Abstract
Background
The doublesex and mab-3 related transcription factor 1 (Dmrt1) is a highly conserved gene across numerous vertebrates and invertebrates in sequence and function. Small aminoacid changes in Dmrt1 are associated with turnovers in sex determination in reptiles. Dmrt1 is upregulated in males during gonadal development in many species, including the painted turtle, Chrysemys picta, a reptile with temperature-dependent sex determination (TSD). Dmrt1 is reported to play different roles during sex determination and differentiation, yet whether these functions are controlled by distinct Dmrt1 spliceoforms remains unclear. While Dmrt1 isoforms have been characterized in various vertebrates, no study has investigated their existence in any turtle.
Methods
We examine the painted turtle to identify novel Dmrt1 isoforms that may be present during urogenital development using PCR, profile their expression by RNA-seq across five embryonic stages at male- and female-producing temperatures, and validate their expression pattern via qPCR with transcript-specific fluorescent probes.
Results
A novel Dmrt1 spliceoform was discovered for the first time in chelonians, lacking exons 2 and 3 (Dmrt1 ΔEx2Ex3). Dmrt1 canonical and ΔEx2Ex3 transcripts were differentialy expressed by temperature at stages 19 and 22 in developing gonads of painted turtles, after the onset of sex determination, and displayed a significant male-biased expression pattern. This transcriptional pattern differs from studies in other turtles and vertebrates that reported Dmrt1 differential expression before or at the onset of sex determination. This study provides the first insight into Dmrt1 transcriptional diversity in turtles and opens the door for future functional studies of the alternative Dmrt1 transcript uncovered here.
Conclusions
The discovery of an isoform in turtles indicate that alternative splicing may be a common feature of Dmrt1 across vertebrates, as isoforms are also found in crocodilians, birds, mammals and fish, and this variation remains unexplained. The relatively late-onset of Dmrt1 expression observed here contrasts with other turtles, indicating that Dmrt1 is not the topmost male sex -determining factor in C. picta. When placed in a phylogenetic context, this discrepancy underscores the divergent regulation of Dmrt1, and of sexual development more generally, across vertebrates.
Keywords: Turtles, Spliceoforms, Chrysemys picta, Dmrt1, Sex determination, Sex differentiation, Transcriptional regulation evolution
Introduction
Sex determination is an important developmental process that contributes to the sexual fate of an individual. There are two extremes in the continuum of sex-determining mechanisms, one based on sexually dimorphic genomic content, called genotypic sex determination (GSD), and another on environmental factors, known as environmental sex determination (ESD) (Valenzuela, Adams & Janzen, 2003; Sarre, Georges & Quinn, 2004). Temperature-dependent sex determination (TSD) is the most common ESD mechanism in vertebrates, a polyphenism where individuals become male or female depending on the incubation temperature experienced by the developing embryo (Valenzuela & Lance, 2004; Bachtrog et al., 2014). TSD is present in many vertebrates, such as all crocodilians and tuatara, most turtles, some lizards and some fish (Valenzuela & Lance, 2004; Tree of Sex Consortium et al., 2014). Fewer examples are known of species with a mixed mechanism between GSD and TSD (Holleley et al., 2015; Yamamoto et al., 2014; Shine, Elphick & Donnellan, 2002) as some reports have been debunked (Mu et al., 2015; Valenzuela et al., 2014).
Many elements of the molecular network that regulate gonadal formation are shared between GSD and TSD species. In therian mammals, this process is triggered by Sry (sex-determining region of the Y chromosome), a gene that activates downstream genes of the male cascade (Eggers, Ohnesorg & Sinclair, 2014). All other vertebrates lack Sry, including TSD taxa, but non-Therians possess downstream genes in the male cascade, such as Sox9, Wt1 and Dmrt1 among others (Shoemaker & Crews, 2009) (Supplementary Material 1), some of which must be responsible for male determination and differentiation in the absence of Sry.
Within this molecular circuitry, an important component shared by vertebrates and invertebrates is Dmrt1 (doublesex and mab-3 related transcription factor 1), a member of the Dmrt gene family which is mainly characterized by the presence of a zinc-finger DNA-binding domain, the DM domain (Raymond et al., 2000). This DM domain was first described in Drosophila (doublesex gene –dsx) and in Caenorhabditis elegans (mab-3 gene) (Volff et al., 2003). Dmrt genes are involved in sexual development, more specifically in male-specific differentiation, being expressed mainly in developing gonads. However, some Dmrt members might also participate in neural and muscular development (Hong, Park & Saint-Jeannet, 2007). Dmrt1 is conserved at the nucleotide and protein sequence level, as well as in its function (Boyer et al., 2002; Bratus & Slota, 2006). Dmrt1 also contains a ‘male-specific domain’ which is conserved across vertebrates and invertebrates (Jeong et al., 2009; Guan, Kobayashi & Nagahama, 2000), and is so called for its discovery in Drosophila where its sex-specific splicing produces dsxm (Burtis & Baker, 1989; Burtis et al., 1991). The dsxm isoform contains the male-specific region that binds to enhancers, promoting tissue-specific genes that repress genes related to female development (Coschigano & Wensink, 1993). A third important Dmrt1 domain is the P/S-rich region, a non-DNA-binding domain involved in the transcription machinery, playing a role in the binding of enhancers and inhibitors that regulate mRNA production (Latchman, 1997). Dmrt1 is not just an important element for vertebrate sexual differentiation, but in some vertebrates it is the top-most sex-determining gene. For instance, Dmrt1 and its orthologs and paralogues were proposed as the male sex-determining gene in chicken (Smith et al., 2009), medaka fish (dmy) (Matsuda et al., 2002) and in the Chinese tongue sole (Cui et al., 2017; Herpin & Schartl, 2015). Notably, chicken Dmrt1 is located in the Z-chromosome and works in a dosage manner (Teranishi et al., 2001). In contrast, the Dmrt1-paralogue DM-W is a female sex-determining gene in the frog Xenopus laevis, acting as an antagonist of Dmrt1 expression (Yoshimoto et al., 2010). In contrast, in some species Dmrt1 functions mainly in sexual differentiation rather than determination. For instance, in the rainbow trout, rtDmrt1 is upregulated in males during testicular differentiation, and during spermatogenesis in adults (Marchand et al., 2000). In Rana rugosa, Dmrt1 is also upregulated only during sex differentiation (Shibata, Takase & Nakamura, 2002). In the pejerrey, Odontesthes bonariensis, a fish with a mixed TSD+GSD system (Yamamoto et al., 2014; Hattori et al., 2012), Dmrt1 is upregulated at male-producing temperatures (MPT) compared to female-producing temperatures (FPT) during gonadal development (Fernandino et al., 2008). Consistently, Dmrt1 exhibits temperature-dependent expression in TSD reptiles, a lineage where the molecular evolution of Dmrt1 is associated with turnovers in sex determination between TSD and GSD (Janes et al., 2014). Indeed, Dmrt1 is upregulated under MPT starting at early embryonic stages in the TSD turtles Trachemys scripta, Lepidochelys olivacea and Chelydra serpentina, as well as the crocodile Crocodylus palustris (also TSD) (Kettlewell, Raymond & Zarkower, 2006; Torres Maldonado et al., 2002; Rhen et al., 2007; Anand et al., 2008). In T. scripta turtles and chicken, this early Dmrt1 male-biased expression precedes the expression of other genes relevant for male development, including Sox9, suggesting that Dmrt1 is upstream of Sox9 in their sex determination cascade (Kettlewell, Raymond & Zarkower, 2006; Smith et al., 2009; Czerwinski et al., 2016). Furthermore, recent studies report that Dmrt1 is required for male development in T. scripta (Ge et al., 2017) and in the GSD turtle Pelosdiscus sinensis (Sun et al., 2017). Thus, Dmrt1 can act either in sex determination (deciding the sexual fate of the bipotential gonad) and in sex differentiation (contributing to the ensuing tissue development of the gonad) depending on its expression profile in particular species (Bratus & Slota, 2006).
So how does Dmrt1 carry out these different roles? Genes may accomplish multiple functions by producing multiple isoform via alternative splicing (Anand et al., 2008), a process that allows a higher proteomic complexity without increasing genomic size. Some isoforms are important in sexual development. For instance, Wt1 has 36 different reported isoforms, two of which (+KTS and –KTS) participate in sexual development (Bandiera et al., 2015). Each isoform encodes for a protein with a distinct DNA binding profile that differs in function, one being essential for male development and the other for survival of the gonadal primordium (Hammes et al., 2001). Because Dmrt1 plays multiple roles in sexual development, including cell fate determination, postnatal Sertoli cells and primordial germ cells maintenance (Capriglione et al., 2010), an open question is whether those functions are undertaken by alternative Dmrt1 isoforms. While Dmrt1 isoforms have been reported in the TSD Crocodylus palustris (Anand et al., 2008) and in other vertebrates such as chicken, mice, zebrafish and rice field eel (Lu et al., 2007; Gao et al., 2005; Huang et al., 2005; Zhao et al., 2007b; Zhao et al., 2007a), no Dmrt1 isoforms have been described in turtles.
Here we test the hypotheses that Dmrt1 isoforms exist in turtles and are differentially regulated by temperature, using the TSD painted turtle (Chrysemys picta) and leveraging RNA-seq data from a parallel study. Initial studies in C. picta profiled the Dmrt1 expression in adrenal-kidney-gonad (AKG) complexes only (Valenzuela, 2010) without distinguishing between canonical transcripts and other isoforms that may exist, and no differential expression was detected. Later transcriptomic approaches (Radhakrishnan et al., 2017) revealed higher Dmrt1 expression in developing testis during the thermosensitive period when gonads were analyzed separately, a pattern that had been masked by the adrenal-kidney (AK) expression when using AKGs (Valenzuela, 2010). These first transcriptomic data from painted turtles (Radhakrishnan et al., 2017) also revealed that RNA-seq is less sensitive than qPCR to detect subtler but significant differential expression, including at early stages of development for other important genes in the sexual development network (Supplementary material 1) such as Wt1, Sf1, Dax1, Sox9, and Aromatase (Valenzuela, Neuwald & Literman, 2013; Valenzuela, 2010; Valenzuela, LeClere & Shikano, 2006; Valenzuela & Shikano, 2007; Valenzuela, 2008a). Thus, here we (1) test for the presence of Dmrt1 isoforms during the gonadal development of C. picta, and (2) test whether the canonical transcript or isoforms of Dmrt1 exhibit differential expression by temperature consistent with Dmrt1’s role in sex determination or sexual differentiation in this TSD turtle using RNA-seq and qPCR validation.
Methods
Eggs collection, incubation, and tissue dissection
Freshly laid eggs were collected from a turtle farm and transported in moist vermiculite to the laboratory for incubation following standard protocols (Valenzuela, 2009). Specifically, eggs were cleaned from excess mud, marked with a unique ID, randomly assigned to boxes with moist sand (30 eggs per box), and placed in incubators at 26 °C (Male Producing Temperature—MPT) and 31 °C (Female Producing Temperature—FPT). Boxes were rotated daily in a clockwise fashion to control for potential temperature gradients within the incubators. Moisture inside the egg boxes was maintained constant by replacing evaporated water weekly. Embryonic development was monitored by egg candling. Embryos and tissues (detailed below) were dissected at stages before (stages 9 and 12), at the onset of (stage 15), in the middle of (stage 19), and at the end of (stage 22) the thermosensitive period (TSP) for C. picta (sensu (Yntema, 1968; Bull & Vogt, 1981)) and stored in RNA later (Invitrogen) at −20 °C until processing. All samples were treated identically.
RNA extraction and cDNA conversion
RNA was extracted from trunks of stage 9 embryos when the gonadal primordium cannot be separated (n = 15 per T° per replicate), adrenal-kidney-gonad (AKG) complex of stage 12 embryos (when genital ridge may be present in C. picta given that urogenital tissue is present by this stage in T. scripta (Spotila, Spotila & Hall, 1998), from AKG of stage 15 embryos (n = 15 per T° per replicate) (when bipotential gonads could not be separated from AK), and from separated gonads from stage 19 (n = 13 per T° per replicate) and stage 22 (n = 12 per T° per replicate) embryos using Qiagen Rneasy™ Mini (stages 9, 12 and 15) and Micro (stages 19 and 22) kits, following the manufacturer’s instructions. RNA was quantified using a NanoDrop Spectrophotomoter and RNA quality was assessed by the presence of ribosomal bands in 1% agarose gels. Extracted RNA was stored at −80 °C until processing. All RNA was extracted from individual embryos and 200 ng to 1 µg of RNA was retro-transcribed to cDNA by RT-PCR using Invitrogen SuperScript™ VILO™ Synthesis kit following the manufacturer’s instructions. cDNA was stored at −20 °C.
Identification of Dmrt1 isoforms by PCR and Sanger sequencing
Primers (Dmrt1-F: 5′ CTT GTT AGC CGA ACC TCT CT 3′ and Dmrt1-R: 5′ AGA ATG CAC TTG ATC TCC TG 3′) were designed at the untranslated region (UTR) of the Dmrt1 gene, based on the C. picta genome (Badenhorst et al., 2015; Shaffer et al., 2013) using Geneious (Kearse et al., 2012). PCR amplification of Dmrt1 transcripts used 1 µL of pooled cDNA (from all stages and temperatures) as template in 15 µL reactions containing 1X Taq buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 µM of each primer (Dmrt1-F and Dmrt1-R), 0.4U Taq polymerase and 10.5 µL water. PCR conditions included an initial denaturing step at 94 °C for 3 min, followed by 35 cycles of denaturing at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 90 s. Amplicons were visualized in 0.8% agarose gel stained with EtBr, and their size estimated using a 1 kb plus ladder (Invitrogen). Amplicon bands were cut from the agarose gel, placed in 50 µL distilled water, incubated 5 min at 65 °C, 10 min at −80 °C and then centrifuged 10 min at 4 °C, after which 1 µL was used as template in a secondary 50 µL PCR reaction using an annealing temperature of 61 °C. PCR products from reactions that yielded a single amplicon were cleaned using Ampure beads, and Sanger sequenced. DNA sequences were analyzed in Geneious (Kearse et al., 2012) to assess their quality, aligned by BLAST to the C. picta genome assembly 3.0.3 (Badenhorst et al., 2015) for annotation and to assess the similarity of any isoforms to the canonical Dmrt1 transcript.
Identification and expression profiling of Dmrt1 isoforms from RNA-seq data
Differential expression analysis of RNA-Seq data
To complement the identification of isoforms by PCR, we leverage RNA-seq data obtained in duplicate for a parallel study from C. picta embryos at five developmental stages, incubated at 26 °C and 31 °C (MPT and FPT, respectively) (see Data Availability). Total RNA was extracted as described above and 20 duplicate mRNA libraries from the same 5 stages and 2 temperatures were constructed using 1 µg total RNA pooled from 11–15 embryos (equal RNA amount per embryo) per stage per temperature per replicate, using the KAPA Stranded mRNA-seq kit (KK8421). Libraries were sequenced using Illumina’s HiSeq 4000 protocol, which generated 50 million 150 bp paired-end cleaned reads per library on average.
The bioinformatics pipeline employed here is illustrated in Supplementary Material 2A and scripts are provided in Supplementary material 3. We used the Chrysemys picta genome version 3.0.3 (NCBI) (Badenhorst et al., 2015) as reference genome. Initial quality control of raw reads was carried out with FASTQC (Andrews, 2010), followed by removal of adapters and low-quality reads using Trimmomatic (Bolger, Lohse & Usadel, 2014). Trinity (Haas et al., 2013) was used for read normalization. We used HISAT2 (Kim, Langmead & Salzberg, 2015) to map the reads to the C. picta reference genome. Mapping was conducted separately by stage and temperature, with 2 replicates each. All steps of the RNA-seq analysis were run at the High Performance Computing facility from Iowa State University.
Then we used Samtools view tool (Li et al., 2009) to select the reads that were uniquely mapped to the reference genome to avoid false positives and enhance the accuracy during the assembly of transcripts. Gene annotations were retrieved from NCBI and used as input along with the uniquely mapped and normalized reads, to assemble the transcripts using StringTie (Pertea et al., 2016). For the differential expression analysis, we pooled together all transcripts from all stages and temperatures produced by StringTie, to assemble an overall transcriptome using the StringTie merge tool.
Read counts were quantified with Kallisto (Bray et al., 2016) (Supplementary Material 4) using the transcriptome generated by StringTie and the normalized reads as input. We used DESEQ2 to assess differential expression based on the read counts, and significance was assessed at an alpha of 0.05.
Because not all Dmrt1 transcripts that were detected by PCR and Sanger sequencing were present in the transcriptome assembled by using the whole genome as a reference, we developed an alternative sub-genomic approach as follows. First, we extracted the scaffolds assigned to chromosome 6 of the C. picta 3.0.3 genome where Dmrt1 is located, plus all the unplaced scaffolds containing the other Dmrt members. We refer to this reference sub-genome as CPI-6U hereafter. This CPI-6U ‘reference sub-genome’ was then used to map the reads and assemble Dmrt1 transcripts. We followed the same steps as with the whole genome approach and compared the results. The results were robust to using the Tuxedo pipeline (Trapnell, Pachter & Salzberg, 2009; Trapnell et al., 2012).
Validation of Dmrt1 isoform identification by splice junction analysis
Additionally, in a third approach, we tested for the presence of all potential Dmrt family isoforms in our RNAseq data by examining the mapping of the RNA-seq reads to all splice junctions of all Dmrt genes using Geneious (Kearse et al., 2012) (Supplementary Material 2B), in order to avoid misidentifying other Dmrt transcripts as Dmrt1 isoforms . First, we mapped reads to a reference containing all Dmrt gene regions (first mapping step), and retained all reads that mapped to Dmrt genes and exons junctions to produce a reduced read dataset that was then mapped to a second reference file containing all possible exon junction combinations for each Dmrt gene. Results were inspected manually to identify any reads mapped to junctions undetected in previous steps and which would indicate the existence of additional isoforms in our RNA-seq data.
qPCR validation
We validated our RNA-seq data results further by using qPCR and Taqman probes, which also permitted us to profile transcript-specific expression during embryonic development at MPT and FPT in individual embryos. Primers and Taqman probes were designed for each Dmrt1 isoform (Table 1), as well as for ß-actin, a housekeeping gene used for normalization of gene expression by qPCR in previous studies of C. picta (Valenzuela, Neuwald & Literman, 2013), and whose expression was steady between temperatures and across stages in our study as determined by ANOVA (Supplementary Material 5). Expression data by qPCR was obtained from 12-15 embryos per temperature (MPT and FPT) per stage (these embryos were different from the embryos used for RNA sequencing). Dmrt1 Taqman probes were designed to match unique exon junctions of each isoform, in order to profile isoform-specific expression. qPCR was carried out in an Mx3000P real time PCR thermal cycler (Stratagene) using IDT Prime Time Gene Expression Mastermix. This mastermix already contains the DNA polymerase, dNTPs, MgCl2, enhancers and stabilizers, in concentrations undisclosed by the manufacturer. Optimization was run for individual genes using 6 different concentrations of primers (100 nM, 200 nM, 300 nM, 400 nM, 500 nM, 600 nM) and a fixed concentration of probe (250 nM) followed by probe optimization, with 6 different concentrations of probes (100 nM, 150 nM, 200 nM, 250 nM, 300 nM, 350 nM) combined with the optimal primer concentration of each gene. Later, multiplex optimization reactions with optimal primers and probes concentrations were tested, containing 5 uL of commercial mastermix, ß-actin forward and reverse primers, ß-actin Taqman probe, Dmrt1 forward and reverse primers for either the canonical or the non-canonical isoform and the corresponding Taqman probe, 2 uL of cDNA and water to 15 ul. Thermal profile was 95 °C for 3min followed by 50 cycles of 95 °C for 30s and 60 °C for 1min. All reactions were run in duplicate. A standard curve was generated for each transcript by pooling RNA from all samples (100 ng per individual), and then diluting the pooled RNA using 1:5 ratio, to obtain a total of eight standards. Standards were included in duplicate in each qPCR plate. Standard curves were used to calculate qPCR efficiency and R2 values.
Table 1. Primer and Taqman probe sequences used for multiplex qPCR and optimal ß-actin concentrations.
| Transcript | Sequences | Optimal ß-actin concentration |
|---|---|---|
| ß-actin | Forward: 5′ TGTGCTGCTTACAGAGG 3′ Reverse: 5′ GTACGACCAGAGGCCTA 3′ Probe: 5′/CY5/GCCAACAGAGAAAAGATGACACAGATC 3′ | 500 nM (c)/200 nM (i) 500 nM (c)/200 nM (i) 250 nM (c)/100 nM (i) |
| Dmrt1 canonical | Forward: 5′ CCAACACATTCAACAAACA 3′ Reverse: 5′ ACTGCTGTAGTAGGTGGAGTC 3′ Probe: 5′/FAM/ATCAGAGGGACGGATGCTCATTCAG 3′ | 500 nM 500 nM 300 nM |
| Dmrt1 isoform | Forward: 5′ TACTCCTCGCCACTGAA 3′ Reverse: 5′ CACTCTGGCCCAGGTAG 3′ Probe: 5′/FAM/TGGCAGCCAGATGAAAAGCACAG 3′ | 600 nM 600 nM 300 nM |
Notes.
- (c)
- ß-actin concentration for PCR of Dmrt1 canonical transcript
- (i)
- ß-actin concentration for PCR of non-canonical isoform
Data analysis
Samples with Cq values deviation >0.5 were discarded from further analysis. All samples displayed coefficient of variation <10% between technical replicates and were thus included in the analyses. Relative expression was calculated using the Pfaffl method (Pfaffl, 2001) (which takes into account differences in reaction efficiencies to calculate relative expression values), with ß-actin as the reference gene for normalization. An ANOVA was used to test for differences in Dmrt1 expression between temperatures across stages, and post hoc t-tests were then used to identify the stages at which differential expression was significant (at an alpha of 0.05).
Results
Here, we investigated the transcriptional dynamics of the Dmrt1 gene, in terms of its alternative splicing and thermosensitive transcription in the TSD turtle Chrysemys picta. First, we investigated whether Dmrt1 produces alternative spliceoforms to the canonical transcript, and second, we profiled the expression of the identified transcripts, to build working hypotheses about the potential role of Dmrt1 in the sexual development of C. picta that could guide future functional assays.
Alternative splicing
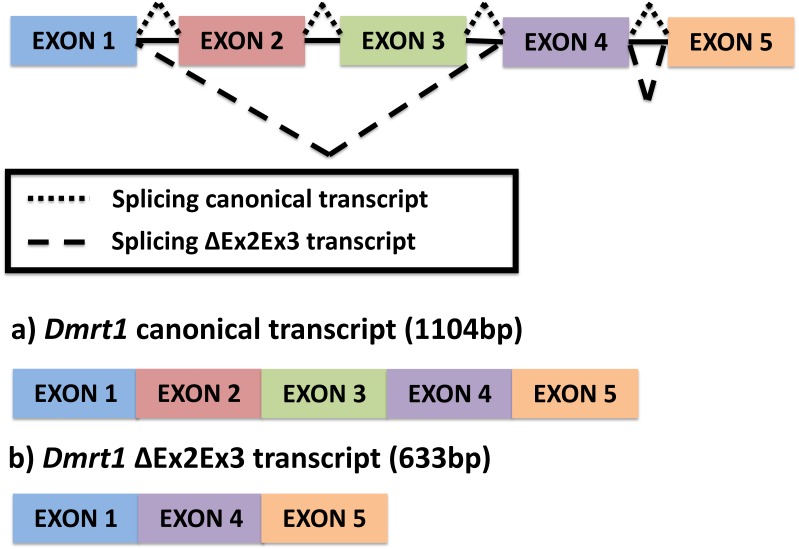
A single novel isoform was detected by PCR amplification using primers at the UTR region of the Dmrt1 gene sequence, followed by Sanger sequencing. In contrast to the canonical Dmrt1 transcript that contains all 5 exons of the Dmrt1 gene, the novel Dmrt1 isoform lacks exons 2 and 3 (Fig. 1) (hereafter referred to as Dmrt1 ΔEx2Ex3). Likewise, our mapping of RNA-seq reads to the full set of potential junctions of all exon pairs for Dmrt1 (and for all other Dmrt genes in the painted turtle genome) detected this unique alternative spliceoform exclusively and no other alternative Dmrt1 transcript.
Figure 1. Alternative splicing of Dmrt1 in Chrysemys picta turtles discovered in the present study.
The full canonical Dmrt1 (hereafter referred to as Dmrt1) cDNA sequence is 1104 bp long. This canonical Dmrt1 transcript encodes a protein containing the three characteristic domains that are conserved in the Dmrt1 of other vertebrates, namely, the DM domain, the male-specific domain, and the proline- (P-) and serine-(S-)rich regions (Adolfi et al., 2015). On the other hand, the novel Dmrt1 ΔEx2Ex3 cDNA is 633 bp long, and lacks the male-specific domain and the P- and S-rich region. Both transcripts were detected by qPCR at all stages and tissues examined, but their expression was accentuated at MPT at stage 19 in the individual gonads, as described below.
Dmrt1 expression profiling by RNA-seq
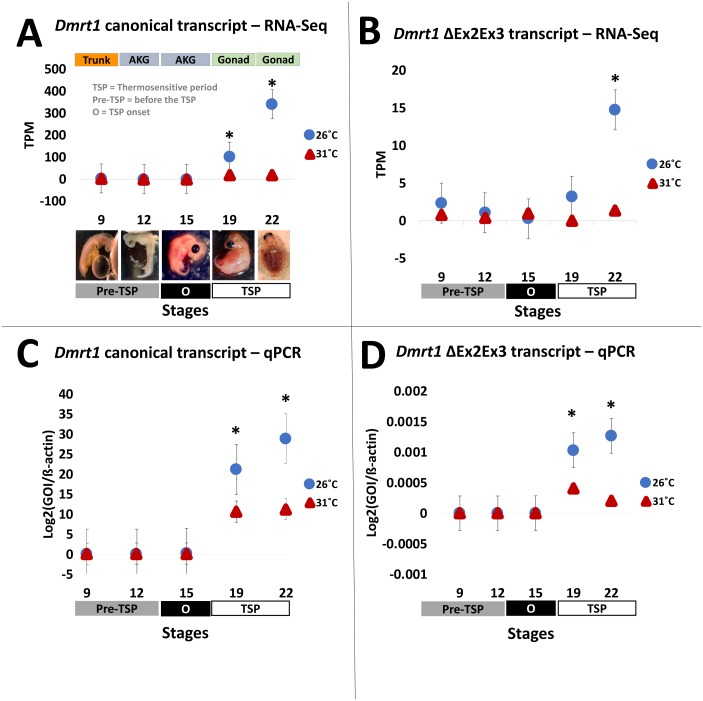
Results from our quantification analysis using duplicate RNA-seq experiments, indicate that the canonical Dmrt1 transcript in the painted turtle exhibits very low and monomorphic expression before the thermosensitive period in the embryonic trunks (stage 9) and AKGs (stages 12 and 15), but it becomes significantly upregulated under MPT in the gonads alone at stages 19 and 22 (Figs. 2A–2B), showing maximal expression towards the end of the thermosensitive period (stage 22). However, the Dmrt1 ΔEx2Ex3 transcript was not detected in RNA-Seq analysis at any stage and temperature when using the whole genome analysis approach. However, when using the CPI-6U reference sub-genome, the Dmrt1 ΔEx2Ex3 transcript was assembled successfully. The sub-genome approach revealed that Dmrt1 ΔEx2Ex3 has quite a similar expression pattern to the canonical Dmrt1 transcript, with monomorphic expression before the thermosensitive period followed by significant differential expression at MPT at stage 22. However, expression levels of Dmrt1 ΔEx2Ex3 were orders of magnitude lower compared to the canonical transcript. Expression levels for ß-actin, used as the normalizer gene, did not differ significantly across stages and temperature treatments (Supplementary Material 5) as accessed by an ANOVA (F = 14.76, p = 0.52).
Figure 2. Transcription of Dmrt1 spliceoforms during embryonic development of Chrysemys picta turtles.
Averge expression level of canonical and Dmrt1 ΔEx2Ex3 transcripts assessed by RNA-Seq (A,B) and by qPCR using TaqMan probes (C,D). Bars represent standard deviations. *, significant differences in expression between male- and female-producing temperatures (p < 0.05).
qPCR validation
All qPCR reactions had standard curves with an R2>0.96 and all negative controls yielded no amplification. The qPCR results mimicked the RNA-seq results obtained for the canonical Dmrt1 transcript. Namely, Dmrt1 was upregulated in embryos incubated at MPT compared to FPT, a pattern that became significant at stage 19 and was accentuated at stage 22 (Figs. 2C–2D). qPCR results revealed that the abundance of the Dmrt1 ΔEx2Ex3 transcript is an order of magnitude lower than for the Dmrt1 canonical transcript, although ΔEx2Ex3 is also upregulated at MPT compared to FTP at stage 19 and 22 (whereas the CPI-6U RNA-Seq analysis detected ΔEx2Ex3 upregulation at MPT only at stage 22).
Discussion
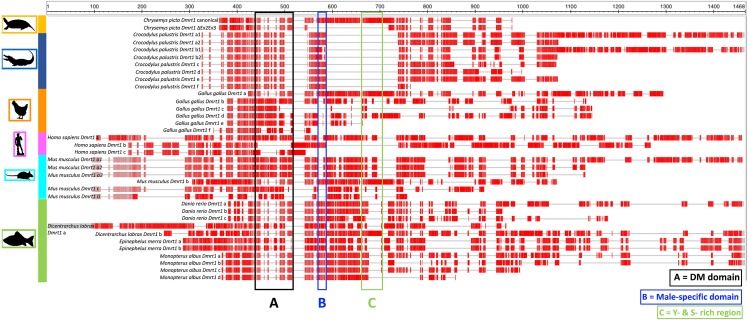
In this study we identified a novel Dmrt1 spliceoform in Chrysemys picta, a TSD turtle, the first report of a Dmrt1 isoform in any turtle irrespective of their sex-determining mechanism. C. picta’s novel isoform is unique in its sequence compared to the Dmrt1 isoforms that have been identified in other vertebrates including fish, crocodilians, birds and mammals (Table 2, Fig. 3). While most Dmrt1 non-canonical transcripts described to date in C. picta and other species retain the highly conserved DM domain (an exception is found in mouse (Lu et al., 2007)), such conservation is not preserved in other domains as described below. Namely, compared to the canonical Dmrt1 transcript, C. picta’s novel Dmrt1 ΔEx2Ex3 isoform retains exon 1 (where the DM domain is located) but it differs at the 3′ end downstream the DM domain where it lacks exons 2 and 3. Changes at the 3′ region downstream of the DM domain are also observed in three different Dmrt1 isoforms in zebrafish (Guo et al., 2005), in the Indian mugger, a TSD crocodilian that produces eight Dmrt1 isoforms (Anand et al., 2008), and in chicken whose Dmrt1 generates six isoforms (Anand et al., 2008; Zhao et al., 2007a). On the other hand, the European sea bass produces two Dmrt1 isoforms that differ by a 78 bp insertion, creating a separation between the Tyrosine (Y)- and the Serine (S)-rich domains (Deloffre et al., 2009), whereas the mouse generates Dmrt1 isoforms that either lack the Y-rich region, lack both Y- and S-rich regions, or lack the DM domain (Lu et al., 2007). The Y- and S-rich domain differs from the P- and S- domain, and are not encountered in all vertebrates that encode Dmrt1. The specific role of the Y- and S-rich domain remains unknown, but some speculate that it may be important for the DM domain protein dimerization (Deloffre et al., 2009). Other vertebrate Dmrt1 isoforms from various taxa analyzed in this study also differ in the presence or absence of the male-specific domain and/or the P- and S-rich region (that are present in exons 2 and 3 of C. picta) (Fig. 3). These isoforms are all male-specific (except for chicken Dmrt1c), and their expression was also lower compared to their respective canonical transcripts. Besides the presence/absence of characteristic domains, the transcription splice sites vary, leading to the formation of isoforms of varying length according to the number of exons that are included/excluded. There is no consensus about the specific function of the diversity of Dmrt1 isoforms, and their expression profiles in other vertebrates led to the still untested hypothesis that they could be involved in regulating the canonical transcript, acting as coregulatory factors, and thus mediating sexual development (Lu et al., 2007; Anand et al., 2008).
Table 2. Number of Dmrt1 isoforms reported to date in vertebrates.
| Group | Species | # of isoforms | Source |
|---|---|---|---|
| Fish | Zebrafish | 3 | Guo et al. (2005) |
| Fish | European sea bass | 2 | Deloffre et al. (2009) |
| Fish | Honeycomb grouper | 2 | Alam et al. (2008) |
| Fish | Rice field eel | 4 | Huang et al. (2005) |
| Turtle | Painted turtle | 2 | This study |
| Crocodilians | Indian mugger | 8 | Anand et al. (2008) |
| Birds | Chicken | 6 | Zhao et al. (2007b) |
| Mammals | Mouse | 4 | Lu et al. (2007) |
| Mammals | Human | 3 | Cheng et al. (2006) |
Figure 3. Protein alignment of canonical and ΔEx2Ex3 Dmrt1 transcripts in Chrysemys picta and isoforms from selected vertebrates.
Protein sequences from the canonical and Dmrt1 isoforms correspond to those listed in Table 2. Red blocks illustrate conserved regions, gray horizontal lines illustrate sequence gaps. Colored vertical boxes denote Dmrt1 domains. See Supplementary Material 6 for full alignment.
Our expression profiling revealed the transcriptional dynamics of the novel turtle isoform. C. picta’s Dmrt1 ΔEx2Ex3 transcript was upregulated at stages 19 and 22 at MPT, but its transcription was an order of magnitude lower compared to the canonical transcript. This is consistent with most Dmrt1 isoforms described in vertebrates which also display male-biased expression and lower expression compared to the canonical transcript (Lu et al., 2007; Guo et al., 2005; Anand et al., 2008; Deloffre et al., 2009; Zhao et al., 2007a). An exception is Dmrt1c in chicken, which is upregulated in females at stage 31 of embryonic development, which corresponds to the time of gonadal differentiation (Zhao et al., 2007a). It has been hypothesized that isoforms derived from alternative splicing might act as transcriptional regulator of the canonical transcript by affecting access to its activators or repressors (Lu et al., 2007; Anand et al., 2008). It is unclear whether the novel Dmrt1 ΔEx2Ex3 isoform identified in C. picta plays a functional role despite its low transcription level as occurs for other genes in other taxa. For instance, differences in isoform abundance of glucocorticoid receptor (GR), some of which are expressed at very low levels, are linked to immune thrombocytopenia (ITP) (Hamed et al., 2015; Ma et al., 2013). In particular, the transcription of isoform GRß is extremely low compared to the isoform GRα, such that GRß protein is undetectable (Ma et al., 2013). However, GRß mRNA transcript works as a regulator of GRα mRNA transcript activity, and disruption of the GRα/GRß ratio may be related to resistance to glucocorticoids in ITP (Hamed et al., 2015), as this ratio predicts how the cell will respond to glucocorticoid treatments. Our data show that the Dmrt1 canonical/ΔEx2Ex3 ratio responds to temperature in stage 15 AKGs of C. picta (Table 3), which corresponds with the onset of the TSP, a critical time for sex determination (though the expression of each individual isoform did not vary significantly by temperature at stage 15). However, the hypothesis that the Dmrt1 canonical/ΔEx2Ex3 ratio plays an important role in the sexual development of C. picta requires functional testing, as well as isolation of gonadal from AK expression before stage 19. Also unknown is whether Dmrt1 thermosensitive transcription in C. picta is epigenetically regulated as it is in the TSD turtle T. scripta (Ge et al., 2018).
Table 3. Ratio of Chrysemys picta’s Dmrt1 transcripts (canonical/ΔEx2Ex3) at the embryonic stages studied.
| Stage 9 | Stage 12 | Stage 15 | Stage 19 | Stage 22 | |
|---|---|---|---|---|---|
| 26 °C | 278428.7 | 278428.7 | 106777.1 | 20465.8 | 22812.3 |
| 31 °C | 28134.7 | 119667.9 | 99133.6 | 26103.9 | 54215.8 |
| p-value | 0.4359 | 0.2416 | 0.02362 | 0.0767 | 0.2464 |
Notes.
Statistical significance between temperatures was assessed by t-tests with alpha = 0.05.
Our results also provide important novel insights into the expression of the canonical transcript of Dmrt1 in C. picta, which was upregulated under MPT at stages 19 and 22. These results corroborate the expression pattern of Dmrt1 observed in C. picta gonads in a previous transcriptomic analysis that used a single replicate (Radhakrishnan et al., 2017) providing a much needed validation. These observations contrast with previous qPCR studies (Valenzuela, 2010) that failed to detect significant differential expression of Dmrt1 between individuals incubated at MPT and FPT at the same developmental stages examined here. The discrepancies are likely due to technical differences between studies. Specifically, in the earlier study (Valenzuela, 2010) expression at stages 19 and 22 was profiled in AKG complexes such that the expression from AK tissue likely masked the expression from gonadal tissue alone, thus obscuring the significant differences between temperatures that we detected here and in Radhakrishnan et al. (2017). The same masking phenomenon was reported in C. picta and other turtles for a number of other genes (Valenzuela, Neuwald & Literman, 2013; Ramsey & Crews, 2007). Likewise, care should be taken when interpreting our results, since extra gonadal tissues were included in our analysis at stage 9 (trunks) and at stages 12 and 15 (AKGs) due to the difficulty of isolating the embryonic gonads at these time points, such that Dmrt1 expression in the genital ridge or bipotential gonad could be confounded with extra-gonadal expression.
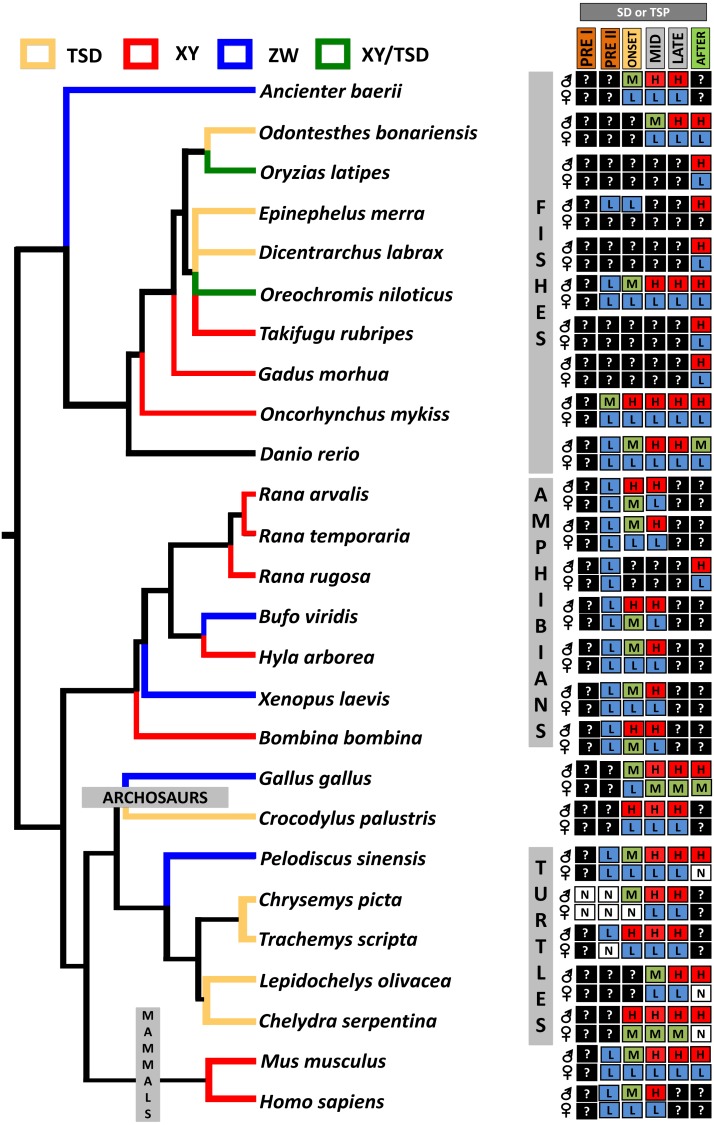
From an evolutionary perspective, it has been hypothesized that the ancestral function of Dmrt1 was to trigger male sexual development and that new functions expanded later to regulate the development of sexual dimorphism (Matson & Zarkower, 2012). This conservation in function of Dmrt1 in male development is reflected in its sexually dimorphic expression pattern across vertebrates, as observed in isolated gonadal tissue in C. picta (this study), and in other TSD and GSD reptiles, such as T. scripta, Pelodiscus sinensis, Lepidochelys olivacea, Chelydra serpentina and Crocodylus palustris (Kettlewell, Raymond & Zarkower, 2006; Sun et al., 2017; Torres Maldonado et al., 2002; Rhen et al., 2007; Ge et al., 2017). However, differences exist in the timing of Dmrt1 expression among species (Fig. 4), reflecting the divergence of its placement in the sex determination/differentiation regulatory network. Indeed, various studies demonstrated Dmrt1 upregulation in developing males (at MPT, or in XY or ZZ male individuals) in most vertebrates investigated to date (Fig. 4). Dmrt1 (and homologs) is required to trigger the male sex determination pathway in the Chinese tongue sole, medaka and chicken (Cui et al., 2017; Nanda et al., 2002; Smith et al., 2009). In other species of fish, amphibians and in mammals, Dmrt1 plays a pivotal role in Sertoli and germ cells differentiation and testicular maintenance, being upregulated in males during the mid or late stages of sex determination or after the sex determination period (Raymond et al., 1999; Jørgensen et al., 2008; Deloffre et al., 2009; Fernandino, Guilgur & Somoza, 2006; Kobayashi et al., 2008; Johnsen et al., 2010; Yamaguchi et al., 2006).
Figure 4. Transcriptional patterns of Dmrt1 during embryonic development of selected vertebrates with different sex-determining mechanisms.
Expression levels are color-coded as: none detected (white, N), low (blue, L), medium (green, M), high (red, H) transcription, and not studied (black, ?). Stages examined correspond to those before, during and after the thermosensitive period (TSP) for TSD taxa or sex differentiation (SD) for GSD taxa, following Valenzuela, Neuwald & Literman, (2013).
In turtles, functional assays demonstrated that Dmrt1 is required for male development in T. scripta and P. sinensis and it sits upstream of Sox9 and AMH in the male sexual development cascade (Supplementary Material 1), two important genes for testis development that are upregulated after Dmrt1 in these two species (Ge et al., 2017; Sun et al., 2017). Likewise, L. olivacea and C. serpentina exhibit Dmrt1 upregulation prior to Sox9 (Rhen et al., 2007; Torres Maldonado et al., 2002). In contrast, our RNA-seq and qPCR results detected Dmrt1 upregulation at MPT during the thermosensistive period, at the same stages (19 and 22) at which AMH is differentially expressed (Radhakrishnan et al., 2017), whereas the male differentiation genes Sox9, Wt1 and Sf1 are upregulated at MPT before Dmrt1 (stage 15) (Valenzuela, Neuwald & Literman, 2013; Radhakrishnan et al., 2017). Again, we note that AKGs were examined at stage 15, and the extragonadal tissue could confound gonadal expression. However, studies in stage 15 T. scripta embryos detected no Dmrt1 expression in AKs (Shoemaker et al., 2007), qualitatively upregulation at MPT in AKGs (Kettlewell, Raymond & Zarkower, 2000), but not quantitative differences in AKGs (Murdock & Wibbels, 2006) or isolated gonads (Shoemaker et al., 2007; Czerwinski et al., 2016). Given that C. picta and T. scripta are closely related species, and assuming that stage 15 AK expression is also low/absent in C. picta, the monomorphic expression we observe in AKGs at stage 15 may reflect actual monomorphic Dmrt1 gonadal expression. Combined, current data indicate that Dmrt1 is not the topmost trigger in the male sexual development cascade in C. picta. However, there is strong evidence suggesting that Dmrt1 contributes to male sexual development in C. picta, given its upregulation at MPT during the TSP and its predominantly conserved function across other taxa. Functional studies are needed to elucidate Dmrt1’s specific role in painted turtles.
We note the disparity of results between the two approaches of RNA-Seq analysis. The use of a subgenome allowed us to assemble the novel transcript identified by PCR and Sanger sequencing, but the same did not happen when using the whole genome as reference for mapping the reads. We believe that using the whole genome contributes to (1) discard transcripts of extremely low expression, as most assembler software packages consider them as “not true” and (2) using a genome-guided assembly can be restrictive in assembling low-expression isoforms. Also, we first identified the Dmrt1 ΔEx2Ex3 transcript using PCR and sequencing and we were able to detect this transcript using probe-based qPCR, using a probe specific of Dmrt1 ΔEx2Ex3 transcript.
Lastly, we performed qPCR in numerous biological replicates to validate the biological conclusions derived from previous RNA-seq data (Radhakrishnan et al., 2017). Earlier qPCR studies of C. picta (Valenzuela, LeClere & Shikano, 2006; Valenzuela, 2008b; Valenzuela, 2010) detected differential expression for Sox9, Wt1 and Sf1 at stages preceding those when RNA-Seq analysis detects upregulation (Radhakrishnan et al., 2017), which is not surprising given the higher sensitivity of qPCR to detect subtle differences that occur at these earlier stages in C. picta. The discordance between qPCR and RNA-seq analysis we observe is not unique. For instance, some studies on oysters found that around 55% (19 out 34) of the results would match between RNA-seq and qPCR (Shi & He, 2014), while other studies on bacteria observed high correlation between RNA-seq and qPCR expression results (Camarena et al., 2010).
Conclusion
Here we identified the first non-canonical Dmrt1 isoform (Dmrt1 ΔEx2Ex3) in any turtle species, and elucidated the response to temperature of the two Dmrt1 transcripts of C. picta. Our data revealed that both Dmrt1 transcripts are upregulated at MPT during the TSP in isolated gonads, consistent with previous studies on canonical Dmrt1 expression, and supporting the notion that Dmrt1 may be a fundamental element for male sexual development in painted turtles. Monomorphic Dmrt1 expression was observed at earlier developmental stages when extragonadal tissues were included that could have confounded Dmrt1 expression from the genital ridge or early bipotential gonad. Nonetheless, compared to T. scripta, our data from stage 15 C. picta suggest that Dmrt1 is unlikely to sit at the top of the male determination cascade in painted turtles, although further research is warranted, particularly in light of the thermosensitive response of the ratio of the canonical transcript to Dmrt1 ΔEx2Ex3 at stage 15. Additional differences in the timing of expression among taxa provide evidence of a divergent regulation of Dmrt1 across vertebrates irrespective of the mode of sex determination (TSD or GSD), unlike Dmrt1’s molecular evolution, which appears linked to transitions between TSD and GSD in terms of the rate at which sequences evolve (Literman et al., 2018) and in terms of shifts in the sequence of amino acids (Janes et al., 2014). Why exactly does Dmrt1 vary in the number of isoforms that are produced in different taxa remains an open question. More broadly, our results contribute to ongoing efforts to characterize isoforms in genetic networks, which are often neglected. Given the role of alternative splicing to increase transcriptomic diversity without demanding greater genomic complexity, it is critical to investigate the presence of isoforms and their function using a combination of approaches as shown here, if we are to gain a comprehensive picture of embryonic development.
Supplemental Information
Sry is present only in Therian mammals. Modified from (Mizoguchi & Valenzuela, 2016)
Reads are separated by developmental stage and incubation temperature (26 °C = Male Producing Temperature, 31 °C = Female Producing Temperature).
Panel A illustrates RNA-Seq results. Panel B illustrates TaqMan qPCR results. Bars represent standard deviations.
Acknowledgments
We thank R. Literman for help with primer and qPCR design, S. Seng for assistance with some of the data collection, and Z. Wu for advise during transcriptome analysis.
Funding Statement
This work was supported by NSF grants MCB-1244355 and IOS-1555999 to Nicole Valenzuela, by a scholarship from Science without Borders/CAPES (Brazil) to Beatriz Mizoguchi, and by Iowa State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Beatriz Mizoguchi conceived and designed the experiments, performed the experiments, analyzed the data, interpreted results, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Nicole Valenzuela conceived and designed the experiments, interpreted results, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The RNAseq data is available in NCBI: study number SRP099173, bioproject PRJNA594037, SRR10674595–SRR10674614.
The scripts are available in the Supplemental File.
References
- Adolfi et al. (2015).Adolfi MC, Carreira AC, Jesus LW, Bogerd J, Funes RM, Schartl M, Sogayar MC, Borella MI. Molecular cloning and expression analysis of dmrt1 and sox9 during gonad development and male reproductive cycle in the lambari fish, Astyanax altiparanae. Reproductive Biology and Endocrinology. 2015;13:1–15. doi: 10.1186/1477-7827-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam et al. (2008).Alam MA, Kobayashi Y, Horiguchi R, Hirai T, Nakamura M. Molecular cloning and quantitative expression of sexually dimorphic markers Dmrt1 and Foxl2 during female-to-male sex change in Epinephelus merra. General and Comparative Endocrinology. 2008;157:75–85. doi: 10.1016/j.ygcen.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Anand et al. (2008).Anand A, Patel M, Lalremruata A, Singh AP, Agrawal R, Singh L, Aggarwal RK. Multiple alternative splicing of Dmrt1 during gonadogenesis in Indian mugger, a species exhibiting temperature-dependent sex determination. Gene. 2008;425:56–63. doi: 10.1016/j.gene.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Andrews (2010).Andrews S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk?/projects/fastqc/ 2010
- Bachtrog et al. (2014).Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman T-L, Hahn MW, Kitano J, Mayrose I, Ming R, Perrin N, Ross L, Valenzuela N, Vamosi JC, The Tree of Sex Consortium Sex determination: why so many ways of doing it? PLOS Biology. 2014;12:e1001899. doi: 10.1371/journal.pbio.1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhorst et al. (2015).Badenhorst D, Hillier LW, Literman R, Montiel EE, Radhakrishnan S, Shen Y, Minx P, Janes DE, Warren WC, Edwards SV, Valenzuela N. Physical mapping and refinement of the painted turtle genome (chrysemys picta) inform amniote genome evolution and challenge turtle-bird chromosomal conservation. Genome Biology and Evolution. 2015;7:2038–2050. doi: 10.1093/gbe/evv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera et al. (2015).Bandiera R, Sacco S, Vidal VP, Chaboissier MC, Schedl A. Steroidogenic organ development and homeostasis: a WT1-centric view. Molecular and Cellular Endocrinology. 2015;408:145–155. doi: 10.1016/j.mce.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer et al. (2002).Boyer A, Saffron D, Daneau I, Lussier J, Silversides DW. Conservation of the function of DMRT1 regulatory sequences in mammalian sex differentiation. Genesis. 2002;34:236–243. doi: 10.1002/gene.10158. [DOI] [PubMed] [Google Scholar]
- Bratus & Slota (2006).Bratus A, Slota E. DMRT1/Dmrt1, the sex determining or sex differentiating gene in Vertebrata. Folia Biologica. 2006;54:81–86. doi: 10.3409/173491606778557563. [DOI] [PubMed] [Google Scholar]
- Bray et al. (2016).Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nature Biotechnology. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Bull & Vogt (1981).Bull JJ, Vogt RC. Temperature-sensitive periods of sex determination in Emydid turtles. Journal of Experimental Zoology. 1981;218:435–440. doi: 10.1002/jez.1402180315. [DOI] [PubMed] [Google Scholar]
- Burtis & Baker (1989).Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Burtis et al. (1991).Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. The EMBO Journal. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarena et al. (2010).Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLOS Pathogens. 2010;6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriglione et al. (2010).Capriglione T, Vaccaro MC, Morescalchi MA, Tammaro S, De Iorio S. Differential DMRT1 expression in the gonads of podarcis sicula (reptilia: lacertidae) Sexual Development. 2010;4:104–109. doi: 10.1159/000289579. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2006).Cheng HH, Ying M, Tian YH, Guo Y, McElreavey K, Zhou RJ. Transcriptional diversity of DMRT1 (dsx- and mab3-related transcription factor 1) in human testis. Cell Research. 2006;16:389–393. doi: 10.1038/sj.cr.7310050. [DOI] [PubMed] [Google Scholar]
- Coschigano & Wensink (1993).Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes & Development. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2017).Cui Z, Liu Y, Wang W, Wang Q, Zhang N, Lin F, Wang N, Shao C, Dong Z, Li Y, Yang Y, Hu M, Li H, Gao F, Wei Z, Meng L, Wei M, Zhu Y, Guo H, Cheng CH, Schartl M, Chen S. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis) Scientific Reports. 2017;7:42213. doi: 10.1038/srep42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerwinski et al. (2016).Czerwinski M, Natarajan A, Barske L, Looger LL, Capel B. A timecourse analysis of systemic and gonadal effects of temperature on sexual development of the red-eared slider turtle Trachemys scripta elegans. Developmental Biology. 2016;420:166–177. doi: 10.1016/j.ydbio.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Deloffre et al. (2009).Deloffre LAM, Martins RST, Mylonas CC, Canario AVM. Alternative transcripts of DMRT1 in the European sea bass: expression during gonadal differentiation. Aquaculture. 2009;293:89–99. doi: 10.1016/j.aquaculture.2009.03.048. [DOI] [Google Scholar]
- Eggers, Ohnesorg & Sinclair (2014).Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nature Reviews Endocrinology. 2014;10:673–683. doi: 10.1038/nrendo.2014.163. [DOI] [PubMed] [Google Scholar]
- Fernandino, Guilgur & Somoza (2006).Fernandino JI, Guilgur LG, Somoza GM. Dmrt1 expression analysis during spermatogenesis in pejerrey, Odontesthes bonariensis 2006.
- Fernandino et al. (2008).Fernandino JI, Hattori RS, Shinoda T, Kimura H, Strobl-Mazzulla PH, Strussmann CA, Somoza GM. Dimorphic expression of dmrt1 and cyp19a1 (ovarian aromatase) during early gonadal development in pejerrey, Odontesthes bonariensis. Sexual Development. 2008;2:316–324. doi: 10.1159/000195681. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2005).Gao S, Zhang T, Zhou X, Zhao Y, Li Q, Guo Y, Cheng H, Zhou R. Molecular cloning, expression of Sox5 and its down-regulation of Dmrt1 transcription in zebrafish. Journal of Experimental Zoology Part B Molecular and Developmental Evolution. 2005;304:476–483. doi: 10.1002/jez.b.21053. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2018).Ge C, Ye J, Weber C, Sun W, Zhang H, Zhou Y, Cai C, Qian G, Capel B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science. 2018;360:645–648. doi: 10.1126/science.aap8328. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2017).Ge C, Ye J, Zhang H, Zhang Y, Sun W, Sang Y, Capel B, Qian G. Dmrt1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development. 2017;144:2222–2233. doi: 10.1242/dev.152033. [DOI] [PubMed] [Google Scholar]
- Guan, Kobayashi & Nagahama (2000).Guan G, Kobayashi T, Nagahama Y. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the Tilapia (Oreochromis niloticus) Biochemical and Biophysical Research Communications. 2000;272:662–666. doi: 10.1006/bbrc.2000.2840. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2005).Guo Y, Cheng H, Huang X, Gao S, Yu H, Zhou R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochemical and Biophysical Research Communications. 2005;330:950–957. doi: 10.1016/j.bbrc.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Haas et al. (2013).Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, MacManes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, LeDuc RD, Friedman N, Regev A. De novo transcript sequence reconstruction from RNA-Seq: reference generation and analysis with Trinity. Nature Protocols. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed et al. (2015).Hamed NA, Aldefrawy M, Elneely D, Ghallab O, Jepngetich B. Expression of Glucocorticoid Receptor Isoforms (α, β, γ, and p) in Egyptian Primary Immune Thrombocytopenia Patients. International Blood Research and Reviews 2015 [Google Scholar]
- Hammes et al. (2001).Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/S0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hattori et al. (2012).Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strussmann CA. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin & Schartl (2015).Herpin A, Schartl M. Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Reports. 2015;16:1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleley et al. (2015).Holleley CE, O’Meally D, Sarre SD, Marshall Graves JA, Ezaz T, Matsubara K, Azad B, Zhang X, Georges A. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature. 2015;523:79–82. doi: 10.1038/nature14574. [DOI] [PubMed] [Google Scholar]
- Hong, Park & Saint-Jeannet (2007).Hong CS, Park BY, Saint-Jeannet J. The function of Dmrt genes in vertebrate development: it is not just about sex. Developmental Biology. 2007;310:1–9. doi: 10.1016/j.ydbio.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2005).Huang X, Guo Y, Shui Y, Gao S, Yu H, Cheng H, Zhou R. Multiple alternative splicing and differential expression of dmrt1 during gonad transformation of the rice field eel. Biology of Reproduction. 2005;73:1017–1024. doi: 10.1095/biolreprod.105.041871. [DOI] [PubMed] [Google Scholar]
- Janes et al. (2014).Janes DE, Organ CL, Stiglec R, O’Meally D, Sarre SD, Georges A, Graves JAM, Valenzuela N, Literman RA, Rutherford K, Gemmell N, Iverson JB, Tamplin JW, Edwards SV, Ezaz T. Molecular evolution of Dmrt1 accompanies change of sex-determining mechanisms in reptilia. Biology Letters. 2014;10:20140809. doi: 10.1098/rsbl.2014.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2009).Jeong HB, Park JG, Park YJ, Takemura A, Hur SP, Lee YD, Kim SJ. Isolation and characterization of DMRT1 and its putative regulatory region in the protogynous wrasse, Halichoeres tenuispinis. Gene. 2009;438:8–16. doi: 10.1016/j.gene.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Johnsen et al. (2010).Johnsen H, Seppola M, Torgersen JS, Delghandi M, Andersen Ø. Sexually dimorphic expression of dmrt1 in immature and mature Atlantic cod (Gadus morhua L.) Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2010;156:197–205. doi: 10.1016/j.cbpb.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Jørgensen et al. (2008).Jørgensen A, Morthorst JE, Andersen O, Rasmussen LJ, Bjerregaard P. Expression profiles for six zebrafish genes during gonadal sex differentiation. Reproductive Biology and Endocrinology. 2008;6:1–12. doi: 10.1186/1477-7827-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlewell, Raymond & Zarkower (2000).Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- Kettlewell, Raymond & Zarkower (2006).Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Folia Biologica. 2006;26:174–178. [PubMed] [Google Scholar]
- Kim, Langmead & Salzberg (2015).Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi et al. (2008).Kobayashi T, Kajiura-Kobayashi H, Guan G, Nagahama Y. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus) Developmental Dynamics. 2008;237:297–306. doi: 10.1002/dvdy.21409. [DOI] [PubMed] [Google Scholar]
- Latchman (1997).Latchman DS. Transcription factors: an overview. The International Journal of Biochemistry & Cell Biology. 1997;29:1305–1312. doi: 10.1016/s1357-2725(97)00085-x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2009).Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. Genome Project Data The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Literman et al. (2018).Literman R, Burrett A, Bista B, Valenzuela N. Putative independent evolutionary reversals from genotypic to temperature-dependent sex determination are associated with accelerated evolution of sex-determining genes in turtles. Journal of Molecular Evolution. 2018;86:11–26. doi: 10.1007/s00239-017-9820-x. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2007).Lu H, Huang X, Zhang L, Guo Y, Cheng H, Zhou R. Multiple alternative splicing of mouse Dmrt1 during gonadal differentiation. Biochemical and Biophysical Research Communications. 2007;352:630–634. doi: 10.1016/j.bbrc.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2013).Ma L, Fang M, Liang Y, Xiang Y, Jia Z, Sun X, Wang Y, Qin J. Low expression of glucocorticoid receptor alpha isoform in adult immune thrombocytopenia correlates with glucocorticoid resistance. Annals of Hematology. 2013;92:953–960. doi: 10.1007/s00277-013-1705-5. [DOI] [PubMed] [Google Scholar]
- Marchand et al. (2000).Marchand O, Govoroun M, D’Cotta H, McMeel O, Lareyre JJ, Bernot A, Laudet V, Guiguen Y. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochimica et Biophysica Acta. 2000;1493:180–187. doi: 10.1016/S0167-4781(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Matson & Zarkower (2012).Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nature Reviews Genetics. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda et al. (2002).Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- Mu et al. (2015).Mu Y, Zhao B, Tang WQ, Sun BJ, Zeng ZG, Valenzuela N, Du WG. Temperature-dependent sex determination ruled out in the Chinese soft-shelled turtle (Pelodiscus sinensis) via molecular cytogenetics and incubation experiments across populations. Sexual Development. 2015;9:111–117. doi: 10.1159/000373903. [DOI] [PubMed] [Google Scholar]
- Murdock & Wibbels (2006).Murdock C, Wibbels T. Dmrt1 expression in response to estrogen treatment in a reptile with temperature-dependent sex determination. Journal of Experimental Zoology Part B Molecular and Developmental Evolution. 2006;306:134–139. doi: 10.1002/jez.b.21076. [DOI] [PubMed] [Google Scholar]
- Nanda et al. (2002).Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea et al. (2016).Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature Protocols. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl (2001).Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan et al. (2017).Radhakrishnan S, Literman R, Neuwald J, Severin A, Valenzuela N. Transcriptomic responses to environmental temperature by turtles with temperature-dependent and genotypic sex determination assessed by RNAseq inform the genetic architecture of embryonic gonadal development. PLOS ONE. 2017;12:e0172044. doi: 10.1371/journal.pone.0172044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey & Crews (2007).Ramsey M, Crews D. Adrenal-kidney-gonad complex measurements may not predict gonad-specific changes in gene expression patterns during temperature-dependent sex determination in the red-eared slider turtle (Trachemys scripta elegans) Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 2007;307:463–470. doi: 10.1002/jez.399. [DOI] [PubMed] [Google Scholar]
- Raymond et al. (1999).Raymond C, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Developmental Biology. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- Raymond et al. (2000).Raymond C, Murphy M, O’Sullivan G, Bardell V, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes and Development. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen et al. (2007).Rhen T, Metzger K, Schroeder A, Woodward R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sexual Development. 2007;1:255–270. doi: 10.1159/000104775. [DOI] [PubMed] [Google Scholar]
- Sarre, Georges & Quinn (2004).Sarre SD, Georges A, Quinn A. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays. 2004;26:639–645. doi: 10.1002/bies.20050. [DOI] [PubMed] [Google Scholar]
- Shaffer et al. (2013).Shaffer HB, Minx P, Warren DE, Shedlock AM, Thomson RC, Valenzuela N, Abramyan J, Amemiya CT, Badenhorst D, Biggar KK, Borchert GM, Botka CW, Bowden RM, Braun EL, Bronikowski AM, Bruneau BG, Buck LT, Capel B, Castoe TA, Czerwinski M, Delehaunty KD, Edwards SV, Fronick CC, Fujita MK, Fulton L, Graves TA, Green RE, Haerty W, Hariharan R, Hernandez O, Hillier LW, Holloway AK, Janes D, Janzen FJ, Kandoth C, Kong L, D KAJ, Li Y, Literman R, McGaugh SE, Mork L, O’Laughlin M, Paitz RT, Pollock DD, Ponting CP, Radhakrishnan S, Raney BJ, Richman JM, St John J, Schwartz T, Sethuraman A, Spinks PQ, Storey KB, Thane N, Vinar T, Zimmerman LM, Warren WC, Mardis ER, Wilson RK. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biology. 2013;14:1–23. doi: 10.1186/gb-2013-14-3-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi & He (2014).Shi Y, He M. Differential gene expression identified by RNA-Seq and qPCR in two sizes of pearl oyster (Pinctada fucata) Gene. 2014;538:313–322. doi: 10.1016/j.gene.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Shibata, Takase & Nakamura (2002).Shibata K, Takase M, Nakamura M. The Dmrt1 expression in sex-reversed gonads of amphibians. General and Comparative Endocrinology. 2002;127:232–241. doi: 10.1016/S0016-6480(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Shine, Elphick & Donnellan (2002).Shine R, Elphick MJ, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecology Letters. 2002;5:486–489. doi: 10.1046/j.1461-0248.2002.00351.x. [DOI] [Google Scholar]
- Shoemaker & Crews (2009).Shoemaker C, Crews D. Analyzing the coordinated gene network underlying temperature-dependent sex determination in reptiles. Seminars in Cell & Developmental Biology. 2009;20:293–303. doi: 10.1016/j.semcdb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker et al. (2007).Shoemaker C, Ramsey M, Queen J, Crews D. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Developmental Dynamics. 2007;236:1055–1063. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2009).Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- Spotila, Spotila & Hall (1998).Spotila LD, Spotila JR, Hall SE. Sequence and expression analysis of WT1 and Sox9 in the red-eared slider turtle, Trachemys scripta. Journal of Experimental Zoology. 1998;284:417–427. doi: 10.1002/(sici)1097-010x(19980801)281:5<417::aid-jez7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2017).Sun W, Cai H, Zhang G, Zhang H, Bao H, Wang L, Ye J, Qian G, Ge C. Dmrt1 is required for primary male sexual differentiation in Chinese soft-shelled turtle Pelodiscus sinensis. Scientific Reports. 2017;7:4433. doi: 10.1038/s41598-017-04938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi et al. (2001).Teranishi M, Shimada Y, Hori T, Nakabayashi O, Kikuchi T, Macleod T, Pym R, Sheldon B, Solovei I, Macgregor H, Mizuno S. Transcripts of the MHM region on the chicken Z chromosome accumulate as non-coding RNA in the nucleus of female cells adjacent to the DMRT1 locus. Chromosome Research. 2001;9:147–165. doi: 10.1023/A:1009235120741. [DOI] [PubMed] [Google Scholar]
- Torres Maldonado et al. (2002).Torres Maldonado LC, Landa Piedra A, Moreno Mendoza N, Marmolejo Valencia A, Meza Martinez A, Merchant Larios H. Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea. General and Comparative Endocrinology. 2002;129:20–26. doi: 10.1016/S0016-6480(02)00511-7. [DOI] [PubMed] [Google Scholar]
- Trapnell, Pachter & Salzberg (2009).Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell et al. (2012).Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree of Sex Consortium et al. (2014).Tree of Sex Consortium. Ashman TL, Bachtrog D, Blackmon H, Goldberg EE, Hahn MW, Kirkpatrick M, Kitano J, Mank JE, Mayrose I, Ming R, Otto SP, Peichel CL, Pennell MW, Perrin N, Ross L, Valenzuela N, Vamosi JC. Tree of sex: a database of sexual systems. Scientific Data. 2014;1:1–8. doi: 10.1038/sdata.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela (2008a).Valenzuela N. Evolution of the gene network underlying gonadogenesis in turtles with temperature-dependent and genotypic sex determination. Integrative and Comparative Biology. 2008a;48:476–485. doi: 10.1093/icb/icn031. [DOI] [PubMed] [Google Scholar]
- Valenzuela (2008b).Valenzuela N. Relic thermosensitive gene expression in a turtle with genotypic sex determination. Evolution. 2008b;62:234–240. doi: 10.1111/j.1558-5646.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela (2009).Valenzuela N. Egg incubation and collection of painted turtle embryos. Cold Spring Harbor Protocols. 2009;2009(7) doi: 10.1101/pdb.prot5238. pdb.prot5238. [DOI] [PubMed] [Google Scholar]
- Valenzuela (2010).Valenzuela N. Multivariate expression analysis of the gene network underlying sexual development in turtle embryos with temperature-dependent and genotypic sex determination. Sexual Development. 2010;4:39–49. doi: 10.1159/000277935. [DOI] [PubMed] [Google Scholar]
- Valenzuela, Adams & Janzen (2003).Valenzuela N, Adams DC, Janzen FJ. Pattern does not equal process: exactly when is sex environmentally determined? The American Naturalist. 2003;161:676–683. doi: 10.1086/368292. [DOI] [PubMed] [Google Scholar]
- Valenzuela et al. (2014).Valenzuela N, Badenhorst D, Montiel EE, Literman R. Molecular cytogenetic search for cryptic sex chromosomes in painted turtles Chrysemys picta. Cytogenetic and Genome Research. 2014;144:39–46. doi: 10.1159/000366076. [DOI] [PubMed] [Google Scholar]
- Valenzuela & Lance (2004).Valenzuela N, Lance V, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, D.C.: 2004. [Google Scholar]
- Valenzuela, LeClere & Shikano (2006).Valenzuela N, LeClere A, Shikano T. Comparative gene expression of steroidogenic factor 1 in Chrysemys picta and Apalone mutica turtles with temperature-dependent and genotypic sex determination. Evolution & Development. 2006;8:424–432. doi: 10.1111/j.1525-142X.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela, Neuwald & Literman (2013).Valenzuela N, Neuwald JL, Literman R. Transcriptional evolution underlying vertebrate sexual development. Developmental Dynamics. 2013;242:307–319. doi: 10.1002/dvdy.23897. [DOI] [PubMed] [Google Scholar]
- Valenzuela & Shikano (2007).Valenzuela N, Shikano T. Embryological ontogeny of aromatase gene expression in Chrysemys picta and Apalone mutica turtles: comparative patterns within and across temperature-dependent and genotypic sex-determining mechanisms. Development Genes and Evolution. 2007;217:55–62. doi: 10.1007/s00427-006-0106-3. [DOI] [PubMed] [Google Scholar]
- Volff et al. (2003).Volff JN, Zarkower D, Bardwell VJ, Schartl M. Evolutionary dynamics of the DM domain gene family in metazoans. Journal of Molecular Evolution. 2003;57(Suppl 1):S241–S249. doi: 10.1007/s00239-003-0033-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi et al. (2006).Yamaguchi A, Lee KH, Fujimoto H, Kadomura K, Yasumoto S, Matsuyama M. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2006;1:59–68. doi: 10.1016/j.cbd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto et al. (2014).Yamamoto Y, Zhang Y, Sarida M, Hattori RS, Strussmann CA. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLOS ONE. 2014;9:e102574. doi: 10.1371/journal.pone.0102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema (1968).Yntema CL. A series of stages in the embryonic development of Chelydra serpentina. Journal of Morphology. 1968;125:219–251. doi: 10.1002/jmor.1051250207. [DOI] [PubMed] [Google Scholar]
- Yoshimoto et al. (2010).Yoshimoto S, Ikeda N, Izutsu Y, Shiba T, Takamatsu N, Ito M. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development. 2010;137:2519–2526. doi: 10.1242/dev.048751. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2007a).Zhao Y, Lu H, Yu H, Cheng H, Zhou R. Multiple alternative splicing in gonads of chicken DMRT1. Development Genes and Evolution. 2007a;217:119–126. doi: 10.1007/s00427-006-0117-0. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2007b).Zhao Y, Lu H, Yu H, Cheng H, Zhou R. Multiple alternative splicing in gonads of chicken DMRT1. Development Genes and Evolution. 2007b;217:119–126. doi: 10.1007/s00427-006-0117-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sry is present only in Therian mammals. Modified from (Mizoguchi & Valenzuela, 2016)
Reads are separated by developmental stage and incubation temperature (26 °C = Male Producing Temperature, 31 °C = Female Producing Temperature).
Panel A illustrates RNA-Seq results. Panel B illustrates TaqMan qPCR results. Bars represent standard deviations.
Data Availability Statement
The following information was supplied regarding data availability:
The RNAseq data is available in NCBI: study number SRP099173, bioproject PRJNA594037, SRR10674595–SRR10674614.
The scripts are available in the Supplemental File.