Abstract
As an anti-tumor modality based on sensitizer photoexcitation by tumor-directed light, photodynamic therapy (PDT) has the advantage of being site-specific compared with conventional chemotherapy or radiotherapy. Like these other therapies, however, PDT is often limited by pre-existing or acquired resistance. One type of resistance, discovered in the author’s laboratory, involves nitric oxide (NO) generated by inducible nitric oxide synthase (iNOS) in tumor cells. Using human breast, prostate, and brain cancer cell lines, we have shown that iNOS is dramatically upregulated after a moderate PDT challenge sensitized by 5-aminilevulinic acid (ALA)-induced protoporphyrin IX. The elevated NO not only elicited a greater resistance to cell photokilling, but also an increase in the growth and migration/invasion rate of surviving cells. Greater iNOS/NO-based resistance was also demonstrated at the in vivo level using a breast tumor xenograft model. More recent studies have shown that NO from PDT-targeted cells can stimulate a pro-growth/migration response in non-targeted bystander cells. These novel effects of NO, their negative impact on PDT efficacy, and possible mitigation thereof by anti-iNOS/NO pharmacologic agents will be discussed.
Graphical Abstract
ALA/light-targeted tumor cells can succumb to 1O2-induced apoptosis or upregulate iNOS/NO to resist it and survive. Surviving cells can also exploit higher levels of NO to proliferate, migrate and invade more aggressively. Moreover, the NO can diffuse to non-targeted bystander cells, stimulating them to proliferate and migrate/invade more aggressively as well.
Introduction
In the mid-1970’s Dr. Tom Dougherty led a group at Roswell Park Memorial Institute that introduced photodynamic therapy (PDT) as a novel anti-tumor modality involving a photosensitizing agent (PS) and non-ionizing radiation (1,2). In 1978 they carried out the first clinical testing of this approach using cutaneous and subcutaneous malignancies sensitized with hematoporphyrin derivative (3). Since then, PDT has increased steadily in clinical use as its advantages over conventional radiotherapy or chemotherapy have become increasingly apparent (4–8). Included among these advantages are (i) site-specificity, i.e. limitation to solid tumors at which light is directed via fiber optic networks, (ii) ability to often overcome tumor resistance to other therapies, and (iii) few (if any) negative side effects of the PS or light alone. Unlike other cancer modalities, PDT requires three operating components: the PS or a metabolic precursor, PS-exciting far-visible to near-infrared light, and molecular oxygen. Upon photoactivation, the PS gives rise to cytotoxic reactive oxygen species (ROS), the most common one being singlet molecular oxygen (1O2) (5–7). In 1996, the first PS to receive FDA approval for certain tumors (e.g. esophageal) was Photofrin®, an oligomeric form of hematoporphyrin (6). Since then, it has been used for many other malignancies, including bladder, prostate, head-and-neck, breast, and brain, some of which exhibit resistance to other treatments. 5-Aminolevulinic acid (ALA)-based PDT is a more recently developed form of PDT in which pro-sensitizer ALA (or ester thereof) is metabolized to the active sensitizer, protoporphyrin IX (PpIX) (9,10). PpIX accumulates initially in mitochondria, making them prominent subcellular targets of photodynamic action.
As indicated above, many cancers display an innate or acquired resistance to chemo- or radiotherapy and it is now clear that resistance mechanisms also exist for PDT. For example, ROS-scavenging cytoprotective enzymes such as glutathione peroxidase and superoxide dismutase have been shown to be upregulated after a PDT challenge (11,12). In the case of ALA-PDT, upregulation of the membrane transporter ABCG2 has been demonstrated, which promotes resistance via efflux of newly synthesized PpIX (13). Another key resistance mechanism was discovered in the author’s laboratory and involves generation of nitric oxide (NO) by inducible NO synthase (iNOS/NOS2), which can be upregulated by PDT stress (14,15).
Nitric oxide and its role in cancer
NO is a short-lived free radical molecule (τ <2 sec in H2O) that is produced by enzymes of the nitric oxide synthase (NOS) family, including nNOS/NOS1 (neuronal), iNOS/NOS2 (inducible), and eNOS/NOS3 (endothelial). These enzymes catalyze the reaction between L-arginine, NADPH, and O2 to give L-citrulline and NO as products (16,17). nNOS-derived NO is known to play a key role in retrograde neurotransmission, whereas eNOS-derived NO is crucial for blood pressure regulation via its vasodilatory action (16). With regard to cancer, NO at low-to-moderate steady state levels (e.g. 0.1–0.5 μM), can increase drug resistance of many different cancer cells and also act as a pro-growth/pro-migration signaling molecule (18–21). This NO is typically generated by endogenous iNOS, which is expressed at relatively high levels in many tumor cells (17,21) and can be upregulated by stress-inducing drugs such as cisplatin (22). The first known studies to describe iNOS/NO-dependent resistance to anti-tumor PDT will be discussed, along with an acquired hyper-aggressiveness of PDT-surviving cells and non-contacting bystander cells.
Effects of NO on PDT efficacy: early studies
How PDT might be affected by NO produced by tumor cells themselves or by proximal vascular cells was examined in an early study involving a rat aorta model (23). The results suggested that PDT somehow impairs NO production by endothelial cells in tumor vascular systems, thereby incapacitating tumor cells via vasoconstriction. In another early study, nNOS in epidermoid cancer cells was found to be rapidly upregulated by PDT and it was assumed that the resulting “high level” NO augmented overall cell killing (24). However the possibility of NO involvement in pro-survival signaling (see below) was not considered. How NO produced by solid tumors might affect PDT efficacy in vivo was investigated in subsequent groundbreaking studies by Henderson et al. (25) and by Korbelik et al. (26). Mice bearing Photofrin®-sensitized syngeneic tumors such as radiation-induced fibrosarcoma (RIF) and breast EMT6 were used in these studies. When L-NAME, a non-specific inhibitor of NOS activity, was administered immediately before or after irradiation, the tumor-suppressing effects of PDT were significantly improved (25,26), implying that endogenous NOS/NO was imposing a cytoprotective resistance. Importantly, the extent of improvement with L-NAME correlated directly with measured NO output, tumors with the highest output responding best to L-NAME and vice-versa (26). These effects were attributed to a reduction in the antagonism between NO dilation of tumor microvessels and PDT’s anti-tumor vasoconstrictive effects. While these studies (25,26) and subsequent related ones by other investigators (27) were the first to identify NO-based resistance to PDT, they left important questions such as the following open: (i) the cellular source(s) of the NO in question, e.g. tumor cells alone, vascular endothelial cells, or possibly both; (ii) whether resistance was imposed by pre-existing NOS/NO or possibly NOS/NO upregulated by PDT stress; and (iii) which NOS isoform was the primary source of resistance NO. These issues have been addressed in the author’s laboratory and key findings will be discussed in a later section.
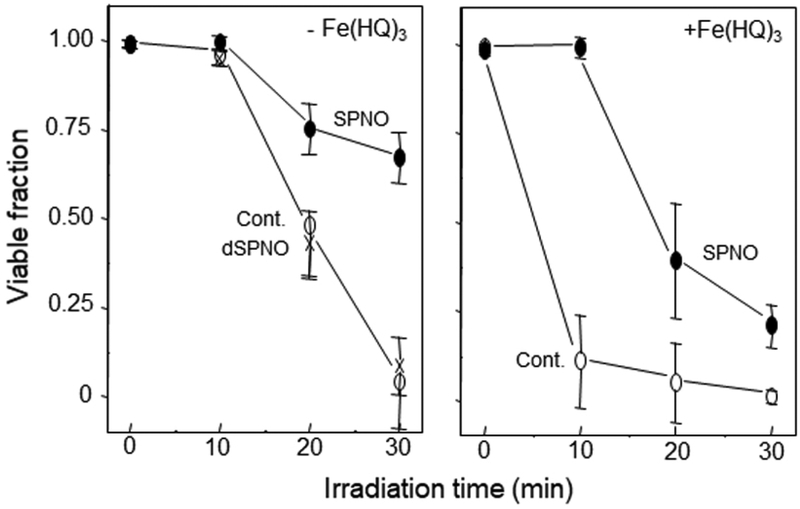
About 15 years ago, we began testing the hypothesis that NO might protect cancer cells against photokilling by acting as a chain-breaking antioxidant, e.g. by intercepting free radical intermediates of lipid peroxidation (28,29). Using chemical donors of NO, we had already observed such effects for unsaturated liposomal lipids undergoing peroxyl radical (LOO•)-mediated chain peroxidation (30). We first chose a breast tumor subline (COH-BR1), which lacks the ability to detoxify membrane lipid hydroperoxides (LOOHs) due to absence of selenoperoxidase GPx4 (31). Photosensitization of these cells with plasma membrane-relocalized ALA-induced PpIX, followed by increasing fluences of broad-band visible light resulted in vigorous membrane lipid peroxidation and necrotic cell death (28,29). When the chemical NO donor spermine NONOate (SPNO) at a non-cytotoxic concentration was introduced immediately before irradiation, the extent of necrosis was substantially reduced relative to a control without the donor (Fig. 1A). This effect was attributed to NO scavenging of chain-carrying lipid peroxyl and oxyl radicals (LOO•, LO•). No cytoprotection was observed when decomposed SPNO was used, showing that the active agent was NO and not some other SPNO-derived species (29). Lipid peroxidation can be stimulated by low levels of lipophilic iron, e.g. ferric 8-hydroxyquinoline [Fe(HQ)3] (30). When Fe(HQ)3 was added to sensitized COH-BR1 cells prior to irradiation, there was a dramatic increase in necrotic photokilling, suggesting more extensive lipid peroxidation, but SPNO once again had a very large protective effect (Fig. 1B). It is important to point out that SPNO does not absorb light in the visible range, so it could not have interfered with PpIX photoexcitation.
Figure 1.
NO-mediated cytoprotection against necrotic photokilling. COH-BR1 cells in serum-free medium were sensitized in plasma membrane with ALA-induced PpIX and irradiated in the absence (Cont.) vs. presence of SPNO (0.4 mM) or decomposed SPNO. After irradiation, cells were switched to serum-containing medium and checked for extent of necrosis 5 h later. Values are means ± deviation (n=2). Reproduced from Ref. 29, with permission.
The results shown in Fig. 1A were confirmed when plasma membrane-sensitized COH-BR1 cells were exposed to lipopolysaccharide-activated RAW264.7 macrophages during irradiation. At 15 h after activation, these macrophages were generating NO, but few (if any) cytotoxic NO metabolites like peroxynitrite. The extent of COH-BR1 necrosis in the presence of RAW cells was substantially less than in their absence, and this difference was abolished by L-NAME, suggesting that NO from these cells was acting cytoprotectively by quenching free radical-mediated lipid peroxidation (32). These effects of NO and those observed with the chemical donor (Fig. 1 A,B) are illustrated in Fig. 2, where NO is shown intercepting chain-propagating radicals (LOO•, LO•) arising downstream of primary 1O2-generated LOOHs. Clearly, NO had to be physically present during the photoreaction for its chain-breaking effects (Fig. 2) to be realized; hence the term “NO-now” was adopted (33). Subsequent studies revealed that NO could signal for a delayed resistance, which materialized when NO was no longer present during the actual photodynamic process. This so-called “NO-then” acted as a signaling mediator which conditioned the cells for greater resistance to a subsequent photochallenge. At least two antioxidant proteins were progressively upregulated after COH-BR1 cells were pre-incubated with SPNO: heme oxygenase-1 (HO-1) and the ferritin H and L chains (32). Our evidence suggested that these upregulations ultimately resulted in iron sequestration which hindered chain peroxidation, thereby increasing resistance to photokilling. To summarize, the effects of “NO now” were mainly due to immediate chemical interception of lipid-derived free radicals, whereas those of “NO then” were due to delayed signaling activity leading to cytoprotective induction of HO-1 and ferritin (32,33).
Figure 2.
NO suppression of free radical-mediated (chain) lipid peroxidation. Scheme shows initiation of peroxidation by photogenerated 1O2 and amplification by iron-catalyzed 1-electron reduction of LOOH intermediates. Arrows show sites of chain breaking by NO.
Effects of NO on PDT efficacy: relatively recent studies
More recent work in the author’s lab demonstrated for the first time that low-level NO produced by PDT-stressed cancer cells themselves played a major role in resistance to photokilling (34–36). In initial studies, COH-BR1 cells were again sensitized with ALA-induced PpIX, but the porphyrin was restricted to mitochondria before irradiation, so these were the primary targets of subsequent photodynamic action. After cells were exposed to a moderate fluence of broad-band visible light (1–2 J/cm2), we observed a rapid and progressive upregulation of iNOS mRNA and iNOS protein (4–5-fold over control), which persisted for at least 20 h after irradiation (36). Other NOS isoforms (nNOS and eNOS) were undetectable or did not increase from a low basal level. When an iNOS activity inhibitor (1400W) on NO scavenger (cPTIO) was present from the outset, there was a striking increase in apoptotic cell death measured at 20 h post-hν, indicating that endogenous iNOS/NO was signaling for greater resistance (35,36). COH-BR1 cells with shRNA-induced iNOS knockdown exhibited a consistent increase in apoptotic photokilling relative to controls, confirming the role of basal and/or stress-induced iNOS in photoresistance (35). Interestingly, the extent and duration of phosphorylation-activation of pro-apoptotic kinases (JNK, p38α) were increased when 1400W or cPTIO was present during a photochallenge (36). Similarly, 1400W or cPTIO raised pro-apoptotic Bax expression and lowered anti-apoptotic Bcl-xL expression, again consistent with a pro-survival function of iNOS/NO. It is important to point out that Rapozzi et al. (37), studying pheophorbide-sensitized melanoma cells, reached the same conclusion about NO for low photooxidative pressures; however, at relatively high pressures, NO switched from being cytoprotective to cytotoxic, reportedly due to modifications in the NF-κB/YY1/RKIP/SNAIL signaling pathway. Whether these intriguing findings also apply to other cancer types remains to be determined.
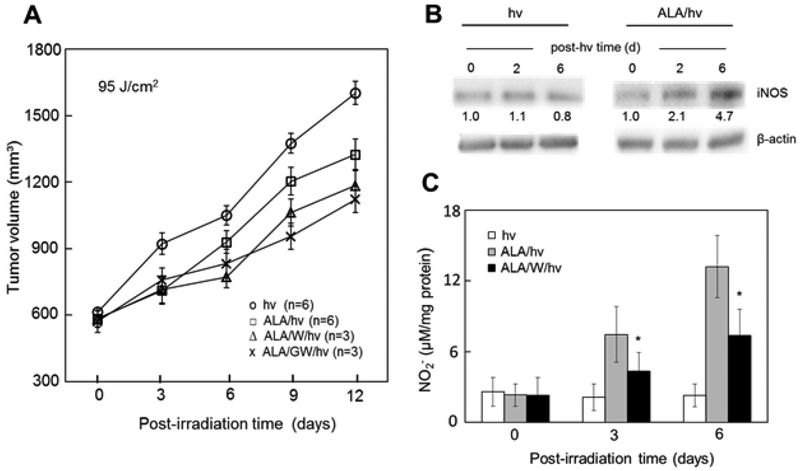
The above evidence obtained by the author’s group was recently validated at the in vivo level using female immunodeficient (SCID) mice bearing triple negative breast MDA-MB-231 tumors (38). After intraperitoneal ALA administration, tumors were irradiated using a 633 nm LED source and light fluence ~95 J/cm2. These animals exhibited a significant reduction in tumor growth relative to light-only controls over a 12-day post-irradiation period. Importantly, an iNOS inhibitor (1400W or GW274150), administered in multiple doses one day apart, reduced tumor growth even further (Fig. 3A), suggesting that iNOS/NO was imposing tumor resistance. This was the first in vivo evidence for iNOS/NO-mediated resistance to PDT. Western blot analyses of tumor samples supported our findings by showing a steady increase in iNOS level to ~5-times that of light-only controls six days after PDT (Fig.3B). Correspondingly, the NO-derived nitrite levels in PDT tumor samples were found to be substantially higher than those in controls, and 1400W suppressed the nitrite increases (Fig. 3C). The iNOS inhibitors described above (1400W, GW274150) had no growth inhibitory effects on control tumors (not shown), meaning that preexisting iNOS (in contrast to photostress-upregulated iNOS) had no significant pro-tumor effects in this system (38).
Figure 3.
Endogenous iNOS/NO-mediated resistance to ALA-PDT in a tumor xenograft model. Breast MDA-MB-231 tumor-bearing SCID mice were injected i.p. with ALA (100 mg/kg) or PBS vehicle (light-only controls), followed by 1400W (10 mg/kg) or GW274150 (25 mg/kg), a 4 h delay, and then 633 nm irradiation (fluence ~95 J/cm2). Each iNOS inhibitor was injected once again every day after irradiation. (A) Tumor volume at increasing post-hν time; values are means ± SEM, n=3–6; P<0.05 for ALA/W/hν or ALA/GW/hν vs. ALA/hν at 6, 9, and 12 days. (B) Immunoblot of tumor iNOS after ALA/hν or hν alone. Each lane represents material from pooled homogenates of duplicate experimental tumors. Band intensities relative to β-actin and normalized to day-zero are shown. (C) Tumor levels of NO-derived nitrite determined by Griess assay; means ± SEM (n=3); *P<0.01 vs. ALA/hν. Reproduced from Ref. 38, with permission.
Because of irregular vascular supply and non-uniform light delivery, not all cells in a given tumor will be equally damaged by PDT and many may survive the challenge. However, certain characteristics of survivors might be altered, e.g. increased proliferation rate, as reported for certain cancers (39,40). The author’s group was the first to address this issue in terms of possible iNOS/NO involvement. Using human prostate carcinoma PC3 cells sensitized in mitochondria with ALA-induced PpIX, they found that iNOS was progressively upregulated after irradiation, reaching ~8-times the control level at 20 h post-hν (Fig. 4A), or at least twice greater than observed for similarly treated COH-BR1 cells (41,42). Apoptotic photokilling of PC3 cells over 24 h post-hν was significantly enhanced by 1400W or cPTIO, implying iNOS/NO-dependent resistance (Fig. 4B). When surviving (attached) cells were monitored from 24–72 h post-hν, a striking 1400W- and cPTIO-inhibitable increase in proliferation rate over a dark control rate was observed (42) (Fig.4B). This increase was attributed to greater S-phase occupancy, as determined by cell cycle analysis (41). Using a gap-closure or “wound-healing” assay, we found (42) that photostressed PC3 cells also migrated significantly faster than controls in 1400W-preventable fashion (Fig. 4C). This boost in migration rate could be mimicked by exposing cells to a low concentration of the NO donor DETA/NO (Fig. 4C), thereby confirming that that NO was the migratory stimulant in photostressed cells. It was also shown that NO stimulated PC3 cell invasion across a basement membrane which simulated an extracellular matrix (Fig. 4D). Accelerated invasion was preceded by changes in proteins know to facilitate this process, e.g. activation of matrix metalloproteinase-9 (MMP-9), downregulation of MMP-9 inhibitor TIMP-1, and upregulation of integrins α6 and β1 (42). Several other human cancer lines have exhibited similar hyper-aggressiveness after ALA/light treatment, including breast MDA-MB-231, prostate DU145, and glioma U87 and U251 (38,42,43). Thus, these previously unrecognized NO-dependent responses to a PDT-like challenge appear to be generally applicable. One key point to be emphasized is that the greater PC3 aggressiveness described in Fig. 4B–D was mainly due to stress-upregulated iNOS, the very low level of preexisting enzyme (Fig. 4A) being inconsequential. The same distinction applied to most of the other cancer lines mentioned. This dominant role of upregulated vs. constitutive iNOS has not been described for other oxidative anti-cancer modalities and may be unique to PDT-type stress. In clinical PDT, the potential switching of surviving cells to a more aggressive phenotype raises concerns and points to a need for safe anti-iNOS pharmacologic agents.
Figure 4.
Elevated resistance and aggressiveness in photodynamically-challenged prostate cancer cells. PC3 cells sensitized in mitochondria with ALA-induced PpIX were irradiated (1 J/cm2) in the absence vs. presence of 25 μM 1400W or cPTIO as indicated. (A) iNOS Western blot at various post-hν times. Below-band numbers are iNOS/β-actin intensity ratios normalized to a dark control (DC). (B) Post-hν cell viability (0–24h) and surviving cell proliferation (24–48h); means ± SEM (n=3). (C) Surviving cell migration at 24h and 48h post-hν; means ± SD (n=6); cells treated with DETA/NO (10 μM) also represented. (D) Surviving cell invasion over 48 h post-hν; means ± SEM (n=3); *P<0.05 vs. ALA/hν. Reproduced from Ref. 42, with permission.
Bystander effects of NO during PDT
Another manifestation of iNOS/NO’s anti-PDT activity is the bystander effect, which was recently described by the author and colleagues (44,45). In general terms, bystander effects occur when cells targeted by stress-inducing chemical or physical agents send pro-survival or pro-death signals to non-targeted counterparts (bystanders). Often, the latter make no physical contact with the former. Most of the research in this area has involved ionizing radiation and a variety of signaling molecules have been proposed, including cytokines, H2O2, and NO (46). PDT-induced bystander effects have been recognized for at least 20 years (47,48), but questions about types of bystander responses and identity of signaling mediators have remained largely unsettled before our findings. Using an innovative approach to distinguish ALA/light-targeted prostate PC3 cells from non-targeted PC3 bystanders, we found that after irradiation, iNOS was upregulated in the former as well as the latter cells, diffusible NO playing the driving role (44,45). Thus, an NO-mediated feed-forward signaling mechanism appeared to be in operation. Like surviving targeted cells, bystanders exhibited a striking spurt in proliferation and migration which could be strongly suppressed by 1400W or cPTIO. Conditioned medium from targeted cells had no effect of bystander proliferation or migration (44), ruling out any signaling activity of molecules longer lived than NO, e.g. H2O2 and cytokines like TNF-α. Underscoring the increased bystander aggressiveness was the activation of at least two kinases, Akt and ERK1/2, and upregulation of cyclooxygenase-2 (COX-2), all of which promote tumor survival/progression (44). For clinical PDT, these findings could raise concerns because they suggest that NO from targeted tumor cells might stimulate growth/migration of non- or poorly-target bystanders, thereby promoting tumor expansion. As indicated above and reiterated below, these negative effects could be alleviated through rational use of iNOS inhibitors as PDT adjuvants.
Summary and conclusions
Oncologists as well as basic researchers involved with PDT are indebted to Tom Dougherty for his pioneering efforts in developing this unique anti-tumor modality and moving it to the clinical level. PDT has many advantages over other anti-cancer therapies, but it, like the others, is often confronted with resistance mechanisms (either preexisting or acquired) which tend to reduce treatment efficacy. NO-mediated signaling is now seen as highly important among these mechanisms. The in vitro and in vivo studies described in this review are groundbreaking in showing that tumor cell iNOS/NO plays a major role not only in PDT resistance, but also in the elevated aggressiveness of surviving targeted cells as well as non-targeted bystanders (Fig. 5). Although both pre-existing and stress-induced iNOS might be implicated in these responses, the induced enzyme plays an almost exclusive role in several different cancer cell types. This evidence is especially noteworthy because most therapy-based studies up to now have considered only pre-existing iNOS/NO and not that possibly upregulated by the treatment itself. Whether PDT is unique in this regard, or whether it might apply to other anti-cancer modalities remains to be determined. Concerns about a more aggressive and potentially more metastatic post-PDT phenotype could be alleviated through pharmacologic intervention, e.g. with inhibitors of iNOS activity (16) or transcription (49), or inhibitors of upstream events preceding iNOS expression (50). Thus, a variety of options are available for improving PDT efficacy and minimizing any negative ramifications produced by iNOS/NO.
Figure 5.
Scheme depicting various ways in which tumor iNOS/NO can antagonize PDT. ALA/light-targeted tumor cells can succumb to 1O2-induced apoptosis or upregulate iNOS/NO to resist it and survive. Surviving cells can also exploit higher levels of NO to proliferate, migrate and invade more aggressively. Moreover, the NO can diffuse to non-targeted bystander cells, stimulating them to proliferate and migrate/invade more aggressively as well. Reproduced from Ref. 45, with permission.
Acknowledgements
Studies in the author’s laboratory were supported by NIH/NCI Grant CA70823, Rock River Pilot Grant FP14869, and grants from the Wisconsin Breast Cancer Showhouse for a Cure and Advancing a Healthier Wisconsin Endowment. Jonathan Fahey, Reshma Bhowmick, Magda Niziolek-Kierecka, Mariusz Zareba, and Jerzy Bazak are thanked for their many valuable contributions to the research described. Witold Kowytowski, Mladen Korbelik, and Neil Hogg are also thanked for their helpful advice and recommendations.
References
- 1.Dougherty TJ (1974) Activated dyes as antitumor agents. J. Natl. Cancer Inst 52, 1333–1336. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR and Boyle DG (1975) Photoradiation therapy II: Cure of animal tumors with hematoporphyrin and light. J. Natl. Cancer Inst 55, 115–121. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle DG and Mittleman A (1978) Photoradiation therapy for the treatment of malignant tumors. Cancer Res 38, 2628–2635. [PubMed] [Google Scholar]
- 4.Dougherty TJ (1989) Photodynamic therapy: status and potential. Oncology (Williston Park) 7, 67–73. [PubMed] [Google Scholar]
- 5.Henderson BW and Dougherty TJ (1992) How does photodynamic therapy work? Photochem. Photobiol 55, 145–157. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J and Peng Q (1998) Photodynamic therapy. J. Natl. Cancer Inst 90, 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC and Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanovsky RL, Bartenstein DW, Rogers GW, Isakoff SJ and Chen ST (2019) Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed doi: 10.1111/phpp.12489. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy JC and Pottier H (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 14, 275–292. [DOI] [PubMed] [Google Scholar]
- 10.Peng Q, Berg K, Moan J, Kongshaug M and Nesland JM (1997) 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol 65, 235–251. [DOI] [PubMed] [Google Scholar]
- 11.Casas A, Perotti C, Fukuda H and del C Batlle AM (2002) Photodynamic therapy of activated and resting lymphocytes and its antioxidant adaptive response. Lasers Med. Sci 17, 42–50. [DOI] [PubMed] [Google Scholar]
- 12.Dolgachev V, Oberley LW, Huang TT, Kraniak JM, Tainsky MA, Hanada K and Separovic D (2005) A role for manganese superoxide dismutase in apoptosis after photosensitization. Biochem. Biophys. Res. Commun 332, 411–417. [DOI] [PubMed] [Google Scholar]
- 13.Palasuberniam P, Yang X, Kraus D, Jones P, Myers KA and Chen B (2015) ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Sci Rep 5, 13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girotti AW (2016) Role of endogenous nitric oxide in hyperaggressiveness of tumor cells that survive a photodynamic therapy challenge. Crit. Rev. Oncog 21, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girotti AW (2016) Modulation of the anti-tumor efficacy of photodynamic therapy by nitric oxide. Cancers (Basel) 8(10), pii: E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderton WK, Cooper CE and Knowles RG (2001) Nitric oxide synthases: structure, function, and inhibition. Biochem. J 357, 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinelli MA, Do HT, Miley GP and Silverman RB (2019) Inducible nitric oxide synthase: regulation, structure, and inhibition. Med. Res. Rev doi: 10.1002/med.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC and Moncada S (1995) Roles of nitric oxide in tumor growth. Proc. Natl. Acad. Sci. U S A 92, 4392–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wink DA and Mitchell JB (1998) Chemical biology of nitric oxide: insights into the regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med 25, 434–456. [DOI] [PubMed] [Google Scholar]
- 20.Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA and Isenberg JS (2006) The biphasic nature of nitric oxide responses in tumor biology. Antiox. Redox Signal 8, 1329–1337. [DOI] [PubMed] [Google Scholar]
- 21.Burke AJ, Sullivan FJ, Giles FJ and Glynn SA (2013) The yin and yang of nitric oxide in cancer progression. Carcinogenesis 34, 503–512. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga T, Yamaji Y, Tomokuni T, Morita H, Morikawa Y, Suzuki A, Yonezawa A, Endo S, Ikari A, Iguchi K, El-Kabbani O, Tajima K and Hara A (2014) Nitric oxide confers cisplatin resistance in human lung cancer cells through upregulation of aldo-keto reductase 1B10 and proteasome. Free Radic. Res 48, 1371–1385. [DOI] [PubMed] [Google Scholar]
- 23.Gilissen MJ, van de Merbel-de Wit LE, Star WM, Koster JF and Sluiter W (1993) Effect of photodynamic therapy on the endothelium-dependent relaxation of isolated rat aortas. Cancer Res 53, 2548–2552. [PubMed] [Google Scholar]
- 24.Gupta S, Ahmad N and Mukhtar H (1993) Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res 53, 2548–2552. [PubMed] [Google Scholar]
- 25.Henderson BW, Sitnik-Busch TM and Vaughn LA (1999) Potentiation of photodynamic therapy antitumor activity in mice by nitric oxide synthase inhibition is fluence rate dependent. Photochem. Photobiol 70, 64–71. [PubMed] [Google Scholar]
- 26.Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford RML and Chaplin DJ (2000) Nitric oxide production by tumor tissue: impact on the response to photodynamic therapy. Br. J. Cancer 82, 1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves KL, Reed MWR and Brown NJ (2010) the role of nitric oxide in the treatment of tumours with aminolaevulinic acid-induced photodynamic therapy. J. Photochem. Photobiol. B 101, 224–232. [DOI] [PubMed] [Google Scholar]
- 28.Niziolek M, Korytowski W and Girotti AW (2003) Nitric oxide inhibition of free radical-mediated lipid peroxidation in photodynamically treated membranes and cells. Free Radic. Biol. Med 34, 997–1005. [DOI] [PubMed] [Google Scholar]
- 29.Niziolek M, Korytowski W and Girotti AW (2003) Chain-breaking antioxidant and cytoprotective action of nitric oxide on photodynamically stressed tumor cells. Photochem Photobiol 78, 262–270. [DOI] [PubMed] [Google Scholar]
- 30.Korytowski W Zareba M and Girotti AW (2000) Inhibition of free radical-mediated cholesterol peroxidation by diazeniumdiolate-derived nitric oxide: effect of release rate on mechanism of action in a membrane system. Chem. Res. Toxicol 13, 1265–1274. [DOI] [PubMed] [Google Scholar]
- 31.Esworthy RS, Baker MA and Chu F-F (1995) Expression of selenium-dependent glutathione peroxidase in human breast tumor cell lines. Cancer Res 55, 957–962. [PubMed] [Google Scholar]
- 32.Niziolek M, Korytowski W and Girotti AW (2006) Nitric oxide-induced resistance to lethal photooxidative damage in a breast cancer cell line. Free Radic. Biol. Med 40, 1323–1331. [DOI] [PubMed] [Google Scholar]
- 33.Niziolek-Kierecka M, Korytowski W and Girotti AW (2007) Tumor cell hyperresistance to photodynamic killing arising from nitric oxide preconditioning. Proc. Of SPIE 6427, 642705. [Google Scholar]
- 34.Bhowmick R and Girotti AW (2009) Signaling events in apoptotic photokilling of 5-aminolevulinic acid-treated tumor cells: inhibitory effects of nitric oxide. Free Radic. Biol. Med 47, 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhowmick R and Girotti AW (2011) Rapid upregulation of cytoprotective nitric oxide in breast tumor cells subjected to a photodynamic therapy-like oxidative challenge. Photochem. Photobiol 87, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhowmick R and Girotti AW (2013) Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic. Biol. Med 57, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapozzi V, Della Pietra E and Bonavida B (2015) Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol 6, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahey JM and Girotti AW (2017) Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: improved outcomes with NOS2 inhibitors. Nitric Oxide 62, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almeida RD, Manadas BJ, Carvalho AP and Duarte CB (2004) Intracellular signaling mechanisms in photodynamic therapy. Biochim. Biophys. Acta 704, 59–86. [DOI] [PubMed] [Google Scholar]
- 40.Edmonds C Hagan S, Gallagher-Colombo SM, Busch TM and Cengel KA 2012) Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3: targeting survival pathways to increase PDT efficacy in ovarian and lung cancer. Cancer Biol. Ther 13, 1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhowmick R and Girotti AW (2014) Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer Lett 343, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahey JM and Girotti AW (2015) Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: role of nitric oxide. Nitric Oxide 49, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahey JM, Emmer JV, Koytowski W, Hogg N and Girotti AW (2016) Antagonistic effects of endogenous nitric oxide in a glioblastoma photodynamic therapy model. Photochem. Photobiol 92, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bazak J, Fahey JM, Wawak K, Korytowski W and Girotti AW (2017) Enhanced aggressiveness of bystander cells in an anti-tumor photodynamic therapy model: role of nitric oxide produced by targeted cells. Free Radic. Biol. Med 102, 111–121. [DOI] [PubMed] [Google Scholar]
- 45.Bazak J, Fahey JM, Wawak K, Korytowski W and Girotti AW (2017) Cancer Cell Microenviron 4: e1511. doi: 10.14800/ccm.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hei TK, Zhou H, chai Y, Ponnaiya B and Ivanov VN (2011) Radiation-induced non-targeted response: mechanism and potential clinical implications. Cur. Mol. Pharmacol 4, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahle J, Bagdonas S, Kaalhus O, Olsen G, Steen HB and Moan J (2000) The bystander effect in photodynamic inactivation of cells. Biochim. Biophys. Acta 1475, 273–280. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty A, Held KD, Prise KM, Liber HL and Redmond RW (2009) Bystander effects induced by diffusing mediators after photodynamic stress. Radiat. Res 172, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fahey JM, Stancill JS, Smith BC and Girotti AW (2018) Nitric oxide antagonism to glioblastoma photodynamic therapy and mitigation thereof by BET bromodomain inhibitor JQ1.J. Biol. Chem 293, 5345–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahey JM, Korytowski W, Girotti AW. (2019) Upstream signaling events leading to elevated production of pro-survival nitric oxide in photodynamically-challenged glioblastoma cells. Free Radic. Biol. Med 137, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]