Abstract
Background
Combination antiemetic therapy has become a common practice for the prevention of postoperative nausea and vomiting (PONV). The aim of the present study was to evaluate the stability and compatibility of ramosetron hydrochloride and midazolam in 0.9% sodium chloride injection when stored at 4°C and 25°C for up to 14 days.
Methods
Admixtures were assessed initially and for 14 days after preparation in polyolefin bags and glass bottles using 0.9% sodium chloride injection as the diluent and stored at 4°C or 25°C. The initial concentrations were 0.3 mg/100 mL ramosetron hydrochloride and 0.5 mg/100 mL midazolam hydrochloride. For all samples, the compatibility parameters (including precipitation, cloudiness, discoloration and pH values) were evaluated. Chemical stability was also determined using high-performance liquid chromatography (HPLC) analysis.
Results
After a 14-day period of storage at 4°C or 25°C, the percent of the initial concentration of ramosetron hydrochloride and midazolam hydrochloride in the various solutions were maintained at a minimum of 97%. All of the mixtures remained clear and colourless throughout the observation period, and no colour change or precipitation was observed.
Conclusion
The results indicate that admixtures of 0.3 mg/100 mL ramosetron hydrochloride and 0.5 mg/100 mL midazolam hydrochloride in normal saline were stable for 14 days at 4°C or 25°C when packaged in polyolefin bags or glass bottles and protected from light.
Keywords: ramosetron, midazolam, drug stability, postoperative nausea and vomiting
Introduction
Postoperative nausea and vomiting (PONV) is one of the most common perioperative concerns, with an incidence of up to 80% in high-risk patients.1 PONV can lead to not only patient discomfort but also increased postoperative pain and medical costs.2 Serotonin receptor antagonist can reduce the incidence of PONV but do not block it completely.3 Considering the multiple risk factors of PONV and the current evidence suggesting the limited efficacy of single antiemetic prophylaxis, combining two or more antiemetics with different mechanisms of action has been shown to be more effective in preventing PONV.4
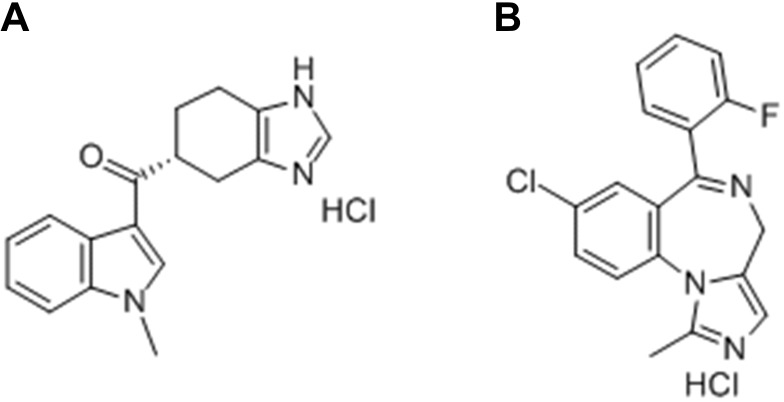
Ramosetron hydrochloride (Figure 1A), (1-methyl-1H-indol-3-yl)[(6R)-4,5,6,7- tetrahydro-1H-benzimidazol-6-yl]methanone hydrochloride, is a newly developed drug with higher 5-HT3 receptor affinity and has been frequently used for the prevention of PONV in high-risk patients due to a longer duration of action than its congeners, such as ondansetron and granisetron.5,6 Midazolam hydrochloride (Figure 1B, 5-a), (1,4) benzodiazepine, 8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo (hy;8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo(1,5-a)(1,4)benzodiazepinehyd), is a medication used for procedural sedation, severe agitation and premedication and co-induction of anaesthesia.7,8 In addition to its known anxiolytic effects, several investigators have demonstrated that midazolam has the effects of prevention and treatment of PONV.9 Randomized controlled trials have evaluated the antiemetic properties of a combination of ramosetron and midazolam in patients undergoing surgery and found that it significantly reduced the incidence of PONV.10–12
Figure 1.
Structures of (A) ramosetron hydrochloride and (B) midazolam hydrochloride.
Currently, no commercially available mixtures of ramosetron and midazolam exist; thus, both infusions had been prepared by the pharmacy in advance using 0.9% sodium chloride injection under aseptic conditions and approved for clinical use.13 Known incompatibilities arising from the combination of therapies in i.v. administration can affect the physical and chemical stability of antiemetic drugs.14 To the best of our knowledge, the stability of ramosetron with midazolam in the solution for PONV administration has not been reported. Therefore, the goal of the present research was to investigate the compatibility and chemical stability of ramosetron hydrochloride in binary admixtures with haloperidol midazolam hydrochloride in 0.9% sodium chloride when stored in polyolefin or glass containers at 4°C and 25°C for a trial period of 14 days.
Methods
Materials and Reagents
The working standards of midazolam (lot 120245, pharmaceutical grade 99.7%) and ramosetron (lot 120421, pharmaceutical grade 99.8%) were obtained from Huadong Chemical Reagent Co., Ltd. (Shanghai, China). Commercially available ampoules of midazolam hydrochloride injection (5 mg/5 mL, lot 17062842) were supplied by Hengrui Medicine Co., Ltd. (Jiangsu, China). Ramosetron hydrochloride injection (0.3 mg/2 mL, lot 20170508) was purchased from Xian Janssen Pharmaceutical Co., Ltd. (Xian, China). The sodium chloride 0.9% injection used to prepare the sample mixtures was purchased from Guoyao Chemical Reagent Co., Ltd. (Beijing, China, lot A1508371). All other chemicals used were of analytical grade and purchased from Guoyao Chemical Reagent Co., Ltd. (Beijing, China) if not otherwise stated.
Instrumentation
The concentrations of midazolam hydrochloride and ramosetron hydrochloride were measured using a chromatographic technique with high-performance liquid chromatography (HPLC). The separation was performed using an Ultimate 3000 HPLC system (Dionex, Germering, Germany) equipped with a quaternary liquid gradient system, WPS-3000RS auto-injector, TCC-100 column oven, and DAD-3000RS UV spectrophotometer. Chromeleon software (version 6.80) was used for the collection of all chromatographic data. The pH of the samples was measured using a model PHS−3C pH meter (Leici Instrument Co, Shanghai, China).
Chromatographic Conditions
Chromatographic separation was achieved using a 1.9 μm Hypersile Gold C18 column (100 mm×2.1 mm) (Thermo Fisher Scientific). The isocratic mobile phase consisted of a mixture of acetonitrile, 50 mM KH2PO4 buffer, and triethylamine (25:74:1 by volume) at a flow rate of 0.5 mL/min. The column compartment was maintained at a temperature of 25°C, and the injection volume was 10 μL. The wavelength selection and detection for midazolam and ramosetron were 220 nm and 230 nm, respectively.15,16
Preparation of Stock and Working Solutions
Stock standard solutions of midazolam hydrochloride injection (0.5 mg/mL) and ramosetron hydrochloride (0.3 mg/mL) were prepared in deionized water and frozen at −20°C. Working mixed standard solutions were prepared daily by mixing the stock solution with deionized water as needed to obtain the required concentration before being used.
Validation of the HPLC Method
A validated stability-indicating HPLC assay method included linearity, accuracy, intra-and inter-day precision (RSD, %), and stability for testing the concentration of the drugs. Linearity was demonstrated by running the standard solutions at six different concentrations and three replicates over the range of 4.0–100 and 1.0–20 μg/mL of midazolam and ramosetron hydrochloride. The calibration curve was performed by plotting peak areas against drug concentrations. The coefficient of determination (r2) was calculated. To determine the accuracy, intraday precision and interday precision, three control samples (QCs) of midazolam hydrochloride (10.0, 30.0, and 60.0 μg/mL) and ramosetron hydrochloride (2.0, 4.0, and 8.0 μg/mL) were prepared and analysed. The recovery and relative standard deviation (RSD%) of the peak area values were calculated from 3 QCs of midazolam hydrochloride and ramosetron hydrochloride, and each concentration was measured 5 times on the same day to estimate the accuracy and intraday or interday precision.
Preparation of Midazolam Hydrochloride and Ramosetron Hydrochloride Mixtures
Midazolam hydrochloride injection (5 mg/5 mL) and ramosetron hydrochloride injection (0.3 mg/2 mL) were diluted in 100-mL PVC bags or glass bottles to make a compatible solution of 5 mg/100 mL midazolam hydrochloride and 0.3 mg/100 mL ramosetron hydrochloride. A rotary shaker was used to agitate the solution after each addition of fluid. The concentration testing of the drugs was performed according to methods used in the current daily practice. Three replicates of this solution were prepared and stored under refrigeration (4±0.5°C) and at room temperature (25±0.5°C).
Stability Study of the Solutions
For the study of the compatibility and storage stability of ramosetron with midazolam in a 0.9% sodium chloride injection, the samples were assessed for their appearance, pH, and drug concentration (0 days, 1 day, 2 days, 3 days, 5 days, 7 days, 10 days, and 14 days) after preparation. At each time point, the pH was measured using a pH meter. For analysis on the same sample, samples were diluted 1:10 in deionized water at room temperature before injection into and elution from the HPLC system. Samples from each bag and bottle were analysed in triplicate (n=3).
Stability Indication
To confirm the separation of degradation products from the parent molecule, a chromatographic method for the concentration detection of degraded samples in 0.9% sodium chloride was used. The midazolam hydrochloride, ramosetron hydrochloride and mixtures of midazolam hydrochloride with ramosetron hydrochloride with injectable solutions of sodium chloride were degraded by heating at 60°C for 5 hrs under the following conditions: 0.1 mol/L hydrochloric acid, 0.1 mol/L sodium hydroxide, and 3% hydrogen peroxide. After completing the degradation preparations, the solutions were assessed by HPLC assay.
Analysis of Data
Data are expressed as the mean ± standard deviation (mean ± sd). The stability of midazolam hydrochloride and ramosetron hydrochloride was determined by evaluating the percent of the initial concentration remaining at each time point. The mixtures were considered stable if they retained more than 90% of their initial concentration. Linear regression analysis was used to assess the drug concentration in the mixed system. Analyses were conducted with the Statistical Package Social Sciences 19.0 system (SPSS Inc., Chicago, Illinois USA).
Results
Validation of the HPLC Method
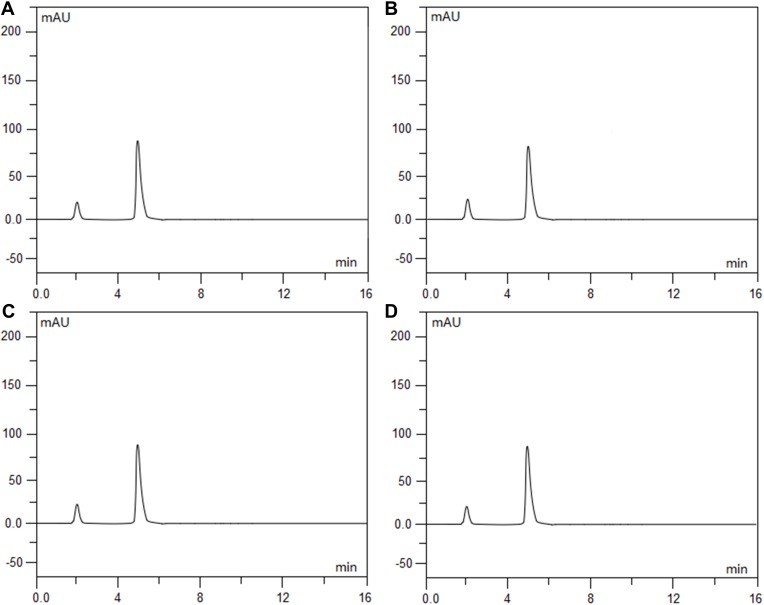
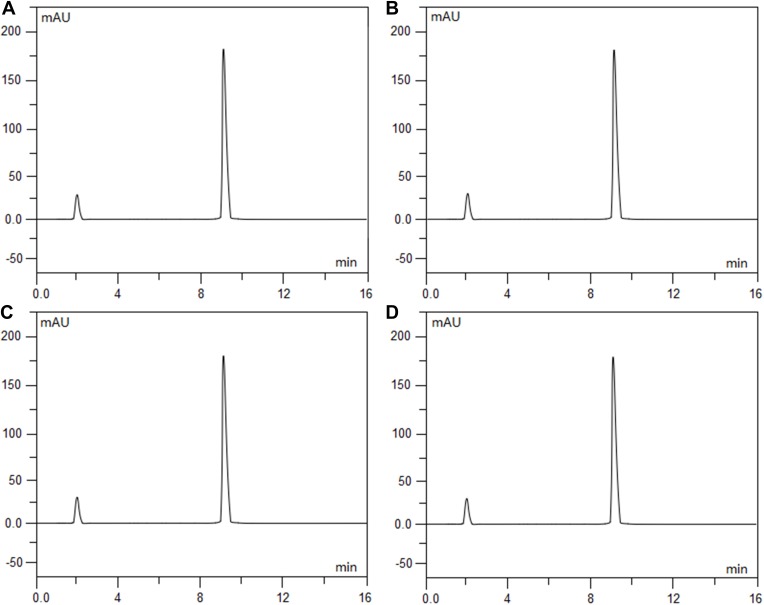
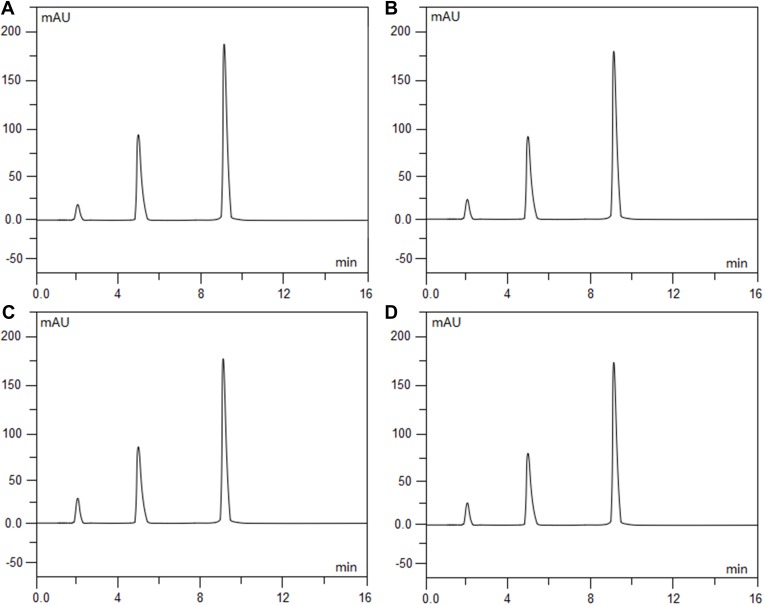
Intraday and interday precision and accuracy for the assay method were determined, and the results of the four analytes are indicated in Table 1. The results of the study showed that the proposed HPLC method is simple, accurate and precise, which is useful for the determination of ramosetron and midazolam in admixtures. Furthermore, it was found that there is a good linear relationship between the peak area and concentration for both drugs (the correlation coefficient (r) reached 0.9999 and above). Under extreme conditions, such as strongly acidic, strongly basic, and in oxidative solutions, each drug and the combination of drugs together showed good stability and compatibility from the degradation study results (Figures 2A–D, 3A–D and 4A–D) (the values for the degradation of compounds and the baselines were both less than 3% isolated from all analytes). The average chromatographic retention times for ramosetron hydrochloride and midazolam hydrochloride were 4.84 mins and 9.08 mins, respectively.
Table 1.
Validation of HPLC Method
| Compound | Measured Concentrations, μg/mL | Accuracy, % (n=3) | Precision RSD, % (n=3) | |
|---|---|---|---|---|
| Intraday | Interday | |||
| Ramosetron hydrochloride | 2.0 | 99.9 | 0.6 | 1.1 |
| 4.0 | 99.8 | 0.5 | 0.7 | |
| 8.0 | 100.2 | 1.2 | 1.6 | |
| Midazolam hydrochloride | 10.0 | 100.2 | 0.8 | 1.7 |
| 30.0 | 99.8 | 0.6 | 0.8 | |
| 60.0 | 101.4 | 1.3 | 1.4 | |
Abbreviations: HPLC, high-pressure liquid chromatography; RSD, relative standard deviation.
Figure 2.
Chromatograms of ramosetron hydrochloride (0.3 mg/100 mL) that was freshly prepared (A), exposed to 0.1 mol/L hydrochloric acid at 60°C for 5 hrs (B), exposed to 0.1 mol/L sodium hydroxide at 60°C for 5 hrs (C), and exposed to 3% hydrogen peroxide at 60°C for 5 hrs (D). Ramosetron hydrochloride eluted at 4.84 min.
Figure 3.
Chromatograms of midazolam hydrochloride (0.5 mg/100 mL) that was freshly prepared (A), exposed to 0.1 mol/L hydrochloric acid at 60°C for 5 hrs (B), exposed to 0.1 mol/L sodium hydroxide at 60°C for 5 hrs (C), and exposed to 3% hydrogen peroxide at 60°C for 5 hrs (D). Midazolam hydrochloride eluted at 9.08 min.
Figure 4.
Chromatograms of ramosetron hydrochloride (0.3 mg/100 mL) and midazolam hydrochloride (0.5 mg/100 mL) admixtures that were freshly prepared (A), exposed to 0.1 mol/L hydrochloric acid at 60°C for 5 hrs (B), exposed to 0.1 mol/L sodium hydroxide at 60°C for 5 hrs (C), and exposed to 3% hydrogen peroxide at 60°C for 5 hrs (D). Ramosetron hydrochloride eluted at 4.84 min (peak 1) and midazolam hydrochloride eluted at 9.08 min (peak 2).
Stability of the Mixtures of Midazolam and Ramosetron Hydrochloride
Visual examinations of each admixture at each sampling time did not reveal any evidence of instability and incompatibility (no colour change, no turbidity or opacity and no precipitation were observed). The initial pH of the admixtures ranged from 3.4 to 4.1, and the changes were less than 0.14 throughout the study in all samples (Table 2). Compatibility data are summarized in Tables 3 and 4, and the results showed that there were no degradation products from the mixtures over the 14-day trial period, and the concentrations of ramosetron and midazolam remaining in the admixtures were all greater than 97%. Compatibility data of the ramosetron hydrochloride plus midazolam hydrochloride solutions revealed that the mixtures were stable for up to 14 days at 4°C and 25°C.
Table 2.
The pH of Ramosetron Hydrochloride in Mixtures with Midazolam Hydrochloride in 0.9% Sodium Chloride Injection After Storage at 4°C and 25°C (Mean ± Sd; n=3)
| Time (Days) | 4°C | 25°C |
|---|---|---|
| 0 day | 3.74±0.1 | 3.84±0.2 |
| 1 day | 3.81±0.2 | 3.82±0.4 |
| 2 days | 3.69±0.5 | 3.71±0.6 |
| 3 days | 3.68±0.2 | 3.78±0.2 |
| 5 days | 3.72±0.4 | 3.79±0.5 |
| 7 days | 3.69±0.3 | 3.83±0.4 |
| 10 days | 3.76±0.2 | 3.82±0.3 |
| 14 days | 3.80±0.1 | 3.79±0.4 |
Table 3.
Amount of the Initial Concentration of Ramosetron Hydrochloride (0.3 Mg/100 mL) and Midazolam Hydrochloride (0.5 Mg/100 mL) Remaining After 14 Days of Storage at 25°C in Polyolefin Bags or Glass Containers (%; Mean ± Sd; n=3)
| Program | Glass Containers | Polyolefin Bags | ||
|---|---|---|---|---|
| Ramosetron Hydrochloride | Midazolam Hydrochloride | Ramosetron Hydrochloride | Midazolam Hydrochloride | |
| Initial concentration(mg/L) | 3.1±0.13 | 5.0±0.24 | 2.9±0.52 | 5.1±0.37 |
| 0 day | 100.01±0.26 | 99.94±0.92 | 99.99±0.24 | 99.95±0.32 |
| 1 day | 100.04±0.51 | 99.73±1.01 | 99.98±0.22 | 98.97±0.25 |
| 2 days | 100.14±0.31 | 99.88±0.36 | 100.14±0.17 | 100.46±0.39 |
| 3 days | 99.97±0.33 | 100.02±0.15 | 100.23±0.34 | 100.32±0.27 |
| 5 days | 99.99±0.18 | 100.24±0.43 | 100.16±0.58 | 99.99±0.21 |
| 7 days | 101.06±0.47 | 100.20±0.52 | 99.96±0.69 | 100.21±0.74 |
| 10 days | 100.23±0.36 | 100.06±0.12 | 99.84±0.47 | 99.97±0.64 |
| 14 days | 99.87±0.82 | 100.01±0.44 | 100.02±0.38 | 98.88±0.18 |
Table 4.
Amount of the Initial Concentration of Ramosetron Hydrochloride (0.3mg/100mL) and Midazolam Hydrochloride (0.5mg/100mL) Remaining After 14 Days of Storage at 4°C in Polyolefin Bags or Glass Containers (%; Mean ± Sd; n=3)
| Program | Glass Containers | Polyolefin Bags | ||
|---|---|---|---|---|
| Ramosetron Hydrochloride | Midazolam Hydrochloride | Ramosetron Hydrochloride | Midazolam Hydrochloride | |
| Initial concentration(mg/L) | 2.9±0.46 | 5.0±0.35 | 3.0±0.36 | 4.9±0.29 |
| 0 day | 99.60±1.22 | 99.78±0.49 | 100.61±0.43 | 99.95±0.48 |
| 1 day | 98.80±0.64 | 99.69±0.22 | 100.22±0.16 | 99.88±0.45 |
| 2 days | 100.24±0.15 | 100.14±0.31 | 100.42±0.35 | 100.42±1.01 |
| 3 days | 99.87±0.46 | 100.26±0.55 | 99.89±0.31 | 99.97±0.31 |
| 5 days | 100.33±0.36 | 100.27±0.24 | 100.20±0.40 | 99.79±0.72 |
| 7 days | 101.23±0.29 | 99.06±0.27 | 100.64±0.44 | 98.73±0.51 |
| 10 days | 100.14±0.18 | 100.18±0.32 | 100.30±0.12 | 99.99±0.42 |
| 14 days | 99.89±0.54 | 100.08±0.31 | 100.14±0.42 | 97.97±0.14 |
Discussion
PONV is a frequent and distressing outcome in patients that have undergone operations.17 It is a common practice in the prevention of PONV to use a combination of drugs.18 Many studies have confirmed that combination therapies (involving antiemetics with different mechanisms of action) may offer a more effective approach to prevent PONV.19 The combination of ramosetron (a 5-HT3 receptor antagonist) with midazolam in the solution for relieving PONV is now accepted, but there have been no reports regarding the physical compatibility and chemical stability of such mixtures for clinical practice.10–12 The present study aimed to address this lack of information.
Previous studies have revealed the satisfactory stability and compatibility of midazolam hydrochloride alone or when mixed with other drugs in a syringe and suggested that midazolam hydrochloride is a very stable drug. RL Hagan (1993) and others published that midazolam hydrochloride is stable for at least 30 days in 5% dextrose injection (USP) or 0.9% sodium chloride injection (USP) at room temperature.20 No signs of the chemical degradation of midazolam were found in previous studies during testing. Moreover, it has also been demonstrated experimentally that other tested drugs, such as fentanyl, hydromorphone hydrochloride, ketamine hydrochloride, morphine sulfate, lorazepam, and pentobarbital sodium, were stable and compatible in the presence of midazolam hydrochloride.21–23 However, Wong et al reported that dexamethasone and midazolam are compatible for only 24 hrs at 4°C or 23°C, and a precipitate was observed at 48 hrs.24 The physical incompatibility of dexamethasone and midazolam is due to the precipitation of midazolam. For ramosetron hydrochloride, there is limited published information regarding the compatibility and stability of it alone or combined with other drugs in the solution for infusion. Song et al studied the stability of ramosetron hydrochloride prepared in 0.9% sodium chloride solution or 5% dextrose injection.25 They reported that 0.3 mg/100 mL ramosetron hydrochloride was stable for 10 hrs when stored at 25°C with protection from light.
In this experiment, the methods for the determination of ramosetron hydrochloride and midazolam were adapted from the published literature.14,16 An alkaline aqueous medium and acetonitrile were selected to start the optimization of the mobile phase composition. Trials showed that an acidic mobile phase with a reverse-phase C18 column gave sharp peaks. In our study, there was no sign of precipitation during the experimental period, and no evidence of chromatographic peak modification in the binary mixtures at either of the temperatures was observed. The pH values of the binary mixtures containing ramosetron hydrochloride with midazolam hydrochloride ranged between 3.4 and 4.1, and their acidity is equal to some organic acids. These values were predictive for the satisfactory stability and compatibility results of ramosetron with midazolam in 0.9% sodium chloride injection in polyolefin bags or glass bottles, which remained stable for up to 14 days when stored at 4°C or 25°C.
When mixing drugs taken from ampoules of sterile solutions, bacterial contamination may occur.26 In the current study, we examined only the stability of compatible solutions, and the issue of microbial contamination was ignored. Hence, it was suggested to be in compliance with the pharmaceutical compounding-sterile preparations, General Chapter <797 (USP, National Prescription) in clinical practice.27 Based on these criteria, the agents we analysed were qualified and reached the standard of low-risk compound sterile products.28 Given that sterility can change according to site, equipment, operator, and procedures used, the following cautions are suggested: (1) the preparation could be stored for 48 hrs at room temperature and 14 days when refrigerated on the basis of standards of the USP; and (2) the infusion can be safely prepared and stored in a hospital pharmacy using aseptic techniques by licensed central intravenous additive (CIVA) services.
Conclusion
The clinical implications of our results are that a diluted infusion solution containing 0.3 mg/100 mL ramosetron hydrochloride mixed with 0.5 mg/100 mL midazolam hydrochloride in 0.9% sodium chloride injection may be pre-prepared and used with confidence up to at least 14 days following preparation when stored in polyolefin bags or glass bottles at 4°C or 25°C and protected from light. Given that this experiment has demonstrated the satisfactory stability of these two agents, it can be concluded that the combined solution can be safely prepared in advance and stored (in the dark) for up to 14 days by CIVA services, which may be convenient in hospitals.
Funding Statement
This work was supported by Research Project of Wuhan Municipal Health Commission (WZ18E06).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li Y, Wei X, Zhang S, et al. A meta-analysis of palonosetron for the prevention of postoperative nausea and vomiting in adults. J Perianesth Nurs. 2015;30(5):398–405. doi: 10.1016/j.jopan.2015.05.116 [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Shim JK, Song JW, et al. Dexmedetomidine added to an opioid-based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: a prospective, randomised controlled trial. Eur J Anaesthesiol. 2016;33(2):75–83. doi: 10.1097/EJA.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 3.Ryu JH, Chang JE, Kim HR, et al. Ramosetron vs. ramosetron plus dexamethasone for the prevention of postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: prospective, randomized, and double-blind study. Int J Surg. 2013;11(2):183–187. doi: 10.1016/j.ijsu.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 4.Kinnavy JJ. Utilization of preoperative risk assessment for management of postoperative nausea and vomiting (PONV). J Peroanes Nur. 2015;30(4):e9–e10. doi: 10.1016/j.jopan.2015.05.030 [DOI] [Google Scholar]
- 5.Sanmukhani JJ, Pawar P, Mittal R. Ramosetron hydrochloride for the prevention of cancer chemotherapy induced nausea and vomiting: the Indian experience. South Asian J Cancer. 2014;3(2):132–137. doi: 10.4103/2278-330X.130466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii Y, Saitoh Y, Tanaka H. A randomized, double-blind, placebo-controlled trial of ramosetron for preventing nausea and vomiting during termination of pregnancy. Int J Obstet Anesth. 2004;13(1):15–18. doi: 10.1016/S0959-289X(03)00101-8 [DOI] [PubMed] [Google Scholar]
- 7.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolapropofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304 [DOI] [PubMed] [Google Scholar]
- 8.Sheta SA, Al-sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth. 2014;24(2):181–189. doi: 10.1111/pan.12287 [DOI] [PubMed] [Google Scholar]
- 9.Yadav G, Pratihary PB, Jain G, et al. A prospective, randomized, double blind and placebo-control study comparing the additive effect of oral midazolam and clonidine for postoperative nausea and vomiting prophylaxis in granisetron premedicated patients undergoing laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2013;29(1):61–65. doi: 10.4103/0970-9185.105800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WJ, Kang H, Shin HY, et al. Ramosetron, midazolam, and combination of ramosetron and midazolam for prevention of postoperative nausea and vomiting: a prospective, randomized, double-blind study. J Int Med Res. 2013;41(4):1203–1213. doi: 10.1177/0300060513485864 [DOI] [PubMed] [Google Scholar]
- 11.Byon HJ, Lee SJ, Kim JT, et al. Comparison of the antiemetic effect of ramosetron and combined ramosetron and midazolam in children: a double-blind, randomised clinical trial. Eur J Anaesth. 2012;29(4):192–196. doi: 10.1097/EJA.0b013e32834fc1fb [DOI] [PubMed] [Google Scholar]
- 12.Park EY, Lee SK, Kang MH, et al. Comparison of ramosetron with combined ramosetron and midazolam for preventing postoperative nausea and vomiting in patients at high risk following laparoscopic gynaecological surgery. J Int Med Res. 2013;41(3):654–663. doi: 10.1177/0300060513487627 [DOI] [PubMed] [Google Scholar]
- 13.Fang BX, Chen FC, Zhu D, et al. Stability of azasetron-dexamethasone mixture for chemotherapy-induced nausea and vomiting administration. Oncotarget. 2017;8(63):106249–106257. doi: 10.18632/oncotarget.22174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P, Chen F, Zhou BH. Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration. Medicine. 2018;97(50):e13698. doi: 10.1097/MD.0000000000013698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SE, Grad HA, Haas DA, Mayer A. Stability of parenteral midazolam in an oral formulation. Anesth Prog. 1997;44(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 16.He G, Zeng F, Lei K, et al. Compatibility of dexamethasone sodium phosphate with 5-HT receptor antagonists in infusion solutions: a comprehensive study. Eur J Hosp Pharm. 2017;24(3):162–166. doi: 10.1136/ejhpharm-2016-000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apfel CC, Stoecklein K, Lipfert P. PONV: a problem of inhalational anaesthesia? Best Pract Res Clin Anaesthesiol. 2005;19(3):485–500. doi: 10.1016/j.bpa.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 18.Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Prevention of PONV with granisetron, droperidol or metoclopramide in patients with postoperative emesis. Can J Anaesth. 1998;45(2):153–156. doi: 10.1007/BF03013255 [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam B, Madan R, Sadhasivam S, et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86(1):84–93. doi: 10.1093/bja/86.1.84 [DOI] [PubMed] [Google Scholar]
- 20.Hagan RL, Jacobs LF, Pimsler M, et al. Stability of midazolam hydrochloride in 5% dextrose injection or 0.9% sodium chloride injection over 30 days. Am J Hosp Pharm. 1993;50(11):2379–2381. [PubMed] [Google Scholar]
- 21.Anderson C, Mackay M. Stability of fentanyl citrate, hydromorphone hydrochloride, ketamine hydrochloride, midazolam, morphine sulfate, and pentobarbital sodium in polypropylene syringes. Pharm. 2015;3(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson KM, Schneider JJ, Ravenscroft PJ. Stability of midazolam and fentanyl in infusion solutions. J Pain Symptom Manage. 1998;16(1):52–58. doi: 10.1016/S0885-3924(98)00024-4 [DOI] [PubMed] [Google Scholar]
- 23.Riggs RM, English BA, Webster AA, et al. Fosphenytoin Y-site stability studies with lorazepam and midazolam hydrochloride. Int J Pharm Compd. 1999;3(3):235. [PubMed] [Google Scholar]
- 24.Wong AH, Law S, Walker SE, et al. Concentration-dependent compatibility and stability of dexamethasone and midazolam. Can J Hosp Pharm. 2000;53(1):24–31. [Google Scholar]
- 25.Song F. Compatibility of ramosetron hydrochloride for injection with four kinds of injections. Anhui Med Pharm J. 2008;12(1):15–16. [Google Scholar]
- 26.Boitquin L, Hecq JD, Evrard JM, et al. Long-term stability of sufentanil citrate with levobupivacaine hydrochloride in 0.9% sodium chloride infusion PVC bags at 4 degrees C. J Pain Symptom Manage. 2004;28(1):4–6. doi: 10.1016/j.jpainsymman.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Eroles AA, Bafalluy IM, Arnaiz JA. Stability of docetaxel diluted to 0.3 or 0.9 mg/mL with 0.9% sodium chloride injection and stored in polyolefin or glass containers. Am J Health Syst Pharm. 2009;66(17):1565–1568. doi: 10.2146/ajhp080482 [DOI] [PubMed] [Google Scholar]
- 28.Chen F, Li P, Zhou B, et al. Stability of an epidural analgesic admixture containing butorphanol tartrate and ropivacaine hydrochloride. Eur J Hosp Pharm. 2015;22(1):7–11. doi: 10.1136/ejhpharm-2014-000450 [DOI] [Google Scholar]