Abstract
Objective
To assess the health economic impact of cervical screening with liquid based cytology (LBC) compared with conventional cytology (CC) in Germany.
Methods
An economic model was constructed depicting the management of a hypothetical cohort of women aged ≥20 years who undergo cervical screening in Germany. The model estimated the cost-effectiveness and cost-benefit of LBC compared with CC at 2017/18 prices over a time-horizon of 70 years.
Results
Performing cervical screens with LBC instead of CC is expected to increase the probability of detecting a true positive over a subject’s lifetime by 73% (0.038 versus 0.022) and of diagnosing a subject with stage 3 cervical intraepithelial neoplasia (CIN3) (0.019 versus 0.011). Women screened with LBC instead of CC are expected to have a 57% reduction in the probability of having undetected CIN3 (0.006 versus 0.014) and to experience a 44% reduction in the probability of transitioning into disease progression (from 0.018 to 0.010). The mean discounted lifetime cost of healthcare resource use associated with performing cervical screens with LBC and CC was estimated at €4852 and €7523 per subject respectively. For every Euro invested in cervical screening with LBC instead of CC, the German healthcare system could potentially save ~€170 over a subject’s lifetime.
Conclusion
Within the study’s limitations, the analysis showed that LBC affords a cost-effective cervical screening test compared with CC in Germany, since it improves detection rates and has the potential to lead to a reduction in disease progression for less cost.
Keywords: cancer, cervical screening, conventional cytology, cost-effectiveness, liquid based cytology, Germany, Papanicolaou
Introduction
Cervical cancer is the fourth most common cancer in women worldwide.1 Hence, this disease is a major cause of morbidity and mortality. In 2018, there were an estimated 569,847 new cases and 311,365 deaths worldwide, with more than 85% of these cases occurring in developing countries.1 In Germany, the incidence of cervical cancer is one of the highest in Western Europe2 with an estimated annual incidence of 11 per 100,000 women3 and around 4600 new cases being diagnosed annually.3 Additionally, there are around 1500 cervical cancer-related deaths each year.4 It has been estimated that there are 35.9 million women over the age of 15 who could be at risk of cervical cancer in Germany.3
The onset of cervical cancer often results from a persistent infection with high-risk Human Papillomavirus (HPV).5 The lifetime probability of women acquiring an HPV infection is 0.85, and the virus is transmitted through sexual contact.6,7 HPV infection can result in changes to cervical cells which over time may become cancerous.8 Patients are asymptomatic in the early stages of the disease, hence the necessity for a screening programme.7
Cervical screening is designed to detect precancerous lesions that have the potential to develop into cancer.7 These precancerous lesions, referred to as cervical intraepithelial neoplasia (CIN) are divided into three grades: CIN1, CIN2 and CIN3.9 A subject is considered to have CIN1 when a third of the thickness of the surface layer of the cervix is affected, but will often return to normal without the need for treatment.9 A subject is considered to have CIN2 when two-thirds of the thickness of the surface layer is affected and to have CIN3 when the full surface layer of the cervix is affected.9 Women with either CIN2 or CIN3 are likely to require treatment.9 Disease progression to cervical cancer is staged from 1 to 4 according to the International Federation of Gynecology and Obstetrics (FIGO) staging system, with increasing stages reflecting the extent of metastasis.10
There are multiple screening techniques including conventional cytology (CC) and liquid based cytology (LBC).2 CC, also called the Papanicolaou or Pap test has been around for over 50 years and has been the standard screening technique in Germany since screening began in the 1970s.2,11,12 The test involves a sample of cells being taken from the cervix and smeared directly onto a glass slide.13 Since then, LBC has been introduced in a number of developed countries, such as New Zealand, the United Kingdom and the United States of America.14–16 With LBC, the sample of cervical cells is collected and rinsed in a vial of preservative solution.11 The vial containing the suspended cells in solution is transferred to a cytology processor that is used to place a uniform layer of cells on a slide.13 It has been reported that LBC yields a better representation of the cells than CC and fewer inadequate results.13,17 LBC also has the advantage that the residual liquid sample can also be used for HPV testing, if required.2
The current German guidelines invite women for an annual cytology cervical screen, although these guidelines are changing.18,19 From 2020, women between 20 and 34 years of age will be offered an annual cytology cervical screen. Women who are 35 years of age and above (with no upper age limit) will be offered a combined cytology and HPV test every three years.19 The objective of this study was to investigate the cost-effectiveness and cost-benefit of cervical screening with LBC compared with CC in Germany on the basis that women are offered an annual cytology test.
Methods
Study Design
This was a modelling study based on a systematic review of published literature and German statistics, to assess the health economic impact of cervical screening in Germany with LBC compared with CC among a hypothetical cohort of women.
Data Sources
A systematic literature review was performed using search terms that included conventional cytology, liquid based cytology, cervical cancer, smear test, Pap test and Papanicolaou test. The searches were limited to English or German studies published in the last 10 years and only human studies were included. Sites searched included PubMed, National Institute for Health and Care Excellence (NICE), The German Federal Ministry of Health (Bundesministerium für Gesundheit, BMG), The Federal Joint Committee (German: Gemeinsamer Bundesausschuss; G-BA), Kassenärztliche Bundesvereingung (KBV), Cochrane database of systematic reviews, World Health Organization, Institut fur Qualitat und Wirschaftlichkeit im Gesundheitswesen (IQWIG) and Office of Technology Assessment of the German Parliament. A manual search was also undertaken.
The literature search yielded 1838 distinct abstracts of which 1412 abstracts were removed because of duplication or lack of relevance. Of the remaining 426 abstracts, a further 338 were excluded as they lacked the necessary information required to construct an economic model (i.e. there was no clear focus on CC and LBC, studies were insufficiently powered, lacked any useful quantitative estimates, characteristics of the study population were unclear). This resulted in 98 articles requiring a full text review, of which 16 contained relevant data which were used to directly inform this modelling study (Figure S1).
Economic Model
The economic analysis was performed using a combined decision and Markov model to depict the lifetime costs and consequences of cervical screening among a cohort of hypothetical women from 20 years of age in Germany.
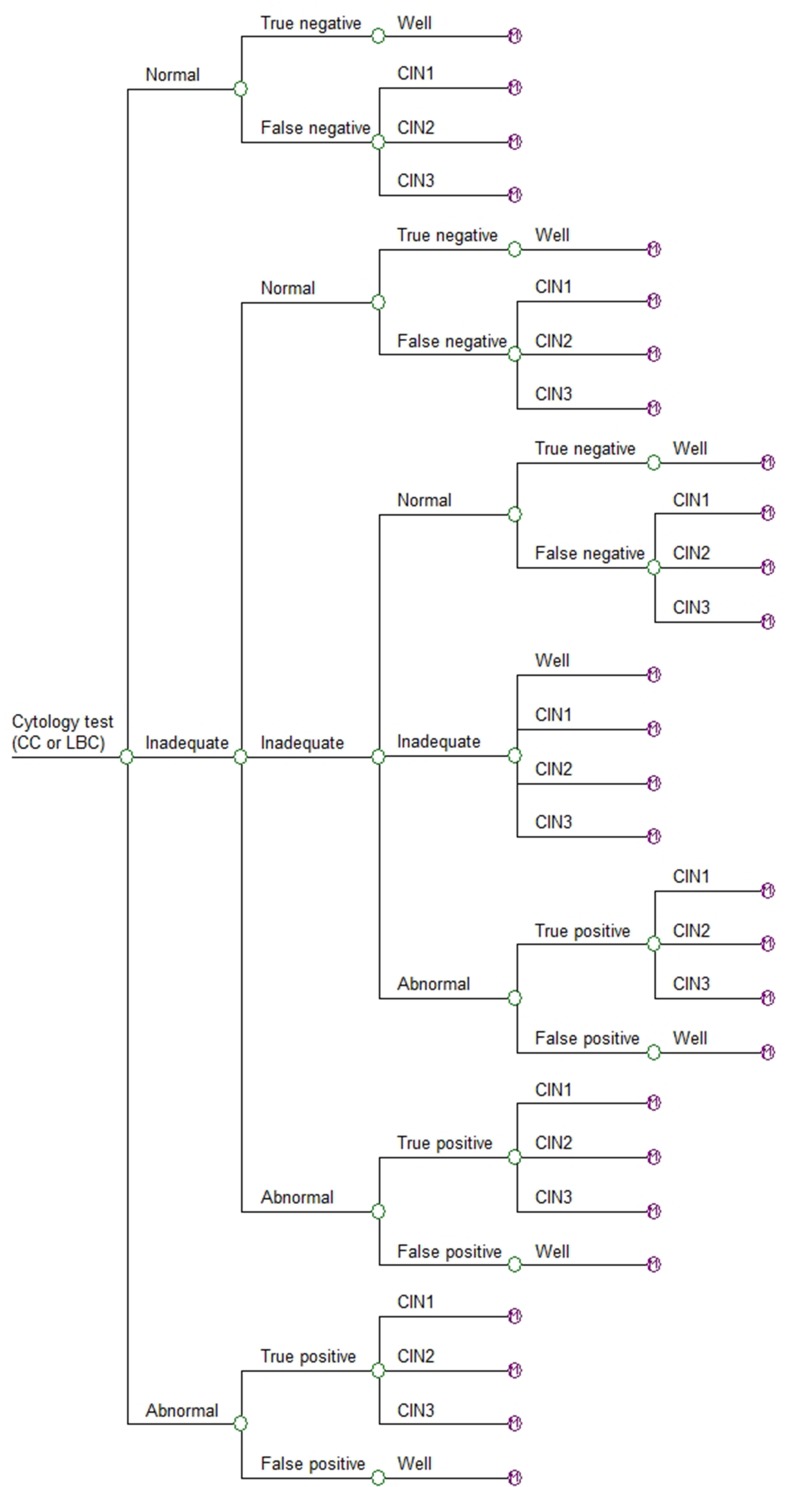
In the first instance a hypothetical 20-year-old woman enters the decision model (Figure 1), which simulated the possible outcomes following a single smear test. These outcomes comprised the probability of a subject receiving either a normal, abnormal or inadequate test result. An inadequate result generally arises from an issue with the quality of the slide, such as obscuration by blood or incorrect spread of cells and does not reflect the likelihood of abnormal cells being present.17 The accuracy of the test is ultimately based on the probability of detecting a true negative or true positive.
Figure 1.
Decision model.
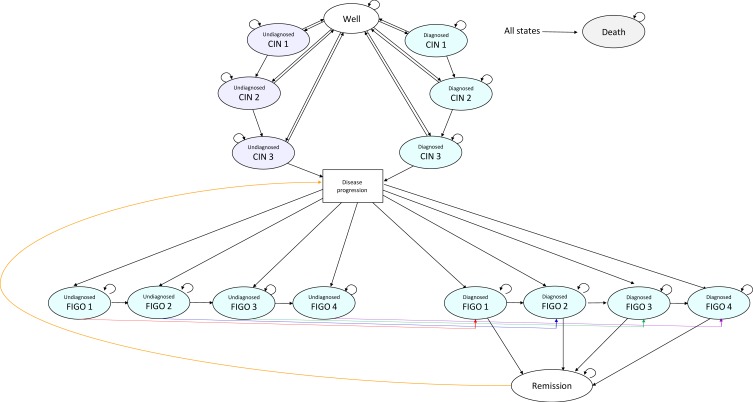
After one smear test, subjects exit the decision model and based on the branch from which they leave, they enter the same state in the Markov Model (Figure 2). The Markov model comprised 16 health states and death; well, diagnosed CIN1, diagnosed CIN2, diagnosed CIN3, undiagnosed CIN1, undiagnosed CIN2, undiagnosed CIN3, diagnosed FIGO 1, diagnosed FIGO 2, diagnosed FIGO 3, diagnosed FIGO 4, undiagnosed FIGO 1, undiagnosed FIGO 2, undiagnosed FIGO 3, undiagnosed FIGO 4 and disease remission. Within the Markov model, women transition annually between being well, the different health states pertaining to pre-cancerous lesions, the different stages of cervical cancer, remission and death.
Figure 2.
Markov model. The Disease Progression box is not a health state per se, and has been inserted to simplify the diagram.
Each year, subjects in the well and undiagnosed CIN health states leave the Markov model and re-enter the decision model for a new screen. However, not all women are screened every year. Hence, the model assumed that 80% of women aged 20 to 65 years and 70% of women over 65 years of age in the well state and the undiagnosed CIN states of the Markov model re-enter the decision model annually to undergo a new smear test. The model assumed that 100% of women in the diagnosed CIN1 or CIN2 health states would re-enter the decision model for a new smear test every 6 months.
Model Inputs
The probabilities of LBC and CC detecting a normal, inadequate, abnormal, true negative and true positive result incorporated into the decision model are shown in Table 1. The transition probabilities incorporated into the Markov model are shown in Table 2. The model assumed that those subjects with a CIN1 diagnosis would be monitored to see whether the lesions ameliorated on their own. The model also assumed that those subjects who were diagnosed with CIN2 or CIN3 would undergo further diagnostic tests (i.e. a colposcopy and biopsy) and be treated with standard care. Within the model, if a subject’s pre-cancerous lesions resolved they would return to the well health state. Accordingly, the model assumed that all subjects with diagnosed CIN1 or diagnosed CIN2 would transition to the well state after a year. Published data were used to estimate subjects’ transition from CIN3 to other states. The probability of subjects in an undiagnosed CIN health state having pre-cancerous lesions is obviously unknown. Hence, the model assumed they would undergo an annual smear test as if they were in the well state.
Table 1.
Decision Model Probabilities
| Variable | CC Values | LBC Values |
|---|---|---|
| Probability of an abnormal result | 0.02936,38 | 0.04736,38 |
| Probability of an inadequate result | 0.02917,36,38 | 0.00817,36,38 |
| Probability of a normal result | 0.94236,38 | 0.94536,38 |
| Probability of a true positive | 0.98212,36,39 | 0.97712,36,39 |
| Probability of a false positive | 0.01812,36,39 | 0.02312,36,39 |
| Probability of a true negative | 0.92012,36,39 | 0.96212,36,39 |
| Probability of a false negative | 0.08012,36,39 | 0.03812,36,39 |
| Proportion of subjects with CIN1 (Normal) | 0.78920 | 0.78920 |
| Proportion of subjects with CIN2 (Normal) | 0.12220 | 0.12220 |
| Proportion of subjects with CIN3 (Normal) | 0.08920 | 0.08920 |
| Proportion of subjects who are Well (Inadequate) | 0.98021,22 | 0.98021,22 |
| Proportion of subjects with CIN1 (Inadequate) | 0.01421,22 | 0.01421,22 |
| Proportion of subjects with CIN2 (Inadequate) | 0.00121,22 | 0.00121,22 |
| Proportion of subjects with CIN3 (Inadequate) | 0.00521,22 | 0.00521,22 |
| Proportion of subjects with CIN1 (Abnormal) | 0.02220 | 0.02220 |
| Proportion of subjects with CIN2 (Abnormal) | 0.47320 | 0.47320 |
| Proportion of subjects with CIN3 (Abnormal) | 0.50520 | 0.50520 |
Table 2.
Markov Model Transition Probabilities
| Variable | CC Values | LBC Values |
|---|---|---|
| Well to CIN1 (undiagnosed) | 0.061* | 0.029* |
| Well to CIN2 (undiagnosed) | 0.009* | 0.004* |
| Well to CIN3 (undiagnosed) | 0.007* | 0.003* |
| CIN1 to Well (undiagnosed) | 0.893* | 0.918* |
| CIN1 to CIN2 (undiagnosed) | 0.009* | 0.004* |
| CIN2 to Well (undiagnosed) | 0.893* | 0.918* |
| CIN2 to CIN3 (undiagnosed) | 0.007* | 0.003* |
| Well to CIN1 (diagnosed) | 0.001* | 0.001* |
| Well to CIN2 (diagnosed) | 0.014* | 0.022* |
| Well to CIN3 (diagnosed) | 0.015* | 0.023* |
| CIN1 to Well (diagnosed) | 1.000** | 1.000** |
| CIN1 to CIN2 (diagnosed) | 0.000** | 0.000** |
| CIN2 to Well (diagnosed) | 1.000** | 1.000** |
| CIN2 to CIN3 (diagnosed) | 0.000** | 0.000** |
| CIN3 to Well (undiagnosed) | 0.0695 | 0.0695 |
| CIN3 to Well (diagnosed) | 0.85640 | 0.85640 |
| CIN3 to disease progression (aged 15–24 years) | 0.0015 | 0.0015 |
| CIN3 to disease progression (aged 25–34 years) | 0.0015 | 0.0015 |
| CIN3 to disease progression (aged 35–38 years) | 0.0305 | 0.0305 |
| CIN3 to disease progression (aged 39–49 years) | 0.0655 | 0.0655 |
| CIN3 to disease progression (aged 50–64 years) | 0.0825 | 0.0825 |
| CIN3 to disease progression (aged ≥65 years) | 0.0835 | 0.0835 |
| Undiagnosed CIN 3 to FIGO 1 | 0.30040 | 0.30040 |
| Diagnosed FIGO 1 to Diagnosed FIGO 2 | 0.2935 | 0.2935 |
| Diagnosed FIGO 2 to Diagnosed FIGO 3 | 0.2795 | 0.2795 |
| Diagnosed FIGO 3 to Diagnosed FIGO 4 | 0.3465 | 0.3465 |
| Undiagnosed FIGO 1 to Diagnosed FIGO 1 | 0.1505 | 0.1505 |
| Undiagnosed FIGO 2 to Diagnosed FIGO 2 | 0.2255 | 0.2255 |
| Undiagnosed FIGO 3 to Diagnosed FIGO 3 | 0.6005 | 0.6005 |
| Undiagnosed FIGO 4 to Diagnosed FIGO 4 | 0.9005 | 0.9005 |
| Diagnosed FIGO 1 to Remission | 0.95041 | 0.95041 |
| Diagnosed FIGO 2 to Remission | 0.50041 | 0.50041 |
| Diagnosed FIGO 3 to Remission | 0.40041 | 0.40041 |
| Diagnosed FIGO 4 to Remission | 0.05041 | 0.05041 |
Notes: *derived from the decision tree. **assumed values.
No published evidence could be found for the distribution of subjects between the well, CIN1, CIN2 and CIN3 health states. There was some evidence for the distribution of subjects between the CIN1, CIN2 and CIN3 health states who were HPV positive or negative.20 These data were used as a proxy by assuming that if a subject had a false negative result then the ensuing probability distribution between the three CIN health states would be similar to those who were HPV negative. Similarly, if a subject had a true positive result then it was assumed the ensuing probability distribution between three CIN health states would be similar to those who were HPV positive. The model assumed that the inadequate smears occurred at random and were not related to the severity of potential pre-cancerous lesions. Therefore, the estimates of disease prevalence were used to assign a probability distribution between the three CIN health states to subjects who received an inadequate result.21,22
No published evidence could be found for the transitions between individual undiagnosed FIGO states. Hence, the Markov model assumed these transitions would be the same as those for the diagnosed FIGO states
Background Death Rate
Within the model, subjects can die at any time. The mortality rate for different age groups was obtained by dividing the number of deaths of women at each age by the number of females in the population at each age in Germany.23,24 This mortality rate was then applied to all the health states in order to calculate the total number of women in the model who died annually.
Unit Costs
Unit costs in Euros at 2017/2018 prices (Table 3) were assigned to the estimates of healthcare resource use in the model (Table 4). Unit costs obtained from previous years were uprated to 2017/2018 prices using the German inflation index. From the second year onwards the costs were discounted at a rate of 3% per annum.25,26 The cost of treatment for those in the diagnosed CIN2 and CIN3 health states in the Markov model was only applied to the first year subjects were in that state, since they were unlikely to receive the same treatment multiple times. These subjects could subsequently receive more extensive cancer management, depending on the health state to which they transition.
Table 3.
Unit Costs
| Resource | Unit Cost |
|---|---|
| CC (per test) | €0.65 |
| LBC (per test) | €3.65 |
| Transportation to laboratory (per sample) | €2.6042 |
| Clinician visit | €9.8042 |
| Colposcopy | €25.0143 |
| Biopsy | €100.0442 |
| Treatment of subject in CIN2 (per year) | €356.1443 |
| Treatment of subject in CIN3 (per year) | €1587.7943 |
| Treatment of FIGO 1 (per year) | €8456.6227 |
| Treatment of FIGO 2 (per year) | €12,763.0327 |
| Treatment of FIGO 3 (per year) | €13,792.1027 |
| Treatment of FIGO 4 (per year) | €11,826.4627 |
| Palliative Care | €8375.1527 |
Table 4.
Resource Use Incorporated into the Models
| Resource | Value |
|---|---|
| Annual screening rate among those aged 20–64 years | 80% |
| Annual screening rate among those aged ≥65 years | 70% |
| Number of re-screens per subject per year in diagnosed CIN1 | 2 |
| Number of re-screens per subject per year in diagnosed CIN2 | 2 |
| Number of annual clinician visits per subject in each diagnosed CIN state | 2 |
| Number of colposcopies per subject in each diagnosed CIN state | 1 |
| Number of biopsies per subject in each diagnosed CIN state | 1 |
| Probability of subjects in each diagnosed CIN state undergoing treatment | 1 |
| Discount rate | 3%25 |
The treatment of FIGO 1 can include conisation with/without pelvic lymph node dissection, simple hysterectomy with/without simple pelvic lymph node dissection, radical trachelectomy with pelvic lymph node dissection, radical hysterectomy with pelvic and paraaortic lymph node dissection with and without adjuvant chemoradiotherapy or chemoradiotherapy. The treatment of FIGO 2 and FIGO 3 can include radical hysterectomy with pelvic and paraaortic lymph node dissection with and without adjuvant chemoradiotherapy or chemoradiotherapy. The treatment of FIGO 4 can include radical hysterectomy with pelvic and paraaortic lymph node dissection with and without adjuvant chemoradiotherapy, exenteration with and without adjuvant chemoradiotherapy, chemoradiotherapy, palliative chemotherapy, hormone replacement therapy and manual lymphatic drainage and venous compression.27
Model Outputs
The outputs of the model comprised the expected lifetime costs of healthcare resource use and the following measures of effectiveness, which were not discounted:
Probability of detecting a true positive (i.e. abnormal cytology) over a subject’s lifetime.
Probability of detecting CIN3 over a subject’s lifetime.
Probability of a subject having undiagnosed CIN3 over their lifetime.
Total probability of a subject having CIN3 over their lifetime.
Probability of a subject moving into disease progression.
Cost-Effectiveness Analysis
The incremental cost-effectiveness of LBC compared with CC was calculated as the difference in the discounted management cost between the two screening strategies divided by the difference in the effectiveness between the two groups (i.e. the probability of detecting a true positive and the probability of detecting CIN3). Hence, the incremental cost-effectiveness of LBC compared with CC was defined as (1) the incremental discounted cost for each additional true abnormality found over a subject’s lifetime and (2) the incremental discounted cost for each additional subject diagnosed with CIN3. If one of the screening strategies improved outcome for less cost, it was the dominant (cost-effective) strategy.
Cost-Benefit Analysis
The incremental cost-benefit of LBC compared with CC was calculated as the difference in the discounted management cost between the two screening strategies divided by the difference in the cost of screening between the two groups to provide an indication of the return on investment with LBC.
Sensitivity Analysis
Probability sensitivity analysis was undertaken to evaluate uncertainty within the model by applying a distribution to all the model inputs. A beta distribution was assigned to the probabilities and a gamma distribution to resource use and costs, by assuming a 5% standard error around the mean values. This enabled the generation of 10,000 iterations of the model by randomly selecting a value from all the different inputs simultaneously. The outputs from these iterations was a distribution of costs and outcomes over a subject’s lifetime. These analyses enabled an estimation of the probability of LBC being cost-effective compared with CC at different willingness to pay thresholds.
Deterministic sensitivity analysis (in the form of a tornado diagram) was also performed to assess the impact of independently varying the values of individual parameters within the model. The parameter estimates were individually varied over plausible ranges by altering them to ±25% around the base case value. However, the probability values were bounded by 0 and 1.
Results
Expected Outcomes
Performing cervical screens with LBC instead of CC is expected to increase the probability of detecting a true abnormality over a subject’s lifetime by 73% (0.038 compared with 0.022). Similarly, the probability of detecting a subject having CIN3 is expected to increase from 0.011 to 0.019 using LBC instead of CC. The model also estimated that a subject would have a 0.014 probability of having undetected CIN3 if they were screened with CC compared with a 0.006 probability if screened with LBC (Table 5). Consequently, the total probability of a subject having CIN3 over their lifetime was 0.025.
Table 5.
Expected Outcomes
| CC | LBC | |
|---|---|---|
| Probability of detecting a true positive (i.e. abnormal cytology) over a subject’s lifetime | 0.022 | 0.038 |
| Probability of detecting CIN3 over a subject’s lifetime | 0.011 | 0.019 |
| Probability of a subject having undiagnosed CIN3 over their lifetime | 0.014 | 0.006 |
| Total probability of a subject having CIN3 over their lifetime | 0.025 | 0.025 |
| Probability of a subject moving into disease progression | 0.018 | 0.010 |
Due to LBC’s greater potential to detect abnormalities, the model estimated the probability of moving into disease progression was decreased by 44% (from 0.018 to 0.010) among subjects who were screened with LBC instead of CC (Table 5).
Expected Healthcare Costs
Cervical screening with LBC instead of CC is expected to detect more abnormalities and thereby reduce the probability of subjects transitioning into disease progression. Consequently, the expected lifetime management costs of healthcare resource use were reduced by 35% among subjects screened with LBC instead of CC (Table 6). Table 6 illustrates that over 65% of the lifetime management costs are attributable to managing subjects in a cancerous state. The screening tests accounted for <5% of the total management cost.
Table 6.
Expected Lifetime Costs of Healthcare Resource Use per Subject (Percentage of Total Cost in Parentheses)
| CC (Discounted) | LBC (Discounted) | CC (Undiscounted) | LBC (Undiscounted) | |||||
|---|---|---|---|---|---|---|---|---|
| Screening tests | € 85.32 | (1%) | € 170.64 | (4%) | € 178.53 | (1%) | € 364.37 | (3%) |
| Diagnostic tests | € 76.70 | (1%) | € 128.46 | (3%) | € 158.81 | (1%) | € 271.53 | (2%) |
| Clinician office visits | € 257.27 | (3%) | € 267.56 | (6%) | € 538.32 | (3%) | € 571.32 | (4%) |
| Management in pre-cancerous states | € 619.72 | (8%) | € 1042.30 | (21%) | € 1322.22 | (6%) | € 2267.48 | (17%) |
| Management in cancerous states | € 4332.82 | (58%) | € 2175.65 | (45%) | € 12,302.95 | (58%) | € 6263.04 | (48%) |
| Palliative care | € 2151.06 | (29%) | € 1067.83 | (22%) | € 6536.77 | (31%) | € 3283.78 | (25%) |
| Total | € 7522.89 | (100%) | € 4852.44 | (100%) | € 21,037.60 | (100%) | € 13,021.52 | (100%) |
Cost-Effectiveness Analysis
Performing cervical screens with LBC instead of CC is expected to result in reduced management costs over a subject’s lifetime (Table 7). However, screening with LBC instead of CC is expected to increase the probability of detecting (1) a true abnormality (i.e. abnormal cytology) and (2) a subject having CIN3. Hence, screening with LBC instead of CC affords the German healthcare system a dominant (cost-effective) cervical screening test, since it improves outcomes for less cost (Table 7).
Table 7.
Cost-Effectiveness Analysis
| Test | Mean Lifetime Cost of Management Per Subject | Probability of Detecting: | Difference in Management Cost | Difference in Probability of Detecting: | Incremental Cost for Each Additional: | ||||
|---|---|---|---|---|---|---|---|---|---|
| A true Positive |
A Subject in CIN3 | A True Positive | A Subject in CIN3 | True Positive Detected | Subject Diagnosed with CIN3 | ||||
| Discounted costs | |||||||||
| CC | €7,523 | 0.022 | 0.011 | ||||||
| LBC | €4,852 | 0.038 | 0.019 | -€2,671 | 0.016 | 0.008 | Dominant (-€166,938) | Dominant (-€333,875) | |
| Undiscounted costs | |||||||||
| CC | €21,038 | 0.022 | 0.011 | ||||||
| LBC | €13,022 | 0.038 | 0.019 | -€8016 | 0.016 | 0.008 | Dominant (-€501,000) | Dominant (-€1,002,000) | |
Cost-Benefit Analysis
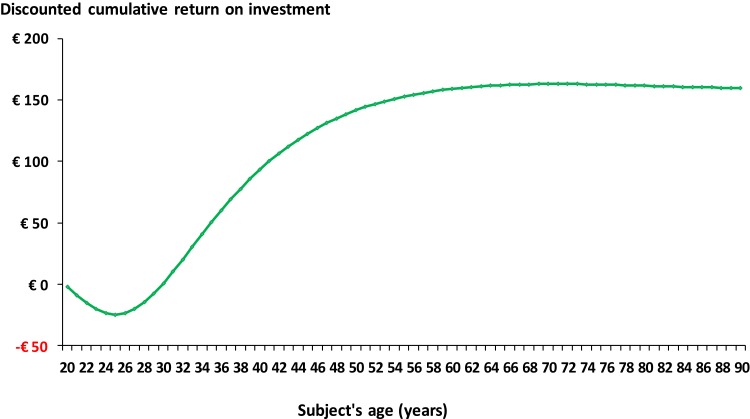
Performing cervical screens with LBC instead of CC is expected to result in a reduced discounted management cost over a subject’s lifetime of €2671 (Table 8). However, the lifetime discounted cost of screening is expected to increase by €16 (Table 8). Hence, the analysis suggests that for every Euro invested in cervical screening with LBC, the German healthcare system would save ~€170 over a subject’s lifetime, irrespective of whether discounted or undiscounted costs were used for the analysis (Table 8). Figure 3 shows that the return on investment starts after ~10 years of screening and then peaks after 30 years (50 years of age) after which it appears to plateau.
Table 8.
Cost-Benefit Analysis
| Mean Lifetime Cost of Management Per Subject | Difference in Management Cost | Mean Lifetime Cost of Screening Per Subject | Difference in Screening Cost | Return on Investment | |
|---|---|---|---|---|---|
| Discounted costs | |||||
| CC | €7,523 | €2,671 | €343 | €167 | |
| LBC | €4,852 | €359 | €16 | ||
| Undiscounted costs | |||||
| CC | €21,038 | €8,016 | €717 | €171 | |
| LBC | €13,022 | €764 | €47 |
Figure 3.
Estimated discounted cumulative cost-benefit of LBC versus CC.
Probabilistic Sensitivity Analysis
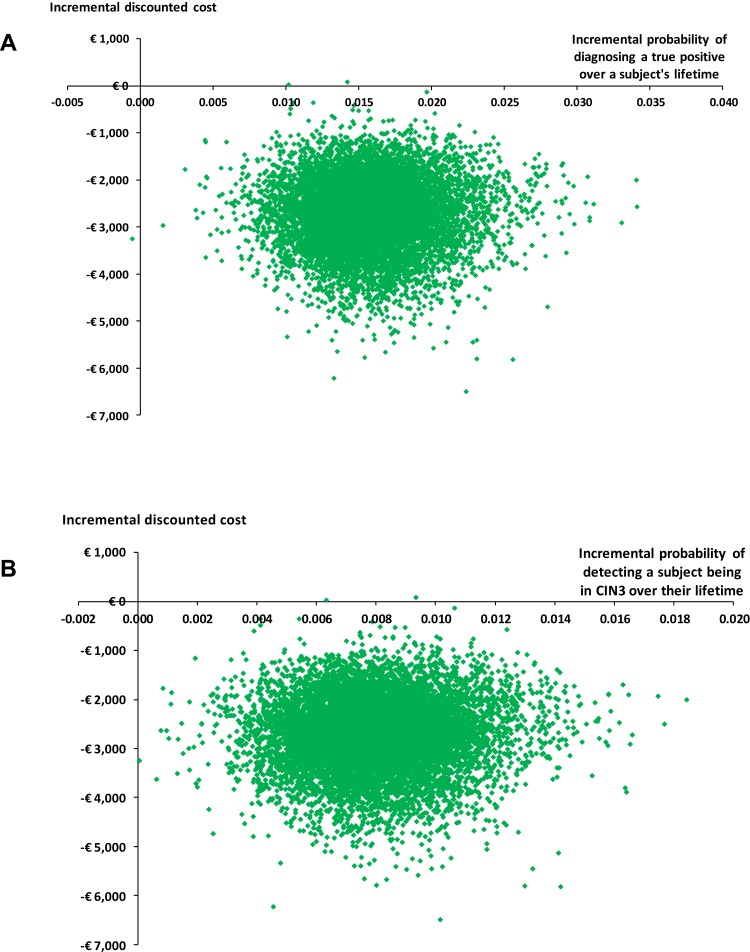
Probabilistic sensitivity analysis highlighted the distribution in the incremental costs and outcomes over a subject’s lifetime, from which all the samples were located in the bottom right (dominant) quadrant (Figure 4). Ouputs from this analysis were used to estimate that there was a >0.99 probability of LBC being cost-effective over a subject’s lifetime when compared with CC.
Figure 4.
(A) Distribution of incremental costs and incremental probabilities of detecting a true positive (i.e. abnormal cytology). (B) Distribution of incremental costs and incremental probabilities of detecting a subject being in CIN3 over their lifetime.
Deterministic Sensitivity Analysis
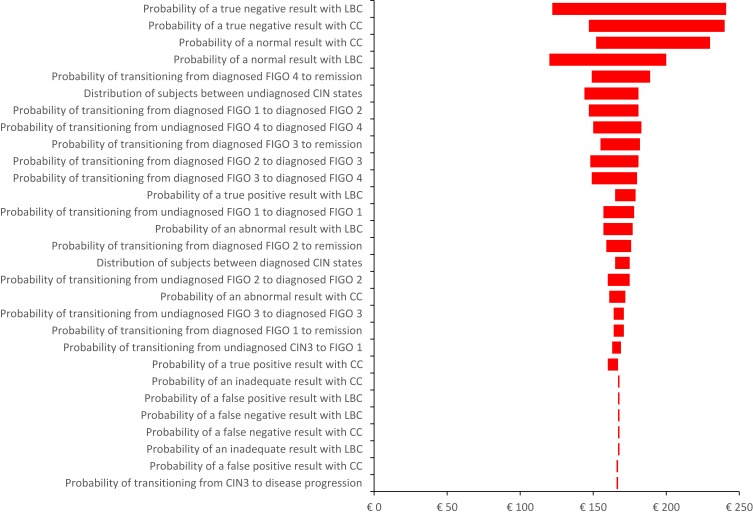
Deterministic sensitivity analysis (Figure 5) showed that the cost-benefit of LBC compared with CC is sensitive to a range of model inputs. In particular, the model was sensitive to (1) the probability of the screening test detecting a normal result, (2) the transition between different FIGO states and (3) the probability of the screening tests detecting an abnormality. However, in all scenarios, screening with LBC compared with CC resulted in a positive net return on investment over a subject’s lifetime. In particular, changing the distribution of subjects between the well and diagnosed CIN health states and between the undiagnosed CIN states had minimal effect on the cost-benefit of LBC compared with CC.
Figure 5.
Tornado diagram of the deterministic sensitivity analysis (range in model inputs is ±25%; probability values were bounded by 0 and 1).
Discussion
Most industrial countries with a cervical screening programme have observed a significant reduction in the incidence and mortality from cervical cancer,28 and LBC is a constituent part of many National Screening Programmes.14–16 Currently all women over 20 years of age in Germany are offered an annual cytology test,18 although this will change in 2020 as previously described.19
This study assessed the health economic impact of undertaking cervical screening with LBC compared with CC from a German healthcare perspective. The authors were unable to find any published German-based studies comparing the cost-effectiveness of these two cytology tests. Therefore, a combination of non-German published data and German statistics were used to populate this study’s model.
The model comprised two components: a decision tree and a Markov chain. The decision tree was considered the most accurate way to depict the short-term outcomes of a smear test and the Markov chain the most representative way to simulate the development of pre-cancerous lesions and disease progression over a subject’s lifetime. A subject’s lifetime was the selected time-horizon of the model in order to reflect the natural history of cervical cancer, which can possibly take up to 20 years to develop.8 The FIGO stages were incorporated into the Markov model to capture the probability of subjects developing cervical cancer and transitioning between the different stages of metastasis. The probabilities of subjects entering remission or dying were also incorporated in separate states. Accordingly, the analysis has included multiple treatments for cervical cancer, which include surgery, radiotherapy and chemotherapy.29,30 There is a paucity of published evidence surrounding patients transitioning between the different diagnosed and undiagnosed FIGO stages of cervical cancer. Hence, inclusion of these health states may have increased inherent uncertainty within the model.
Most economic studies on cervical screening published in the last decade appear to have focussed on HPV testing, which was outside the scope of this study. Therefore, the literature search was extended to encompass the last fifteen years to find cost-effectiveness analyses comparing LBC with CC. The resulting publications were discordant as they reported contradictory results. The findings from our study are consistent with those reported in an Australian31 and UK study.32 However, they contradict the findings from studies conducted in The Netherlands33 and France.34
NICE approved LBC for use within the UK’s National Health Service (NHS).14 One year later, Karnon et al performed a systematic review and economic analysis which provided more certainty around LBC’s cost-effectiveness compared with CC in the United Kingdom.32 Neville and Quinn used an American model, created by the Medicare Services Advisory Committee, in an Australian setting and found that screening with LBC was cost-effective (dominant) compared with CC.31 However, this model appears to have excluded subjects transitioning to CIN1 and therefore the benefits of LBC may have been underestimated.31
The Netherlands-based study reported that switching cervical screening from CC to LBC was only cost-effective under certain circumstances.33 These were the total cost of LBC should not be €3.20 more than that of CC, the sensitivity of LBC should be at least 7% better than that of CC, there should be a larger decrease in quality of life while the women are in the follow-up period and there should be fewer inadequate results with LBC than with CC.33 The authors reported that in the Netherlands there was already a low rate of inadequate smears with CC, and therefore there was little benefit in using LBC.33 However, the authors also stated that a possible reason for the low rate of inadequate results in The Netherlands was attributable to a difference in criteria with other countries in evaluating whether a CC test result was unsatisfactory.33 This present study found that the probability of an inadequate result was halved when using LBC compared with CC. However, the model was not sensitive to the probability of an inadequate result and therefore this parameter was unlikely to have had a large effect on this study’s results.
The findings from the French study34 were consistent with those of the Netherlands-based study.33 The authors of the French study found no clinical benefit for cervical screening with LBC compared with CC and that LBC was more expensive. The cost-effectiveness analysis, which was based on a trial in France, found that the proportion of inadequate results was higher with LBC than with CC.34 This finding is different from those of other studies and may be due to the clinical trial protocol, as the same cervical swab appears to have been used for both the CC and LBC tests.34 CC was performed as normal, however after the slide had been produced the same spatula appears to have then been used to create the LBC sample.34 Consequently, the size of the sample left on the spatula would have been smaller and this may have accounted for a higher number of inadequate results with LBC.
One benefit of LBC is that it involves preserving cells in solution rather than making-up a slide that is fixed at a healthcare practitioner’s surgery. Hence, the suspended cells required for LBC can be potentially used for further testing. This includes using the same sample for both cytology and HPV when co-testing in Germany starts in 2020.19 This is important since if there is an issue with the way a slide has been produced then another slide can be created from the same sample without asking the subject to undergo another smear, as is the case with CC. Additionally, if LBC yields a positive result, the sample could be immediately tested for HPV, whereas subjects would be required to undergo another test if CC was being used. These opportunity costs in terms of healthcare practitioner time and additional tests have not been considered in our analysis. Neither has the costs and consequences of HPV testing, since this was beyond the scope of the model. Consequently, the cost-effectiveness and cost-benefit of LBC may have been underestimated in our analysis.
Study Limitations
The study was subject to several limitations. One such limitation was that data were obtained from indirect and multiple sources, including clinical trials and assumptions. The various studies may have had different protocols, such as differing definitions of inadequate or abnormal results. There were limited sources which documented the probabilities of normal and abnormal results. Increasing the amount of published evidence would inevitably reduce uncertainty and improve the accuracy of the probability values in our model.
The model simulated the pathways for an “average individual”, consequently the probabilities, clinical outcomes and resource use values used in the model may not reflect the dispersion of women in the general public. This analysis excluded health-related quality of life. Also excluded were societal costs and direct costs to subjects. Women may have to take time off work, such as annual leave, to attend a cervical screening examination; there are also costs incurred by subjects for travelling to and from a clinician’s office. These costs will be the same irrespective of which test a woman undergoes. However, the total cost incurred may be greater among women who undergo CC because of the propensity for repeat smears for reasons previously outlined. Moreover, the model assumed that 80% of eligible women would be screened every year until 65 years of age and 70% of women would be screened annually, thereafter. In practice, the annual percentages of women who undergo cervical screening may be lower.
The possible psychological impact of the test results on subjects has not been included in this analysis. There is evidence that an inadequate result can cause a negative impact on women’s well-being in the short-term.35 Women are reported to experience a level of anxiety with an inadequate result similar to that if they received a diagnosis of having CIN1.35 Receiving an inadequate result may also increase a women’s concern about the next smear test.35 Consequently, as the probability of an inadequate result is lower with LBC than with CC, the number of women experiencing such anxiety may be reduced.12,17,36–38
The literature search was unable to identify any evidence pertaining to the distribution of subjects between CIN1, CIN2 and CIN3. It was assumed that the distribution would vary and be dependent on the cytology test result, however no evidence could be found to support this. The distribution of subjects between CIN1, CIN2 and CIN3 after a normal or inadequate test result did not appear to impact on the results from our model. However, the distribution after an abnormal result could potentially affect the incremental cost-effectiveness ratio. Proxy estimates were used to provide these distributions, however the estimates cannot be as accurate as using reliable data. Additionally, minimal published evidence was found on the transition from diagnosed and undiagnosed CIN3 to disease progression, transition between undiagnosed to diagnosed FIGO states and transition between different diagnosed FIGO states. Furthermore, no evidence was found on the transition between different undiagnosed FIGO states, so the model assumed it was the same as for diagnosed FIGO States. Hence, further research is required to inform the estimates used to populate this study’s model and to reduce its inherent uncertainty.
Incorporating HPV testing was beyond the remit of this study. Nevertheless, the prevailing view in the published literature is that primary HPV testing is more cost-effective than either LBC or CC. Consequently, while we found LBC to be more cost-effective than CC, HPV testing could be even more cost-effective. Hence, the broader impact of this study is limited by this exclusion.
This study modelled the German guidelines that were in practice at the time of constructing the economic model (i.e. women were invited for an annual cytology cervical screen).18,19 From 2020, women between 20 and 34 years of age will be offered an annual cytology cervical screen, but women who are 35 years of age and above (with no upper age limit) will be offered a combined cytology and HPV test every three years.19 Modelling the 2020 guidelines was beyond the remit of this study. However, extending the screening interval to three years is unlikely to substantially alter cervical cancer incidence or mortality rates, but will inevitably lead to a reduction in the number of cytology tests and colposcopy procedures. Consequently, extending the screening interval could potentially provide safety benefits by reducing the potentially harmful risks of tests and procedures for abnormalities that may otherwise regress. Nevertheless, it would be speculative to comment on whether LBC would be more cost-effective than CC when including a HPV test and extending the screening interval to three years, since it is not possible to quantify the magnitude of the ensuing potential risks and benefits with this study’s model.
In conclusion, within the study’s limitations, the analysis showed that LBC affords a cost-effective cervical screening test compared with CC in Germany. It has the potential to increase the number of true abnormalities by 73% and to decrease the probability of a subject transitioning into disease progression by >40% for less cost. Therefore, policy makers should view LBC as the cytology test of choice.
Author Contributions
Both authors contributed to data analysis, drafting or revising the article, gave final approval of this version to be published, and agree to be accountable for all aspects of the work.
Funding
This paper was funded by Hologic Ltd, Wythenshawe, Manchester, UK.
Disclosure
The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript. The authors have no other conflicts of interest that are directly relevant to the content of this manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Klug SJ, Neis KJ, Harlfinger W, et al. A randomized trial comparing conventional cytology to liquid-based cytology and computer assistance. Int J Cancer. 2013;132(12):2849–2857. doi: 10.1002/ijc.27955 [DOI] [PubMed] [Google Scholar]
- 3.Bruni LAG, Serrano B, Mena M, et al. Human Papillomavirus and related diseases in Germany. Summary report 10 December 2018. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre).
- 4.Robert Koch Institut. HPV-Prävalenzstudie 2017/18; 2018. Available from: https://www.rki.de/DE/Content/Infekt/Impfen/Forschungsprojekte/HPV-Praevalenzstudie/HPV_node.html. Accessed January24, 2020 2019.
- 5.Sroczynski G, Schnell-Inderst P, Muhlberger N, et al. Cost-effectiveness of primary HPV screening for cervical cancer in Germany–a decision analysis. Eur J Cancer. 2011;47(11):1633–1646. doi: 10.1016/j.ejca.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–664. doi: 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.England NHS. Overviw of cervical cancer. 2018. Available from: https://www.nhs.uk/conditions/cervical-cancer/. Accessed January24, 2020 2019.
- 8.NHS England. Cervical Cancer, Causes. 2018. Available from: https://www.nhs.uk/conditions/cervical-cancer/causes/. Accessed January24, 2020 2019.
- 9.Macmillan Cancer Support. Colposcopy and CIN. 2018. Available from: https://www.macmillan.org.uk/information-and-support/diagnosing/how-cancers-are-diagnosed/cervical-screening/cin.html. Accessed January24, 2020 2019.
- 10.Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–135. doi: 10.1002/ijgo.12749 [DOI] [PubMed] [Google Scholar]
- 11.Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009;302(16):1757–1764. doi: 10.1001/jama.2009.1569 [DOI] [PubMed] [Google Scholar]
- 12.Rimiene J, Petronyte J, Gudleviciene Z, Smailyte G, Krasauskaite I, Laurinavicius A. A Shandon PapSpin liquid-based gynecologicl test: a split-sample and direct-to-vial test with histology follow-up study. Cytojournal. 2010;7(2). doi: 10.4103/1742-6413.61200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozemeijer K, Penning C, Siebers AG, et al. Comparing SurePath, ThinPrep, and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27(1):15–25. doi: 10.1007/s10552-015-0678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Health and Care Excellence. Guidance on the use of liquid-based cytology for cervical screening. 2003. Available from: https://www.nice.org.uk/guidance/ta69/chapter/1-Guidance. Accessed January24, 2020 2019.
- 15.National Screening Unit. HPV primary screening. 2017. Available from: https://www.nsu.govt.nz/health-professionals/national-cervical-screening-programme/hpv-primary-screening. Accessed January24, 2020 2019.
- 16.Hing E, Saraiya M, Roland K. Liquid-based cytology test use by office-based physicians: United States, 2006–2007. National Health Statistics Reports. 2011. Vol. 40. [PubMed] [Google Scholar]
- 17.Castle PE, Bulten J, Confortini M, et al. Age-specific patterns of unsatisfactory results for conventional Pap smears and liquid-based cytology: data from two randomised clinical trials. BJOG. 2010;117(9):1067–1073. doi: 10.1111/j.1471-0528.2010.02650.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zentrum Fur Krebsregisterdaten. Cervical cancer. 2017. Available from: https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Gebaermutterhalskrebs/gebaermutterhalskrebs_node.html;jsessionid=0D20C7CBCF9B6114207230D754414EBE.2_cid298. Accessed January24, 2020 2019.
- 19.Bundesausschuss G. Early detection of cervical cancer in the future as an organized program. Available from: https://www.g-ba.de/institution/presse/pressemitteilungen/774/. Accessed January24, 2020 2019.
- 20.Iftner T, Becker S, Neis KJ, et al. Head-to-head comparison of the RNA-based aptima human papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol. 2015;53(8):2509–2516. doi: 10.1128/JCM.01013-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer. 2003;88(10):1570–1577. doi: 10.1038/sj.bjc.6600918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheider AH, Lotz,B H. Screening for high-grade cervical intra-epithelial, neoplasia and cancer by testing for high-risk, routine cytology or colposcopy. Int J Cancer. 2000;89(6):529–534. [doi:89(6):529-34]. doi: [DOI] [PubMed] [Google Scholar]
- 23.Destatis Statistisches Bundesamt. Population projection. 2018. Available from: https://www.destatis.de/EN/FactsFigures/SocietyState/Population/PopulationProjection/PopulationProjection.html. Accessed January24, 2020 2019.
- 24.Destatis Statistisches Bundesamt. Deaths, life expectancy. 2018. Available from: https://www.destatis.de/EN/FactsFigures/SocietyState/Population/Deaths/Deaths.html. Accessed January24, 2020 2019.
- 25.Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745–758. doi: 10.1007/s40273-018-0672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). General methods for the assessment of the relation of benefits to costs. 2009. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwiL4_SFjfLmAhXSTBUIHUy0C50QFjABegQIBhAC&url=https%3A%2F%2Fwww.iqwig.de%2Fdownload%2FGeneral_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf&usg=AOvVaw1Muo_-bYcWW-Dug3fRKlcR. Accessed January24, 2020.
- 27.Damm O, Horn J, Mikolajczyk RT, et al. Cost-effectiveness of human papillomavirus vaccination in Germany. Cost Eff Resour Alloc. 2017;15:18. doi: 10.1186/s12962-017-0080-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petry KU, Wormann B, Schneider A. Benefits and risks of cervical cancer screening. Oncol Res Treat. 2014;37(Suppl 3):48–57. doi: 10.1159/000365059 [DOI] [PubMed] [Google Scholar]
- 29.Tomao F, Corrado G, Peccatori FA, et al. Fertility-sparing options in young women with cervical cancer. Curr Treat Options Oncol. 2016;17(1):5. doi: 10.1007/s11864-015-0386-9 [DOI] [PubMed] [Google Scholar]
- 30.Brucker SY, Ulrich UA. Surgical treatment of early-stage cervical cancer. Oncol Res Treat. 2016;39(9):508–514. doi: 10.1159/000448794 [DOI] [PubMed] [Google Scholar]
- 31.Neville AM, Quinn MA. An alternative cost effectiveness analysis of ThinPrep in the Australian setting. Aust N Z J Obstet Gynaecol. 2005;45(4):289–294. doi: 10.1111/j.1479-828X.2005.00413.x [DOI] [PubMed] [Google Scholar]
- 32.Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N. Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis. Health Technol Assess. 2004;8(20):iii,1–78. doi: 10.3310/hta8200 [DOI] [PubMed] [Google Scholar]
- 33.de Bekker-grob EW, de Kok IM, Bulten J, et al. Liquid-based cervical cytology using ThinPrep technology: weighing the pros and cons in a cost-effectiveness analysis. Cancer Causes Control. 2012;23(8):1323–1331. doi: 10.1007/s10552-012-0011-1 [DOI] [PubMed] [Google Scholar]
- 34.Cochand-Priollet M, Cartier I, de Cremoux P, et al. Cost-effectiveness of liquid-based cytology with or without hybrid-capture II HPV test compared with conventional Pap Smears: a study by the french society of clinical cytology. Diagn Cytopathol. 2004;33(5):338–343. doi: 10.1002/dc.20283 [DOI] [PubMed] [Google Scholar]
- 35.French DP, Maissi E, Marteau TM. Psychological costs of inadequate cervical smear test results. Br J Cancer. 2004;91(11):1887–1892. doi: 10.1038/sj.bjc.6602224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beerman H, van Dorst EB, Kuenen-Boumeester V, Hogendoorn PC. Superior performance of liquid-based versus conventional cytology in a population-based cervical cancer screening program. Gynecol Oncol. 2009;112(3):572–576. doi: 10.1016/j.ygyno.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 37.Rahimi S, Carnovale-Scalzo C, Marani C, Renzi C, Malvasi A, Votano S. Comparison of conventional Papanicolaou smears and fluid-based, thin-layer cytology with colposcopic biopsy control in central Italy: a consecutive sampling study of 461 cases. Diagn Cytopathol. 2009;37(1):1–3. doi: 10.1002/dc.20947 [DOI] [PubMed] [Google Scholar]
- 38.Sigurdsson K. Is a liquid-based cytology more sensitive than a conventional Pap smear? Cytopathology. 2013;24(4):254–263. doi: 10.1111/cyt.12037 [DOI] [PubMed] [Google Scholar]
- 39.Yanoh K, Norimatsu Y, Munakata S, et al. Evaluation of endometrial cytology prepared with the Becton Dickinson SurePath method: a pilot study by the Osaki study group. Acta Cytol. 2014;58(2):153–161. doi: 10.1159/000357769 [DOI] [PubMed] [Google Scholar]
- 40.Del Mistro A, Matteucci M, Insacco EA, et al. Long-term clinical outcome after treatment for high-grade cervical lesions: a retrospective monoinstitutional cohort study. Biomed Res Int. 2015;2015:984528. doi: 10.1155/2015/984528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancer Research UK. 2019. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/survival?_ga=2.51202347.346490256.1559984656-841381996.1559984656. Accessed March 2019.
- 42.Kassenarztliche Bundesvereininung (KBV). Kassenarztliche Bundesvereininung (KBV). 2019. Available from: Available from: https://www.kbv.de/html/index.php. Accessed January24, 2020.
- 43.Schobert D, Remy V, Schoeffski O. Cost-effectiveness of vaccination with a quadrivalent HPV vaccine in Germany using a dynamic transmission model. Health Econ Rev. 2012;2:19. doi: 10.1186/2191-1991-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Robert Koch Institut. HPV-Prävalenzstudie 2017/18; 2018. Available from: https://www.rki.de/DE/Content/Infekt/Impfen/Forschungsprojekte/HPV-Praevalenzstudie/HPV_node.html. Accessed January24, 2020 2019.