Abstract
Background
Prostate cancer (PCa) is a common malignant tumor of the urinary system in males. LncRNA taurine-upregulated gene 1 (TUG1) has been verified to play a crucial role in progression and prognosis of PCa. However, the functional mechanism of TUG1 remains unclear with radiosensitivity of PCa.
Methods
Quantitative real-time PCR (qRT-PCR) was conducted to measure the transcription levels of genes. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and flow cytometry analysis were employed to assess cell proliferation and apoptosis, respectively. Moreover, colony formation assay was used to measure colony survival. Western blot was performed to detect the relative proteins expression. The interaction among variables was predicted by online tool starbase, and then confirmed using the dual luciferase reporter assay. A xenograft mouse model was constructed to investigate the effect of TUG1 on tumor growth in vivo.
Results
The levels of lncRNA TUG1 and SMC1A were remarkably increased, while miR-139-5p was downregulated in PCa. Patients with high expression of TUG1 showed a lower survival rate and poor prognosis. Knockdown of TUG1 inhibited PCa cell proliferation and colony survival fraction, and promoted apoptosis. Downregulation of miR-139-5p reversed the effects of TUG1 deletion on proliferation, apoptosis and colony survival fraction in PCa cells treated with 4 Gy of X-ray radiation. Moreover, TUG1 sponged miR-139-5p to regulate SMC1A expression. SMC1A deletion blocked the effects of TUG1 on the progression of PCa cells treated with 4 Gy of X-ray radiation. The tumor volume and weight were illustriously reduced with radiation and TUG1 silencing in xenograft model.
Conclusion
Knockdown of lncRNA TUG1 enhanced radiosensitivity in PCa via the TUG1/miR-139-5p/SMC1A axis. It may become a promising target for PCa treatment.
Keywords: TUG1, miR-139-5p, SMC1A, prostate cancer, radiosensitivity
Introduction
The prostate is a crucial organ in male health. In recent years, prostate cancer (PCa) has become a significant clinical disease all over the world. Many patients are always clinically diagnosed with PCa at the average age of approximately 66 years old.1 Patients were always diagnosed as PCa at advanced stage and treated with the conventional treatments with poor prognosis.2 Radiotherapy is a commonly adjuvant therapy of surgery and is also a treatment for the localized PCa patients. Although radiotherapy is a crucial therapeutic opinion for patients with regionally unresectable advanced PCa, the therapeutic efficiency is eventually reduced in most PCa patients caused by resistance to radiation.3 Thus, it is increasingly important to search an effective therapeutic strategy for clinical medical research.
Long non-coding RNAs (LncRNAs), composed of almost 200 nucleotides without ability to code protein, are a kind of transcript with extremely conserved sequence. Accumulating evidence indicated that lncRNAs could play a crucial regulatory role in tumor pathogenesis and progression through complex regulation mechanisms. LncRNAs knockdown promoted cell apoptosis and the resistance of radiotherapy in human tumor, such as colorectal cancer, non-small cell lung cancer and PCa.4–6 Overexpression of lncRNA taurine-upregulated gene 1 (TUG1) aggravated progression and was associated with the poor prognosis in PCa.2,7-9 TUG1 knockdown suppressed HMGB1 expression to improve the radiosensitivity of bladder cancer.10 However, the effect of TUG1 on radiosensitivity of PCa is not yet well understood.
MicroRNAs (miRNAs) are a class of endogenous RNAs with about 18–25 nucleotides in length without coding protein sequence, and miRNAs have been discovered regulatory functions in eukaryotes. As known, miRNAs fixed to the 3ʹ UTR of target mRNA and formed a silent complex to inhibit target genes activity. Accumulating researches revealed that miRNAs acted as tumor suppressors or oncogenes, and participated in tumorigenesis and cancer progression. MiR-17-3p attenuated the activation of antioxidants and enhanced the radiosensitivity of PCa.11 In ovarian cancer cells, miR-142-5p enhanced cisplatin-induced apoptosis by targeting multiple anti-apoptotic genes12 and facilitated tumor metastasis through directly regulating CYR61 expression.13 MiR-449a enhanced radiosensitivity through modulating pRb/E2F1 pathway in PC-3 cells.14 MiR-139-5p downregulation promoted tumor progression by regulating of SOX5 in PCa,15 and elevated cell proliferation by targeting Notch1, but it had no consequence on apoptosis.16 Pajic et al found that miR-139-5p enhanced the radiosensitivity of breast cancer by suppressing DNA repair networks and ROS protection.17 However, the regulatory effects of miR-139-5p on the radiosensitivity of PCa remains undefined.
Structural maintenance of chromosomes protein 1A (SMC1A) is a crucial subunit of the cohesion protein complex, and it is essential for sister chromatid cohesion in chromosome dynamics. The abnormal expression of SMC1A or mutation is closely related to tumorigenesis and tumor progress. LncRNA NEAT1 elevation restrained cell proliferation and advanced apoptosis by regulating SMC1A in acute myeloid leukemia cells.18 SMC1A expression was meaningfully increased in androgen-independent PCa cells, and knockdown of SMC1A inhibited cell growth, proliferation, colony formation, and migration, but had no effects on biochemical recurrence and distant metastasis.19 The recent reports indicated that SMC1A deletion enhanced the efficacy of radiotherapy by important regulation pathway through regulating the colony formation ability, the epithelial-mesenchymal transition (EMT), the cancer stem-like cell (CSC) population, DNA repair capacity and ROS.20
In the current report, the target relationship among TUG1, miR-139-5p and SMC1A in PCa was verified. Our results revealed that TUG1 regulated SMC1A by sponging miR-139-5p, and SMC1A expression was positively correlated with TUG1. TUG1 deletion enhanced the radiosensitivity of PCa cell lines, and miR-139-5p inhibitor could partially restore this effect. Overexpression of SMC1A reversed the effects of TUG1 knockdown on tumor progress, including cell proliferation, metastasis, viability and apoptosis. To be brief, we aimed to explore the underlying molecular mechanism of TUG1 in the radiosensitivity of PCa and provided new potential therapeutic targets for PCa treatment.
Methods
Patient Tissue Samples and Cell Lines
Fifty pairs of PCa pathological samples and the adjacent normal tissue samples were collected from China-Japan Union Hospital of Jilin University. The clinicopathologic features of these patients were presented in Table 1. Before using these clinical materials, ratification from the Ethics Committee of China-Japan Union Hospital of Jilin University and written informed consents of all patients were obtained. And our study was conducted in line with the guidelines and principles of the Helsinki Declaration.
Table 1.
Association of TUG1 Expression with Clinicopathological Factors in PCa
| Clinicopathological Features | Number of Cases | lncRNA TUG1 Expression | P value | |
|---|---|---|---|---|
| Low (n) | High (n) | |||
| Age | ||||
| >60 years | 30 | 13 | 17 | 0.248 |
| ≤60 years | 20 | 12 | 8 | |
| Tumor size (cm) | ||||
| >2.5 | 28 | 12 | 16 | 0.254 |
| ≤2.5 | 22 | 13 | 9 | |
| Gleason score | ||||
| >7 | 37 | 15 | 22 | 0.024 |
| ≤7 | 13 | 10 | 3 | |
| Clinical stage | ||||
| I+II | 33 | 20 | 13 | 0.037 |
| III+IV | 17 | 5 | 12 | |
| Preoperative PSA level (ng/mL) | ||||
| >10 | 36 | 13 | 23 | 0.002 |
| ≤10 | 14 | 12 | 2 | |
| Lymph node metastasis | ||||
| Positive | 21 | 6 | 15 | 0.010 |
| Negative | 29 | 19 | 10 | |
Human normal prostatic stromal immortalized cell line (WPMY-1 (Dulbecco’s Modified Eagle’s Medium)) and PCa cells (LNCaP (RPMI 1640), 22RV1 (RPMI 1640), PC-3 (F-12K) and DU145 (Eagle’s Minimum Essential Medium)) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). WPMY-1 and DU145 cells were incubated with Dulbecco’s Modified Eagle’s Medium (DMEM), and LNCaP and 22RV1 cells were incubated with Roswell Park Memorial Institute-1640 (RPMI-1640) medium, as well as PC-3 cells were incubated with F12K medium, respectively. All cells were incubated with these medium supplemented with 10% fetal bovine serum (FBS) and maintained in a humid 5% CO2 incubator at 37°C. Pharmaceutical reagents were purchased from Gibco (Rockville, MD, USA). Radiation was carried out using a Faxitron 43855F X-ray Irradiator (Faxitron Bioptics LLC, Tucson, AZ, USA) and 2, 4, 6, and 8 Gy radiation were used to treat PCa cells as previously described.21
Quantitative Real-Time PCR
TRIzol reagent kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract total RNA from PCa tissues and cells according to the manufacturer’s instructions. All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia, FulenGen, China) was used for the synthesis of complementary DNA (cDNA). QRT-PCR reaction solution was prepared using SYBR Premix Ex Taq II (TaKaRa, Dalian, China), qRT-PCR quantitation was used to analyze the mRNA expression level using Applied Biosystems 7500 Real-Time PCR Systems (Applied Biosystems, Foster City, CA) by adopting 2−ΔΔct method.22 U6 and GAPDH were used to standardize miRNA and mRNA, respectively. The primers were the following: TUG1: 5ʹ-CTGAAGAAAGGCAACATC-3ʹ and 5ʹ-GTAGGCTACTACAGGATTTG-3ʹ; miR-139-5p: 5ʹ-CCTCTACAGTGCACGTGTCTC-3ʹ and 5ʹ-CGCTGTTCTCATCTGTCTCGC-3ʹ; U6: 5ʹ-CTCGCTTCGGCAGCAGCACATATA-3ʹ and 5ʹ-AAATTGGAACGCTTCACGA-3ʹ; SMC1A: 5ʹ-TACCTCAGTTCTCGGGCGTA-3ʹ and 5ʹ-TGACTTACCAGAGCCATTGGG-3ʹ; GAPDH: 5ʹ-GGAGCGAGATCCCTCCAAAAT-3ʹ and 5ʹ-GGCTGTTGTCATACTTCTCATGG-3ʹ.
Cell Transfection
The negative scramble controls (si-NC) and two TUG1 siRNAs (si-TUG1 #1 and si-TUG1 32) were synthesized from GenePharma (Shanghai, China). The sequences of TUG1 siRNAs were the following: si-TUG1#1: CAGUCCUGGUGAUUUAGACAGUCUU; si-TUG1#2: CCCAGAAGUUGUAAGUUCACCUUGA. PC-3 and DU-145 cells were both transfected with si-NC, si-TUG1#1 and si-TUG1#2 to determine the knockdown efficiency of TUG1. To explore the relationship among TUG1, miR-139-5p and SMC1A, PC-3 and DU-145 cells were co-transfected with TUG1 siRNA and pcDNA, pcDNA-SMC1A (Invitrogen), inhibitor-NC and miR-139-5p inhibitor (Ambion, Austin, TX, USA), respectively. All transfected cells were admixed with Lipofectamine 2000 reagent (Invitrogen) for the following experiments.
MTT Assay
MTT assay was conducted to detect cell viability. Cells were seeded into 96-well plates at a density of 2.0×103 per well and incubated in DMEM containing 10% FBS for 3–5 days. Then, 20 μL MTT were added and cells were incubated for another 4 h at 37°C, the formazan crystals were dissolved by 150 µL dimethyl sulfoxide (DMSO; Sigma). Finally, the cell viability was measured at 490 nm using Multiskan Ascent 354 microplate reader (Thermo Labsystems, Waltham, MA, USA).
Western Blot Analysis
Protein samples were separated by 10% SDS-PAGE, and then proteins were delivered to nitrocellulose membranes (GE Healthcare, Westborough, MA, USA). After being treated with 5% non-fat milk, the nitrocellulose membranes were incubated with primary antibody: anti-Bcl-2 (ab692, Abcam), anti-cleaved-caspase-3 (ab49822, Abcam), anti-Bax (ab32503, Abcam), or anti-GAPDH (ab8245, Abcam) at 4°C overnight. The membranes were washed twice by TBST and incubated with the secondary antibody with horseradish peroxidase (HRP) conjugated at 37°C for 1 h. The bands were emerged by the ECL detection system (Thermo Fisher Scientific).
Flow Cytometry Assay
Cells were harvested at logarithmic period and washed twice with phosphate-buffered saline buffer (PBS). Each sample was re-suspended by 400 μL Binding Buffer (Biomiga, San Diego, CA, USA) at a density of 1.0×106 per well and stained with 5 μL AnnexinV-fluorescein isothiocyanate (AnnexinV-FITC) for 15 min at 4°C. Subsequently, 10 μL propidium iodide (PI) (BD Pharmingen, San Diego, CA, USA) was added. After transfected for 1 h, the positive cells were measured by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) with the dual excitation wavelength of 488 nm and 510 nm.
Dual-Luciferase Reporter Assay
Prospective binding sites of miR-139-5p were predicted using MiRcode and starBase 2.0 online. PC-3 and DU145 cells were co-transfected with WT/MUT-TUG1 or SMC1A 3ʹUTR-WT/MUT with miR-139-5p mimics or negative control miRNA (mimics-NC) for 48 h. The luciferase activity was visualized by a Dual-luciferase Reporter Assay system (Promega Corporation, Madison, WI, USA).
Colony Formation Assay
Transfected PC-3 and DU145 cells were exposed to 0, 2, 4, 6 and 8 Gy radiation and continuously cultured for about two weeks until appearing visible colony in a humid incubator containing 5% CO2 at 37°C. Cells were washed using PBS, and soaked in pure methanol for 30 min and stained with Giemsa (Chemicon International, Temecula, CA, USA) for 15 min. The stained colonies were slowly washed with running water and dried in the air. Finally, the ability of colony formation was observed through a fluorescence microscope, and the clonogenic survival curve was established.23
Xenograft Mouse Model
Animal testing was approved by the Institutional Animal Care and Use Committee of China-Japan Union Hospital of Jilin University, and was in compliance with the recommended procedures of National Institutes of Health guide for the care and use of laboratory animals. PC-3 and DU145 cells stably transfected with sh-NC or sh-TUG1 were subcutaneously injected into the right-back of male nude mice at about 6-week-old (n = 5 per group, Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China). To evaluate the radio-resistance of PCa in vivo, when the tumors in the mice reached an average volume of approximately 100 mm3, the mice were consecutively exposed to radiation (4 Gy) for 5 days. Tumor volume was measured once every 7 d. After 35 d, the mice were euthanized and tumor weight was measured.
Statistical Analysis
Tentative data were preserved with a mean ± standard deviation (SD), every group contained the least three independent duplicate tests. The difference was analyzed using software SPSS 19.0. The Student’s t-test was used to analyze the difference between two groups, while the difference among 3 or more groups by one-way analysis of variance (ANOVA). P value <0.05 was considered statistically significant.
Results
Radiation Enhanced High Expression of TUG1 in PCa Cells
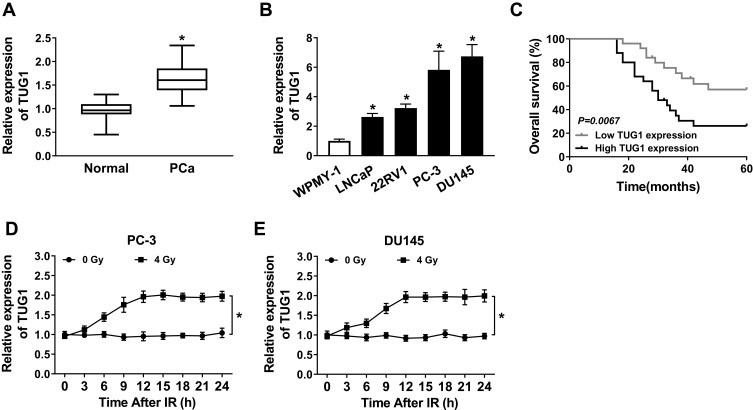
To indicate the function of TUG1 in PCa, qRT-PCR was performed to detect the expression of TUG1 in PCa tissues and cells. Analysis of qRT-PCR showed that the expression level of TUG1 was significantly increased in PCa tissues compared with the corresponding normal tissues (Figure 1A). Meanwhile, we detected the TUG1 expression in human normal prostate matrix immortalized cells (WPMY-1) and PCa cells (LNCaP, 22RV1, PC-3 and DU145 cells), TUG1 expression level was dramatically unregulated in PCa cell lines, especially PC-3 and DU145 (Figure 1B). Additionally, the Kaplan-Meier analysis presented that PCa patients with high expression of TUG1 had a poor prognosis compared with the patients with low expression of TUG1 (Figure 1C). To further investigate whether radiation could affect TUG1 expression in PCa cells, PC-3 and DU145 cells were treated with 4 Gy X-ray radiation, and TUG1 expression was measured every 3 h. Our results indicated that TUG1 expression was markedly enhanced in both PC-3 and DU145 cells since 12 h under4 Gy X-ray radiation (Figure 1D and E). These results illustrated that TUG1 played an important role in radiosensitivity of PCa.
Figure 1.
Expression of TUG1 and its effect on prognosis and radiosensitivity in PCa. (A) The expression of TUG1 was measured by qRT-PCR analysis in PCa tissues (n = 50) or the adjacent non-cancer tissues. (B) The expression of TUG1 was measured by qRT-PCR analysis in human normal prostatic stromal immortalized cell line (WPMY-1) and PCa cell lines (LNCaP, 22RV1, PC-3 and DU145). (C) Survival was analyzed and compared between patients with high and low levels of TUG1 using Kaplan–Meier analysis. (D and E) TUG1 expression was detected in PC-3 and DU145 cells every 3 hrs after 0 or 4 Gy of radiation treatment. *P < 0.05.
Knockdown of TUG1 Enhanced Radiosensitivity of PCa Cells Through Suppressing Cell Proliferation, Survival Fraction and Promoting Cell Apoptosis
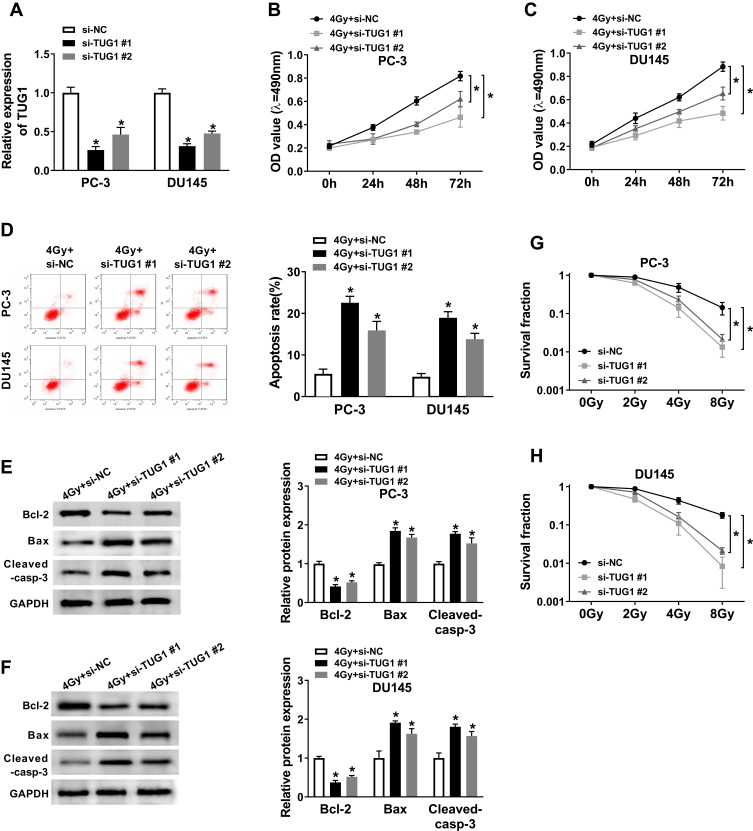
To explore the effects of TUG1 on the radiosensitivity of PCa cells, we performed siRNA-mediated TUG1 knockdown in PC-3 and DU145 cells. Quantitative result (Figure 2A) showed that the successful interference efficiency of si-TUG1#1 and si-TUG1#2 in PCa cells. To investigate how si-TUG1 affected cell proliferation, apoptosis and radiosensitivity in PCa cells, si-TUG1 or si-NC was transfected into PC-3 and DU145 cells with 4 Gy Gy irradiation treatment. MTT assay revealed that proliferation of PC-3 and DU145 cells were both significantly controlled by downregulating TUG1 compared with the si-NC group (Figure 2B and C). Flow cytometry analysis indicated that apoptosis rates of PC-3 and DU145 cells were promoted by TUG1 deletion (Figure 2D). And we found that knockdown of TUG1 dramatically decreased the expression of Bcl-2 and increased the expression of Bax and Cleaved-casp-3 in both PC-3 and DU145 cells treated with 4 Gy X-ray radiation (Figure 2E and F). Furthermore, PC-3 and DU145 cells were exposed to 2–8 Gy X-ray irradiation to induce cell injury, and the data showed that TUG1 downregulation induced a significant decrease of colony survival fraction in both PC-3 and DU145 cells (Figure 2G and H).
Figure 2.
Knockdown of TUG1 enhanced radiosensitivity of PCa cell lines. (A) TUG1 expression in PC-3 and DU145 cells transfected with si-NC or si-TUG1 was detected by qRT-PCR analysis. (B and C) The cell viability of transfected PC-3 and DU145 cells at 0 hrs, 24 hrs, 48 hrs and 72hrs after radiation treatment. (D) Cell apoptosis was determined in transfected PC-3 and DU145 cells at 24 hrs after 4 Gy of radiotherapy. (E and F) The expression levels of apoptosis-related proteins (Bcl-2, Bax and cleaved-casp-3) were examined by Weston blot in PC-3 and DU145 cells transfected with si-NC or si-TUG1 at 24 hrs after 4 Gy of radiotherapy. (G and H) The clonogenic survival curve was established in Clonogenic assay after transfected PC-3 and DU145 cells subjected to 0, 2, 4, 6 and 8 Gy of irradiation. *P < 0.05.
The Expression of miR-139-5p Was Decreased and Negatively Related to TUG1 in PCa Samples and Cell Lines
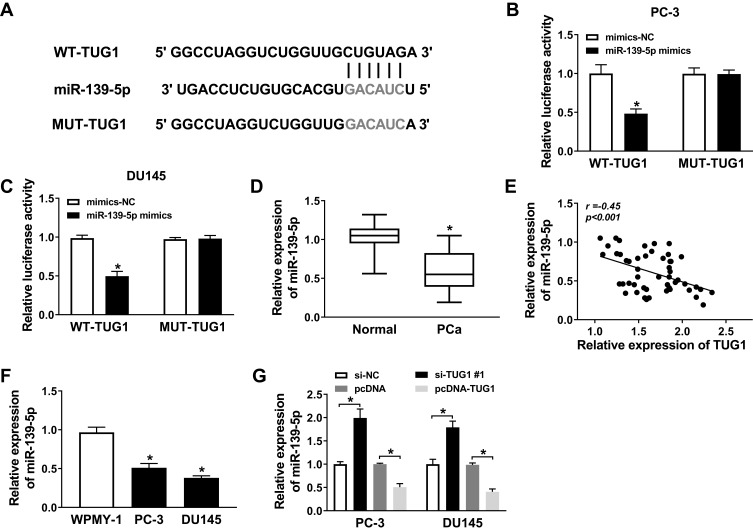
To explore the role of miR-139-5p in PCa cells, we conducted bioinformatics prediction and luciferase reporter assay. The MiRcode prediction program showed that WT-TUG1 contained binding sites to miR-139-5p (Figure 3A). Luciferase reporter assay was performed for further demonstration. PC-3 and DU145 cells were transfected with mimics-NC and miR-139-5p mimics. The luciferase reporter assay suggested that the luciferase activity of WT-TUG1 was decreased by miR-139-5p mimics (Figure 3B and C). Moreover, qRT-PCR showed that miR-139-5p expression was downregulated and negatively related to TUG1 expression in PCa tissues samples (Figure 3D and E). Moreover, we found that the expression of miR-139-5p was lower in PC-3 and DU145 cells compared with WPMY-1 (Figure 3F). Furthermore, we indicated that the miR-139-5p expression was negatively regulated by TUG1 expression in PC-3 and DU145 cell lines (Figure 3G). These findings confirmed that TUG1 acted as a molecular sponge for miR-139-5p.
Figure 3.
The expression of miR-139-5p was downregulated and negatively correlated with TUG1 in PCa samples and cell lines. (A) The presumed binding sites of miR-139-5p with TUG1 were predicted. (B and C) The luciferase activity in PC-3 and DU145 cells co-transfected with miR-139-5p mimics or mimics-NC and TUG1-WT or TUG1-MUT. (D) The expression of miR-139-5p was detected by qRT-PCR in PCa tissue samples. (E) The expression of miR-139-5p was negatively correlated with TUG1 in PCa samples by qRT-PCR. (F) The expression of miR-139-5p was detected by qRT-PCR. (G) The expression of miR-139-5p in PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, pcDNA and pcDNA-TUG1 was measured by qRT-PCR. *P < 0.05.
miR-139-5p Inhibitor Relieved the Effects of TUG1 Knockdown on Cell Proliferation, Survival Fraction, Apoptosis and Radiosensitivity of PCa Cell
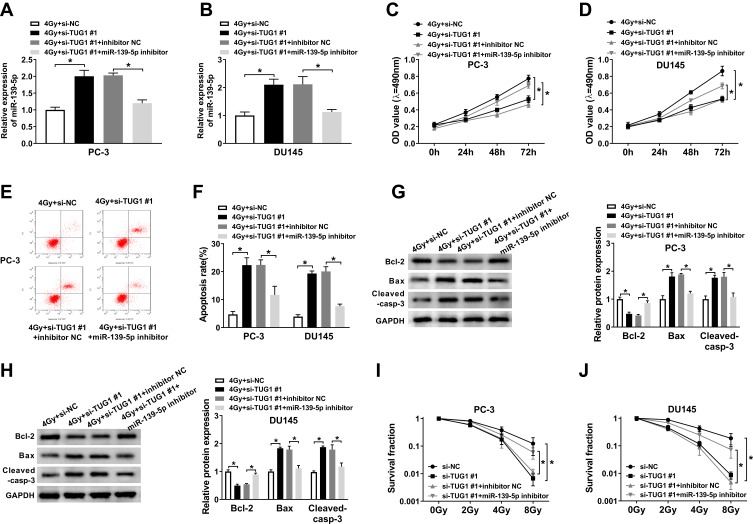
Based on the regulatory role of TUG1 on miR-139-5p level, we further investigated the effect of this axis on radiosensitivity of PCa cells. PC-3 and DU145 cells transfected with si-NC, si-TUG#1, si-TUG#1+ inhibitor NC or si-TUG#1+ miR-139-5p inhibitor were exposed to 4 Gy X-ray irradiation. TUG1 deletion significantly enhanced the expression of miR-1339-5p in PCa cells under irradiation while miR-139-5p inhibitor completely blocked this effect (Figure 4A and B). MTT assay suggested that TUG1 knockdown obviously reduced cell proliferation, while this effect was reversed by the inhibitor of miR-139-5p in both PC-3 and DU145 cells (Figure 4C and D). Annexin V-FITC/PI double staining flow assay demonstrated that the apoptosis rates in PC-3 and DU145 cells were dramatically elevated with knockdown of TUG1; however, the apoptosis rates were reduced by miR-139-5p inhibitor (Figure 4E and F). Moreover, knockdown of TUG1 downregulated the protein level of Bcl-2 and upregulated the protein level of Bax and Cleaved-casp-3, and these effects of TUG1 downregulation on apoptosis-related proteins were partially reversed by miR-139-5p inhibitor (Figure 4G and H). In addition, the survival fraction was reduced by knockdown of TUG1 in PC-3 and DU145 cells under 4 Gy irradiation, which was absolutely restored by miR-139-5p inhibitor (Figure 4I and J). In summary, TUG1 knockdown improved radiosensitivity of PCa cells by sponging miR-139-5p.
Figure 4.
The inhibitor of miR-139-5p mitigated the effect of si-TUG1 on radiosensitivity, cell apoptosis, and survival fraction under 4 Gy radiation treatment. PC-3 and DU145 cells treated with irradiation were transfected with si-NC, si-TUG1#1, si-TUG1#1+ inhibitor NC or si-TUG1#1+ miR-139-5p inhibitor. (A and B) The expression of miR-139-5p was detected by qRT-PCR. (C and D) MTT assay detected the viability of PC-3 and DU145 cells. (E and F) Flow cytometry determined cell apoptosis of PC-3 and DU145 cells. (G and H) The expression level of apoptosis-related proteins (Bcl-2, Bax and cleaved-casp-3) was examined in PC-3 and DU145 cells. (I and J) The clonogenic survival curve was proven in PC-3 and DU145 cells, which were transfected with si-NC, si-TUG1#1, si-TUG1#1+ inhibitor NC or si-TUG1#1+ miR-139-5p inhibitor and subjected to 0, 2, 4, 6 and 8 Gy of irradiation. *P < 0.05.
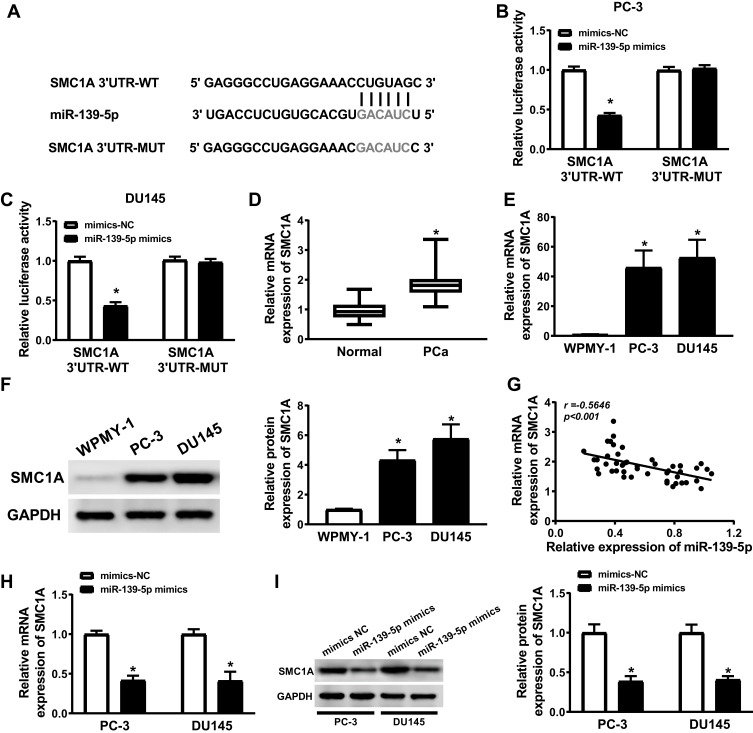
SMC1A Was a Target Gene of miR-139-5p and High Express in PCa Cells
StarBase2.0 predicted the binding sites between SMC1A and miR-139-5p, WT-SMC1A has the binding sites with miR-139-5p (Figure 5A). The luciferase activity was significantly decreased in PC-3 and DU145 cells co-transfected with SMC1A 3ʹUTR WT and miR-139-5p mimics, but not changed in PC-3 and DU145 cells co-transfected with SMC1A 3ʹUTR MUT and miR-139-5p mimics compared with the corresponding control groups (Figure 5B and C). Western blot and qRT-PCR analysis illustrated that the expression levels of SMC1A were increased in PCa tissues and cell lines (Figure 5D, F and G), and SMC1A expression level was negatively correlated with the expression level of miR-139-5p (Figure 5E). PC-3 and DU145 cells were transfected with mimics-NC and miR-139-5p mimics, miR-139-5p overexpression decreased the protein and mRNA expression levels of SMC1A (Figure 5H and I). These evidences indicated that the expression of SMC1A was regulated by miR-139-5p.
Figure 5.
SMC1A was a target gene of miR-139-5p and the expression of SMC1A was upregulated in PCa cells. (A) The presumed binding sites of miR-139-5p with SMC1A were predicted. (B and C) The luciferase activity in PC-3 and DU145 cells simultaneously transfected with miR-139-5p mimics or mimics-NC and TUG1-WT or TUG1-MUT. (D) The expression of SMC1A was upregulated in PCa samples (n=50). (E) The relationship between SMC1A and miR-139-5p was analyzed by Kaplan–Meier analysis (r = −0.5646, P < 0.001). (F and G) The expression levels of SMC1A mRNA and protein were overexpressed in PC-3 and DU145 cells comparing to human normal prostatic stromal immortalized cell line (WPMY-1). (H and I) The mRNA and protein expression of SMC1A in PC-3 and DU145 cells transfected with miR-139-5p mimics or mimics-NC were detected by qRT-PCR and Weston blot, respectively. *P < 0.05.
Overexpression SMC1A Restored the Consequence of TUG1 Knockdown on Proliferation, Apoptosis and Radiosensitivity of PCa Cells Through miR-139-5p
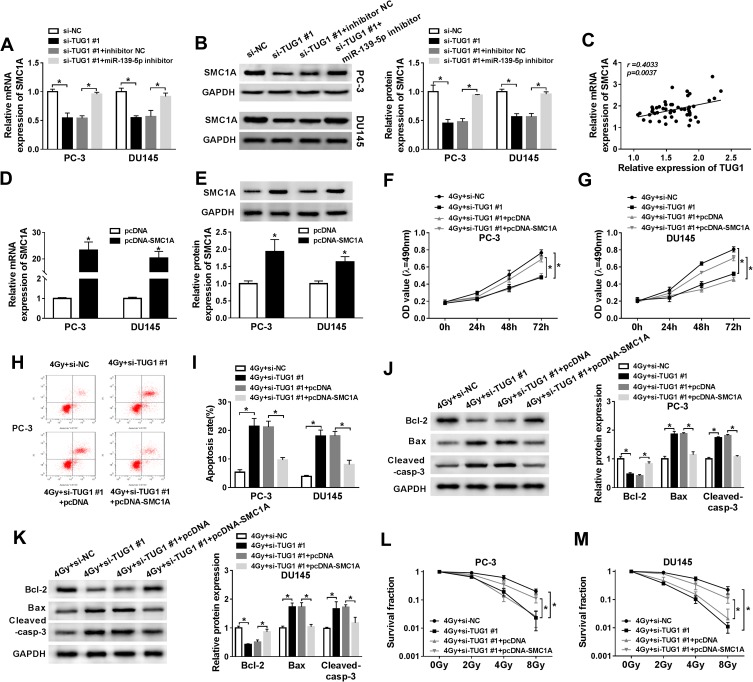
To validate the relationship between lncRNA TUG1, miR-139-5p, SMC1A, we firstly detected the protein and mRNA expression levels of SMC1A in PC-3 and DU145 cells transfected with si-NC, si-TUG#1, si-TUG#1+ inhibitor NC or si-TUG#1+miR-139-5p inhibitor. Western blot and qRT-PCR analysis showed the expression of SMC1A was downgraded by TUG1 knockdown, while miR-139-5p inhibitor restored SMC1A expression (Figure 6A and B). Additionally, the expression of SMC1A was positively correlated with TUG1 (Figure 6C). These results indicated that TUG1 sponged miR-139-5p to regulate the expression of SMC1A.
Figure 6.
Restoration of HMGB1 expression reversed the improved radiosensitivity induced by TUG1 knockdown in PCa cells. (A and B) SMC1A mRNA and protein expression were detected by qRT-PCR and Weston blot in PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, si-TUG1#1+ inhibitor NC or si-TUG1#1+ miR-139-5p inhibitor at 24 hrs. (C) SMC1A mRNA expression was positively correlated with TUG1 by qRT-PCR. (D and E) The expression of SMC1A was measured by qRT-PCR. (F and G) MTT assay detected the viability of PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, si-TUG1#1+ pcDNA or si-TUG1#1+ pcDNA-SMC1A at the indicated time points (0 hrs, 24 hrs, 48 hrs and 72 hrs) after 4 Gy of radiation. (H and I) Flow cytometry determined cell apoptosis rate of PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, si-TUG1#1+ pcDNA or si-TUG1#1+ SMC1A at 24 hrs after 4 Gy radiation. (J and K) The expression level of apoptosis-related proteins (Bcl-2, Bax and cleaved-casp-3) was tested by Weston blot in PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, si-TUG1#1+ pcDNA or si-TUG1#1+ SMC1A at 24 hrs after 4 Gy radiation. (L and M) The survival fraction was established by Kaplan–Meier and log-rank in PC-3 and DU145 cells transfected with si-NC, si-TUG1#1, si-TUG1#1+ pcDNA or si-TUG1#1+ SMC1A and subjected to 0, 2, 4, 6 and 8 Gy of radiation. *P < 0.05.
To explore the effects of the regulatory network TUG1/miR-139-5p/SMC1A on radiosensitivity of PCa cells, PC-3 and DU145 cells transfected with si-NC, si-TUG#1, si-TUG#1+ pcDNA or si-TUG#1+pcDNA-SMC1A were exposed to 4 Gy irradiation. We firstly detected the mRNA and protein expression of SMC1A in PCa cells transfected with pcDNA or SMC1A and found that SMC1A showed a successful overexpression efficacy in PCa cells (Figure 6D and E). TUG1 deletion inhibited cell proliferation of PCa, but overexpression SMC1A restored this inhibitory effect of TUG1 deletion (Figure 6F and G). In the same treatment, knockdown of TUG1 facilitated cell apoptosis in PCa, while this effect was suppressed by SMC1A upregulation (Figure 6H and I). Moreover, the downregulated effect on Bcl-2 and upregulated effects on Bax and cleaved-casp-3 were reversed by overexpression SMC1A (Figure 6J and K). Clonogenic assay unveiled TUG1 knockdown dramatically inhibited the survival fraction of PC-3 and DU145 cells at 4Gy of radiation, while overexpression of SMC1A partially restored this inhibition (Figure 6L and M). Collectively, we concluded that TUG1 regulated radiosensitivity of PCa by overexpressing SMC1A expression through targeting miR-139-5p.
TUG1 Knockdown Facilitated the Radiosensitivity of PCa Cells in vivo
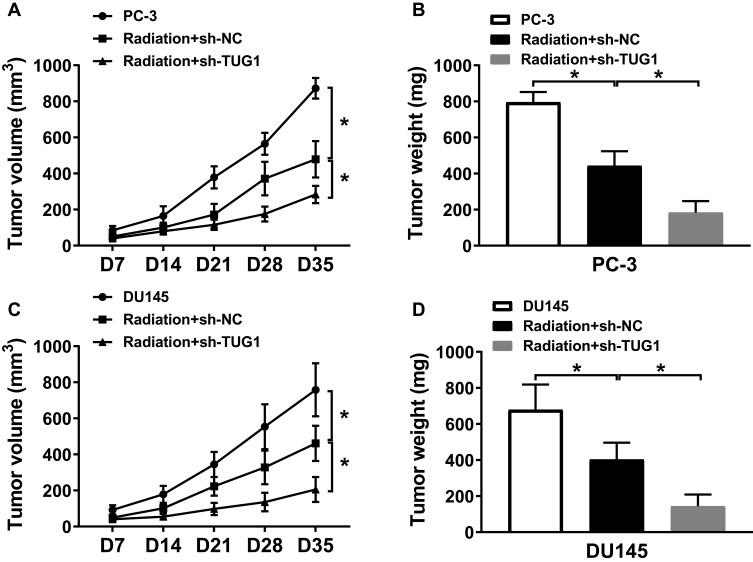
To further determine the effect of TUG1 knockdown on the radiosensitivity of PCa in vivo, PC-3 and DU145 cells stably transfected with sh-NC or sh-TUG1 were injected subcutaneously into the right side of nude mice back, which were treated with X-ray radiation for 5 days. As represented in Figure 7A–D, we found that radiation could significantly inhibit tumor volume and weight, and TUG1 downregulation suppressed tumor volume and weight compared with the radiation group. The results demonstrated that TUG1 knockdown enhanced the radiosensitivity of PCa cells in vivo.
Figure 7.
Knockdown of TUG1 enhanced the radiosensitivity of PCa cells in vivo. PC-3 and DU145 cells were transfected with sh-NC or sh-TUG1 and subcutaneously injected into the back of male nude mice (n = 5 per group). When the tumors reached an average volume of approximately 100 mm3, all mice were treated with 4 Gy radiation every 7 days. (A and C) Tumor volume was measured every 7 days after the first radiation. (B and D) Tumor weight was recorded after cultivating 35 days. *P < 0.05.
Discussion
PCa is the second mortality-related malignant tumor in males in many western countries, and the incidence of PCa is growing in China.24 In 1995, the China Anti-Cancer Association advocated the launch of the National Cancer Prevention and Awareness Week to allow more people to understand tumors and prevent tumors. At present, radiotherapy and chemotherapy are conventional treatments of tumors. Nevertheless, radioresistance is the main difficulty limiting the treatment effects of radiotherapy for PCa, followed by tumor recurrence and metastasis. Aberrant expression of lncRNAs was confirmed to be associated with tumorigenesis, progression and poor prognosis of tumors. In the present study, our discoveries suggested that knockdown of lncRNA TUG1 enhanced the radiosensitivity of PCa cells by regulating SMC1A.
Long non-coding RNAs are a group of small RNA molecules that involved in the transcription. Previous studies indicated that lncRNAs mediated the radiosensitivity of multiple cancers. Wu et al proved that lncRNA PVT1 expression was increased in non-small cell lung cancer, knockdown of PVT1 decreased cell proliferation, colony formation rates and increased cell apoptosis of lung cancer under irradiation.5 Liu et al concluded that the expression of lncRNA FAM201A was increased in non-small cell lung cancer, the silence of FAM201A inhibited cell proliferation and promoted apoptosis and radiosensitivity of NSCLC cells both in vitro and in vivo.25 TUG1 expression was upregulated in many tumors. The overexpression of TUG1 could promote progression and adriamycin resistance in urothelial carcinoma.26 TUG1 aggravated PCa progression and predicted poor prognosis in a multi-complex regulatory network.2,7-9 Thus, we proposed that TUG1 might be related to the radiosensitivity of PCa. In the current research, the expression of TUG1 was dramatically boosted in PCa tissues and cells, which was consistent with the previous studies. TUG1 expression was increased by the radiation treatment, insinuating that TUG1 might participate in regulating radiosensitivity of PCa.
Up to now, multiple miRNAs have been confirmed to be important regulators of radiosensitivity in varieties of cancers, including PCa. For example, miR-205 sensitized radiation sensitivity of prostate cancer cells through inhibiting PKCε and ZEB1.27 MiR-875-5p was demonstrated to counteract epithelial-to-mesenchymal transition and facilitate radiation response in prostate cancer via repressing the EGFR-ZEB1 axis.28 MiR-139-5p was previously verified to be significantly downregulated and function as a tumor suppressor in PCa.15 Our study suggested that the expression of miR-139-5p was reduced in PCa tissues and cells. In addition, our study indicated that TUG1 could sponge miR-139-5p in PCa cells. In the functional qualification tests, TUG1 knockdown inhibited cell proliferation, survival fractions and induced apoptosis under radiation treatment, while miR-139-5p inhibitor reversed these effects. All these findings demonstrated that knockdown of TUG1 enhanced the radiosensitivity in PCa through controlling miR-139-5p.
SMC1A was discovered to regulate tumor growth and progress. In colorectal cancer, overexpression of SMC1A contributed to colorectal cancer development, poor prognosis and metastasis;29–31 while downregulation of SMC1A inhibited growth and increased apoptosis and chemosensitivity.32 Knockdown of SMC1A suppressed cell proliferation and caused G2/M arrest in glioblastoma.33,34 Moreover, SMC1A knockdown regulated cell growth, colony formation and migration in PCa.19,20 This study firstly verified the correlation among TUG1, miR-139-5p and SMC1A expression. SMC1A was a sponge gene of miR-139-5p, overexpression SMC1A could restore the effects of TUG1 knockdown on proliferation, apoptosis and radiosensitivity of PCa cells. Therefore, we explored that TUG1 regulated the radiosensitivity by stimulating SMC1A expression in PCa cells. A previous document revealed that SMC1A was associated with radioresistance in PCa by attenuating the NHEJ and HR pathways.20 Further research is necessary to investigate whether there is a link between these two signal paths.
In summary, TUG1 expression was increased in PCa tissues and cell lines. TUG1 downregulation enhanced the radiosensitivity of PCa by inhibiting SMC1A expression. Our findings implied that TUG1 could act as a promising therapeutic target for PCa, the treatment strategy that TUG1 knockdown with radiation may be a better approach for PCa patients.
Disclosure
The authors declare that they have no financial conflicts of interest in this work.
References
- 1.Diamond AM. Selenoproteins of the human prostate: unusual properties and role in cancer etiology. Biol Trace Elem Res. 2019;192(1):51–59. doi: 10.1007/s12011-019-01809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu T, Liu CL, Li T, et al. LncRNA TUG1 aggravates the progression of prostate cancer and predicts the poor prognosis. Eur Rev Med Pharmacol Sci. 2019;23(11):4698–4705. doi: 10.26355/eurrev_201906_18062. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Yang Y, An W, et al. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J Gastroenterol Hepatol. 2017;32(4):837–845. doi: 10.1111/jgh.13606. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Li Y, Zhang H, et al. Knockdown of lncRNA PVT1 enhances radiosensitivity in non-small cell lung cancer by sponging Mir-195. Cell Physiol Biochem. 2017;42(6):2453–2466. doi: 10.1159/000480209. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Hao T, Sun J, et al. Long noncoding RNA GAS5 modulates alpha-solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed Pharmacother. 2019;112:108656. doi: 10.1016/j.biopha.2019.108656. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Tang X, Wang Z, et al. TUG1 promotes prostate cancer progression by acting as a ceRNA of miR-26a. Biosci Rep. 2018;38(5). doi: 10.1042/BSR20180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo BH, Zhao Q, Li HY. TUG1 promotes the development of prostate cancer by regulating RLIM. Eur Rev Med Pharmacol Sci. 2019;23(5):1926–1933. doi: 10.26355/eurrev_201903_17230. [DOI] [PubMed] [Google Scholar]
- 9.Yang XL, Wei C, Zhang YB, et al. Long noncoding RNA TUG1 promotes progression via upregulating DGCR8 in prostate cancer. Eur Rev Med Pharmacol Sci. 2019;23(6):2391–2398. doi: 10.26355/eurrev_201903_17385. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Hu X, Zhang H, et al. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat Oncol. 2017;12(1):65. doi: 10.1186/s13014-017-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Zhang Y, Ding J, et al. miR-17-3p downregulates mitochondrial antioxidant enzymes and enhances the radiosensitivity of prostate cancer cells. Mol Ther Nucleic Acids. 2018;13:64–77. doi: 10.1016/j.omtn.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Chen W, Jin Y, et al. miR-142-5p enhances cisplatin- induced apoptosis in ovarian cancer cells by targeting multiple anti- apoptotic genes. Biochem Pharmacol. 2019;161:98– 112. doi: 10.1016/j.bcp.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, Yang B, Lin S, et al. Downregulation of miR-142-5p promotes tumor metastasis through directly regulating CYR61 expression in gastric cancer. Gastric Cancer. 2019;22(2):302–313. doi: 10.1007/s10120-018-0872-4. [DOI] [PubMed] [Google Scholar]
- 14.Mao A, Liu Y, Wang Y, et al. miR-449a enhances radiosensitivity through modulating pRb/E2F1 in prostate cancer cells. Tumour Biol. 2016;37(4):4831–4840. doi: 10.1007/s13277-015-4336-8. [DOI] [PubMed] [Google Scholar]
- 15.Yang B, Zhang W, Sun D, et al. Downregulation of miR-139-5p promotes prostate cancer progression through regulation of SOX5. Biomed Pharmacother. 2019;109:2128–2135. doi: 10.1016/j.biopha.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Weng D, Li K, et al. MicroRNA-139-5P inhibits human prostate cancer cell proliferation by targeting Notch1. Oncol Lett. 2018;16(1):793–800. doi: 10.3892/ol.2018.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajic M, Froio D, Daly S, et al. miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense. Cancer Res. 2018;78(2):501–515. doi: 10.1158/0008-5472.CAN-16-3105. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Wang S, Zhao Y, et al. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J Cell Physiol. 2019;234(5):6161–6172. doi: 10.1002/jcp.27393. [DOI] [PubMed] [Google Scholar]
- 19.Pan XW, Gan SS, Ye JQ, et al. SMC1A promotes growth and migration of prostate cancer in vitro and in vivo. Int J Oncol. 2016;49(5):1963–1972. doi: 10.3892/ijo.2016.3697. [DOI] [PubMed] [Google Scholar]
- 20.Yadav S, Kowolik CM, Lin M, et al. SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties. Mol Carcinog. 2019;58(1):113–125. doi: 10.1002/mc.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoey C, Ray J, Jeon J, et al. MiRNA-106a and prostate cancer radioresistance: a novel role for LITAF in ATM regulation. Mol Oncol. 2018;12(8):1324–1341. doi: 10.1002/1878-0261.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liva KKJ, Schmitt Gen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Taeb S, Jahangiri S, et al. MiRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013;73(23):6972–6986. doi: 10.1158/0008-5472.CAN-13-1657. [DOI] [PubMed] [Google Scholar]
- 24.Gang Zhu KZ. Chinese prostate cancer screening: current situation and challenges. J Shandong Univ. 2019;57(1):11–15. doi: 10.6040/j.issn.1671-7554.0.2018.1053. [DOI] [Google Scholar]
- 25.Liu AM, Zhu Y, Huang ZW, et al. Long noncoding RNA FAM201A involves in radioresistance of non-small-cell lung cancer by enhancing EGFR expression via miR-370. Eur Rev Med Pharmacol Sci. 2019;23(13):5802–5814. doi: 10.26355/eurrev_201907_18319. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Huang G, Cheng H. Transcription factor Nrf2 induces the up-regulation of lncRNA TUG1 to promote progression and adriamycin resistance in urothelial carcinoma of the bladder. Cancer Manag Res. 2019;11:6079–6090. doi: 10.2147/CMAR.S200998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Bezawy R, Tinelli S, Tortoreto M, et al. MiR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCε and ZEB1 inhibition. J Exp Clin Cancer Res. 2019;38(1):51. doi: 10.1186/s13046-019-1060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Bezawy R, Cominetti D, Fenderico NR, et al. MiR-875-5p counteracts epithelial-to-mesenchymal transition and enhances radiation response in prostate cancer through repression of the EGFR-ZEB1 axis. Cancer Lett. 2017;395:53–62. doi: 10.1016/j.canlet.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Sarogni P, Palumbo O, Servadio A, et al. Overexpression of the cohesin-core subunit SMC1A contributes to colorectal cancer development. J Exp Clin Cancer Res. 2019;38(1):108. doi: 10.1186/s13046-019-1116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Yu S, Cui L, et al. Role of SMC1A overexpression as a predictor of poor prognosis in late stage colorectal cancer. BMC Cancer. 2015;15:90. doi: 10.1186/s12885-015-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou P, Xiao N, Wang J, et al. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett. 2017;385:39–45. doi: 10.1016/j.canlet.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Feng W, Chen L, et al. Downregulation of SMC1A inhibits growth and increases apoptosis and chemosensitivity of colorectal cancer cells. J Int Med Res. 2016;44(1):67–74. doi: 10.1177/0300060515600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Zhang Z, Wang R, et al. siRNA-mediated knockdown of SMC1A expression suppresses the proliferation of glioblastoma cells. Mol Cell Biochem. 2013;381(1–2):209–215. doi: 10.1007/s11010-013-1704-9. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Lin M, Li K, et al. Knocking down SMC1A inhibits growth and leads to G2/M arrest in human glioma cells. Int J Clin Exp Pathol. 2013;6(5):862–869. [PMC free article] [PubMed] [Google Scholar]