Abstract
In recent years, the exploration for potential inhibitors of HIV has been increased due to the development of drug resistant HIV strains in infected people undergoing antiretroviral agents treatment. In this report, we studied the anti-HIV properties of ethyl gallate (EG) against a panel of HIV-1 strains in in vitro conditions. Different clinical isolates and laboratory strains of HIV-1 were tested for their sensitivity with EG. We found that EG exhibited non-toxic nature over a wide range of cell lines from different tissue origin. Serum proteins have little to no impact on the antiviral activity of EG. In the present study, EG was found to be a promising anti-HIV agent.
Keywords: Anti-HIV, Cytotoxicity, Ethyl gallate, HIV-1 clinical isolates, Serum shift
Introduction
AIDS (Acquired immunodeficiency syndrome) is the consequence of human immunodeficiency virus (HIV) infection with reflective immunosuppression [3]. The usual treatment contains a combination of no less than 2 or 3 drugs (normally termed “highly active antiretroviral therapy” or HAART) which chase to conquer the HIV cycle, and decrease the possibility of drug resistance. The latency of HIV to develop resistant to ARV drug is an alarming issue since it was first described [4]. Regardless of the various treatment regimes, this infection is unable to treat permanently and the epidemic remains. Antiretrovirals every so often cause mild to severe cytoxicity [17]. Drug combinations that target diverse viral strains decrease the risk of emerging, or delay the progress of drug resistance [12].
The progress of HIV drug resistant strains in patients under the management of ARV’s prompted the exploration for potential HIV inhibitors. Plants are the rich source for many diverse molecules. A diversity of bioactive molecules from plants has exposed sensible to better anti-HIV property. Phenolic compounds are known antivirals in many viral systems, as mode of action fits with the many scaffolds of viral/host proteins that subsequently hinder the one or more steps in viral cycle that leads to antivirals [26]. Repandusinic acid from the plant Phyllanthus niruri (Family Euphorbiaceae) reported to be an inhibitor of HIV-1 RT (reverse transcriptase), and catechin isolated from Detarium microcarpum (Family: Caesalpiniaceae) demonstrated as an anti-HIV molecules by abrogating the gp-120 binding to CD 4 cells and decreases RT activity [29].
In recent times, much development was seen in the finding of effective anti-HIV agents from plants. Plants are known source for the finding of novel compounds, due to scaffold selectivity and minimal toxicity to the cells [2]. Although plant derived drugs are not in the management of AIDS, there is much need to explore this area. Calanolide A from Calophyllum spp. is in clinical phase II trials for valuation of its durable anti-HIV activity [20]. It is also under trail to establish Calanolide A in combinatorial approach to govern its anti-HIV potential. Similarly the phenolic groups of gallic acid (GA) is also under experimentation/study for its free radical scavenging activity [16]. The consideration in this class of molecules is owing to its biological action as antioxidants. GA’s are known to have possible defensive and beneficial effects in the treatment of many diseases i.e., cancer, cardiovascular diseases, neurodegenerative disorders and age-related disorders [10]. As a result of these events, GA and its derivatives could be used as a hopeful main molecule for drug discovery. Many studies reported that prodelphinidin B-2 3-O-gallate and epigallocatechin-3-gallate have anti-viral potency counter to a variety of viruses [14, 15, 25, 28]. But then again there are limited reports about the anti-viral activity of n-alkyl esters of gallic acid. Previous reports on the potency of methyl GA specified that it prevents plaque development of herpes simplex virus type 1 or 2, but not effective on influenza virus [9]. Existing HAART is based on combination treatments with substances from diverse classes to fight the drug resistance of HIV viruses. Inappropriately, HAART-associated toxicity was main reason to halt or modify antiretroviral therapy. In this scenario, antioxidant agent with antiviral activity might be a hope for cytoprotection during multi-drug combinational ARV therapy. So, we attempted a study on the anti-HIV properties of ethyl gallate (EG) against a panel of HIV-1 strains in in vitro conditions.
Materials and methods
Viral propagation
MT-2 cells and MT-4 cell lines procured from the NIH ARP (NIH AIDS Reagent Program). The cells such as HepG2, Colo-205, HEK-293, HT29, A549 and U87 MG utilized in cytotoxicity are procured from ATCC. Proviral HIV-1 NL4-3 clone and its bevirimat resistant mutant (V370A variant) are kind gift of Prof. Feng Li, South Dakota State University. Laboratory-adapted HIV-1 strain IIIB and B—sub type strains 92HT599 and 92US657 were obtained from NIH ARP. Indian HIV-1 sub type C clinical isolates NARI-VB 28, NARI-VB 30 and NARI-VB 40 were obtained from National AIDS Research Institute (Pune, India).
Cell culture
MT-2 and MT-4 cell lines were cultured two times per week in RPMI 1640 (Sigma-Aldrich) and HepG2, Colo-205, HEK-293, HT-29, A-549 and U-87MG in DMEM along with 1X antibacterial and antimycotic solution (Thermo Fisher Scientific) and 10% fetal bovine serum (FBS), PBMC (Peripheral blood mononuclear cells) were isolated from healthy volunteer by Ficoll-Hypaque reagent (Sigma-Aldrich). Centrifugation was carried out by density gradient method using heparinized blood. PBMC’s were maintained in RPMI 1640 comprising 20% FBS, 1X antibacterial and antimycotic solution and 1 ng/ml of recombinant human IL-2 (Sigma-Aldrich) and treated with phyto hemagglutinin (Sigma-Aldrich) at 2–4 μg/ml for 3 days prior to use. MT-2 cells cultivated in RPMI 1640 medium comprising above mentioned concentrations of antibiotics, 10% FBS and 2 mM l-glutamine.
Titers of viral stocks
Viral stocks were estimated in specific cells (PBMC for CCR5 virus and MT-2 for CXCR4 virus), using p24 as antigen and ELISA (ABL Inc., USA) as an end point assay. The infective dose (TCID50) of virus/ml was determined through Spearman–Karber method [7].
Antiviral assay
MT-2 cells were infected with IIIB or 92HT 599. PBMC cells were infected with either 92US657 or C-sub type clinical isolates. Cells and viral titer were mixed in specified medium, kept for 1 h at 5% CO2 and 37 °C, and mixed to test compounds in 96-well microplates at an ultimate concentration of 1 × 103 cells/well. EG and control compounds were consecutively diluted threefolds in DMSO and maintained in 1% DMSO throughout the assay. All the experiments comprised 1% DMSO as a negative control and employed in data analysis. After 96 h of incubation, viral yields were determined by p24 yield using p24 ELISA kit.
Cytotoxicity of EG
Different concentrations of EG were used to determine the cell toxicity (CC50s) in various cell lines by means of MTS reduction method (MTS; Sigma) as per the manufacturer’s protocol [23] as follows. Human cells from different tissue origins (HepG2—Liver, Colo-205—Colon, HEK-293—Kidney, HT29—Colorectal, A549—Lung and U87 MG—Brain) were plated at 3 × 103 cells per well in 96 well plate. Ethyl Gallate (0–200 µg) was added to the micro plate in defined format with the final concentration of DMSO (vehicle) is not more than 1%. Incubation was carried out in CO2 incubator for 96 h. By the end of incubation, 100 μl of supernatant was discarded from each well and added 10 µl of MTT (5 mg/ml) and kept for 4 h incubation in CO2 (5%) incubator. After 4 h of incubation, 200 µl of stop solution (1 N acidic isopropanol) was mixed to terminate the assay and the absorbance was read at 590 nm in multimode microplate instrument (Tecan Infinite® M1000, Switzerland). CC50 values were calculated using graph pad prism software (GraphPad v 5.1).
Serum effects on antiviral effect
Serum effect on antiviral activity of EG were estimated by supplementing the DMEM (10% FBS) medium with different concentrations of serum components as variable experimental parameters such as 40% HS (human serum) and 27 mg/ml of extra HSA (human serum albumin), 40% HS plus 1 mg/ml of AGP (α-acid glycoprotein), 45 mg/ml of HSA, or 45 mg/ml of HSA plus 1 mg/ml of extra AGP. Impact of serum and its components was estimated as the fold change in EC50 against EC50 without extra serum.
Antiviral effect on drug resistant provirus
According to Petropoulos et al. [18], briefly, 1.5 µg of pNL 4-3 DNA (an infectious HIV-1 proviral clone) or its bevirimat (1st generation maturation inhibitor) resistant variant 1.5 µg of V370A were transfected (DAE-Dextran method) into MT4 cells (60–70% confluence; 100 mm culture dish). Transfected cells were dispensed in microplates at the number of 20,000 cells/well. EG and other test compounds were logarithmically diluted (threefold) and established 1% final DMSO concentration. DMSO (1%) as a negative control and data analyzed. After 96 h days of incubation at 5% CO2 and 37° C, viral yields were estimated by p24 yield using p24 ELISA kit.
Results and discussion
Antiviral activity of EG against different HIV isolates
We examined the efficiency of EG on the inhibition of HIV1 infectivity through a range of HIV subtypes and clinical strains (Fig. 1). As per dose, EG inhibited HIV-1 p24 antigen (mature HIV-1 virus) levels in laboratory-adapted strain, IIIB (IC50 of 22 µM), laboratory-adapted subtype B strains, 92HT 599 and 92US657 (IC50 range 12–20 µM), clinical isolate subtype C, NARI-VB 28, NARI-VB 30 and NARI-VB 40 (IC50 range 13–42 µM), and the reference compound (AZT) considerably inhibited the total panel of isolates. These isolates displayed inhibition of HIV1 p24 antigen due to EG from 12 to 42 µM and also likely susceptible to AZT under these experimental conditions. EG was found to be strongly abrogated both tested B and C clade strains of HIV-1. EG was established to be more potent for the strain 92US657, compared to clinical isolates NARI-VB 40.
Fig. 1.
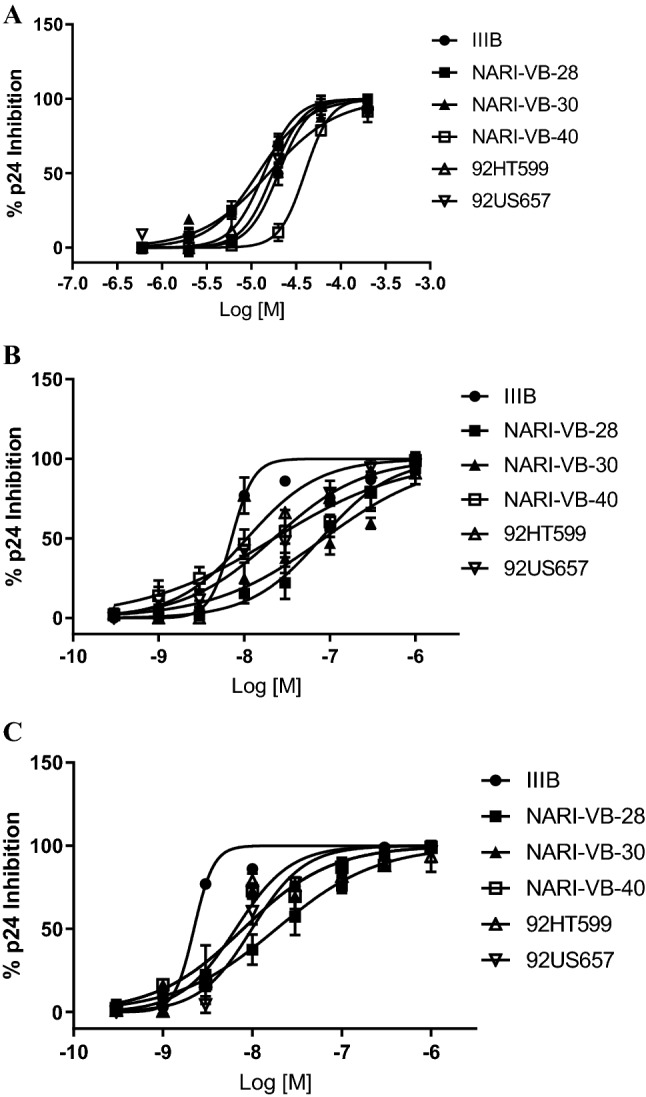
Effect of a ethyl gallate b bevirimat c AZT on different HIV-1 isolates. The host cells for HIV-1 strains with CXCR4 and HIV-1 strains with CCR5 were MT2 and PBMC respectively. The mean values ± standard deviations are representative of three independent experiments
Cytotoxic profile of EG
EG does not have any impact on the viability of human lymphocytes and doesn’t showed cytotoxicity, determined by the MTS assay, and minimal cytotoxicity was observed by EG up to 200 µM. At this concentration, viability of cells was not affected up to 72 h of incubation with EG (Table 1). Synthetic antiviral drugs like AZT exhibits toxicity to bone marrow, low therapeutic index as a result of cellular polymerases inhibition, less half-life in blood which needs frequent AZT management to continue a therapeutic drug dose. Even though AZT efficiently inhibits the HIV-1 replication, but it’s less effective on HIV latency [24]. Furthermore, patients lower than continuing therapy fail to regulate the infection because of the development of resistant variants of the virus. Gallic acid derivatives like EG are well known as natural antioxidant, moreover we confirmed its non-toxic nature over a wide range of cell lines from different tissue origin.
Table 1.
In vitro cytotoxicity of EG against cell lines of different tissue origin. Values were expressed in cytotoxicity concentration 50%—CC50 (µM)
| Tissue | Cell line | EG (µM) | Doxorubicin (µM) |
|---|---|---|---|
| Liver | HepG2 | > 200 | 5.10 |
| Colon | Colo-205 | > 200 | 3.00 |
| Kidney | HEK-293 | > 200 | 4.10 |
| Colon | HT29 | > 200 | 4.00 |
| Lung | A549 | > 200 | 5.00 |
| Brain | U87 MG | > 200 | 2.20 |
| Blood | MT4 | > 200 | 1.20 |
| Blood | MT2 | > 200 | 1.57 |
| Blood | PBMC | > 200 | 1.50 |
Human serum effect on antiviral activity of EG
The impact of serum components on antiviral effectiveness of EG was evaluated in MT-2 cells infected with HT599T by means of p24 antigen as the endpoint assay (Fig. 2). To find the impact of serum devoid of affecting cell viability, complete medium was supplemented with additional serum (40% HS and 27 mg/ml HSA). Under these settings (40% HS plus 27 mg/ml of HSA), EG exhibited 1.9-fold decrease of antiviral potency compared to that with 10% FBS. HSA appeared to be moderately attribute to the serum impact, since it made a 1.9 fold decrease in activity when it’s levels are 45 mg/ml. A comparable 1.2-fold shift was detected with 40% HS alone. On the other hand, the influence of AGP is minimum (EC50 16 µM versus 17 µM for 10% FBS). These results propose that serum components have little to no impact on the antiviral activity of EG. First generation maturation inhibitor BVM exhibited high human serum binding, which was main cause for the failure of BVM in clinical studies [22].
Fig. 2.
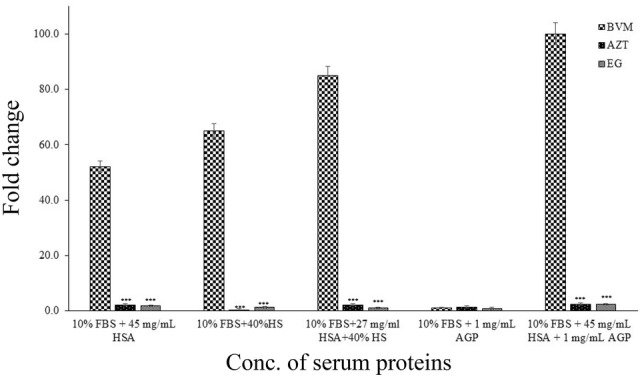
Influence of serum and its components on antiviral activity of EG (± SD, n = 3. ***P < 0.0001). Fold change values were calculated as the EC50 change with human serum components versus without human serum components. HS human serum, HSA human serum albumin, AGP α-acid glycoprotein
Anti-HIV activity against a drug-resistant HIV provirus
EG showed inhibitory activity against WT NL4-3 and the SP1-V370A (V7A). The IC50’s of EG against WT NL4-3 were primarily in the range of 6.5 µM. Against the V7A variant, EG have IC50 of 1.8 µM. The antiviral activity of EG against the V7A variant was more potent when compare to wild type NL4-3 (Fig. 3). The importance of this enrichment in effectiveness is emphasized by prominent polymorphisms which are relatively associated more with HIV-1 subtype B. The frequencies of drug resistant mutants are V370A (12.4%), V362I (9.8%), and V370M (4.4%), as per the database of LANL 2018 (Los Alamos National Laboratory). EG displayed potential activity against the variant V7A in the tested panel.
Fig. 3.
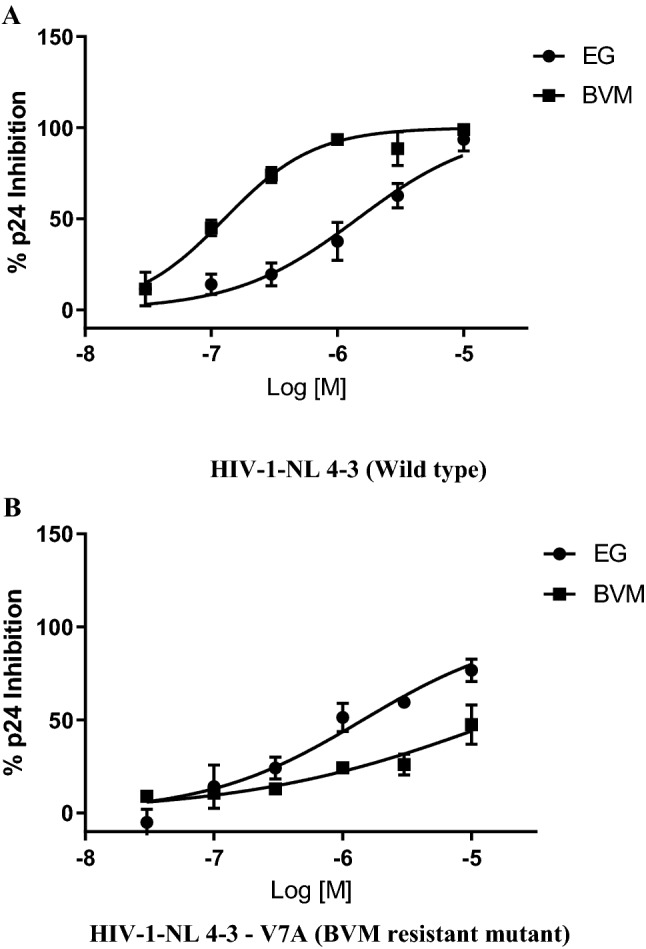
Antiviral activities of EG against the a HIV-1-NL 4-3 (Wild type) virus and the b HIV-1-NL 4-3—V7A (BVM resistant mutant) variant in MT-4 cells
Many natural products from plants are established as antiviral molecules including anti-HIV. Gallate analogues are well known for antiviral properties [6, 11, 30]. In the current study, we have established the EG-influenced control of HIV-1 infectivity. EG exerted a dose-related inhibition of mature virus release over a broad spectrum of HIV-1 subtypes. HIV-1 p24 levels were considerably controlled by EG in different HIV-1 strains. This comprehensive effect of EG for HIV-1 subtype put forwarded that EG may be an effective alternative therapeutic agent against infection of HIV-1 in combinatorial therapy. Both the normal resistance of the diverse viruses in drug-naive persons and the tendency of the virus for emerging drug resistance after treatment are key concern. The cell viability of human lymphocytes and other cell lines of diverse tissue origin was not inhibited by EG over a range of cell lines. Gallic acid and its methyl ester are known to have anti-inflammatory [8], antioxidant [27], and anti-cancer properties [5]. Additionally, Ahn et al. [1] proposed that the galloyl function group plays a main role in inhibiting 3’-processing of HIV-1 integrase of these compounds. Rivero-Buceta et al. [19] reported that gallic acid analogues from green tea has enhanced antiviral effect against HIV-1. Furthermore, methyl gallate inhibited HIV-1 integrase, reverse transcriptase and viral replication activities [21]. Mishra et al. [13] described that tea polyphenols comprising gallic acid could inhibit the pass of HIV-1 into target cells by hindering envelope-mediated membrane fusion. In the current study, we explored the anti-HIV effect of ethyl gallate against a wide range of HIV-1 clinical as well as laboratory strains. In addition to antioxidant properties, EG exhibited antiviral activity against different HIV-1 isolates. Attempts are in progress to evaluate the EG in combinational approach with known ARVs for better anti-HIV activities and also to hinder the development of resistance. EG could be a promising anti-HIV supplement for future development without any cytotoxicity.
In addition to antioxidant property, EG possessed anti-HIV activity against different laboratory strains as well as clinical isolates. Unlike other ARV drugs, EG found to be non-toxic to the cells of different tissue origin. These results suggest that EG could be a promising therapeutic agent for the next generation cytoprotective anti-HIV molecules in combinational therapy, and further studies concerning the cytotprotection are in progress.
Acknowledgements
Authors acknowledge the generous support received from Dr. K. Ratnakar Reddy, Director Hetero Research Foundation, Hyderabad 500018, India for this research work. We also thank management, VFSTR (Deemed to be University) for the support and encouragement.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahn MJ, Kim CY, Lee JS, Kim TG, Kim SH, Lee CK, et al. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002;68:457–459. doi: 10.1055/s-2002-32070. [DOI] [PubMed] [Google Scholar]
- 2.Akram M, Tahir IM, Shah SMA, Mahmood Z, Altaf A, Ahmad K, Munir N, Daniyal M, Nasir S, Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res. 2018;32:811–822. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- 3.Cos P, Hermans N, De BT, Apers S, Sindambiwe JB, Witvrouw M, De CE, Vanden BD, Pieters L, Vlietinck AJ. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1) Phytomedicine. 2002;9(1):62–68. doi: 10.1078/0944-7113-00083. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. Antiviral therapy for human immunodeficiency virus infections. Clin Microbiol Rev. 1995;8:200–239. doi: 10.1128/CMR.8.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–613. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 6.Govea Salas M, Rivas Estilla A, Morlett Chavez J, Lozano Sepulveda S, Rodriguez Herrera R, Aguilar GC. P420 gallic acid has antiviral effect against hepatitis C virus (HCV), which is mediated by its antioxidant activity. J Hepatol. 2014;60:208. doi: 10.1016/S0168-8278(14)60582-1. [DOI] [Google Scholar]
- 7.Hubert J. Spearman–Karber method. Bioassay. 2. Dubuque: Hunt Publishing; 1984. pp. 65–66. [Google Scholar]
- 8.Jeong JJ, Lee YS, Kim DH. Cytotoxic effects of gallic acid and its derivatives against HIV-I-infected microglia. J Bacteriol Virol. 2016;46(4):239–247. doi: 10.4167/jbv.2016.46.4.239. [DOI] [Google Scholar]
- 9.Kane CJ, Menna JH, Sung CC, Yeh YC. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci Rep. 1998;8:95–102. doi: 10.1007/BF01128976. [DOI] [PubMed] [Google Scholar]
- 10.Karamaæ MA, Kosinska PR. Comparison of radical-scavenging activities of selected phenolic acids. Pol J Food Nutr Sci. 2005;14:165–170. [Google Scholar]
- 11.Lee J, Oh M, Seok J, Kim S, Lee D, Bae G, et al. Antiviral effects of black raspberry (Rubus coreanus) seed and its gallic acid against influenza virus infection. Viruses. 2016;6:E157. doi: 10.3390/v8060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meintjes G, Maartens G, Boulle A, Conradie F, Goemaere E, Hefer E, Johnson D, Mathe M, Moosa Y, Osih R, Rossouw T, Van Cutsem G, Variava E, Venter F, Spencer D. Guidelines for antiretroviral therapy in adults. S Afr J HIV Med. 2012;13:114–130. doi: 10.4102/sajhivmed.v13i3.125. [DOI] [Google Scholar]
- 13.Mishra NN, Kesharwani A, Agarwal A, Polachira SK, Nair R, Gupta SK. Herbal gel formulation developed for anti-human immunodeficiency virus (HIV)-1 activity also inhibits in vitro HSV-2 infection. Viruses. 2018;10(11):580. doi: 10.3390/v10110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Yand Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991;44:181–186. doi: 10.7883/yoken1952.44.181. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M, Nagata K, Kato A, Ishihama A. Interferon-inducible mouse Mx1 protein that confers resistance to influenza virus is GTPase. J Biol Chem. 1991;266:21404–21408. doi: 10.1128/JVI.01682-12. [DOI] [PubMed] [Google Scholar]
- 16.Nikolic KM. Theoretical study of phenolic antioxidants properties in reaction with oxygen-centered radicals. J Mol Struc THEOCHEM. 2006;774:95–105. doi: 10.9734/IRJPAC/2016/27385. [DOI] [Google Scholar]
- 17.Pereira D, Antoni M, Danielson A, Simon T, Efantis-Potter J, Carver CS, O’Sullivan M. Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med. 2003;65:427–434. doi: 10.1037/a0028160. [DOI] [PubMed] [Google Scholar]
- 18.Petropoulos CJ, Parkin NT, Limoli KL, Lie YS, Wrin T, Huang W, Tian H, Smith D, Winslow GA, Capon DJ, Whitcomb JM. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44:920–928. doi: 10.1128/AAC.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivero-Buceta E, Carrero P, Doyaguez EG, Madrona A, Quesada E, Camarasa MJ, et al. Linear and branched alkyl-esters and amides of gallic acid and other (mono-, di- and tri-) hydroxy benzoyl derivatives as promising anti-HCV inhibitors. Eur J Med Chem. 2015;92:656–671. doi: 10.1016/j.ejmech.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Singh IP, Bharate SB, Bhutani K. Anti-HIV natural products. Curr Sci. 2005;89:269–290. [Google Scholar]
- 21.Siwe-Noundou X, Thommas M, Musyoka VM, Derek TN, Dumisani M, Heinrich H, Özlem B, Krause R. Anti-HIV-1 integrase potency of methylgallate from Alchornea cordifolia using in vitro and in silico approaches. Sci Rep. 2019;9:4718. doi: 10.1038/s41598-019-41403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PF, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, Allaway GP, Martin DE. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother. 2007;51:3574–3581. doi: 10.1128/aac.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;36:47–50. doi: 10.1039/a809656b. [DOI] [Google Scholar]
- 24.Turk G, Moroni G, Pampuro S, Brion MC, Salomon H. Antiretroviral activity and cytotoxicity of novel zidovudine (AZT) derivatives and the relation to their chemical structure. Int J Antimicrob Agents. 2002;20(4):282–288. doi: 10.1016/S0924-8579(02)00191-7. [DOI] [PubMed] [Google Scholar]
- 25.Uozaki M, Yamasaki H, Katsuyama Y, Higuchi M, Higuti T, Koyama AH. Antiviral effect of octyl gallate against DNA and RNA viruses. Antivir Res. 2006;73:85–91. doi: 10.1016/j.antiviral.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Calvo A, de Oya NJ, Martin-Acebes MA, Garcia-Moruno E, Saiz JC. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika virus, and dengue virus. Front Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CR, Zhou R, Ng TB, Wong JH, Qiao WT, Liu F. First report on isolation of methyl gallate with antioxidant, anti-HIV-1 and HIV-1 enzyme inhibitory activities from a mushroom (Pholiota adiposa) Environ Toxicol Pharmacol. 2014;37:626–637. doi: 10.1016/j.etap.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir Res. 2003;58:167–173. doi: 10.1016/S0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 29.Yadav P, Fitzgerald MM, Sajadi B, Gilliam MK, Lafferty R, Redfield W. Increased expression of suppressor of cytokine signaling-1 (SOCS-1): a mechanism for dysregulated T helper-1 responses in HIV-1 disease. Virology. 2009;385(1):126–133. doi: 10.1016/j.virol.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Xia Q, Yang G, Zhu D, Shao Y, Zhang J, Cui Y, Wang R, Zhang L. The anti-HIV-1 activity of polyphenols from Phyllanthus urinaria and the pharmacokinetics and tissue distribution of its marker compound, gallic acid. J Tradit Chin Med Sci. 2017;4(2):158–166. doi: 10.1016/j.jtcms. [DOI] [Google Scholar]


