Here we report a rapid CRISPR/Cas9-mediated gene knock-in strategy that utilizes Cas9 ribonucleoprotein and 5’ modified dsDNA donors with 50 bp homology arms and achieved unprecedented 65%/40% KI rates for 0.7 Kb/2.5 Kb inserts, respectively, in HEK293T cells. The identified 5’ end modification led to up to 5-fold increase in gene KI rates at various genomic loci in human cancer and stem cells.
CRISPR/Cas9-induced double-strand breaks (DSBs) enable various genome editing applications including gene knock-out (KO), gene knock-in (KI), and introduction of point mutations1–3. Gene KI has been explored for a wide variety of purposes ranging from functional genomics4, 5 to gene therapies6. Despite its enormous potential in biotechnology and pharmaceutical industry, gene KI is very inefficient in human cells, especially in stem cells (typical gene KI rate in human stem cells is lower than 0.1% without selection)7. To realize the ultimate potential of gene KI in biotechnology and for therapeutic development, we set out to improve the efficiency of CRISPR/Cas9-mediated gene KI in human cells.
Initially we decided to investigate gene KI strategies that utilize linear dsDNA as donor templates (herein referred to as “dsDNA donors”). This decision was based on a lack of correlation between gene KI efficiency and Cas9- induced insertion and deletion (indel) rate (Supplementary Fig. 1a–c). On the other hand, gene KI efficiency was shown to be sensitive to variations in donor concentration, homology arm (HA) length, and types of donor templates (Supplementary Fig.2a–c). Moreover, end modifications of ssDNA donors by replacing the phosphate (PP) linkages with the more stable phosphorothioate (PS) linkages was shown to increase gene editing efficiency8. As a result, we sought to identify additional end modifications of dsDNA donors that improve gene KI efficiency.
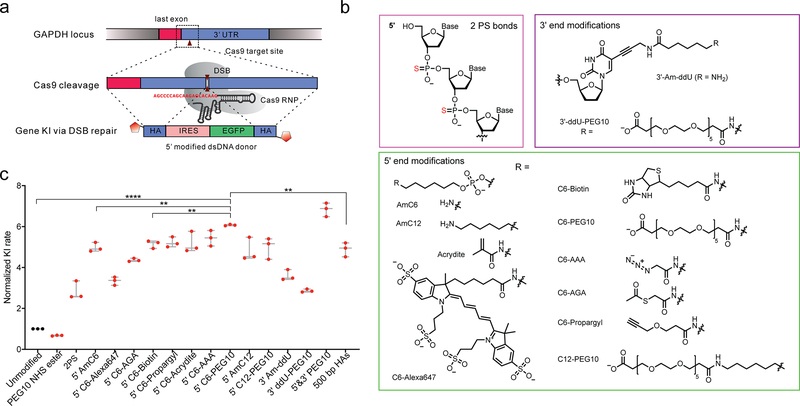
We selected 13 modifications and tested their effect on dsDNA donor-mediated gene KI, including 5’ end modifications, 3’ end modifications, and 2 PS linkages (Fig. 1a–b). Primers with 5’ modifications including amine group with a C6 linker (AmC6) or C12 linker (AmC12) were synthesized (Fig. 1b). Other secondary modifications were added to AmC6/AmC12 linkers by conjugating the amine group with their N-hydroxysuccinimide (NHS) esters (Supplementary Fig. 3). These 5’ modifications were easily incorporated into dsDNA donors by PCR reaction. Enzymatic installation of 3’ end modifications was limited by the scale, so we focused on testing 5’ modifications.
Figure 1. Gene KI strategy using Cas9 RNP and end-modified dsDNA donors.
(a) Gene KI rate report system9. Double strand break was created at the 3’ UTR of GAPDH gene by Cas9 RNP. Gene KI was mediated by linear dsDNA donor template that contains internal ribosomal entry site (IRES), EGFP sequence and HAs. Red hexagon represents end modification in dsDNA donors. (b) Structures of end modifications tested in this study including 2PS linkages at 5’ ends, 5’ end modifications and 3’ end modifications. These modifications were incorporated as described in online methods. (c) KI rate in HCT116 cells using 2.2 pmol dsDNA donors. PEG10 NHS ester: 1 mM PEG10 NHS ester was added to the transfection reaction; 5’&3’ PEG10: both 5’ C6-PEG10 and 3’ ddU-PEG10 were added. The absolute KI rate for unmodified dsDNA donor is 2.1 ± 0.13%. The size, concentration, and preparation of dsDNA donors were described in Supplementary Table 2. Transfection conditions were described in online methods. Data were collected 7 days post-transfection. p value was calculated by two-tailed unpaired t-test, *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; n = 3 biologically independent samples. Error bars represent mean ± s.d.
We first tested the effect of these end-modifications on gene KI using a published gene KI report system at GAPDH locus in HCT116 cells9. A 1.8 ± 0.36-fold increase in KI rate for was observed dsDNA donors with PS bonds (Fig. 1c). Interestingly, the 5’ modifications led to more substantial enhancements in KI rates (Fig. 1c), ranging from 2.3-fold to 5.1-fold at 2.2 pmol donor concentration (Fig. 1c). AmC6/C12 linkers brought 4.0 ± 0.18 /3.8 ± 0.47-fold increase in KI rate, and no C6/C12 linked modifications showed decrease KI rates (Fig. 1c). C6-Azidoacetic acid (AAA) and C6-Polyethylene glycol (PEG10) provided additional stimulating effect compared to AmC6, while C6-Alexa647 and C6-Acetylthioglycolic acid (AGA) showed an opposite effect (Fig. 1c). Modifications at 3’ ends like Am-ddU and ddU-PEG10 also caused 2.6 ± 0.21-fold and 1.9 ± 0.06-fold increase in gene KI rate. A slight additive effect (0.13 ± 0.05-fold increase) was observed when 3’ ddU-PEG10 was added to 5’ C6-PEG10 modified dsDNA donors (Fig. 1c). By incorporating end modifications, the need for long HAs to maximize gene KI rate is eliminated at GAPDH locus (Fig. 1c and Supplementary Fig. 2b). A slightly higher KI rate was observed for 5’ C6-PEG10 modified dsDNA donors with 50 bp HAs compared to unmodified dsDNA donor with 500 bp HAs (Fig. 1c). Moreover, the background signal from the templates was greatly reduced when short HAs were used (Supplementary Fig. 4a). The stimulating effect on KI rate was retained for all 5’ modifications tested at a saturating concentration of dsDNA donors (Supplementary Fig. 4b). No significant increase was observed when 5’ C6-PEG10 modified dsDNA donor with 500 bp HAs was used (Supplementary Fig. 4c). Due to much better scalability and high KI efficiency, 5’-C6-PEG10 modified dsDNA donors with 50 bp HAs were tested in following studies.
Next, we compared the efficiency of our strategy to previous studies using donors with short HAs. For GAPDH locus, dsDNA donors with 5’ C6-PEG10 modifications resulted in a 6.6 ± 0.45% KI rate in hESC H1 (Fig. 2a), whereas a 1.7% KI rate was reported for double-cut NHEJ donors using the same report system9. A recent study demonstrated that using PCR fragments with 33 bp HAs as donor templates, a 15% KI rate for a 0.7 Kb EGFP sequence at lamin A/C locus was achieved in HEK293T10. Although a 13-fold less Cas9 was used in this study, a 30.4 ± 0.96% gene KI rate was observed using conditions described (Fig. 2b). Simply by adding 5’ C6-PEG10 modifications to this dsDNA donor, KI rate was increased to 65.4 ± 0.44% (Fig. 2b), which is the highest pre-selection gene KI rate reported for insertion of longer than 500 bp despite the use of short HAs (Supplementary Table 3). It is worth to mention that other factors besides donor modification might contribute to the increase in gene KI rate, such as Cas9 ribonucleoprotein (RNP) delivery optimization.
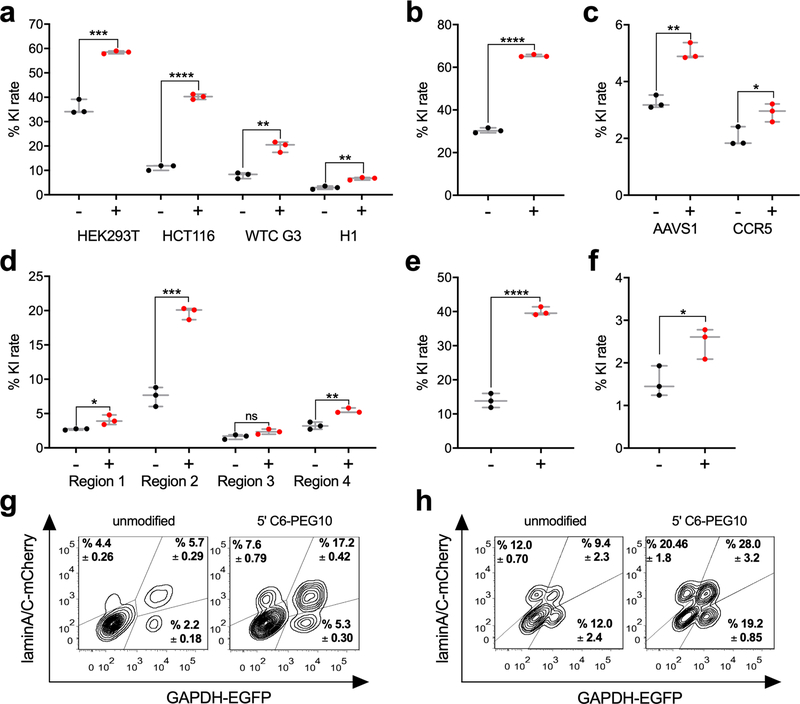
Figure 2. End modified dsDNA donors resulted in enhanced gene KI rate in various applications.
Gene KI rate with (+) or without (−) 5’ C6-PEG10 modifications at (a) GAPDH locus in HCT116, HEK293T, hiPSC WTC G3 and hESC H1; (b) lamin A/C locus in HEK293T; (c) AAVS1 locus and CCR5 locus in hiPSC WTC G3 cells; (d) heterochromatin region 1–4 in HCT116 cells; (e) AAVS1 locus in HEK293T using a 2.5 Kb dsDNA donor; (f) Cpf1-mediated gene KI at GAPDH locus in HEK293T; (g) Duplexed gene KI at GAPDH locus and lamin A/C locus in HEK293T; (h) Allelic-specific gene KI rate at lamin A/C locus in HEK293T. An aliquot of 0.5 M cancer cells or 1.5 M stem cells, 18.8 pmol Cas9, 56.3 pmol gRNA and indicated amount dsDNA donors were used in 100 μL electroporation buffer; for (h), 71.5 pmol Cpf1 and 215 pmol crRNA were used instead. The target sites tested were listed in Supplementary Table 1. The size, concentration, and preparation of dsDNA donors were described in Supplementary Table 2. Transfection conditions were described in online methods. Data were collected 7 days post-transfection (for donors with no promoter) or 10 days post-transfection (for donors contains promoter). p value was calculated by two-tailed unpaired t-test, *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; n = 3 biologically independent samples. Error bars represent mean ± s.d.
To demonstrate its generality, we tested this strategy in diverse applications. For GAPDH locus, KI rate after applying 5’-C6 PEG10 modifications was increased from 35.7 ± 2.5% to 59.4 ± 0.50% in HEK293T, 8.0 ± 0.98% to 19.8 ± 1.8% in hiPSC WTC G3 and 2.9 ± 0.55% to 6.6 ± 0.45% in hESC H1 (Fig. 2a). Gene KI at safe harbor sites such as AAVS1 and CCR5 were evaluated because these sites are preferred for predictable gene integration6. 5’ C6-PEG10 modifications of dsDNA donor improved its KI rate from 3.3 ± 0.19% to 5.0 ± 0.24% at AAVS1 site, 2.0 ± 0.28% to 2.9 ± 0.26% at CCR5 site in hiPSC WTC G3 (Fig. 2c). Gene KI at heterochromatin regions can be applied in the study of chromatin structure, function, and dynamics11. With 5’ C6-PEG10 end-modification, we achieved 0.5 to 1.6-fold increase in gene KI rate at 4 predicted heterochromatin loci11, with an remarkable 19.7 ± 0.72% KI rate at region 2 (Fig. 2d). In addition, we were able to achieve a 40.0 ± 1.0% gene KI rate by using a 2.5 Kb 5’ PEG10 modified dsDNA donor at AAVS1 locus in HEK293T cell (Fig. 2e), which showed that our strategy could be used in many other applications besides reporter gene KI. Moreover, a 0.64 ± 0.15-fold increase was observed at GAPDH locus in Cpf1-mediated gene KI rate with 5’ C6-PEG10 modification (Fig. 2f). To examine the potential of multiplexing, dual gene KI of GAPDH and lamin A/C was tested. An increase in double positive population from 5.7 ± 0.29% to 17.2 ± 0.42% was observed for dsDNA donors with 5’-C6 PEG10 modification (Fig. 2g). Allele-specific gene editing are potentially useful in allelic gene functional studies. Using 5’ C6-PEG10 modified donors contains EGFP or mCherry, EGFP/mCherry double positive population was promoted from 9.4 ± 2.3% to 28.0 ± 3.2% at lamin A/C locus (Fig. 2h). Taken together, these results demonstrated the versatility of this strategy in challenging situations.
To determine the editing accuracy of this strategy, we examined the indels in KI junction regions. A 7.3 ± 0.77% to 4.2 ± 0.36% and a 5.9 ± 0.40% to 3.4 ± 0.59% decrease in indel rates were measured by TIDE analysis12 at 5’ and 3’ junctions respectively with 5’-C6 PEG10 modification (Supplementary Fig. 5a–b). The indels in the KI junctions are usually caused by non-homologous end joining (NHEJ)-mediated KI, while homology directed recombination (HDR)-mediated KI generally results in precise editing10. NHEJ-KI rate was evaluated using dsDNA donors with no HAs, and no increase in NHEJ-KI rate was observed as donor concentration increased (Supplementary Fig. 5c). Together, these results indicated that the increase in gene KI rate by end modification is not caused by promoting error-prone NHEJ-mediated repair. Gene integration copy number was determined for individual KI clones to investigate if there is significant concatemer formation at KI site. We examined a total of 66 clones from KI experiments performed with dsDNA donors with 5’-C6 PEG10 modification targeting 6 different sites. Out of these clones, 61 showed 1 copy gene integration at one allele or both alleles of the target genes (Supplementary Fig. 6a–c). Only 3 clones showed concatemer formation at the target sites, and 2 clones showed off-target integration (either Cas9-guided or random integration, Supplementary Fig. 6a–c). These results are consistent with the recent study which used 5’ biotin modified dsDNA donors to reduce NHEJ-based KI and concatemer formation13. They also indicate that the random integration rate is at a low level (≤2 out of 66) in gene KI experiments performed with end-modified donors.
In summary, we described a simple and effective strategy for gene KI combining Cas9 RNP with 5’ modified dsDNA donors with short HAs. The present method exhibits the advantages of enhanced KI efficiency, shorter workflow, and high accuracy. We screened 13 modifications with diverse structures and identified 5’ C6-PEG10 as the most beneficial one. 5’ C6-PEG10 modified dsDNA resulted in up to 5-fold increase in gene KI efficiency, which resulted in the highest gene KI rate (65.4 ± 0.44%) in human cells to the best of our knowledge. In addition, lower indel rates in the KI junctions were observed with end modified dsDNA donors. Therefore, end modified dsDNA donors with short HAs could be used as an appealing alternative to traditional plasmid donor templates. We tested 13 modifications in this study, and further screening may reveal more potent chemical moieties. Moreover, it might be interesting to study the mechanism underneath this phenomenon. Finally, as the stimulating effect of 5’ C6-PEG10 modification was verified at diverse genomic loci including safe harbor sites and heterochromosome regions, and also in different cell lines including human cancer and stem cell lines, we envision our strategy may benefit a wide range of studies such as gene functional studies, allele-specific gene expression, chromatin structure studies, and genetic disease treatment.
Methods
Generation of plasmids, dsDNA, Cas9 RNP, and Cpf1 RNP
Plasmids used as templates for dsDNA donor PCR were assembled by Gibson assembly using pCRIS-PITChv2-FBL (Addgene, plasmid #63672) as backbone. The insertion sequences were between BamHI and NheI sites and listed in Supplementary Table 4. dsDNA donors were obtained by PCR amplification using Q5 High-Fidelity DNA polymerase (New England BioLabs). The 0 to 50 bp HA sequences were incorporated in the primers listed in Supplementary Table 5. For KI at GAPDH locus, donors with longer HAs were amplified from a plasmid donor with 500 bp HAs (IRES-GFP donor plasmid, GAPDH, 500 bp HAs). PCR products were gel-purified or PCR-purified depending on their purities using QIAquick gel extraction kit or QlAquick PCR purification kit (Qiagen). The typical yield from a 500 μL reaction is around 15–30 μg. gRNAs and crRNA used for transfections were obtained using GeneArt™ Precision gRNA Synthesis Kit (Thermo Fisher Scientific) using primers listed in Supplementary Table 5. gRNAs/crRNAs were eluted in TE buffer and annealed using a PCR thermocycler using the following program: 95 °C for 5 min, and then cooled down to 25 °C at 0.1 °C/s. All primers were ordered from IDT (Integrated DNA Technologies, Coralville, IA). Cas9 protein was purchased from IDT or purified as described14. Cpf1 protein was purified as described15. Cas9/Cpf1 protein was expressed in E. coli Rosetta 2 (DE3) cells using Cas9 expression plasmid (Addgene, plasmid #47327), or Cpf1 expression plasmid (Addgene, plasmid #79007).
Oligonucleotide labelling
For 5’ modification and PS bond modification: primers with 2PS bonds, Acrydite™, C6-Alex 647, amine C6 and amine C12 were ordered from IDT. Bis-PEG10-NHS ester was purchased from Broadpharm (BP-22588). Biotin NHS ester (H1759), azidoacetic acid NHS ester (900919), s-acetylthioglycolic acid NHS ester (A9043), and propargyl NHS ester (764221) were purchased from Sigma-Aldrich. NHS esters were incubated with 10 μM primers with 5’ amine C6 or C12 group at 1 mM concentration in 1 x borate buffer (Thermo Fisher, 28341) overnight at room temperature. Primers were then desalted using Bio-Spin 30 columns (Bio-rad). The labelling efficiencies were measured by HPLC and MALDI-TOF mass spectrometry (Supplementary Fig. 3). For 3’ Am-ddU modification: An aliquot of 10 μg dsDNA donors, 20 μM amino-11-ddUTP (Lumiprobe), 50 μM CoCl2, and 1 U TdT polymerase (New England Biolabs), 1 x TdT reaction buffer were incubated in a 50 μl reaction at 37 °C for 4 h. Amino-11-ddUTP modified dsDNA was purified using QlAquick PCR purification kit (Qiagen) after stopping the reaction with 10 μl 0.2 M EDTA (PH = 8.0). The resulting product was then used to generate 3’ ddU-PEG10 modified dsDNA by incubation with Bis-PEG10-NHS ester at conditions described above.
Cell culture and transfection
HCT116, HEK293T, H1, WTC G3 cells were cultured as described on ATCC website. Cell transfection was performed using the Amaxa™ Nucleofector™ II device (Lonza): Cell Line Nucleofector™ kit V and Program D-032 were used for HCT116 and HEK293T, Human Stem Cell Nucleofector® Kit 2 and Program B-016 were used for H1 and WTC G3. The cells were expanded to 70–90% confluence, and then dissociated using TypLE™ (ThermoFisher Scientific) or Accutase™ (StemCell Technologies) and washed twice with PBS. Cas9 and gRNA were incubated in 10 μL electroporation solution for 15 min, and then added to 0.5 million cancer cells or 1.5 million stem cells resuspended in 90 μL electroporation solution. The donor templates were added into the tubes before the electroporation. The components were added in the amount specified for each experiment.
Flow cytometry analysis, cell sorting and KI clone isolation
For each experiment, 5,000–50,000 cells were analyzed using a BD LSR II or a BD LSR Fortessa flow cytometer (BD biosciences). For cell sorting, cells inside the defined gates were sorted using BD FACS ARIA II (BD biosciences). KI clone isolation was performed either by direct sorting of EGFP positive single cells onto a 96-well plate or use of micropipette tips to pick single colonies from a 10 cm plate seeded with 1000 EGFP positive cells16.
Genomic PCR and DNA Sequencing
Genomic DNA from cell pellets was extracted using QuickExtract DNA solution 1.0 (Epicenter). Genomic PCR was performed using Q5 High-Fidelity DNA polymerase (New England BioLabs) with primers listed in the Supplementary Table 5. The PCR products were subjected to direct DNA sequencing service (ACGT, Wheeling, IL).
TIDE analysis
Genomic DNA from cell pellets was extracted using QuickExtract DNA Extraction Solution 1.0 (Epicentre). Amplification of the junction regions surrounding gene KI sites in edited cells was carried out by PCR using Q5 High-Fidelity DNA polymerase (New England BioLabs) and 100 ng genomic DNA as template in a 50 μL reaction. PCR products were purified using QlAquick PCR purification kit (Qiagen) and then subjected to direct DNA sequencing service (ACGT, Wheeling, IL). The indel rates were analyzed by the online software12 (http://tide.nki.nl) using sequence without indels as reference.
Droplet Digital PCR (ddPCR)
ddPCR17 was used to determine the copy number of target gene integration using ACTB gene as a reference. Primers specific for reference ACTB gene and the EGFP sequence in the insert were used and primer sequences are listed in Supplement Table 5. Isolated KI clones were expanded in 24-well plates and cells were harvested at 90% confluency. Genomic DNA was purified by Quick-DNA microprep kit (Zymo Research), and then digested overnight with EcoRI (New England Biolabs). ddPCR mixture was prepared in PCR tubes by adding 10.5 μL 2 × EvaGreen Supermix (Bio-Rad), 1.05 μL 4 μM forward and reverse primers, 1 μL digested genomic DNA (12.5 ng/μL), and 7.4 μL ddH2O. For each ddPCR reaction, around 15,000 nanoliter-sized water emulsion droplets were generated using a QX200 Automated Droplet Generator with DG8 Cartridges (186–4007, Bio-Rad) according to the manufacturer’s instructions. The resulting droplet mixture was transferred to a 96-well PCR plate and gene amplification was performed using the following program: 95 °C for 5 min, 40 cycles of 95 °C for 30 s and 59.1 °C for 60 s, 4 °C for 5 min, 90 °C for 5 min and then cooling down to 4° C. The amplified products were analyzed using QX200 droplet reader (Bio-Rad) and the concentration of target genes was analyzed using QuantaSoft™ software (Bio-Rad, version 1.7.4.019). The copy number of the gene integration was calculated based on the ratio of the EGFP copy number to the ACTB copy number.
Supplementary Material
Acknowledgements
This work was supported by U.S. National Institutes of Health (1UM1HG009402 and 1U54DK107965) (H.Z.). The authors thank B. Pilas and B. Balhan (Flow Cytometry Facility, Biotechnology Center, University of Illinois at Urbana Champaign, Urbana, IL 61801, USA) for cell sorting experiments. We thank S. Long and K. Sobanska for help with ddPCR experiments. We thank W. Tang, T. Si, J. Lian, C. Field and S. Tsai for helpful suggestions for manuscript writing and experimental designs.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Data availability. The data that support the findings of this study are available from the corresponding author upon request.
References
- 1.Ran FA et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ran FA et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doudna JA et al. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Leonetti MD et al. A scalable strategy for high-throughput GFP tagging of endogenous human proteins. Proc Natl Acad Sci USA 113, 3501–3508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diao YR et al. A tiling-deletion-based genetic screen for cis-regulatory element identification in mammalian cells. Nat Methods 14, 629–633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens CJ et al. Targeted in vivo knock-in of human alpha-1-antitrypsin cDNA using adenoviral delivery of CRISPR/Cas9. Gene Ther 25, 139–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merkle FT et al. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell Rep 11, 875–883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renaud JB et al. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell Rep 14, 2263–2272 (2016). [DOI] [PubMed] [Google Scholar]
- 9.He X et al. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res 44, e85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paix A et al. Precision genome editing using synthesis-dependent repair of Cas9-induced DNA breaks. Proc Natl Acad Sci USA 114, 10745–10754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasan I et al. CRISPR/Cas9-mediated knock-in of an optimized TetO repeat for live cell imaging of endogenous loci. Nucleic Acids Res, 46, e100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkman EK et al. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42, e168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez-Triana JA et al. Efficient single-copy HDR by 5’ modified long dsDNA donors. eLife 7, e39468 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeWitt MA et al. Genome editing via delivery of Cas9 ribonucleoprotein. Methods 121–122, 9–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur JK et al. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat Biotechnol 34, 807–808 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Strukov YG et al. Development of mammalian cell lines with lac operator-tagged chromosomes. Cold Spring Harbor Protoc 2008; doi: 10.1101/pdb.prot4903 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Hindson BJ et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83, 8604–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.