Abstract
OBJECTIVE
Glucosamine is a widely used supplement typically taken for osteoarthritis and joint pain. Emerging evidence suggests potential links of glucosamine with glucose metabolism, inflammation, and cardiometabolic risk. We prospectively analyzed the association of habitual glucosamine use with risk of type 2 diabetes (T2D) and assessed whether genetic susceptibility and inflammation status might modify the association.
RESEARCH DESIGN AND METHODS
This study analyzed 404,508 participants from the UK Biobank who were free of diabetes, cancer, or cardiovascular disease at baseline and completed the questionnaire on supplement use. Cox proportional hazards models were used to evaluate the association between habitual use of glucosamine and risk of incident T2D.
RESULTS
During a median of 8.1 years of follow-up, 7,228 incident cases of T2D were documented. Glucosamine use was associated with a significantly lower risk of T2D (hazard ratio 0.83, 95% CI 0.78–0.89) after adjustment for age, sex, BMI, race, center, Townsend deprivation index, lifestyle factors, history of disease, and other supplement use. This inverse association was more pronounced in participants with a higher blood level of baseline C-reactive protein than in those with a lower level of this inflammation marker (P-interaction = 0.02). A genetic risk score for T2D did not modify this association (P-interaction = 0.99).
CONCLUSIONS
Our findings indicate that glucosamine use is associated with a lower risk of incident T2D.
Introduction
Glucosamine is a widely used supplement typically taken for osteoarthritis and joint pain (1). It has been estimated that ∼20% of middle-aged adults use this supplement in the U.S., U.K., and Australia (2–4), although studies on the effectiveness of glucosamine on osteoarthritis have reported conflicting results (5).
In a recent study, we found that habitual glucosamine use was significantly associated with lower risks of cardiovascular diseases (4). Notably, previous studies have also linked glucosamine use with glucose metabolism, although the evidence is not consistent. Several in vitro studies reported that glucosamine altered glucose metabolism and induced insulin resistance (6–8); however, these findings have not been confirmed in human studies (9–12). In contrast, a long-term clinical trial (3 years) of glucosamine intervention in 212 participants showed that a slightly glucose-lowering effect was observed in glucosamine users (13). In addition, previous studies suggest that glucosamine may affect inflammation status (14–16), which also has been related to glucose metabolism, insulin resistance, and risk of type 2 diabetes (T2D) (17,18). We therefore hypothesized that habitual glucosamine use may be related to T2D risk. No study has analyzed the association between habitual glucosamine use and incident T2D in prospective cohorts.
In this study, we prospectively investigated the association of habitual glucosamine use with risk of T2D in 404,508 adults from the UK Biobank study. We particularly examined the potential effect modification of inflammation on the associations. In addition, because previous studies, including ours (19,20), suggest that the genetic variations may modify the associations between dietary/lifestyle factors and disease risk, we also assessed the interaction between glucosamine use and genetic susceptibility on the associations.
Research Design and Methods
Study Population
The UK Biobank is a large population-based cohort study comprising >0.5 million participants in the UK. The details of the study design have been described in previous studies (21). All participants provided written informed consent, and the study was approved by the North West Multi-centre Research Ethics Committee and the Tulane University (New Orleans, LA) Biomedical Committee Institutional Review Board. In the main analysis of this study, we included 404,508 participants who had complete data on the use of glucosamine and were free of diabetes, cardiovascular disease, or cancer at baseline.
Genetic data were available for 360,550 white participants in this study after excluding participants with sex discordance or high missingness/heterozygosity and those who had a genetic relatedness with others. C-reactive protein (CRP) data were available for 381,165 participants in this study after further excluding participants with missing data.
Exposure Assessment
Habitual glucosamine information was collected through a baseline touch-screen questionnaire. Participants were asked, “Do you regularly take any of the following?” Participants selected more than one answer from a list of supplements through the touch-screen questionnaire. We defined glucosamine users as follows: 0, no; 1, yes. Two repeated surveys (the first visit was completed from 2012 to 2013; the second visit was conducted in 2014+) conducted in 16,687 and 30,972 participants, respectively, were used to assess the reproducibility and validity of glucosamine use with the same touch-screen questionnaire. In the first repeated survey, 56.4% (2,183 of 3,872) of baseline glucosamine users reported that they continued to take glucosamine (Spearman r = 0.55), and 42.0% (2,711 of 6,455) of baseline glucosamine users reported that they continued to take glucosamine (Spearman r = 0.43) in the second repeated survey (2014+).
Blood samples were collected at baseline (2006–2010). CRP (mg/L) was measured by immunoturbidimetric assay on a Beckman Coulter AU5800, glucose (mmol/L) was measured by hexokinase analysis on a Beckman Coulter AU5800, and HbA1c (mmol/mol) was measured by high-performance liquid chromatography analysis on a Bio-Rad VARIANT II Turbo. Calibration and quality control were conducted according to the manufacturer’s recommendations. In addition, nearly half of the CRP values fell between 0 and 1, and the distribution was right-skewed; thus, log (units + 1) of CRP values was used in all analyses (22). Further details of these measurements can be found at the UK Biobank website (https://biobank.ctsu.ox.ac.uk/showcase).
We created a genetic risk score (GRS) for T2D using 102 single nucleotide polymorphisms (SNPs),which passed quality control, based on the previous study (23) (Supplementary Table 1). A weighted method was used to calculate the T2D-GRS. Each SNP was recoded as 0, 1, or 2 according to the number of risk alleles, and each SNP was multiplied by a weighted risk estimate (β-coefficient) on T2D obtained from the previous genome-wide association study. The genetic risk score was calculated by using the equation: GRS = (β1 × SNP1 + β2 × SNP2 +…+ β102 × SNP102) × (102/sum of the β-coefficients), where SNPn is the risk allele number of each SNP (24). The T2D-GRS ranged from 73.8 to 133.0, with higher scores indicating a higher genetic predisposition to T2D. Detailed information about genotyping, imputation, and quality control in the UK Biobank study has been described previously (25).
Ascertainment of Outcomes
The prevalent diabetes was assessed based on the UK Biobank algorithms for the diagnosis of diabetes (26) (Supplementary Table 2). Incident T2D was ascertained using hospital inpatient records containing data on admissions and diagnoses obtained from the Hospital Episode Statistics for England, Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales. T2D was defined by the ICD-10 code E11. Information on timing of incident T2D was collected through cumulative medical records of hospital diagnoses (until 31 March 2017). Detailed information on the ascertainment of T2D is available online at https://biobank.ctsu.ox.ac.uk/showcase/label.cgi?id=2000.
Statistical Analysis
Cox proportional hazards models were used to calculate the hazard ratios (HRs) comparing incident T2D rates in participants who did and did not use glucosamine. The proportional hazards assumption was tested using Schoenfeld residuals. A general linear model was used to evaluate the association of glucosamine use with glucose levels, CRP levels, and HbA1c levels. Several potential confounders were adjusted in these models, including age (years), sex, race (white, mixed, Asian, black, others), assessment centers, BMI (<18.5, 18.5–24.9 or 25–29.9, ≥30 kg/m2), Townsend deprivation index (quintiles), smoking status (current, past, never), physical activity (≥600 MET min/week or <600 MET min/week, which was calculated according to the International Physical Activity Questionnaire short form) (27), moderate drinking (women: >0 and ≤14 g/day, men: >0 and ≤28 g/day, https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/appendix-9/), healthy diet, family history of diabetes, hypertension, high cholesterol, osteoarthritis or joint pain, aspirin use, nonaspirin nonsteroidal anti-inflammatory drug (NSAID) use, vitamin supplement use, and nonvitamin supplement use. Details of the assessment of covariates are described in the Supplementary Materials. For analyses of genetic data, we also adjusted for the first 10 genetic principal components and genotyping array (106 batches); for analyses that included glucose levels, we also adjusted for fasting time. In addition, we created an overall healthy lifestyle score based on BMI (<30 kg/m2), physical activity (≥600 MET min/week), smoking (never), and healthy diet (yes). Missing data were coded as a missing indicator category for categorical variables and with mean values for continuous variables.
We performed stratified analysis to evaluate potential modification effects by the following factors: sex (women or men), age (<55 or ≥55 years), BMI (<30 or ≥30 kg/m2), physical activity (<600 MET min/week or ≥600 MET min/week), smoking (never, past, current), healthy diet (no or yes), overall healthy lifestyle score (0–1, 2–3, and 4), Townsend deprivation index (<median or ≥median), hypertension (no or yes), high cholesterol (no or yes), osteoarthritis or joint pain (no or yes), vitamin supplement use (no or yes), nonvitamin supplement use (no or yes), aspirin use (no or yes), and nonaspirin NSAID use (no or yes). To evaluate interactions between glucosamine use and potential T2D risk factors, CRP levels and T2D-GRS, multiplicative interaction was assessed by adding interaction terms to the Cox models.
In sensitivity analyses, we first addressed the issues of potential undiagnosed diabetes at baseline by removing participants with HbA1c levels ≥48 mmol/mol (6.5%) or glucose levels ≥7.0 mmol/L and repeated the main analyses. Second, to minimize the influence of reverse causation, we also did a sensitivity analysis by excluding participants with limited follow-up years (≤2 years). Third, to minimize the influence of other regular inflammatory drug use, we also did a sensitivity analysis by excluding participants who regularly took an anti-inflammatory drug. Fourth, we also further adjusted for glucose levels, HbA1c levels, or CRP levels in the multivariable model. Finally, because the status of glucosamine might have changed in the follow-up period, we further evaluated the association of habitual glucosamine use with risk of T2D.
All statistical analyses were conducted using SAS 9.4 software (SAS Institute) and SPSS 22.0 software. All statistical tests were two sided, and we considered P < 0.05 to be statistically significant.
Data and Resource Availability
The genetic and phenotypic UK Biobank data are available on application to the UK Biobank (www.ukbiobank.ac.uk/).
Results
Baseline Characteristics of Participants According to Glucosamine Use
Table 1 reports baseline characteristics of study participants according to the use of glucosamine. At baseline, 19.4% of the study population reported glucosamine use. Glucosamine users were older, more likely to be women, and less likely to be current smokers. They tended to have a healthy diet, were more likely to have higher physical activity level, a lower Townsend deprive index, higher prevalence of hypertension, and high cholesterol compared with nonusers. Glucosamine users also tended to have a history of osteoarthritis or joint pain and took other supplements and anti-inflammatory drugs.
Table 1.
Basic characteristics of participants by use of glucosamine in the UK Biobank cohort
| Glucosamine nonuser | Glucosamine user | |
|---|---|---|
| Participants | 325,920 (80.6) | 78,588 (19.4) |
| Age, years, mean (SD) | 55.1 (8.2) | 58.6 (7.2) |
| Women | 174,537 (53.6) | 49,587 (63.1) |
| White ethnicity | 306,882 (94.2) | 75,446 (96.0) |
| BMI, kg/m2 | ||
| <18.5 | 1,856 (0.6) | 301 (0.4) |
| 18.5–24.9 | 111,539 (34.2) | 26,756 (34.0) |
| 25–29.9 | 140,213 (43.0) | 34,550 (44.0) |
| ≥30 | 72,312 (22.2) | 16,981 (21.6) |
| MET ≥600 min/week | 257,650 (79.1) | 65,379 (83.2) |
| Current smoker | 36,740 (11.3) | 5,097 (6.5) |
| Moderate drinker | 149,088 (45.7) | 37,534 (47.8) |
| Healthy diet | 196,821 (60.4) | 55,946 (71.2) |
| Family history of diabetes | 67,284 (20.6) | 15,615 (19.9) |
| Townsend deprivation index, mean (SD) | −1.3 (3.1) | −1.8 (2.8) |
| Regular anti-inflammatory drugs use | ||
| Aspirin | 26,634 (8.2) | 7,810 (9.9) |
| Nonaspirin NSAIDs | 47,967 (14.7) | 15,253 (19.4) |
| Supplement use | ||
| Vitamin supplements | 85,241 (26.2) | 43,695 (55.6) |
| Mineral supplements | 95,090 (29.1) | 54,424 (69.3) |
| Other diseases status | ||
| History of osteoarthritis or joint pain | 46,118 (14.2) | 24,287 (30.9) |
| Hypertension | 161,476 (49.5) | 40,892 (52.0) |
| High cholesterol | 37,595 (11.5) | 10,309 (13.1) |
Data are n (%) unless otherwise indicated.
After adjustment for covariates including age, sex, race, BMI, assessment center, physical activity, smoking status, moderate drinking, healthy diet, Townsend deprivation index, family history of diabetes, aspirin use, nonaspirin NSAID use, osteoarthritis or joint pain, hypertension, high cholesterol, vitamin supplement use, nonvitamin supplement use, and fasting hours (except for HbA1c analysis), glucosamine use showed slightly inverse association with glucose levels (β = −0.01, P < 0.001) or HbA1c levels (β = −0.03, P = 0.13) at baseline.
Association Between Glucosamine Use and Risk of Incident T2D
During a median of 8.1 years of follow-up, we documented 7,228 incident cases of T2D. Glucosamine use was significantly associated with a lower risk of incident T2D. In the age- and sex-adjusted analyses, the HR associated with glucosamine use was 0.82 (95% CI 0.77–0.87). After further adjustment for race, BMI, assessment center, physical activity, smoking status, moderate drinking, healthy diet, Townsend deprivation index, family history of diabetes, aspirin use, nonaspirin NSAID use, osteoarthritis or joint pain, hypertension, and high cholesterol, the HR associated with glucosamine use was 0.81 (95% CI 0.76–0.86). The results did not appreciably alter after further adjustment for vitamin supplement use and nonvitamin supplement use (HR 0.83, 95% CI 0.78–0.89) (Table 2).
Table 2.
HRs of glucosamine use for T2D in the UK Biobank
| Nonglucosamine user | Glucosamine user | P value | |
|---|---|---|---|
| Cases, n (%) | 5,930 (1.8) | 1,298 (1.7) | |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 0.82 (0.77–0.87) | <0.001 |
| Model 1 | 1 [Reference] | 0.81 (0.76–0.86) | <0.001 |
| Model 2 | 1 [Reference] | 0.83 (0.78–0.89) | <0.001 |
Model 1: Results were adjusted for age, sex, race, center, BMI, physical activity, smoking, moderate drinking, healthy diet, Townsend deprivation index, aspirin use, nonaspirin NSAID use, family history of diabetes, osteoarthritis or joint pain, hypertension, and high cholesterol. Model 2: further adjustment for vitamin supplement use and mineral supplement use on the basis of model 1.
Similar associations of glucosamine use with risk of incident T2D were observed if we further excluded participants with glucose levels ≥7.0 mmol/L or participants with HbA1c levels ≥48 mmol/mol (6.5%) or excluded participants with limited follow-up years (≤2 years), or excluded participants who regularly took anti-inflammatory drugs or further adjusted for glucose levels, HbA1c levels, or CRP levels (Supplementary Tables 4–8).
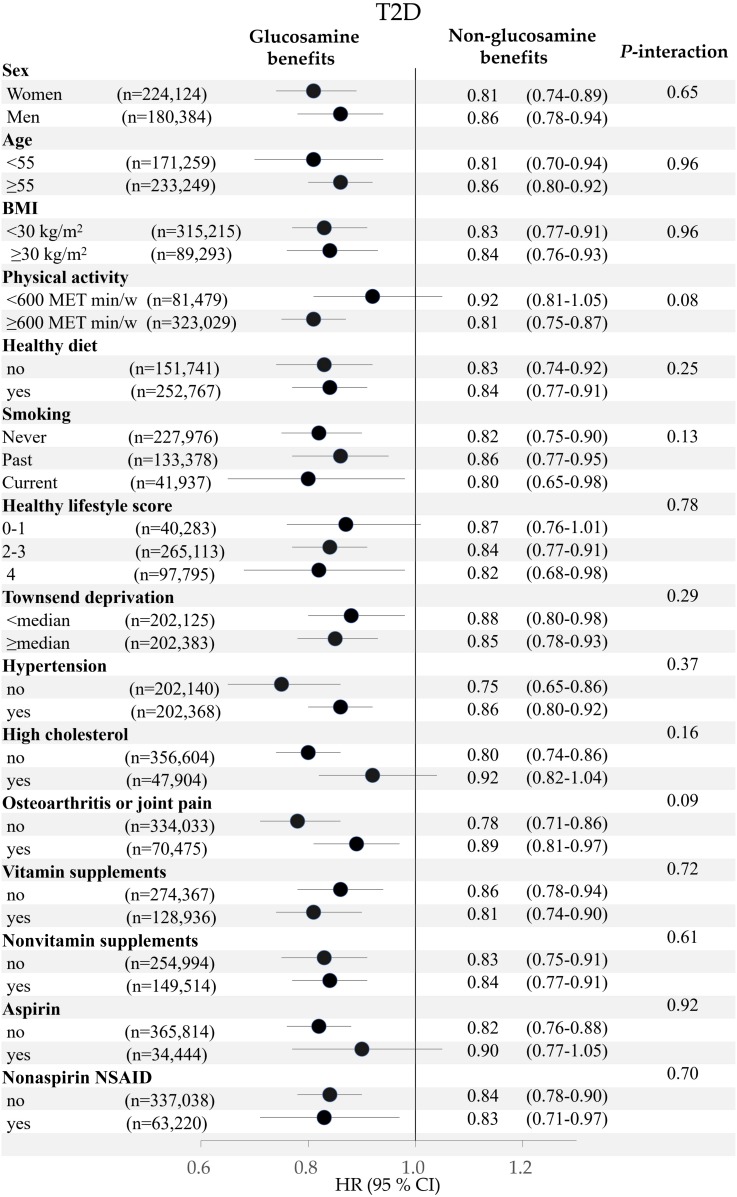
We also evaluated the associations of habitual glucosamine use with risk of T2D. We conducted these analyses in participants who provided information in the “Vitamin/mineral supplements yesterday” questionnaire at least once during the follow-up period. After participants who developed T2D before the last survey were excluded, a higher degree of habitual glucosamine was associated with a lower risk of T2D (P-trend <0.001). In the multivariable-adjusted analyses, compared with nonglucosamine users, the HR was 0.85 (95% CI 0.73–0.99) for participants who indicated they took glucosamine only once at baseline or during the follow-up (one baseline survey + four times of “Vitamin/mineral supplements yesterday” surveys); the HR was 0.75 (0.63–0.88) for participants who indicated they took glucosamine at least two times at baseline or during the follow-up period (Supplementary Table 9). To evaluate whether T2D risk factors modify the relation between glucosamine use and risk of T2D, we performed stratified analyses according to the potential T2D risk factors. We did not find significant interactions between glucosamine use and these risk factors on risk of incident T2D. Because the inverse association might be due to an overall healthier lifestyle in glucosamine users, we also examined the interaction between the overall healthy life score and glucosamine use and did not find a significant interaction (P-interaction = 0.78) (Fig. 1).
Figure 1.
Adjusted HR (95% CI) for the use of glucosamine supplements and risk of T2D stratified by potential risk factors. Results were adjusted for age, sex, race, center, BMI, physical activity, smoking, healthy diet, moderate drinking, Townsend deprivation index, aspirin use, nonaspirin NSAID use, family history of diabetes, osteoarthritis or joint pain, hypertension, high cholesterol, vitamin supplement use, and mineral supplement use.
Interaction Between Glucosamine Use and CRP Levels on Risk of T2D
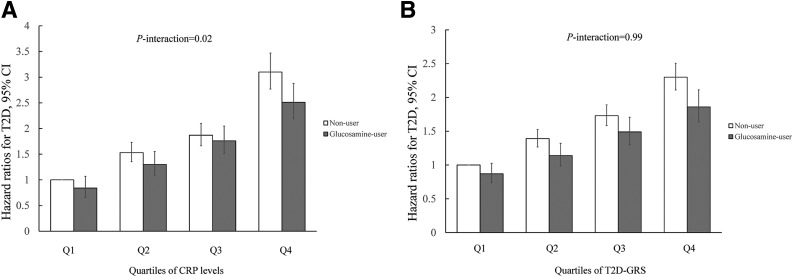
We also examined the interaction between glucosamine use and CRP levels on risk of T2D. After adjustment for covariates, including age, sex, race, BMI, assessment center, physical activity, smoking status, moderate drinking, healthy diet, Townsend deprivation index, family history of diabetes, aspirin use, nonaspirin NSAID use, osteoarthritis or joint pain, hypertension, high cholesterol, vitamin supplement use, and nonvitamin supplement use, the baseline CRP level (mg/L) was significantly lower in glucosamine users than in nonusers (β = −0.02, P < 0.001). We found that the inverse associations between glucosamine use and risk of T2D were more pronounced in participants with high CRP levels than in those with low CRP levels. Glucosamine use was associated with an 18.8% decreased risk in participants with the highest quartile of CRP levels. The interaction between glucosamine use and CRP levels on the risk of T2D was statistically significant (P-interaction = 0.02) (Fig. 2A).
Figure 2.
The joint association of glucosamine use and quartiles of CRP (A) and T2D-GRS (B) in relation to risk of T2D. Results were adjusted for age, sex, race (only for A), center, BMI, physical activity, smoking, healthy diet, Townsend deprivation index, moderate drinking, aspirin use, nonaspirin NSAID use, family history of diabetes, osteoarthritis or joint pain, hypertension, high cholesterol, vitamin supplement use and mineral supplement use, the first 10 genetic principal components (only for B), and genotyping array (only for B).
Similar interaction patterns were observed if we further excluded participants with a high level of glucose (≥7.0 mmol/L), or participants with a high level of HbA1c (≥48 mmol/mol [6.5%]), or participants with limited follow-up years (≤2 years) (P-interaction = 0.04, P-interaction = 0.02, and P-interaction = 0.03, respectively) (Supplementary Fig. 1A–C). However, after excluding 96,136 participants who took any other anti-inflammatory drugs, the P value for interaction did not reach the significant level (P-interaction = 0.09) (Supplementary Fig. 1D).
Interaction Between Glucosamine Use and T2D-GRS on Risk of T2D
The genetic data were available for 360,550 participants in this study. After adjustment for all of the variables listed in Table 1, the first 10 genetic principal components, and genotyping array, higher T2D-GRS was significantly associated with increased risk of incident T2D (the HR of T2D comparing extreme quartiles was 2.27 [95% CI 2.11–2.46]) (Supplementary Table 3).
In the joint analysis, glucosamine use was significantly associated with risk of T2D independent of T2D-GRS.We did not find significant interaction between glucosamine use and T2D-GRS on the risk of T2D (P-interaction = 0.99) (Fig. 2B).
Conclusions
In this prospective study, we observed that habitual use of glucosamine, a widely used supplement for relieving osteoarthritis and joint pain, was associated with a 17% lower risk of incident T2D. This association was independent of traditional risk factors for T2D, socioeconomic factors, and use of other supplements.
To the best of our knowledge, the current study is the first to investigate the association of glucosamine use with risk of incident T2D in a prospective cohort. Of note, several previous studies showed that short-term administration of high-dose glucosamine had adverse effects on glucose tolerance and insulin sensitivity in animals or humans (6–8,28). However, a group of clinical trials found that long-term glucosamine use did not show adverse effects on glucose metabolism in both healthy individuals and patients with diabetes at the oral dose level (9–12,29). On the contrary, in a long-term controlled trial that involved 212 participants with arthritis, glucosamine produced a slightly glucose-lowering effect in participants who received a 3-year intervention (13). Similar results were observed in a meta-analysis of the clinical trials (30). Consistent with this finding, we found in the current study that habitual glucosamine use was slightly associated with lower levels of glucose at baseline. The contradictory results between animal and human studies may be partly because the dosages of glucosamine used in animal studies were much higher (100–200 times) than the usual oral dosage in humans (30).
Even though the biological mechanisms underlying the inverse association of glucosamine use with risk of T2D remain to be explored, several lines of evidence support the potentially protective effects of glucosamine on the disease. First, several previous studies have shown that glucosamine has anti-inflammatory properties (14,15). Consistent with a previous study in the National Health and Nutrition Examination Survey (NHANES) (31), we found that circulating levels of CRP, a marker of low-grade systemic inflammation, were significantly lower in glucosamine users compared with nonglucosamine users. A randomized clinical trial also demonstrated that glucosamine and chondroitin use significantly reduced circulating CRP concentrations compared with the placebo group (32). Given the detrimental roles of inflammation in the development of diabetes (17,18), we assumed that glucosamine might lower the risk of T2D at least partly through its anti-inflammatory effect. Interestingly, our findings showed that the inverse association of glucosamine use with risk of T2D tended to be stronger in participants with higher baseline CRP levels, and a suggestive interaction between glucosamine use and baseline CRP levels was observed. These results lend support to our postulation, whereas further studies are needed to verify these findings. Second, a previous animal study showed that glucosamine could improve blood glucose levels and extend life span through decreasing glycolysis and increasing amino acid catabolism, and this process was described as mimicking a low-carbohydrate diet (33). A low-carbohydrate diet has been associated with lower risk of T2D in previous studies, probably due to its direct impact on glucose metabolism (34–38). Other mechanisms may be also involved, and future studies are needed to explore the functional roles of glucosamine in glucose metabolism.
Consistent with a previous study (39), T2D-GRS was significantly positively associated with incident T2D in our study. However, we did not observe significant interaction between glucosamine use and T2D-GRS on the risk of incident T2D, suggesting that the potentially beneficial effects of glucosamine on T2D might be consistent among people with different genetic makeups.
The major strengths of this study include the large sample size, the well-validated measures of biomarkers, and the consistent results in several sensitivity analyses. Several potential limitations should be carefully considered in this study. First, we could not exclude the possibility that glucosamine use is a marker for a healthy lifestyle. However, we carefully controlled for lifestyle factors and did not find any significant modification of lifestyle factors on the association of glucosamine use with risk of T2D, suggesting that the observed association of glucosamine use with risk of T2D was less likely due to its correlation with the healthier lifestyle. Second, our results regarding the interaction of glucosamine and CRP should be interpreted with caution. We could not rule out the possibility that these results were due to chance. The study used a single measurement of CRP at baseline, which might only reflect short-term inflammation status, and other inflammatory markers should be assessed in further studies. In addition, we did not observe the stronger inverse associations in other high inflammation statuses, such as smoking and obesity. Third, some detailed information on glucosamine use, such as the dosage, the formulation (i.e., some glucosamine supplements contain chondroitin, methyl-sulfonyl-methane, or others), and the duration of glucosamine use was not collected in this study. Further studies are warranted to evaluate the effects of these factors on results. Fourth, some incident T2D cases were diagnosed by the secondary diagnosis, thus the incident time of T2D that was used in this study may be later than the actual onset time in this study. However, similar results were observed if logistical models were used (odds ratio 0.83, 95% CI 0.78–0.89). Finally, because of the observational nature of this study, causality could not be derived in our study, and randomized clinical trials are needed to verify our findings.
Our findings indicate that habitual glucosamine use, a common supplement used for osteoarthritis and joint pain, is associated with a lower risk of T2D. These findings provide support that glucosamine may act as a potential supplement for preventing T2D. Further clinical trials are needed to test this hypothesis.
Supplementary Material
Article Information
Funding. L.Q. was supported by grants from the National Heart, Lung, and Blood Institute (HL-071981, HL-034594, HL-126024) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-115679, DK-091718, DK-100383, DK-078616). This study was conducted using the UK Biobank resource, approved project number 29256.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.M. performed the statistical analysis. H.M. and L.Q. contributed to the concept and design; acquired, analyzed, or interpreted the data; and drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. L.Q. attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. L.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1836/-/DC1.
References
- 1.Jordan KM, Arden NK, Doherty M, et al.; Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT . EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008;(12):1–23. [PubMed] [Google Scholar]
- 3.Sibbritt D, Adams J, Lui CW, Broom A, Wardle J. Who uses glucosamine and why? A study of 266,848 Australians aged 45 years and older. PLoS One 2012;7:e41540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H, Li X, Sun D, et al. Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ 2019;365:l1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795–808 [DOI] [PubMed] [Google Scholar]
- 6.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 1991;266:4706–4712 [PubMed] [Google Scholar]
- 7.Patti ME, Virkamäki A, Landaker EJ, Kahn CR, Yki-Järvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 1999;48:1562–1571 [DOI] [PubMed] [Google Scholar]
- 8.Robinson KA, Sens DA, Buse MG. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor. Diabetes 1993;42:1333–1346 [DOI] [PubMed] [Google Scholar]
- 9.Scroggie DA, Albright A, Harris MD. The effect of glucosamine-chondroitin supplementation on glycosylated hemoglobin levels in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized clinical trial. Arch Intern Med 2003;163:1587–1590 [DOI] [PubMed] [Google Scholar]
- 10.Albert SG, Oiknine RF, Parseghian S, Mooradian AD, Haas MJ, McPherson T. The effect of glucosamine on serum HDL cholesterol and apolipoprotein AI levels in people with diabetes. Diabetes Care 2007;30:2800–2803 [DOI] [PubMed] [Google Scholar]
- 11.Muniyappa R, Karne RJ, Hall G, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes 2006;55:3142–3150 [DOI] [PubMed] [Google Scholar]
- 12.Tannis AJ, Barban J, Conquer JA. Effect of glucosamine supplementation on fasting and non-fasting plasma glucose and serum insulin concentrations in healthy individuals. Osteoarthritis Cartilage 2004;12:506–511 [DOI] [PubMed] [Google Scholar]
- 13.Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251–256 [DOI] [PubMed] [Google Scholar]
- 14.Largo R, Alvarez-Soria MA, Díez-Ortego I, et al. Glucosamine inhibits IL-1β-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2003;11:290–298 [DOI] [PubMed] [Google Scholar]
- 15.Yomogida S, Hua J, Sakamoto K, Nagaoka I. Glucosamine suppresses interleukin-8 production and ICAM-1 expression by TNF-α-stimulated human colonic epithelial HT-29 cells. Int J Mol Med 2008;22:205–211 [PubMed] [Google Scholar]
- 16.Azuma K, Osaki T, Wakuda T, et al. Suppressive effects of N-acetyl-d-glucosamine on rheumatoid arthritis mouse models. Inflammation 2012;35:1462–1465 [DOI] [PubMed] [Google Scholar]
- 17.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972–978 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ. Is type 2 diabetes mellitus a vascular condition? Arterioscler Thromb Vasc Biol 2003;23:1715–1716. [DOI] [PubMed]
- 19.Qi L, Liang J. Interactions between genetic factors that predict diabetes and dietary factors that ultimately impact on risk of diabetes. Curr Opin Lipidol 2010;21:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med 2008;8:519–532 [DOI] [PubMed] [Google Scholar]
- 21.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett MS. The use of transformations. Biometrics 1947;3:39–52 [PubMed] [Google Scholar]
- 23.Scott RA, Scott LJ, Mägi R, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2017;66:2888–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripatti S, Tikkanen E, Orho-Melander M, et al. A multilocus genetic risk score for coronary heart disease: case-control and prospective cohort analyses. Lancet 2010;376:1393–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ∼500,000 UK Biobank participants [article online]. bioRxiv 2017. Available from https://www.biorxiv.org/content/10.1101/166298v1.full.pdf. Accessed 19 December 2019 [Google Scholar]
- 26.Eastwood SV, Mathur R, Atkinson M, et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS One 2016;11:e0162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Global Recommendations on Physical Activity for Health. Geneva, Switzerland, World Health Organization, 2010 [PubMed] [Google Scholar]
- 28.Monauni T, Zenti MG, Cretti A, et al. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes 2000;49:926–935 [DOI] [PubMed] [Google Scholar]
- 29.Simon RR, Marks V, Leeds AR, Anderson JW. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab Res Rev 2011;27:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol 2005;43:187–201 [DOI] [PubMed] [Google Scholar]
- 31.Kantor ED, Lampe JW, Vaughan TL, Peters U, Rehm CD, White E. Association between use of specialty dietary supplements and C-reactive protein concentrations. Am J Epidemiol 2012;176:1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro SL, White E, Kantor ED, et al. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS One 2015;10:e0117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weimer S, Priebs J, Kuhlow D, et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun 2014;5:3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–2081 [DOI] [PubMed] [Google Scholar]
- 35.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 2004;140:778–785 [DOI] [PubMed] [Google Scholar]
- 36.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–2090 [DOI] [PubMed] [Google Scholar]
- 37.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142:403–411 [DOI] [PubMed] [Google Scholar]
- 38.Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2017;5:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langenberg C, Sharp SJ, Franks PW, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 2014;11:e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.