Abstract
OBJECTIVE
Most individuals with two or more islet autoantibodies progress to clinical type 1 diabetes. However, in some individuals, autoantibodies are subsequently lost. Here, our objectives were to determine the frequency of autoantibody loss (reversion) in multiple-autoantibody–positive individuals and to determine the association between reversion and progression to clinical disease.
RESEARCH DESIGN AND METHODS
We analyzed multiple-autoantibody–positive individuals participating in TrialNet’s Pathway to Prevention Study for reversion and determined the effect of reversion on progression to clinical disease using a Cox regression analysis.
RESULTS
Of 3,284 multiple-autoantibody–positive subjects, reversion occurred in 134 (4.1%) and was associated with reduced incidence of clinical disease. Reversion occurred more frequently with older age, lower autoantibody titers, and fewer positive autoantibodies.
CONCLUSIONS
Although reversion of multiple-autoantibody positivity is rare, when it occurs, the risk of progressing to clinical disease is reduced. This suggests unknown mechanisms promoting immune remission in some individuals.
Introduction
Islet autoantibodies are measured to distinguish type 1 diabetes from other forms of diabetes and to predict disease progression. Large-scale prospective studies of relatives of individuals with type 1 diabetes revealed that nearly all with two or more (multiple) autoantibodies progress to clinical diabetes (1,2). On the basis of these observations, the presence of multiple autoantibodies prior to symptomatic disease is now recognized as the first stage of type 1 diabetes (2). However, it has been observed that autoantibody titers fluctuate, sometimes dropping below the threshold for positivity (3–8). The implications of loss of multiple-autoantibody positivity (reversion) on disease development are not known. To address these gaps in knowledge, we analyzed multiple-autoantibody–positive at-risk subjects in TrialNet’s Pathway to Prevention Study for frequency of reversion, and then we determined the impact of reversion on clinical disease development.
Research Design and Methods
Study Population
Relatives of subjects with type 1 diabetes (n = 201,617) were screened for autoantibodies between 2004 and 2018 through TrialNet’s Pathway to Prevention (PTP) Study (ClinicalTrials.gov identifier NCT00097292) (9) (Supplementary Fig. 1). Multiple-autoantibody positivity was defined as two or more islet autoantibodies (islet cell antibody [ICA], insulin antibody [MIAA], GAD65 antibody [GADA], IA2 antibody [IA2A], or zinc transporter 8 antibody [ZNT8A]) confirmed on two occasions within 12 months. Participants diagnosed with diabetes within 12 months of confirmed multiple-autoantibody positivity were excluded because they were diagnosed before autoantibody maintenance status could be established.
Autoantibody Assays
Autoantibodies were measured as previously described (10). ZNT8A testing was introduced in 2012. Harmonized assay results were used when both standard and harmonized assay results were available (11). Titer analysis was performed on the first visit used to establish multiple-autoantibody positivity.
Statistical Analysis
Demographic variables were summarized by mean ± SD or count (%), and they were compared using a χ2 test or Student t test. Kaplan-Meier plots were used to estimate the cumulative incidence of disease. After multiply imputing missing values, the risk of diabetes diagnosis was estimated using a Cox proportional hazards model. Analyses were performed using R statistical software.
Results
Frequency and Baseline Characteristics of Reverters
We defined a “reverter” as an individual who demonstrated a loss of multiple-autoantibody positivity to one or zero autoantibodies on two consecutive encounters within 12 months, irrespective of later reestablishment of multiple-autoantibody status. A “maintainer” was any individual who maintained two or more autoantibodies throughout follow-up. Of the 3,284 multiple-autoantibody–positive individuals in TrialNet’s PTP Study, there were 134 (4.1%) reverters and 3,150 maintainers (Table 1). Reverters were more likely to be older at the time of confirmed multiple-autoantibody positivity (19.4 vs. 13.1 years old; P < 0.001), with longer follow-up (5.2 vs. 2.1 years; P < 0.001). Reverters also had a lower number of autoantibodies (2.1 vs. 3.1; P < 0.001). On the basis of a univariable Cox model, each additional positive autoantibody decreased the risk of reversion by a factor of 5.0 (95% CI 4.1–6.0).
Table 1.
Baseline characteristics and summary statistics by maintenance group and overall
| Maintainer (N = 3,150) | Reverter (N = 134) | Overall (N = 3,284) | P value | |
|---|---|---|---|---|
| Age at positivity (years) | 13.1 ± 10.9 | 19.4 ± 14.4 | 13.3 ± 11.1 | <0.001 |
| Age, range (years) | 0.6–49.0 | 1.3–45.6 | 0.6–49.0 | |
| Follow-up (years) | 2.1 ± 2.3 | 5.2 ± 2.8 | 2.2 ± 2.4 | <0.001 |
| Age group | <0.001 | |||
| 0–8 years | 1,213 (38.5) | 31 (23.1) | 1,244 (37.9) | |
| 9–12 years | 763 (24.2) | 28 (20.9) | 791 (24.1) | |
| 13–18 years | 612 (19.4) | 28 (20.9) | 640 (19.5) | |
| >18 years | 562 (17.8) | 47 (35.1) | 609 (18.5) | |
| Sex, male | 1,649 (53.3) | 64 (49.2) | 1,713 (53.1) | 0.414 |
| BMI, kg/m2 | 20.1 ± 6.0 | 21.9 ± 7.1 | 20.2 ± 6.0 | 0.001 |
| BMI (z score) | 0.45 ± 1.15 | 0.47 ± 1.09 | 0.45 ± 1.15 | 0.824 |
| Number of positive AAB | 3.1 ± 1.0 | 2.1 ± 0.4 | 3.0 ± 1.0 | <0.001 |
| OGTT interpretation | 0.704 | |||
| Normal glucose tolerance | 1,772 (70.5) | 84 (73.7) | 1,856 (70.6) | |
| Abnormal glucose tolerance | 706 (28.1) | 28 (24.6) | 734 (27.9) | |
| Diabetic range* | 37 (1.5) | 2 (1.8) | 39 (1.5) | |
| Protective HLA† | 93 (3.2) | 8 (6.1) | 101 (3.4) | 0.124 |
| HLA-DR3 | 1,327 (46.1) | 58 (44.3) | 1,385 (46.0) | 0.750 |
| HLA-DR4 | 1,823 (63.3) | 75 (57.3) | 1,898 (63.1) | 0.187 |
| HLA-DR3 or -DR4 | 2,442 (84.9) | 109 (83.2) | 2,551 (84.8) | 0.698 |
| HLA-DR3 and -DR4 | 708 (24.6) | 24 (18.3) | 732 (24.3) | 0.125 |
| Positive AAB at study enrollment‡ | ||||
| MIAA | 1,796 (57.0) | 44 (32.8) | 1,840 (56.0) | <0.001 |
| ICA | 2,410 (76.6) | 94 (70.1) | 2,504 (76.3) | 0.106 |
| GADA | 2,907 (92.3) | 129 (96.3) | 3,036 (92.4) | 0.123 |
| IA2A | 1,820 (57.8) | 27 (20.1) | 1,847 (56.2) | <0.001 |
| ZNT8A | 1,507 (61.3) | 15 (16.7) | 1,522 (59.7) | <0.001 |
Data are n (%) or mean ± SD. AAB, autoantibody; OGTT, oral glucose tolerance test.
Participants who had a single OGTT in the diabetic reference range but subsequent tests reverted to abnormal or normal glucose tolerance.
HLA-DQB1*0602.
Positive results from both the standard and harmonized assays were included. If both standard and harmonized assay results were available at a particular time point, results from the harmonized assay were used.
Autoantibody titers were lower for reverters compared with maintainers for IA2A, ZNT8A, and ICA (P < 0.001 for each) (Supplementary Fig. 2A) but not for GADA (P = 0.927) or MIAA (P = 0.143). However, among reverters who started with GADAs, those who lost GADAs had lower titers than those who retained it (P < 0.001). This was also true for MIAAs (P = 0.01). Thus, while reversion was observed to be associated with lower median titers for all islet autoantibodies, reversion also occurred at higher titers (Supplementary Fig. 2A). Among reverters, ICAs were most likely to be lost, with 85 of the 87 (98%) who started with a positive ICA losing positivity (Supplementary Fig. 3). In contrast, reverters were unlikely to lose GADAs, with only 19/129 (15%) reverters who had GADAs, losing GADA positivity.
Diabetes Incidence in Reverters
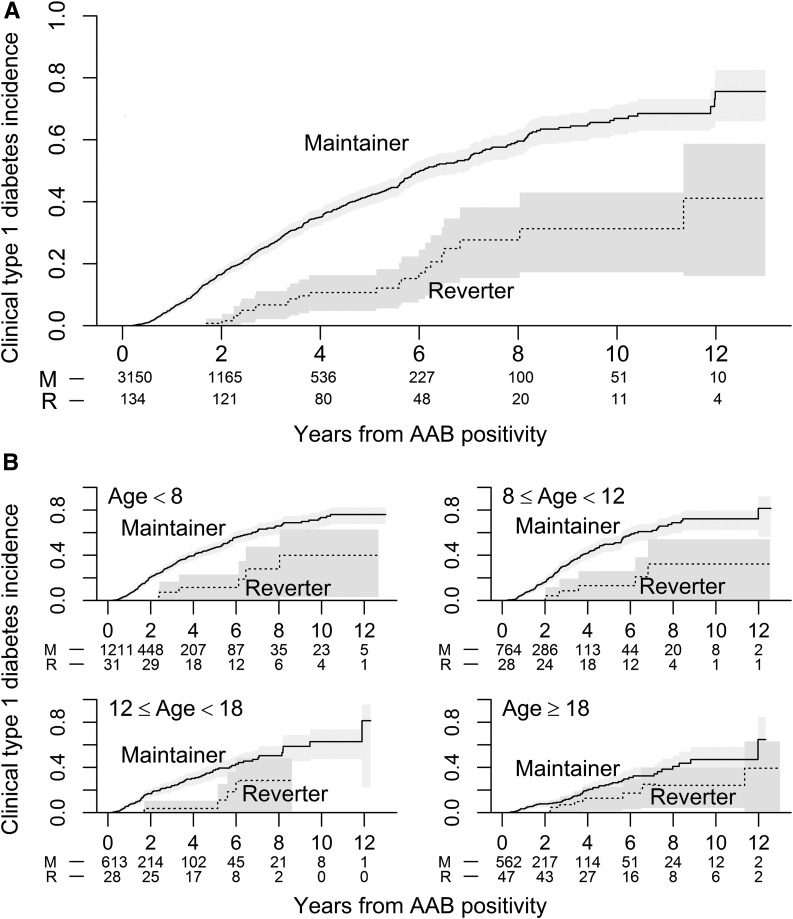
The incidence of diabetes was significantly lower for reverters compared with maintainers (Fig. 1A). This remained significant in a multivariable model (Supplementary Table 1) (P= 0.005). The estimated cumulative 5-year risk of type 1 diabetes was 42% (95% CI 39–45%) for the maintainers and 11% for the reverters (95% CI 6–18%). The difference in diabetes incidence between reverters and maintainers was largest in the youngest participants, with the difference between groups diminishing with increasing age (Fig. 1B). An exploratory analysis revealed that this diminishing difference was driven by the reverters, who demonstrated a low and stable rate of diabetes incidence with increasing age, compared with the typical inverse relationship between age and rate of diabetes incidence seen in the maintainers (Supplementary Fig. 4).
Figure 1.
Stratified estimated cumulative incidence of clinical type 1 diabetes. Lines represent Kaplan-Meier estimates, and shaded regions represent pointwise 95% CIs. The numbers below the x-axis are the number of subjects remaining at risk for developing clinical type 1 diabetes at the indicated time points. A: Cumulative incidence of type 1 diabetes diagnosis by autoantibody maintenance group (maintainers versus reverters). B: Cumulative incidence of type 1 diabetes diagnosis by autoantibody maintenance group stratified by age. M, maintainers; R, reverters; AAB, autoantibody.
Reverters Who Regain Multiple-Autoantibody Status
During follow-up, 41 of 134 (31%) reverters regained multiple-autoantibody status. There was no significant difference in diabetes incidence (P = 0.99) between the reverters who regained their multiple-autoantibody status (7/41, 17%) and those who did not (16/93, 17%). Nor was there a significant difference in distribution of autoantibody titers (Supplementary Fig. 2B).
Conclusions
Understanding the impact of reversion on the risk of diabetes progression is critical for insight into the natural history of the disease, and it has the potential to affect trial recruitment strategies especially for prevention trials that enroll at-risk individuals with multiple autoantibodies (12). Here, we show that reversion in multiple-autoantibody–positive individuals is infrequent and associated with a reduced incidence of clinical diabetes. To our knowledge, this is the first study characterizing the impact of autoantibody loss in multiple-autoantibody–positive individuals. Previous studies assessing autoantibody reversion have reported it almost exclusively in single-autoantibody–positive individuals; these prospective birth cohorts had insufficient numbers of multiple-autoantibody–positive individuals who reverted to understand the implications of this phenomenon (4–8,13). Our unique study design defined reversion as a loss of multiple-autoantibody positivity rather than a loss of all autoantibodies, taking into account the low disease risk of single-autoantibody–positive individuals. The previously reported 5-year cumulative risk for single-autoantibody–positive individuals who do not progress to multiple autoantibodies is only 5.7% (14). Our finding that multiple-autoantibody–positive individuals who revert had a significantly lower 5-year cumulative risk of clinical diabetes (11%) compared with maintainers (42%) was novel. Collectively, this suggests individuals who seroconvert to multiple autoantibodies and then revert remain at a higher risk than seroconverters who never become multiple-autoantibody positive but at a substantially lower risk than those who maintain multiple autoantibodies.
While the absolute number of reverters is small, this is the largest study of reverters to date, highlighting the rarity of this phenomenon in multiple-autoantibody–positive individuals. Furthermore, though maintainers demonstrated a shorter follow-up time, this was consistent with their expected, high rate of disease progression. Additional exploratory studies are needed to address the role of baseline characteristics (age, number of antibodies) in reversion.
The mechanisms behind the inverse relationship between age and risk of disease progression are currently unknown. Therefore, of interest is the reverters’ relatively stable rate of progression across the age groups compared with the usual inverse relationship seen in maintainers. This points to potential regulatory mechanisms in reverters that may partly counteract the usual autoimmune process. The occurrence of reversion at higher autoantibody titers affirms that this phenomenon is not solely driven by autoantibody levels hovering around the titer cutoff, highlighting the need for mechanistic studies explaining the biology of reversion.
Collectively, our findings provide new information on the baseline characteristics of reverters, and importantly implicate autoantibody loss in limiting or delaying disease progression. Notably, reverters represent a unique cohort worth targeting for further mechanistic investigations to understand the immunologic and metabolic drivers of disease heterogeneity in type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the subjects and families, as well as the many TrialNet clinical teams who conducted study visits and recruited participants. The authors thank Rachel Hartley (Benaroya Research Institute) for data cleanup and members of the TrialNet Coordinating Center, especially Sarah Muller and Ilma Asif, for data sharing. The authors also thank Alyssa Ylescupidez and Anne Hocking (Benaroya Research Institute) for reviewing the manuscript.
Funding. This work was funded by JDRF under an Innovations grant (1-INO-2018-641-A-N) to C.J.G. We acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085453, U01-DK-085461, U01-DK-085466, U01-DK-085499, U01-DK-085504, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-085476, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-106994, U01-DK-107013, U01-DK-107014, UC4-DK-106993, and JDRF.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or JDRF.
Duality of Interest. C.J.G. reports grants from Janssen Research and Development during the conduct of the study and personal fees from Novo Nordisk, outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S. wrote the manuscript and contributed to the data analysis. C.O. analyzed the data and contributed to the manuscript. C.J.G. and C.S. designed the study, contributed to discussion, reviewed the data analysis, and reviewed and edited the manuscript. H.T.B. contributed to discussion, reviewed the data analysis, and reviewed and edited the manuscript. C.S. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1731/-/DC1.
M.S. and C.O. contributed equally.
References
- 1.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer KM, Tarn A, Dean BM, Lister J, Bottazzo GF. Fluctuating islet-cell autoimmunity in unaffected relatives of patients with insulin-dependent diabetes. Lancet 1984;1:764–766 [DOI] [PubMed] [Google Scholar]
- 4.Kimpimäki T, Kulmala P, Savola K, et al. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab 2002;87:4572–4579 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Yu L, Bugawan TL, et al. Transient antiislet autoantibodies: infrequent occurrence and lack of association with “genetic” risk factors. J Clin Endocrinol Metab 2000;85:2421–2428 [DOI] [PubMed] [Google Scholar]
- 6.Colman PG, Steele C, Couper JJ, et al. Islet autoimmunity in infants with a Type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia 2000;43:203–209 [DOI] [PubMed] [Google Scholar]
- 7.Kulmala P, Rahko J, Savola K, et al. Stability of autoantibodies and their relation to genetic and metabolic markers of Type I diabetes in initially unaffected schoolchildren. Diabetologia 2000;43:457–464 [DOI] [PubMed] [Google Scholar]
- 8.Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 9.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Boulware DC, Beam CA, et al.; Type 1 Diabetes TrialNet Study Group . Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 2012;35:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krischer JP; Type 1 Diabetes TrialNet Study Group . The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 2013;56:1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vehik K, Lynch KF, Schatz DA, et al.; TEDDY Study Group . Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care 2016;39:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bingley PJ, Boulware DC, Krischer JP; Type 1 Diabetes TrialNet Study Group . The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia 2016;59:542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.