficits seen on the Trail Making Test in our study; although some research has found correlations between test performance and these biomarkers (e.g., Ismail et al., 2017b).
4.1. Urinary TCPy associated with job titles
Applicators had higher GM urinary TCPy concentrations than either engineers or technicians at Sessions 1–3, while the urinary TCPy concentrations of Engineers and Technicians did not differ significantly (Fig. 3). This establishes that there are two different levels of exposure associated with the job titles and thus establishes a basis for defining a dose response for performance differences in participants in those job titles. Data from University employees not working in agriculture suggest that “control” exposure levels are at the midpoint of Session 1 levels in the engineers and technicians and below that of the applicators.
4.2. Blood cholinesterase measures
AChE activity did not differ significantly among job classifications over the time from pre-application (Session 1) to during application (Session 3) to post-application (Sessions 4 and 5), but all three job titles showed the same decrease of ~2.7 % for successive sessions (e.g., 2.7 % reduction from Session 1 to Session 3; 2.7 % reduction from Session 3 to Session 5), and this decrease was significant. The magnitude of these changes are lower than those seen in the same population in 2008, the year prior to the year these data were collected; applications were believed to be more extensive in 2008 based on anecdotal reports of participants (Singleton et al., 2015).
BuChE activity decreased more for applicators than for the other two occupations when comparing Session 3 (during exposure) against Session 1 (pre-exposure); technicians and engineers had the same BuChE profiles over time, although these two job classifications also showed a decrease compared to the pre-application time (Session 1). BuChE levels were uniformly lower in all job type groups than in MOA controls sampled in 2010 over Sessions 1, 2, 3. The lower confidence limit for these controls was higher than the upper limit of the levels of the application job types indicating that BuChE activity did reflect exposures from recent applications (Fig. 4, B).
Our data are consistent with the Garabrant et al. (2009) report demonstrating that plasma BuChE activity, which constitutes 99 % of human plasma B-esterase activity (van Gemert et al., 2001), is a measure of exposure that can be inhibited at exposure levels below those required to inhibit red blood cell AChE.
4.3. Differences in trail making performance by job title
For both Trail Making A and B, the changes in performance observed over time were similar across all job titles (viz., no significant job x time interaction) as demonstrated by parallel performance profiles over time (Fig. 5). The ordering was always the same: Applicators were slowest, followed by technicians, with engineers being fastest among those working on the pesticide application teams. The MOA controls were always fastest, compared to the pesticide application team members, and on the more challenging Trail Making part B, they performed significantly faster than all other groups at all time points. There also were differences in urinary TCPy measures between the job titles (Table 3, 4.1). Even after adjusting for age, there were Trail Making Test differences between job categories, and those differences remained constant across all testing sessions (as indicated by parallel performance profiles) both during and after CPF exposures in the fields. Age, adjusted for in the analyses as noted above, was a significant factor and was associated with a 13 s increase in completion time for each 10 years of increasing age on Trail Making B. After accounting for occupation and age, years of education and years of work experience were not significant predictors of performance (as noted in section 3.4).
These results establish that occupational exposures to CPF were associated with dose-dependent deficits in human performance. This is established by the differences between the applicators (whose exposure was the highest but whose Trail Making performance was the lowest), the engineers/technicians (whose exposure was lower than that of the applicators and higher than controls, but whose Trail Making performance was better than the applicators and worse than the controls) and the controls (with the lowest exposures and the best Trail Making performance) as seen in Figs. 3, 4, and 6. This was true both during CPF exposures and after exposures had ended.
This has been a point of contention for some authors who doubted the evidence that exposures to CPF affect behavioral test performance because the studies did not have data from exposures over time primarily due to CPF (i.e., there were many pesticide exposures involved) or there was only one exposed group, or both, as noted by Meyer-Baron et al. (2015). In the present study, we report one group with higher and two groups with lower exposure levels, plus controls with much lower exposure levels; the controls’ exposures may have been due to home exposures as CPF is widely available commercially, and was reportedly used for insect control in homes (F. M. Farahat et al., 2011; Lein et al., 2012).
4.4. Results from studies in Egypt: relationships between CPF exposures and test performance
There were no relationships between classic biomarkers of OP exposure and Trail Making performance in the present study, but some of the other studies of Egyptian adolescent pesticide applicator teams have reported such relationships. The present study did see an association between AChE activity and improved performance (faster times to complete) on Trail Making B (p = 0.03), but a marginal association was also seen with slower performance on Trail Making A (p = 0.07). Since the changes were in the opposite direction, the associations do not create a compelling case. Further, there was no relationship of the other widely-used measures of recent OP exposures, urinary TCPy levels or blood BuChE activity (Crane et al., 2013; Singleton et al., 2015), with Trail Making performance. T. M. Farahat et al. (2003) also recruited adults but relationships between biomarker data and Trail Making performance (the test was administered individually in a quiet room) was not correlated with AChE. This is summarized in Table 5.
Table 5.
Evidence of Adverse Effects on the Trail Making Test in Studies of Chlorpyrifos-Exposed Adult and Adolescent Samples and Correlations of Trail Making Results with Biomarkers.
| Study | Age of sample (y) | Exposure of C v. E | Deficit on A | Deficit on B | Correl. with TCPy | Correl. with AChE | Correl. with BuChE |
|---|---|---|---|---|---|---|---|
| Present | 48–54 | TCPy: 5.5–6.3 (C) v. 12 (T&E) v. 21 μg/g creat. (A) BuChE: 0.86 (A) v. 0.94 (T&E) U/ml * | Yes | Yes | No | No | No |
| Farahat et al. (2003) | 42 | AChE: 108 v. 87 U/ml | Yes | Yes | • | No | • |
| Abdel Rasoul et al. (2008) | 9–18 | AChE: 283 v. 240 IU/L | Yes | Yes | • | Yes | • |
| Rohlman et al. (2016) | 16–17 | TCPy:<1559 (C) v. > 1.6 ng/g creat. | Yes | Yes | • | • | • |
| Ismail et al. (2017a) | 15 | No comparisons | • | • | Yes (B) | Yes (A) | No |
| Ismail et al. (2017b) | 15 | 33–42 % of applicators had BuChE depression vs. 5–13 % of non-applicators | No | Yes | • | • | Yes |
Notes: C= control; T = technician; E =engineer; A =applicator.
“•” indicates tests were not reported in the article. Data rounded to nearest whole number to facilitate visual inspection of the results.
No difference in AChE between applicators and technicians or engineers. 2017a = JTEH article. 2017b = PLOSone article. Creat. = creatinine
The remaining studies recruited adolescents. Abdel Rasoul et al. (2008)’s adolescent applicators had significantly lower AChE activity (240 IU/L) than the children from the same village who did not work in the cotton fields who served as controls (283 IU/L). The participants were divided into older (16–18 years) and younger (9–15 years) groups, and applicators had significant deficits in Trail Making A and B performance compared to controls in both age groups. There were significant correlations between Trail Making B performance and AChE activity, days worked during the current season, and years worked as an applicator, all differing from the current study (Table 5).
Ismail et al. (2017b) recruited adolescent applicators and controls from the same community who had not worked in the cotton fields. The groups were combined for regression analyses of biomarkers and TMT performance. Data were drawn from four sessions, two during CPF application periods and two prior to application periods, over two years. There was a significant relationship between higher urinary TCPy concentrations and performance deficits in Trail Making B, and AChE activity was negatively correlated with Trail Making A performance (Table 5).
Ismail et al. (2017a) analyzed data from adolescent pesticide applicators and non-applicators from two years (2005 and 2009). Group differences on Trail Making were not reported, but mixed model correlation analyses revealed correlations between Trail Making performance and urinary TCPy levels (B only) and blood AChE activity (A only), but not blood BuChE activity (Table 5). Thus, studies of adolescents have found correlations between Trail Making test performance and the standard biomarkers of recent exposure. One study of adult agricultural workers with diverse undocumented exposures in the US also reported correlations between AChE and several behavioral test measures, though none was Trail Making (Rothlein et al., 2006).
Since the Ismail et al. (2017b) study tested adolescents from the same population as our adult participants (cotton field applicators/teams working for the Ministry of Agriculture in the Nile Delta), though using a slightly different analytic approach, we took a more focused look at the association between Trail Making A and B performance (Sessions 2–5) and log(TCPy) concentration from Sessions 2–5. Following the Ismail et al. (2017b) methodology, we used a mixed-effect model for the response (Trail Making A and B latency; no transformation) with testing session as a factor and contemporaneous log(TCPy) as predictors. Ismail et al. (2017b) made further adjustment for education rather than age of individual, so we ran models with both age and education even though years of education appeared to be non-significant in the Ismail et al. reporting of Trail Making A and B performance (the authors reported that they only include one of these variables in the analysis because age and education were highly correlated). Our models for Trail Making A and B each used 138 participants (30 Applicators, 51 Engineers, 57 technicians); data from one technician is lost if education rather than age is used for the analysis.
Considering Trail Making B adjusted for age, the log(TCPy) effect was −0.85 (95 % CI: −7.96 to 6.26; p = 0.815) and was −1.32 (95 % CI:8.46 to 5.82; p = 0.717) when adjusted instead for education in our adult sample (results for A were similarly non-significant). Thus, using these same models (mixed models vs GEE; using transformed TCPy as a predictor instead of occupation) we still reach the conclusion that there is no association between TCPy and Trail Making B (or A) performance. The effect for Trail Making B reported by Ismail et al. (2017b) translates to ~ 2.1 (95 % CI: 0.7–3.4) s worse performance for each doubling in TCPy concentration. To relate these concentrations to the present study, a cohort of adolescents having geometric mean (GM) concentration of 30 μg TCPy/g creatinine would be about 2.1 s slower than a cohort with GM concentration of 15 μg TCPy/g creatinine (the difference between the approximate GM concentration of TCPy in our applicator group [30] and that of our controls [15] per Fig. 3). This same performance reduction would occur when comparing any two cohorts where average concentration is separated 2-fold.
Overall, the present study identifies a dose-related effect based on job title associated with CPF exposure measures (of the groups as noted in 4.2 and 4.3) and Trail Making performance. However, a dose response relationship cannot be based on the classic measures of recent CPF exposure (viz., TCPy and AChE or BuChE; Singleton et al., 2015; Crane et al., 2013) and Trail Making performance in adults, though there are correlations between Trail Making performance and biomarkers of recent CPF exposure in adolescents, which we cannot explain. These data suggest that the classic CPF exposure measures that reflect recent exposures are not predictive of persistent effects detected at times well beyond the exposure period in adults, although they may be used to identify those individuals who will, after an extended period of such exposures, be at risk of reduced cognitive function. A similar conclusion was reached by Meyer-Baron et al. (2015).
The reason for the disconnect between the observation that deficits in Trail Making performance are correlated with job titles that differ in chlorpyrifos exposure as determined by urinary TCPy values, and the lack of significant correlation between individual urinary TCPy levels and behavioral outcomes is unknown. Perhaps it is due to the fact that urinary TCPy levels increase rapidly initially and then remain fairly steady throughout the exposure period while behavioral deficits manifest only after chronic exposures and remain after urinary TCPy levels return to baseline. In other words, there is a temporal disconnect between these two measures. It may also indicate that CPF exposures differentially affect peripheral systemic processes (e.g., hepatic metabolism of CPF to TCPy) and central nervous system processes. The lack of correlation between AChE and BChE activity and behavior supports the hypothesis that these two outcomes are mechanistically unrelated (a conclusion also offered by Rohlman et al., 2011). Terry (2012) and Zurlinden and Reisfeld (2018) propose a similar hypothesis, the latter asserting that cognitive outcomes following chlorpyrifos exposure “may be more sensitive markers of adverse health effects than AChE inhibition” page 047009–2.
4.5. Causal relationship between CPF exposure and trail making performance deficits
The identification of a dose-response relationship for CPF effects on the Trail Making Test is an important addition to the evaluation of whether there is a causal relationship between CPF exposures and Trail Making Test performance. With the addition of these new data, CPF meets all of the 9 Bradford Hill criteria for concluding that an exposure has a causative effect on the performance measure based on evidence from epidemiologic studies (Table 6).
Table 6.
The Nine Bradford-Hill (Hill, 1965) Criteria and the Evidence that Supports or Meets Each Criterion.
| Criterion | Supporting Evidence |
|---|---|
| 1) Strength (e.g., effect size) | The effect size (d) of the score differences between exposed and controls in the T. M. Farahat et al. (2003) study was 0.55 for Trail Making A and 0.61 for B; both are moderate effect sizes per Cohen (1992). In the present study of a workforce from the same population, applicators completed the Trail Making A task an average of 19.7 (95% CI: 3.8 – 35.6; p = 0.015; d ~ 0.38) s slower than engineers and 12.7 (95% CI: 1.1 – 24.4; p = 0.032; d ~ 0.24) s slower than technicians. For Trail Making B, applicators were 30 (95% CI: 6.5 – 53; p = 0.012; d ~ 0.46) s slower than engineers and 3.2 s slower than technicians (p = 0.747; d ~ 0.05); technicians were 26 (95% CI: 9 – 44; p = 0.003; d ~ 0.41) s slower than engineers at each of the testing sessions. The effect sizes are model-based rather than derived from the mean and standard deviation as developed by (Cohen, 1992). |
| 2) Consistency (e.g., reproducibility from multiple studies) | Demonstrated by the Munoz-Quezada et al. (2016) review identifying 8 studies that documented poorer exposed group performance on the Trail Making Test compared to control groups. The present study adds a ninth that replicates and extends one prior finding in the same population of pesticide applicators (T. M. Farahat et al., 2003). |
| 3) Specificity (e.g., there is a specific population at a specific site with no other likely explanation) | Demonstrated by two studies (the present study and T. M. Farahat et al., 2003) with the same result in different samples from the same population primarily exposed occupationally to only one neurotoxic pesticide, CPF. There is limited evidence that exposure to pyrethroids, to which this population is also exposed, reduces performance on some cognitive tests (Hansen et al., 2017) though not those with a motor component as seen in the present studies. |
| 4) Temporality (e.g., does the exposure precede the effect) | Demonstrated for chronic exposures by the differences between the controls and exposed and for recent exposures by Rohlman et al. (2016) in adolescent pesticide applicators in this same population that had better performance on the Trail Making test before and after applications by these young applicators (e.g., as a well-paying summer job). |
| 5) Biological gradient (e.g., greater exposure should generally lead to greater incidence of the effect, or dose response) | Dose-related effect based job title demonstrated for the first time in multiple levels by the present study, as applicators, who had the greatest CPF exposure, had the poorest performance, while engineers/technicians who had CPF exposures lower than applicators but higher than controls, had intermediate performance and controls who had the lowest CPF exposures had the best performance. This is supported by the Meyer-Baron et al. (2015) meta-analysis of 22 studies relating duration of OP pesticide exposure in years (history/range of OP pesticides not documented) to performance deficits on a range of behavioral tests (including Trail Making). |
| 6) Plausibility (e.g., a plausible mechanism of cause and effect) | While ACHE inhibition is known to mediate the acute neurotoxic effects of OPs, considerable evidence in the human and animal literature point to non-cholinergic mechanisms of neurotoxicity following chronic exposures (Voorhees et al., 2016). How chronic OP exposures cause behavioral deficits remains an area of active research, but there is evidence implicating neuroinflammation and oxidative stress: chronic OP exposures has been shown to trigger neuroinflammation and oxidative stress (Guignet and Lein, 2019; Naughton and Terry, 2018) and both of these neuropathological responses have been linked to behavioral deficits in humans and animals. Thus there is a plausible mechanism to link chronic OP exposures to behavioral deficits. |
| 7) Coherence (viz., does not conflict with known facts of biological effects of OP chemicals) | As indicated under criterion 6 above, there is significant evidence in the experimental literature that under conditions of well-controlled exposures, OPs, including CPF, cause behavioral deficits via non-cholinergic mechanisms (Voorhees et al., 2016). Thus, the human data are coherent with the known facts of biological effects of OP chemicals. |
| 8) Experiment (viz., agrees with experimental studies) | Studies in a rat model with exposures based on those reported for Egyptian pesticide workers in this study demonstrated behavioral deficits in a cued learning test in two contexts or settings associated with CPF exposures (as related to attention also measured by the Trail Making Test) that appeared only after repeated exposures (viz., 15 exposures but not after 5 or 10 exposures). The series of studies also presents evidence that AChE is a poor biomarker of the effects of repeated exposures (Lattal et al., 2010). Experimental studies suggest that CPF-induced behavioral deficits appear to be causally related to oxidative stress (Lein, unpublished data) |
| 9) Analogy (e.g., similar evidence from related compounds or conditions) | The Munoz-Quezada et al. (2016) systematic review of studies of chronic OP exposure in adults revealed that 24 of 35 studies found adverse neurobehavioral effects, including 8 that used the Trail Making Test. There is independent evidence in children who apply the same pesticide in Egypt of adverse behavioral effects of CPF (Rohlman et al., 2016). |
Thus, all 9 of the Bradford-Hill criteria are met convincingly by experimental evidence to support a causative effect of CPF exposures on neurobehavioral deficits, specifically on the Trail Making Test. This establishes CPF as a neurotoxic substance, underscoring the need to implement exposure controls to prevent chronic exposures that can lead to neurotoxicity.
4.6. Limitations
We did not attempt to measure exposures to pyrethroids, the insecticide(s) used between the two longer periods of chlorpyrifos applications. As reported in a recent systematic review of pyrethroid neurotoxicity (Chrustek et al., 2018), oral administration in animals led to motor dysfunction and tremors in one experimental study, and in motor coordination and learning deficits in rats in another study, but there is limited evidence from adult human case studies that dermal exposures to pyrethroids may be associated with neurotoxic damage (viz. tremors). Thus, while we cannot rule out the possibility that pyrethyroid exposure contributes to the lack of association between biomarkers of CPF exposure and behavioral effects noted in this population, it is difficult to determine how much of a confounding factor this is given that the evidence for their occupational neurotoxicity in humans is quite limited.
The Trail Making Test was administered in a relatively quiet small room in 2009 but in a larger room with other activities going on in 2010, which provided a less optimal setting for the test and led us to avoid behavioral test comparisons between the years. Often there were several people in the room completing surveys waiting for their turn for the test, so conversation and interactions may have sometimes distracted those taking the test. Controls were tested in a room with other testing activities which would be expected to be more distracting circumstances. While not ideal testing circumstances, it was typical of the culture to have frequent interactions with others, and individual rooms were not available for testing the large numbers recruited for the study.
Our sample of applicators was drawn from three MOA stations and may not have been representative of overall exposures in Egypt or in other countries.
While the pesticide applicator teams we studied did not apply pesticides to any other crop in their occupation, they did in some cases state that they took on side jobs involving episodic pesticide application such as for farmers who grew crops other than cotton (Ismail et al., 2012; Lein et al., 2012).
5. Conclusion
In sum, there is ample evidence that Trail Making performance deficits are associated with job title, and job title is associated with different levels of chlorpyrifos exposure. The applicators who had the highest chlorpyrifos exposures had the greatest performance deficit, while the engineers who had the lowest exposures had the least deficit. Controls who did not work in or next to the fields, and had the lowest CPF exposures, had the best Trail Making performance. This is the first evidence of a dose-related effect for behavioral deficits associated with occupational exposures to CPF based on job title, establishing it as a neurotoxic substance in adult humans.
Because Trail Making performance was not associated with the classic biomarkers of recent chlorpyrifos exposure in the adults we studied, and not consistently associated with those biomarkers in other studies of children from this population (Table 5), we conclude that they are not mechanistically relevant biomarkers of effect caused by chronic or repeated, long-term exposure to chlorpyrifos in adults. We believe it is the long-term exposures that produced behavioral changes, especially since they were not correlated with biomarkers of recent exposure, and because the group differences persisted when exposures ended. The lack of correlation of behavioral performance deficits with the acute exposure biomarkers should not be considered evidence that the effects are specious or lacking in rigor as some have suggested (as documented by Meyer-Baron et al., 2015).
The present data suggest that the OP chlorpyrifos joins 6 other chemicals with demonstrated neurotoxic effects in humans by providing a key Bradford-Hill criterion indicative of strong evidence of a cause-effect relationship: Lead (Lanphear et al., 2005), methylmercury (Debes et al., 2006), manganese (Bowler et al., 2007; Claus Henn et al., 2010), polychlorinated biphenyls (PCBs) (Lin et al., 2008; Stewart et al., 2008), arsenic (Wasserman et al., 2004) and styrene (Benignus et al., 2005). While the adverse effects of lead, methylmercury, manganese and PCB are directly and proportionately associated with biomarker concentrations (blood lead at time of testing, cord blood mercury at birth, blood manganese at time of testing, PCBs in placental tissue at birth, respectively), arsenic and styrene are associated with a relevant exposure marker (arsenic concentrations in well water consumed and airborne exposure levels in the chamber at the time of testing, respectively). Thus, this association of Trail Making performance with adults’ TCPy associated with job type is more direct and thus convincing than is the case with arsenic and styrene but slightly less so than that for lead, methylmercury, manganese and PCBs.
Acknowledgements
Appreciation is extended to the several hundred Egyptian cotton field pesticide application teams and controls working at the Ministry of Agriculture (MOA) and the Menoufia University participated in our study and pilots that preceded it. Field sample collection and testing was performed by Nashat N, Farag N, Eleseregy F, Ahmed N, Shalash S, AbdulFattah B, Ahmed M, Raya I, Hashad M, Haikal M, Mahmoud, Hamza M, AlSakka A. Appreciation is also extended to members of the MOA in Menoufia Governorate, including AlElemi M, Mostafa S, Deyab Y, Bayoumi M, AlAraby R, for arranging access to the population and providing testing and sample preparation space. Barb McGarrigle performed most of the TCPy analyses at the University of Buffalo. Matt Bonner of the University of Buffalo and Kit Galvin of the University of Washington provided critical input to the surveys used in this project. Appreciation is also extended to Dr. Phil Bushnell of the USEPA, who provided guidance and counsel during the formative stages of the project.
The project protocol and consent forms were approved by the Menoufia University and Oregon Health & Science University IRBs.
Funding
This work was supported by National Institute of Environmental Health Sciences, United States, NIH R01 ES01630.
Footnotes
Declaration of Competing Interest
The following authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Pamela J. Lein, PhD
Michael Lasarev, MS
Fayssal Farahat, MD, PhD
Taghreed Farahat, PhD
These authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Wyndham Kent Anger, PhD
Diane S. Rohlman, PhD
James R. Olson, PhD
OHSU and Drs. Anger and Rohlman have a significant financial interest in Northwest Education Training and Assessment [or NwETA], a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU and the University of Iowa.
Dr. Olson has served as an expert witness for the plaintiffs in legal actions relating to the health effects of PCBs.
References
- Abdel Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, Ismail AA, 2008. Effects of occupational pesticide exposure on children applying pesticides. NeuroToxicology 29 (5), 833–838. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18662718. [DOI] [PubMed] [Google Scholar]
- Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, Baker BA, 2006. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J. Expo. Sci. Environ. Epidemiol. 16 (5), 447–456. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16570094. [DOI] [PubMed] [Google Scholar]
- Benignus VA, Geller AM, Boyes WK, Bushnell PJ, 2005. Human neurobehavioral effects of long-term exposure to styrene: a meta-analysis. Environ. Health Perspect. 113 (5), 532–538. 10.1289/ehp.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc-Lapierre A, Bouvier G, Gruber A, Leffondre K, Lebailly P, Fabrigoule C, Baldi I, 2013. Cognitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am. J. Epidemiol. 177 (10), 1086–1096. 10.1093/aje/kws346. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. , 2007. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med. 64 (3), 167–177. 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA, 2013. Pesticide exposure and neurodevelopmental outcomes: review of the epidemiologic and animal studies. J. Toxicol. Environ. Health B Crit. Rev. 16 (3–4), 127–283. 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrustek A, Holynska-Iwan I, Dziembowska I, Bogusiewicz J, Wroblewski M, Cwynar A, Olszewska-Slonina D, 2018. Current research on the safety of pyrethroids used as insecticides. Medicina Kaunas (Kaunas) 54 (4). 10.3390/medicina54040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. , 2010. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21 (4), 433–439. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/20549838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Am. Psychol. 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Cole DC, Carpio F, Julian J, Leon N, Carbotte R, De Almeida H, 1997. Neurobehavioral outcomes among farm and nonfarm rural ecuadorians. Neurotoxicol. Teratol. 19 (4), 277–286. [DOI] [PubMed] [Google Scholar]
- Crane AL, Abdel Rasoul G, Ismail AA, Hendy O, Bonner MR, Lasarev MR, et al. , 2013. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J. Expo. Sci. Environ. Epidemiol. 23 (4), 356–362. 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P, 2006. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 28 (5), 536–547. 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Duff K, Callister C, Dennett K, Tometich D, 2012. Practice effects: a unique cognitive variable. Clin. Neuropsychol. 26 (7), 1117–1127. 10.1080/13854046.2012.722685. [DOI] [PubMed] [Google Scholar]
- Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK, 2003. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup. Environ. Med. 60 (4), 279–286. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12660376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Ellison EA, Bonner MR, McGarricle BP, Crane AL, Fenske RA, et al. , 2011. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ. Health Perspect. 119, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Farahat FM, Galvin K, Fenske EK, Olson JR, 2012. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int. J. Occup. Environ. Health 18 (3), 198–209. 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ballesteros R, Zamarron MD, Tarraga L, 2005. Learning potential: a new method for assessing cognitive impairment. Int. Psychogeriatr. 17 (1), 119–128. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/15945596. [DOI] [PubMed] [Google Scholar]
- Fiedler N, Kipen H, McNeil K, Fenske R, 1997. Long-term use of organophosphates and neuropsychological performance. Am. J. Ind. Med. 5, 487–496. [DOI] [PubMed] [Google Scholar]
- Garabrant DH, Aylward LL, Berent S, Chen Q, Timchalk C, Burns CJ, et al. , 2009. Cholinesterase inhibition in chlorpyrifos workers: characterization of biomarkers of exposure and response in relation to urinary TCPy. J. Expo. Sci. Environ. Epidemiol. 19 (7), 634–642. 10.1038/jes.2008.51. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B, Lacasana M, Aguilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, Hernandez AF, 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett. 230 (2), 104–121. 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Guignet M, Lein PJ, 2019. Organophosphates In: In: Aschner M, Costa LG (Eds.), Advances in Neurotoxicology: Role of Inflammation in Environmental Neurotoxicity, vol. 3 Academic Press, Cambridge, MA, pp. 35–79. [Google Scholar]
- Hansen MRH, Jors E, Lander F, Condarco G, Debes F, Bustillos NT, Schlunssen V, 2017. Neurological deficits after long-term pyrethroid exposure. Environ. Health Insights 11, 1178630217700628. 10.1177/1178630217700628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB, 1965. The environment and disease: association or causation? Proc. R. Soc. Med. 58, 295–300. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/14283879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AA, Bodner TE, Rohlman DS, 2012. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup. Environ. Med. 69 (7), 457–464. 10.1136/oemed-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AA, Bonner MR, Hendy O, Abdel Rasoul G, Wang K, Olson JR, Rohlman DS, 2017a. Comparison of neurological health outcomes between two adolescent cohorts exposed to pesticides in Egypt. PLoS One 12 (2), e0172696. 10.1371/journal.pone.0172696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AA, Wang K, Olson JR, Bonner MR, Hendy O, Abdel Rasoul G, Rohlman DS, 2017b. The impact of repeated organophosphorus pesticide exposure on biomarkers and neurobehavioral outcomes among adolescent pesticide applicators. J. Toxicol. Environ. Health A 80 (10–12), 542–555. 10.1080/15287394.2017.1362612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W, 2008. Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int. J. Occup. Med. Environ. Health 21 (2), 121–132. 10.2478/v10001-008-0014-z. [DOI] [PubMed] [Google Scholar]
- Korsak RJ, Sato MM, 1977. Effects of chronic organophosphate pesticide exposure on the Central Nervous System. Clin. Toxicol. 11 (1), 83–95. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. , 2005. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ. Health Perspect. 113 (7), 894–899. 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal M, Yang D, Bruun D, Anger WK, Lein PJ, 2010. Subchronic chlorpyrifos exposure alters appetitive behavior and contextual fear conditioning in rats. Toxicologist 114 (1), 264. [Google Scholar]
- Lein PJ, Bonner MR, Farahat FM, Olson JR, Rohlman DS, Fenske RA, et al. , 2012. Experimental strategy for translational studies of organophosphorus pesticide neurotoxicity based on real-world occupational exposures to chlorpyrifos. NeuroToxicology 33 (4), 660–668. 10.1016/j.neuro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, 1995. Neuropsychological Assessment. Oxford University Press, New York. [Google Scholar]
- Lezak M, Howieson D, Loring D, 2004. Neuropsychological Assessment, 4th ed Oxford University Press, New York. [Google Scholar]
- Li AA, Lowe KA, McIntosh LJ, Mink PJ, 2012. Evaluation of epidemiology and animal data for risk assessment: chlorpyrifos developmental neurobehavioral outcomes. J. Toxicol. Environ. Health B Crit. Rev. 15 (2), 109–184. 10.1080/10937404.2012.645142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KC, Guo NW, Tsai PC, Yang CY, Guo YL, 2008. Neurocognitive changes among elderly exposed to PCBs/PCDFs in Taiwan. Environ. Health Perspect. 116 (2), 184–189. 10.1289/ehp.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero B, Ceballos PA, Munoz-Quezeda MT, Reynaldos C, Saracomo C, Baumert BO, 2019. Validity and reliability of an assessment tool for the screening of neurotoxic effects in agricultural workers in Chile. Biomed Res. Int. 2019, 1–11. 10.1155/2019/7901760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V, 2010. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol. Teratol. 32 (4), 452–459. 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Cedillo L, Keifer M, Palomo MR, 1992. Monitoring organophosphate insecticide-exposed workers for cholinesterase depression. New technology for office or field use. J. Occup. Med. 34 (1), 34–37 Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1552378. [PubMed] [Google Scholar]
- Meyer-Baron M, Knapp G, Schaper M, van Thriel C, 2015. Meta-analysis on occupational exposure to pesticides–neurobehavioral impact and dose-response relationships. Environ. Res. 136, 234–245. 10.1016/j.envres.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Munoz-Quezada MT, Lucero BA, Iglesias VP, Munoz MP, Cornejo CA, Achu E, et al. , 2016. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int. J. Occup. Environ. Health 22 (1), 68–79. 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton SX, Terry AV Jr., 2018. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 408, 101–112. 10.1016/j.tox.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Quandt SA, Summers P, Morgan TM, Chen H, Walker FO, et al. , 2015. Learning Ability as a Function of Practice: Does It Apply to Farmworkers? J. Occup. Environ. Med. 57 (6), 676–681. 10.1097/JOM.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R, 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 119 (8), 1196–1201. 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter ED, Chuwers P, Levy Y, Gordon M, Grauer F, Marzouk J, et al. , 1992. Health effects from exposure to organophosphate pesticides in workers and residents in Israel. Isr. J. Med. Sci. 28 (8–9), 584–598. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1330977. [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ, 2011. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology 32 (2), 268–276. 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, Bonner MR, Hendy O, Mara K, et al. , 2016. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex 74, 383–395. 10.1016/j.cortex.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan-Tapia L, Nieto-Escamez FA, del Aguila EM, Laynez F, Parron T, Sanchez-Santed F, 2006. Neuropsychological sequelae from acute poisoning and long-term exposure to carbamate and organophosphate pesticides. Neurotoxicol. Teratol. 28 (6), 694–703. 10.1016/j.ntt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K, 1991. Chronic central nervous system effects of acute organophosphate pesticide intoxication. The Pesticide Health Effects Study Group. Lancet 338 (8761), 223–227. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O, 2013. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and metaanalytic review. Crit. Rev. Toxicol. 43 (1), 21–44. 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L, 2006. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ. Health Perspect. 114, 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G, 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. [Google Scholar]
- Singleton ST, Lein PJ, Farahat FM, Farahat T, Bonner MR, Knaak JB, Olson JR, 2014. Characterization of alpha-cypermethrin exposure in Egyptian agricultural workers. Int. J. Hyg. Environ. Health 217 (4–5), 538–545. 10.1016/j.ijheh.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton ST, Lein PJ, Dadson OA, McGarrigle BP, Farahat FM, Farahat T, et al. , 2015. Longitudinal assessment of occupational exposures to the organophosphorous insecticides chlorpyrifos and profenofos in Egyptian cotton field workers. Int. J. Hyg. Environ. Health 218 (2), 203–211. 10.1016/j.ijheh.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczak EE, Stanczak EM, Awadalla AW, 2001. Development and initial validation of an Arabic version of the expanded Trail making Test: implications for cross-cultural assessment. Arch. Clin. Neuropsychol. 16, 141–149. [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T, 2008. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 116 (10), 1416–1422. 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr., 2012. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol. Ther. 134 (3), 355–365. 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, 2004. Trail making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. [DOI] [PubMed] [Google Scholar]
- van Gemert M, Dourson M, Moretto A, Watson M, 2001. Use of human data for the derivation of a reference dose for chlorpyrifos. Regul. Toxicol. Pharmacol. 33, 110–116. [DOI] [PubMed] [Google Scholar]
- Voorhees JR, Rohlman DS, Lein PJ, Pieper AA, 2016. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front. Neurosci. 10, 590 10.3389/fnins.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Z, Zhang J, Wu Y, Sun H, 2016. Chlorpyrifos exposure in farmers and urban adults: metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ. Res. 149, 164–170. 10.1016/j.envres.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, et al. , 2004. Water arsenic exposure and children’s intellectual function in Araihazar. Bangladesh. Environ Health Perspect 112 (13), 1329–1333. 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling C, Keifer M, Ahlbom A, McConnell, Moon JD, Rosenstock K, Hogstedt C, 2002. Long-term neurobehavioral effects of mild poisoning with organophosphate and n-Methyl carbamate pesticides among banana workers. Int. J. Occup. Environ. Health 8, 27–34. [DOI] [PubMed] [Google Scholar]
- Zurlinden TJ, Reisfeld B, 2018. A novel method for the development of environmental public health indicators and benchmark dose estimation using a health-based end point for chlorpyrifos. Environ. Health Perspect. 126 (4), 047009. 10.1289/EHP1743. [DOI] [PMC free article] [PubMed] [Google Scholar]