Abstract
Background and Purpose
Short-term central venous catheterization (CVC) is one of the commonly used invasive interventions in ICU and other patient-care areas. Practice and management of CVC is not standardized, varies widely, and need appropriate guidance. Purpose of this document is to provide a comprehensive, evidence-based and up-to-date, one document source for practice and management of central venous catheterization. These recommendations are intended to be used by critical care physicians and allied professionals involved in care of patients with central venous lines.
Methods
This position statement for central venous catheterization is framed by expert committee members under the aegis of Indian Society of Critical Care Medicine (ISCCM). Experts group exchanged and reviewed the relevant literature. During the final meeting of the experts held at the ISCCM Head Office, a consensus on all the topics was made and the recommendations for final document draft were prepared. The final document was reviewed and accepted by all expert committee members and after a process of peer-review this document is finally accepted as an official ISCCM position paper.
Modified grade system was utilized to classify the quality of evidence and the strength of recommendations. The draft document thus formulated was reviewed by all committee members; further comments and suggestions were incorporated after discussion, and a final document was prepared.
Results
This document makes recommendations about various aspects of resource preparation, infection control, prevention of mechanical complication and surveillance related to short-term central venous catheterization. This document also provides four appendices for ready reference and use at institutional level.
Conclusion
In this document, committee is able to make 54 different recommendations for various aspects of care, out of which 40 are strong and 14 weak recommendations. Among all of them, 42 recommendations are backed by any level of evidence, however due to paucity of data on 12 clinical questions, a consensus was reached by working committee and practice recommendations given on these topics are based on vast clinical experience of the members of this committee, which makes a useful practice point. Committee recognizes the fact that in event of new emerging evidences this document will require update, and that shall be provided in due time.
Abbreviations list
ABHR: Alcohol-based hand rub; AICD: Automated implantable cardioverter defibrillator; BSI: Blood stream infection; C/SS: CHG/silver sulfadiazine; Cath Lab: Catheterization laboratory (Cardiac Cath Lab); CDC: Centers for Disease Control and Prevention; CFU: Colony forming unit; CHG: Chlorhexidine gluconate; CL: Central line; COMBUX: Comparison of Bedside Ultrasound with Chest X-ray (COMBUX study); CQI: Continuous quality improvement; CRBSI: Catheter-related blood stream infection; CUS: Chest ultrasonography; CVC: Central Venous Catheter; CXR: Chest X-ray; DTTP: Differential time to positivity; DVT: Deep venous thrombosis; ECG: Electrocardiography; ELVIS: Ethanol lock and risk of hemodialysis catheter infection in critically ill patients; ER: Emergency room; FDA: Food and Drug Administration; FV: Femoral vein; GWE: Guidewire exchange; HD catheter: Hemodialysis catheter; HTS: Hypertonic saline; ICP: Intracranial pressure; ICU: Intensive Care Unit; IDSA: Infectious Disease Society of America; IJV: Internal jugular vein; IPC: Indian penal code; IRR: Incidence rate ratio; ISCCM: Indian Society of Critical Care Medicine; IV: Intravenous; LCBI: Laboratory confirmed blood stream infection; M/R: Minocycline/rifampicin; MBI-LCBI: Mucosal barrier injury laboratory-confirmed bloodstream infection; MRSA: Methicillin-resistant Staphylococcus aureus; NHS: National Health Service (UK); NHSN: National Healthcare Safety Network (USA); OT: Operation Theater; PICC: Peripherally-inserted central catheter; PIV: Peripheral intravenous line; PL: Peripheral line; PVI: Povidone-iodine; RA: Right atrium; RCT: Randomized controlled trial; RR: Relative risk; SCV/SV: Subclavian vein; ScVO2: Central venous oxygen saturation; Sn: Sensitivity; SOP: Standard operating procedure; SVC: Superior vena cava; TEE: Transesophageal echocardiography; UPP: Useful Practice Points; USG: Ultrasonography; WHO: World Health Organization
How to cite this article
Javeri Y, Jagathkar G, Dixit S, Chaudhary D, Zirpe KG, Mehta Y, et al. Indian Society of Critical Care Medicine Position Statement for Central Venous Catheterization and Management 2020. Indian J Crit Care Med 2020;24(Suppl 1):S6–S30.
Keywords: Central venous catheterization, CRBSI, Infection control, Position statement, Surveillance
PREAMBLE
Present document is an evidence based approach towards management of CVC in clinical practice. The recommendations presented in this document are based upon:
Latest available literature on the topic
Various patient safety and institutional manuals
Guidelines policy of national and global bodies
Consensus and experience of expert committee on the topic
After evaluation of the available literature, this position statement is developed by a representative committee of Indian Society of Critical Care Medicine (ISCCM). This document's recommendations suggest a preferred approach for management of CVC in healthcare settings. Simultaneously these recommendations are intended to be flexible and should be rationalized to the clinical context. This document is designed as a “position paper”, as use of CVCs is not just limited to ICUs and extended to other specialties and patient care areas. However, in its limited sense it should serve an important purpose to provide standardized and high level of safe patient care. After completion of thorough reviews by the committee, this document is officially endorsed by ISCCM.
INTRODUCTION
Central venous catheterization is one of the regularly used invasive procedures in various areas of patient care like intensive care unit, operating room, and emergency department. The practice of CVC varies widely. This document is an attempt to suggest a safe and preferred strategy for CVC.
For standardization in this document, National Healthcare Safety Network (NHSN) definition of central line has been adopted. NHSN defines “Central line (CL), as an intravascular catheter that terminates at or close to the heart, or in one of the great vessels that is used for infusion, withdrawal of blood, or hemodynamic monitoring.”1
For all practical purposes in this present document, “Central Venous Catheter” is defined here as a central line placed in one the large venous great vessel, which include internal jugular vein (IJV), brachiocephalic vein, subclavian vein (SCV), superior vena cava (SVC), iliac vein, femoral veins, inferior vena cava (IVC).” This definition has been simplified for easy understanding.
Central venous catheterization have been classified by various ways like the vessels it occupies, site of insertion, duration of use, its path from skin to vessel, material, special coatings, physical length, number of lumens or some special characteristic and uses of catheter. During the discussion all of these aspects have been considered but this document remained focused on short-term use of CVCs in adult patients. During the discussion all long term CVCs, tunneled catheters, HD catheter, PICC, Hickman catheter, peripheral cannula, pediatric patient population and catheter terminating in a systemic artery and pulmonary arteries were not reviewed.
PURPOSE AND SCOPE
The purpose of this document is to provide a preferred strategy for:
Judicious use of CVC
CVC placement and management
Reduce mechanical, infectious, and thrombotic complications
Provide guidance to improve CVC care quality
These recommendations are intended for use by critical care physicians and other professionals involved in care of patients with central venous line. This also serves as a resource for other care areas who manage patients with short-term central venous catheters.
METHODS AND EVIDENCE DEVELOPMENT
This position statement for CVC is framed by expert committee members under the aegis of ISCCM. After multiple rounds of meeting of team of experts in the field, the consensus was derived on the scope and questions that needed to be answered in the formulation of this document. The team of experts was assigned the task of literature review in divided sections of the document. Search for relevant literature was performed by probing various electronic databases including Google Scholar, PubMed, and Embase. Following keywords were used to formulate search strategy: Arterial catheter, antibiotic lock, bacteremia, central lines, central venous catheter, catheter-related cultures, endocarditis, implanted catheter, management, non-tunneled, outbreak. Peripheral, suppurative thrombophlebitis, and treatment—other references from articles and major contemporary guidelines on the topic were also reviewed.
Experts in each group then exchanged and reviewed the relevant literature. During the final meeting of the experts held at the ISCCM head office, a consensus on all the topics was made and the recommendations for final document draft were prepared.
Modified grade system was utilized to classify the quality of evidence and the strength of recommendations (Table 1). For semantic separation of strong and weak recommendations, the working group has introduced each strong (grade A) recommendation by “we recommend” and each weak (grade B) recommendation by “we suggest” terminologies.
Table 1.
Criteria for level of evidence and grading of strength of recommendations used in formulation of present document.
| Quality of evidence | Level |
| Evidence from ≥1 good quality and well-conducted randomized control trial(s) or meta-analysis of RCT's | 1 |
| Evidence from at least 1 RCT of moderate quality, or well-designed clinical trial without randomization; or from cohort or case-controlled studies | 2 |
| Evidence from descriptive studies, or reports of expert committees, or opinion of respected authorities based on clinical experience | 3 |
| Not backed by sufficient evidence; however, a consensus reached by the working group, based on clinical experience and expertise | Useful Practice Point (UPP) |
| Strength of recommendations | Grade |
| Strong recommendations to do (or not to do) where the benefits clearly outweigh the risk (or vice versa) for most, if not all patients | A |
| Weak recommendations, where benefits and risk are more closely balanced or are more uncertain | B |
The draft document thus formulated was reviewed by all committee members; further comments and suggestions were incorporated after discussion, and a final document was prepared. The final document was reviewed and accepted by all expert committee members and after a process of peer review this document is finally accepted as an official ISCCM position paper.
RECOMMENDATIONS AND EVIDENCE STATEMENTS
Table 2 here provides summary of recommendation provided in this documents. A detailed description of all recommendations is in the following discussion.
Table 2.
Recommendations list of Indian Society of Critical Care Medicine Position Statement for Central Venous Catheterization and Management 2020.
| S/no. | Statement | GOR, LOE |
| 1. Resource preparation A. Indications of central venous catheterization | ||
| 1 | We recommend central venous catheterization after understanding clear indication | A, 3 |
| 2 | We suggest CVC when hyperosmolar and locally irritant agents are to be administered | B, UPP |
| 3 | We recommend CVC use for vasoactive drugs unless the risk outweighs benefit of placing a CVC and delaying the therapy | A, 3 |
| B. CVC catheterization in locations other than ICU | ||
| 1 | We recommend that care areas, where CVC is utilized should have a central venous cannulation and maintenance SOP in accordance with recommendations made in this document | A, UPP |
| 2 | We recommend that all units performing central venous cannulation should have a quality improvement program in place with follow-up of outcomes | A, UPP |
| 3 | We recommend that daily review for the necessity of CVC should be done at all care sites | A, 2 |
| C. Central venous catheter site selection | ||
| 1 | We recommend In emergency scenarios, insertion site selection should be based on patient factors, clinical need, practitioner judgment, experience and skills | A, 3 |
| 2 | We suggest subclavian insertion site should be preferred over IJV and femoral for central venous catheterization to decrease infectious and thrombotic complications | B, 2 |
| 3 | We recommend subclavian vein to be avoided in patient with coagulopathy, distorted anatomy, and who may have high chances of mechanical complications | A, 2 |
| 4 | We recommend that in case of burns, extensive skin loss and superficial infections, CVC insertion should be done where the skin is intact | A, UPP |
| 5 | We suggest Internal Jugular CVC lines could safely be inserted in adult neurocritical care patients | B, 2 |
| D. Catheter selection | ||
| 1 | We suggest to use a CVC with the minimum number lumens needed for patient management | B, 3 |
| 2 | No recommendation can be made for designated lumen for parenteral nutrition. Unresolved issue | B, 3 |
| 2. CVC—Infection control A. Site selection | ||
| 1 | We suggest evaluating risk-to-benefit ratio of infectious and mechanical complications before choosing a particular insertion site | B, 2 |
| 2 | We recommend avoiding using femoral vein for the routine placement of central venous catheters | A, 2 |
| B. Hygiene practices, barrier precautions, and skin preparation | ||
| 1 | We recommend mandatory hand hygiene practice, either by washing hands with conventional soap and water or with alcohol-based hand rub (ABHR), before and after any interventions or contact with CVC | A, 2 |
| 2 | We recommend maintaining aseptic technique for insertion and maintenance of CVC | A, 2 |
| 3 | We recommend maximal sterile barrier (MSB) precautions before any insertion (de novo or exchange over guidewire) of CVC | A, 1 |
| 4 | We recommend wearing either clean or sterile gloves when handling or dressing the CVC | A, 3 |
| 5 | We recommend preparation and cleaning of the skin site with an alcoholic chlorhexidine solution containing a concentration more than 0.5% chlorhexidine and 70 % alcohol before central venous catheter insertion and during dressing changes | A, 1 |
| 6 | We suggest to use tincture of iodine, an iodophor, or 70 % alcohol use as alternatives if chlorhexidine is contraindicated | B, 3 |
| 7 | We recommend allowing the skin antiseptic to dry completely before catheter insertion | A, 2 |
| C. CVC Fixation | ||
| 1 | No recommendation can be made for preference of securing system and operator or local practice based decision should be taken. | B, 3 |
| D. Port utilization and maintenance | ||
| 1 | We recommend disinfecting catheter hubs, needleless connectors, taps and injection ports before accessing the catheter using an alcoholic chlorhexidine preparation or 70 % alcohol | A, 2 |
| 2 | We recommend wearing either clean or sterile gloves when handling the hub and catheter | A, 3 |
| E. Prophylactic antibiotics and antiseptics | ||
| 1 | No recommendation can be made for or against the use of antiseptic solutions (Aqueous chlorhexidine or aqueous povidone-iodine] for routine CVC site care | A, 3 |
| 2 | We recommend the use of chlorhexidine soaked sponge or dressing at the catheter exit site to prevent CRBSI | A, 1 |
| 3 | We recommend daily Chlorhexidine Bed Bath[sponging] for patients in ICU to reduce CRBSI incidence | A, 1 |
| 4 | We suggest antibiotic lock solutions to prevent CRBSI only in selected conditions, which are as follows:
|
B, 2 |
| 5 | We recommend against systemic intravenous antibiotics in prevention of CRBSI | A, 1 |
| F. Removal of Central line | ||
| 1 | We recommend removing central venous catheter as soon as its indication ceases | A, UPP |
| 2 | We suggest not routinely replacing or relocating the central venous lines unless clinically indicated | B, UPP |
| 3 | We recommend each institute to have central venous catheter removal protocol and only staff trained in the same should remove central line | A, UPP |
| G. Catheters impregnated with antiseptics and antibiotics | ||
| 1 | We recommend using M/R or C/SS coated CVCs when catheter is expected to be in use for more than five days and the CLABSI rate is not decreasing to the institutional target benchmark even after implementing comprehensive strategy program. Comprehensive strategy should include education and training, maximal barrier precaution and aseptic skin preparation while insertion of CVC. | A, 1 |
| 3. Prevention of mechanical complications A. Role of sonography | ||
| 1 | Wherever available we recommend US guidance to improve success rate, patient safety and procedural quality and reduce mechanical complications during CVC placement | A, 2 |
| B. Guidewire exchange | ||
| 1 | We suggest exchange of malfunctioning CVC over guidewire in selected patients with no evidence of infection | B, 2 |
| C. Tip positioning | ||
| 1 | We recommend post-procedure, position of the catheter tip must be assessed | A, UPP |
| 2 | We recommend IJ and SCV catheter tip should be placed in the lower one-third of the SVC near the SVC/RA junction | A, 2 |
| 3 | We recommend the use of chest X-ray to assess the CVC catheter tip position | A, 2 |
| 4. Surveillance A. Infection control | ||
| 1 | We recommend against routine replacement of CVCs to prevent catheter-related infections | A, 1 |
| 2 | We recommend prompt removal of CVC when it is not essential | A, 2 |
| 3 | We recommend against routine catheter tip cultures for purpose of surveillance | A, 2 |
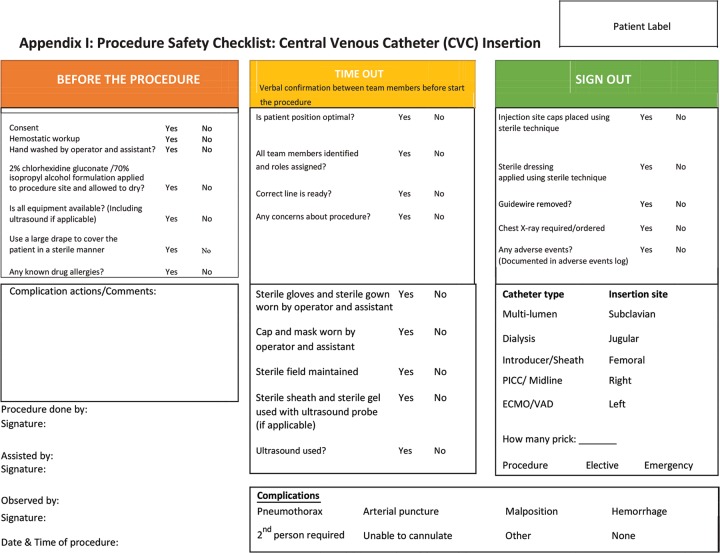
| 4 | We recommend that routine practice bundle (Appendix II) should be followed to reduce CVC-related infections. | A, 1 |
| B. Surveillance of mechanical complications | ||
| 1 | We recommend Chest X-ray post insertion of IJ and SC central line | A, 2 |
| 2 | We suggest that ultrasound guidance can be used for early identification of mechanical complication | B, 2 |
| C. Education, training, CQI initiatives, and audit Education and training | ||
| 1 | We recommend that a healthcare education and training program should be in place wherever CVCs are inserted and maintained for overall quality improvement | A, 1 |
| 2 | We recommend that a mechanism should be in place to assess knowledge and compliance with guidelines of all the personnel involved in care related to CVC | A, 1 |
| 3 | We suggest providing appropriate and adequate nursing care to improve CVC-related outcomes | B, 2 |
| CQI initiatives | ||
| 1 | We recommend using institutional CQI initiatives with bundled approach for performance improvement | A, 2 |
| Audit tools | ||
| 1 | We recommend conducting surveillance to determine CLABSI rates, monitor its trends and identify lapses in infection control practices | A, 1 |
| 2 | We do not recommended routine culture of catheter tip for purpose of surveillance | A, 1 |
| 3 | DTTP is the recommended method of diagnosis for CVC-related infections in patients | A, 2 |
| 4 | We suggest recording the operator, date and time of catheter insertion and removal and dressing changes on a standardized form | A, UPP |
| D. Consent and medicolegal issues | ||
| 1 | We suggest that a structured Credentialing process be in place for personnel involved in insertion and maintenance of CVC | B, UPP |
RESOURCE PREPARATION
Indications of Central Venous Catheterization
Evidence Statement
Central venous catheterization is a vital procedure in care of a critically ill patient. There are various indications for use of CVC and we reviewed each indication systematically for purpose of standardization and uniformity. The recommended indications are as below, but the list is not exclusive and judicious justification by user is recommended.
Central venous catheterization as means of vascular access
Central venous cannulation is indicated as means of vascular access for patients with difficult intravenous access, those requiring multiple attempts for peripheral access, obese patients with difficult peripheral access and patients with other chronic conditions.2
Central venous cannulation for vasopressors and inotropes administration
Central venous access should be chosen for administration of vasopressors and inotropes required for prolonged duration (>4 hours).3
Few small descriptive observational studies and review articles suggest safe use of peripherally administered vasopressors; however, a systematic review of descriptive studies which included 85 articles, had 270 patients and 325 events of local tissue injury and extravasations occurred.3 On further evaluation, it was noted that majority of events occurred when vasopressors used with peripheral lines (318/325 events). Out of which 204 events had local injury and 114 events had extravasation. These events were not generally associated with major disability and harm, as they occurs in <5% chances, with mortality chances of <2.2%. In further description it was found that 85.3% local tissue injury occurred when vasopressor were given via a distal peripheral venous lines than the popliteal or antecubital fossae (e.g., dorsal hands, forearms, feet), and in 96.8% occurred when infusion continued for 4 hours or more. Same results were observed for extravasation, as 75% events occurred due to a distal peripheral line. Hence, based on these findings of studies and meta-analysis, vasopressors infusions cannot be recommended to be used via peripheral line.
However, the expert committee recognizes that in compelling situations, vasopressor infusions for a short duration (<4 hours) via a more proximal vein (in antecubital fossa or external jugular vein) can be used without causing major harm, especially when a CVC is planned and risk of delaying therapy outweighs the benefit.3-7
Central venous cannulation for administration of parenteral nutrition
High osmolarity liquids cause damage of endothelium and further lead to thrombophlebitis of peripheral vein hence central venous cannulation can be considered for administration of parenteral nutrition with osmolarity >900 mOsm/L. However, commercially available parenteral nutrition with osmolarity of up to 900 mOsm/L can be administered and tolerated peripherally.8
Drugs to be given through central venous cannulation
Need for central venous cannulation for any drug administration depends on factors like osmolarity of the drug, pH of drug, and direct cytotoxicity of drug. Central venous catheterization should be considered for infusion of drugs with osmolarity of >600 mOsm/L and pH of less than 5 or greater than 9, if requiring continuous infusion at high rate or repeated infusions.9
3% NaCl and 20% mannitol administration
3% NaCl and 20% mannitol both are drugs with high osmolarity as 1,026 mOsm/L and 1,098 mOsm/L, respectively. Mandatory central venous cannulation for administration of these drugs is institutional practice, as central line insertion might be time consuming specifically in some time sensitive situations like patient with head injury with cerebral edema.
Although some studies suggest safe use of 3% NaCl through large bore peripheral cannula (16–20 G) at maximum rate of 50 mL/hour.10 In one prospective study patients were subjected to 3% hypertonic saline (HTS) infusions for prolong time for several days which resulted in infiltration (6%), and thrombophlebitis (3%). These studies indicate that concern regarding peripheral intravenous (PIV) 3% HTS infusion may be exaggerated. Further it carries a low risk of serious limb, or life-threatening complications, and hence the committee recognizes that a patient can be subjected to it if the benefit outweighs the risk.11 However, further research is needed for safe peripheral infusion of 20% mannitol, and this issue was unresolved.
Potassium infusion
Potassium infusion of maximum concentration 10 mEq/100 mL can be given via peripheral IV lines. Replacement via CVC may be required at higher concentrations and at higher rate for better fluid tolerance and rapid correction. Available potassium ampoule contains concentrated potassium solution at osmolarity of approximately 4,000 mOsm/L (2 mEq/mL of potassium). This needs to be diluted and infused slowly via central venous cannulation (maximum recommended concentration 20 mEq/50 mL). Administration through central cannula helps to thoroughly dilute the solution in blood stream and also decrease the risk of extravasations and avoid pain and phlebitis associated with peripheral administration.
Amiodarone administration
Practice of administration of IV amiodarone infusion also depends upon institutional protocols and has many issues. Injection amiodarone has pH of 3.5–4.5 which is highly irritant to peripheral veins. Amiodarone has tendency to form precipitates at normal pH of blood and responsible for thrombophlebitis when infusion given through peripheral vein. For these reasons when amiodarone infusion is required for more than 1 hour and at concentration of more than 2 mg/mL, central venous cannulation is prescribed by some institutions.
CVC can be considered for other drugs such as continuous infusion of sodium bicarbonate solution (osmolality of ~2000 mOsm/kg), continuous infusion of >20% dextrose infusions (osmolarity > 1200 mOsm/L), etc. An institutional protocol for such infusions is recommended.
CONCLUSION
In general CVC is indicated for vascular access, vasopressor infusions, hyperosmolar and irritant infusions. However, the drafting committee unanimously agrees to the fact that, whenever a CVC is planned, documentation of its indication is necessary to be maintained in clinical records and benefit of placing a CVC should outweighs its risk.
Recommendations.
We recommend CVC after documenting its indication [A, 3].
We suggest CVC when hyperosmolar and locally irritant agents are to be administered [B, UPP].
We recommend CVC's use for vasoactive drugs unless the risk outweighs benefit of placing a CVC and delaying the therapy [A, 3].
CVC Catheterization in Locations other than ICU
Evidence Statement
Central venous catheterization is done frequently in many other locations like emergency rooms (ER), operation theaters (OT), cardiac catheterization labs (Cath Labs), procedure rooms, etc. The practice and various outcomes related to CVC vary widely according to the user location in hospital.
CVC placement in emergency department
Central venous catheter placement in ER is used for various indications such as resuscitation of sepsis in patients to guide CVP and ScVO2 monitoring,12-14 hyperosmotic infusions and difficulty in securing a peripheral venous access.15 However, in the recent years, growing evidence has questioned the validity of all of the above-mentioned indications in ER.
Recently performed major trials have challenged the role of EGDT refuting the routine monitoring of CVP and ScVO2 while resuscitating a septic patient.16 Likewise, assessment for preload and its responsiveness has moved far from CVP.17 There is also the fear of breach of infection control practices in an emergency and a possible higher incidence of CLABSI.18,19 The greater use of ultrasound for peripheral intravenous access has further obviated the need for central lines20 in instances of difficult access.
Also as evidence have been evaluated in previous section of indications, it seems prudent to state that hyperosmolar or vasoactive infusion are also fairly safe while administered in appropriate dilution and for short periods through a well-placed wide bore peripheral venous access.21,22
Most of the available literature with regards to CVCs placed in the ER is datasets which are not globally representative. Needless to say, the outlook for CVC practice in an ER depends on various factors, notably, the ER infrastructure, staffing pattern, experience of emergency physician, presence of SOPs, etc.23 Generating a strong evidence-based guideline as to whether placing CVCs in the ER is warranted, is virtually impossible. While the importance of the “Golden Hour” and “Initial Stabilization” cannot be neglected, practices and concepts have perhaps moved away from the CVC for reasons discussed earlier.16-22
Moreover, the paradigm shift from landmark guided to USG-guided cannulation24-26 and requirement for stringent infection prevention measures27 raises caution in resource limited and emergent settings.
It may be prudent to avoid CVCs in the ER, unless strongly indicated. The same applies to CVC placement in other areas including ICU. Those units which perform central venous cannulation should have a rigorous quality improvement program with ongoing training and follow-up of outcomes.19,28,29 Due consideration should be given for prompt removal of an emergently placed CVC if breach of aseptic precautions have occurred.30
CVC placement in ward
Placement of central venous catheters in the ward/rooms is fraught with multiple challenges including—(1) monitoring during insertion, (2) adherence to infection prevention bundles, (3) availability of bedside ultrasound, (4) availability of trained assistant/nurse, etc.
Furthermore, ambulatory patients present with more unique challenges to post-insertion care. The common indications in this patient population are prolonged antibiotic therapy, difficult peripheral venous access, or for chemotherapy.31 We presume that the complication rates, both infective and non-infective could be higher for CVCs inserted outside acute care areas as no large studies or database exists to support this conviction. Surveillance and benchmark data for out-of-ICU CLABSI are gradually emerging.32
Site of insertion should be guided by factors such as (1) level of care, (2) resource availability, (3) patient population, etc.
At this time point, it may be difficult to restrict CVC insertion to acute care areas. CVCs may be inserted in the wards provided all necessary precautions are strictly adhered to.33 Daily documentation for the need to continue CVC is to be mandated as a means of avoiding unnecessary line days and CLABSI.34,35 Recommendations pertaining to monitoring, infection, prevention and management, audit tools and medicolegal liabilities described elsewhere in this document will apply universally even to CVCs placed in the wards.
CVC placement in procedure rooms
There is insufficient evidence at this juncture to suggest if CVC placement in dedicated procedure rooms has clinical implications. The whole idea of procedure rooms revolves around the concept of providing a comfortable and organized environment where aseptic procedures can be safely performed. However, standardized infection control practices, equipment, and monitoring facilities are mandatory for procedure rooms.36-39
CONCLUSION
Central venous catheterization is done frequently in many other locations like emergency rooms (ER), operation theaters (OT), cardiac catheterization labs (Cath Labs), and procedure rooms etc. Institutional policy with standard operating procedure (SOP) should be available. Adequate preparation and training is desired for all those involved in insertion and maintenance of CVCs.
Recommendations.
We recommend that all site of care, where CVC is utilized should have a central venous cannulation and maintenance SOP in accordance with recommendations made in this document [A, UPP].
We recommend that all units performing central venous cannulation should have a quality improvement program in place with follow-up of outcomes [A, UPP].
We recommend that daily review for the necessity of CVC should be done in the wards [A, 2].
Central Venous Catheter Site Selection
Evidence Statement
As already mentioned, CVC can be placed in veins in the neck (IJV), chest (SCV or axillary vein), groin (femoral vein). These sites can be selected on the basis of ease of placement, individual expertise and on the associated procedural risk, and other clinical variables. We examined the evidences for comparison of each site.
Site Selection in Emergent Conditions
3SITES study, a RCT, randomized approximately 3,000 patients to different groups to have CVC placed in the subclavian, jugular, or femoral veins.40 They concluded that lines in SCV were associated with a lower CRBSI risk and thrombotic complications then lines in IJV or femoral vein. Simultaneously, they also found that SCV lines were also associated with highest mechanical complications among the three sites. SCV lines are associated with three times more risk of pneumothorax then the IJV option, whereas femoral lines eliminates pneumothorax risk, and comparable to the IJ in infection risk, but has significant DVT risk.
Although, SCV lines are safer in terms of infection and thrombotic complication risks but have commoner mechanical complications which may have serious consequences.41 Although choosing SCV is preferable for any catheter intended to be used for more than 5 days, but in emergent conditions such as severe hypoxia or coagulopathies, femoral CVC are associated with an acceptable complications rate, especially if strict aseptic techniques are used while insertion.42
IJV versus subclavian central venous cannulation
While comparing IJV and SCV sites for CVC cannulation the committee reviewed ease of access, mechanical complication rates, malposition and its complications, and infectious complications closely.
Literature review concludes that in comparison to SCV, IJV access is much easier and less technically demanding with the use of USG guidance.41 Malpositioning with IJV approach is less common then in SCV approach.43 Simultaneously, a meta-analysis by Ruesch et al. including six trials, 1,299 catheter, conclude that malposition events were significantly less common and less serious with jugular approach.44 In same analysis, SCV approach had less frequent accidental arterial punctures then IJV. However, control over carotid artery bleeding is easier than subclavian artery. In this analysis, hemothorax or pneumothorax complications were similar with both IJV and SCV approach. However, in other recent prospective study by Iovino et al. there was significantly low risk of pneumothorax in IJV approach then in SCV approach. These results are similar to the results of three sites study where mechanical complications were far more common with SCV then IJV.40,45,46
Thrombosis of IJV was more common than thrombosis of SCV. Large bore CVC insertion has increased risk of thrombosis and subsequent occlusion especially in subclavian vein.
As quoted before, 3SITES study concluded that infectious complications were less with SCV than IJV and hence, the SCV approach is preferred for CVC placements.
IJV and SVC versus femoral lines
As the local flora density is different in each common site of insertion for CVC, site selection also influences the risk of infection. In a randomized study of 270 catheters found that femoral site has a higher colonization rate in comparison to SCV (RR: 6.4 [95% CI: 1.9–21.2] but without any increase in BSI (RR: 2.0 [95% CI: 0.2–22.1]). However, multivariate analysis of several prospective studies reported frequent infectious complications when using femoral or IJV sites.47
The subcutaneous course of the SCV CVC is longer than for the other sites and it has lowest bacterial bio-burden and it is better protected against dislodgement of dressing. SCV catheters are also associated with less chances of venous thrombosis.48
Femoral catheterization was associated with a higher incidence rate of overall infectious complications, as well as of overall thrombotic complications and complete vessel thrombosis.
In this study, SCV site was associated with less infectious complications (1.3 compared to 2.7 per 1,000 catheter days for other sites, incidence density ratio 0.50, 95% CI (0.33–0.74), p < 0.001).48
Incidence of mechanical complications—pneumothorax in subclavian catheterization:
Subclavian vein catheterization was associated with a higher risk of pneumothorax than femoral vein catheterization.40
Special situations
Severe coagulopathy
During SCV catheterization, bleeding from the SC artery is very difficult to control by compression and often it goes undetected because hematoma may track into the mediastinum or pleural cavity. Hence, SCV is generally the least suitable approach for CVC in patients who are on anticoagulant therapy, and other sites may be preferred.49
Patients with burn, extensive skin loss and superficial infections
In case of burn patients, extensive skin loss and superficial infections, and site of CVC insertion should be preferred where skin is intact.
In a study, even femoral approach for CVC placement does not increase the incidence of CVC colonization in massive burn patients. However, use of anti-microbial impregnated CVCs may be helpful in reducing colonization.50
CVC in adult neurocritical care patients who are at risk of high intracranial pressure
Many practitioners used to believe that IJV CVC can increase the risk raised ICP in neurocritical care patients. Major physiological reason is that right side IJV serves as the major drainage point for cerebral blood flow, and any kind of obstruction by thrombosis or hematoma or vascular spasm can lead to reduced cerebral blood drainage, poor CSF absorption in cerebral venous blood and so raised ICP.51,52
A study Goetting et al. failed to demonstrate any significant rise in ICP with use of jugular bulb catheterization in 37 pediatric neurocritical care patients many other small studies demonstrated the safety of unilateral IJV CVC in neurocritical care patients.53
CONCLUSION
The committee recognizes the fact that emergent situation can arise at any location and all due precautions should be taken to maintain a sepsis. We suggest that femoral line is preferred and safer route for CVC insertion during emergency situation for a short-term use.
In elective scenarios, SCV insertion has an edge over other sites as it has low risk of infectious and thrombotic complication. The expected duration of catheter use is also important, as cumulative risk of infections and vessel thrombosis increases with increasing number of days.
One should consider the fact that mechanical complications can be controlled by use of USG guidance and physician experience but infectious and thrombotic complications cannot be diminished. Thus an ideal site for CVC insertion does not exist and decisions for the choice of insertion site should be considered on a case-to-case basis by the operator.
For neurocritical care patients, current evidence does not reject use of unilateral IJV CVC.
Recommendations.
We recommend in emergency scenarios, insertion site selection should be based on clinical need, patient factors, practitioner judgment, experience, and skills [A, 3].
We suggest subclavian insertion site should be preferred over IJV and femoral for central venous catheterization to decrease infectious and thrombotic complications [B, 2].
We recommend subclavian vein to be avoided in patient with coagulopathy, distorted anatomy, and who may have high chances of mechanical complications [A, 2].
We recommend that in case of burn, extensive skin loss and superficial infections, CVC insertion should be done where the skin is intact [A, UPP].
We suggest internal jugular CVC lines could safely be inserted in adult neurocritical care patients [B, 2].
Catheter Selection
Evidence Statement
Central venous catheters have physical variations like length, coating, number of lumens, and types of material. The committee here reviewed the relevant literature to suggest its recommendations.
Central venous catheters are commercially available from single lumen to five lumens for various needs of patients. This fact that there may be positive correlation with infection risk to number of lumen has been evaluated in many observational studies. Hilton et al. (1988), they compared infection rates of 502 catheters and found out that infection rate of single lumen catheter was 8% whereas triple lumen catheter was 32%.54 In another randomized observational study, a total of 204 patients and CVC catheters were observed for infectious outcomes. Only 177 were able to complete 7 days of therapy, among those 78 patients were randomized to single lumen catheter and 99 patients were randomized for triple lumen catheters, for administration of total parenteral nutrition. Incidence of catheter-related sepsis was 2.6% in single lumen to 13.1% in triple lumen.55 In other prospective, observational studies reported here the researcher ultimately concluded that number of lumen have a correlation with infectious complication with CVC.56,57
CONCLUSION
After reviewing the relevant literature the committee suggested that CVC with minimum number of lumens required should be inserted.
Recommendations.
We suggest using a CVC with minimum number of lumens needed for patient management whenever feasible [B, 3].
No recommendation can be made for a dedicated lumen for parenteral nutrition. Unresolved issue [B, 3].
CVC—INFECTION CONTROL
Site Selection
Evidence Statement
As discussed in previously, infection is one of the most important care defining outcomes.58,59 Infection control strategies ranges from the modifications in insertion technique to use of USG and selecting a site less prone to colonization and infection.60 As this is one of the challenging task to reduce CL-related BSI in ICU, the committee here examined and complied various practice- related topics to reduce infectious complications in CVC.
As various evidences examined in subheading C of Section 1, it is by now clear that SCV approach have least risk of local skin and CVC colonization and also related BSI.47 This may be attributed to longer subcutaneous course or lowest bacterial bio-burden related to SCV approach.48 Femoral approach has a higher incidence rate of overall infectious complications and of major infectious complications.48 However, the preferred site for the placement of a central venous catheter depends on multiple factors such as availability of the selected site, operator expertise, infrastructure and availability of ultrasound, clinical urgency, and risk factors for complications like coagulopathy, pneumothorax, etc.
In 2011, the Centers for Disease Control and Prevention (CDC) had given a class 1A recommendation against the use of femoral vein for central venous cannulation to decrease the risk of CRBSI.30 However, a systematic review and meta-analysis by Paul E Marik questioned the grading of this recommendation and concluded that there was no difference in the rates of CRBSI between the three commonly used sites for CVC placement.61 In 2015, a French multicenter randomized controlled study comparing the three different sites suggested a reduction in the incidence of CRBSI when subclavian vein was used as compared to the femoral vein. This trial also highlighted the higher risk of pneumothorax with subclavian vein cannulation, a finding similar to other studies.40 A recent meta-analysis by Kostoula Arvaniti showed inconclusive evidence on the CRBSI risk for various sites.62
CONCLUSION
In light of recent evidences suggesting reduced or comparable infectious complication with femoral approach, the committee still upheld the previous view of discouraging femoral lines to reduce CRBSI burden. As the supporting data is pooled form western world and its relevance in Indian scenarios is very questionable. After a thorough discussion and evaluation of all the available literature, the committee was able to formulate following recommendations related to site selection in context to infectious complications.
Recommendations.
We suggest to evaluate risk-to-benefit ratio of infectious and mechanical complications before choosing a particular site for catheter insertion [B, 2].
We recommend to avoid using femoral vein for the routine placement of central venous catheters [A, 2].
Hygiene Practices, Barrier Precautions, and Skin Preparation
Evidence Statement
Standard hygiene practices, barrier precautions and preparation before inserting a CVC is one of the most crucial step towards infection control.
Hygiene practices
Hand hygiene and aseptic techniques, before insertion or during maintenance of CVC, is most important for prevention of infections.63 Adequate hand hygiene can also be achieved by the use of either an alcohol-based hand rub (ABHR)64 or with standard soap and water rinsing.65-67
Barrier precautions
Maximum sterile barrier (MSB) precautions are primary requirements during each CVC placements. MSB precautions are defined as wearing a cap, sterile gown, sterile gloves and face mask, and also using a full body drape (similar to the drapes used in the operating room). MSB have been compared in a RCT with small drape and sterile gloves, where MSB has been proven to better in term of catheter colonization and CRBSI rates. With MSB-CRBSI developed much later with gram-negative organisms C/F to sterile gloves and small drape group.68 Simultaneously, many other studies revealed that MSB help in reducing skin colonization, and ultimately reduced CSB.69-72
Skin preparation
Study by Yasuda et al.73 compared 3 groups, 0.5% alcohol/CHG solution with 79% ethanol, 1.0% alcohol/CHG solution with 79% ethanol, and 10% aqueous povidone-iodine (PVI). In this study catheter-tip colonization incidence (per 1,000 catheter-days) was found to be 3.7, 3.9, and 10.5 events, respectively (p = 0.03). Pairwise comparison between groups showed that the risk was significantly higher in the 10% PVI group. However, there were no significant differences in colonization risk in both CHG groups. Catheter kept for ≥72 hours had a greater risk of colonization in the 10% PVI group than that in the 0.5% CHG group. However, there were no statistical significant differences for probability of developing CRBSI among the groups.73
Current CDC guidelines for preventing CRBSI published in 201130 also recommend skin preparation with >0.5% chlorhexidine gluconate solution with alcohol before insertion of a CVC.
CONCLUSION
Needless to state, why these precautions are now standard of practice worldwide? These practices have become the basic ethics of procedural interventions. With respect to CVC and prevention of infectious complication these practices along with education and training makes the corner stone of CRBSI prevention bundle. Other contemporary international literature also recommends its mandatory implementation before trying any novel approach to reduce CRBSI rates.
Recommendations.
Hygiene practices
We recommend mandatory hand hygiene practice, either by washing hands with conventional soap and water or with ABHR, before and after any interventions or contact with CVC [A, 2].
We recommend maintaining aseptic technique for insertion and maintenance of CVC [A, 2].
Barrier precautions
We recommend MSB precautions before any insertion (de novo or exchange over guidewire) of CVC [A, 1].
We recommend wearing either clean or sterile gloves when handling or dressing the CVC [A, 3].
Skin preparation
We recommend preparation and cleaning of the skin with an alcoholic chlorhexidine solution containing a concentration more than 0.5% chlorhexidine and 70% alcohol before CVC insertion and during dressing changes [A, 1].
We suggest to use tincture of iodine, an iodophor, or 70% alcohol use as alternatives if chlorhexidine is contraindicated [B, 3].
We recommend allowing the skin antiseptic being used to dry completely before catheter insertion [A, 2].
CVC Fixation
Evidence Statement
Traditionally, short-term CVCs are fixed to skin with the help of sutures. Skin sutures are responsible for local microbial colonization and later on infectious complications. Commercial devices are available for securement purpose like anchor devices, staples, catheter holders, adhesive tapes, locking device, etc., hence it needs a critical evaluation to compare them in terms of related complications, safety, and feasibility.
In 2015, Cochrane review for dressing and securement devices, 22 studies with 7,436 patients were included.74 These patients had a CVC and nine different types of securement device or dressing. After a multiple treatment meta-analysis, in this review authors concluded that suture less securement devices are likely to be the most effective at reducing CRBSI though the quality of evidence is very low and most of these studies were conducted in intensive care unit (ICU) settings.
A common perception indeed exists that infections risk are low but malposition and dislodgement risks increases with suture less systems. A recently published international RCT, 186 patients who were treated with help of a CVC, randomized to receive suture (n = 87) or suture free (n = 97) securing device.75 They were analyzed for CVC migration and unplanned CVC removals. This study concludes that these two systems performed similarly.
Similarly in a Spanish RCT, study group found that complications rate was higher with suture group (47.2%) versus suture less group (21.3%), and also there were significantly higher local complications like signs of infection, oozing and CRBSI in suture group. Also dressing change for local bleeding was lesser in adhesive device group and staff preference was very high towards adhesive device.76
In another interesting in vitro study, comparing various suturing methods only the observer found that “finger trap fixation” by suture technique increases dislodgement force significantly.77
CONCLUSION
We recognize that all the presented literature has an element of significant observer bias and there is no concrete and consistent data supporting or refuting suture based or suture less technique in all clinical contexts, and further good quality research is needed to evaluate this aspect of care. In presently available literature, at one side infectious and local complication were lower with suture less techniques, on another side risk of malposition and dislodgement increases without sutures. In such a scenario where dislodgement is one of a serious concern then “finger trap fixation” by suture can be considered.
Recommendation.
No recommendation can be made for preference of securing system in each setting and an operator or local practice-based decision should be taken into consideration [B, 3].
Port Utilization and Maintenance
Definition of hub refers to the end of the CVC that connects to the blood lines or cap.
The hubs on CVCs are a common source of bacterial colonization and can result in CLABSI.78
There are two important aspect of care, to reduce intraluminal contamination; one is to ensure MSB precautions during catheter insertion and two is adequate hub disinfection prior to administration of intravenous medication.79,80 Adequate hub disinfection here refers to, rubbing hub for at least 10 seconds with chlorhexidine, povidone iodine, an iodophor, or 70% alcohol followed by a drying time of 30 seconds.81 Study by Helder et al. demonstrated 35% nurse's compliance with the 30 seconds drying time after hub disinfection.82
For prevention of hub contamination, an antiseptic barrier cap was developed. This device optimizes needleless connector disinfection via cleaning of catheter hub, through keeping it in continuous contact with disinfectant.83,84
CONCLUSION
For prevention of late CRBSI, along with other preventive interventions, disinfection of hub is an important step. Hence, it is widely advised to disinfect hub in contemporary guidelines and literature. Here also committee decided to make recommendation for port utilization and maintenance.
Recommendations.
We recommend disinfecting hubs, needleless connectors, taps, and injection ports, before accessing catheter, with 70% alcohol or an alcoholic chlorhexidine preparation [A, 2].
We recommend wearing either clean or sterile gloves when handling the hub and catheter [A, 3].
Prophylactic Antibiotics and Antiseptics
Evidence Statement
The committee here in this section attempt to provide evidence based approach for infection prevention and maintenance.
Extra-luminal [skin flora] source predominantly causes CRBSI during short-term [5–7 days] use of central venous catheters.85-91 Thus, its logical that measures like maximizing skin antisepsis and preventing contamination during insertion remains the most important step to prevent CRBSI. Povidone-iodine or polysporin triple ointment application at HD catheter site [after insertion as well as after each dialysis] has been shown in multiple studies to prevent exit site infection and CRBSI.92-95 Cochrane meta-analysis published in 2016 could find very low quality data in support of skin antiseptics used for CVC catheter care, with chlorhexidine gluconate better than povidone-iodine. However, authors’ concluded that this low-quality evidence is insufficient at the moment to recommend use of antiseptic solutions for routine care of CVC site. Use of Mupirocin at local site is discouraged, as study documented high incidence of resistance among skin flora, especially staphylococci, in the unit after its routine use for this purpose.96 Caution advised with use of any ointment containing polyethylene glycol as base at catheter exit site, at its interaction with catheter material is known to weaken the catheter, leading to spontaneous rupture of the catheter.97,98
Daily chlorhexidine bath to decontaminate skin of ICU patients has been shown to reduce CRBSI and is strongly recommended as cost-effective measure aimed at CRBSI prevention.99-104 Chlorhexidine soaked sponge or polyurethane dressing at insertion site to prevent penetration of skin flora to CVC exit site has been proven to reduce catheter colonization and CRBSI.105-111 However, use of sponge at exit site in patients treated with daily chlorhexidine bath in ICU as add on intervention is questioned.112
Beyond second week the intraluminal microbiological source predominantly causes CRBSI. Contamination of catheter hub(s) is common source of colonization and subsequent intraluminal biofilm formation. One study on intraluminal bacterial biofilm formation showed up to 15% surface has biofilm in less than ten days, but up to >40% surface if left in situ for >30 days.88 The incidence and incidence density of late-onset CRBSI in long-term oncology catheters is 22.5% and 1.6/1,000 catheter days in tunneled vs. 3.6% and 0.1/1000 catheter days in totally implanted catheters [ports], pointing finger at effect of handling the catheter hub as a key risk factor for hub contamination. Improper handling of hubs and difficult to clean needleless port valves has been proven to increase CRBSI. Emerging evidence shows role of needle less connectors in reducing CRBSI rates. Antibiotic lock solutions, though effective in treating uncomplicated tunneled long term CVC catheter CRBSI, are not recommended for prevention of CRBSI due to fear of emergence of antibiotic resistance with few exceptions.112-117 In patients with long-term tunneled catheters, who have limited venous access and repeated CRBSIs despite adequate preventive measures and are at high risk of sequela from CRBSI like recently implanted intravascular devices, such as prosthetic heart valve or aortic graft, pacemaker or AICD, antibiotic lock may be used as a CVC catheter salvage method. Here adequate CRBSI prevention measures include at least three measures, namely education about CRBSI bundles to staff who insert and maintain CVC, use of maximal aseptic precautions during insertion and use of >0.5% Chlorhexidine in alcohol solution for CVC site preparation.
In absence of distant infection causing blood stream infection, seeding from blood stream is an uncommon mechanism of CRBSI. Antibiotics Injected systemically thus unlikely to have significant concentration to alter skin or exit site flora [causing early CRBSI] or within the intraluminal or extra luminal biofilm [causing late CRBSI] to have any effect in preventing CRBSI. This has been proven in multiple studies, that prophylactic antibiotics injected at the time of insertion or continued use during its use is unlikely to reduce CRBSI.118-122 One Cochrane review showed low-quality evidence on use of prophylactic antibiotics in cancer patients with long-term implantable catheters, neutropenia post-chemotherapy with baseline CLABSI rate >15%.123 However, this evidence should not be extrapolated to routine ICU patients not having any of these high-risk conditions for whom prophylactic antibiotics if used will be ineffective and promote antibiotic resistance in the unit.30,124-126
CONCLUSION
During this evaluation of literature the committee was able to reach a consensus on many daily practice points, and recommendation made here in these sections can be bundled together for routine CVC care in many patient care settings.
Recommendations.
No recommendation can be made for or against the use of antiseptic solutions [Aqueous chlorhexidine or aqueous povidone-iodine] for routine CVC site care [A, 3].
We recommend the use of chlorhexidine soaked sponge or dressing at the catheter exit site to prevent CRBSI [A, 1].
We recommend daily chlorhexidine bed bath (sponging) for patients in ICU to reduce CRBSI incidence [A, 1].
-
We suggest use of antibiotic lock solutions to prevent CRBSI only in selected conditions as follows [B, 2]:
– Limited or difficult venous access and a history of recurrent CRBSI
– At high risk of severe sequela from a CLABSI (e.g., recently implanted intravascular devices, such as pacemaker or AICD, prosthetic heart valve, or aortic graft).
– When CRBSI rate is high despite all measures to reduce it are implemented stringently.
We recommend against systemic intravenous antibiotics use for prevention of CRBSI [A, 1].
Removal of Central line
Evidence Statement
Catheter colonization and intra- or extra-luminal biofilm increases with each passing day.127,128 Thus CVC should be removes as soon as need ceases, however, routine change or relocating the central lines is not recommended. Removal of the central venous line also involves risks of air embolism, catheter fracture and embolism, dislodgement or thrombus or fibrin sheath hemorrhage, scars in addition to pain and discomfort during removal. Trendelenburg's position, use of Valsalva maneuver, application of pressure at puncture site long enough to collapse the tract followed by application of occlusive dressing and monitoring the patient for complications for reasonable period is required after removal of central venous accesses. A CVC removal protocol and training is desirable.129
CONCLUSION
Although literature related to removal of central line protocol is scarce and mostly based upon expert opinion and low-quality observational studies, the committee unanimously agrees to recommendation present below, and considers them as useful practice points.
Recommendations.
We recommend removing central venous accesses as soon as its indication ceases [A, UPP].
We suggest not having policy to routinely replace relocate the central venous lines unless clinically indicated [B, UPP].
We recommend each institute to have central venous catheter removal protocol and only staff trained in the same to remove central venous accesses [A, UPP].
Catheters Impregnated with Antiseptics and Antibiotics
Evidence Statement
For preventing CRBSI, several different variations of antibiotic- and antiseptic-coated catheter have been marketed in previous years. These coatings include silver, platinum/silver, CHG-silver sulfadiazine of first and second generation, minocycline/rifampicin.
Silver- and platinum/silver-coated catheters have been studied in comparison noncoated catheters where equivocal results were produced for catheter colonization.130-134
Chlorhexidine gluconate/silver sulfadiazine (C/SS) coated catheter were studies in many studies and analysis.135-140 First generation of such CVCs had coating only on outer surface whereas second generation additionally had CHG inner coating extending till the hubs. Second generation devices have higher CHG concentration on outer surface. There are several RCTs and meta-analysis that shows superiority of second generation over first generation and first generation over noncoated CVC in terms to prevent colonization; however, these studies produced equivocal findings for CRBSI.138-140
Minocycline/rifampicin (M/R) impregnated catheters with coating on both sides, studied in comparison with first generation C/SS-coated CVC, found to be having low colonization and CRBSI rates.141-143 The beneficial effect begins after 6 days of catheterization. In a recent before and after study comparing M/R and second generation C/SS catheter also found that M/R CVC significantly reduced colonization and CLABSI rates.144 However, the study reported no significant change in microbial profile and no increase in resistance pattern.144
Gold salt preparations (Auranofin) releasing CVC have been developed. It claims to be having antibacterial and antibiofilm property.145 However, pending human trials these cannot be recommended at any point soon.
CONCLUSION
Plethora of literature suggests antibiotic/antimicrobial coated CVC reduces CRBSI rates, however, studies also suggest that maximal benefit is seen after the sixth day and more so in a long-term CVC. Using these devices although seems lucrative in first flush, but this comes with serious cost implications and apprehension of developing antibiotics resistance. Committee stresses on the fact that any novel approach cannot substitute for basic infection control practices, hence recommend use of antibiotic- or antiseptic-coated CVC only when CLABSI rates are not comparable to benchmark even after successful implementation of such strategy.
Recommendation.
We recommend using M/R- or C/SS-coated CVCs when catheter is expected to be in use for more than 5 days and the CLABSI rate is not decreasing to the institutional target benchmark even after implementing comprehensive strategy program. Comprehensive strategy should include education and training, maximal barrier precaution and aseptic skin preparation while insertion of CVC [A, 1].
PREVENTION OF MECHANICAL COMPLICATIONS
Role of Sonography
Evidence Statement
US-guidance facilitates safe CVC placement.146 There are concrete evidences now, that USG offers advantage of safety and quality during IJV-CVC placement. However, for the SCV and femoral routes, the gain of safety and quality is very small.147
A meta-analysis and systematic review by Lalu, et al. comparing US and landmark technique for SCV catheterization and concluded that USG-guided SCV catheterization reduced the frequency of adverse events compared with the landmark technique.148
However, it might be technically difficult to prove benefit of USG for CVC placement in the subclavian vein, because US-guided approach is technically more challenging. USG shows less benefit for the femoral route as mechanical complications other than arterial puncture occur infrequently. Altered anatomy of femoral vein often demands USG guidance.
The American Society of Echocardiography and the Society of Cardiovascular Anaesthesiologists in 2012149 strongly recommended real time US-guided CVC insertion, however, the level of evidence for SCV and femoral CVC insertion was not as strong as that of IJV.
Similarly, the Association of Anaesthetists of Great Britain and Ireland also recommends US-guided CVC placement in IJV. This document recommends US-guided CVC placement for other sites as well but recognizes that evidence, at present is limited for them.150
CONCLUSION
The committee unanimously agrees that USG guidance improves procedural safety. The evidence for same is strong for IJV access and relatively low for SCV and femoral access. Although the technology needs further dissemination and many practitioners need to acquire the US skills. The committee also recognizes the fact that in a resource-limited setting where stringent legal issues exist in acquiring US technology, it is not prudent to recommend it for every clinical situation and for every type of healthcare setup. Hence recommendation in favor of US use is rewritten in accordance with our local medicolegal obligations.
Recommendation.
Wherever available we recommend US guidance to improve success rate, patient safety and procedural quality and reduce mechanical complications during CVC placement.[A, 2]
Guidewire Exchange
Evidence Statement
CVC can either be inserted “de novo” or placed by guidewire exchange (GWE). In spite of availability of real-time US guidance, there are certain conditions where central vein patency is diminished in ICU patients. In those few selective conditions, it may be necessary to exchange CVC using a guidewire.151-155
Shimada et al.156 described a technique for catheter exchange where an outer sheath is placed over an existing catheter, subsequently the sheath removed after placement of a new catheter.
Whereas in guidewire exchange, a guidewire is passed through the distal port of the CVC and old catheter is removed, while a new one is placed over the existing guidewire.157
Cook et al. included 12 randomized control studies in their meta-analysis and assessed CVC management through routine replacement of CVC over guidewire. This analysis failed to demonstrate any reduce rate of CRBSI and routine replacement over a guidewire is not recommended at least in functioning catheters and have no evidence of causing local or systemic complications.151 However, guidewire exchange is less discomforting and have significantly low mechanical complications.153 Exchange over wire can be of use in limited venous access scenarios, with high success rates and very low mechanical complication rates but should not be done in confirm or clinical suspicion of BSI, as tract colonization is the usual source of BSI.69,153 Coagulopathy is also one of the conditions warrant guidewire exchange for CVC placement in critically ill patients.
The ELVIS trial, a multicenter, randomized, double blind trial, had 16 participating ICU units and enrolled 1460 critically-ill patients that needed dialysis catheter replacement. A guidewire exchange does not pose significant risk for CRBSI but predispose for catheter dysfunction. This dysfunction is inability to maintain extracorporeal blood flow.158-160
CONCLUSION
Guidewire exchange technique may be useful in many clinical scenarios, where chances of mechanical complication can be very high such as in emergent situations, unavailability of resources such as USG, expert personnel, etc. The evidences in present topic suggest exchange can be used in these limited settings, only if there is no evidence or suspicion of BSI.
Recommendation.
We suggest exchange of malfunctioning CVC over guidewire in selected patients with no evidence of infection.[B, 2]
Tip Positioning
Evidence Statement
Primary central venous catheter (CVC) tip malposition is a common occurrence with incidence of around 6.8%.161 However, much higher rates of catheter malposition, of up to 10–30%, have been reported when the CVCs are placed without any radiological guidance.162-164
Correct positioning of CVC tip is vital to prevent complications associated with CVC insertion. The most dreaded complication of CVC insertion is cardiac tamponade, which is associated with high mortality.165,166 The complication is more frequent in distally placed CVC tips and may occur secondary to perforation of the vein or the cardiac chamber. The other common complication of intra-atrial tip positioning is arrhythmias. Hence, the US Food and Drug Administration (FDA) has recommended that the CVC tip should not be placed in or allowed to migrate into the heart.167 On the other hand, CVC catheters, if placed more proximally in the superior vena cava (SVC), increase the risk of venous thrombosis, migration and malfunction.168,169
It is prudent to check and document the tip position after CVC placement.170 Several methods have been tried to place the CVC tip in the correct position. These include clinician devised formulas like the Peres164 and Andropoulos,171 or the use of radiological techniques such as the chest X-ray (CXR), ultrasonography (USG), real-time fluoroscopy or the right atrial (RA) electrocardiography (ECG) and transesophageal echocardiography (TEE). Newer methods such as proximity of cardiac motion method, have also been tried to ensure the correct placement.172
Several landmarks on a CXR have been suggested as an ideal site for positioning the CVC tip, like the site between the fifth and sixth thoracic vertebrae173 and the site below the inferior border of the clavicles.174 However, these anatomic landmarks are not in the same plane as the SVC and hence, on a CXR, it results in a parallax effect, leading to significant errors in positioning of the CVC tip.162 Hence, an anatomical landmark, which is closer to the plane in which the SCV lies, has been suggested.163 Carina lies about 3 cm above the SVC/RA junction and can be easily identified in a CXR.175 Hence, CVC tips should be positioned above the carina, which can be considered as a ‘safe’ area.163
To prevent endoluminal injury, and resultant thrombosis and perforation, the course of the catheter should be parallel to the wall of the SVC and the tip of the catheter should move freely within the vascular lumen. Such a position is best achieved when right internal jugular vein (IJV) is used for CVC insertion as the catheter runs a straight course into the lower SVC. Chances of these complications secondary to CVCs abutting the catheter wall are more with the left-sided catheters. As the CVCs inserted from the left side must turn a 90° corner to enter the SVC, if the CVC length is short, the distal tip may get positioned against the lateral wall of the SVC. Hence, positioning the tip of such catheters in the lower segment of the SVC may be preferred, where it tends to lie parallel to the vessel wall.164
It is also essential to keep in mind that even after catheter placement; distal catheter tip may exhibit a range of movement, up to 2–3 cm, in majority of patients. This range of movement may depend upon several factors such as the site of insertion and the patient's body habitus. The position of the CVC tip also varies with respiration and position of the patient. Because of this, it may commonly migrate into upper part of SVC, IJV, RA, right ventricle, subclavian or the innominate vein. Hence, the position of the CVC and its distal tip should be frequently monitored as long as the catheter is in situ. 176
CONCLUSION
Tip positioning is vital to avoid many delayed mechanical complications of CVC. Post-procedure chest X-ray is considered a standard practice for tip positioning although other modalities can also be used for same purpose. The committee suggests chest X-ray for routine documentation and for complication surveillance.
Recommendations.
We recommend post-procedure, position of the catheter tip must be assessed [A, UPP].
We recommend IJ and SCV catheter tip should be placed in the lower one third of the SVC near the SVC/RA junction [A, 2].
We recommend the use of chest X-ray to assess the CVC catheter tip position [A, 2].
SURVEILLANCE
Infection Control
Evidence Statement
Surveillance of CVC-related infection control practices is important quality improvement strategy. In this section, the committee evaluated the literature and made its recommendation on various surveillance approaches. Checklist for insertion and maintenance phase of CVC was debated.
Routine removal
Several prospective observational studies found that more the days of use of central venous catheter higher the incidence of positive catheter tip culture.177-180 Nonrandomized comparative studies indicate that durations of CVC use has a positive correlation with rates of catheter colonization, and infection. These observation lead to routine removal of central venous catheter to prevent central venous catheter-related bloodstream infection (CRBSI). However, randomized controlled trials reported equivocal findings regarding differences in catheter tip colonization when catheters are changed at three versus seven day intervals.181,182 One recent multi-centric study observed that early removal of CVC even in diagnosed CRBSI did not decrease mortality.183 A subgroup analysis of two phase III, multicenter, double-blind, randomized, controlled trials examined the effects of early CVC removal (within 24 or 48 hours after treatment initiation) on the outcomes of 842 patients with candidemia. It found that early CVC removal was not associated with any clinical benefit.184 Another study of 78 patients with CRBSI caused by multidrug resistance antimicrobials reported increased mortality, if infected CVC was not removed.185
Catheter tip culture
Clinical findings alone are not reliable method for diagnosing device-related infection and most of these clinical findings are not enough sensitive or specific. Traditionally, culturing catheter tip has been advocated as a definite method to diagnose CRBSI.186 But practice of sending a tip culture is not used widely. Clinicians are mostly influenced by CDC and NHSN surveillance definitions for CLBSI as it does not consider TC results.187 There is reported poor positive predictive value of catheter tip cultures in making diagnosis of CRBSIs188 further guidelines limits tip culture sampling to high probability cases only thus may have decreased a practice of over-culturing of CVC tips.186
The trend of sampling a catheter tip is declining and only used in research scenarios. Although a tip culture is advised for diagnosis of CRBSI, in one study many users did not even started appropriate therapy for CRBSI while culturing catheter tips. Moreover, positive tip cultures are difficult to be interpreted as a source or outcome.189
Daily checklist to prevent CRBSI
In a quasi-control study, a total of 444 central catheters corresponding to 390 patients were observed. It was possible to observe a 54.5% decrease in the rate of central catheter infection when compared with the control group with the help of various prevention strategies.190 Another multi-centric study observed decreased incidence of CRBSI from 3.9 per 1000 catheter days to 1.0 per 1000 catheter days with the help of educational program teaching of hand hygiene, standards of catheter care, and preparation of intravenous drugs.191 A multimodal central line associated bloodstream infection (CLABSI) risk reduction strategy consisting of: 2% chlorhexidine in 70% alcohol solution for skin preparation before CVC insertion, standardized CVC insertion packs, CVC insertion guidelines, and nursing education regarding CRBSI care significantly reduced incidence rate ration of CRBSI. A systematic review and meta-analysis of 79 studies found that strict adherence to CRBSI prevention bundle reduced its incidence significantly from median 6·4 per 1000 catheter-days (IQR 3·8–10·9) to 2·5 per 1000 catheter-days (1·4–4·8) (IRR 0·44, 95% CI 0·39–0·50, p < 0·0001; I2 = 89%).192
CONCLUSION
After evaluating the relevant literature for surveillance of infection control, the committee unanimously agrees to the fact that following a bundle of highly recommended practices makes use and maintenance of CVC safer. It was also able to determine the fact that long recommended practice of culturing and defining CVC-related infection also needs timely modification, and so is followed in recommendations made by the committee.
Recommendations.
We recommend against routine replacement of CVCs to prevent catheter-related infections [A, 1].
We recommend prompt removal of CVC when it is not essential [A, 2].
We recommend against routine catheter-tip cultures for purpose of surveillance [A, 2].
We recommend that routine practice bundle (Appendix II) should be followed to reduce CVC-related infections [A, 1].
Surveillance of Mechanical Complications
Evidence Statement
Surveillance for mechanical complication is necessary as these may cause immediate and delayed threat to life. These mechanical complications are pneumothorax, hemothorax, malposition of CVC, arterial puncture and hematoma formation. Surveillance should be continued even, if CVC was tried but could not be secured.
Till date, the post-procedural chest X-ray has been considered as the reference standard to detect mechanical complications. Some studies suggest that it should not be considered a reliable procedure for detecting complications in the absence of clinical symptoms. In addition, reading of a bedside CXR alone is not very accurate to identify intra-atrial tip position. The exceedingly low complication rate after right internal jugular vein catheterization suggests that, to detect pneumothorax and intra-atrial malposition, routine post-procedure CXR is neither necessary nor accurate and causes delay until catheter use.
Due to the developing knowledge and techniques in ultrasound, it can be suggested that it would be a suitable method to replace CXR in the role of detecting pneumothorax and identifying CVC tip position.
Pneumothorax
Ablordeppey et al.193 in their systematic review and meta-analysis demonstrates that bedside ultrasound accurately identifies pneumothorax after CVC insertion. The sensitivity and specificity of ultrasound for identification of post-procedure pneumothorax was nearly 100% in most of the literature. Previous literature to this systematic review also states that the superiority of ultrasound when compared to chest radiography for pneumothorax detection.194-198
A meta-analysis done by Alrajab et al,199 who reviewed 13 studies, demonstrated a pooled sensitivity of 78.6% and specificity of 98.4% for chest ultrasonography (CUS), while these rates were 39.8% and 99.3% for CXR, respectively.
Recently a Prospective Observational Study (COMBUX-study)200 is in final phase of completion which is designed to compare bedside ultrasound with chest X-ray to detect CVC-related mechanical complications. This may likely to confirm the superiority of ultrasonography as a diagnostic modality to standard chest X-ray to detect various mechanical complications.
Thrombotic Complications
Central venous catheterized patients are at high risk for catheter-related thrombosis. Used routinely, ultrasonography with color Doppler imaging could detects venous thrombosis in 33% of patients in medical ICU patients and in approximately 15% of these patients the thrombosis is catheter-related.201
Arterial puncture
In a patient with normal blood pressure and normal arterial oxygen tension, arterial puncture is usually easy to identify by the pulsatile flow into the syringe and the bright-red color of the blood. However, in patients with profound hypotension or marked arterial desaturation, these findings may not be present. If location of the catheter in the vein is uncertain, measuring intraluminal pressure, with a transducer prior to dilation aids in recognizing arterial puncture.
Hemothorax
Perforations of arteries or veins inside the chest can result in hemothorax, if the perforation communicates with the pleural space. While the mediastinum has relatively little potential space, the potential pleural space is up to approximately 3 liters, as the lung is completely compressible. Clearly, a catheter that perforates an artery or a vein, and also perforates and communicates with the pleural space rapidly can result in life-threatening hemorrhage. Diagnosis can begin with a simple chest X-ray to locate the tip of the catheter. Injection of contrast into the catheter under fluoroscopic examination also may be helpful in determining the location of the catheter.
Catheter tip malposition/migration
Ablordeppey et al.193 in their systematic review and meta-analysis stated that in CVC malpositioning, bedside ultrasound will identify four out of every five malpositioning. Importantly, ultrasound provides results regarding catheter position and pneumothorax faster than chest radiography. They recommend that bedside ultrasound be used as first-line confirmation method to determine catheter malposition. If the CVC is found to be mal-positioned in a venous structure, the CVC can be expediently addressed without obtaining a chest radiograph first. However, if the CVC malposition is not detected by ultrasound and concern is high for malposition, such as in the case of multiple cannulation attempts or incomplete/inadequate ultrasound confirmation technique, chest radiography should be performed to rule out catheter malposition.
After review and analysis of the current literature, best practice for ultrasound-guided confirmation of CVC positioning includes a focused vascular and cardiac ultrasound with rapid non-agitated saline flush.
CONCLUSION
Given the relative benefits of ultrasound with respect to image feasibility and efficiency, this modality needs further dissemination and implementation into clinical practice. USG guidance for surveillance of complication needs learning curve and is very subjective and operator dependent. In present scenarios, the committee abstains from recommending USG for routine use of post-procedure surveillance.
Recommendations.
We recommend chest X-ray post-insertion of IJ AND SC central line [A, 2].
We suggest that ultrasound guidance can be used for early identification of mechanical complication [B, 2].
Education, Training, CQI initiatives and Audit
Evidence Statement: Education and Training
The committee while drafting this document felt strongly that CVC care cannot be improved if an education and training CQI initiative is not implemented.
It is essential to have well-organized training program for overall process and outcome improvement. Ample amount of data accumulated in past several decades that suggest that risk of colonization and infection reduces with standardized aseptic care.202-208 Data also suggest that rate of infectious and other complication increases with untutored persons handling the CVC.205,209 Proper staffing level and care also suggested being an important factor in overall quality of CVC care.210-221 Staffing with regular critical care nurses and reduced pool nurses improves CVC-related outcomes.222,223
CONCLUSION
The committee unanimously agrees to the fact that education and training on best practices for healthcare professionals is imperative for overall quality improvement. Committee finds it prudent to guide about appropriate staffing pattern and level of training.
Recommendations.
We recommend that a healthcare education and training program should be in place wherever CVCs are inserted and maintained for overall quality improvement [A, 1].
We recommend that a mechanism should be in place to assess knowledge and compliance with guidelines of all the personnel involved in care related to CVC [A, 1].
We suggest maintaining appropriate and adequate nursing level at all times to improve CVC-related outcomes [B, 2].
Evidence Statement: CQI Initiatives
In our country where CVC care practices are heterogeneous, the committee decided to revalidate and roll out the standards of CQI to help practitioner maintain a uniform practice. Also committee prescribed some measures to audit the process and outcome. Audit for infectious complications as a result of central venous cannulation should be a routine in any organization to prevent or at least reduce the rate of these complications.
Formulating and implementing a uniform evidence-based practice is high priority. However data suggest that CRBSI preventive strategy adherence in American hospitals is suboptimal.224,225 Even use of universally recommended strategy like hand washing, maximal barrier precaution and CHG skin preparation is suboptimal.30,226 Large amount of data in form of quality improvement studies has been published in past few years, which include research on various strategies such as education training, staffing, feedback program, organizational change.227-236 Several before and after studies and controlled trials for educational programs and training (e.g. training for maximal barrier precaution, site selection, line-care protocol, prompt removal strategy, etc.) reported significant improvement in CRBSI outcomes.205,232,237 Bundling multiple evidence-based approaches was also tested in many studies and had statistically significant improvement in CRBSI outcomes with an ease of implementation.205,231,232,238 This “bundle and checklist” approach for preparation, insertion, maintenance and removal also provides opportunity to improve performance, both at individual and unit's level and help in quality improvement.
Recommendation.
We recommend using institutional CQI initiatives with bundled approach for performance improvement [A, 2].
Evidence Statement: Audit Tools
Tools to detect CLABSI
For the purpose of standardization universally accepted National Healthcare Safety Network (NHSN), CDC definitions for BSI surveillance are used in this document and provided here in Appendix III.4 The committee recommends continuous audit and surveillance with these definitions to allow comparison with benchmark.1
Tools for performance measures
Standard tools for performance measures are accepted and adopted here in this document also for benchmarking and uniformity purpose. The internal reporting requirement tools are presented in, ‘strategies to prevent central line associated blood stream infections in acute care hospitals-2014 update.’33
Internal reporting is used to support internal CQI initiatives. These process and outcomes measures are derived from other contemporary literature and guidelines. These measures should be reported to internal stake holders such as clinicians, hospital quality administration and nursing leadership.
Recommendations.
We recommend conducting surveillance to determine CLABSI rates, monitor its trends and identify lapses in Infection Control Practices [A, 1].
We do not recommended routine culture of catheter tip for purpose of surveillance [A, 1].
DTTP is the recommended method of diagnosis for CVC-related infections in patients [A, 2].
We suggest recording the operator, date and time of catheter insertion and removal and dressing changes on a standardized form [A, UPP].
Consents and Medicolegal Issues
Evidence Statement
As with any other procedure in medical practice, CVC also have serious medicolegal implications. This section does not need any recommendation as these are governed by the law of the land of India, and absolute compliance to these laws is the only recommendation the committee could make.
Vicarious liability
Person who has been trained and qualified to perform central venous pressure would be responsible for procedure-related consequences. Trainee/nursing staff/technician-performing procedure must perform under supervision of trained person and he/she would be accountable under vicarious liability.
Contents of consent
‘Informed consent’ is mandatory.
Consent form must be filled by the doctor (one who either performs procedure himself or is a part of team who performs procedure), in one sitting, preferably without changing pen
Consent must be carried out in a manner and language the patient/proxy can understand.
Patient name and his identification number must be mentioned clearly
Consent must not be clubbed with consents for procedure(s) other than Central venous catheter insertion
Consent must mention medically recognized alternative measures relating to diagnosis or treatment, including measures that may be considered less desirable by the physician
Consent should include consequences of the patient's/proxy's decision to decline or refuse treatment
Consent must be taken from patient, however if patient in not capable to give consent it can be taken from proxy with mentioning of valid reason(s) for incapacity of patient to consent.
Credentialing
To limit the disparity and haphazard practice, and purpose of standardization it is mandatory that every institution has a policy and credentialing process. These can be flexible according to local needs, but absolute compliance to medicolegal issues and standard practice in accordance with this document and local medical council guidelines is suggested.
Recommendation.
We suggest that a structured credentialing process be in place for personnel involved in insertion and maintenance of CVC [B, UPP].
APPENDIX II
Routine Practice Bundle
Strict adherence to following practices to prevent catheter-related bloodstream infection (CRBSI) should be implemented for routine care and maintenance:
Comply with hand hygiene requirements.
Bathe ICU patients with a chlorhexidine preparation on a daily basis.
Scrub the access port or hub with friction for 10 seconds, immediately prior to each use with an appropriate antiseptic (chlorhexidine, povidone iodine, an iodophor, or 70% alcohol).
Use only sterile devices to access catheters.
Immediately replace dressings that are wet, soiled, or dislodged.
-
Perform routine dressing changes using aseptic technique with clean or sterile gloves.
– Change gauze dressings at least every 2 days or semipermeable dressings at least every 7 days.
-
Change administrations sets for continuous infusions no more frequently than every 4 days, but at least every 7 days.
– If blood or blood products or fat emulsions are administered change tubing every 24 hours.
– If propofol is administered, change tubing every 6–12 hours or when the vial is changed.
Promptly remove unnecessary central lines. Perform daily audits to assess whether each central line is still needed.
APPENDIX III
CDC and NHSN Surveillance Definitions
Primary bloodstream infection (BSI): A laboratory-confirmed bloodstream infection (LCBI) that is not secondary to a bacterial infection at another body site.
Secondary BSI: A BSI that is thought to be seeded from a site-specific infection at another body site.
Eligible BSI organism: Any organism that is eligible for use to meet LCBI or mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) criteria.
Catheter-related bloodstream infection (CRBSI): CRBSI attributed to an intravascular catheter by quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture specimens. This definition is primarily used in research.
Central line-associated BSI (CLABSI): It is an LCBI where an eligible BSI organism is identified and an eligible central line (CL) in a patient is present within 48 hours period before the development of the BSI, and that is not related to an infection at another site.
The case definition for the diagnosis of CLABSI (LCBI) as per CDC is as following (must meet one of the three criteria).
LCBI-1: Patient has recognized pathogen cultured from one or more blood cultures and Organism cultured from blood is not related to infection at another site.
LCBI-2: Patient has at least one of the following signs and symptoms: Fever (>38°C), chills, or hypotension, and
Signs and symptoms and positive laboratory findings are not related to infection at other site, and
Common skin contaminants are cultured from two or more blood cultures drawn on separate occasions.
-
LCBI-3: Patient of age less than or equal to 1 year has at least one of the following signs and symptoms: Fever (>38°C) core, hypothermia (<36°C) core temperature, apnea, or bradycardia, and
Signs and symptoms and positive laboratory findings are not related to infection at other site, and
Common skin contaminants are cultured from two or more blood cultures drawn on separate occasions.
Central Line
It is an intravascular access device or catheter that terminates at or close to the heart, or in one of the great vessels.
The line may be used for infusion or hemodynamic monitoring and may be inserted centrally or peripherally (PICC line).
Once a line has been designated a CL it continues to be a CL, regardless of migration (Migrated CL), until removed from the body or patient is discharged, whichever is earlier.
A non-lumened intravascular catheter that terminates at or close to the heart or in a great vessel which is not used for infusion, withdrawal of blood or hemodynamic monitoring is not considered a CL.
Eligible CL: The one that has been in place for more than two consecutive days (on or after CL day 3), following the first access of the central line, in an inpatient location, during the current admission. Such lines are eligible for CLABSI events.
Central line days: The number of days a CL has been accessed to determine if LCBI is a CLABSI.
Denominator count days: The count of CL on an inpatient unit that is recorded in the monthly denominator summary data.
Types of central lines
There are following three types of CL for CLABSI surveillance.
-
Permanent CL: It includes—
Tunneled catheters including tunneled dialysis catheters
Implanted catheters including ports.
Temporary CL: These are non-tunneled and non-implanted catheter.
Umbilical catheter: These are vascular catheter inserted through the umbilical artery or vein in a neonate. All umbilical catheters are considered as CL.
Access: It is defined as entering the CL with a needle or needleless device for infusion, withdrawal of blood, or hemodynamic monitoring.
The day of insertion is considered as day of access and hence, counted as first central line day.
If an outpatient patient comes with a CL already in place and it is the only CL in body, then the first day access in inpatient location begins the central line day count (CL day 1).
De-accessing any type of CL, e.g. removing the port needle but port remains in the body, simply does not remove the patient from CLABSI surveillance.
Acknowledgments
Dr Simant Jha, Dr Rohit Yadav, Dr Prasad Padwal, Dr Ashima Katyal, Dr Prajkta Wankhede
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.National healthcare safety network (NHSN) patient safety component manual. Jan’2020. https://www.cdc.gov/nhsn/pdfs/pcsmanualcurrent.pdf https://www.cdc.gov/nhsn/pdfs/pcsmanualcurrent.pdf Accessed from,
- 2.Nancy Moureau, Chopra Vineet. Indications for peripheral, midline and central catheters: summary of MAGIC recommendations. British Journal of Nursing, 2016. [DOI] [PubMed]
- 3.Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care. 2015;653(3):e-9e17. doi: 10.1016/j.jcrc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Medlej K1, Kazzi AA, El Hajj Chehade A, Saad Eldine M, Chami A, Bachir R, et al. Complications from administration of vasopressors through peripheral venous catheters: an observational study, J. Emerg Med. 2018;(1):54. 47–53. doi: 10.1016/j.jemermed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Lewis T, Merchan C, Altshuler D, Papadopoulos J. Safety of the peripheral administration of vasopressor agents. J Intensive Care Med. 2019;34(1):26–33. doi: 10.1177/0885066616686035. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas-Garcia J, Schaub KF, Belchikov YG, Narasimhan M, Koenig SJ, Mayo PH. Safety of peripheral intravenous administration of vasoactive medication. Journal of Hospital Medicine. 2015;10:581–585. doi: 10.1002/jhm.2394. [DOI] [PubMed] [Google Scholar]
- 7.Craig Cocchio. Do vasopressors really need a central line? Pharmacy times. 2016 Jan; [Google Scholar]
- 8.Boullata JI, Gilbert K, Sacks G, Labossiere RJ, Crill C, Goday P. A.S.P.E.N. JPEN J Parenter Enteral Nutr. 2014;38(3):334–377. doi: 10.1177/0148607114521833. Clinical Guidelines: Parenteral nutrition ordering, order review, compounding, labeling and dispensing. [DOI] [PubMed] [Google Scholar]
- 9.Marc Stranz, Pharm D. Adjusting pH and osmolarity levels to fit standards and practices. JVAD Fall. 2002:12–15. [Google Scholar]
- 10.Dillon RC, Merchan C, Altshuler D, Papadopoulos J. Incidence of adverse events during peripheral administration of sodium chloride 3. J Intensive Care Med. 2018;33(1):48–53. doi: 10.1177/0885066617702590. [DOI] [PubMed] [Google Scholar]
- 11.Perez CA, Figueroa SA. Complication rate of 3% hypertonic saline infusion through peripheral intravenous access. J Neurosci Nurs. 2017;49(3):191–195. doi: 10.1097/JNN.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 12.Suter RE. Emergency medicine in the United States: A systemic review. World J Emerg Med. 2012;3(1):5–10. doi: 10.5847/wjem.j.issn.1920-8642.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard DW, Reed ME, Rauchwerger AS, Chettipally UK, Offerman SR, Mark DG. Emergency physician perspectives on central venous catheterization in the emergency department: A survey – based study. Academic Emerg Med. 2014;26(6):623–630. doi: 10.1111/acem.12386. [DOI] [PubMed] [Google Scholar]
- 14.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B., et al. Early Goal – Directed Therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Akaraborworm O. A review in emergency central venous catherization. Chinese J of Traumatology. 2017;20:137–140. doi: 10.1016/j.cjtee.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESSant the ProMISe investigators. Int Care Med. 2015;41(9):1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 17.Eskesen TG, Wetterslev M, Perner A. Systematic review including re-analysis of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Int Care Med. 2016;42(3):324–342. doi: 10.1007/s00134-015-4168-4. [DOI] [PubMed] [Google Scholar]
- 18.LeMaster CH, Schuur JD, Pandya D, Pallin DJ, Silvia J, Yokoe D, et al. Infection and natural history of emergency department – placed central venous catheters. Ann Emerg Med. 2010;56:492–497. doi: 10.1016/j.annemergmed.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Theodoro D, Olsen MA, Warren DK, McMullen KM, Asaro P, Henderson A, et al. Emergency department central line associated blood stream infections (CLABSI): Incidence in the era of prevention practices. Acad Emerg Med. 2015;22(9):1048–1055. doi: 10.1111/acem.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan DF, Cassidy DD, Leech S. Significant reduction of emergency department central venous catheter placement after implementation of nursing education on ultrasound guided peripheral vein canalization (Abstract publication 51). Annals of Emerg Med. 2013;62(4):S21. [Google Scholar]
- 21.Cardenas-Garcia J, Schaub KF, Belchikov YG, Narasimhan M, Koenig SJ, Mayo PH. Safety of peripheral venous administration of vasoactive medication. J Hosp Med. 2015;10(9):581–585. doi: 10.1002/jhm.2394. [DOI] [PubMed] [Google Scholar]
- 22.Perez CA, Figueroa SA. Complication rates of 3% hypertonic saline infusion through peripheral intravenous access. J Neurosci Nurs. 2017;49(3):191–195. doi: 10.1097/JNN.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 23.LeMaster CH, Hoffart N, Chafe T, Benzer T, Schuur JD. Implementing the central venous catheter infection prevention bundle in the emergency department: experiences among early adopters. Ann Emerg Med. 2014;63(3):340–350. doi: 10.1016/j.annemergmed.2013.09.006. e1. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson P, Boyle A, Robinson S, Campbell-Hewson G. Should ultrasound guidance be used for central venous cannulations in the emergency department? Emerg Med J. 2005;22:158–164. doi: 10.1136/emj.2003.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S, Ling Q, Cao L, Wang J, Xu MX, Zeng WA. Realtime ultrasound guidance for central venous cannulation: A meta-analysis. Anesthesiology. 2013;118:361–375. doi: 10.1097/ALN.0b013e31827bd172. [DOI] [PubMed] [Google Scholar]
- 26.Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: A structured review and recommendations for clinical practice. Critical Care. 2017;21:225–236. doi: 10.1186/s13054-017-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling ML, Apisamthanarak A, Jaggi N, Harrington G, Morikane K, Le Thi Anh Thu, et al. APSIC guide for prevention of central line associated blood stream infections (CLABSI). Antimicrob Resist Infect Control. 2016;5:16–25. doi: 10.1186/s13756-016-0116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: The Keystone Intensive Care Unit Project. Am J Infect Control. 2008;36:S171. doi: 10.1016/j.ajic.2008.10.008. e1-5. [DOI] [PubMed] [Google Scholar]
- 29.Liang SY, Theodoro DL, Schuur JD, Marschall J. Infection prevention in emergency department. Ann Emerg Med. 2014;64(3):299–313. doi: 10.1016/j.annemergmed.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum J, McGrath C, Modi A, Saxena S, Shah A, Sidhu N, et al. Assessing the burden of unnecessary central venous catheters in patients on Medical-Surgical floors (2017). http://jdc.jefferson.edu/patientsafetyposters/34 House staff quality improvement and patient safety posters. Poster 34. [Google Scholar]
- 32.Marschall J. Cather-associated bloodstream infections: Looking outside of the ICU. Am J Infect Control. 2008;36:S172.e5–S172.e8. doi: 10.1016/j.ajic.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, et al. Strategies to prevent central line associated bloodstream infections in acute care hospitals 2014 update. Infection Control and Hospital Epidemiology. 2014;35(7):753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 34.Quan KA, Cousins SM, Porter DD. Electronic health record solutions to reduce central line associated blood stream infections by enhancing documentation of central line insertion practices, line days and daily line necessity. Am J Infect Control. 2016;44:438–443. doi: 10.1016/j.ajic.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 35.The Joint Commission. http://www.jointcommission.org/CLABSIToolkit http://www.jointcommission.org/CLABSIToolkit Preventing Central line-Associated Bloodstream Infections: Useful tools, an international perspective. No 20, 2013. Accessed [June 29th 2018].
- 36.MMWR recommendations and reports. Guidelines for environmental infection control in health – care facilities, recommendations od CDC and the HICPAC. MMWR. 2003;53(RR10):1–42. [PubMed] [Google Scholar]
- 37.International Health Facility Guidelines. Part D – Infection Control 2017;Version 5: 6.
- 38.Fenik Y, Celebi N, Wagner R. Prepackaged central line kits reduce procedural mistakes during central line insertion: a randomised controlled prospective trial. BMC Medical Education. 2013;13:60–67. doi: 10.1186/1472-6920-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen GB, Miller V, Nicholas C, Hess S, Cordes MK, Fortune JB, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line – associated bloodstream infections. Am J Infect Control. 2014;42(6):643–648. doi: 10.1016/j.ajic.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Parienti J-J, Nicolas M, Mégarbane B, Mira J-P, Kalfon P, Gros A, et al. (2015). The New England journal of medicine. 2015;373:1220–1229. doi: 10.1056/NEJMoa1500964. Intravascular Complications of Central Venous Catheterization by Insertion Site. DOI: [DOI] [PubMed] [Google Scholar]
- 41.Timsit JF. What is the best site for central venous catheter insertion in critically ill patients? Crit Care. 2003;7(6):397–399. doi: 10.1186/cc2179. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–707. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- 43.Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med. 1999;27:1819–1823. doi: 10.1097/00003246-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Ruesch S, Walder B, Tramèr MR. Complication of central venous catheters: Internal Jugular versus subclavian access–A systematic review. Crit Care Med. 2002;30:454–460. doi: 10.1097/00003246-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 45.Iovino F, Pittiruti M, Buononato M, Lo Schiavo F. Central venous catheterization: complications of different placements [in French] Ann Chir. 2001;126:1001–1006. doi: 10.1016/S0003-3944(01)00653-8. DOI: [DOI] [PubMed] [Google Scholar]
- 46.Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Arch Intern Med. 1986;146:259–261. doi: 10.1001/archinte.146.2.259. Failure and complication rates by three percutaneous approaches. DOI: [DOI] [PubMed] [Google Scholar]
- 47.Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. doi: 10.1186/cc8853. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700–707. doi: 10.1001/jama.286.6.700. DOI: [DOI] [PubMed] [Google Scholar]
- 49.Timsit, J. What is the best site for central venous catheter insertion in critically ill patients? Crit Care. 2003;7:397. doi: 10.1186/cc2179. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse A, Schick MA. Treasure Island (FL): StatPearls Publishing; Central Line Placement. [Updated 2019 Feb 23]. In: StatPearls [Internet]. 2019 Jan-. Available from: [PubMed] [Google Scholar]
- 51.Rubenstein JS, Hageman JR. Monitoring of critically ill infants and children. Crit Care Clin. 1988;4:621–639. [PubMed] [Google Scholar]
- 52.Epstein HM, Linde HW, Crampton AR, Ciric IS, Eckenhoff JE. The vertebral venous plexus as a major cerebral venous outflow tract. Anesthesiology. 1970;32:332–337. doi: 10.1097/00000542-197004000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Goetting MG, Preston G. Jugular bulb catheterization does not increase intracranial pressure. Intensive Care Med. 1991;17:195–198. doi: 10.1007/BF01709876. [DOI] [PubMed] [Google Scholar]
- 54.Hilton E, Haslett TM, Borenstein MT, Tucci V, Isenberg HD, Singer C. Central catheter infections: single-versus triple-lumen catheters. Am J Med. 1988;84:667–672. doi: 10.1016/0002-9343(88)90102-7. Influence of guidewires on infection rates when used for replacement of catheters. [DOI] [PubMed] [Google Scholar]
- 55.Clark-Christoff N, Watters VA, Sparks W, Snyder P, Grant JP. Use of triple-lumen subclavian catheters for administration of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1992;16:403–407. doi: 10.1177/0148607192016005403. [DOI] [PubMed] [Google Scholar]
- 56.Early TF, Gregory RT, Wheeler JR, Snyder SO, Jr., Gayle RG. Increased infection rate in double-lumen versus single-lumen Hickman catheters in cancer patients. South Med J. 1990;83:34–36. doi: 10.1097/00007611-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Yeung C, May J, Hughes R. Infection rate for single lumen v triple lumen subclavian catheters. Infect Control Hosp Epidemiol. 1988;9:154–158. doi: 10.1086/645820. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: Systematic review and meta-analysis. Infection. 2015;43:29–36. doi: 10.1007/s15010-014-0689-y. [DOI] [PubMed] [Google Scholar]
- 59.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 60.Million Lives Campaign Getting Started Kit—How-to Guide: Prevent Central Line- Associated Bloodstream Infection Cambridge, MA: Institute for Healthcare Improvement; (2006) http://www.ihi.org/IHI/Programs/Campaign/CentralLineInfection.htm. http://www.ihi.org/IHI/Programs/Campaign/CentralLineInfection.htm Available: Accessed 2009 May 26.
- 61.Marik PE, Flemmer M, Harrison W. The risk of catheter-related bloodstream infection with femoral venous catheters as compared to subclavian and internal jugular venous catheters: A systematic review of the literature and meta-analysis. Crit Care Med. 2012;40:2479–2485. doi: 10.1097/CCM.0b013e318255d9bc. [DOI] [PubMed] [Google Scholar]
- 62.Arvaniti K, Lathyris D, Blot S, Apostolidou-Kiouti F, Koulenti D, Haidich AB. Cumulative Evidence of Randomized Controlled and Observational Studies on Catheter – Related Infection Risk of Central Venous Catheter Insertion Site in ICU Patients : A Pairwise and Network Meta – Analysis. Crit Care Med. 2017;45:e437, e448. doi: 10.1097/CCM.0000000000002092. [DOI] [PubMed] [Google Scholar]
- 63.Coopersmith CM, Rebmann TL, Zack JE, Ward MR, Corcoran RM, Schallom ME, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. doi: 10.1097/00003246-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Pittet D, Hugonnet S, Harbath S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet. 2000;356:1307–1309. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 65.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23:S3–40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 66.Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med. 2000;160:1017–1021. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
- 67.Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999;159:821–826. doi: 10.1001/archinte.159.8.821. [DOI] [PubMed] [Google Scholar]
- 68.Raad II, Hohn DC, Gilbreath BJ, Suleiman N, Hill LA, Bruso, PA, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control HospEpidemiol. 1994;15:231–238. [PubMed] [Google Scholar]
- 69.Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91:197S, 205. doi: 10.1016/0002-9343(91)90369-9. [DOI] [PubMed] [Google Scholar]
- 70.Sherertz RJ, Ely EW, Westbrook DM, Gledhill KS, Streed SA, Kiger B, et al. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med. 2000;132:641–648. doi: 10.7326/0003-4819-132-8-200004180-00007. [DOI] [PubMed] [Google Scholar]
- 71.Carrer S, Bocchi A, Bortolotti M, Braga N, Gilli G, Candini M, et al. Effect of different sterile barrier precautions and central venous catheter dressing on the skin colonization around the insertion site. Minerva Anestesiol. 2005;71:197–206. [PubMed] [Google Scholar]
- 72.Abi-Said D, Raad I, Umphrey J, Gonzalez V, Richardson D, Marts K, et al. Infusion therapy team and dressing changes of central venous catheters. Infect Control Hosp Epidemiol. 1999;20:101–105. doi: 10.1086/501597. [DOI] [PubMed] [Google Scholar]
- 73.Yasuda H, Sanui M, Abe T, Shime N, Komuro T, Hatakeyama J, et al. Comparison of the efficacy of three topical antiseptic solutions for the prevention of catheter colonization: a multicenter randomized controlled study. Crit Care. 2017;21:320. doi: 10.1186/s13054-017-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ullman AJ, Cooke ML, Mitchell M. Dressings and securement devices for central venous catheters (CVC). Cochrane Database Syst Rev. 2015;(9) doi: 10.1002/14651858.CD010367.pub2. CD010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karpanen TJ, Casey AL, Whitehouse T, Timsit JF, Mimoz O, Palomar M, et al. A clinical evaluation of two central venous catheter stabilization systems. Ann Intensive Care. 2019;49(1) doi: 10.1186/s13613-019-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molina-Mazón CS, Martín-Cerezo X, Domene-Nieves de la Vega G, Asensio-Flores S, Adamuz-Tomás J. Comparative study on fixation of central venous catheter by suture versus adhesive device. Enferm Intensiva. 2018-112;103(3) doi: 10.1016/j.enfi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Struck MF, Friedrich L, Schleifenbaum S, Kirsten H, Schummer W, Winkler BE. Effectiveness of different central venous catheter fixation suture techniques: An in vitro crossover study. PLoS ONE. 2019;14(9):e0222463. doi: 10.1371/journal.pone.0222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29–36. doi: 10.1007/s15010-014-0689-y. PMID: [DOI] [PubMed] [Google Scholar]
- 79.Salzman MB, Isenberg HD, Shapiro JF, Lipsitz PJ, Rubin LG. A Prospective Study of the Catheter Hub as the Portal of Entry for Microorganisms Causing Catheter-Related Sepsis in Neonates. J Infect Dis. 1993;167(2):487–490. doi: 10.1093/infdis/167.2.487. [DOI] [PubMed] [Google Scholar]
- 80.Salzman MB, Isenberg HD, Rubin LG. Use of disinfectants to reduce microbial contamination of hubs of vascular catheters. J ClinMicrobiol. 1993;31(3):475–479. doi: 10.1128/jcm.31.3.475-479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong H, Morrow DF, Sandora TJ, Priebe, GP. Disinfection of needleless connectors with chlorhexidine-alcohol provides long-lasting residual disinfectant activity. Am. J. Infect. Control. 2013;41(8):e77–79. doi: 10.1016/j.ajic.2012.10.018. PMID: [DOI] [PubMed] [Google Scholar]
- 82.Helder OK, Kornelisse RF, Reiss IK, Ista E. Disinfection practices in intravenous drug administration. Am. J. Infect. Control. 2016;44(6):721–723. doi: 10.1016/j.ajic.2015.12.036. PMID: [DOI] [PubMed] [Google Scholar]
- 83.Menyhay SZ, Maki DG. Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap. Infect Control Hosp Epidemiol. 2006;27(1):23–27. doi: 10.1086/500280. PMID: [DOI] [PubMed] [Google Scholar]
- 84.Menyhay SZ, Maki DG. Preventing central venous catheter-associated bloodstream infections: development of an antiseptic barrier cap for needleless connectors. Am. J. Infect. Control. 2008;36(10):S174e1, e5. doi: 10.1016/j.ajic.2008.10.006. PMID: [DOI] [PubMed] [Google Scholar]
- 85.Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with non-cuffed short-term central venous catheters. Intensive Care Med. 2004;30:62–67. doi: 10.1007/s00134-003-2045-z. [DOI] [PubMed] [Google Scholar]
- 86.Segura M, Lladó L, Guirao X, Piracés M, Herms R, Alia C, et al. A prospective study of a new protocol for ‘in situ'diagnosis of central venous catheter related bacteremia. Clin Nutr. 1993;12:103–107. doi: 10.1016/0261-5614(93)90059-d. [DOI] [PubMed] [Google Scholar]
- 87.Douard MC, Clementi E, Arlet G, Marie O, Jacob L, Schremmer B, et al. Negative catheter-tip culture and diagnosis of catheter-related bacteremia. Nutrition. 1994;10:397–404. [PubMed] [Google Scholar]
- 88.Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: A quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993;168:400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- 89.Mermel L. A. What is the predominant source of intravascular catheter infections? CID. 2011;52:211. doi: 10.1093/cid/ciq108. [DOI] [PubMed] [Google Scholar]
- 90.Beekmann SE, Henderson DK. Infections caused by percutaneous intravascular devices. Principles and Practice of Infectious Disease. In: Mandell GL, Bennett JE, Dolin R, . Vol 16th ed. Philadelphia, PA: Elsevier; 2005. pp. 3347–3361. editors., eds. [Google Scholar]
- 91.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 92.Levin A, Mason AJ, Jindal KK, Fong IW, Goldstein MB. Prevention of hemodialysis subclavian vein catheter infections by topical povidone-iodine. Kidney Int. 1991;40(5):934–938. doi: 10.1038/ki.1991.297. [DOI] [PubMed] [Google Scholar]
- 93.Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW, Conly J. Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol. 2003;14(1):169–179. doi: 10.1097/01.asn.0000038688.76195.a4. [DOI] [PubMed] [Google Scholar]
- 94.Fong IW. Prevention of haemodialysis and peritoneal dialysis catheter related infection by topical povidone-iodine. Postgrad Med J. 1993;69(suppl 3):S15, S17. [PubMed] [Google Scholar]
- 95.Battistella M, Bhola C, Lok CE. Long-term follow-up of the Hemodialysis Infection Prevention with Polysporin Ointment (HIPPO) Study: a quality improvement report. Am J Kidney Dis. 2011;57(3):432–441. doi: 10.1053/j.ajkd.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Zakrzewska-Bode A, Muytjens HL, Liem KD, Hoogkamp-Korstanje JA. Mupirocin resistance in coagulase-negative staphylococci, after topical prophylaxis for the reduction of colonization of central venous catheters. Hosp Infect. 1995;31(3):189–193. doi: 10.1016/0195-6701(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 97.Riu S, Ruiz CG, Martinez-Vea A, Peralta C, Oliver JA. Spontaneous rupture of polyurethane peritoneal catheter: a possible deleterious effect of mupirocin ointment. Nephrol Dial Transplant. 1998;13(7):1870–1871. doi: 10.1093/ndt/13.7.1870. [DOI] [PubMed] [Google Scholar]
- 98.Hemodialysis Vascular access and peritoneal dialysis Catheters, a book by Claudio Ronco, Nathan Levin: Karger; 2004:139–140. [Google Scholar]
- 99.Lai NM, Lai NA, O'Riordan E, Chaiyakunapruk N, Taylor JE, Tan K. Skin antisepsis for reducing central venous catheter-related infections. Cochrane Database of Systematic Reviews. 2016;2016(7):CD010140. doi: 10.1002/14651858.CD010140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167(19):2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 101.O'Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol. 2012;33(3):257–267. doi: 10.1086/664496. [DOI] [PubMed] [Google Scholar]
- 102.Montecalvo MA, McKenna D, Yarrish R, Mack L, Maguire G, Haas J, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505–511. doi: 10.1016/j.amjmed.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 103.Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Musuuza JS, Guru PK, O'Horo JC, Bongiorno CM, Korobkin MA, Gangnon RE, et al. The impact of chlorhexidine bathing on hospital-acquired bloodstream infections: a systematic review and meta-analysis. BMC Infect Dis. 2019;416(1) doi: 10.1186/s12879-019-4002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levy I, Katz J, Solter E, Samra Z, Vidne B, Birk E, et al. Chlorhexidine-impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatr Infect Dis J. 2005;24(8):676–679. doi: 10.1097/01.inf.0000172934.98865.14. [DOI] [PubMed] [Google Scholar]
- 106.Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. Antimicrob Chemother. 2006;58(2):281–287. doi: 10.1093/jac/dkl234. [DOI] [PubMed] [Google Scholar]
- 107.Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S, et al. Chlorhexidine impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 108.Ruschulte H, Franke M, Gastmeier P, Zenz S, Mahr KH, Buchholz S, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Ann Hematol. 2009;88(3):267–272. doi: 10.1007/s00277-008-0568-7. [DOI] [PubMed] [Google Scholar]
- 109.Camins BC, Richmond AM, Dyer KL, Zimmerman HN, Coyne DW, Rothstein M, et al. A crossover intervention trial evaluating the efficacy of a chlorhexidine impregnated sponge in reducing catheter-related bloodstream infections among patients undergoing hemodialysis. Infect Control Hosp Epidemiol. 2010;31(11):1118–1123. doi: 10.1086/657075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J RespirCrit Care Med. 2012;186(12):1272–1278. doi: 10.1164/rccm.201206-1038OC. [DOI] [PubMed] [Google Scholar]
- 111.Ullman AJ, Cooke ML, Mitchell M, Lin F, New K, Long DA, et al. Dressings and securement devices for central venous catheters (CVC). Cochrane Database Syst Rev. 2015;9:CD010367. doi: 10.1002/14651858.CD010367.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter-related bacteraemia with an antimicrobial lock solution: a meta-analysis of prospective randomized trials. Nephrol Dial Transplant. 2008;23(5):1666–1672. doi: 10.1093/ndt/gfm847. [DOI] [PubMed] [Google Scholar]
- 113.Carratala J, Niubo J, Fernandez-Sevilla A, Juvé E, Castellsagué X, Berlanga J, et al. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob Agents Chemother. 1999;43(9):2200–2204. doi: 10.1128/aac.43.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henrickson KJ, Axtell RA, Hoover SM, Kuhn SM, Pritchett J, Kehl SC, et al. Prevention of central venous catheter-related infections and thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double-blind trial. J Clin Oncol. 2000;18(6):1269–1278. doi: 10.1200/JCO.2000.18.6.1269. [DOI] [PubMed] [Google Scholar]
- 115.Safdar N, Maki DG. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trials. Clin Infect Dis. 2006;43(4):474–484. doi: 10.1086/505976. [DOI] [PubMed] [Google Scholar]
- 116.Snaterse M, Ruger W, Scholte Op Reimer WJ, Lucas C. Antibiotic-based catheter lock solutions for prevention of catheter- related bloodstream infection: a systematic review of randomized controlled trials. Hosp Infect. 2010;75(1):1–11. doi: 10.1016/j.jhin.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 117.Pittiruti M, Bertoglio S, Scoppettuolo G, Biffi R, Lamperti M, Dal Molin A, et al. Evidence-based criteria for the choice and the clinical use of the most appropriate lock solutions for central venous catheters (excluding dialysis catheters): a GAVeCeLTconsensus. J Vasc Access. 2016;17(6):453–464. doi: 10.5301/jva.5000576. [DOI] [PubMed] [Google Scholar]
- 118.James MT, Conley J, Tonelli M, Manns BJ, MacRae J, Hemmelgarn BR. Meta-analysis: antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med. 2008;148(8):596–605. doi: 10.7326/0003-4819-148-8-200804150-00004. [DOI] [PubMed] [Google Scholar]
- 119.McKee R, Dunsmuir R, Whitby M, Garden OJ. Does antibiotic prophylaxis at the time of catheter insertion reduce the incidence of catheter-related sepsis in intravenous nutrition? J Hosp Infect. 1985;6(4):419–425. doi: 10.1016/0195-6701(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 120.Ranson MR, Oppenheim BA, Jackson A, Kamthan AG, Scarffe JH. Double-blind placebo controlled study of vancomycin prophylaxis for central venous catheter insertion in cancer patients. J Hosp Infect. 1990;15(1):95–102. doi: 10.1016/0195-6701(90)90025-j. [DOI] [PubMed] [Google Scholar]
- 121.Sandoe JA, Kumar B, Stoddart B, Milton R, Dave J, Nair UR, et al. Effect of extended perioperative antibiotic prophylaxis on intravascular catheter colonization and infection in cardiothoracic surgery patients. J Antimicrob Chemother. 2003;52(5):877–879. doi: 10.1093/jac/dkg442. [DOI] [PubMed] [Google Scholar]
- 122.Karanlik H, Kurul S, Saip P, Unal ES, Sen F, Disci R, et al. The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg. 2011;202(1):10–15. doi: 10.1016/j.amjsurg.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Van de Wetering MD, van Woensel JBM, Lawrie TA. Prophylactic antibiotics for preventing Gram positive infections associated with long-term central venous catheters in oncology patients. Cochrane Database of Systematic Reviews. 2013;11:CD003295. doi: 10.1002/14651858.CD003295.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rajgopaln RE. Guidelines for Prevention of infections with vascular catheters used in Indian ICUS: Section on Central Line associated infections. IJCCM. 2003;7(Suppl 1) [Google Scholar]
- 125.Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, et al. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 126.Bell T, O'Grady NP. Prevention of Central Line-Associated Bloodstream Infections. Infect Dis Clin North Am. 2017;31(3):551–559. doi: 10.1016/j.idc.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mermel LA. What is the predominant source of intravascular catheter infections? CID. 2011;52:211. doi: 10.1093/cid/ciq108. [DOI] [PubMed] [Google Scholar]
- 128.Moureau NL, Marsh N, Zhang L, Bauer MJ, Larsen E, Mihala G, et al. Evaluation of Skin Colonisation And Placement of vascular access device Exit sites (ESCAPE Study). J Infect Prev. 2019;20(1):51–59. doi: 10.1177/1757177418805836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamilton H, Bodenham AR (eds). Cetnral Venous Catheters.: Wiley-Blackwell Publications; 2009. Chapter on Removal of Central Venous Access Devices. [Google Scholar]
- 130.Bong JJ, Kite P, Wilco MH, McMahon MJ. Prevention of catheter-related bloodstream infection by silver iontophoretic central venous catheters: A randomised controlled trial. J Clin Pathol. 2003;56:731–735. doi: 10.1136/jcp.56.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boswald M, Lugauer S, Regenfus A, Braun GG, Martus P, Geis C, Scharf J, Bechert T, Greil J, Guggenbichler J-P. Reduced rates of catheter-associated infection by use of a new silver-impregnated central venous catheter. Infection. 1999;27:56–60. doi: 10.1007/BF02561621. [DOI] [PubMed] [Google Scholar]
- 132.Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter related bloodstream infections in critically ill patients: A comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26:752–758. doi: 10.1097/EJA.0b013e32832a3a84. [DOI] [PubMed] [Google Scholar]
- 133.Harter C, Salwender HJ, Bach A, Egerer G, Goldschmidt H, Ho AD. Catheter-related infection and thrombosis of the internal jugular vein in hematologic-oncologic patients undergoing chemotherapy: A prospective comparison of silver-coated and uncoated catheters. Cancer. 2002;94:245–251. doi: 10.1002/cncr.10199. [DOI] [PubMed] [Google Scholar]
- 134.Kalfon P, de Vaumas C, Samba D, Boulet E, Lefrant JY, Eyraud D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35:1032–1039. doi: 10.1097/01.CCM.0000259378.53166.1B. [DOI] [PubMed] [Google Scholar]
- 135.Mermel LA. Prevention of intravascular catheter-related infections (Erratum: Ann Intern Med. 2000;133:395). Ann Intern Med. 2000;132:391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- 136.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999;281:261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 137.Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. A randomized, controlled trial. [DOI] [PubMed] [Google Scholar]
- 138.Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30:837–843. doi: 10.1007/s00134-004-2221-9. [DOI] [PubMed] [Google Scholar]
- 139.Ostendorf T, Meinhold A, Harter C, Salwender H, Egerer G, Geiss HK, et al. Chlorhexidine and silver-sulfadiazine coated central venous catheters in haematological patients–a double-blind, randomised, prospective, controlled trial. Support Care Cancer. 2005;13:993–1000. doi: 10.1007/s00520-005-0812-9. [DOI] [PubMed] [Google Scholar]
- 140.Rupp ME, Lisco SJ, Lipsett PA, Perl TM, Keating K, Civetta JM, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143:570–580. doi: 10.7326/0003-4819-143-8-200510180-00007. [DOI] [PubMed] [Google Scholar]
- 141.Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, et al. A comparison of two antimicrobial-impregnated central venous catheters. N Engl J Med. 1999;340:1–8. doi: 10.1056/NEJM199901073400101. Catheter Study Group. [DOI] [PubMed] [Google Scholar]
- 142.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. Ann Intern Med. 1997;127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. [DOI] [PubMed] [Google Scholar]
- 143.Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol. 2004;22:3163–3171. doi: 10.1200/JCO.2004.04.124. [DOI] [PubMed] [Google Scholar]
- 144.Bonne S, Mazuski JE, Sona C, Schallom M, Boyle W, Buchman TG, et al. Effectiveness of Minocycline/RifampinVsChlorhexidine/Silver Sulfadiazine Impregnated Central Venous Catheters In Preventing Central Line-Associated Bloodstream Infection In A High-Volume Academic Intensive Care Unit: A Before And After Trial. J. Am Coll Surg. 2015;221(3):739–747. doi: 10.1016/j.jamcollsurg.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 145.Liu H, Shukla S, Vera-González N, Tharmalingam N, Mylonakis E, Fuchs BB, et al. Auranofin releasing Antibacterial and Antibiofilm Polyurethane intravascular catheter coatings. Front. Cell. Infect. Microbiol. 2019;9:37. doi: 10.3389/fcimb.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hilty WM, Hudson PA, Levitt MA, Hall JB. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29:331. doi: 10.1016/s0196-0644(97)70344-5. [DOI] [PubMed] [Google Scholar]
- 147.Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38:1105–1117. doi: 10.1007/s00134-012-2597-x. [DOI] [PubMed] [Google Scholar]
- 148.Lalu MM, Fayad A, Ahmed O, Bryson GL, Fergusson DA, Barron CC, et al. Ultrasound-guided subclavian vein catheterization: a systematic review and meta-analysis. Crit Care Med. 2015;43:1498–1507. doi: 10.1097/CCM.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 149.Troianos CA, Hartman GS, Glas KE, Skubas NJ, Eberhardt RT, Walker JD, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114:46–72. doi: 10.1213/ANE.0b013e3182407cd8. [DOI] [PubMed] [Google Scholar]
- 150.Bodenham Chair A, Babu S, Bennett J, Binks R, Fee P, Fox B, et al. Association of Anaesthetists of Great Britain and Ireland: safe vascular access 2016. Anaesthesia. 2016;71:573–585. doi: 10.1111/anae.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cook D, Randolph A, Kernerman P, Cupido C, King D, Soukup C, et al. Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med. 1997;25(8):1417–1424. doi: 10.1097/00003246-199708000-00033. [DOI] [PubMed] [Google Scholar]
- 152.Bonadimani B, Sperti C, Stevanin A, Cappellazzo F, Militello C, Petrin P, et al. Central venous catheter guidewire replacement according to the Seldinger technique: usefulness in the management of patients on total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1987;11(3):267–270. doi: 10.1177/0148607187011003267. [DOI] [PubMed] [Google Scholar]
- 153.Cobb DK, High KP, Sawyer RG, Sable CA, Adams RB, Lindley DA, et al. A controlled trial of scheduled replacement of central venous and pulmonary-artery catheters. N Engl J Med. 1992;327(15):1062–1068. doi: 10.1056/NEJM199210083271505. [DOI] [PubMed] [Google Scholar]
- 154.Shaffer D. Catheter-related sepsis complicating long-term, tunnelled central venous dialysis catheters: management by guidewire exchange. Am J Kidney Dis. 1995;25(4):593–596. doi: 10.1016/0272-6386(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 155.Hagley MT, Martin B, Gast P, Traeger SM. Infectious and mechanical complications of central venous catheters placed by percutaneous venipuncture and over guidewires. Crit Care Med. 1992;20(10):1426–1430. doi: 10.1097/00003246-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 156.Shimada M, Matsumata T, Wakiyama S, Maeda T, Kanematsu T, Sugimachi K. A safe and simple technique for exchanging central venous catheters. Postgrad Med J. 1993;69(808):139–140. doi: 10.1136/pgmj.69.808.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frassinelli P, Pasquale MD, Cipolle MD, Rhodes M. Utility of chest radiographs after guidewire exchanges of central venous catheters. Crit Care Med. 1998;26(3):611–615. doi: 10.1097/00003246-199803000-00040. [DOI] [PubMed] [Google Scholar]
- 158.Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling CR, Walther SM, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care Lond Engl. 2015;19:221. doi: 10.1186/s13054-015-0920-y. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Souweine B, Traore O, Aublet-Cuvelier B, Badrikian L, Bret L, Sirot J, et al. Dialysis and central venous catheter infections in critically ill patients: results of a prospective study. Crit Care Med. 1999;27:2394–2398. doi: 10.1097/00003246-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 160.Chatzinikolaou I, Finkel K, Hanna H, Boktour M, Foringer J, Ho T, et al. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115:352–357. doi: 10.1016/s0002-9343(03)00367-x. [DOI] [PubMed] [Google Scholar]
- 161.Smit JM, Raadsen R, Blans MJ, Petjak M, Van de Ven PM, Tuinman PR. Bedside ultrasound to detect central venous catheter misplacement and associated iatrogenic complications: a systematic review and meta-analysis. Critical Care. 2018;22:65. doi: 10.1186/s13054-018-1989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aslamy Z, Dewald CL, Heffner JE. MRI of central venous anatomy. Implications for central venous catheter insertion. Chest. 1998;114:820–826. doi: 10.1378/chest.114.3.820. [DOI] [PubMed] [Google Scholar]
- 163.Rutherford JS, Merry AF, Occleshaw CJ. Depth of central venous catheterization: an audit of practice in a cardiac surgical unit. Anaesth Intensive Care. 1994;22:267–271. doi: 10.1177/0310057X9402200303. [DOI] [PubMed] [Google Scholar]
- 164.Peres PW. Positioning central venous catheters: a prospective study. Anaesth Intensive Care. 1990;18:536–539. doi: 10.1177/0310057X9001800422. [DOI] [PubMed] [Google Scholar]
- 165.Karnauchow PN. Cardiac tamponade from central venous catheterization. Can Med Assoc J. 1986;135:1145–1147. [PMC free article] [PubMed] [Google Scholar]
- 166.Booth SA, Norton B, Mulvey DA. Central venous catheterization and fatal cardiac tamponade. Br J Anaesth. 2001;87:298–302. doi: 10.1093/bja/87.2.298. [DOI] [PubMed] [Google Scholar]
- 167.Vesely TM. Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol. 2003;14:527–534. doi: 10.1097/01.rvi.0000071097.76348.72. [DOI] [PubMed] [Google Scholar]
- 168.Gibson F, Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 169.Cadman A, Lawrance JAL, Fitzsimmons L, Spencer-Shaw A, Swindell R. To clot or not to clot? Clin Radiol. 2004;59:349–355. doi: 10.1016/j.crad.2003.11.015. That is the question in central venous catheters. [DOI] [PubMed] [Google Scholar]
- 170.Gibson E, Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 171.Andropoulos DB, Bent ST, Skjonsby B, Stayer SA. The optimal length of insertion of central venous catheters for pediatric patients. Anesth Analg. 2001;93:883–886. doi: 10.1097/00000539-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 172.Konings MK, van Schelven LJ, Meijer RC, Westra AH, Snijder RA, van Boxtel AJ. Correct positioning of central venous catheters using a new electric method. J Vasc Access. 2015;16:327–332. doi: 10.5301/jva.5000361. [DOI] [PubMed] [Google Scholar]
- 173.Defalque RJ, Campbell C. Cardiac tamponade from central venous catheters. Anesthesiology. 1979;50:249–252. doi: 10.1097/00000542-197903000-00021. [DOI] [PubMed] [Google Scholar]
- 174.Greenall MJ, Blewitt RW, McMahon MJ. Cardiac tamponade and central venous catheters. BMJ. 1975;2:595–597. doi: 10.1136/bmj.2.5971.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mahlon MA, Yoon HC. CT angiography of the superior vena cava: normative values and implications for central venous catheter position. J Vasc Interv Radiol. 2007;18:1106–1110. doi: 10.1016/j.jvir.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 176.Chan TY, England A, Meredith SM, McWilliams RG. Radiologist variability in assessing the position of the cavoatrial junction on chest radiographs. Br J Radiol. 2016;89(1065):20150965. doi: 10.1259/bjr.20150965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Richet H, Hubert B, Nitemberg G, Andremont A, Buu-Hoi A, Ourbak P, et al. Prospective multicenter study of vascular-catheter-related complications and risk factors for positive central-catheter cultures in intensive care unit patients. J Clin Microbiol. 1990;28:2520–2525. doi: 10.1128/jcm.28.11.2520-2525.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moro ML, Viganò EF, Cozzi Lepri A. Risk factors for central venous catheter-related infections in surgical and intensive care units. Infect Control Hosp Epidemiol. 15(253):1994–264. The Central Venous Catheter-Related Infections Study Group. [PubMed] [Google Scholar]
- 179.Heard SO, Wagle M, Vijayakumar E, McLean S, Brueggemann A, Napolitano LM, et al. Influence of triple-lumen central venous catheters coated with chlorhexidine and silver sulfadiazine on the incidence of catheter-related bacteremia. Arch Intern Med. 1998;158:81–87. doi: 10.1001/archinte.158.1.81. [DOI] [PubMed] [Google Scholar]
- 180.Gil RT, Kruse JA, Thill-Baharozian MC, Carlosn RW. Triple- vs single-lumen central venous catheters. Arch Intern Med. 1989;149:1139–1143. A prospective study in a critically ill population. [PubMed] [Google Scholar]
- 181.Bonawitz SC, Hammell EJ, Kirkpatrick JR. Prevention of central venous catheter sepsis: A prospective randomized trial. Am Surg. 1991;57:618–623. [PubMed] [Google Scholar]
- 182.Kowalewska-Grochowska K, Richards R, Moysa GL, Lam K, Costerton JW, King EG. Guidewire catheter change in central venous catheter biofilm formation in a burn population. Chest. 1991;100:1090–1095. doi: 10.1378/chest.100.4.1090. [DOI] [PubMed] [Google Scholar]
- 183.Lorente L, Martín MM, Vidal P, Rebollo S, Ostabal MI, Solé-Violán J, et al. Should central venous catheter be systematically removed in patients with suspected catheter related infection? Crit Care. 2014;18(5):5, 564. doi: 10.1186/s13054-014-0564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nucci M, Anaissie E, Betts RF, Dupont BF, Wu C, Buell DN, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 51:295;, 2010–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
- 185.Lee YM, Moon C, Kim YJ, Lee HJ, Lee MS, Park KH. Clinical impact of delayed catheter removal for patients with central-venous-catheter-related Gram-negative bacteraemia. J Hosp Infect. 106(1):2018–113. doi: 10.1016/j.jhin.2018.01.004. 99; [DOI] [PubMed] [Google Scholar]
- 186.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) Patient Safety Component Manual. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_curret.pdf. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_curret.pdf Available at: Accessed January 2019.
- 188.O'Flaherty N, Crowley B. How to use central venous catheter tip cultures. Arch Dis Child Educ Pract Ed. 2015;100:69–74. doi: 10.1136/archdischild-2013-305096. [DOI] [PubMed] [Google Scholar]
- 189.Lai YL, Adjemian J, Ricotta EE, Mathew L, O'Grady NP, Kadri SS. Dwindling utilization of central venous catheter tip cultures: an analysis of sampling trends and clinical utility at 128 us hospitals, 2009-2014. Clinical Infectious Diseases. 2019;69:797–1800. doi: 10.1093/cid/ciz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement central line bundle. Am J Surg. 2014;207(6):817–823. doi: 10.1016/j.amjsurg.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 191.Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 37:2009–2173. doi: 10.1097/CCM.0b013e3181a02d8f. 2167; [DOI] [PubMed] [Google Scholar]
- 192.Ista E, van der Hoven B, Kornelisse RF, van der Starre C, Vos MC, Boersma E, et al. Effectiveness of insertion and maintenance bundles to prevent central line associated blood stream infection in critically ill patients all age: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:724–734. doi: 10.1016/S1473-3099(15)00409-0. [DOI] [PubMed] [Google Scholar]
- 193.Ablordeppey EA, Drewry AM, Beyer AB, Theodoro DL, Fowler SA, Fuller BM, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: A systematic review and meta-analysis. Critical care medicine. 2017;45(4):715–724. doi: 10.1097/CCM.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 195.Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859–866. doi: 10.1378/chest.10-2946. [DOI] [PubMed] [Google Scholar]
- 196.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703–708. doi: 10.1378/chest.11-0131. [DOI] [PubMed] [Google Scholar]
- 198.Ebrahimi A, Yousefifard M, Mohammad Kazemi H, Reza Rasouli H, Asady H, Moghadas Jafari A, et al. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos. 2014;13(4):29–40. [PMC free article] [PubMed] [Google Scholar]
- 199.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Comparison of Bedside Ultrasound With Chest X-ray for Confirmation of Central Venous Catheter Position (COMBUX): Clinical Trials. gov identifier (NCT number): NCT02959203
- 201.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335–337. [PubMed] [Google Scholar]
- 202.Yoo S, Ha M, Choi D, Pai H. Effectiveness of surveillance of central catheter-related bloodstream infection in an ICU in Korea. Infect Control Hosp Epidemiol. 2001;22:433–436. doi: 10.1086/501930. [DOI] [PubMed] [Google Scholar]
- 203.Coopersmith CM, Rebmann TL, Zack JE, Ward MR, Corcoran RM, Schallom ME, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. doi: 10.1097/00003246-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 204.Sherertz RJ, Ely EW, Westbrook DM, Gledhill KS, Streed SA, Kiger B, et al. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med. 2000;132:641–648. doi: 10.7326/0003-4819-132-8-200004180-00007. [DOI] [PubMed] [Google Scholar]
- 205.Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355:1864–1868. doi: 10.1016/S0140-6736(00)02291-1. [DOI] [PubMed] [Google Scholar]
- 206.Sanders RA, Sheldon GF. Septic complications of total parenteral nutrition. Am J Surg. 1976;132:214–220. doi: 10.1016/0002-9610(76)90050-7. A five year experience. [DOI] [PubMed] [Google Scholar]
- 207.Ryan JA, Jr., Abel RM, Abbott WM, Hopkins CC, Chesney TM, Colley R, et al. Catheter complications in total parenteral nutrition. N Engl J Med. 1974;290:757–761. doi: 10.1056/NEJM197404042901401. A prospective study of 200 consecutive patients. [DOI] [PubMed] [Google Scholar]
- 208.Murphy LM, Lipman TO. Central venous catheter care in parenteral nutrition: a review. JPEN J Parenter Enteral Nutr. 1987;11:190–201. doi: 10.1177/0148607187011002190. [DOI] [PubMed] [Google Scholar]
- 209.Armstrong CW, Mayhall CG, Miller KB, Newsome HH, Jr, Sugerman HJ, Dalton HP, et al. Prospective study of catheter replacement and other risk factors for infection of hyperalimentation catheters. J Infect Dis. 1986;154:808–816. doi: 10.1093/infdis/154.5.808. [DOI] [PubMed] [Google Scholar]
- 210.Nehme AE. Nutritional support of the hospitalized patient. JAMA. 1980;243:1906–1908. The team concept. [PubMed] [Google Scholar]
- 211.Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: a randomized controlled trial. Arch Intern Med. 1998;158:473–477. doi: 10.1001/archinte.158.5.473. [DOI] [PubMed] [Google Scholar]
- 212.Tomford JW, Hershey CO, McLaren CE, Porter DK, Cohen DI. Intravenous therapy team and peripheral venous catheter-associated complications. Arch Intern Med. 1984;144:1191–1194. A prospective controlled study. [PubMed] [Google Scholar]
- 213.Scalley RD, Van CS, Cochran RS. The impact of an i.v. J Intraven Nurs. 1992;15:100–109. team on the occurrence of intravenous-related phlebitis. A 30-month study. [PubMed] [Google Scholar]
- 214.Palefski SS, Stoddard GJ. The infusion nurse and patient complication rates of peripheral-short catheters. J Intraven Nurs. 2001;24:113–123. A prospective evaluation. [PubMed] [Google Scholar]
- 215.Miller JM, Goetz AM, Squier C, Muder RR. Reduction in nosocomial intravenous device-related bacteremias after institution of an intravenous therapy team. J Intraven Nurs. 1996;19:103–106. [PubMed] [Google Scholar]
- 216.Hunter MR. Development of a Vascular Access Team in an acute care setting. J Infus Nurs. 2003;26:86–91. doi: 10.1097/00129804-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 217.Hawes ML. A proactive approach to combating venous depletion in the hospital setting. J Infus Nurs. 2007;30:33–44. doi: 10.1097/00129804-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 218.Brunelle D. Impact of a dedicated infusion therapy team on the reduction of catheter-related nosocomial infections. J Infus Nurs. 2003;26:362–366. doi: 10.1097/00129804-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 219.Bosma TL, Jewesson PJ. An infusion program resource nurse consult service: our experience in a major Canadian teaching hospital. J Infus Nurs. 2002;25:310–315. doi: 10.1097/00129804-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 220.Pierce CA, Baker JJ. A nursing process model: quantifying infusion therapy resource consumption. J InfusNurs. 2004;27:232–244. doi: 10.1097/00129804-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 221.Fridkin SK, Pear SM, Williamson TH, Galgiani JN, Jarvis WR. The role of understaffing in central venous catheter-associated bloodstream infections. Infect Control HospEpidemiol. 1996;17:150–158. doi: 10.1086/647262. [DOI] [PubMed] [Google Scholar]
- 222.Alonso-Echanove J, Edwards JR, Richards MJ, Brennan P, Venezia RA, Keen J, et al. Effect of nurse staffing and antimicrobial-impregnated central venous catheters on the risk for bloodstream infections in intensive care units. Infect Control Hosp Epidemiol. 2003;24:916–925. doi: 10.1086/502160. [DOI] [PubMed] [Google Scholar]
- 223.Robert J, Fridkin SK, Blumberg HM, Anderson B, White N, Ray SM. The influence of the composition of the nursing staff on primary bloodstream infection rates in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2000;21:12–17. doi: 10.1086/501690. [DOI] [PubMed] [Google Scholar]
- 224.Safdar N, Maki DG. Lost in translation. Infect Control Hosp Epidemiol. 2006;27:3–7. doi: 10.1086/500282. [DOI] [PubMed] [Google Scholar]
- 225.Warren DK, Yokoe DS, Climo MW, Herwaldt LA, Noskin GA, Zuccotti G, et al. Preventing catheter-associated bloodstream infections: a survey of policies for insertion and care of central venous catheters from hospitals in the prevention epicenter program. Infect Control Hosp Epidemiol. 2006;27:8–13. doi: 10.1086/499151. [DOI] [PubMed] [Google Scholar]
- 226.Krein SL, Hofer TP, Kowalski CP, Olmsted RN, Kauffman CA, Forman JH, et al. Use of central venous catheter-related bloodstream infection prevention practices by US hospitals. Mayo Clin Proc. 2007;82:672–678. doi: 10.4065/82.6.672. [DOI] [PubMed] [Google Scholar]
- 227.Warren DK, Zack JE, Cox MJ, Cohen MM, Fraser VJ. An educational intervention to prevent catheter-associated bloodstream infections in a non-teaching community medical center. Crit Care Med. 2003;31:1959–1963. doi: 10.1097/01.CCM.0000069513.15417.1C. [DOI] [PubMed] [Google Scholar]
- 228.Warren DK, Zack JE, Mayfield JL, Chen A, Prentice D, Fraser VJ, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126:1612–1618. doi: 10.1378/chest.126.5.1612. [DOI] [PubMed] [Google Scholar]
- 229.Warren DK, Cosgrove SE, Diekema DJ, Zuccotti G, Climo MW, Bolon MK, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol, 2006;27:662–669. doi: 10.1086/506184. [DOI] [PubMed] [Google Scholar]
- 230.Higuera F, Rosenthal VD, Duarte P, Ruiz J, Franco G, Safdar N. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med. 2005;33:2022–2027. doi: 10.1097/01.ccm.0000178190.89663.e5. [DOI] [PubMed] [Google Scholar]
- 231.ronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 232.Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 233.Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201:349–358. doi: 10.1016/j.jamcollsurg.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 234.Lobo RD, Levin AS, Gomes LM, Cursino R, Park M, Figueiredo VB, et al. Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical intensive care unit in Brazil. Am J Infect Control. 2005;33:83–87. doi: 10.1016/j.ajic.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 235.Marschall J, Leone C, Jones M, Nihill D, Fraser VJ, Warren DK. Catheter-associated bloodstream infections in general medical patients outside the intensive care unit: a surveillance study. Infect Control Hosp Epidemiol. 2007;28:905–909. doi: 10.1086/519206. [DOI] [PubMed] [Google Scholar]
- 236.Rosenthal VD, McCormick RD, Guzman S, Villamayor C, Orellano PW. Effect of education and performance feedback on handwashing: the benefit of administrative support in Argentinean hospitals. Am J Infect Control. 2003;31:85–92. doi: 10.1067/mic.2003.63. [DOI] [PubMed] [Google Scholar]
- 237.Gastmeier P, Geffers C. Prevention of catheter-related bloodstream infections: analysis of studies published between 2002 and 2005. J Hosp Infect. 2006;64:326–335. doi: 10.1016/j.jhin.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 238.Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM., et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]