Abstract
Objectives:
Network analyses of psychopathology examine the relationships between individual symptoms in an attempt to establish the causal interactions between symptoms that may give rise to episodes of psychiatric disorders. We conducted a network analysis of mood symptoms in adolescents with or at risk for bipolar spectrum disorders.
Methods:
The sample consisted of 272 treatment-seeking adolescents with or at high risk for bipolar disorder who had at least subsyndromal depressive or (hypo)manic symptoms. Based on symptom scores assessed via semi-structured interviews, we constructed the network of depressive and manic symptoms and identified the most central symptoms and symptom communities within the network. We used bootstrapping analyses to determine the reliability of network parameters.
Results:
Symptoms within the depressive and manic mood poles were more related to each other than to symptoms of the opposing mood pole. Four communities were identified, including a depressive symptom community and three manic symptom communities. Fatigue and depressed mood were the strongest individual symptoms within the overall network (ie the most highly correlated with other symptoms), followed by motor hyperactivity. Mood lability and irritability were found to be “bridge” symptoms that connected the two mood poles.
Conclusions:
Symptoms of activity/energy (ie fatigue and hyperactivity) and depressed mood are the most prominent mood symptoms among youth with bipolar spectrum disorders. Mood lability and irritability represent potential warning signs of emergent episodes of either polarity. Targeting these central and bridge symptoms would lead to more efficient assessments and therapeutic interventions for bipolar disorder.
Keywords: bootstrap, centrality, depression, mania, pediatric, strength
1 |. INTRODUCTION
Bipolar I and II disorders affect about 2% of the world’s population and subthreshold forms of the illness affect another 2%.1,2 Despite a large genetic basis for bipolar disorder, no identifiable “common cause” has been found for the illness.3,4 Traditional methods of analyzing psychopathy have examined the top-down latent structure of disorders via factor analysis and latent class analyses.5–7 An alternative approach that seeks to elucidate the heterogeneity of paths that lead to psychiatric disorders as well as the heterogeneity of symptoms within disorders is through network analysis, which examines the symptom-to-symptom relationships that give rise to mental illness.8 In this study, we used a network approach to examine bipolar mood symptoms in adolescents with or at high risk for bipolar disorder.
Rather than conceptualizing mental disorders as latent entities that give rise to symptoms, network theory posits that when an individual symptom is elicited or becomes more severe within an individual, this increases the probability that a connected symptom will arise and that episodes of illness will occur.9 This perspective of symptoms is consistent with how most clinicians conceptualize mental illness,10 and fits with the underlying principles of common psychosocial therapies like cognitive-behavioral therapy.11,12 To model symptom interactions, network analyses examine the partial correlations between each symptom and create a visual depiction of the symptom-to-symptom relationships (termed a “network”).9 From these analyses, the most central experiences of a particular disorder, symptom communities (ie symptoms that are more related to each other than to other symptoms), and bridge symptoms (ie symptoms that connect communities) can be identified. The elucidation of symptom-to-symptom interactions may inform the development of adaptive and more efficient clinical assessments as well as more targeted and effective therapeutic interventions.13–15 For example, simulated single-node interventions in which central symptoms are “treated”/deactivated have a significant effect on diminishing network strength and symptom relationships.16
Network approaches have been used to model a range of psychiatric symptoms in adult populations, including anxiety, depression, posttraumatic stress disorder, autism, psychosis, and alcohol-use disorders.17–20 Most relevant to this study are network models of depressive and manic symptoms. Among depressive symptoms, sad mood, anhedonia, and low energy/fatigue consistently replicate as most central to depressive networks.21–24 These symptoms also account for more unique variance in psychosocial impairment compared to other depressive symptoms.21 Findings from these studies suggest that individual symptoms of depression have differential impact on clinical and psychosocial functioning, and indicate that total mood scores obfuscate important differences between depressive symptoms.14 Additionally, longitudinal analyses of depressive symptoms have found that more densely connected networks at baseline are associated with a more persistent course of depression over time, indicating that more pronounced relationships between symptoms may predict a poorer prognosis.25
To our knowledge, only one study has examined the network of bipolar mood symptoms. In a cross-sectional examination of 125 patients with bipolar disorder who were separated into three longitudinal clinical courses (minimally impaired, depressed, and cycling), Koenders and colleagues26 found that mood symptoms were most strongly interconnected in patients with the more severe courses of illness. Central symptoms were inconsistent across subgroups of illness severity, however, and network relationships were not presented for the overall sample. No studies to date have conducted network analyses to examine the relationship between mood symptoms in adolescents. Considering that the majority of patients with bipolar illness experience their first mood episode in adolescence,27 it is important to understand the associations between symptoms at that time. Clarifying the centrality (strength, betweenness, closeness) of individual depressive and manic symptoms may generate hypotheses about what symptoms are primary in the development of mood episodes and point to assessment and treatment strategies that are most efficient and effective.
The current study constructed the network of bipolar mood symptoms in adolescents with bipolar I and II disorder and adolescents at high risk for bipolar disorder (BD). We then examined whether there were clusters of symptoms (“communities”) within the network via community detection analysis. Based on prior network modeling,22,23 we expected the two mood poles (mania and depression) to represent separable communities of symptoms. Additionally, given that mood lability is robustly associated with conversion to bipolar disorder among youth with bipolar parents and that irritability is a symptom commonly occurring in both manic and depressive episodes,28 we predicted these symptoms would form a unique community that would bridge manic and depressive symptoms. Finally, we analyzed the centrality of each symptom via indices of node strength, closeness, and betweenness. We expected sad mood, anhedonia, and fatigue to be most central within the depressive symptoms. Based on prior research,29–31 we expected elevated mood and symptoms of unusually energetic and motor hyperactivity to be most central among manic symptoms. Bootstrapping analyses via sampling with replacement were performed to examine the accuracy and stability of the centrality indices.
2 |. METHODS
2.1 |. Participants
We utilized baseline data from two separate family treatment trials–a trial for adolescents at high risk for bipolar disorder and a trial for adolescents with bipolar I or II disorder (BD I/II). These two samples were combined for this study for three primary reasons. First, both populations were characterized by early onset (ie age at onset ≤18) depressive and/or (hypo)manic symptoms. Second, 45% of youth with bipolar disorder, not otherwise specified who have a familial risk of bipolar disorder “convert” to bipolar I or II disorder over a 5-year period.32,33 Third, the two samples used the same measures of psychiatric symptoms. Network analyses are considered adequately powered if there are at least five participants per node in the network,34 so we sought to capitalize on a larger sample size to increase statistical power and reliability of the study results.
High-risk youth were between the ages of 9 years, 0 months and 17 years, 11 months with a first- or second-degree relative with BD I/II. Participants were recruited at the University of Colorado, the Stanford University School of Medicine, or the UCLA Semel Institute. The studies were approved by the human subjects review boards of all three institutions. Following recruitment, each participant provided informed written consent after receiving a complete description of the study. Participants were eligible if they: (a) spoke English; (b) had at least one first- or second-degree degree relative who met DSM-IV-TR criteria for BD I/II, based on the Mini-International Neuropsychiatric Interview (MINI)35 or, when the relative could not be interviewed directly, via secondary report from a first-degree relative using the Family History Screen36; and (c) met DSM-IV-TR criteria for a lifetime diagnosis of BD not otherwise specified (NOS) or major depressive disorder (MDD) and had significant current affective symptoms (1-week Young Mania Rating Scale [YMRS] score >11 or 2-week Children’s Depression Rating Scale, Revised [CDRS-R] score >29).37,38 Diagnostic criteria for BD-NOS included having a distinct period of abnormally elevated, expansive, or irritable mood plus two (three, if irritable only) DSM-IV-TR symptoms of mania that caused a change in functioning, lasted ≥4 hours in a day, and occurred for a total of 4 or more days across the child’s lifetime. If the main diagnosis was MDD, the youth must have had a full DSM-IV major depressive episode (MDE) within the previous 2 years.
BD I/II participants were between the ages of 12 years, 0 months and 18 years, 1 month, and were recruited at the University of Colorado, the University of Pittsburgh School of Medicine, and the Cincinnati Children’s Hospital Medical Center. Participants were eligible if they had: (a) a DSM-IV-TR diagnosis of bipolar I or II disorder; (b) a hypomanic/manic or mixed episode lasting at least 1 week or a MDE lasting at least 2 weeks within the 3 months; (c) symptoms of at least moderate severity (a score of ≥17 on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) Mania Rating Scale (MRS)39 or a score of ≥16 on the K-SADS Depression Rating Scale (DRS)40 for at least 1 week over the previous month). BD I/II participants also had to be willing to engage in pharmacotherapy with a study psychiatrist. Exclusion criteria for both groups included meeting a current DSM-IV-TR diagnosis for a substance use or pervasive developmental disorder or being in a family where the child was a victim of current physical or sexual abuse (in which case appropriate notifications to the Department of Child Services and referrals for care were made).
2.2 |. Measures
To assess bipolar mood symptoms, participants were interviewed with at least one parent using the K-SADS MRS and DRS by trained MA/MD/PhD level diagnosticians. The MRS and DRS were used to rate the worst week over the past month. The ratings represent unfiltered symptoms, meaning that symptoms were rated based solely on their presence and independent of mood state or episode. When there were discrepancies between parent and youth, they were interviewed conjointly. For each item, consensus ratings were made by best clinical judgment. The MRS consists of 15 items and the DRS consists of 20 items. Items are rated on a 6-point scale of severity, from 0 (“Not at All”) to 6 (“Extreme”). Only 13 items for the MRS and 13 items for the DRS are used to compose the total mania and depression scores, respectively, as some items have redundancy (eg Number of Suicidal Acts, Suicidal Acts–Seriousness, Suicidal Acts–Medical Lethality). Due to the large number of K-SADS mood items relative to the sample size, we used only the 26 core items (13 for each the MRS and DRS) that compose the total mania and depression mood ratings for this study. Network analyses estimate a large number of parameters (ie the partial correlation of each variable with every other variable in the network), so increasing the sample size-to-node ratio helps improve the reliability of results.41 For the high-risk participants, reliability (intraclass r values) across the three sites was 0.89 for DRS scores and 0.97 for MRS scores. For the BD I/II participants, reliabilities across the three sites were 0.89 for DRS scores and 0.81 for MRS scores.
2.3 |. Statistical analyses
2.3.1 |. Network construction
All analyses were conducted using the “R” statistical software. Using the qgraph package,42 the network was constructed with the 26 K-SADS MRS and DRS symptoms (represented as “nodes”). The connections between nodes (“edges”) represent the partial correlation between the two nodes. The strength of the relationship between two nodes is referred to as the “edge-weight.” The initial correlational matrix was estimated using polychoric correlations, which estimate the association between two variables that are theorized to be continuous and normally distributed but are measured on ordinal scales. A Graphical gaussian model (GGM)43 was used to estimate the networks. To control for these spurious connections (false positives) when estimating parameter as well as to estimate a more parsimonious model, the GGM was regularized using the graphical LASSO glasso R package (glasso).44 Glasso regularization uses a tuning parameter, which controls the sparsity of the model via extended Bayesian information criteria (EBIC). Thus, the glasso algorithm corrects for Type I error and reduces the overall strength of parameter estimates by shrinking all parameters and minimizing small edge values to exactly 0. The regularization tuning parameter for this study was set at γ = 0.5 (the default tuning).45 Finally, the Fruchterman-Reingold (“spring”) algorithm was used to determine node placement within the network, which places nodes with stronger average associations toward the center of the network.46
Within the visual representation of the model, green edges indicate a positive partial correlation between variables; red edges indicate negative partial association between variables. Additionally, the wider the edge, the stronger the association between variables.42 While the network is constructed based on cross-sectional partial correlations, the edges are purported to have predictive effects. The direction of causation is not elucidated from network analyses; however, the presence of a causal effect is assumed.8,9,17 See Table S1 for a list of introductory network terms.
2.3.2 |. Community detection analysis
Community detection within the network was examined using the well-established spin glass test in R package igraph.47,48 The spin glass method uses a top-down approach whereby the network is initially considered to be one community. A community is then detected when the average edge-weight and number of edges between nodes are significantly greater than the average edge-weight and number of edges within another group of nodes.49 To increase reliability of results, a seed was set to run the algorithm 1000 times. The median number of clusters/communities was used as the final result.
2.3.3 |. Network centrality analysis
To analyze the networks, node centrality was measured based on three indices–strength, closeness, and betweenness. Strength measures the sum of edge-weights (ie sum of partial correlations) connected to a node. Thus, strength signifies the total weight/“involvement” a node has in the network. Closeness measures the average distance from the node to all other nodes in the network, indicating which nodes represent a greater “risk” for eliciting other nodes. Betweenness measures the number of times that a node lies on the shortest path between two other nodes, indicating which nodes serve as a “hub” between other nodes. These values are standardized as z-scores (indicating the relative effect size of that node’s centrality parameter) for ease of interpretation.
2.3.4 |. Bootstrapping centrality network parameters
Bootstrapping analyses of the centrality indices assess the stability of these indices and determine whether the ordering of centrality values is reliable. This was done using the bootnet R package and following methods outlined by Epskamp and colleagues.41 Centrality stability is computed by taking decreasingly smaller (case-dropping) subsets of the data and calculating the correlations between the centrality indices of the subsetted data and the original full sample. A total of 2500 bootstrapped tests were performed and the default bootnet bootstrapping technique was applied, which uses a non-parametric approach whereby observations in the data are resampled with replacement to obtain new datasets. The stability of the centrality coefficients can be quantified using the CS-coefficient, with a cutoff of 0.25 indicating modest stability and 0.5 indicating strong/high metric stability.41 The CS-coefficient calculates the maximum proportion of cases that can be dropped (with 95% certainty) to maintain a correlation greater than 0.7 with the original centrality value.
2.3.5 |. Sensitivity test
As a test of sensitivity, we examined whether the network of the full sample was different from the network of individuals with bipolar spectrum conditions (BSDs, excluding participants who had MDD only). The network of mood symptoms for participants with BSDs was constructed following the same methods outlined above. The full sample and the smaller sample were then compared based on their centrality indices as well as on their overall strength and edge-weight differences using the R package Network Comparison Test (NCT).50 The NCT is a two-tailed permutation test which conducts repeated calculations of differences between two networks (100 000 repetitions) for randomly regrouped individuals within each sample. The repeated comparisons create a distribution under the null hypothesis (assuming that the two groups are equal), to test for differences between groups. A significant difference between groups is set at an alpha level of .05.
3 |. RESULTS
3.1 |. Demographic characteristics
The total sample consisted of 272 participants, of which 77 had BD-I, 68 with BD-II, 50 were at high risk with BD-NOS, and 77 were at high risk with MDD. The sample consisted primarily of White, non-Hispanic, middle-class youth (see Table 1). Of note, all participants entered the study with at least subthreshold mood symptoms.
TABLE 1.
Characteristics of study participants
| Total sample (N = 272) | Bipolar 1 disorder (n = 77) | Bipolar II disorder (n = 68) | Bipolar disorder NOS (n = 52) | Major depressive disorder (n = 75) | p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years), mean (SD) | 14.5 (2.4) | 15.4a (1.5) | 15.9a (1.4) | 12.6b(2.6) | 13.6c(2.3) | <.001 |
| Hollingshead SES*, mean (SD) | 3.6(1.2) | 3.6(1.2) | 3.7(1.2) | 3.9 (0.9) | 3.4 (1.0) | .2 |
| Female participants, N (%) | 149 (54.8) | 39 (50.6) | 28 (41.2) | 37(71.2) | 45 (60.0) | .1 |
| Non-White participants, N (%) | 47 (17.3) | 13 (16.9) | 11 (16.2) | 10 (19.2) | 13 (17.3) | .9 |
| Hispanic participants, N (%) | 35 (12.9) | 6 (7.8) | 6 (8.8) | 10 (19.2) | 13 (17.3) | .1 |
| Clinical characteristics, mean (SD) | ||||||
| K-SADS MRS | 22.0 (12.7) | 31.4a (11.5) | 25.6b (10.4) | 20.3c (8.4) | 10.1d (7.3) | <.001 |
| K-SADS DRS | 24.0 (10.1) | 23.9a (12.0) | 27.4b (9.0) | 20.8a (9.6) | 23.4a (8.1) | .01 |
| Comorbid disorders, N (%) | ||||||
| Anxiety disorder | 138 (50.7) | 25 (32.5)a | 32 (47.1)a,b | 34(65.3)c | 47 (62.7)b,c | <.001 |
| ADHD | 97 (35.7) | 27 (35.1) | 21 (30.9) | 22 (42.3) | 27 (36.0) | .6 |
| ODD/CD | 74 (27.2) | 27(35.1) | 15 (22.1) | 17 (32.7) | 15 (20.0) | .1 |
| Medications, N (%) | ||||||
| Any psychotropic | 189 (69.5) | 73 (94.8)a | 49 (72.1)b | 28 (53.8)b,c | 39 (52.0)c | <.001 |
| Anticonvulsant | 33(12.1) | 11 (14.3)a,b,c | 5 (7.4)c | 12 (23.1)b | 5 (6.7)a,c | .01 |
| Antidepressant | 71 (26.1) | 13 (16.9)a | 14 (20.6)a,b | 16 (30.8)b,c | 28 (37.3)c | .006 |
| Antipsychotic | 121 (44.5) | 61 (79.2)a | 31 (45.6)b | 16 (30.8)b,c | 14 (18.7)c | <.001 |
| Lithium | 25 (9.2) | 17 (22.1)a | 7 (10.3)a | 0 (0.0)b | 1 (1.3)b | <.001 |
| Stimulant | 43(15.8) | 12(15.6) | 8 (11.8) | 9 (17.3) | 14 (18.7) | .6 |
Note: Each superscript letter within a row denotes classes whose column proportions or means do not significantly differ from each other at the P < .05 level.
Abbreviations: ADHD, attention-deficit hyperactivity disorder; CD/ODD, conduct disorder/oppositional defiant disorder; K-SADS DRS, Kiddie Schedule for Affective Disorders and Schizophrenia Depression Rating Scale; K-SADS MRS, Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale; NOS, not otherwise specified.
Higher values indicate higher education, income, and occupation; a value of 3 indicates middle class.
3.2 |. Network composition
A total of 119 edges (out of a possible total of 325; 36.6%) were calculated to be greater than zero (see Figure 1; the values of the centrality plot are presented in Table S2). Overall, the network revealed mostly independent relationships within the manic and depressive mood poles, meaning that symptoms organized more closely with symptoms in the same mood pole rather than with symptoms of the opposing mood pole. The community detection analysis confirmed relative independence between mood poles, and revealed a total of four communities within the data. The largest two communities consisted of manic symptoms and depressive symptoms, respectively. Within the manic pole, two communities were separable from the bulk of manic symptoms–a symptom cluster consisting of irritability and anger [MRS2] and mood lability [MRS13] and another cluster consisting of psychotic symptoms (hallucinations [MRS11] and delusions [MRS12]).
FIGURE 1.
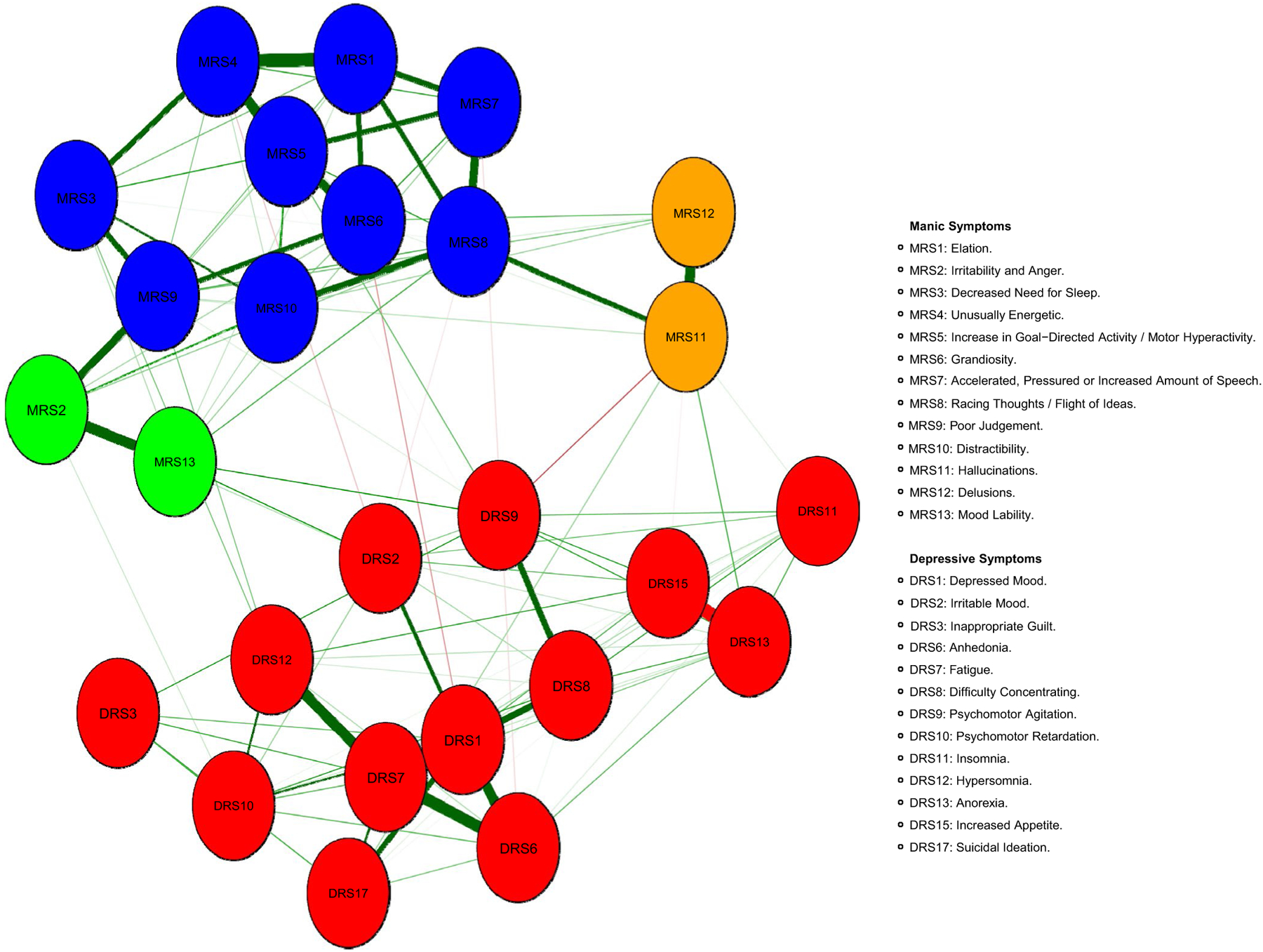
Network of 26 bipolar mood symptoms in adolescents with bipolar I/II disorder or at high risk for bipolar disorder (N = 272)
Examination of the strength centrality index (Figure 2) indicated that depressed mood [DRS1] and fatigue [DRS7] were the strongest nodes, with z-scores of 2.0 and 1.8, respectively. Both symptoms organized in the relative center of the depressive symptom mood pole. Depressed mood [DRS1] connected most strongly to anhedonia [DRS6], suicidal ideation [DRS17], difficulties concentrating [DRS8], and irritable mood [DRS2]. Fatigue [DRS7] also connected most strongly to anhedonia [DRS6] and difficulties concentrating [DRS8] as well as hypersomnia [DRS12]. Insomnia [DRS11] and inappropriate guilt [DRS3] were the weakest nodes in the network.
FIGURE 2.
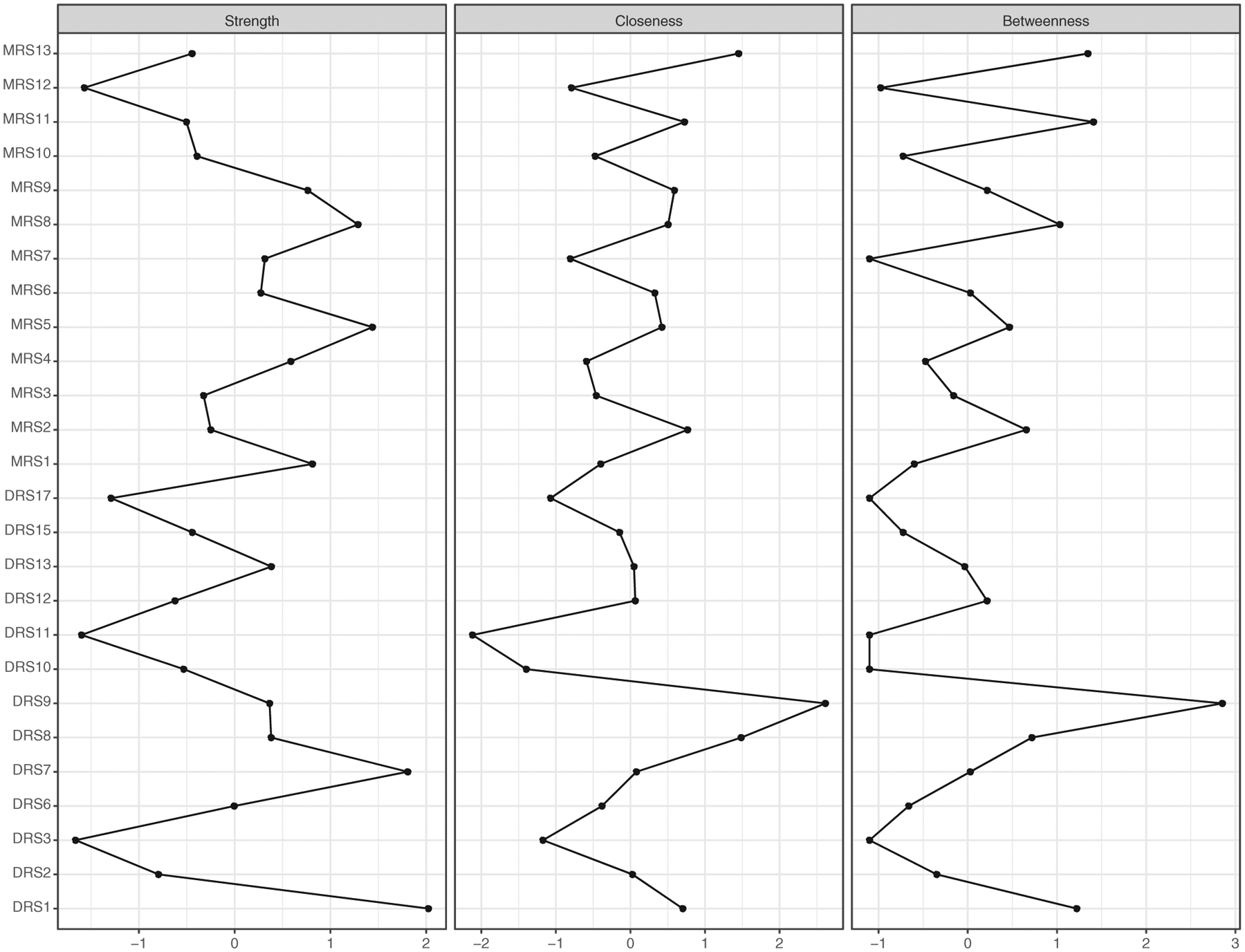
Centrality indices of strength, closeness, and betweenness (values are shown as standardized z-scores) in adolescents with bipolar I/II disorder or at high risk for bipolar disorder (N = 272)
Among manic symptoms, increase in goal-directed activity/motor hyperactivity [MRS5] (henceforth, “motor hyperactivity”) and racing thoughts/flight of ideas [MRS8] had the greatest strength, with z-scores of 1.4 and 1.3, respectively. Motor hyperactivity [MRS5] organized in the relative center of the manic symptoms and most strongly related to symptoms of unusually energetic [MRS4] and grandiosity [MRS6]. Racing thoughts/flight of ideas [MRS8] organized at the relative edge of the manic symptoms, most strongly connecting to accelerated, pressured or increased amount of speech [MRS7], distractibility [MRS10], and hallucinations [MRS11]. Surprisingly, elation [MRS1] was only the third strongest node among the manic symptoms (z = 0.8).
The centrality indices of closeness and betweenness were highly correlated (r = .92, P < .001). Thus, the results of these centrality indices are presented together. Psychomotor agitation [DRS9] stood out among symptoms within the centrality plot with a closeness z-score of 2.6 and a betweenness z-score of 2.8. Mood lability [MRS13] had the next highest closeness and betweenness values. Psychomotor agitation [DRS9] and mood lability [MRS13] organized in the relative center of the network, and were the most central symptoms connecting the two mood poles via symptoms of irritability [MRS2 & DRS2], difficulties concentrating [DRS8], and motor hyperactivity [MRS5]. Insomnia [DRS11] and psychomotor retardation [DRS10] had the lowest closeness and betweenness values in the network.
3.3 |. Centrality stability
The case-dropping bootstrap of the centrality indices is shown in Figure 3. The stability of node strength is relatively strong (CS[cor = 0.7] = 0.52) and meets the cutoff for metric stability. This result indicates that the node strength values are stable and the order of node strength is reliable across 2500 bootstrapping models. However, the stability of node closeness and betweenness dropped below the necessary cutoff. The CS-coefficients indicated that betweenness (CS[cor = 0.7) = 0.05) and closeness (CS[cor = 0.7] = 0.05) were not stable in the case-dropping method, suggesting that the ordering of the betweenness and closeness indices should be taken with caution.
FIGURE 3.
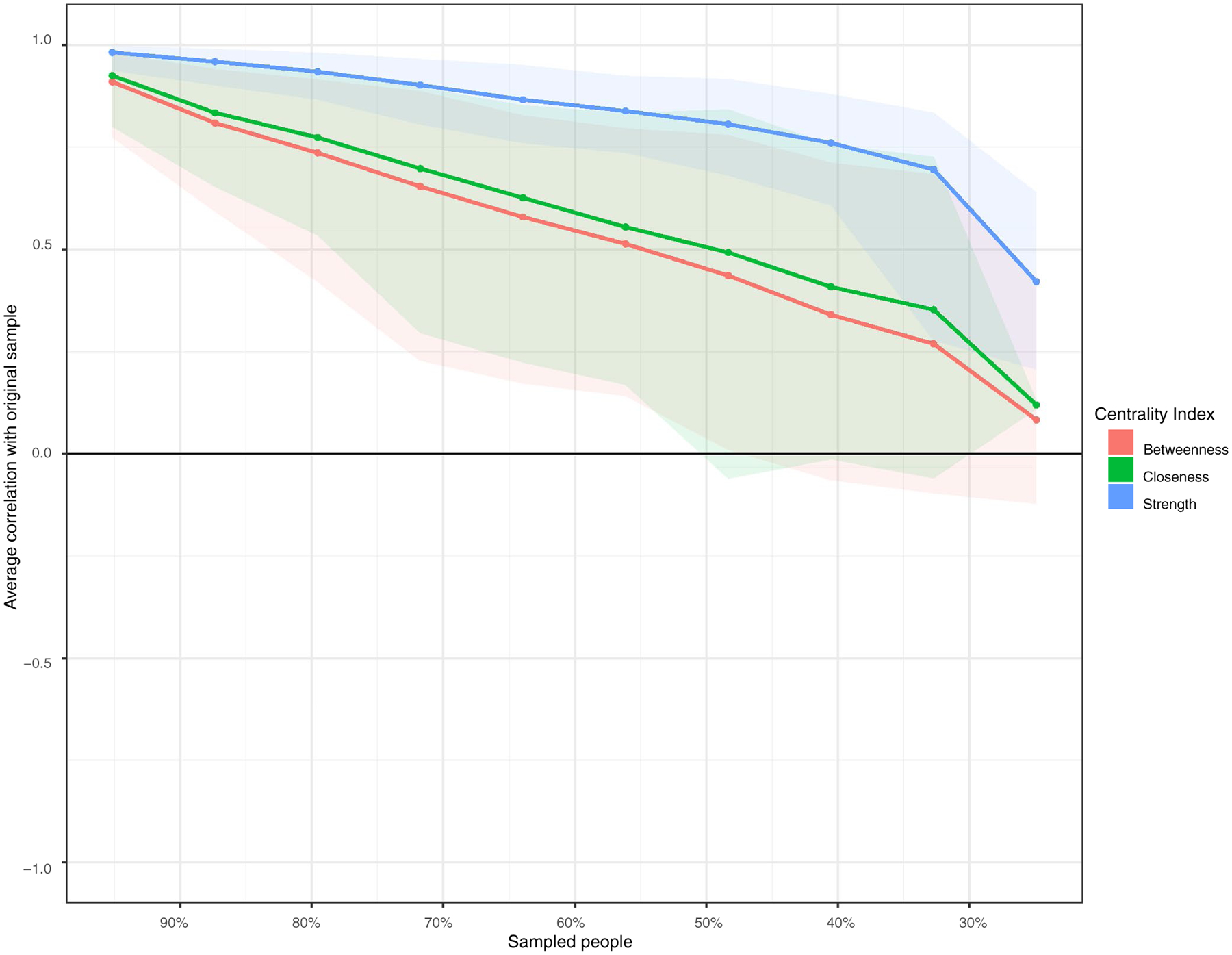
Centrality stability plot–the lines indicate the means of the average correlation between centrality indices of networks sampled with persons dropped and the original sample. Shaded areas around the lines represent the range of correlations from the 2.5th quantile to the 97.5th quantile
3.4 |. Sensitivity analyses
The network for individuals with BSDs (excluding high-risk individuals with MDD) had a total of 100 edges (out of a possible total of 325; 30.8%) that were calculated to be greater than zero (see Figure S1). Even more so than the network of the full sample, the two poles of the BSD network were mostly independent of each other. The community analyses revealed a total of five communities that closely matched the full sample. The manic symptoms formed three communities, which were (a) psychotic symptoms (hallucinations [MRS11] and delusions [MRS12]), (b) irritability and anger [MRS2] and mood lability [MRS13], and (c) the remainder of the manic symptoms. The bulk of depressive symptoms formed their own community with increased appetite [DRS15] forming an isolated community separate from all other symptoms.
As in the full sample, the centrality indices indicated that depressed mood [DRS1] and fatigue [DRS7] were the two strongest symptoms in the network, followed by unusually energetic [MRS4], motor hyperactivity [MRS5], and racing thoughts [MRS8]. The case-dropping bootstrap analyses for the bipolar spectrum network showed modest stability for the strength indices (CS[cor = 0.7] = 0.28); the closeness and betweenness indices did not show any stability (CS[cor = 0.7] = 0.0 for both; see Figure S2).
The NCT indicated no significant differences between the overall global strength (gs) of the two networks (gs of full sample = 9.7, gs of BSD sample = 8.5; P = .46) and no significant difference between edge-weights of the two networks (maximum difference in edge-weights = 0.1; P = 1.0), suggesting that the fully syndromal and high-risk BD spectrum samples had similar network structures.
4 |. DISCUSSION
This study characterized the network of bipolar (depressive and manic) mood symptoms in adolescents with and at risk for bipolar disorder. Overall, symptoms tended to be most interrelated with symptoms of the same mood pole. Additionally, a total of four communities of symptoms within the network were detected: a community of depressive symptoms, a community representing the majority of manic symptoms (including elated mood and symptoms related to energy/activity), a smaller community consisting of mood lability and irritability/anger, and, finally, psychotic symptoms. Within the overall network, depressed mood and fatigue were the most central symptoms in terms of their node strength. The centrality of depressed mood among mood symptoms is consistent with previous work of depression networks,22–24 as well as the general consensus that depressed mood is a core symptom of depression. Contrary to predictions, anhedonia was not ranked among the strongest symptoms in the network, although it did have a strong and close connection to depressed mood. Among manic symptoms, increase in goal-directed activity/motor hyperactivity and racing thoughts/flight of ideas ranked strongest, followed by elation. Of note, the strongest depressive and manic symptoms did not correlate with each other. This lack of correlation is consistent with findings from the network analysis of adults with both modest and more severe bipolar illness presentations.23
Interestingly, symptoms measuring the levels of energy/activity were among the most central to the bipolar mood network, including fatigue and motor hyperactivity. Characterizing increased vs decreased energy/activity as central symptoms of bipolar disorder is relatively novel, as the core symptoms of the disorder have historically been depressed and elated mood; however, increased activation is increasingly garnering attention and empirical support within the literature.51 Consistent with other network approaches, we found that fatigue/low energy were among the most central symptoms to depression.22–24 Among manic symptoms, some prior work has established symptoms of increased goal-directed activity and motor hyperactivity as central features of mania.29 These findings also support the recent addition of “increased energy or activity” as part of criterion A for the diagnosis of bipolar disorder in the DSM-5.31 Just as depressed mood and elation may increase the likelihood of fatigue and motor hyperactivity being activated, respectively, network theory posits fatigue and hyperactivity could have an impact on mood.
The elucidation of symptoms’ strength within the network has implications for determining risk and prognosis of adolescent bipolar disorder. Previous work has found that individual symptoms do not equally predict diagnosis and have different impacts on patient impairment and psychosocial functioning (ie there is symptom inequivalence).14,52 In models of depression, low mood and anhedonia have been found to outperform other individual depressive symptoms and sum scores of all depressive symptoms in predicting a diagnosis of depression and psychosocial impairment.21,53 Surprisingly, anhedonia in this study was only average in network strength among bipolar mood symptoms, which suggests it may not play a large role in bipolar disorder diagnosis or outcomes among adolescents with the disorder. Because symptoms of fatigue and motor hyperactivity, as well as depressed mood and elation, were among the strongest symptoms within the network, network theory suggests they would be particularly important in predicting diagnosis and prognostic outcomes of adolescent bipolar disorder. Further, these findings support a disaggregated approach to symptom measurement that weights each mood symptom based on its centrality in the bipolar network when conducting assessments of risk/diagnosis and predicting clinical outcomes.
Community analysis of the network demonstrated that bipolar mood symptoms coalesce most strongly and closely with symptoms of the same mood pole, suggesting relative independence of depressive and manic symptoms. Two communities within manic symptoms diverged from the manic cluster, including psychotic symptoms (delusions and hallucinations) and a community containing mood lability and irritability and anger. The community of mood lability and irritability represents potential “bridge symptoms” based on their positioning between the two mood poles, their significant connections “bridging” the mood poles, and the high closeness and betweenness values (particularly for mood lability). Mood lability and irritability have been identified as hallmark symptoms in the phenomenology of at-risk and bipolar youth,28,54 and there is evidence to suggest that they pose risk for switching from major depression or unspecified bipolar disorder to bipolar I or II disorder.55 According to network theory, bridge symptoms indicate which symptoms pose the greatest risk for activating disparate parts of the network.10 Based on this theory, mood lability and irritability may pose risk for switching or transitioning to the opposing mood pole. Additionally, the presence of mood lability and irritability may increase the likelihood of developing or maintaining mixed episodes.
The findings have implications for testing and developing clinical assessments. Network theory posits that incorporating central symptoms as initial or primary symptoms of assessment would likely improve efficiency and accuracy of psychiatric diagnosing. Thus, depressed mood, elated mood, fatigue, and motor hyperactivity represent central symptoms to assess first in adolescent bipolar disorder. Mood lability and irritability also represent important symptoms and appear to be particularly important to assess for risk of conversion from depression to mania and/or mixed features. These central symptoms and the elucidation of connections between these symptoms (eg the relationship between depressed mood, irritability, and mood lability) could be used in the creation of interactive, adaptive diagnostic testing.13 Since stronger relationships between symptoms suggest a higher probability of the two symptoms being present, adaptive tests could assess for symptoms that are most strongly connected in succession to more quickly arrive at a diagnosis. For example, an adolescent who endorses depressed mood would next be assessed for irritability and then mood lability in an assessment for risk or presence of bipolar disorder. Conversely, symptoms that were not central in the network (eg insomnia and guilt) may not be necessary for briefer clinical assessments.
Based on network theory, central symptoms and pathways also represent efficient and effective avenues for change within adolescent bipolar patients.16 Psychosocial and pharmacological treatments have been shown to be efficacious in reducing and stabilizing depressed and manic mood symptoms in bipolar adults and youth.56 The centrality of symptoms related to activation (eg fatigue and motor hyperactivity) suggests that intervening specifically on these symptoms would also be effective and efficient in treating bipolar illness. Recent work has found that increased motor activity, but not mood, predicts circadian and sleep dysregulation within bipolar disorder, suggesting that intervening on motor activity and energy may be more efficacious for these symptoms than targeting mood.57 Preliminary findings suggest that behavioral interventions for the management of sleep and insomnia–which often address levels of daily or nightly activation–reduce rates of bipolar relapse and improve general functioning.58,59 Behavioral activation has large effects on anhedonia and fatigue, in addition to depressed mood60; however, this work has not been tested in depressed or adolescent bipolar populations. Testing the efficacy of treatments that seek to increase behavioral engagement and energy in depression and manage activity/energy in mania seems warranted.
The bootstrapping analyses indicated that the strength centrality index was fairly robust, meaning that the node strengths are reliable and stable parameters that can be compared among each other. Consistent with prior work,41 the centrality indices of closeness and betweenness were not very stable, so the results related to closeness and betweenness must be taken with caution and require replication in a larger sample before firm conclusions can be made. Importantly, the construction of the network using LASSO regularization requires sufficiently strong partial associations between nodes for edge-weights to be produced.41 As such, LASSO regularization creates a sparse network model. The network analyses in this study revealed a relatively interconnected model within mood poles, but more sparse connections between mood poles. This would suggest that mood symptoms within the same mood pole strongly relate to one another, but that only a select few symptoms play a role in influencing the opposing mood pole.8,26 All the relationships shown in the network are significant and suggest some causal relationship between these symptoms under the network theory. However, the directionality of causation is unclear, and true tests of causation via pre-post data were not possible. In a cross-sectional network, relationships between nodes may result from shared item content, a reciprocal effect (ie co-occurrence of symptoms), or the effect of an unmodeled variable.61 These limitations of cross-sectional network analyses, in addition to controversy over the replicability of network analyses, underscore the importance of not assigning causal directionality in cross-sectional networks.62
There are other limitations of the study. First, the study is based on a combination of at-risk and pediatric bipolar disorder samples which, while a strength for identifying early representations of the symptom network, limits our conclusions about symptoms relationships in patients with more chronic presentations of bipolar disorder. Mania scores in adult samples would have been more severe and variable. It is possible that the lower range of symptom severity of patients in this study, especially our high-risk sample, limited how strongly interconnected the network would be. Second, the relatively small sample sizes limited our ability to include more variables (including comorbid psychiatric symptoms) within the network, compare edge-weights of the network, and interpret the closeness and betweenness indices with confidence. The limited sample size precluded reliable estimation and comparison of networks for subsamples of the study (high-risk vs fully syndromal BD). Future investigation and comparison of the relationships between mood symptoms and comorbid psychiatric symptoms across various stages of bipolar illness will more clearly elucidate the dynamic symptom relationships that may give rise to the disorder. Third, we do not report goodness-of-fit tests for the networks as these metrics for network analyses do not yet exist.63 Finally, this study assessed symptoms in a relatively simplified manner, as network nodes were measured cross-sectionally. Dynamic models that measure symptoms longitudinally to examine how networks change/evolve over time are at the vanguard of network analyses, but require very large datasets to reliably compare network parameters. Future work would also benefit from investigation of underlying mechanisms/processes that may give rise to and/or mediate symptoms.
This study examined the networks of bipolar mood symptoms in syndromal and subsyndromal adolescents. Considering the preponderance of work on network analyses have relied on self-report measures, a significant strength of this study is the use of clinician-rated assessments measured in adolescents with recently emerging bipolar mood symptoms. Overall, this study provides an initial examination of the central symptoms and pathways in the early courses of the illness. Future research should seek greater specificity within the bipolar mood network (eg examining bipolar mood subtypes), differences between symptom pathways to determine which pose the highest risks, and whether bipolar mood networks are consistent over a longer course of the illness.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the following individuals for their assistance in data collection, data management, and treatment: Adrine Biuckians, PhD, Jedediah Bopp, PhD, Victoria Cosgrove, PhD, L. Miriam Dickinson, PhD, Dana Elkun, MA, MFA, Elizabeth George, PhD, Chris Hawkey, PhD, Jessica Lunsford-Avery, PhD, Zachary Millman, PhD, Aimee Sullivan, PhD, Dawn Taylor, PhD, and Marianne Wamboldt, MD at the University of Colorado, Boulder and University of Colorado Denver Health Center, Denver, CO; Kiki Chang, MD, Manpreet Singh, MD, Tenah Acquaye, BA, Daniella DeGeorge, BA, Kathryn Goffin, BA, Meghan Howe, LCSW, Jennifer Pearlstein, MA, Donna Roybal, M.D., and Meagan Whitney, BA, at the Stanford University School of Medicine; Mary Beth Hickey at the University of Pittsburgh Medical Center, Pittsburgh, PA; and David Axelson, MD, and Robert Kowatch, MD, The Ohio State University College of Medicine, Columbus, OH, USA. The authors also thank William HB McAuliffe, PhD, for his support.
Funding information
Financial Support was provided by National Institute of Mental Health (NIMH) grants R01MH093676, R01MH093666, R01MH073871, R01MH073817, and R01MH074033. The sponsors named above did not have any role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
CONFLICT OF INTEREST
None. Dr Schneck has received research support from the National Institute of Mental Health and the Ryan White HIV/AIDS Treatment Extension Act. Dr Miklowitz has received research funding from the National Institute of Mental Health, Brain and Behavior Research Foundation, Attias Family Foundation, Danny Alberts Foundation, Carl and Roberta Deutsch Foundation, Kayne Family Foundation, Max Gray Foundation, American Foundation for Suicide Prevention and the AIM for Mental Health nonprofit organization. He has received book royalties from Guilford Press and John Wiley & Sons.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Jin R, He J-P, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. The Lancet. 2009;373(9659):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendler KS. Toward a philosophical structure for psychiatry. Am J Psychiatry. 2005;162(3):433–440. [DOI] [PubMed] [Google Scholar]
- 5.Serretti A, Olgiati P. Profiles of “manic” symptoms in bipolar I, bipolar II and major depressive disorders. J Affect Disord. 2005;84(2–3):159–166. [DOI] [PubMed] [Google Scholar]
- 6.Pan PM, Salum GA, Gadelha A, et al. Manic symptoms in youth: dimensions, latent classes, and associations with parental psychopathology. J Am Acad Child Adolesc Psychiatry. 2014;53(6):625–634.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy F, Forest K, Murry E, Carroll BJ. A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry. 1998;55(1):27–32. [DOI] [PubMed] [Google Scholar]
- 8.Borsboom D A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9(1):91–121. [DOI] [PubMed] [Google Scholar]
- 10.Kim NS, Ahn W-K. Clinical psychologists’ theory-based representations of mental disorders predict their diagnostic reasoning and memory. J Exp Psychol: Gen. 2002;131(4):451. [PubMed] [Google Scholar]
- 11.Beck AT, Haigh EA. Advances in cognitive theory and therapy: the generic cognitive model. Annu Rev Clin Psychol. 2014;10:1–24. [DOI] [PubMed] [Google Scholar]
- 12.Clark DA, Steer RA. Empirical status of the cognitive model of anxiety and depression In: Salkovskis Paul M, ed. Frontiers in Cognitive Therapy. New York: Guilford Press; 1996: 75–96. [Google Scholar]
- 13.Gibbons RD, Weiss DJ, Frank E, Kupfer D. Computerized adaptive diagnosis and testing of mental health disorders. Annu Rev Clin Psychol. 2016;12:83–104. [DOI] [PubMed] [Google Scholar]
- 14.Fried EI, Nesse RM. Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Med. 2015;13(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borsboom D, Cramer AO, Schmittmann VD, Epskamp S, Waldorp LJ. The small world of psychopathology. PLoS ONE. 2011;6(11):e27407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinaugh DJ, Millner AJ, McNally RJ. Identifying highly influential nodes in the complicated grief network. J Abnorm Psychol. 2016;125(6):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNally RJ, Robinaugh DJ, Wu GW, Wang L, Deserno MK, Borsboom D. Mental disorders as causal systems: A network approach to posttraumatic stress disorder. Clin Psychol Sci. 2015;3(6):836–849. [Google Scholar]
- 18.Ruzzano L, Borsboom D, Geurts HM. Repetitive behaviors in autism and obsessive–compulsive disorder: new perspectives from a network analysis. J Autism Dev Disord. 2015;45(1):192–202. [DOI] [PubMed] [Google Scholar]
- 19.Isvoranu A-M, Borsboom D, van Os J, Guloksuz S. A network approach to environmental impact in psychotic disorder: brief theoretical framework. Schizophr Bull. 2016;42(4):870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anker JJ, Forbes MK, Almquist ZW, et al. A network approach to modeling comorbid internalizing and alcohol use disorders. J Abnorm Psychol. 2017;126(3):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried EI, Nesse RM. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS ONE. 2014;9(2):e90311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, Borsboom D. What are ‘good’ depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J Affect Disord. 2016;189:314–320. [DOI] [PubMed] [Google Scholar]
- 23.Beard C, Millner AJ, Forgeard MJC, et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol Med. 2016;46(16):3359–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried EI, van Borkulo CD, Epskamp S, Schoevers RA, Tuerlinckx F, Borsboom D. Measuring depression over time. Or not? Lack of unidimensionality and longitudinal measurement invariance in four common rating scales of depression. Psychol Assess. 2016;28(11):1354. [DOI] [PubMed] [Google Scholar]
- 25.van Borkulo C, Boschloo L, Borsboom D, Penninx BW, Waldorp LJ, Schoevers RA. Association of symptom network structure with the course of depression. JAMA Psychiatry. 2015;72(12):1219–1226. [DOI] [PubMed] [Google Scholar]
- 26.Koenders M, De Kleijn R, Giltay E, Elzinga B, Spinhoven P, Spijker A. A network approach to bipolar symptomatology in patients with different course types. PLoS ONE. 2015;10(10):e0141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlis RH, Smoller JW, Fava M, Rosenbaum JF, Nierenberg AA, Sachs GS. The prevalence and clinical correlates of anger attacks during depressive episodes in bipolar disorder. J Affect Disord. 2004;79(1–3):291–295. [DOI] [PubMed] [Google Scholar]
- 28.Hafeman DM, Merranko J, Goldstein TR, et al. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk. JAMA Psychiatry. 2017;74(8):841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiskal H, Azorin J, Hantouche E. Proposed multidimensional structure of mania: beyond the euphoric-dysphoric dichotomy. J Affect Disord. 2003;73(1–2):7–18. [DOI] [PubMed] [Google Scholar]
- 30.Cheniaux E, Silva RAD, Santana CM, Filgueiras A. Changes in energy and motor activity: core symptoms of bipolar mania and depression? Braz J Psychiatry. 2018;40(3):233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- 32.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1001–1016.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigman JT, de Vos S, Wichers M, van Os J, Bartels-Velthuis AA. A transdiagnostic network approach to psychosis. Schizophr Bull. 2016;43(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IVand ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):2233. [PubMed] [Google Scholar]
- 36.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–682. [DOI] [PubMed] [Google Scholar]
- 37.Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R). Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 38.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Brit J Psychiatry. 1978;133(5):429–435. [DOI] [PubMed] [Google Scholar]
- 39.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie schedule for affective disorders and schizophrenia for school-age children mania rating scale for children and adolescents. J Child Adolesc Psychopharm. 2003;13(4):463–470. [DOI] [PubMed] [Google Scholar]
- 40.Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42(7):696–702. [DOI] [PubMed] [Google Scholar]
- 41.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: A tutorial paper. Behav Res Methods. 2018;50(1):195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: Network visualizations of relationships in psychometric data. J Stat Softw. 2012;48(4):1–18. [Google Scholar]
- 43.Lauritzen SL, Wermuth N. Graphical models for associations between variables, some of which are qualitative and some quantitative. Ann Stat. 1989;17(1):31–57. [Google Scholar]
- 44.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foygel R, Drton M. Extended Bayesian information criteria for Gaussian graphical models. Paper presented at: advances in neural information processing systems; 2010. [Google Scholar]
- 46.Fruchterman TM, Reingold EM. Graph drawing by force-directed placement. Software Pract Exper. 1991;21(11):1129–1164. [Google Scholar]
- 47.Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E. 2004;69(2):026113. [DOI] [PubMed] [Google Scholar]
- 48.Csardi G, Nepusz T. The igraph software package for complex network research. Inter J Complex Syst. 2006;1695(5):1–9. [Google Scholar]
- 49.Heeren A, McNally RJ. An integrative network approach to social anxiety disorder: the complex dynamic interplay among attentional bias for threat, attentional control, and symptoms. J Anxiety Disord. 2016;42:95–104. [DOI] [PubMed] [Google Scholar]
- 50.Van Borkulo CD, Epskamp S, Millner A. Network comparison test: permutation-based test of differences in strength of networks. R Package. 2016;2(1). https://github.com/cvborkulo/NetworkComparisonTest [Google Scholar]
- 51.Scott J, Murray G, Henry C, et al. Activation in bipolar disorders: a systematic review. JAMA Psychiatry. 2017;74(2):189–196. [DOI] [PubMed] [Google Scholar]
- 52.Schmittmann VD, Cramer AO, Waldorp LJ, Epskamp S, Kievit RA, Borsboom D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas Psychol. 2013;31(1):43–53. [Google Scholar]
- 53.Rosenström T, Elovainio M, Jokela M, et al. Concordance between composite international diagnostic interview and self-reports of depressive symptoms: a re-analysis. Int J Methods Psychiatric Res. 2015;24(3):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birmaher B, Goldstein BI, Axelson DA, et al. Mood lability among offspring of parents with bipolar disorder and community controls. Bipolar Disord. 2013;15(3):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akiskal HS, Maser JD, Zeller PJ, et al. Switching from ‘unipolar’ to bipolar II: an 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry. 1995;52(2):114–123. [DOI] [PubMed] [Google Scholar]
- 56.Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merikangas KR, Swendsen J, Hickie IB, et al. Real-time mobile monitoring of the dynamic associations among motor activity, energy, mood, and sleep in adults with bipolar disorder. JAMA Psychiatry. 2019;76(2):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harvey AG. The adverse consequences of sleep disturbance in pediatric bipolar disorder: implications for intervention. Child Adolesc Psychiatric Clin North Am. 2009;18(2):321–338. [DOI] [PubMed] [Google Scholar]
- 59.Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. J Consult Clin Psychol. 2015;83(3):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuijpers P, Van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27(3):318–326. [DOI] [PubMed] [Google Scholar]
- 61.Costantini G, Epskamp S, Borsboom D, et al. State of the aRt personality research: a tutorial on network analysis of personality data in R. J Res Personal. 2015;54:13–29. [Google Scholar]
- 62.Forbes MK, Wright AG, Markon KE, Krueger RF. Evidence that psychopathology symptom networks have limited replicability. J Abnorm Psychol. 2017;126(7):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolaczyk ED, Csárdi G. Statistical analysis of network data with R. Vol 65 Washington, DC: Springer; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


