Abstract
Methods
A retrospective follow-up study was conducted among clients on ART from 2012 to 2017. Data were collected using checklists. The Kaplan-Meier curve was employed to compare survival rates. The Cox proportional hazard model was applied to identify predictors of time to development of anemia.
Results
A total of 490 ART patients were followed. The overall incidence of anemia was 27/100 person-years. The incidence was highest in the second year (18.7/100 PY) of starting ART when compared with the first year (13.8/100 PY) and third year (18.1/100 PY) of ART initiation. The independent predictors show an association for time to development of anemia and were as follows: being female (AHR = 2.94, 95%CI = 2.15–4.0), pulmonary tuberculosis positive (AHR = 2.98, 95%CI = 1.62–5.51), baseline weight < 60 kg (AHR = 1.51, 95%CI = 1.19-1.92), and severe acute malnutrition (AHR = 2.0, 95%CI = 1.39-2.89).
Conclusion
Most of the anemia cases occurred after the first year of ART initiation. Pulmonary tuberculosis, baseline weight, nutritional status, and sex were predictors for anemia. Clients with low baseline weight and abnormal nutritional status need to get close follow-up to prevent the risk of early development of anemia.
1. Background
Anemia is one of the world's most widespread health problems especially among HIV-infected individuals. It may result from the indirect effects of HIV infection, such as adverse reactions of medications such as zidovudine, opportunistic infections, neoplasms, and nutritional abnormalities arising from anorexia, malabsorption, or metabolic disorders [1–3].
Use of highly active antiretroviral therapy (HAART) is associated with an increase in hemoglobin concentrations and a decrease in the incidence of anemia. Moreover, there is wide variation in the incidence of anemia among HIV/AIDS patients in all over the world [4].
Anemia is a frequent complication that occurs in 20-80% of HIV-infected persons and is associated with faster disease progression and shorter survival. It is a major public health concern in children and adolescents in the developing countries [4, 5].
It is believed that, in developing countries, survival and risk factors of anemia among patients living with HIV depend on a variety of factors, which may also vary greatly with economic, demographic, behavioral risk, and health factors. Being female, lower CD4 count, higher HIV viral loads, and coinfection with tuberculosis (TB) were strongly associated with moderate and severe anemia [6, 7].
Studies conducted in India, Nigeria, and South Africa indicated that HIV-infected patients with age > 30 years, male gender, hemoglobin level < 11 g/dl, body weight < 45 kg, coinfection with HIV and TB, and CD4 count < 100/μl at baseline had significantly higher risk for anemia [8–10].
In Ethiopia, ART service began in 2003 and free ART was launched in 2005. An estimated number of 738,976 Ethiopians are currently living with HIV, and all of them require ART; however, only 426,000 are currently taking ART [11].
According to the 2016 Ethiopian Demographic and Health Survey (EDHS), the prevalence of HIV among women and men aged 15-49 in Ethiopia is 0.9%; HIV prevalence is higher among women than men (1.2% versus 0.6) [12]. Hence, the aim of this study was to assess time to development of anemia and predictors among HIV/AIDS patients initiating ART.
2. Methods
The study was conducted in Bahir Dar city at Felege Hiwot Referral Hospital from June 1 to July 31, 2018, among 490 study participants. Bahir Dar city is found in northwest Ethiopia, and it is 560 km away from Addis Ababa. According to the Bahir Dar city health department office report in 2017, the total population of Bahir Dar city was 348,429 [13]. In the city, there were 2 hospitals and 4 health centers in the governmental sector which provide free ART service for HIV/AIDS patients.
A retrospective follow-up study was conducted in Felege Hiwot Referral Hospital to assess time to development of anemia and predictors among HIV/AIDS patients initiating ART.
All HIV/AIDS patients aged greater than or equal to 15 years who were taking ART at Felege Hiwot Referral Hospital from January 2012 to December 2017 were the study population.
Patients were eligible for inclusion if they were HIV positive, aged 15 years or older, and taking ART and if their follow-up time was fixed. The key exclusion criteria considered if the diagnosis was made outside the health institution or if the patients were transferred in were as follows: women who were pregnant before the time of ART initiation or lactating mothers; HIV patients with incomplete intake form, registration form, and follow-up form; and patients who already had anemia at baseline or before the start of the follow-up period.
The total records in the hospital among eligible study population were 4,000. All the records of study subjects on ART follow-up during the five consecutive years with complete information were used to determine incidence and predictors of anemia among patients on ART. Each study participant was selected by using systematic random sampling method. From one random number in the patient's ART unique identification numbers as a starting point, every eighth record was taken as a study participant.
According to WHO classification, anemic patients were categorized into mild, moderate, and severe anemia: mild anemia (hemoglobin 9.0–10.9 g/dl), moderate anemia (hemoglobin 7.0–8.9 g/dl), and severe anemia (hemoglobin less than 7.0 g/dl).
Data were entered to Epi Info 7 and analyzed by using SPSS version 23. Anemia was confirmed by reviewing the patient card and medical registration in the ART clinic. Finally, the outcome of each subject was dichotomized into censored or event.
Kaplan-Meier method was used to compare survival curves. A range of measures of goodness-of-fit was checked using Schoenfeld's global test. Cox proportional hazard regression was employed to calculate adjusted hazard rate and then determine independent predictors of time to event.
2.1. Ethical Consideration
Ethical approval and clearance was given by the Institutional Review Board of Bahir Dar University, College of Medicine and Health Sciences. Permission was also obtained from the concerned bodies of Felege Hiwot Referral Hospital. To maintain confidentiality of people living with HIV/AIDS (PLWHA), health professionals working in the ART clinic were abstracting the data. In addition, no personal identifier was extracted on medical records and the recorded data was not accessed by a third person.
3. Results
3.1. Sociodemographic Characteristics
A total of 490 HIV/AIDS patients who were taking ART at Felege Hiwot Referral Hospital were included in the study. Among the participants, 306 (62.4%), 296 (60.4%), and 305 (62.2%) were females, orthodox, and living in urban residence, respectively (Table 1).
Table 1.
Sociodemographic characteristics of HIV-positive adults at initiation of HAART at Felege Hiwot Referral Hospital (January 2012 to December 2017) (n = 490).
| Variables | Category | n (%) |
|---|---|---|
| Sex | Male | 184 (37.6) |
| Female | 306 (62.4) | |
|
| ||
| Age (years) | 15–24 | 114 (23.3) |
| 25–34 | 133 (27.1) | |
| 35–44 | 130 (26.5) | |
| >45 | 113 (23.1) | |
|
| ||
| Ethnic group | Amhara | 465 (94.9) |
| Oromia | 8 (1.6) | |
| Tigray | 17 (3.5) | |
|
| ||
| Educational status | No education | 151 (30.8) |
| Primary school | 128 (26.1) | |
| Secondary school | 148 (30.2) | |
| Higher education | 63 (12.9) | |
|
| ||
| Marital status | Single | 142 (29.0) |
| Married | 209 (42.7) | |
| Divorced | 74 (15.1) | |
| Widowed | 33 (6.7) | |
| Separated | 32 (6.5) | |
|
| ||
| Residence | Rural | 185 (37.8) |
| Urban | 305 (62.2) | |
|
| ||
| Occupational status | Farmer | 75 (15.3) |
| Merchant | 132 (26.9) | |
| Governmental | 66 (13.5) | |
| Daily laborer | 50 (10.2) | |
| Driver | 32 (6.5) | |
| Unemployed | 49 (10) | |
| NGO | 66 (13.5) | |
| Others | 20 (4.1) | |
3.2. Baseline Clinical, Laboratory, and ART Information of the Study Subjects
Two hundred eighty-nine (59%) of patients were on WHO stage II at the time of HAART initiation, and WHO stage IV accounts for the lowest frequency among others, that is, 19 (3.9%). The mean ± SD of baseline CD4 count and weight for the study participants were 370 cells/μl ± 235 and 57 ± 11 kg, respectively. 255 (52%) participants used different prophylaxis, among them 140 (28.6%) received cotrimoxazole and 179 (36.5%) of the patients had mild acute malnutrition.
The predominant HAART regimen initially prescribed for 271 (55.3%) patients was a combination of stavudine, lamivudine, and efavirenz (1b = (40) d4t (40)/-3Tc-EFV) (Table 2).
Table 2.
Baseline clinical, biological and ART information of HIV-positive adults at initiation of HAART at Felege Hiwot Referral Hospital (January 2012 to December 2017) (n = 490).
| Variables | Category | n (%) |
|---|---|---|
| Weight at baseline | ≤60 | 187 (38.2) |
| >60 | 303 (61.8) | |
|
| ||
| BMI | Overweight | 60 (12.2) |
| Normal | 288 (58.8) | |
| Underweight | 142 (29.0) | |
|
| ||
| Functional status at baseline | Working | 224 (45.7) |
| Ambulatory | 202 (41.2) | |
| Bedridden | 64 (13.1) | |
|
| ||
| WHO staging at baseline | Stage 1 | 94 (19.2) |
| Stage 2 | 289 (59.0) | |
| Stage 3 | 88 (18.0) | |
| Stage 4 | 19 (3.9) | |
|
| ||
| Past prophylaxis | No | 235 (48.0) |
| Yes | 255 (52.0) | |
|
| ||
| CD4 count at baseline | <200 | 142 (29.0) |
| ≥200 | 348 (71.0) | |
|
| ||
| Baseline ART regimen | 1a = (30) d4t(30)/-3Tc-NVP | 6 (1.2) |
| 1a = (40) d4t(40)/-3Tc-NVP | 68 (13.9) | |
| 1b = (30) d4t(30)/-3Tc-EFV | 101 (20.6) | |
| 1b = (40) d4t(40)/-3Tc-EFV | 271 (55.3) | |
| 1c = AZT-3Tc-NVP | 27 (5.5) | |
| 1d = AZT-3Tc-EFV | 17 (3.5) | |
|
| ||
| Nutritional status | Severe acute malnutrition (BMI < 16 kg/m2) | 56 (11.4) |
| Moderate acute | 69 (14.1) | |
| Mild acute | 179 (36.5) | |
| Normal (BMI 18.5-24.9 kg/m2) | 186 (38) | |
BMI: body mass index; d4t: stavudine; 3Tc: lamivudine; NVP: nevirapine; EFV: efavirenz; AZT: zidovudine.
3.3. Incidence and Development of Anemia
A total of 490 study participants followed retrospectively for the last five years of whom 288 (58.7%) had developed anemia.
The study subjects had experienced anemia in 12,349 person-months (PM) of observations.
The overall incidence of anemia was 27/100 person-years (PY). The incidence was highest in the second year (18.7/100 PY) of starting ART when compared with the first year (13.8/100 PY) and third year (18.1/100 PY) of ART initiation. The median time to develop anemia was 32 months with IQR (29-34 months).
3.4. Predictors for Time to Development of Anemia
Based on the multivariable Cox regression analysis, sex, past exposure to pulmonary TB, baseline weight, and nutritional status showed a significant association (Table 3). Patients who are females had higher risk for developing anemia early compared to those who are males (AHR = 2.9, 95% CI: 2.15-4.0). Patients who had baseline weight ≤ 60 kg had higher risk for developing anemia early compared to those > 60 kg (AHR = 1.51 95% CI: 1.19-1.92). Severe acute malnourished patients had higher risk for developing anemia early compared to those patients who had normal nutritional status (AHR = 2.0 95% CI: 1.39-2.89). Patients who had past pulmonary TB had higher risk for developing anemia early compared to those who had no past pulmonary TB (AHR = 2.98, 95% CI: 1.61-5.51) (Figures 1 and 2).
Table 3.
Predictors of time to development of anemia among adult HIV-positive patients on HAART regimen at Felege Hiwot Referral Hospital (January 2012 to December 2017) (n = 490).
| Variables | Survival status | CHR (95% CI) | AHR (95% CI) | |
|---|---|---|---|---|
| Event | Censored | |||
| Sex | ||||
| Male | 49 | 135 | 1 | 1 |
| Female | 239 | 67 | 2.97 (2.18-4.05) | 2.94 (2.15-4.0) |
| Past toxoplasmosis | ||||
| No | 281 | 191 | 1 | 1 |
| Yes | 7 | 11 | 1.73 (0.82-3.68) | 1.68 (0.75-3.74) |
| Past pulmonary TB | ||||
| No | 277 | 200 | 1 | 1 |
| Yes | 11 | 2 | 3.02 (1.65-5.52) | 2.98 (1.61-5.51) |
| Baseline weight | ||||
| >60 | 159 | 144 | 1 | 1 |
| ≤60 | 129 | 58 | 1.45 (1.15-1.84) | 1.5 (1.19-1.92) |
| Current diarrhea | ||||
| No | 228 | 178 | 1 | 1 |
| Yes | 60 | 24 | 1.29 (0.97-1.72) | 1.16 (0.87-1.56) |
| Nutritional status | ||||
| Severe acute malnutrition | 46 | 10 | 2.37 (1.66-3.40) | 2.0 (1.39-2.89) |
| Moderate acute malnutrition | 44 | 25 | 1.86 (1.29-2.67) | 1.49 (1.03-2.16) |
| Mild acute malnutrition | 109 | 70 | 1.41 (1.06-1.87) | 1.2 (0.9-1.6) |
| Normal | 89 | 97 | 1 | 1 |
| Current extra pulmonary TB | ||||
| No | 279 | 196 | 1 | 1 |
| Yes | 9 | 6 | 1.78 (0.91-3.47) | 1.22 (0.62-2.42) |
| TB prophylaxis | ||||
| No | 275 | 185 | 1.71 (0.98-2.99) | 1.64 (0.93-2.91) |
| Yes | 13 | 17 | 1 | 1 |
Figure 1.
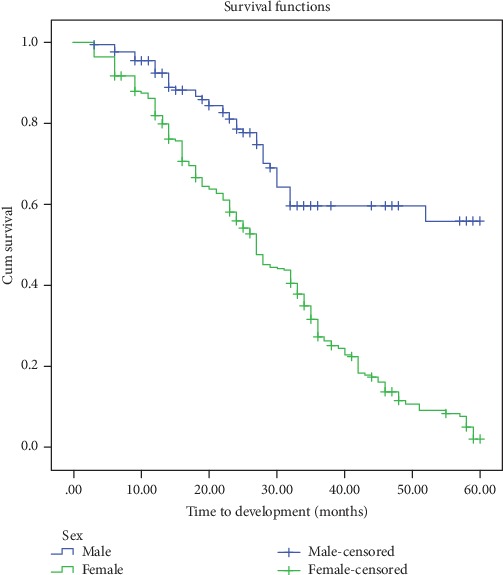
Kaplan-Meier curves for time to development of anemia, in months, among HIV patients on ART, Felege Hiwot Referral Hospital, 2012-2017, classified based on sex.
Figure 2.
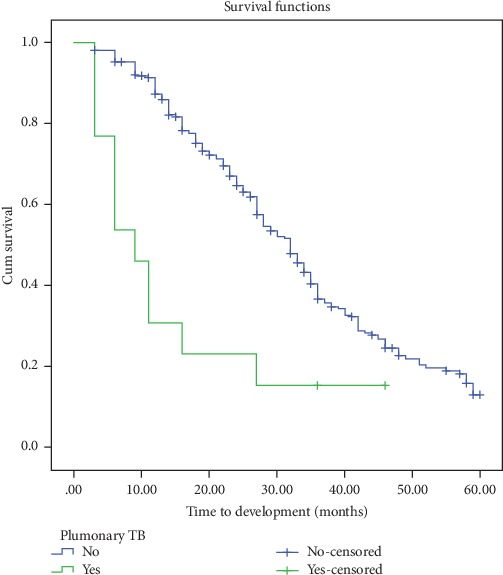
Kaplan-Meier curves for time to development of anemia, in months, among HIV patients on ART, Felege Hiwot Referral Hospital, 2012-2017, classified by past pulmonary tuberculosis exposure.
4. Discussion
This study determined the incidence rate, time to development of anemia, and its predictors among adult HIV patients taking ART at Felege Hiwot Referral Hospital.
From the total study subjects, 288 (58.8%) of them developed anemia. Of these events, 68 (13.8%) occurred in the first year, 92 (18.7%) in the second year, 89 (18.1%) in the third year, and the remaining in the fourth and fifth years. It was higher than the studies done in multicenters (35.5%) [14], University of Gondar (35%) [4], and southwest Ethiopia (16.2%) [15]. The possible reason for why the variation occurred is because some studies calculated the overall prevalence and others included HAART-naive patients.
The overall incidence of anemia was 27/100 PY with the highest value after 1 year of follow-up. This finding is supported by a study done in the Democratic Republic of the Congo which reported that the incidence of anemia was higher after one year of follow-up [16]. However, a study conducted in Addis Ababa and South Africa revealed that the highest incidence was observed after three years and six months of ART initiation, respectively [10, 17].
The difference between the current study and the previous studies mentioned above might be due to the fact that some of them studied young patients, where such patients need a long period of time to develop anemia. As the age is increased from time to time, the chance of developing anemia early also increased, and some of these studies had a long period of follow-up unlike the current study which is about 5 years.
In this study, female patients had 2.9 (95%CI = 2.15-4.0) times higher risk of anemia than male patients. This was supported by other studies done in Ethiopia [18], South Africa [7], and southern India [16].
This finding was opposite with the study conducted in Zewditu Memorial Hospital, Addis Ababa [1]. In which male patients had 1.55 times higher risk of anemia than patients who are female. The possible reason for the difference might be that in the current study the majority of participants were females, which may be largely attributed to menstrual blood loss. The other reason might be that in the study the majority of participants were old-aged males; evidences showed that early development of anemia would be high as the age increased [17].
Patients who had pulmonary TB in the past had 2.98 (95%CI = 1.6-5.5) times higher risk for developing anemia early than patients who had no previous pulmonary TB. Many studies done in different countries also supported this finding [19]. The study in South Africa showed that patients with pulmonary TB in the past had 1.4 times higher risk for developing anemia early as compared to patients who had no pulmonary TB in the past [10].
A study done in India revealed that patients who had pulmonary TB in the past also confirmed the association but with lower risk than the current study [20]. In the current study area, nutritional abnormality and opportunistic infections are common. So, patients had a low immunity system, and these are aggravated factors for early development of anemia.
Patients who had baseline weight ≤ 60 kg had 1.5 (95%CI = 1.19-1.92) times higher risk for developing anemia early compared to patients who had baseline weight > 60 kg. Other studies done in Southern Ethiopia also supported this finding in which the weight of patients increased by 1 kg and the risk of early development of anemia is decreased by 0.97 [21].
Another study done in Ethiopia, Kombat and Hadiya zones, supported this finding and explained that patients whose weight > 65 kg had a lower risk of developing anemia (0.55) [22].
The possible reason might be that patients who had low body weight are exposed to different types of infections. Due to this reason, they have no resistance for not developing anemia than those patients' weight > 65 kg.
Patients who had severe acute malnutrition had 2.0 (95%CI = 1.39-2.8) times higher risk for developing anemia than patients with moderate and mild malnutrition. And also, patients who had moderate and mild malnutrition had 1.5 and 1.2 times higher risk for developing anemia than patients who are normal, respectively.
The study done in Singapore also supported the current study where patients who had severe acute malnutrition had higher risk for developing anemia than patients with moderate and mild acute malnutrition [23].
Other studies done in Lebanon and Boston showed that patients who had malnutrition had higher risk to develop anemia since malnutrition, deficiencies of micronutrients, and complications with metabolism and body composition are common in HIV/AIDS-infected patients after initiation of ART [24].
4.1. Limitation of the Study
Using secondary data in which some important variables were not documented well and many opportunistic infections were presumed diagnoses, future studies will need to be carried out to develop interventions aimed at reducing the incidence of anemia in HIV-infected patients, and additional studies with longer follow-up time should be considered.
Hemoglobin measurement time of some study participants was not constant.
5. Conclusion
The overall incidence rate of anemia was high in the follow-up of patients in Felege Hiwot Referral Hospital.
A significant development of anemia was observed after the first year of starting HAART. Sex, past pulmonary tuberculosis exposure, baseline weight, and nutritional status showed a significant association with time to development of anemia.
Effective screening for anemia and giving enough advice for those people living with HIV/AIDS, especially among women and TB-exposed patients, should be designed. A well-integrated nutritional and HIV care system should be demanded to ensure the negative effect of low baseline weight and opportunistic infections like tuberculosis.
Acknowledgments
We would like to acknowledge all database workers, data collectors, and supervisor.
Abbreviations
- AIDS:
Acquired immunodeficiency syndrome
- ART:
Antiretroviral therapy
- AHR:
Adjusted hazard ratio
- CPT:
Cotrimoxazole prophylaxis therapy
- HAART:
Highly active antiretroviral therapy
- OI:
Opportunistic infection
- PY:
Person-year.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors (YM, AA, and EWM) have substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Assefa M., Abegaz W. E., Shewamare A., Medhin G., Belay M. Prevalence and correlates of anemia among HIV infected patients on highly active anti-retroviral therapy at Zewditu Memorial Hospital, Ethiopia. BMC Hematology. 2015;15(1):p. 6. doi: 10.1186/s12878-015-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zunke P., Ghat V., Waran M., Tyagi A. A study of prevalence of anemia among HIV patients and its correlation with clinical stage of aids, Cd4 count and antiretroviral therapy. International Journal of Medical Science and Clinical Inventions. 2017;4(2):2698–2701. [Google Scholar]
- 3.Lakhotia M., Tyagi A., Srivastava A. K. A study of prevalence of anemia by sociodemographic, clinical, and laboratory characteristics among HIV-positive patients. Asian Pacific Journal of Health Sciences. 2017;4(4):43–47. doi: 10.21276/apjhs.2017.4.4.12. [DOI] [Google Scholar]
- 4.Ferede G., Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Hematology. 2013;13(1):p. 8. doi: 10.1186/2052-1839-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunda D. W., Nkandala I., Kilonzo S. B., Kilangi B. B., Mpondo B. C. Prevalence and risk factors of mortality among adult HIV patients initiating ART in rural setting of HIV care and treatment services in North Western Tanzania: a retrospective cohort study. Journal of Sexually Transmitted Diseases. 2017;2017:8. doi: 10.1155/2017/7075601.7075601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obirikorang C., Yeboah F. A. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting. Journal of Biomedical Science. 2009;16(1):p. 102. doi: 10.1186/1423-0127-16-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerkhoff A. D., Wood R., Vogt M., Lawn S. D. Predictive value of Anemia for tuberculosis in HIV-infected patients in sub-Saharan Africa. Journal of Acquired Immune Deficiency Syndromes. 2014;66(1):33–40. doi: 10.1097/QAI.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaya Chakravarty N. K. T., Prasad S. R., Shukla S., Tiwari A., Mishra R. N., Sundar S. Determinants of survival in adult HIV patients on antiretroviral therapy in Eastern Uttar Pradesh: a prospective study. Indian Journal of Medical Research. 2014;140(4):491–500. [PMC free article] [PubMed] [Google Scholar]
- 9.Ndu A. C., Arinze-Onyia S. U., Aguwa E. N., Obi I. E. Prevalence of depression and role of support groups in its management: a study of adult HIV/AIDS patients attending HIV/AIDS clinic in a tertiary health facility in South-eastern Nigeria. Journal of Public Health and Epidemiology. 2011;3(4):182–186. [Google Scholar]
- 10.Takuva S., Maskew M., Brennan A. T., Sanne I., MacPhail A. P., Fox M. P. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. Journal of Tropical Medicine. 2013;2013:6. doi: 10.1155/2013/162950.162950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FMOH. National guidelines for comprehensive HIV prevention, care and treatment. Federal Ministry of health; 2017. [Google Scholar]
- 12.EDHS. Demographic and health survey 2016: key indicators report. The DHS Program ICF; 2016. [Google Scholar]
- 13.Bahir Dar city health department office. Bahir Dar city adminstration health department office annual report Bahir Dar. Bahir Dar city health department office; 2017. [Google Scholar]
- 14.Mildvan D., Creagh T., Leitz G., for the Anemia Prevalence Study Group Prevalence of anemia and correlation with biomarkers and specific antiretroviral regimens in 9690 human immunodeficiency virus-infected patients: findings of the anemia prevalence study. Current Medical Research and Opinion. 2007;23(2):343–355. doi: 10.1185/030079906X162683. [DOI] [PubMed] [Google Scholar]
- 15.Gedefaw L., Yemane T., Sahlemariam Z., Yilma D. Anemia and risk factors in HAART naïve and HAART experienced HIV positive persons in South West Ethiopia: a comparative study. PLoS One. 2013;8(8, article e72202) doi: 10.1371/journal.pone.0072202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaraman R. Anemia among HIV-infected individuals in South India. Yale Medicine Thesis Digital Library; 2007. [Google Scholar]
- 17.Lerebo W. T., Melaku Y. A., Girmay K. H., Wolde H. M. Incidence and risk factors of anemia among HIV/AIDS patients taking anti-retroviral therapy at tertiary hospitals in Addis Ababa, Ethiopia: a retrospective cohort study. Journal of HIV/AIDS and Infectious Diseases. 2013;2:1–6. doi: 10.17303/jaid.2014.303. [DOI] [Google Scholar]
- 18.Daka D., Lelissa D., Amsalu A. Prevalence of anaemia before and after the initiation of antiretroviral therapy at ART centre of Hawassa University Referral Hospital, Hawassa, South Ethiopia. Scholarly Journal of Medicine. 2013;3(1):1–6. [Google Scholar]
- 19.Kiragga A. N., Castelnuovo B., Nakanjako D., Manabe Y. C. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. Journal of the International AIDS Society. 2010;13(1):p. 42. doi: 10.1186/1758-2652-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbaraman R., Devaleenal B., Selvamuthu P., et al. Factors associated with anaemia in HIV-infected individuals in southern India. International journal of STD & AIDS. 2009;20(7):489–492. doi: 10.1258/ijsa.2008.008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hailemariam S., Tenkolu G., Tadese H., Vata P. K. Determinants of survival in HIV patients: a retrospective study of Dilla University Hospital HIV cohort. International Journal of Virology and AIDS. 2016;3(2):p. 23. doi: 10.23937/2469-567X/1510023. [DOI] [Google Scholar]
- 22.Ayele W., Mulugeta A., Desta A., Rabito F. A. Treatment outcomes and their determinants in HIV patients on anti-retroviral treatment program in selected health facilities of Kembata and Hadiya zones, Southern Nations, Nationalities and Peoples Region, Ethiopia. BMC Public Health. 2015;15(1):p. 826. doi: 10.1186/s12889-015-2176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paton N., Sangeetha S., Earnest A., Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Medicine. 2006;7(5):323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 24.Hendricks K., Gorbach S. Nutrition issues in chronic drug users living with HIV infection. Addiction Science & Clinical Practice. 2009;5(1):16–23. doi: 10.1151/ascp095116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


